Venetoclax Initiation in Chronic Lymphocytic Leukemia: International Insights and Innovative Approaches for Optimal Patient Care
Abstract
:Simple Summary
Abstract
1. Introduction
2. Clinical Trials and Real-World Studies of Venetoclax in Patients with CLL
2.1. Efficacy
2.2. Safety
2.3. Risk of Tumor Lysis Syndrome
2.4. On-Label Venetoclax Initiation
3. International Insights and Innovative Approaches Illustrated by Hypothetical Patient Scenarios
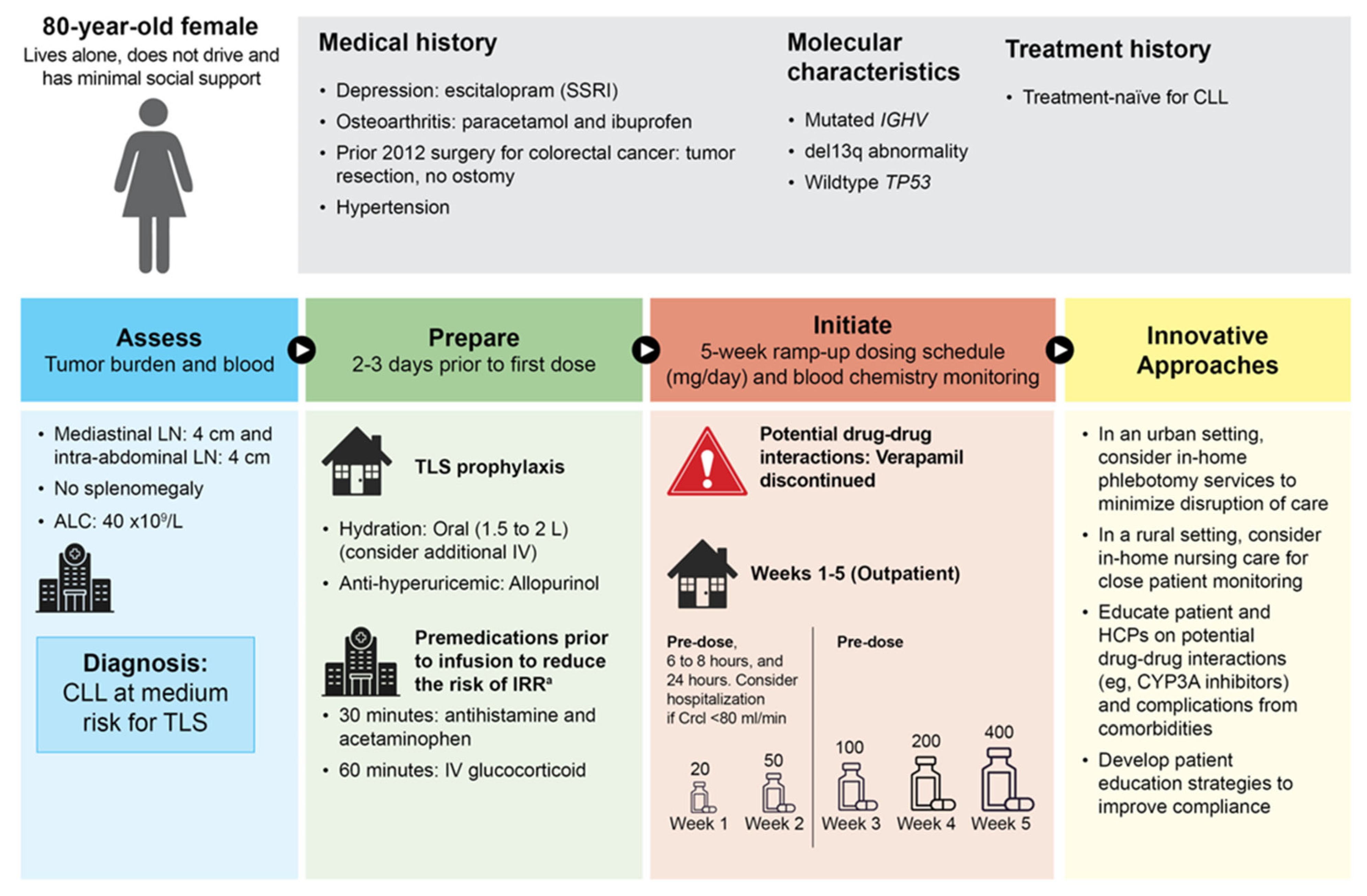
3.1. Patient Case 1—High Risk for TLS and Renal Failure
3.2. Patient Case 2—Medium Risk for TLS with Potential Drug-to-Drug Interactions and Infusion Reactions
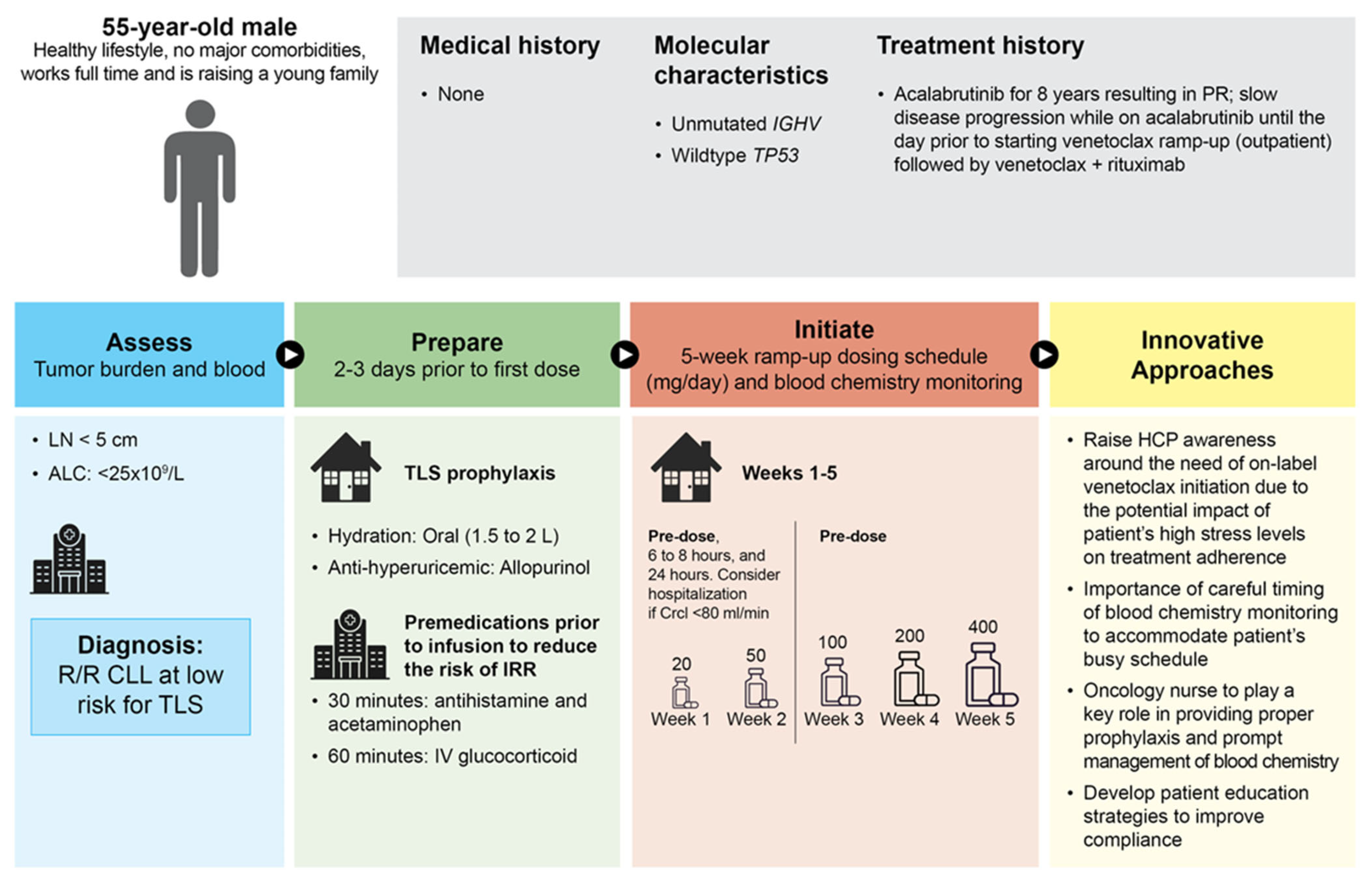
3.3. Patient Case 3—Low Risk for TLS and Busy Working Patient
4. Patient Journey
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Salvaris, R.; Opat, S. An update of venetoclax and obinutuzumab in chronic lymphocytic leukemia. Future Oncol. 2021, 17, 371–387. [Google Scholar] [CrossRef]
- Anderson, M.A.; Deng, J.; Seymour, J.F.; Tam, C.; Kim, S.Y.; Fein, J.; Yu, L.; Brown, J.R.; Westerman, D.; Si, E.G.; et al. The BCL2 selective inhibitor venetoclax induces rapid onset apoptosis of CLL cells in patients via a TP53-independent mechanism. Blood 2016, 127, 3215–3224. [Google Scholar] [CrossRef]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef]
- Borg, M.A.; Clemmons, A. Venetoclax: A Novel Treatment for Patients with del(17p) Chronic Lymphocytic Leukemia. J. Adv. Pract. Oncol. 2017, 8, 647–652. [Google Scholar]
- Stilgenbauer, S.; Eichhorst, B.; Schetelig, J.; Hillmen, P.; Seymour, J.F.; Coutre, S.; Jurczak, W.; Mulligan, S.P.; Schuh, A.; Assouline, S.; et al. Venetoclax for Patients With Chronic Lymphocytic Leukemia With 17p Deletion: Results From the Full Population of a Phase II Pivotal Trial. J. Clin. Oncol. 2018, 36, 1973–1980. [Google Scholar] [CrossRef]
- Stilgenbauer, S.; Eichhorst, B.; Schetelig, J.; Coutre, S.; Seymour, J.F.; Munir, T.; Puvvada, S.D.; Wendtner, C.M.; Roberts, A.W.; Jurczak, W.; et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: A multicentre, open-label, phase 2 study. Lancet Oncol. 2016, 17, 768–778. [Google Scholar] [CrossRef]
- VENCLEXTA (venetoclax tablets). Prescribing Information 2022; AbbVie, Inc.: North Chicago, IL, USA, 2022. [Google Scholar]
- VENCLYXTO (venetoclax tablets). Summary of Product Characteristics 2021; AbbVie Deutschland GmbH & Co.: Ludwigshafen, Germany, 2021. [Google Scholar]
- Kater, A.P.; Owen, C.; Moreno, C.; Follows, G.; Muir, M.; Levin, M.D.; Benjamini, O.; Janssens, A.; Osterborg, A.; Roabk, T.; et al. Fixed-duration ibrutinib-venetoclax in patients with chronic lymphocytic leukemia and comorbidities. N. Engl. J. Med. Evid. 2022, 1, EVIDoa2200006. [Google Scholar] [CrossRef]
- Wierda, W.G.; Tambaro, F.P. How I manage CLL with venetoclax-based treatments. Blood 2020, 135, 1421–1427. [Google Scholar] [CrossRef]
- Brem, E.A.; O’Brien, S. Is a BTKi or BCL2i preferable for first “novel” therapy in CLL? The case for BTKis. Blood Adv. 2022, 6, 1361–1364. [Google Scholar] [CrossRef]
- Seymour, J.F. Is BTKi or BCL2i preferable as first novel therapy in patients with CLL? The case for BCL2i. Blood Adv. 2022, 6, 1365–1370. [Google Scholar] [CrossRef]
- Seymour, J.F.; Gribben, J.G.; Davids, M.S.; Mato, A.R.; Sharman, J.P.; Cyr, A.; Bartkus, C.; Pena, G.E.; Boyer, M.; Sharmokh, S.; et al. Assessment of Tumor Lysis Syndrome in Patients with Chronic Lymphocytic Leukemia Treated with Venetoclax in the Clinical Trial and Post-Marketing Settings. Blood 2020, 136, 37–38. [Google Scholar] [CrossRef]
- Sharman, J.P.; Biondo, J.M.L.; Boyer, M.; Fischer, K.; Hallek, M.; Jiang, D.; Kater, A.P.; Porro Lura, M.; Wierda, W.G. A review of the incidence of tumor lysis syndrome in patients with chronic lymphocytic leukemia treated with venetoclax and debulking strategies. EJHaem 2022, 3, 492–506. [Google Scholar] [CrossRef]
- Deeks, E.D. Venetoclax: First Global Approval. Drugs 2016, 76, 979–987. [Google Scholar] [CrossRef]
- Seymour, J.F.; Kipps, T.J.; Eichhorst, B.; Hillmen, P.; D’Rozario, J.; Assouline, S.; Owen, C.; Gerecitano, J.; Robak, T.; De la Serna, J.; et al. Venetoclax-Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2018, 378, 1107–1120. [Google Scholar] [CrossRef]
- Seymour, J.F.; Kipps, T.J.; Eichhorst, B.F.; D’Rozario, J.; Owen, C.J.; Assouline, S.; Lamanna, N.; Robak, T.; de la Serna, J.; Jaeger, U.; et al. Enduring undetectable MRD and updated outcomes in relapsed/refractory CLL after fixed-duration venetoclax-rituximab. Blood 2022, 140, 839–850. [Google Scholar] [CrossRef]
- Sobon, A.; Drozd-Sokolowska, J.; Paszkiewicz-Kozik, E.; Poplawska, L.; Morawska, M.; Tryc-Szponder, J.; Bolkun, L.; Rybka, J.; Pruszczyk, K.; Juda, A.; et al. Clinical efficacy and tolerability of venetoclax plus rituximab in patients with relapsed or refractory chronic lymphocytic leukemia-a real-world analysis of the Polish Adult Leukemia Study Group. Ann. Hematol. 2023, 102, 2119–2126. [Google Scholar] [CrossRef]
- Fischer, K.; Al-Sawaf, O.; Bahlo, J.; Fink, A.M.; Tandon, M.; Dixon, M.; Robrecht, S.; Warburton, S.; Humphrey, K.; Samoylova, O.; et al. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2019, 380, 2225–2236. [Google Scholar] [CrossRef]
- Al-Sawaf, O.; Zhang, C.; Robrecht, S.; Kotak, A.; Chang, N.; Fink, A.M.; Tausch, E.; Schneider, C.; Ritgen, M.; Kreuzer, K.; et al. Venetoclax-obinutuzumab for previously untreated chronic lymphocytic leukemia: 5-year results of the randomized CLL14 study. HemaSphere 2022, 41, 49–50. [Google Scholar] [CrossRef]
- Al-Sawaf, O.; Zhang, C.; Lu, T.; Liao, M.Z.; Panchal, A.; Robrecht, S.; Ching, T.; Tandon, M.; Fink, A.M.; Tausch, E.; et al. Minimal Residual Disease Dynamics after Venetoclax-Obinutuzumab Treatment: Extended Off-Treatment Follow-up From the Randomized CLL14 Study. J. Clin. Oncol. 2021, 39, 4049–4060. [Google Scholar] [CrossRef]
- IMBRUVICA (ibrutinib). Prescribing Information 2023; Pharmacyclics LLC: South San Francisco, CA, USA; Janssen Biotech, Inc.: Horsham, PA, USA, 2023. [Google Scholar]
- Tam, C.S.; Allan, J.N.; Siddiqi, T.; Kipps, T.J.; Jacobs, R.; Opat, S.; Barr, P.M.; Tedeschi, A.; Trentin, L.; Bannerji, R.; et al. Fixed-duration ibrutinib plus venetoclax for first-line treatment of CLL: Primary analysis of the CAPTIVATE FD cohort. Blood 2022, 139, 3278–3289. [Google Scholar] [CrossRef]
- Eichhorst, B.; Niemann, C.U.; Kater, A.P.; Furstenau, M.; von Tresckow, J.; Zhang, C.; Robrecht, S.; Gregor, M.; Juliusson, G.; Thornton, P.; et al. First-Line Venetoclax Combinations in Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2023, 388, 1739–1754. [Google Scholar] [CrossRef]
- Figueroa-Mora, R.; Rampotas, A.; Halperin, D.; Worth, T.; Vidler, J.; Melotti, D.; Ferguson, P.; Elmusharaf, N.; Preston, G.; Furtado, M.; et al. Venetoclax ramp-up strategies for chronic lymphocytic leukaemia in the United Kingdom: A real world multicentre retrospective study. Br. J. Haematol. 2023, 202, 48–53. [Google Scholar] [CrossRef]
- Herishanu, Y.; Goldschmidt, N.; Itchaki, G.; Levi, I.; Aviv, A.; Fineman, R.; Dally, N.; Tadmor, T.; Ruchlemer, R.; Abadi, U.; et al. World Efficacy of Venetoclax-Based Regimens in Patients with Chronic Lymphocytic Leukemia in Israel: A Multicenter Prospective Study. Blood 2021, 138, 3727. [Google Scholar] [CrossRef]
- Mato, A.R.; Roeker, L.E.; Eyre, T.A.; Nabhan, C.; Lamanna, N.; Hill, B.T.; Brander, D.M.; Barr, P.M.; Lansigan, F.; Cheson, B.D.; et al. A retrospective comparison of venetoclax alone or in combination with an anti-CD20 monoclonal antibody in R/R CLL. Blood Adv. 2019, 3, 1568–1573. [Google Scholar] [CrossRef]
- Ysebaert, L.; Troussard, X.L.V.; Le Calloch, R.; Guieze, R.; Laribi, K.; Lepretre, S.; Michallet, A.S.; Leblond, V.; Feugier, P.; Lahjibi, E.; et al. Real-World Efficacy and Safety of Venetoclax Alone and in Combination with Rituximab in Patients with Chronic Lymphocytic Leukemia: Interim Results of the Verone Study. Blood 2022, 140, 12395–12396. [Google Scholar] [CrossRef]
- Roeker, L.E.; Fox, C.P.; Eyre, T.A.; Brander, D.M.; Allan, J.N.; Schuster, S.J.; Nabhan, C.; Hill, B.T.; Shah, N.N.; Lansigan, F.; et al. Tumor Lysis, Adverse Events, and Dose Adjustments in 297 Venetoclax-Treated CLL Patients in Routine Clinical Practice. Clin. Cancer Res. 2019, 25, 4264–4270. [Google Scholar] [CrossRef]
- Zakeri, M.; Emechebe, N.; Chowdhery, R.; Jawaid, D.; Alhasani, H.; Manzoor, B.S. Real-World Effectiveness and Treatment Patterns of Venetoclax-Based Regimens Among Patients with Chronic Lymphocytic Leukemia (CLL) Treated in Community Settings. Value Health 2023, 28, S54–S55. [Google Scholar] [CrossRef]
- Davids, M.S.; Hallek, M.; Wierda, W.; Roberts, A.W.; Stilgenbauer, S.; Jones, J.A.; Gerecitano, J.F.; Kim, S.Y.; Potluri, J.; Busman, T.; et al. Comprehensive Safety Analysis of Venetoclax Monotherapy for Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia. Clin. Cancer Res. 2018, 24, 4371–4379. [Google Scholar] [CrossRef]
- Mato, A.R.; Sharman, J.P.; Biondo, J.M.L.; Wu, M.; Mun, Y.; Kim, S.Y.; Humphrey, K.; Boyer, M.; Zhu, Q.; Seymour, J.F. The impact of early discontinuation/dose modification of venetoclax on outcomes in patients with relapsed/refractory chronic lymphocytic leukemia: Post-hoc analyses from the phase III MURANO study. Haematologica 2022, 107, 134–142. [Google Scholar] [CrossRef]
- Yang, Y.; Shu, Y.; Chen, G.; Yin, Y.; Li, F.; Li, J. A real-world pharmacovigilance study of FDA Adverse Event Reporting System (FAERS) events for venetoclax. PLoS ONE 2022, 17, e0278725. [Google Scholar] [CrossRef]
- Initiating CLL/SLL Patients on Venclexta. Available online: https://www.venclextahcp.com/content/dam/gene/venclextahcp/cll/pdfs/VENCLEXTA-Treatment-Guide.pdf (accessed on 29 August 2023).
- Cartron, G.; de Guibert, S.; Dilhuydy, M.S.; Morschhauser, F.; Leblond, V.; Dupuis, J.; Mahe, B.; Bouabdallah, R.; Lei, G.; Wenger, M.; et al. Obinutuzumab (GA101) in relapsed/refractory chronic lymphocytic leukemia: Final data from the phase 1/2 GAUGUIN study. Blood 2014, 124, 2196–2202. [Google Scholar] [CrossRef]
- Goede, V.; Fischer, K.; Busch, R.; Engelke, A.; Eichhorst, B.; Wendtner, C.M.; Chagorova, T.; de la Serna, J.; Dilhuydy, M.S.; Illmer, T.; et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N. Engl. J. Med. 2014, 370, 1101–1110. [Google Scholar] [CrossRef]
- Kater, A.P.; Kersting, S.; van Norden, Y.; Dubois, J.; Dobber, J.A.; Mellink, C.H.; Evers, L.M.; Croon-de Boer, F.; Schreurs, J.; van der Spek, E.; et al. Obinutuzumab pretreatment abrogates tumor lysis risk while maintaining undetectable MRD for venetoclax + obinutuzumab in CLL. Blood Adv 2018, 2, 3566–3571. [Google Scholar] [CrossRef]
- Kersting, S.; Dubois, J.; Nasserinejad, K.; Dobber, J.A.; Mellink, C.; van der Kevie-Kersemaekers, A.F.; Evers, L.M.; de Boer, F.; Koene, H.R.; Schreurs, J.; et al. Venetoclax consolidation after fixed-duration venetoclax plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (HOVON 139/GiVe): Primary endpoint analysis of a multicentre, open-label, randomised, parallel-group, phase 2 trial. Lancet Haematol. 2022, 9, e190–e199. [Google Scholar] [CrossRef]
- Roberts, A.W.; Davids, M.S.; Pagel, J.M.; Kahl, B.S.; Puvvada, S.D.; Gerecitano, J.F.; Kipps, T.J.; Anderson, M.A.; Brown, J.R.; Gressick, L.; et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016, 374, 311–322. [Google Scholar] [CrossRef]
- Waggoner, M.; Katsetos, J.; Thomas, E.; Galinsky, I.; Fox, H. Practical Management of the Venetoclax-Treated Patient in Chronic Lymphocytic Leukemia and Acute Myeloid Leukemia. J. Adv. Pract. Oncol. 2022, 13, 400–415. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. Version 3. 2023. Available online: https://www.nccn.org/guidelines/category_1 (accessed on 13 February 2024).
- Sharman, J.P.; Andorsky, D.; Melear, J.M.; Manda, S.; Anz, B.M.; Kolibaba, K.S.; Yimer, H.A.; Burke, J.M.; Fanning, S.R.; Courtright, J.; et al. Debulking Eliminates Need for Hospitalization Prior to Initiating Frontline Venetoclax Therapy in Previously Untreated CLL Patients: A Phase 3b Study. Blood 2019, 134, 3042. [Google Scholar] [CrossRef]
- Chohan, S. Safety and efficacy of febuxostat treatment in subjects with gout and severe allopurinol adverse reactions. J. Rheumatol. 2011, 38, 1957–1959. [Google Scholar] [CrossRef]
- Cheah, C.Y.; Lew, T.E.; Seymour, J.F.; Burbury, K. Rasburicase causing severe oxidative hemolysis and methemoglobinemia in a patient with previously unrecognized glucose-6-phosphate dehydrogenase deficiency. Acta Haematol. 2013, 130, 254–259. [Google Scholar] [CrossRef]
- Tosi, P.; Barosi, G.; Lazzaro, C.; Liso, V.; Marchetti, M.; Morra, E.; Pession, A.; Rosti, G.; Santoro, A.; Zinzani, P.L.; et al. Consensus conference on the management of tumor lysis syndrome. Haematologica 2008, 93, 1877–1885. [Google Scholar] [CrossRef]
- Baliakas, P.; Jeromin, S.; Iskas, M.; Puiggros, A.; Plevova, K.; Nguyen-Khac, F.; Davis, Z.; Rigolin, G.M.; Visentin, A.; Xochelli, A.; et al. Cytogenetic complexity in chronic lymphocytic leukemia: Definitions, associations, and clinical impact. Blood 2019, 133, 1205–1216. [Google Scholar] [CrossRef]
- Hampel, P.J.; Parikh, S.A. Chronic lymphocytic leukemia treatment algorithm 2022. Blood Cancer J. 2022, 12, 161. [Google Scholar] [CrossRef]
- Cozad, M.; Stump, S.E.; Buhlinger, K.; Collins, J.t.; Muir, M.; Coombs, C.C.; Muluneh, B. Evaluation of an interdisciplinary venetoclax initiation process in minimizing risk of tumor lysis syndrome. Leuk. Lymphoma 2022, 63, 1831–1838. [Google Scholar] [CrossRef]
- Gribben, J.G. Practical management of tumour lysis syndrome in venetoclax-treated patients with chronic lymphocytic leukaemia. Br. J. Haematol. 2020, 188, 844–851. [Google Scholar] [CrossRef]
- Selby, P.; Popescu, R.; Lawler, M.; Butcher, H.; Costa, A. The Value and Future Developments of Multidisciplinary Team Cancer Care. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 332–340. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Shared decision making. NICE Guideline (NG197). June 2021. Available online: https://www.nice.org.uk/guidance/ng197 (accessed on 13 February 2024).
- Molica, S. Venetoclax: A real game changer in treatment of chronic lymphocytic leukemia. Int. J. Hematol. Oncol. 2020, 9, IJH31. [Google Scholar] [CrossRef]
- Josfeld, L.; Keinki, C.; Pammer, C.; Zomorodbakhsch, B.; Hubner, J. Cancer patients’ perspective on shared decision-making and decision aids in oncology. J. Cancer Res. Clin. Oncol. 2021, 147, 1725–1732. [Google Scholar] [CrossRef]
- Veenstra, C.M.; Hawley, S.T. Incorporating patient preferences into cancer care decisions: Challenges and opportunities. Cancer 2020, 126, 3393–3396. [Google Scholar] [CrossRef]


| Trial | Phase | N | Patients | Regimen a | Efficacy b | Most Common Grade ≥ 3 AEs | Grade ≥ 3 TLS c |
|---|---|---|---|---|---|---|---|
| NCT01889186 [6] (M13-982) | 2 | 107 | R/R CLL with del(17p) | PO Ven step-up 20–400 mg qd over 4–5 wk; then PO Ven 400 mg qd to PD or D/C | ORR 79.4% CR/CRi 7.5% 18/45 (40%) uMRD 12-mo PFS 72.0% | Neutropenia 40.2% Anemia 17.8% Thrombocytopenia 15.0% | 4.7% d |
| NCT02005471 [16,17] (MURANO) | 3 | 194 (Ven + R) | R/R CLL with 1–3 prior therapies | PO Ven step-up 20–400 mg qd over 5 wk; then IV R (375 mg/m2 C1D1, then 500 mg/m2 D1 C2–6) and 400 mg PO Ven for 2 yr, PD or unacceptable toxicity | ORR 92.3% CR/Cri 26.8% e 83.5% uMRD f mPFS 53.6 mo 5-yr OS 82.1% | Neutropenia 57.7% Infections/infestations 17.5% Anemia 10.8% | 3.1% d < 1% fg [16] |
| 195 (BR) | IV B 70 mg/m2 D1, D2 for 6 cycles, and IV R (375 mg/m2 C1D1, then 500 mg/m2 D1 C2–6) | ORR 72.3% CR/Cri 8.2% e 23.1% uMRD f mPFS 17.0 mo 5-yr OS 62.2% | Neutropenia 38.8% Infections/infestation 21.8% Anemia 13.8% | 1.1% d < 1% fg [16] | |||
| NCT02242942 [19,20,21] (CLL14) | 3 | 216 (Ven + Obi) | Previously untreated CLL with CIRS ≥ 6 | Obi IV D1 (100 mg C1D1, 900 mg C1D2, 1000 mg C1D8 and C1D15, then 1000 mg D1 of C2–6), and PO Ven on C1D22, 20–400 mg 5-week ramp-up, then 400 mg daily through C12 | ORR 84.7% CR 49.5% mPFS NR 5-yr PFS 62.6% 5-yr OS 81.9% 4-yr MRD 18.1% | Neutropenia 52.8% Thrombocytopenia 13.7% IRR 9.0% | 1.4% d,h,i [19] |
| 216 (Clb + Obi) | Clb 0.5 mg/kg D1 and D15 of C1–12, and PO Ven on C1D22, 20–400 mg 5-week ramp-up, then 400 mg daily through C12 | ORR 71.3% CR 23.1% mPFS 36.4 mo 5-yr PFS 27.0% 5-yr OS 77.0% 4-yr MRD 1.9% | Neutropenia 48.1% Thrombocytopenia 15.0% IRR 10.3% | 2.3% d,i [19] | |||
| NCT03462719 [9] (GLOW) | 3 | 106 (Ibr + Ven) | Previously untreated CLL in older patients and/or those with comorbidities | 3 cycles of Ibr lead-in at 420 mg once daily followed by 12 cycles of Ibr + Ven then Ven on C4, 20–400 mg 5-week ramp-up, then 400 mg daily on C5 onward | PFS HR 0.216; p < 0.001 f 24-mo PFS rate 84.4% 30-mo PFS rate 80.5% 55.7% best uMRD j 84.5% sustained uMRD k CR/CRi 38.7% | Neutropenia 34.9% Infections and infestations 17.0% Thrombocytopenia 5.7% | 0 |
| 105 (Clb + Obi) | Obi IV 1000 mg C1D1 (or 100 mg D1 and 900 mg D2), C2D8, and C1D15 and D2 of C2-C6 + Clb 0.5mg/kg on D1 and D15 and 15 of each cycle | 24-mo PFS rate 44.1% 30-mo PFS rate 35.8% 21.0% best uMRD j 29.3% sustained uMRD j CR/CRi 11.4% e | Neutropenia 49.5% Infections and infestations 10.5% Thrombocytopenia 20.0% | 5.7% | |||
| NCT02910583 [23] (CAPTIVATE) | 2 | 159 | Previously untreated CLL | 3 cycles of Ibr lead-in then 12 cycles of Ibr plus Ven (oral ibrutinib [420 mg/d]; oral venetoclax [5-week ramp-up to 400 mg/d]). | CR 55% uMRD 77% 2-year PFS 95% 2-year OS 98% | Neutropenia 33% Hypertension 6% | 0 |
| Reference | N | Countries | Patients | Regimen | Efficacy | Most Common Grade ≥ 3 AEs | TLS a |
|---|---|---|---|---|---|---|---|
| Mato et al. [27] | 270 | US, UK | R/R CLL | Ven | ORR 81% CR 34% mPFS NR b | Neutropenia 40.4% Thrombocytopenia 30.8% Neutropenic fever 8.6% | 11.5% |
| 51 | Ven + R or Obi | ORR 84% CR 32% mPFS NR b | Neutropenia 34% Thrombocytopenia 23% Neutropenic fever 2.3% | 5.8% | |||
| Roeker et al. [29] | 297 | US, UK | CLL (96% R/R) | Ven or Ven combo | N/A | Neutropenia 39.6% Thrombocytopenia 29.2% Febrile neutropenia 7.9% | 2.7% c 5% d |
| Zakeri et al. [30] | 254 | US | 1L or 2L CLL | Ven-based | mTTNT-D NR e (Ven-Obi, Ven-R); mTTNT-D 13.5 mo d (Ven) | N/A | N/A |
| Herishanu et al. [26] | 83 | Israel | TN CLL | Ven-Obi or Ven-R | ORR 89.5% CR 68.4% uMRD: 12/14 (85.7%) 12-mo PFS 90.9% | Neutropenia 19.2% Infections 4.8% Febrile neutropenia 2.4% | 1.2% c |
| 116 | R/R CLL | Ven-based | ORR 67.6% (BCRi-exposed); ORR 85.7% (BCRi-naïve) uMRD: 26/38 (68.4%) 12-mo PFS 81.1% | Neutropenia 17.2% Infections 21.5% Febrile neutropenia 2.6% | 3.4% c 2.6% d | ||
| Ysebaert et al. [28] | 121 | France | CLL | Ven | BORR (2 yr) 91.7% 2-yr PFS 71.7% 2-yr OS 79.6% | N/A | 8.8% |
| 70 | CLL | Ven + R | BORR (2 yr) 94.3% 2-yr PFS 77.9% 2-yr OS 80.6% | N/A | 9.6% | ||
| Figueroa-Mora et al. [25] | 170 | UK | R/R CLL | Ven-based | ORR 85% CR/CRi 45.9% | N/A | 1.1% c 2.4% d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, M.A.; Walewska, R.; Hackett, F.; Kater, A.P.; Montegaard, J.; O’Brien, S.; Seymour, J.F.; Smith, M.; Stilgenbauer, S.; Whitechurch, A.; et al. Venetoclax Initiation in Chronic Lymphocytic Leukemia: International Insights and Innovative Approaches for Optimal Patient Care. Cancers 2024, 16, 980. https://doi.org/10.3390/cancers16050980
Anderson MA, Walewska R, Hackett F, Kater AP, Montegaard J, O’Brien S, Seymour JF, Smith M, Stilgenbauer S, Whitechurch A, et al. Venetoclax Initiation in Chronic Lymphocytic Leukemia: International Insights and Innovative Approaches for Optimal Patient Care. Cancers. 2024; 16(5):980. https://doi.org/10.3390/cancers16050980
Chicago/Turabian StyleAnderson, Mary Ann, Renata Walewska, Fidelma Hackett, Arnon P. Kater, Josie Montegaard, Susan O’Brien, John F. Seymour, Matthew Smith, Stephan Stilgenbauer, Ashley Whitechurch, and et al. 2024. "Venetoclax Initiation in Chronic Lymphocytic Leukemia: International Insights and Innovative Approaches for Optimal Patient Care" Cancers 16, no. 5: 980. https://doi.org/10.3390/cancers16050980
APA StyleAnderson, M. A., Walewska, R., Hackett, F., Kater, A. P., Montegaard, J., O’Brien, S., Seymour, J. F., Smith, M., Stilgenbauer, S., Whitechurch, A., & Brown, J. R. (2024). Venetoclax Initiation in Chronic Lymphocytic Leukemia: International Insights and Innovative Approaches for Optimal Patient Care. Cancers, 16(5), 980. https://doi.org/10.3390/cancers16050980





