Targeting STAT3 and NF-κB Signaling Pathways in Cancer Prevention and Treatment: The Role of Chalcones
Abstract
:Simple Summary
Abstract
1. Introduction
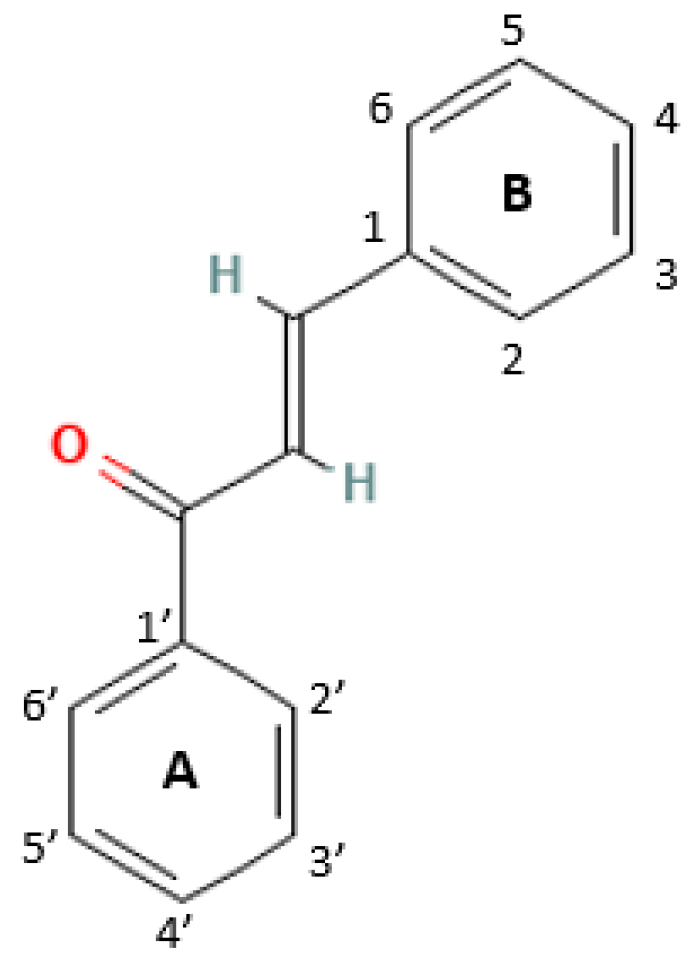
2. Structures and Mechanism of Action of Chalcones
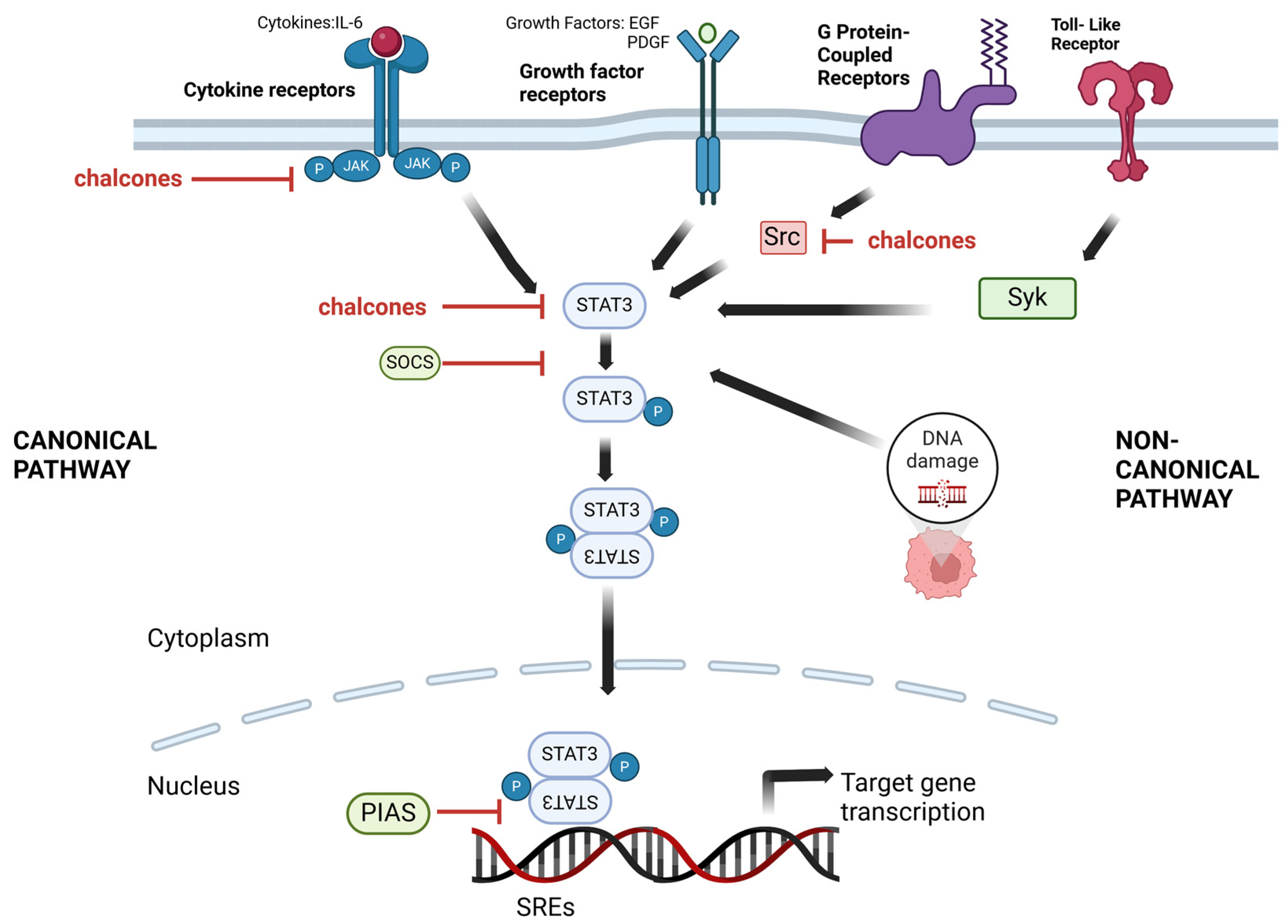
3. STAT and Chalcones
3.1. STAT Signaling Pathways as a Target
3.2. Chalcones as STAT Inhibitors
| Name of Chalcones | In Vitro/In Vivo Models | Effect on STAT3 | References |
|---|---|---|---|
| Butein Semecarpus anacardium | liver cancer cell line (HepG2) | ↓ phosphorylation of STAT3 ↓ nuclear translocation of STAT3 | [40] |
| multiple myeloma cell line (U266) | ↓ phosphorylation of STAT3 ↓ STAT3 DNA-binding activity | [61] | |
| lung cancer cell line (A549) breast cancer cell line (MDA-MB-231) | ↓ phosphorylation of STAT3 | [49] | |
| Cardamonin Alpinia rafflesiana | colon cancer cell lines (HT-29 and SW-460) | ↓ phosphorylation of STAT3 | [35] |
| mice C57BL/6 | |||
| ovarian cancer cell line (SKOV3), monocytes (THP-1) | ↓ phosphorylation of STAT3 | [62] | |
| glioblastoma stem cell line (CD133+) | ↓ phosphorylation of STAT3 ↓ expression of STAT3 ↓ nuclear translocation of STAT3 | [53] | |
| prostate cancer cell line (DU145) | ↓ phosphorylation of STAT3 ↓ STAT3 DNA-binding activity ↓ nuclear level of STAT3 | [58] | |
| Flavokawain B Piper methysticum | hepatocellular carcinoma cell line (HepG2) | ↓ mRNA level of STAT3 | [52] |
| Geranyl dihydrochalcone Artocarpus altilis | prostate cancer cell line (DU145) | ↓ phosphorylation of STAT3 | [63] |
| Isoliquiritigenin Glycyrrhiza glabra | breast cancer cell lines (Hs-578T and MDA-MB-231) | No changes in phosphorylation of STAT3 ↓ STAT3 DNA-binding activity ↑ PIAS3 level | [59] |
| multiple myeloma cell line (U266) | ↓ phosphorylation of STAT3 | [41] | |
| Caki renal carcinoma cell line | ↓ phosphorylation of STAT3 | [56] | |
| Licochalcone A Glycyrrhiza glabra | hematopoietic cell line (Ba/F3) | ↓ phosphorylation of STAT3 ↓ nuclear translocation of STAT3 | [64] |
| Licochalcone B Glycyrrhiza glabra | esophageal cancer cell lines (KYSE450 and KYSE510) | ↓ phosphorylation of STAT3 ↓ STAT3 DNA-binding activity | [55] |
| Licochalcone C Glycyrrhiza glabra | oral squamous cell carcinoma lines (HN22 and HSC4) | ↓ phosphorylation of STAT3 | [36] |
| Licochalcone D Glycyrrhiza glabra | oral squamous cell carcinoma lines (HN22 and HSC4) | ↓ phosphorylation of STAT3 | [37] |
| Licochalcone H | skin cancer cell lines (A375 and A431) | ↓ phosphorylation of STAT3 | [38] |
| oral squamous cell carcinoma cell lines (HN22 and HSC4) | ↓ phosphorylation of STAT3 | [65] | |
| Phloretin Manchurian apricot | hepatocellular carcinoma cell lines (HepG2, SK-Hep1, Hep3B2.1-7, Huh7 and PLC-5) | ↓ phosphorylation of STAT3 ↓ STAT3 activity | [39] |
| mice xenografts (HepG2SR and Huh7SR) | |||
| pancreatic cancer cell lines (PaTu-8988T and PANC-1) | ↓ phosphorylation of STAT3 | [66] | |
| Xanthohumol Humulus lupulus | breast cancer cell line (MCF-7) adriamycin (doxorubicin)-resistant breast cancer cell line (MCF-7/ADR) | ↓ expression of STAT3 | [50] |
| choliangiocarcinoma cell lines (M139 and M214) | ↓ expression of STAT3 | [51] | |
| mice xenografts (KKU-M214) | |||
| (E)-1–(1-hydroxy-4,5,8-trimethoxynaphthalen-2-yl)-3-(quinolin-6-yl) prop-2-en-1-one | gastric cancer cell line (MKN1) | ↓ phosphorylation of STAT3 | [43] |
| (E)-3-(7-(3,4-dimethoxyphenyl)-2- phenylpyrazolo[1,5-a]pyrimidin-5-yl)-1- (3,4,5-trimethoxyphenyl) prop-2-en-1-one | lung cancer cell line (A549) | ↓ phosphorylation of STAT3 | [45] |
| (E)-1-(2,4-dimethoxyphenyl)-3-(4-hydroxy-3,5-dimethoxyphenyl) prop-2-en-1-one | breast cancer cell line (MDA-MB-231) | ↓ phosphorylation of STAT3 | [46] |
| (E)-3-(4-bromo-3,5-dimethoxyphenyl)-1-(3-hydroxyphenyl) prop-2-en-1-one | melanoma cell lines (Sk-Mel-5 and Sk-Mel-28) | ↓ phosphorylation of STAT3 | [42] |
| N-(4-(3-(4-methoxyphenyl)acryloyl) phenyl)-2-((5-(3,4,5-trimethoxy- phenyl)-1,3,4-oxadiazol-2-yl)thio) acetamide | leukemia cell line (K-562) | ↓ STAT3 activity | [60] |
| 4,3′,4′,5′-tetramethoxychalcone | ovarian cancer cell lines (A2780 and SKOV3) | ↓ phosphorylation of STAT3 | [44] |
| ZE-2-(4-(4-chlorophenyl)-6-(4-nitrophenyl)pyrimidin-2-ylthio)- N-(4-(3-(3,4-dimethoxyphenyl) acryloyl)phenyl)acetamide | pancreatic cancer cell line (PANC-1) | ↓ phosphorylation of STAT3 | [47] |
| 3-(4-methylthiophene)-1-(3-bromo-4,5-dimethoxyphenyl)prop-2-en-1-on and 3-(3-methoxy-4-methylthiophenyl)-1- (3-bromo-5-methoxy-4- methylthiophen)prop-2-en-1-on | colorectal carcinoma cell lines (DLD-1 and HCT116) | ↓ phosphorylation of STAT3 ↓ nuclear levels of STAT3 ↓ binding of STAT3 to DNA | [48] |
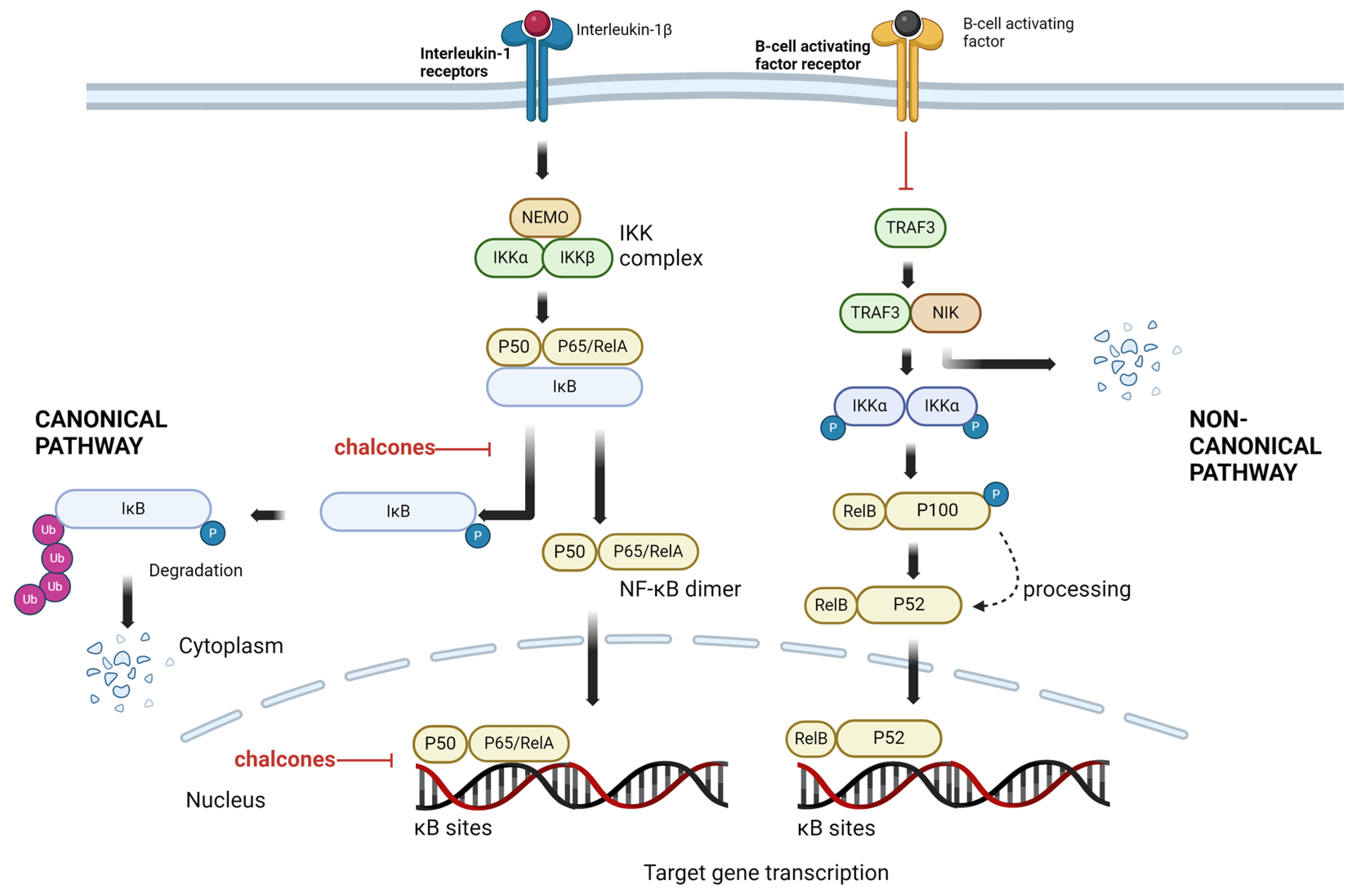
4. NF-κB and Chalcones
4.1. NF-κB Signaling Pathways as a Target
4.2. Chalcones as NF-κB Inhibitors
| Name of Chalcones | In Vitro/In Vivo Models | Effect on NF-κB | References |
|---|---|---|---|
| Butein Semecarpus anacardium | myelogenous leukemia cell line (KBM-5), multiple myeloma cell line (U266) | ↓ IKK activity ↓ phosphorylation and degradation of IκBα ↓ NF-κB p65 activity | [72] |
| oral squamous cell carcinoma line (KB) tongue squamous cell carcinoma (SAS) | ↓ NF-κB activity | [81] | |
| prostate cancer cell line (LNCaP) | ↓ level of NF-κB, IKKα ↓ phosphorylation and degradation of IκBα | [86] | |
| Cardamonin Alpinia rafflesiana | ovarian cancer cell line (SKOV3) | ↓ phosphorylation of NF-κB ↓ level of NF-κB, IKKα/β, IKKβ | [87] |
| mice BALB/c | |||
| hepatoblastoma cell line (HepG2) | ↓ phosphorylation of NF-κB p65 ↓ level of IKKβ | [82] | |
| ICR mice | |||
| nasopharyngeal carcinoma cell line (CNE-2) | ↓ nuclear level of NF-κB p65 ↓ phosphorylation of NF-κB p65 ↓ level of IKKα/β ↓ phosphorylation of IκBα | [73] | |
| colon cancer cell line (5-FU-resistant HCT116) | ↓ level of NF-κB p65 | [83] | |
| Isoliquiritigenin Glycyrrhiza glabra | hepatoblastoma cell line (HepG2) | ↓ nuclear level of NF-κB p65 ↑ level of IĸBα ↓ phosphorylation of IκBα ↓ NF-κB nuclear activity | [74] |
| Isoliquiritigenin 2′-methyl ether Caesalpinia sappan | oral squamous cell carcinoma cell lines (HN4 and HN12) | ↑ phosphorylation of IκBα ↑ degradation of IκBα ↑ NF-κB p65 nuclear activity | [88] |
| Licochalconce A Glycyrrhiza glabra | hepatocellular carcinoma cell line (SK-Hep-1) | ↓ nuclear level of NF-κB p65 ↓phosphorylation of IκBα | [75] |
| Licochalconce B Glycyrrhiza glabra | bladder carcinoma cell line (T24) | ↓ phosphorylation of NF-κB p65 ↓ nuclear level of NF-κB p65 ↓ phosphorylation of IκBα | [76] |
| Phloretin Manchurian apricot | ICR mice with skin carcinogenesis | ↓ DNA binding of NF-κB | [77] |
| lung epithelial cell line (A549) | ↓ NF-κB p65 translocation into the nucleus ↑nuclear level of NF-κB p65 ↓ phosphorylation and degradation of IκBα | [84] | |
| Xanthohumol Humulus lupulus | pancreatic cancer cell line (PANC-1) | ↓ mRNA level of NF-κB p65 and NF-κB p50 ↓ nuclear level of NF-κB p65 ↓ NF-κB p65 activity | [80] |
| pancreatic cancer cell lines (BxPC-3, MIA PaCa-2 and AsPC-1) | ↓ NF-κB p65 activity | [85] | |
| mice BALB/c | |||
| hepatoblastoma cell line (HepG2) | ↓ level of NF-κB | [89] | |
| α-2-bromo-N-(1-methyl-3-(3-oxo-3-(pyridin-4-yl)prop-1-en-1-yl)-1H-indol-5-yl)acrylamide | human melanoma cell line (SK-MEL-1) | ↓ phosphorylation of NF-κB p65 | [90] |
| 2-hydroxy-3′,5,5′-trimenthoxychalcone | breast cancer cell line (MDA-MB-231) | ↓ phosphorylation of NF-κB p65 ↓ phosphorylation of IKKα/β and IĸB ↓ nuclear level of NF-κB p65 ↓ NF-κB activity | [78] |
| 2′-hydroxy-4-methylsulfonylchalcone and 4′-chloro-2′-hydroxy-4- methylsulfonylchalcone | prostate cancer cell line (PC-3) | ↓ NF-κB nuclear activity | [91] |
| 2′,4′,6′-tris(methoxymethoxy) chalcone | pancreatic acinar cells from the C57BL/6 mice | ↓ degradation of IĸBα ↓ NF-κB activity | [79] |
| (2E,2′E)-1,1′-(5,5′-(piperazine-1,4-diylbis(methyl ene))bis(4-hydroxy-3-methoxy-5,1-phenylene))bis(3-ph enylprop-2-en-1-one) | nasopharyngeal carcinoma cell line (NPC-TW 039) | ↓ phosphorylation of NF-κB ↓ nuclear level of NF-κB p65 | [92] |
| 3-(4-methylthiophene)-1-(3-bromo-4,5-dimethoxyphenyl)prop-2-en-1-on and 3-(3-methoxy-4-methylthiophenyl)-1-(3-bromo-5-methoxy-4-methylthiophene)prop-2-en-1-on | colorectal carcinoma cell lines (DLD-1 and HCT116) | ↓ level of the nuclear level of NF-κB p50 and sNF-κB p65 ↓ transcript level of NF-κB p50 and NF-κB p65 | [48] |
5. Targeting the NF-κB and STATs/STAT3 Pathways by Chalcones and Their Other Combination
| Combination | In Vitro/In Vivo Models | Synergistic Effect | References |
|---|---|---|---|
| Butein + radiotherapy | gastric cancer cell line (MKN-45) | ↑ radiosensitivity | [103] |
| Butein + doxorubicin | liver cancer cell line (HepG2) | ↑ chemosensitivity | [40] |
| Butein + paclitaxel | |||
| Isoliquiritigenin + gemcitabine | pancreatic cancer cell lines (PANC1, MIA PaCa-2) | ↑ chemosensitivity | [98] |
| Isoliquiritigenin +5-fluorouracil | gastric cancer cell line (MKN45) gastric cancer mice xenografts | ↑ chemosensitivity | [99] |
| Licochalcone A + paclitaxel | squamous cell carcinoma cell line (SCC-15) | ↑ chemosensitivity | [105] |
| Phloretin + tamoxifen | breast cancer cell lines (MCF7, MDA-MB-231) | ↑ chemosensitivity | [100] |
| Phloretin + doxorubicin | |||
| Phloretin + radiotherapy | Lewis lung cancer cell line | ↑ radiosensitivity | [104] |
| C57BL/6J mice | |||
| Xanthohumol + radiotherapy | breast cancer cell line (MCF-7) adriamycin (doxorubicin)-resistant breast cancer cell line (MCF-7/ADR) | ↑ radiosensitivity | [50] |
| Xanthohumol + doxorubicin | ↑ chemosensitivity | [106] | |
| Xanthohumol + 7-ethyl-10-hydroxycamptothecin | colorectal cancer cell line (SW480) | ↑ chemosensitivity | [101] |
| Isoxanthohumol + paclitaxel | melanoma cell lines (B16 and A375) | ↑ chemosensitivity | [102] |
| C57BL/6 mice |
6. Targeting the Crosstalk between NF-κB and STAT3/STATs Pathways by Chalcones
7. Clinical Trials with Chalcones
8. Nanoformulations as a Future of Chalcones
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Singh, N.; Baby, D.; Rajguru, J.; Patil, P.; Thakkannavar, S.; Pujari, V. Inflammation and Cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Quispe, C.; Chamkhi, I.; El Omari, N.; Balahbib, A.; Sharifi-Rad, J.; Bouyahya, A.; Akram, M.; Iqbal, M.; Docea, A.O.; et al. Pharmacological Properties of Chalcones: A Review of Preclinical Including Molecular Mechanisms and Clinical Evidence. Front. Pharmacol. 2021, 11, 592654. [Google Scholar] [CrossRef]
- Michalkova, R.; Mirossay, L.; Gazdova, M.; Kello, M.; Mojzis, J. Molecular Mechanisms of Antiproliferative Effects of Natural Chalcones. Cancers 2021, 13, 2730. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Pasinetti, G.M. Flavonoids and Isoflavonoids: From Plant Biology to Agriculture and Neuroscience. Plant Physiol. 2010, 154, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, I.S.; Moon, A. 2-Hydroxychalcone and Xanthohumol Inhibit Invasion of Triple Negative Breast Cancer Cells. Chem. Biol. Interact. 2013, 203, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Mojzis, J.; Varinska, L.; Mojzisova, G.; Kostova, I.; Mirossay, L. Antiangiogenic Effects of Flavonoids and Chalcones. Pharmacol. Res. 2008, 57, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Kuo, P.L.; Tzeng, W.S.; Lin, C.C. Chalcone Inhibits the Proliferation of Human Breast Cancer Cell by Blocking Cell Cycle Progression and Inducing Apoptosis. Food Chem. Toxicol. 2006, 44, 704–713. [Google Scholar] [CrossRef]
- Tabata, K.; Motani, K.; Takayanagi, N.; Nishimura, R.; Asami, S.; Kimura, Y.; Ukiya, M.; Hasegawa, D.; Akihisa, T.; Suzuki, T. Xanthoangelol, a Major Chalcone Constituent of Angelica Keiskei, Induces Apoptosis in Neuroblastoma and Leukemia Cells. Biol. Pharm. Bull. 2005, 28, 1404–1407. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Kumar, A.; Kumar, M.; Sarkar, J.; Sinha, S. Synthesis and in Vitro Evaluation of Novel Coumarin-Chalcone Hybrids as Potential Anticancer Agents. Bioorg. Med. Chem. Lett. 2010, 20, 7205–7211. [Google Scholar] [CrossRef]
- Orlikova, B.; Tasdemir, D.; Golais, F.; Dicato, M.; Diederich, M. Dietary Chalcones with Chemopreventive and Chemotherapeutic Potential. Genes Nutr. 2011, 6, 125–147. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.d.F.; Pruccoli, L.; Morroni, F.; Sita, G.; Seghetti, F.; Viegas, C.; Tarozzi, A. The Keap1/Nrf2-ARE Pathway as a Pharmacological Target for Chalcones. Molecules 2018, 23, 1803. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Massiah, M.A.; Bozak, R.E.; Hicks, R.J.; Talalay, P. Potency of Michael Reaction Acceptors as Inducers of Enzymes That Protect against Carcinogenesis Depends on Their Reactivity with Sulfhydryl Groups. Proc. Natl. Acad. Sci. USA 2001, 98, 3404–3409. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Batista, A.d.J.; Philipon, C.I.M.S.; de Souza Albernaz, M.; Pinto, S.R.; Rossi-Bergmann, B.; Santos-Oliveira, R. New Chalcone Compound as a Promising Antileishmanial Drug for an Old Neglected Disease: Biological Evaluation Using Radiolabelled Biodistribution. J. Glob. Antimicrob. Resist. 2018, 13, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.U.; Chung, H.C.; Kim, C.; Hwang, J.K. Oral Intake of Boesenbergia Pandurata Extract Improves Skin Hydration, Gloss, and Wrinkling: A Randomized, Double-Blind, and Placebo-Controlled Study. J. Cosmet. Dermatol. 2017, 16, 512–519. [Google Scholar] [CrossRef]
- Santarsiero, A.; Pappalardo, I.; Rosa, G.M.; Pisano, I.; Superchi, S.; Convertini, P.; Todisco, S.; Scafato, P.; Infantino, V. Mitochondrial Role in Intrinsic Apoptosis Induced by a New Synthesized Chalcone in Hepatocellular Carcinoma Cells. Biomedicines 2022, 10, 3120. [Google Scholar] [CrossRef] [PubMed]
- Rozmer, Z.; Perjési, P. Naturally Occurring Chalcones and Their Biological Activities. Phytochem. Rev. 2016, 15, 87–120. [Google Scholar] [CrossRef]
- Vesaghhamedani, S.; Ebrahimzadeh, F.; Najafi, E.; Shabgah, O.G.; Askari, E.; Shabgah, A.G.; Mohammadi, H.; Jadidi-Niaragh, F.; Navashenaq, J.G. Xanthohumol: An Underestimated, While Potent and Promising Chemotherapeutic Agent in Cancer Treatment. Prog. Biophys. Mol. Biol. 2022, 172, 3–14. [Google Scholar] [CrossRef]
- Constantinescu, T.; Lungu, C.N. Anticancer Activity of Natural and Synthetic Chalcones. Int. J. Mol. Sci. 2021, 22, 11306. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, J.; Chen, X.; Fu, X.; Sun, S.; Wu, Q. Chalcone Derivatives: Role in Anticancer Therapy. Biomolecules 2021, 11, 894. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762. [Google Scholar] [CrossRef]
- Gomes, M.N.; Muratov, E.N.; Pereira, M.; Peixoto, J.C.; Rosseto, L.P.; Cravo, P.V.L.; Andrade, C.H.; Neves, B.J. Chalcone Derivatives: Promising Starting Points for Drug Design. Molecules 2017, 22, 1210. [Google Scholar] [CrossRef] [PubMed]
- Leite, F.F.; de Sousa, N.F.; de Oliveira, B.H.M.; Duarte, G.D.; Ferreira, M.D.L.; Scotti, M.T.; Filho, J.M.B.; Rodrigues, L.C.; de Moura, R.O.; Mendonça-Junior, F.J.B.; et al. Anticancer Activity of Chalcones and Its Derivatives: Review and In Silico Studies. Molecules 2023, 28, 4009. [Google Scholar] [CrossRef]
- Dhaliwal, J.S.; Moshawih, S.; Goh, K.W.; Loy, M.J.; Hossain, M.S.; Hermansyah, A.; Kotra, V.; Kifli, N.; Goh, H.P.; Dhaliwal, S.K.S.; et al. Pharmacotherapeutics Applications and Chemistry of Chalcone Derivatives. Molecules 2022, 27, 7062. [Google Scholar] [CrossRef]
- Wang, H.Q.; Man, Q.W.; Huo, F.Y.; Gao, X.; Lin, H.; Li, S.R.; Wang, J.; Su, F.C.; Cai, L.; Shi, Y.; et al. STAT3 Pathway in Cancers: Past, Present, and Future. MedComm 2022, 3, e124. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Schwartz, D.M.; Villarino, A.V.; Gadina, M.; McInnes, I.B.; Laurence, A. The JAK-STAT Pathway: Impact on Human Disease and Therapeutic Intervention. Annu. Rev. Med. 2015, 66, 311–328. [Google Scholar] [CrossRef]
- Böhmer, F.-D.; Friedrich, K. Protein Tyrosine Phosphatases as Wardens of STAT Signaling. Jak-Stat 2014, 3, e28087. [Google Scholar] [CrossRef]
- Seif, F.; Khoshmirsafa, M.; Aazami, H.; Mohsenzadegan, M.; Sedighi, G.; Bahar, M. The Role of JAK-STAT Signaling Pathway and Its Regulators in the Fate of T Helper Cells. Cell Commun. Signal. 2017, 15, 23. [Google Scholar] [CrossRef]
- Du, Z.; Lovly, C.M. Mechanisms of Receptor Tyrosine Kinase Activation in Cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.; DiGiovanni, J. Non-Canonical Stat3 Signaling in Cancer. Mol. Carcinog. 2016, 55, 1889–1898. [Google Scholar] [CrossRef]
- Luu, K.; Greenhill, C.J.; Majoros, A.; Decker, T.; Jenkins, B.J.; Mansell, A. STAT1 Plays a Role in TLR Signal Transduction and Inflammatory Responses. Immunol. Cell Biol. 2014, 92, 761–769. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Bowie, A.G. The Family of Five: TIR-Domain-Containing Adaptors in Toll-like Receptor Signalling. Nat. Rev. Immunol. 2007, 7, 353–364. [Google Scholar] [CrossRef]
- Yadav, V.R.; Prasad, S.; Sung, B.; Aggarwal, B.B. The Role of Chalcones in Suppression of NF-ΚB-Mediated Inflammation and Cancer. Int. Immunopharmacol. 2011, 11, 295–309. [Google Scholar] [CrossRef]
- Jiang, W.; Zhao, S.; Xu, L.; Lu, Y.; Lu, Z.; Chen, C.; Ni, J.; Wan, R.; Yang, L. The Inhibitory Effects of Xanthohumol, a Prenylated Chalcone Derived from Hops, on Cell Growth and Tumorigenesis in Human Pancreatic Cancer. Biomed. Pharmacother. 2015, 73, 40–47. [Google Scholar] [CrossRef]
- Hou, S.; Yuan, Q.; Yu, N.; Liu, B.; Huang, G.; Yuan, X. Cardamonin Attenuates Chronic Inflammation and Tumorigenesis in Colon. Cell Cycle 2019, 18, 3275–3287. [Google Scholar] [CrossRef]
- Oh, H.N.; Seo, J.H.; Lee, M.H.; Kim, C.; Kim, E.; Yoon, G.; Cho, S.S.; Cho, Y.S.; Choi, H.W.; Shim, J.H.; et al. Licochalcone C Induced Apoptosis in Human Oral Squamous Cell Carcinoma Cells by Regulation of the JAK2/STAT3 Signaling Pathway. J. Cell. Biochem. 2018, 119, 10118–10130. [Google Scholar] [CrossRef]
- Seo, J.H.; Choi, H.W.; Oh, H.N.; Lee, M.H.; Kim, E.; Yoon, G.; Cho, S.S.; Park, S.M.; Cho, Y.S.; Chae, J.I.; et al. Licochalcone D Directly Targets JAK2 to Induced Apoptosis in Human Oral Squamous Cell Carcinoma. J. Cell. Physiol. 2019, 234, 1780–1793. [Google Scholar] [CrossRef]
- Park, K.H.; Joo, S.H.; Seo, J.H.; Kim, J.; Yoon, G.; Jeon, Y.J.; Lee, M.H.; Chae, J., II; Kim, W.K.; Shim, J.H. Licochalcone H Induces Cell Cycle Arrest and Apoptosis in Human Skin Cancer Cells by Modulating JAK2/STAT3 Signaling. Biomol. Ther. 2022, 30, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Saraswati, S.; Alhaider, A.; Abdelgadir, A.M.; Tanwer, P.; Korashy, H.M. Phloretin Attenuates STAT-3 Activity and Overcomes Sorafenib Resistance Targeting SHP-1-Mediated Inhibition of STAT3 and Akt/VEGFR2 Pathway in Hepatocellular Carcinoma. Cell Commun. Signal. 2019, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Ong, T.H.; Chen, L.; Li, F.; Shanmugam, M.K.; Vali, S.; Abbasi, T.; Kapoor, S.; Sharma, A.; Kumar, A.P.; et al. Suppression of Signal Transducer and Activator of Transcription 3 Activation by Butein Inhibits Growth of Human Hepatocellular Carcinoma in Vivo. Clin. Cancer Res. 2011, 17, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, Y.; Jiang, Y.; Zhou, Y.; Wang, Y.; Yao, Y.; Yi, C.; Gou, L.; Yang, J. Isoliquiritigenin Inhibits the Growth of Multiple Myeloma via Blocking IL-6 Signaling. J. Mol. Med. 2012, 90, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Tang, L.; Tang, L.; Long, J.; Long, J.; Long, J.; Li, K.; Li, K.; Li, K.; Zhang, X.; et al. A Novel Chalcone Derivative Suppresses Melanoma Cell Growth through Targeting Fyn/Stat3 Pathway. Cancer Cell Int. 2020, 20, 256. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Yang, J.; Yu, W.; Li, H.; Cai, M.; Xu, J.L.; Xu, H.D.; Shi, Y.F.; Guan, X.; Cheng, X.; et al. Discovery of Benzochalcone Derivative as a Potential Antigastric Cancer Agent Targeting Signal Transducer and Activator of Transcription 3 (STAT3). J. Enzym. Inhib. Med. Chem. 2022, 37, 2004–2016. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Liu, M.; Liu, Y.; Zhang, M.; Yang, G. Tetramethoxychalcone, a Chalcone Derivative, Suppresses Proliferation, Blocks Cell Cycle Progression, and Induces Apoptosis of Human Ovarian Cancer Cells. PLoS ONE 2014, 9, e106206. [Google Scholar] [CrossRef] [PubMed]
- Bagul, C.; Rao, G.K.; Makani, V.K.K.; Tamboli, J.R.; Pal-Bhadra, M.; Kamal, A. Synthesis and Biological Evaluation of Chalcone-Linked Pyrazolo[1,5-: A] Pyrimidines as Potential Anticancer Agents. Medchemcomm 2017, 8, 1810–1816. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.X.; Mei, Y.N.; Shen, Z.; Zhu, J.F.; Xing, S.H.; Yang, H.M.; Liang, G.; Zheng, X.H. A Chalcone-Syringaldehyde Hybrid Inhibits Triple-Negative Breast Cancer Cell Proliferation and Migration by Inhibiting CKAP2-Mediated FAK and STAT3 Phosphorylation. Phytomedicine 2022, 101, 154087. [Google Scholar] [CrossRef]
- Lamie, P.F.; Philoppes, J.N. 2-Thiopyrimidine/Chalcone Hybrids: Design, Synthesis, ADMET Prediction, and Anticancer Evaluation as STAT3/STAT5a Inhibitors. J. Enzym. Inhib. Med. Chem. 2020, 35, 864–879. [Google Scholar] [CrossRef]
- Papierska, K.; Krajka-Kuźniak, V.; Kleszcz, R.; Stefański, T.; Kurczab, R.; Kubicki, M. The Synthesis of Novel Thioderivative Chalcones and Their Influence on NF-ΚB, STAT3 and NRF2 Signaling Pathways in Colorectal Cancer Cells. Sci. Rep. 2022, 12, 14915. [Google Scholar] [CrossRef]
- Sulaiman, S.; Arafat, K.; Al-Azawi, A.M.; Almarzooqi, N.A.; Lootah, S.N.A.H.; Attoub, S. Butein and Frondoside-a Combination Exhibits Additive Anti-Cancer Effects on Tumor Cell Viability, Colony Growth, and Invasion and Synergism on Endothelial Cell Migration. Int. J. Mol. Sci. 2022, 23, 431. [Google Scholar] [CrossRef]
- Kang, Y.; Park, M.A.; Heo, S.W.; Park, S.Y.; Kang, K.W.; Park, P.H.; Kim, J.A. The Radio-Sensitizing Effect of Xanthohumol Is Mediated by STAT3 and EGFR Suppression in Doxorubicin-Resistant MCF-7 Human Breast Cancer Cells. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 2638–2648. [Google Scholar] [CrossRef]
- Dokduang, H.; Yongvanit, P.; Namwat, N.; Pairojkul, C.; Sangkhamanon, S.; Yageta, M.S.; Murakami, Y.; Loilome, W. Xanthohumol Inhibits STAT3 Activation Pathway LeaDing to Growth Suppression and Apoptosis Induction in Human Cholangiocarcinoma Cells. Oncol. Rep. 2016, 35, 2065–2072. [Google Scholar] [CrossRef]
- Malami, I.; Alhassan, A.M.; Adamu, A.A.; Bello, M.B.; Muhammad, A.; Imam, M.U. Cytotoxic Flavokawain B Inhibits the Growth and Metastasis of Hepatocellular Carcinoma through UCK2 Modulation of the STAT3/Hif-1α/VEGF Signalling Pathway. Curr. Drug Targets 2023, 24, 919–928. [Google Scholar] [CrossRef]
- Wu, N.; Liu, J.; Zhao, X.; Yan, Z.; Jiang, B.; Wang, L.; Cao, S.; Shi, D.; Lin, X. Cardamonin Induces Apoptosis by Suppressing STAT3 Signaling Pathway in Glioblastoma Stem Cells. Tumor Biol. 2015, 36, 9667–9676. [Google Scholar] [CrossRef]
- Bose, S.; Banerjee, S.; Mondal, A.; Chakraborty, U.; Pumarol, J.; Croley, C.R.; Bishayee, A. Targeting the JAK/STAT Signaling Pathway Using Phytocompounds for Cancer Prevention and Therapy. Cells 2020, 9, 1451. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Yoon, G.; Choi, J.S.; Kim, E.; Liu, X.; Oh, H.N.; Chae, J., II; Lee, M.H.; Shim, J.H. Janus Kinase 2 Inhibition by Licochalcone B Suppresses Esophageal Squamous Cell Carcinoma Growth. Phyther. Res. 2020, 34, 2032–2043. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Park, J.E.; Chae, I.G.; Park, G.; Lee, S.Y.; Chun, K.S. Isoliquiritigenin Inhibits the Proliferation of Human Renal Carcinoma Caki Cells through the ROS-Mediated Regulation of the Jak2/STAT3 Pathway. Oncol. Rep. 2017, 38, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Krebs, D.L.; Hilton, D.J. SOCS Proteins: Negative Regulators of Cytokine Signaling. Stem Cells 2001, 19, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sikka, S.; Siveen, K.S.; Lee, J.H.; Um, J.Y.; Kumar, A.P.; Chinnathambi, A.; Alharbi, S.A.; Basappa; Rangappa, K.S.; et al. Cardamonin Represses Proliferation, Invasion, and Causes Apoptosis through the Modulation of Signal Transducer and Activator of Transcription 3 Pathway in Prostate Cancer. Apoptosis 2017, 22, 158–168. [Google Scholar] [CrossRef]
- Ning, S.; Ma, X.; Zhu, D.; Shen, Z.; Liu, J.; Liu, Y.; Chen, J.; Li, Z. Isoliquiritigenin Attenuates MiR-21 Expression via Induction of PIAS3 in Breast Cancer Cells. RSC Adv. 2017, 7, 18085–18092. [Google Scholar] [CrossRef]
- Fathi, M.A.A.; Abd El-Hafeez, A.A.; Abdelhamid, D.; Abbas, S.H.; Montano, M.M.; Abdel-Aziz, M. 1,3,4-Oxadiazole/Chalcone Hybrids: Design, Synthesis, and Inhibition of Leukemia Cell Growth and EGFR, Src, IL-6 and STAT3 Activities. Bioorg. Chem. 2019, 84, 150–163. [Google Scholar] [CrossRef]
- Pandey, M.K.; Bokyung, S.; Kwang, S.A.; Aggarwal, B.B. Butein Suppresses Constitutive and Inducible Signal Transducer and Activator of Transcription (Stat) 3 Activation and Stat3-Regulated Gene Products through the Induction of a Protein Tyrosine Phosphatase SHP-1. Mol. Pharmacol. 2009, 75, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, S.; Niu, P.; Zhu, Y.; Zhou, J.; Jiang, L.; Li, D.; Shi, D. Cardamonin Suppresses Pro-Tumor Function of Macrophages by Decreasing M2 Polarization on Ovarian Cancer Cells via MTOR Inhibition. Mol. Ther. Oncolytics 2022, 26, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.J.; Jung, S.N.; Chang, H.; Yun, J.; Lee, C.W.; Lee, J.; Choi, S.; Nash, O.; Han, D.C.; Kwon, B.M. Artocarpus Altilis (Parkinson) Fosberg Extracts and Geranyl Dihydrochalcone Inhibit STAT3 Activity in Prostate Cancer DU145 Cells. Phyther. Res. 2015, 29, 749–756. [Google Scholar] [CrossRef]
- Funakoshi-Tago, M.; Tago, K.; Nishizawa, C.; Takahashi, K.; Mashino, T.; Iwata, S.; Inoue, H.; Sonoda, Y.; Kasahara, T. Licochalcone A Is a Potent Inhibitor of TEL-Jak2-Mediated Transformation through the Specific Inhibition of Stat3 Activation. Biochem. Pharmacol. 2008, 76, 1681–1693. [Google Scholar] [CrossRef]
- Oh, H.N.; Oh, K.B.; Lee, M.H.; Seo, J.H.; Kim, E.; Yoon, G.; Cho, S.S.; Cho, Y.S.; Choi, H.W.; Chae, J.I.I.; et al. JAK2 Regulation by Licochalcone H Inhibits the Cell Growth and Induces Apoptosis in Oral Squamous Cell Carcinoma. Phytomedicine 2019, 52, 60–69. [Google Scholar] [CrossRef]
- Ruan, Q.; Wen, C.; Jin, G.; Yuan, Z.; Yang, X.; Wen, Z.; Huang, G.; Li, G.; Deng, J.; Bai, Y. Phloretin-Induced STAT3 Inhibition Suppresses Pancreatic Cancer Growth and Progression via Enhancing Nrf2 Activity. Phytomedicine 2023, 118, 154990. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. NF-ΚB, the First Quarter-Century: Remarkable Progress and Outstanding Questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef]
- Karin, M.; Ben-neriah, Y. Phosphorylation Meets Ubiquitination: The Control of NF-JB Activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef]
- Sun, S.-C. The Non-Canonical NF-ΚB Pathway in Immunity and Inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Rius-Pérez, S.; Pérez, S.; Martí-Andrés, P.; Monsalve, M.; Sastre, J. Nuclear Factor Kappa B Signaling Complexes in Acute Inflammation. Antioxid. Redox Signal. 2020, 33, 145–165. [Google Scholar] [CrossRef]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Anti-Cancer Chalcones: Structural and Molecular Target Perspectives. Eur. J. Med. Chem. 2015, 98, 69–114. [Google Scholar] [CrossRef]
- Pandey, M.K.; Sandur, S.K.; Sung, B.; Sethi, G.; Kunnumakkara, A.B.; Aggarwal, B.B. Butein, a Tetrahydroxychalcone, Inhibits Nuclear Factor (NF)-ΚB and NF-ΚB-Regulated Gene Expression through Direct Inhibition of IκBα Kinase β on Cysteine 179 Residue. J. Biol. Chem. 2007, 282, 17340–17350. [Google Scholar] [CrossRef]
- Li, Y.; Qin, Y.; Yang, C.; Zhang, H.; Li, Y.; Wu, B.; Huang, J.; Zhou, X.; Huang, B.; Yang, K.; et al. Cardamonin Induces Ros-Mediated G2/m Phase Arrest and Apoptosis through Inhibition of Nf-Κb Pathway in Nasopharyngeal Carcinoma. Cell Death Dis. 2017, 8, e3024-10. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Kuo, P.L.; Lin, L.T.; Lin, C.C. Isoliquiritigenin Inhibits Cell Proliferation and Induces Apoptosis in Human Hepatoma Cells. Planta Med. 2005, 71, 130–134. [Google Scholar] [CrossRef]
- Tsai, J.P.; Hsiao, P.C.; Yang, S.F.; Hsieh, S.C.; Bau, D.T.; Ling, C.L.; Pai, C.L.; Hsieh, Y.H. Licochalcone a Suppresses Migration and Invasion of Human Hepatocellular Carcinoma Cells through Downregulation of MKK4/JNK via NF-ΚB Mediated Urokinase Plasminogen Activator Expression. PLoS ONE 2014, 9, e86537. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yuan, X.; Jiang, J.; Wang, P.; Sun, X.; Wang, D.; Zheng, Q. Antimetastatic Effects of Licochalcone B on Human Bladder Carcinoma T24 by Inhibition of Matrix Metalloproteinases-9 and NF-ΚB Activity. Basic Clin. Pharmacol. Toxicol. 2014, 115, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Kundu, J.K.; Surh, Y.J. Phloretin Inhibits Phorbol Ester-Induced Tumor Promotion and Expression of Cyclooxygenase-2 in Mouse Skin: Extracellular Signal-Regulated Kinase and Nuclear Factor-ΚB as Potential Targets. J. Med. Food 2012, 15, 253–257. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, D.H.; Jung, J.Y.; Koh, D.; Kim, G.S.; Ahn, Y.S.; Lee, Y.H.; Lim, Y.; Shin, S.Y. A Synthetic Chalcone Derivative, 2-Hydroxy-3′,5,5′-Trimethoxychalcone (DK-139), Suppresses the TNFα-Induced Invasive Capability of MDA-MB-231 Human Breast Cancer Cells by Inhibiting NF-ΚB-Mediated GROα Expression. Bioorg. Med. Chem. Lett. 2016, 26, 203–208. [Google Scholar] [CrossRef]
- Kim, T.H.; Bae, G.S.; Oh, H.J.; Kim, M.S.; Park, K.C.; Koo, B.S.; Kim, B.J.; Yang, Y.S.; Park, D.E.; Lee, J.H.; et al. 2′,4′,6′-Tris(Methoxymethoxy) Chalcone (TMMC) Attenuates the Severity of Cerulein-Induced Acute Pancreatitis and Associated Lung Injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, 694–706. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Cykowiak, M.; Szaefer, H.; Kleszcz, R.; Baer-Dubowska, W. Combination of Xanthohumol and Phenethyl Isothiocyanate Inhibits NF-ΚB and Activates Nrf2 in Pancreatic Cancer Cells. Toxicol. Vitr. 2020, 65, 104799. [Google Scholar] [CrossRef]
- Bordoloi, D.; Monisha, J.; Roy, N.K.; Padmavathi, G.; Banik, K.; Harsha, C.; Wang, H.; Kumar, A.P.; Arfuso, F.; Kunnumakkara, A.B. An Investigation on the Therapeutic Potential of Butein, a Tretrahydroxychalcone against Human Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2019, 20, 3437–3446. [Google Scholar] [CrossRef]
- Badroon, N.; Majid, N.A.; Al-Suede, F.S.R.; Mansoureh, N.V.; Giribabu, N.; Majid, A.M.S.A.; Eid, E.E.M.; Alshawsh, M.A. Cardamonin Exerts Antitumor Effect on Human Hepatocellular Carcinoma Xenografts in Athymic Nude Mice through Inhibiting Nf-Κβ Pathway. Biomedicines 2020, 8, 586. [Google Scholar] [CrossRef]
- Lu, S.; Lin, C.; Cheng, X.; Hua, H.; Xiang, T.; Huang, Y.; Huang, X. Cardamonin Reduces Chemotherapy Resistance of Coloncancer Cells via the TSP50/NF-ΚB Pathway in Vitro. Oncol. Lett. 2018, 15, 9641–9646. [Google Scholar] [CrossRef]
- Huang, W.C.; Wu, S.J.; Tu, R.S.; Lai, Y.R.; Liou, C.J. Phloretin Inhibits Interleukin-1β-Induced COX-2 and ICAM-1 Expression through Inhibition of MAPK, Akt, and NF-ΚB Signaling in Human Lung Epithelial Cells. Food Funct. 2015, 6, 1960–1967. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Matsuo, Y.; Imafuji, H.; Okubo, T.; Maeda, Y.; Sato, T.; Shamoto, T.; Tsuboi, K.; Morimoto, M.; Takahashi, H.; et al. Xanthohumol Inhibits Angiogenesis by Suppressing Nuclear Factor-ΚB Activation in Pancreatic Cancer. Cancer Sci. 2018, 109, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Adhami, V.M.; Afaq, F.; Mukhtar, H. Butein Induces Apoptosis and Inhibits Prostate Tumor Growth in Vitro and in Vivo. Antioxid. Redox Signal. 2012, 16, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Ruibin, J.; Bo, J.; Danying, W.; Jianguo, F.; Linhui, G. Cardamonin Induces G2/M Phase Arrest and Apoptosis through Inhibition of NF-ΚB and MTOR Pathways in Ovarian Cancer. Aging 2020, 12, 25730–25743. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Jeong, G.S.; Lim, H.D.; An, R.B.; Kim, Y.C.; Kim, E.C. Isoliquiritigenin 2′-Methyl Ether Induces Growth Inhibition and Apoptosis in Oral Cancer Cells via Heme Oxygenase-1. Toxicol. Vitr. 2010, 24, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jiang, K.; Liang, B.; Huang, X. Anticancer Effect of Xanthohumol Induces Growth Inhibition and Apoptosis of Human Liver Cancer through NF-B/P53-Apoptosis Signaling Pathway. Oncol. Rep. 2016, 35, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, I.; Saavedra, E.; Del Rosario, H.; Perdomo, J.; Quintana, J.; Prencipe, F.; Oliva, P.; Romagnoli, R.; Estévez, F. Apoptosis Pathways Triggered by a Potent Antiproliferative Hybrid Chalcone on Human Melanoma Cells. Int. J. Mol. Sci. 2021, 22, 3462. [Google Scholar] [CrossRef] [PubMed]
- Ismail, B.; Ghezali, L.; Gueye, R.; Limami, Y.; Pouget, C.; Leger, D.Y.; Martin, F.; Beneytout, J.L.; Duroux, J.L.; Diab-Assaf, M.; et al. Novel Methylsulfonyl Chalcones as Potential Antiproliferative Drugs for Human Prostate Cancer: Involvement of the Intrinsic Pathway of Apoptosis. Int. J. Oncol. 2013, 43, 1160–1168. [Google Scholar] [CrossRef]
- Lu, H.L.; Chen, S.S.; Hsu, W.T.; Lu, Y.C.; Lee, C.C.; Wu, T.S.; Lin, M.L. Suppression of Phospho-P85α–GTP-Rac1 Lipid Raft Interaction by Bichalcone Analog Attenuates Cancer Cell Invasion. Mol. Carcinog. 2016, 55, 2106–2120. [Google Scholar] [CrossRef]
- Cioce, M.; Canino, C.; Pulito, C.; Muti, P.; Strano, S.; Blandino, G. Butein Impairs the Pro-Tumorigenic Activity of Malignant Pleural Mesothelioma Cells. Cell Cycle 2012, 11, 132–140. [Google Scholar] [CrossRef]
- Jia, D.; Tan, Y.; Liu, H.; Ooi, S.; Li, L.; Wright, K.; Bennett, S.; Addison, C.L.; Wang, L. Cardamonin Reduces Chemotherapy-Enriched Breast Cancer Stem-like Cells in Vitro and in Vivo. Oncotarget 2016, 7, 771–785. [Google Scholar] [CrossRef]
- Nourbakhsh, M.; Noori, S.; Aminzade, Z.; Bayanati, M.; Alemi, M.; Zarghi, A. Attenuation of Inflammatory Responses in Breast and Ovarian Cancer Cells by a Novel Chalcone Derivative and Its Increased Potency by Curcumin. Mediat. Inflamm. 2023, 2023, 5156320. [Google Scholar] [CrossRef] [PubMed]
- Cykowiak, M.; Kleszcz, R.; Kucińska, M.; Paluszczak, J.; Szaefer, H.; Plewiński, A.; Piotrowska-Kempisty, H.; Murias, M.; Krajka-Kuźniak, V. Attenuation of Pancreatic Cancer in Vitro and in Vivo via Modulation of Nrf2 and Nf-Κb Signaling Pathways by Natural Compounds. Cells 2021, 10, 3556. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Koh, K.N.; Park, C.J.; Jang, S.; Im, H.J.; Nayoung, K.I.M. The Combination of Flavokawain B and Daunorubicin Induces Apoptosis in Human Myeloid Leukemic Cells by Modifying NF-ĸB. Anticancer Res. 2018, 38, 2771–2778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, W.Q.; Zhang, S.Q.; Bai, J.X.; Liu, B.; Yung, K.K.L.; Ko, J.K.S. Isoliquiritigenin Inhibits Pancreatic Cancer Progression through Blockade of P38 MAPK-Regulated Autophagy. Phytomedicine 2022, 106, 154406. [Google Scholar] [CrossRef]
- Lee, C.H.; Tsai, H.Y.; Chen, C.L.; Chen, J.L.; Lu, C.C.; Fang, Y.P.; Wu, D.C.; Huang, Y.B.; Lin, M.W. Isoliquiritigenin Inhibits Gastric Cancer Stemness, Modulates Tumor Microenvironment, and Suppresses Tumor Growth through Glucose-Regulated Protein 78 Downregulation. Biomedicines 2022, 10, 1350. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Gowd, V.; Wang, M.; Chen, F.; Cheng, K.W. The Apple Dihydrochalcone Phloretin Suppresses Growth and Improves Chemosensitivity of Breast Cancer Cells: Via Inhibition of Cytoprotective Autophagy. Food Funct. 2021, 12, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Scagliarini, A.; Mathey, A.; Aires, V.; Delmas, D. Xanthohumol, a Prenylated Flavonoid from Hops, Induces DNA Damages in Colorectal Cancer Cells and Sensitizes SW480 Cells to the SN38 Chemotherapeutic Agent. Cells 2020, 9, 932. [Google Scholar] [CrossRef]
- Krajnović, T.; Kalucrossed, D.; Signerović, G.N.; Wessjohann, L.A.; Mijatović, S.; Maksimović-Ivanić, D. Versatile Antitumor Potential of Isoxanthohumol: Enhancement of Paclitaxel Activity in Vivo. Pharmacol. Res. 2016, 105, 62–73. [Google Scholar] [CrossRef]
- Habibi-Kelishomi, Z.; Goliaei, B.; Nikoofar, A. Butein Combined with Radiotherapy Enhances Radioresponse of Gastric Cancer Cell by Impairing DNA Damage Repair. Biochem. Biophys. Res. Commun. 2021, 570, 35–40. [Google Scholar] [CrossRef]
- Tang, J.; Gong, Y. Synergistic Effect of Phloretin Combined with Radiotherapy on Lung Cancer. Int. J. Radiat. Oncol. 2021, 111, e238. [Google Scholar] [CrossRef]
- Mostafa, M.O. Apoptotic and Anti-Proliferative Effects of Licorice Extract (Licochalcone A) and Paclitaxel Chemotherapy on Human Oral Squamous Cell Carcinoma Cell Line (In Vitro Study); Cairo University: Giza, Egypt, 2017; Volume 12, pp. 1–32. [Google Scholar] [CrossRef]
- Liu, M.; Yin, H.; Qian, X.; Dong, J.; Qian, Z.; Miao, J. Xanthohumol, a Prenylated Chalcone from Hops, Inhibits the Viability and Stemness of Doxorubicin-Resistant MCF-7/ADR Cells. Molecules 2017, 22, 36. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Mao, R.; Yang, J. NF-ΚB and STAT3 Signaling Pathways Collaboratively Link Inflammation to Cancer. Protein Cell 2013, 4, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Karin, M. Dangerous Liaisons: STAT3 and NF-ΚB Collaboration and Crosstalk in Cancer. Cytokine Growth Factor Rev. 2010, 21, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B. Diverse Molecular Targets for Chalcones with Varied Bioactivities. Med. Chem. 2015, 5, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Jandial, D.; Blair, C.; Zhang, S.; Krill, L.; Zhang, Y.-B.; Zi, X. Molecular Targeted Approaches to Cancer Therapy and Prevention Using Chalcones. Curr. Cancer Drug Targets 2014, 14, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.J.; Seo, Y.H. A Novel Chalcone-Based Molecule, BDP Inhibits MDA-MB-231 Triple-Negative Breast Cancer Cell Growth by Suppressing Hsp90 Function. Oncol. Rep. 2017, 38, 2343–2350. [Google Scholar] [CrossRef]
- Jeong, C.H.; Park, H.B.; Jang, W.J.; Jung, S.H.; Seo, Y.H. Discovery of Hybrid Hsp90 Inhibitors and Their Anti-Neoplastic Effects against Gefitinib-Resistant Non-Small Cell Lung Cancer (NSCLC). Bioorg. Med. Chem. Lett. 2014, 24, 224–227. [Google Scholar] [CrossRef]
- Saber, S.; El-Fattah, E.E.A.; Abdelhamid, A.M.; Mourad, A.A.E.; Hamouda, M.A.M.; Elrabat, A.; Zakaria, S.; Haleem, A.A.; Mohamed, S.Z.; Elgharabawy, R.M.; et al. Innovative Challenge for the Inhibition of Hepatocellular Carcinoma Progression by Combined Targeting of HSP90 and STAT3/HIF-1α Signaling. Biomed. Pharmacother. 2023, 158, 114196. [Google Scholar] [CrossRef] [PubMed]
- Hertlein, E.; Wagner, A.J.; Jones, J.; Lin, T.S.; Maddocks, K.J.; Towns, W.H.; Goettl, V.M.; Zhang, X.; Jarjoura, D.; Raymond, C.A.; et al. 17-DMAG Targets the Nuclear Factor-ΚB Family of Proteins to Induce Apoptosis in Chronic Lymphocytic Leukemia: Clinical Implications of HSP90 Inhibition. Blood 2010, 116, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.M.; Ferraldeschi, R.; Armstrong, H.K.; Centenera, M.M.; Workman, P. Maximizing the Therapeutic Potential of HSP90 Inhibitors. Mol. Cancer Res. 2015, 13, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.; Macedo, A.S.; Costa Lima, S.A.; Reis, S.; Soares, R.; Fonte, P. Evaluation of the Antitumour and Antiproliferative Effect of Xanthohumol-Loaded Plga Nanoparticles on Melanoma. Materials 2021, 14, 6421. [Google Scholar] [CrossRef]
- Harish, V.; Tewari, D.; Mohd, S.; Govindaiah, P.; Babu, M.R.; Kumar, R.; Gulati, M.; Gowthamarajan, K.; Madhunapantula, S.R.V.; Chellappan, D.K.; et al. Quality by Design Based Formulation of Xanthohumol Loaded Solid Lipid Nanoparticles with Improved Bioavailability and Anticancer Effect against PC-3 Cells. Pharmaceutics 2022, 14, 2403. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.W.; Wang, L.H.; Meng, D.L.; Che, X. A Green and Facile Preparation Approach, Licochalcone A Capped on Hollow Gold Nanoparticles, for Improving the Solubility and Dissolution of Anticancer Natural Product. Oncotarget 2017, 8, 105673–105681. [Google Scholar] [CrossRef]
- Wang, G.; Yu, Y.; Wang, Y.Z.; Yin, P.H.; Xu, K.; Zhang, H. The Effects and Mechanisms of Isoliquiritigenin Loaded Nanoliposomes Regulated AMPK/MTOR Mediated Glycolysis in Colorectal Cancer. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1231–1249. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajka-Kuźniak, V.; Belka, M.; Papierska, K. Targeting STAT3 and NF-κB Signaling Pathways in Cancer Prevention and Treatment: The Role of Chalcones. Cancers 2024, 16, 1092. https://doi.org/10.3390/cancers16061092
Krajka-Kuźniak V, Belka M, Papierska K. Targeting STAT3 and NF-κB Signaling Pathways in Cancer Prevention and Treatment: The Role of Chalcones. Cancers. 2024; 16(6):1092. https://doi.org/10.3390/cancers16061092
Chicago/Turabian StyleKrajka-Kuźniak, Violetta, Marta Belka, and Katarzyna Papierska. 2024. "Targeting STAT3 and NF-κB Signaling Pathways in Cancer Prevention and Treatment: The Role of Chalcones" Cancers 16, no. 6: 1092. https://doi.org/10.3390/cancers16061092
APA StyleKrajka-Kuźniak, V., Belka, M., & Papierska, K. (2024). Targeting STAT3 and NF-κB Signaling Pathways in Cancer Prevention and Treatment: The Role of Chalcones. Cancers, 16(6), 1092. https://doi.org/10.3390/cancers16061092






