Paracrine Regulation and Immune System Pathways in the Inflammatory Tumor Microenvironment of Lung Cancer: Insights into Oncogenesis and Immunotherapeutic Strategies
Abstract
Simple Summary
Abstract
1. Introduction
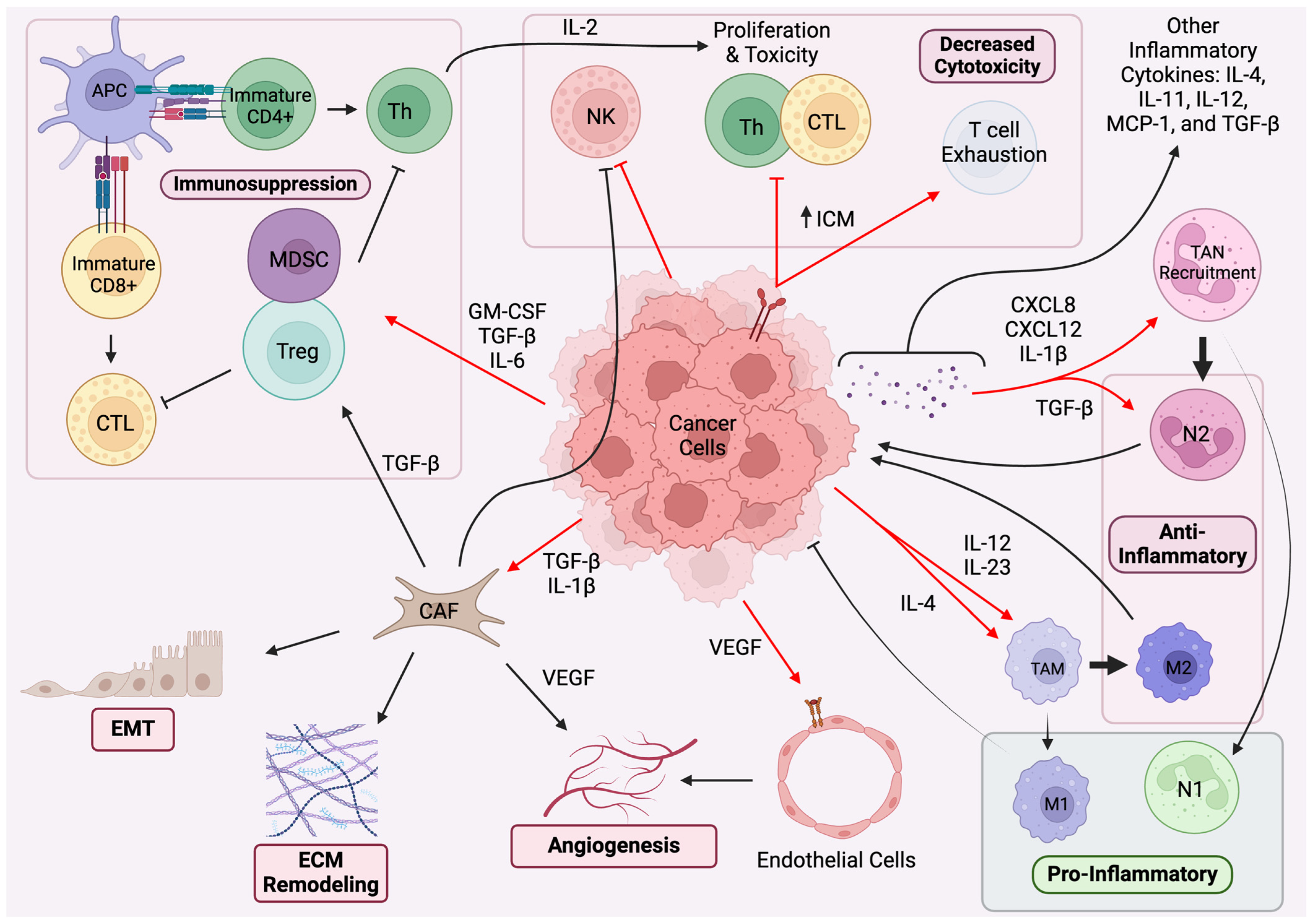
2. Paracrine Regulation and Immune System Pathways
2.1. Paracrine Regulation in TME
2.2. Immune System Pathways
3. Molecular Mechanisms of Oncogenesis
3.1. COX-2/PGE2
3.2. TGF-β
3.3. EGF
3.4. FGF
3.5. Factors of Angiogenesis: VEGFA, HIF-1α, CSF, and PDGF
3.6. Other Cytokines
| Cell | Cytokine Released | Effect |
|---|---|---|
| CAF | FGF | PD-L1 expression TAM recruitment Treg generation T cell depletion MDSC survival EMT VEGF- and PDGF-mediated angiogenesis Apoptosis regulation in angiogenesis via bcl-2 |
| TGF-β | EMT Angiogenesis via MMP9 M2 and N2 polarization of TAM and TAN Treg recruitment NK cell inhibition | |
| PGE2 | ZEB1-, Snail-, and MMP2-mediated ECM dysfunction immunosuppression of DCs Stabilization of Treg | |
| IL-6 | STAT3-mediated cancer cell proliferation and invasion | |
| VEGF | Angiogenesis | |
| TAM (M2) | IL-1β | EMT Invasion VEGF- and COX-2-mediated angiogenesis MDSC-induced immunosuppression TGF-β stimulation Induction of TP53 mutations |
| TGF-β | EMT Angiogenesis via MMP9 M2 and N2 polarization of other TAM and TAN Treg recruitment | |
| IL-10 | Immunosuppression | |
| IL-6 | Stimulation of pro-cancer fibroblast signaling Angiogenesis Proliferation Immunosuppression Apoptotic evasion EMT Invasion Metastasis | |
| TNF-α | Chronic inflammation NF-κB-mediated tumor cell proliferation Apoptotic evasion Release of angiogenic factors | |
| TAN (N2) | MMP9 VEGF IL-8 | Production of pro-angiogenic factors |
| Treg | IL-10 | Immunosuppression |
| TGF-β | Treg recruitment | |
| MDSC | IL-10 | Immunosuppression |
| Cancer Cells | PGE2 | ZEB1-, Snail-, and MMP2-mediated ECM dysfunction immunosuppression of DCs Stabilization of Treg |
| TGF-β | “reverse Warburg effects” in CAF EMT Aangiogenesis via MMP9 M2 and N2 polarization of TAM and TAN Treg recruitment NK cell inhibition | |
| EGF | Apoptotic evasion via survivin, bcl-2, and BAX Production of angiogenic factors | |
| FGF | TAM recruitment MDSC survival EMT Angiogenesis T cell depletion Treg generation | |
| CSF | WBC proliferation Angiogenesis Increased tumor aggression M2 polarization Increasing MDSC and Treg phenotypes Metastasis via Ly6G+Ly6C+ granulocyte mobilization | |
| VEGF | Aangiogenesis | |
| IL-1β | Protumorigenic signaling in CAF TAN recruitment Angiogenesis Induction of MDSC Leukocyte adhesion on endothelial cells IL-22 production | |
| IL-4 | Regulation of immune response | |
| IL-6 | Treg and MDSC upregulation Immunosuppressive modulation of NK, neutrophil, and T cell activity Suppression of apoptosis Stimulation of CAF growth factor release | |
| IL-8 | Recruitment of MDSC and immunosuppressive neutrophils EMT Angiogenesis | |
| IL-12/23 | TAN recruitment |
3.7. Key Intracellular Signals
4. Immunotherapeutic Strategies
4.1. Immune Checkpoint Inhibitors
4.2. CAR T Cell Therapy
4.3. CAFs, TAMs, and TANs
4.4. Oncolytic Viruses
4.5. Tumor-Infiltrating Lymphocytes (TILs)
4.6. IL-1β
4.7. NF-kB
4.8. IL-6
4.9. STAT3
4.10. TNF-α
4.11. IL-8
4.12. IL-10
5. Challenges and Future Directions
5.1. Case-Specific Variations in Efficacy
5.2. Resistance Mechanisms
5.3. Bringing Scientific Success into the Clinic Going Forward
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fang, J.; Lu, Y.; Zheng, J.; Jiang, X.; Shen, H.; Shang, X.; Lu, Y.; Fu, P. Exploring the Crosstalk between Endothelial Cells, Immune Cells, and Immune Checkpoints in the Tumor Microenvironment: New Insights and Therapeutic Implications. Cell Death Dis. 2023, 14, 586. [Google Scholar] [CrossRef] [PubMed]
- Poncette, L.; Bluhm, J.; Blankenstein, T. The Role of CD4 T Cells in Rejection of Solid Tumors. Curr. Opin. Immunol. 2022, 74, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Veeramachaneni, N. Targeting Interleukin-1β and Inflammation in Lung Cancer. Biomark. Res. 2022, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Nishikawa, H. Mechanisms of Regulatory T Cell Infiltration in Tumors: Implications for Innovative Immune Precision Therapies. J. Immunother. Cancer 2021, 9, e002591. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.-L.; Lin, Y.-C.; Wang, C.-H.; Huang, Y.-C.; Liao, W.-Y.; Wang, S.-S.; Chen, J.J.W.; Yang, P.-C. Autocrine and Paracrine Regulation of Interleukin-8 Expression in Lung Cancer Cells. Am. J. Respir. Cell Mol. Biol. 2005, 32, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.K.; Wong, B.H.S.; Poh, Z.S.; Udayakumar, A.; Verma, R.; Goh, R.K.J.; Duggan, S.P.; Shelat, V.G.; Chandy, K.G.; Grigoropoulos, N.F. Obstacles for T-Lymphocytes in the Tumour Microenvironment: Therapeutic Challenges, Advances and Opportunities beyond Immune Checkpoint. eBioMedicine 2022, 83, 104216. [Google Scholar] [CrossRef] [PubMed]
- Shang, G.-S.; Liu, L.; Qin, Y.-W. IL-6 and TNF-α Promote Metastasis of Lung Cancer by Inducing Epithelial-Mesenchymal Transition. Oncol. Lett. 2017, 13, 4657–4660. [Google Scholar] [CrossRef]
- Chen, W.-J.; Ho, C.-C.; Chang, Y.-L.; Chen, H.-Y.; Lin, C.-A.; Ling, T.-Y.; Yu, S.-L.; Yuan, S.-S.; Louisa Chen, Y.-J.; Lin, C.-Y.; et al. Cancer-Associated Fibroblasts Regulate the Plasticity of Lung Cancer Stemness via Paracrine Signalling. Nat. Commun. 2014, 5, 3472. [Google Scholar] [CrossRef]
- Wong, K.Y.; Cheung, A.H.; Chen, B.; Chan, W.N.; Yu, J.; Lo, K.W.; Kang, W.; To, K.F. Cancer-associated Fibroblasts in Nonsmall Cell Lung Cancer: From Molecular Mechanisms to Clinical Implications. Int. J. Cancer 2022, 151, 1195–1215. [Google Scholar] [CrossRef]
- Mishra, P.; Banerjee, D.; Ben-Baruch, A. Chemokines at the Crossroads of Tumor-Fibroblast Interactions That Promote Malignancy. J. Leukoc. Biol. 2011, 89, 31–39. [Google Scholar] [CrossRef]
- Ren, Y.; Jia, H.-H.; Xu, Y.-Q.; Zhou, X.; Zhao, X.-H.; Wang, Y.-F.; Song, X.; Zhu, Z.-Y.; Sun, T.; Dou, Y.; et al. Paracrine and Epigenetic Control of CAF-Induced Metastasis: The Role of HOTAIR Stimulated by TGF-SS1 Secretion. Mol. Cancer 2018, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Kogue, Y.; Kobayashi, H.; Nakamura, Y.; Takano, T.; Furuta, C.; Kawano, O.; Yasuma, T.; Nishimura, T.; D’Alessandro-Gabazza, C.N.; Fujimoto, H.; et al. Prognostic Value of CXCL12 in Non-Small Cell Lung Cancer Patients Undergoing Tumor Resection. Pharmaceuticals 2023, 16, 255. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.M.; Webster, S.J.; Flower, D.; Woll, P.J. Interleukin-8/CXCL8 Is a Growth Factor for Human Lung Cancer Cells. Br. J. Cancer 2004, 91, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Inoue, C.; Miki, Y.; Saito, R.; Hata, S.; Abe, J.; Sato, I.; Okada, Y.; Sasano, H. PD-L1 Induction by Cancer-Associated Fibroblast-Derived Factors in Lung Adenocarcinoma Cells. Cancers 2019, 11, 1257. [Google Scholar] [CrossRef]
- Erdogan, B.; Webb, D.J. Cancer-Associated Fibroblasts Modulate Growth Factor Signaling and Extracellular Matrix Remodeling to Regulate Tumor Metastasis. Biochem. Soc. Trans. 2017, 45, 229–236. [Google Scholar] [CrossRef]
- Yang, J.; Yan, J.; Liu, B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front. Immunol. 2018, 9, 978. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Z.; Xu, X.; Yu, Z.; Mi, J. The Influence of Microenvironment on Tumor Immunotherapy. FEBS J. 2019, 286, 4160–4175. [Google Scholar] [CrossRef]
- de Rodas, M.L.; Nagineni, V.; Ravi, A.; Datar, I.J.; Mino-Kenudson, M.; Corredor, G.; Barrera, C.; Behlman, L.; Rimm, D.L.; Herbst, R.S.; et al. Role of Tumor Infiltrating Lymphocytes and Spatial Immune Heterogeneity in Sensitivity to PD-1 Axis Blockers in Non-Small Cell Lung Cancer. J. Immunother. Cancer 2022, 10, e004440. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A Guide to Cancer Immunotherapy: From T Cell Basic Science to Clinical Practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Garcia-Lora, A.; Algarra, I.; Garrido, F. MHC Class I Antigens, Immune Surveillance, and Tumor Immune Escape. J. Cell. Physiol. 2003, 195, 346–355. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt Signal Transduction for Cancer Therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xu, C. Immune Checkpoint Signaling and Cancer Immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Sanz, L.; Muñoz, P. Tumor-Infiltrating Immunosuppressive Cells in Cancer-Cell Plasticity, Tumor Progression and Therapy Response. Cancer Microenviron. 2019, 12, 119–132. [Google Scholar] [CrossRef]
- Anichini, A.; Perotti, V.E.; Sgambelluri, F.; Mortarini, R. Immune Escape Mechanisms in Non Small Cell Lung Cancer. Cancers 2020, 12, 3605. [Google Scholar] [CrossRef] [PubMed]
- Pawelczyk, K.; Piotrowska, A.; Ciesielska, U.; Jablonska, K.; Glatzel-Plucinska, N.; Grzegrzolka, J.; Podhorska-Okolow, M.; Dziegiel, P.; Nowinska, K. Role of PD-L1 Expression in Non-Small Cell Lung Cancer and Their Prognostic Significance According to Clinicopathological Factors and Diagnostic Markers. Int. J. Mol. Sci. 2019, 20, 824. [Google Scholar] [CrossRef]
- Hung, C.-N.; Chen, M.; DeArmond, D.T.; Chiu, C.H.-L.; Limboy, C.A.; Tan, X.; Kusi, M.; Chou, C.-W.; Lin, L.-L.; Zhang, Z.; et al. AXL-Initiated Paracrine Activation of pSTAT3 Enhances Mesenchymal and Vasculogenic Supportive Features of Tumor-Associated Macrophages. Cell Rep. 2023, 42, 113067. [Google Scholar] [CrossRef]
- Tan, Z.; Xue, H.; Sun, Y.; Zhang, C.; Song, Y.; Qi, Y. The Role of Tumor Inflammatory Microenvironment in Lung Cancer. Front. Pharmacol. 2021, 12, 688625. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor Microenvironment Complexity and Therapeutic Implications at a Glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, L.; Li, Z.; Wang, X.-Y.; Yi, H. Understanding the Multifaceted Role of Neutrophils in Cancer and Autoimmune Diseases. Front. Immunol. 2018, 9, 2456. [Google Scholar] [CrossRef]
- Fang, Y.; Li, X.; Jiang, Y.; Ge, Z. Blocking TGF-β Expression Attenuates Tumor Growth in Lung Cancers, Potentially Mediated by Skewing Development of Neutrophils. J. Oncol. 2022, 2022, 3447185. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, A.; Salvi, V.; Soriani, A.; Laffranchi, M.; Sozio, F.; Bosisio, D.; Sozzani, S. Dendritic Cell Subsets in Cancer Immunity and Tumor Antigen Sensing. Cell Mol. Immunol. 2023, 20, 432–447. [Google Scholar] [CrossRef] [PubMed]
- Bronte, G.; Petracci, E.; De Matteis, S.; Canale, M.; Zampiva, I.; Priano, I.; Cravero, P.; Andrikou, K.; Burgio, M.A.; Ulivi, P.; et al. High Levels of Circulating Monocytic Myeloid-Derived Suppressive-Like Cells Are Associated With the Primary Resistance to Immune Checkpoint Inhibitors in Advanced Non-Small Cell Lung Cancer: An Exploratory Analysis. Front. Immunol. 2022, 13, 866561. [Google Scholar] [CrossRef]
- Bronte, G.; Calabrò, L.; Olivieri, F.; Procopio, A.D.; Crinò, L. The Prognostic Effects of Circulating Myeloid-Derived Suppressor Cells in Non-Small Cell Lung Cancer: Systematic Review and Meta-Analysis. Clin. Exp. Med. 2023, 23, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Bayik, D.; Lee, J.; Lathia, J.D. The Role of Myeloid-Derived Suppressor Cells in Tumor Growth and Metastasis. In Interaction of Immune and Cancer Cells; Klink, M., Szulc-Kielbik, I., Eds.; Experientia Supplementum; Springer International Publishing: Cham, Switzerland, 2022; pp. 189–217. ISBN 978-3-030-91311-3. [Google Scholar]
- Zhou, Y.; Cheng, L.; Liu, L.; Li, X. NK Cells Are Never Alone: Crosstalk and Communication in Tumour Microenvironments. Mol. Cancer 2023, 22, 34. [Google Scholar] [CrossRef] [PubMed]
- Castelao, J.E.; Bart, R.D.; DiPerna, C.A.; Sievers, E.M.; Bremner, R.M. Lung Cancer and Cyclooxygenase-2. Ann. Thorac. Surg. 2003, 76, 1327–1335. [Google Scholar] [CrossRef]
- Ochiai, M.; Oguri, T.; Isobe, T.; Ishioka, S.; Yamakido, M. Cyclooxygenase-2 (COX-2) mRNA Expression Levels in Normal Lung Tissues and Non-Small Cell Lung Cancers. Jpn. J. Cancer Res. 1999, 90, 1338–1343. [Google Scholar] [CrossRef]
- Dohadwala, M.; Batra, R.K.; Luo, J.; Lin, Y.; Krysan, K.; Põld, M.; Sharma, S.; Dubinett, S.M. Autocrine/Paracrine Prostaglandin E2 Production by Non-Small Cell Lung Cancer Cells Regulates Matrix Metalloproteinase-2 and CD44 in Cyclooxygenase-2-Dependent Invasion. J. Biol. Chem. 2002, 277, 50828–50833. [Google Scholar] [CrossRef]
- Gomperts, B.N.; Spira, A.; Massion, P.P.; Walser, T.C.; Wistuba, I.I.; Minna, J.D.; Dubinett, S.M. Evolving Concepts in Lung Carcinogenesis. Semin. Respir. Crit. Care Med. 2011, 32, 32–43. [Google Scholar] [CrossRef]
- Dohadwala, M.; Yang, S.-C.; Luo, J.; Sharma, S.; Batra, R.K.; Huang, M.; Lin, Y.; Goodglick, L.; Krysan, K.; Fishbein, M.C.; et al. Cyclooxygenase-2–Dependent Regulation of E-Cadherin: Prostaglandin E2 Induces Transcriptional Repressors ZEB1 and Snail in Non–Small Cell Lung Cancer. Cancer Res. 2006, 66, 5338–5345. [Google Scholar] [CrossRef]
- Baratelli, F.; Lin, Y.; Zhu, L.; Yang, S.-C.; Heuzé-Vourc’h, N.; Zeng, G.; Reckamp, K.; Dohadwala, M.; Sharma, S.; Dubinett, S.M. Prostaglandin E2 Induces FOXP3 Gene Expression and T Regulatory Cell Function in Human CD4+ T Cells1. J. Immunol. 2005, 175, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Põld, M.; Zhu, L.X.; Sharma, S.; Burdick, M.D.; Lin, Y.; Lee, P.P.N.; Põld, A.; Luo, J.; Krysan, K.; Dohadwala, M.; et al. Cyclooxygenase-2-Dependent Expression of Angiogenic CXC Chemokines ENA-78/CXC Ligand (CXCL) 5 and Interleukin-8/CXCL8 in Human Non-Small Cell Lung Cancer. Cancer Res. 2004, 64, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Gavin, M.A.; Rasmussen, J.P.; Fontenot, J.D.; Vasta, V.; Manganiello, V.C.; Beavo, J.A.; Rudensky, A.Y. Foxp3-Dependent Programme of Regulatory T-Cell Differentiation. Nature 2007, 445, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Stolina, M.; Yang, S.-C.; Baratelli, F.; Lin, J.F.; Atianzar, K.; Luo, J.; Zhu, L.; Lin, Y.; Huang, M.; et al. Tumor Cyclooxygenase 2-Dependent Suppression of Dendritic Cell Function. Clin. Cancer Res. 2003, 9, 961–968. [Google Scholar] [PubMed]
- Krysan, K.; Merchant, F.H.; Zhu, L.; Dohadwala, M.; Luo, J.; Lin, Y.; Heuze-Vourc’h, N.; Põld, M.; Seligson, D.; Chia, D.; et al. COX-2-Dependent Stabilization of Survivin in Non-Small Cell Lung Cancer. FASEB J. 2004, 18, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.-S.; Jen, J. TGF-β Signaling and the Role of Inhibitory Smads in Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2010, 5, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Horie, M.; Nagase, T. TGF-β Signaling in Lung Health and Disease. Int. J. Mol. Sci. 2018, 19, 2460. [Google Scholar] [CrossRef]
- Seoane, J.; Gomis, R.R. TGF-β Family Signaling in Tumor Suppression and Cancer Progression. Cold Spring Harb. Perspect. Biol. 2017, 9, a022277. [Google Scholar] [CrossRef]
- Ahmadi, A.; Najafi, M.; Farhood, B.; Mortezaee, K. Transforming Growth Factor-β Signaling: Tumorigenesis and Targeting for Cancer Therapy. J. Cell. Physiol. 2019, 234, 12173–12187. [Google Scholar] [CrossRef]
- Shi, X.; Yang, J.; Deng, S.; Xu, H.; Wu, D.; Zeng, Q.; Wang, S.; Hu, T.; Wu, F.; Zhou, H. TGF-β Signaling in the Tumor Metabolic Microenvironment and Targeted Therapies. J. Hematol. Oncol. 2022, 15, 135. [Google Scholar] [CrossRef]
- Saito, A.; Suzuki, H.I.; Horie, M.; Ohshima, M.; Morishita, Y.; Abiko, Y.; Nagase, T. An Integrated Expression Profiling Reveals Target Genes of TGF-β and TNF-α Possibly Mediated by microRNAs in Lung Cancer Cells. PLoS ONE 2013, 8, e56587. [Google Scholar] [CrossRef]
- Peng, D.; Fu, M.; Wang, M.; Wei, Y.; Wei, X. Targeting TGF-β Signal Transduction for Fibrosis and Cancer Therapy. Mol. Cancer 2022, 21, 104. [Google Scholar] [CrossRef] [PubMed]
- Shintani, Y.; Kimura, T.; Funaki, S.; Ose, N.; Kanou, T.; Fukui, E. Therapeutic Targeting of Cancer-Associated Fibroblasts in the Non-Small Cell Lung Cancer Tumor Microenvironment. Cancers 2023, 15, 335. [Google Scholar] [CrossRef]
- Pavlides, S.; Whitaker-Menezes, D.; Castello-Cros, R.; Flomenberg, N.; Witkiewicz, A.K.; Frank, P.G.; Casimiro, M.C.; Wang, C.; Fortina, P.; Addya, S.; et al. The Reverse Warburg Effect: Aerobic Glycolysis in Cancer Associated Fibroblasts and the Tumor Stroma. Cell Cycle 2009, 8, 3984–4001. [Google Scholar] [CrossRef]
- Ngaha, T.Y.S.; Zhilenkova, A.V.; Essogmo, F.E.; Uchendu, I.K.; Abah, M.O.; Fossa, L.T.; Sangadzhieva, Z.D.; Sanikovich, D.V.; Rusanov, S.A.; Pirogova, Y.N.; et al. Angiogenesis in Lung Cancer: Understanding the Roles of Growth Factors. Cancers 2023, 15, 4648. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-C.; Jin, X.; Wang, Y.; Wang, K. Role of Epidermal Growth Factor Receptor in Lung Cancer and Targeted Therapies. Am. J. Cancer Res. 2017, 7, 187–202. [Google Scholar]
- Siegelin, M.D.; Borczuk, A.C. Epidermal Growth Factor Receptor Mutations in Lung Adenocarcinoma. Lab. Investig. 2014, 94, 129–137. [Google Scholar] [CrossRef]
- Takata, S.; Takigawa, N.; Segawa, Y.; Kubo, T.; Ohashi, K.; Kozuki, T.; Teramoto, N.; Yamashita, M.; Toyooka, S.; Tanimoto, M.; et al. STAT3 Expression in Activating EGFR-Driven Adenocarcinoma of the Lung. Lung Cancer 2012, 75, 24–29. [Google Scholar] [CrossRef]
- Busser, B.; Sancey, L.; Josserand, V.; Niang, C.; Khochbin, S.; Favrot, M.C.; Coll, J.-L.; Hurbin, A. Amphiregulin Promotes Resistance to Gefitinib in Nonsmall Cell Lung Cancer Cells by Regulating Ku70 Acetylation. Mol. Ther. 2010, 18, 536–543. [Google Scholar] [CrossRef]
- Tsao, M.S.; Zhu, H.; Viallet, J. Autocrine Growth Loop of the Epidermal Growth Factor Receptor in Normal and Immortalized Human Bronchial Epithelial Cells. Exp. Cell Res. 1996, 223, 268–273. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, L.; Xu, W.; Zhang, G. EGFR May Participate in Immune Evasion through Regulation of B7-H5 Expression in Non-small Cell Lung Carcinoma. Mol. Med. Rep. 2018, 18, 3769–3779. [Google Scholar] [CrossRef] [PubMed]
- Bruns, C.J.; Solorzano, C.C.; Harbison, M.T.; Ozawa, S.; Tsan, R.; Fan, D.; Abbruzzese, J.; Traxler, P.; Buchdunger, E.; Radinsky, R.; et al. Blockade of the Epidermal Growth Factor Receptor Signaling by a Novel Tyrosine Kinase Inhibitor Leads to Apoptosis of Endothelial Cells and Therapy of Human Pancreatic Carcinoma. Cancer Res. 2000, 60, 2926–2935. [Google Scholar] [PubMed]
- Schelch, K.; Vogel, L.; Schneller, A.; Brankovic, J.; Mohr, T.; Mayer, R.L.; Slany, A.; Gerner, C.; Grusch, M. EGF Induces Migration Independent of EMT or Invasion in A549 Lung Adenocarcinoma Cells. Front. Cell Dev. Biol. 2021, 9, 634371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zeng, Y.; Du, W.; Zhu, J.; Shen, D.; Liu, Z.; Huang, J.-A. The EGFR Pathway Is Involved in the Regulation of PD-L1 Expression via the IL-6/JAK/STAT3 Signaling Pathway in EGFR-Mutated Non-Small Cell Lung Cancer. Int. J. Oncol. 2016, 49, 1360–1368. [Google Scholar] [CrossRef]
- Behrens, C.; Lin, H.Y.; Lee, J.J.; Raso, M.G.; Hong, W.K.; Wistuba, I.I.; Lotan, R. Immunohistochemical Expression of Basic Fibroblast Growth Factor and Fibroblast Growth Factor Receptors 1 and 2 in the Pathogenesis of Lung Cancer. Clin. Cancer Res. 2008, 14, 6014–6022. [Google Scholar] [CrossRef]
- Ruan, R.; Li, L.; Li, X.; Huang, C.; Zhang, Z.; Zhong, H.; Zeng, S.; Shi, Q.; Xia, Y.; Zeng, Q.; et al. Unleashing the Potential of Combining FGFR Inhibitor and Immune Checkpoint Blockade for FGF/FGFR Signaling in Tumor Microenvironment. Mol. Cancer 2023, 22, 60. [Google Scholar] [CrossRef]
- Hegab, A.E.; Ozaki, M.; Kameyama, N.; Gao, J.; Kagawa, S.; Yasuda, H.; Soejima, K.; Yin, Y.; Guzy, R.D.; Nakamura, Y.; et al. Effect of FGF/FGFR Pathway Blocking on Lung Adenocarcinoma and Its Cancer-Associated Fibroblasts. J. Pathol. 2019, 249, 193–205. [Google Scholar] [CrossRef]
- Murakami, M.; Simons, M. Fibroblast Growth Factor Regulation of Neovascularization. Curr. Opin. Hematol. 2008, 15, 215–220. [Google Scholar] [CrossRef]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental Regulation of Tumour Angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis. Genes. Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Fontanini, G.; Boldrini, L.; Vignati, S.; Chinè, S.; Basolo, F.; Silvestri, V.; Lucchi, M.; Mussi, A.; Angeletti, C.A.; Bevilacqua, G. Bcl2 and P53 Regulate Vascular Endothelial Growth Factor (VEGF)-Mediated Angiogenesis in Non-Small Cell Lung Carcinoma. Eur. J. Cancer 1998, 34, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Markovic, M.; Mitrovic, S.; Dagovic, A.; Jovanovic, D.; Nikolic, T.; Ivosevic, A.; Milosavljevic, M.Z.; Vojinovic, R.; Petrovic, M. Does the Expression of Vascular Endothelial Growth Factor (VEGF) and Bcl-2 Have a Prognostic Significance in Advanced Non-Small Cell Lung Cancer? Healthcare 2023, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Pezzuto, A.; Carico, E. Role of HIF-1 in Cancer Progression: Novel Insights. A Review. Curr. Mol. Med. 2018, 18, 343–351. [Google Scholar] [CrossRef]
- Li, T.; Qiao, T. Unraveling Tumor Microenvironment of Small-Cell Lung Cancer: Implications for Immunotherapy. Semin. Cancer Biol. 2022, 86, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, L.A.; Semenza, G.L. HIF and the Lung. Am. J. Respir. Crit. Care Med. 2011, 183, 152–156. [Google Scholar] [CrossRef]
- Karagiannidis, I.; Salataj, E.; Said Abu Egal, E.; Beswick, E.J. G-CSF in Tumors: Aggressiveness, Tumor Microenvironment and Immune Cell Regulation. Cytokine 2021, 142, 155479. [Google Scholar] [CrossRef]
- Kowanetz, M.; Wu, X.; Lee, J.; Tan, M.; Hagenbeek, T.; Qu, X.; Yu, L.; Ross, J.; Korsisaari, N.; Cao, T.; et al. Granulocyte-Colony Stimulating Factor Promotes Lung Metastasis through Mobilization of Ly6G+Ly6C+ Granulocytes. Proc. Natl. Acad. Sci. USA 2010, 107, 21248–21255. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- KATOH, M. FGFR Inhibitors: Effects on Cancer Cells, Tumor Microenvironment and Whole-Body Homeostasis (Review). Int. J. Mol. Med. 2016, 38, 3–15. [Google Scholar] [CrossRef]
- Zeltz, C.; Primac, I.; Erusappan, P.; Alam, J.; Noel, A.; Gullberg, D. Cancer-Associated Fibroblasts in Desmoplastic Tumors: Emerging Role of Integrins. Semin. Cancer Biol. 2020, 62, 166–181. [Google Scholar] [CrossRef]
- Garon, E.B.; Chih-Hsin Yang, J.; Dubinett, S.M. The Role of Interleukin 1β in the Pathogenesis of Lung Cancer. JTO Clin. Res. Rep. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Zhang, W.; Borcherding, N.; Kolb, R. IL-1 Signaling in Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1240, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kaplanov, I.; Carmi, Y.; Kornetsky, R.; Shemesh, A.; Shurin, G.V.; Shurin, M.R.; Dinarello, C.A.; Voronov, E.; Apte, R.N. Blocking IL-1β Reverses the Immunosuppression in Mouse Breast Cancer and Synergizes with Anti-PD-1 for Tumor Abrogation. Proc. Natl. Acad. Sci. USA 2019, 116, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Landvik, N.E.; Hart, K.; Skaug, V.; Stangeland, L.B.; Haugen, A.; Zienolddiny, S. A Specific Interleukin-1B Haplotype Correlates with High Levels of IL1B mRNA in the Lung and Increased Risk of Non-Small Cell Lung Cancer. Carcinogenesis 2009, 30, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Essogmo, F.E.; Zhilenkova, A.V.; Tchawe, Y.S.N.; Owoicho, A.M.; Rusanov, A.S.; Boroda, A.; Pirogova, Y.N.; Sangadzhieva, Z.D.; Sanikovich, V.D.; Bagmet, N.N.; et al. Cytokine Profile in Lung Cancer Patients: Anti-Tumor and Oncogenic Cytokines. Cancers 2023, 15, 5383. [Google Scholar] [CrossRef] [PubMed]
- Medrano, R.F.V.; Hunger, A.; Mendonça, S.A.; Barbuto, J.A.M.; Strauss, B.E. Immunomodulatory and Antitumor Effects of Type I Interferons and Their Application in Cancer Therapy. Oncotarget 2017, 8, 71249–71284. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.S.; Femel, J.; Breazeale, A.P.; Loo, C.P.; Thibault, G.; Kaempf, A.; Mori, M.; Tsujikawa, T.; Chang, Y.H.; Lund, A.W. IFNγ-Activated Dermal Lymphatic Vessels Inhibit Cytotoxic T Cells in Melanoma and Inflamed Skin. J. Exp. Med. 2018, 215, 3057–3074. [Google Scholar] [CrossRef] [PubMed]
- Werner, E.R.; Bitterlich, G.; Fuchs, D.; Hausen, A.; Reibnegger, G.; Szabo, G.; Dierich, M.P.; Wachter, H. Human Macrophages Degrade Tryptophan upon Induction by Interferon-Gamma. Life Sci. 1987, 41, 273–280. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in Tumor Progression and Regression: A Review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Vahl, J.M.; Friedrich, J.; Mittler, S.; Trump, S.; Heim, L.; Kachler, K.; Balabko, L.; Fuhrich, N.; Geppert, C.-I.; Trufa, D.I.; et al. Interleukin-10-Regulated Tumour Tolerance in Non-Small Cell Lung Cancer. Br. J. Cancer 2017, 117, 1644–1655. [Google Scholar] [CrossRef]
- Tazzyman, S.; Lewis, C.E.; Murdoch, C. Neutrophils: Key Mediators of Tumour Angiogenesis. Int. J. Exp. Pathol. 2009, 90, 222–231. [Google Scholar] [CrossRef]
- Ramachandran, S.; Verma, A.K.; Dev, K.; Goyal, Y.; Bhatt, D.; Alsahli, M.A.; Rahmani, A.H.; Almatroudi, A.; Almatroodi, S.A.; Alrumaihi, F.; et al. Role of Cytokines and Chemokines in NSCLC Immune Navigation and Proliferation. Oxid. Med. Cell. Longev. 2021, 2021, 5563746. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; Groth, C.; Lasser, S.; Arkhypov, I.; Petrova, V.; Altevogt, P.; Utikal, J.; Umansky, V. IL-6 as a Major Regulator of MDSC Activity and Possible Target for Cancer Immunotherapy. Cell. Immunol. 2021, 359, 104254. [Google Scholar] [CrossRef]
- Rašková, M.; Lacina, L.; Kejík, Z.; Venhauerová, A.; Skaličková, M.; Kolář, M.; Jakubek, M.; Rosel, D.; Smetana, K.; Brábek, J. The Role of IL-6 in Cancer Cell Invasiveness and Metastasis—Overview and Therapeutic Opportunities. Cells 2022, 11, 3698. [Google Scholar] [CrossRef]
- Benoot, T.; Piccioni, E.; De Ridder, K.; Goyvaerts, C. TNFα and Immune Checkpoint Inhibition: Friend or Foe for Lung Cancer? Int. J. Mol. Sci. 2021, 22, 8691. [Google Scholar] [CrossRef] [PubMed]
- Guttridge, D.C.; Albanese, C.; Reuther, J.Y.; Pestell, R.G.; Baldwin, A.S. NF-kappaB Controls Cell Growth and Differentiation through Transcriptional Regulation of Cyclin D1. Mol. Cell. Biol. 1999, 19, 5785–5799. [Google Scholar] [CrossRef]
- Chen, C.; Edelstein, L.C.; Gélinas, C. The Rel/NF-κB Family Directly Activates Expression of the Apoptosis Inhibitor Bcl-xL. Mol. Cell. Biol. 2000, 20, 2687–2695. [Google Scholar] [CrossRef] [PubMed]
- Karin, M. Nuclear Factor-kappaB in Cancer Development and Progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Mi, Z.; Jiang, X.; Sun, L.; Zheng, B.; Wang, J.; Meng, M.; Zhang, L.; Wang, Z.; et al. STAT3/miR-135b/NF-κB Axis Confers Aggressiveness and Unfavorable Prognosis in Non-Small-Cell Lung Cancer. Cell Death Dis. 2021, 12, 493. [Google Scholar] [CrossRef]
- Rébé, C.; Ghiringhelli, F. STAT3, a Master Regulator of Anti-Tumor Immune Response. Cancers 2019, 11, 1280. [Google Scholar] [CrossRef]
- Rasmi, R.R.; Sakthivel, K.M.; Guruvayoorappan, C. NF-κB Inhibitors in Treatment and Prevention of Lung Cancer. Biomed. Pharmacother. 2020, 130, 110569. [Google Scholar] [CrossRef] [PubMed]
- Bonizzi, G.; Karin, M. The Two NF-kappaB Activation Pathways and Their Role in Innate and Adaptive Immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar] [CrossRef]
- Tabruyn, S.P.; Griffioen, A.W. NF-κB: A New Player in Angiostatic Therapy. Angiogenesis 2008, 11, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.S. Regulation of Cell Death and Autophagy by IKK and NF-κB: Critical Mechanisms in Immune Function and Cancer. Immunol. Rev. 2012, 246, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Helin, K. Regulation of Cell Proliferation by the E2F Transcription Factors. Curr. Opin. Genet. Dev. 1998, 8, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Hunter, T.; Pines, J. Cyclins and Cancer. II: Cyclin D and CDK Inhibitors Come of Age. Cell 1994, 79, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Deveraux, Q.L.; Reed, J.C. IAP Family Proteins—Suppressors of Apoptosis. Genes Dev. 1999, 13, 239–252. [Google Scholar] [CrossRef]
- Gyrd-Hansen, M.; Meier, P. IAPs: From Caspase Inhibitors to Modulators of NF-kappaB, Inflammation and Cancer. Nat. Rev. Cancer 2010, 10, 561–574. [Google Scholar] [CrossRef]
- Fan, Y.; Mao, R.; Yang, J. NF-κB and STAT3 Signaling Pathways Collaboratively Link Inflammation to Cancer. Protein Cell 2013, 4, 176–185. [Google Scholar] [CrossRef]
- Huynh, J.; Chand, A.; Gough, D.; Ernst, M. Therapeutically Exploiting STAT3 Activity in Cancer—Using Tissue Repair as a Road Map. Nat. Rev. Cancer 2019, 19, 82–96. [Google Scholar] [CrossRef]
- Waldner, M.J.; Foersch, S.; Neurath, M.F. Interleukin-6—A Key Regulator of Colorectal Cancer Development. Int. J. Biol. Sci. 2012, 8, 1248–1253. [Google Scholar] [CrossRef]
- Grivennikov, S.; Karin, M. Dangerous Liaisons: STAT3 and NF-κB Collaboration and Crosstalk in Cancer. Cytokine Growth Factor. Rev. 2010, 21, 11–19. [Google Scholar] [CrossRef]
- Mitochondrial STAT3 Supports Ras-Dependent Oncogenic Transformation. Available online: https://pubmed.ncbi.nlm.nih.gov/19556508/ (accessed on 25 October 2023).
- Lahiri, A.; Maji, A.; Potdar, P.D.; Singh, N.; Parikh, P.; Bisht, B.; Mukherjee, A.; Paul, M.K. Lung Cancer Immunotherapy: Progress, Pitfalls, and Promises. Mol. Cancer 2023, 22, 40. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Paz-Ares, L.G.; Ciuleanu, T.-E.; Cobo, M.; Bennouna, J.; Schenker, M.; Cheng, Y.; Juan-Vidal, O.; Mizutani, H.; Lingua, A.; Reyes-Cosmelli, F.; et al. First-Line Nivolumab Plus Ipilimumab With Chemotherapy Versus Chemotherapy Alone for Metastatic NSCLC in CheckMate 9LA: 3-Year Clinical Update and Outcomes in Patients With Brain Metastases or Select Somatic Mutations. J. Thorac. Oncol. 2023, 18, 204–222. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Sezer, A.; Kilickap, S.; Gümüş, M.; Bondarenko, I.; Özgüroğlu, M.; Gogishvili, M.; Turk, H.M.; Cicin, I.; Bentsion, D.; Gladkov, O.; et al. Cemiplimab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer with PD-L1 of at Least 50%: A Multicentre, Open-Label, Global, Phase 3, Randomised, Controlled Trial. Lancet 2021, 397, 592–604. [Google Scholar] [CrossRef]
- Gogishvili, M.; Melkadze, T.; Makharadze, T.; Giorgadze, D.; Dvorkin, M.; Penkov, K.; Laktionov, K.; Nemsadze, G.; Nechaeva, M.; Rozhkova, I.; et al. Cemiplimab plus Chemotherapy versus Chemotherapy Alone in Non-Small Cell Lung Cancer: A Randomized, Controlled, Double-Blind Phase 3 Trial. Nat. Med. 2022, 28, 2374–2380. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Johnson, M.L.; Cho, B.C.; Luft, A.; Alatorre-Alexander, J.; Geater, S.L.; Laktionov, K.; Kim, S.-W.; Ursol, G.; Hussein, M.; Lim, F.L.; et al. Durvalumab With or Without Tremelimumab in Combination With Chemotherapy as First-Line Therapy for Metastatic Non–Small-Cell Lung Cancer: The Phase III POSEIDON Study. JCO 2023, 41, 1213–1227. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Mei, Q.; Chen, L.; Zhou, J. Chimeric Antigen Receptor (CAR)-T-Cell Therapy in Non-Small-Cell Lung Cancer (NSCLC): Current Status and Future Perspectives. Cancer Immunol. Immunother. 2021, 70, 619–631. [Google Scholar] [CrossRef]
- Chen, X.W.; Yu, T.J.; Zhang, J.; Li, Y.; Chen, H.L.; Yang, G.F.; Yu, W.; Liu, Y.Z.; Liu, X.X.; Duan, C.F.; et al. CYP4A in Tumor-Associated Macrophages Promotes Pre-Metastatic Niche Formation and Metastasis. Oncogene 2017, 36, 5045–5057. [Google Scholar] [CrossRef]
- Ford, K.; Hanley, C.J.; Mellone, M.; Szyndralewiez, C.; Heitz, F.; Wiesel, P.; Wood, O.; Machado, M.; Lopez, M.-A.; Ganesan, A.-P.; et al. NOX4 Inhibition Potentiates Immunotherapy by Overcoming Cancer-Associated Fibroblast-Mediated CD8 T-Cell Exclusion from Tumors. Cancer Res. 2020, 80, 1846–1860. [Google Scholar] [CrossRef] [PubMed]
- Knaapen, A.M.; Güngör, N.; Schins, R.P.F.; Borm, P.J.A.; Van Schooten, F.J. Neutrophils and Respiratory Tract DNA Damage and Mutagenesis: A Review. Mutagenesis 2006, 21, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Andzinski, L.; Kasnitz, N.; Stahnke, S.; Wu, C.-F.; Gereke, M.; von Köckritz-Blickwede, M.; Schilling, B.; Brandau, S.; Weiss, S.; Jablonska, J. Type I IFNs Induce Anti-Tumor Polarization of Tumor Associated Neutrophils in Mice and Human. Int. J. Cancer 2016, 138, 1982–1993. [Google Scholar] [CrossRef] [PubMed]
- Schott, A.F.; Goldstein, L.J.; Cristofanilli, M.; Ruffini, P.A.; McCanna, S.; Reuben, J.M.; Perez, R.P.; Kato, G.; Wicha, M. Phase Ib Pilot Study to Evaluate Reparixin in Combination with Weekly Paclitaxel in Patients with HER-2-Negative Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 5358–5365. [Google Scholar] [CrossRef]
- Taucher, E.; Taucher, V.; Fink-Neuboeck, N.; Lindenmann, J.; Smolle-Juettner, F.-M. Role of Tumor-Associated Neutrophils in the Molecular Carcinogenesis of the Lung. Cancers 2021, 13, 5972. [Google Scholar] [CrossRef]
- Zhang, H.; Yue, X.; Chen, Z.; Liu, C.; Wu, W.; Zhang, N.; Liu, Z.; Yang, L.; Jiang, Q.; Cheng, Q.; et al. Define Cancer-Associated Fibroblasts (CAFs) in the Tumor Microenvironment: New Opportunities in Cancer Immunotherapy and Advances in Clinical Trials. Mol. Cancer 2023, 22, 159. [Google Scholar] [CrossRef]
- Eli Lilly and Company. A Phase 1b/2 Dose Escalation and Cohort Expansion Study of the Safety, Tolerability and Efficacy of a Novel Transforming Growth Factor-Beta Receptor I Kinase Inhibitor (Galunisertib) Administered in Combination With Anti-PD-1 (Nivolumab) in Advanced Refractory Solid Tumors (Phase 1b) and in Recurrent or Refractory Non-Small Cell Lung Cancer or Hepatocellular Carcinoma (Phase 2). 2021. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02423343 (accessed on 28 January 2024).
- Peters, S.; Paz-Ares, L.; Herbst, R.S.; Reck, M. Addressing CPI Resistance in NSCLC: Targeting TAM Receptors to Modulate the Tumor Microenvironment and Future Prospects. J. ImmunoTherapy Cancer 2022, 10, e004863. Available online: https://jitc.bmj.com/content/10/7/e004863 (accessed on 28 October 2023). [CrossRef]
- Oliva, M.; Chepeha, D.; Araujo, D.V.; Diaz-Mejia, J.J.; Olson, P.; Prawira, A.; Spreafico, A.; Bratman, S.V.; Shek, T.; de Almeida, J.; et al. Antitumor Immune Effects of Preoperative Sitravatinib and Nivolumab in Oral Cavity Cancer: SNOW Window-of-Opportunity Study. J. Immunother. Cancer 2021, 9, e003476. [Google Scholar] [CrossRef] [PubMed]
- Leal, T.A.; Berz, D.; Rybkin, I.; Iams, W.T.; Bruno, D.; Blakely, C.; Spira, A.; Patel, M.R.; Waterhouse, D.M.; Richards, D.; et al. 1191O MRTX-500: Phase II Trial of Sitravatinib (Sitra) + Nivolumab (Nivo) in Patients (Pts) with Non-Squamous (NSQ) Non-Small Cell Lung Cancer (NSCLC) Progressing on or after Prior Checkpoint Inhibitor (CPI) Therapy. Ann. Oncol. 2021, 32, S949. [Google Scholar] [CrossRef]
- Borghaei, H.; de Marinis, F.; Dumoulin, D.; Reynolds, C.; Theelen, W.S.M.E.; Percent, I.; Gutierrez Calderon, V.; Johnson, M.L.; Madroszyk-Flandin, A.; Garon, E.B.; et al. SAPPHIRE: Phase III Study of Sitravatinib plus Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. Ann. Oncol. 2024, 35, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Neal, J.W.; Kundu, P.; Tanaka, T.; Enquist, I.; Patel, S.; Balestrini, A.; Wang, J.; Newsom-Davis, T.; Goto, Y.; Pavlakis, N.; et al. CONTACT-01: A Phase III, Randomized Study of Atezolizumab plus Cabozantinib versus Docetaxel in Patients with Metastatic Non-Small Cell Lung Cancer (mNSCLC) Previously Treated with PD-L1/PD-1 Inhibitors and Platinum-Containing Chemotherapy. JCO 2021, 39, TPS9134. [Google Scholar] [CrossRef]
- Neal, J.; Pavlakis, N.; Kim, S.-W.; Goto, Y.; Lim, S.M.; Mountzios, G.; Fountzilas, E.; Mochalova, A.; Christoph, D.C.C.; Bearz, A.; et al. 60 CONTACT-01: Efficacy and Safety from a Phase III Study of Atezolizumab (Atezo) + Cabozantinib (Cabo) vs Docetaxel (Doc) Monotherapy in Patients (Pts) with Metastatic NSCLC (mNSCLC) Previously Treated with Checkpoint Inhibitors and Chemotherapy. J. Thorac. Oncol. 2023, 18, S39–S40. [Google Scholar] [CrossRef]
- Santos Apolonio, J.; Lima de Souza Gonçalves, V.; Cordeiro Santos, M.L.; Silva Luz, M.; Silva Souza, J.V.; Rocha Pinheiro, S.L.; de Souza, W.R.; Sande Loureiro, M.; de Melo, F.F. Oncolytic Virus Therapy in Cancer: A Current Review. World J. Virol. 2021, 10, 229–255. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.J.; Peng, K.-W.; Bell, J.C. Oncolytic Virotherapy. Nat. Biotechnol. 2012, 30, 658–670. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, Y.; Zou, Q.; He, Q.; La, Z.; Yang, L.; Hu, Y. Adenovirus-Mediated Wild-Type P53 Gene Transfer in Combination with Bronchial Arterial Infusion for Treatment of Advanced Non-Small-Cell Lung Cancer, One Year Follow-Up. J. Zhejiang Univ. Sci. B 2009, 10, 331–340. [Google Scholar] [CrossRef]
- Wang, S.; Sun, J.; Chen, K.; Ma, P.; Lei, Q.; Xing, S.; Cao, Z.; Sun, S.; Yu, Z.; Liu, Y.; et al. Perspectives of Tumor-Infiltrating Lymphocyte Treatment in Solid Tumors. BMC Med. 2021, 19, 140. [Google Scholar] [CrossRef]
- Ratto, G.B.; Zino, P.; Mirabelli, S.; Minuti, P.; Aquilina, R.; Fantino, G.; Spessa, E.; Ponte, M.; Bruzzi, P.; Melioli, G. A Randomized Trial of Adoptive Immunotherapy with Tumor-Infiltrating Lymphocytes and Interleukin-2 versus Standard Therapy in the Postoperative Treatment of Resected Nonsmall Cell Lung Carcinoma. Cancer 1996, 78, 244–251. [Google Scholar] [CrossRef]
- Creelan, B.C.; Wang, C.; Teer, J.K.; Toloza, E.M.; Yao, J.; Kim, S.; Landin, A.M.; Mullinax, J.E.; Saller, J.J.; Saltos, A.N.; et al. Tumor-Infiltrating Lymphocyte Treatment for Anti-PD-1 Resistant Metastatic Lung Cancer: A Phase I Trial. Nat. Med. 2021, 27, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Nokihara, H.; Yamamoto, A.; Goto, H.; Ogawa, H.; Kanematsu, T.; Miki, T.; Uehara, H.; Saijo, Y.; Nukiwa, T.; et al. Multifunctional Interleukin-1beta Promotes Metastasis of Human Lung Cancer Cells in SCID Mice via Enhanced Expression of Adhesion-, Invasion- and Angiogenesis-Related Molecules. Cancer Sci. 2003, 94, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Saijo, Y.; Tanaka, M.; Miki, M.; Usui, K.; Suzuki, T.; Maemondo, M.; Hong, X.; Tazawa, R.; Kikuchi, T.; Matsushima, K.; et al. Proinflammatory Cytokine IL-1 Beta Promotes Tumor Growth of Lewis Lung Carcinoma by Induction of Angiogenic Factors: In Vivo Analysis of Tumor-Stromal Interaction. J. Immunol. 2002, 169, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ong, S.L.; Tran, L.M.; Jing, Z.; Liu, B.; Park, S.J.; Huang, Z.L.; Walser, T.C.; Heinrich, E.L.; Lee, G.; et al. Chronic IL-1β-Induced Inflammation Regulates Epithelial-to-Mesenchymal Transition Memory Phenotypes via Epigenetic Modifications in Non-Small Cell Lung Cancer. Sci. Rep. 2020, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, D.; Wauters, E.; Boeckx, B.; Aibar, S.; Nittner, D.; Burton, O.; Bassez, A.; Decaluwé, H.; Pircher, A.; Van den Eynde, K.; et al. Phenotype Molding of Stromal Cells in the Lung Tumor Microenvironment. Nat. Med. 2018, 24, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Watari, K.; Shibata, T.; Kawahara, A.; Sata, K.; Nabeshima, H.; Shinoda, A.; Abe, H.; Azuma, K.; Murakami, Y.; Izumi, H.; et al. Tumor-Derived Interleukin-1 Promotes Lymphangiogenesis and Lymph Node Metastasis through M2-Type Macrophages. PLoS ONE 2014, 9, e99568. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Shapiro, B.; Vucic, E.A.; Vogt, S.; Bar-Sagi, D. Tumor Cell-Derived IL1β Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer. Cancer Res. 2020, 80, 1088–1101. [Google Scholar] [CrossRef]
- Sandler, A.; Gray, R.; Perry, M.C.; Brahmer, J.; Schiller, J.H.; Dowlati, A.; Lilenbaum, R.; Johnson, D.H. Paclitaxel-Carboplatin Alone or with Bevacizumab for Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2006, 355, 2542–2550. [Google Scholar] [CrossRef]
- Reck, M.; von Pawel, J.; Zatloukal, P.; Ramlau, R.; Gorbounova, V.; Hirsh, V.; Leighl, N.; Mezger, J.; Archer, V.; Moore, N.; et al. Phase III Trial of Cisplatin plus Gemcitabine with Either Placebo or Bevacizumab as First-Line Therapy for Nonsquamous Non-Small-Cell Lung Cancer: AVAil. J. Clin. Oncol. 2009, 27, 1227–1234. [Google Scholar] [CrossRef]
- Davies, A.M.; Chansky, K.; Lara, P.N.; Gumerlock, P.H.; Crowley, J.; Albain, K.S.; Vogel, S.J.; Gandara, D.R. Bortezomib plus Gemcitabine/Carboplatin as First-Line Treatment of Advanced Non-Small Cell Lung Cancer: A Phase II Southwest Oncology Group Study (S0339). J. Thorac. Oncol. 2009, 4, 87–92. [Google Scholar] [CrossRef]
- Ando, K.; Takahashi, F.; Kato, M.; Kaneko, N.; Doi, T.; Ohe, Y.; Koizumi, F.; Nishio, K.; Takahashi, K. Tocilizumab, a Proposed Therapy for the Cachexia of Interleukin6-Expressing Lung Cancer. PLoS ONE 2014, 9, e102436. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.L.; Soo, R.A.; Tan, D.S.; Lee, S.C.; Lim, J.S.; Marban, P.C.; Kong, L.R.; Lee, Y.J.; Wang, L.Z.; Thuya, W.L.; et al. Phase I and Biomarker Study of OPB-51602, a Novel Signal Transducer and Activator of Transcription (STAT) 3 Inhibitor, in Patients with Refractory Solid Malignancies. Ann. Oncol. 2015, 26, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Paik, P.K.; Luo, J.; Ai, N.; Kim, R.; Ahn, L.; Biswas, A.; Coker, C.; Ma, W.; Wong, P.; Buonocore, D.J.; et al. Phase I Trial of the TNF-α Inhibitor Certolizumab plus Chemotherapy in Stage IV Lung Adenocarcinomas. Nat. Commun. 2022, 13, 6095. [Google Scholar] [CrossRef]
- Spigel, D.; Jotte, R.; Nemunaitis, J.; Shum, M.; Schneider, J.; Goldschmidt, J.; Eisenstein, J.; Berz, D.; Seneviratne, L.; Socoteanu, M.; et al. Randomized Phase 2 Studies of Checkpoint Inhibitors Alone or in Combination With Pegilodecakin in Patients With Metastatic NSCLC (CYPRESS 1 and CYPRESS 2). J Thorac Oncol 2021, 16, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Shukla, S. Limitations of Immunotherapy in Cancer. Cureus 2022, 14, e30856. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Cancer Immunotherapy, Part 3: Challenges and Future Trends. Pharm. Ther. 2017, 42, 514–521. [Google Scholar]
- Chu, X.; Niu, L.; Xiao, G.; Peng, H.; Deng, F.; Liu, Z.; Wu, H.; Yang, L.; Tan, Z.; Li, Z.; et al. The Long-Term and Short-Term Efficacy of Immunotherapy in Non-Small Cell Lung Cancer Patients With Brain Metastases: A Systematic Review and Meta-Analysis. Front. Immunol. 2022, 13, 875488. [Google Scholar] [CrossRef]
- Wang, F.; Wang, S.; Zhou, Q. The Resistance Mechanisms of Lung Cancer Immunotherapy. Front. Oncol. 2020, 10, 568059. [Google Scholar] [CrossRef]
- Mamdani, H.; Matosevic, S.; Khalid, A.B.; Durm, G.; Jalal, S.I. Immunotherapy in Lung Cancer: Current Landscape and Future Directions. Front. Immunol. 2022, 13, 823618. [Google Scholar] [CrossRef]
- Hamilton, G.; Rath, B. Immunotherapy for Small Cell Lung Cancer: Mechanisms of Resistance. Expert. Opin. Biol. Ther. 2019, 19, 423–432. [Google Scholar] [CrossRef]
- Tostes, K.; Siqueira, A.P.; Reis, R.M.; Leal, L.F.; Arantes, L.M.R.B. Biomarkers for Immune Checkpoint Inhibitor Response in NSCLC: Current Developments and Applicability. Int. J. Mol. Sci. 2023, 24, 11887. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Shafique, M. Combination Checkpoint Inhibitors for Treatment of Non-Small-Cell Lung Cancer: An Update on Dual Anti-CTLA-4 and Anti-PD-1/PD-L1 Therapies. Drugs Context 2020, 9, 2019-9-2. [Google Scholar] [CrossRef] [PubMed]
| Target | Treatment | Mechanism | Phase | Clinical Trial ID |
|---|---|---|---|---|
| CTLA-4 | Iplimumab | Immune Checkpoint Inhibitor | 3 | NCT00527735 |
| PD-1 | Nivolumab | Immune Checkpoint Inhibitor | 2 | NCT02998528 |
| EGFR | N/A | CAR T Cell Therapy | 2 | NCT01869166 |
| N/A | CAR T Cell Therapy | 1 | NCT0415379 | |
| TGF-β | Galunisertib | CAF Inhibition | 1b/2 | NCT02423343 |
| Receptor Tyrosine Kinase | Siravatinib, Nivolumab | TAM Inhibition | 3 | NCT03906071 |
| Tyrosine Kinase | Cabozantinib, Atezolizumab | TAM Inhibition | 3 | NCT04471428 |
| IL-1β | Canakinumbad, Pembrolizumab, Chemotherapy | IL-1β Inhibition | 3 | NCT03631199 |
| Canakinumbad, Chemotherapy | 3 | NCT03626545 | ||
| Canakinumbad, Pebrolizumab | 2 | NCT03968419 | ||
| Canakinumbad | 3 | NCT03447769 | ||
| Canakinumbad, PDR001 | 1b | NCT02900664 | ||
| Canakinumbad, PDR001+ | 1b | NCT03064854 | ||
| P53 Gene | N/A | Oncolytic Virus | 2 | NCT01574729 |
| N/A | N/A | TIL | 3 | N/A |
| N/A | N/A | TIL | 1 | NCT03215810 |
| NF-kB | Bevacimuzab | VEGF Inhibition | 3 | NCT00021060 |
| NF-kB | Bevacimuzab | VEGF Inhibition | 3 | NCT00806923 |
| NF-kB | Bortezomib | NF-kB Inhibition | 2 | NCT00075751 |
| IL-6 | Tocilizumab | IL-6 Antibody | 2 | NCT04940299 |
| IL-6 | Tocilizumab | IL-6 Antibody | 1/2 | NCT04691817 |
| STAT3 | OPB-51602 | STAT3 Inhibitor | 1 | NCT01184807) |
| STAT3 | Danvatirsen, Durvulamab | STAT3 Inhibitor | 2 | NCT02983578 |
| TNF-α | Certizolumab | TNF-α Inhibitor | 1 | NCT02120807 |
| IL-8 | BMS-986253 | IL-8 inhibitor | 2 | NCT04123379 |
| N/A | Pegilodecakin | Recombinant IL-10 | 2 | NCT03382899 |
| N/A | Pegilodecakin | Recombinant IL-10 | 2 | NCT03382912 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batrash, F.; Shaik, A.; Rauf, R.; Kutmah, M.; Zhang, J. Paracrine Regulation and Immune System Pathways in the Inflammatory Tumor Microenvironment of Lung Cancer: Insights into Oncogenesis and Immunotherapeutic Strategies. Cancers 2024, 16, 1113. https://doi.org/10.3390/cancers16061113
Batrash F, Shaik A, Rauf R, Kutmah M, Zhang J. Paracrine Regulation and Immune System Pathways in the Inflammatory Tumor Microenvironment of Lung Cancer: Insights into Oncogenesis and Immunotherapeutic Strategies. Cancers. 2024; 16(6):1113. https://doi.org/10.3390/cancers16061113
Chicago/Turabian StyleBatrash, Firas, Adnan Shaik, Rayaan Rauf, Mahmoud Kutmah, and Jun Zhang. 2024. "Paracrine Regulation and Immune System Pathways in the Inflammatory Tumor Microenvironment of Lung Cancer: Insights into Oncogenesis and Immunotherapeutic Strategies" Cancers 16, no. 6: 1113. https://doi.org/10.3390/cancers16061113
APA StyleBatrash, F., Shaik, A., Rauf, R., Kutmah, M., & Zhang, J. (2024). Paracrine Regulation and Immune System Pathways in the Inflammatory Tumor Microenvironment of Lung Cancer: Insights into Oncogenesis and Immunotherapeutic Strategies. Cancers, 16(6), 1113. https://doi.org/10.3390/cancers16061113






