Intracranial Efficacy of Atezolizumab, Bevacizumab, Carboplatin, and Paclitaxel in Real-World Patients with Non-Small-Cell Lung Cancer and EGFR or ALK Alterations
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Management Criteria
2.2. Definitions
2.3. Statistical Methods
3. Results
3.1. Patient Characteristics
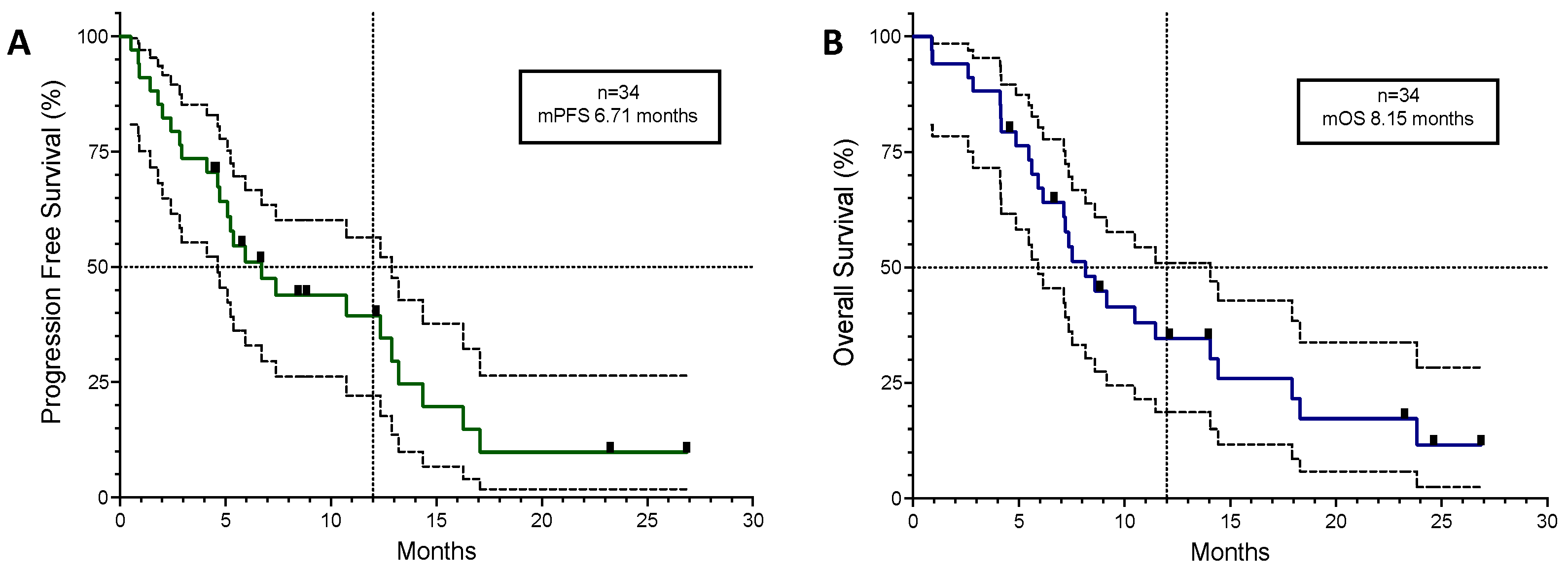
3.2. Efficacy and Tolerability
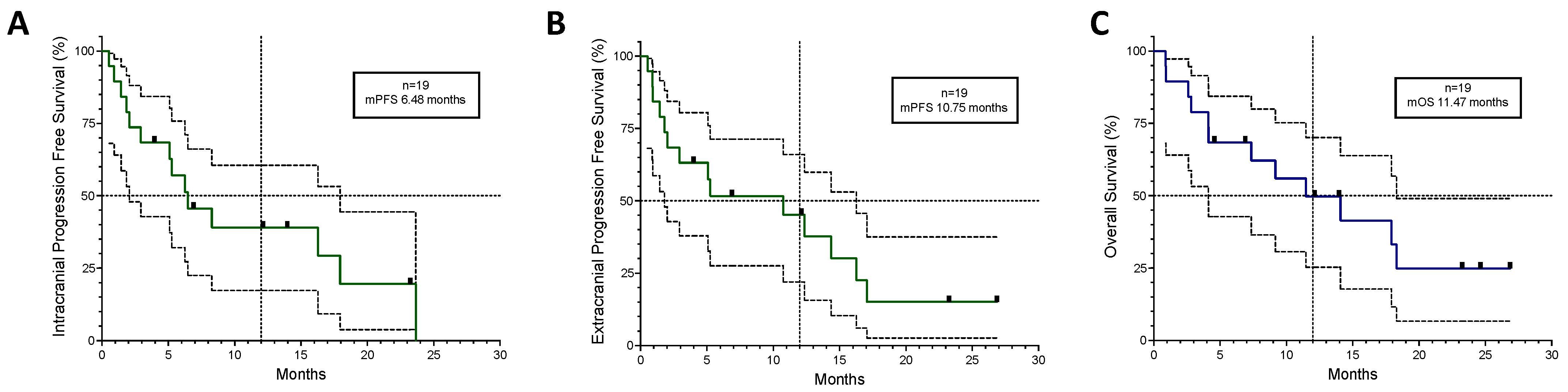
3.3. Patients with CNS Involvement
3.4. ALK+ Lung Cancer
4. Discussion
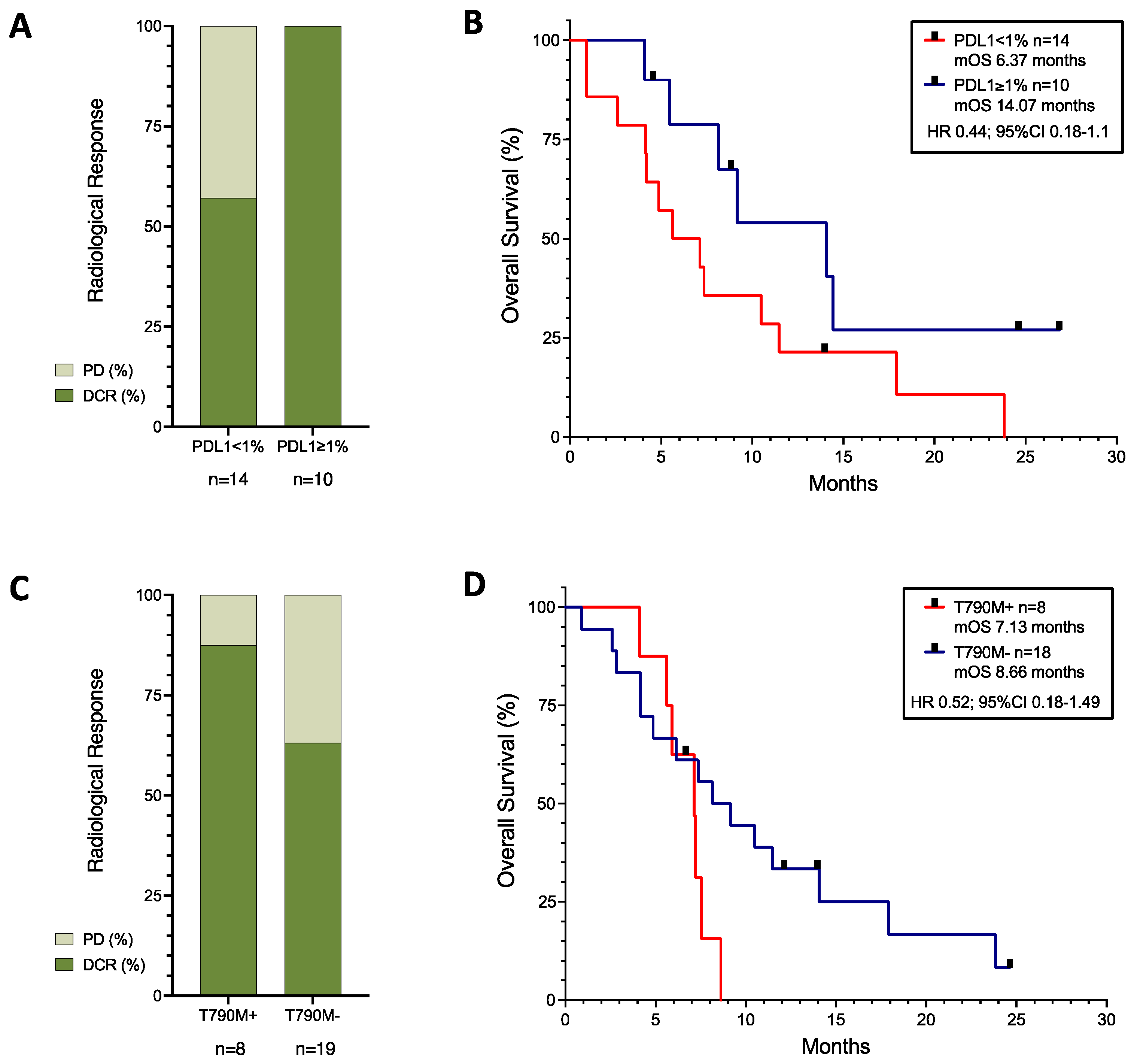
4.1. PDL1 Was a Positive Biomarker of Response
4.2. Patients with EGFR-Mutated Tumours
4.3. Patients with CNS Involvement
4.4. Patients with ALK-Translocated Tumours
4.5. Paclitaxel versus Pemetrexed
4.6. Tolerability
4.7. Clinical Implications and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Yoon, B.W.; Chang, B.; Lee, S.H. High pd-l1 expression is associated with unfavorable clinical outcome in egfr-mutated lung adenocarcinomas treated with targeted therapy. Onco Targets Ther. 2020, 13, 8273–8285. [Google Scholar] [CrossRef]
- Chapman, A.M.; Sun, K.Y.; Ruestow, P.; Cowan, D.M.; Madl, A.K. Lung cancer mutation profile of EGFR, ALK and KRAS: Meta-analysis and comparison of never and ever smokers. Lung Cancer 2016, 102, 122–134. [Google Scholar] [CrossRef]
- Shin, D.Y.; Na, I., II; Kim, C.H.; Park, S.; Baek, H.; Yang, S.H. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J. Thorac. Oncol. 2014, 9, 195–199. [Google Scholar] [CrossRef]
- Gillespie, C.S.; Mustafa, M.A.; Richardson, G.E.; Alam, A.M.; Lee, K.S.; Hughes, D.M.; Escriu, C.; Zakaria, R. Genomic Alterations and the Incidence of Brain Metastases in Advanced and Metastatic NSCLC: A Systematic Review and Meta-Analysis. J. Thorac. Oncol. 2023, 18, 1703–1713. [Google Scholar] [CrossRef]
- Escriu, C. Role and evidence for targeted therapies in surgically resectable non-small cell lung cancer: A narrative review. Video-Assist. Thorac. Surg. 2022, 7. [Google Scholar] [CrossRef]
- Yu, H.A.; Sima, C.S.; Huang, J.; Solomon, S.B.; Rimner, A.; Paik, P.; Pietanza, M.C.; Azzoli, C.G.; Rizvi, N.A.; Krug, L.M.; et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J. Thorac. Oncol. 2013, 8, 346–351. [Google Scholar] [CrossRef]
- Le Rhun, E.; Guckenberger, M.; Smits, M.; Dummer, R.; Bachelot, T.; Sahm, F.; Galldiks, N.; de Azambuja, E.; Berghoff, A.S.; Metellus, P.; et al. EANO–ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann. Oncol. 2021, 32, 1332–1347. [Google Scholar] [CrossRef]
- Ferreira, M.; Reckamp, K.L. Editorial: Impact of immunotherapy in lung cancer. Front. Oncol. 2022, 12, 12–14. [Google Scholar] [CrossRef]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 10–12. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csöszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes with Pembrolizumab versus Chemotherapy for Metastatic Non–Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score ≥ 50%. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef]
- Velcheti, V.; Hu, X.; Yang, L.; Pietanza, M.C.; Burke, T. Long-Term Real-World Outcomes of First-Line Pembrolizumab Monotherapy for Metastatic Non-Small Cell Lung Cancer with ≥50% Expression of Programmed Cell Death-Ligand 1. Front. Oncol. 2022, 12, 834761. [Google Scholar] [CrossRef]
- Hendriks, L.E.L.; Henon, C.; Auclin, E.; Mezquita, L.; Ferrara, R.; Audigier-Valette, C.; Mazieres, J.; Lefebvre, C.; Rabeau, A.; Le Moulec, S.; et al. Outcome of Patients with Non–Small Cell Lung Cancer and Brain Metastases Treated with Checkpoint Inhibitors. J. Thorac. Oncol. 2019, 14, 1244–1254. [Google Scholar] [CrossRef]
- Magee, D.E.; Hird, A.E.; Klaassen, Z.; Sridhar, S.S.; Nam, R.K.; Wallis, C.J.D.; Kulkarni, G.S. Adverse event profile for immunotherapy agents compared with chemotherapy in solid organ tumors: A systematic review and meta-analysis of randomized clinical trials. Ann. Oncol. 2020, 31, 50–60. [Google Scholar] [CrossRef]
- To, K.K.W.; Fong, W.; Cho, W.C.S. Immunotherapy in Treating EGFR-Mutant Lung Cancer: Current Challenges and New Strategies. Front. Oncol. 2021, 11, 635007. [Google Scholar] [CrossRef]
- Jahanzeb, M.; Lin, H.M.; Pan, X.; Yin, Y.; Baumann, P.; Langer, C.J. Immunotherapy Treatment Patterns and Outcomes Among ALK-Positive Patients with Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2021, 22, 49–57. [Google Scholar] [CrossRef]
- Gainor, J.F.; Shaw, A.T.; Sequist, L.V.; Fu, X.; Azzoli, C.G.; Piotrowska, Z.; Huynh, T.G.; Zhao, L.; Fulton, L.; Schultz, K.R.; et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: A retrospective analysis. Clin. Cancer Res. 2016, 22, 4585–4593. [Google Scholar] [CrossRef]
- Addeo, A.; Passaro, A.; Malapelle, U.; Luigi Banna, G.; Subbiah, V.; Friedlaender, A. Immunotherapy in non-small cell lung cancer harbouring driver mutations. Cancer Treat. Rev. 2021, 96, 102179. [Google Scholar] [CrossRef]
- Lisberg, A.; Cummings, A.; Goldman, J.W.; Bornazyan, K.; Reese, N.; Wang, T.; Coluzzi, P.; Ledezma, B.; Mendenhall, M.; Hunt, J.; et al. A Phase II Study of Pembrolizumab in EGFR-Mutant, PD-L1+, Tyrosine Kinase Inhibitor Naïve Patients with Advanced NSCLC. J. Thorac. Oncol. 2018, 13, 1138–1145. [Google Scholar] [CrossRef]
- Hastings, K.; Yu, H.A.; Wei, W.; Sanchez-Vega, F.; Deveaux, M.; Choi, J.; Rizvi, H.; Lisberg, A.; Truini, A.; Lydon, C.A.; et al. EGFR mutation subtypes and response to immune checkpoint blockade treatment in non-small-cell lung cancer. Ann. Oncol. 2019, 30, 1311–1320. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. KEYNOTE 189 (Adeno): Pembrolizumab plus Chemotherapy (Carbo/Pemetrexed) in Metastatic Non–Small-Cell Lung Cancer (Adeno). N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Ciuleanu, T.E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.J.; Stroyakovskiy, D.; Nogami, N.; Rodriguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Overall survival (OS) analysis of IMpower150, a randomized Ph 3 study of atezolizumab (atezo) + chemotherapy (chemo) ± bevacizumab (bev) vs. chemo + bev in 1L nonsquamous (NSQ) NSCLC. J. Clin. Oncol. 2018, 36, 9002. [Google Scholar] [CrossRef]
- Reck, M.; Mok, T.S.; Nishio, M.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): Key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir. Med. 2019, 7, 387–401. [Google Scholar] [CrossRef]
- Trummer, A.; Bethge, A.; Dickgreber, N.; Dittrich, I.; Golpon, H.; Hoffknecht, P.; Overbeck, T.R.; Wesseler, C.; Reck, M. NSCLC with uncommon EGFR mutations treated with atezolizumab plus bevacizumab and chemotherapy. Lung Cancer 2022, 174, 141–145. [Google Scholar] [CrossRef]
- Jeene, P.M.; de Vries, K.C.; van Nes, J.G.H.; Kwakman, J.J.M.; Wester, G.; Rozema, T.; Braam, P.M.; Zindler, J.D.; Koper, P.; Nuyttens, J.J.; et al. Survival after whole brain radiotherapy for brain metastases from lung cancer and breast cancer is poor in 6325 Dutch patients treated between 2000 and 2014. Acta Oncol. 2018, 57, 637–643. [Google Scholar] [CrossRef]
- Serizawa, T.; Yamamoto, M.; Nagano, O.; Higuchi, Y.; Matsuda, S.; Ono, J.; Iwadate, Y.; Saeki, N. Gamma Knife surgery for metastatic brain tumors. J. Neurosurg. 2008, 109, 118–121. [Google Scholar] [CrossRef]
- Kraft, J.; Zindler, J.; Minniti, G.; Guckenberger, M.; Andratschke, N. Stereotactic Radiosurgery for Multiple Brain Metastases. Curr. Treat. Options Neurol. 2019, 21, 6. [Google Scholar] [CrossRef]
- Yamamoto, M.; Serizawa, T.; Shuto, T.; Akabane, A.; Higuchi, Y.; Kawagishi, J.; Yamanaka, K.; Sato, Y.; Jokura, H.; Yomo, S.; et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014, 15, 387–395. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef]
- Clinical Cancer Clinical Trial Eligibility Criteria: Brain Metastases. Final 2020. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cancer-clinical-trial-eligibility-criteria-brain-metastases (accessed on 10 March 2024).
- Rebuzzi, S.E.; Prelaj, A.; Friedlaender, A.; Cortellini, A.; Addeo, A.; Genova, C.; Naqash, A.R.; Auclin, E.; Mezquita, L.; Banna, G.L. Prognostic scores including peripheral blood-derived inflammatory indices in patients with advanced non-small-cell lung cancer treated with immune checkpoint inhibitors. Crit. Rev. Oncol. Hematol. 2022, 179, 103806. [Google Scholar] [CrossRef]
- Park, K.; Yang, J.C.-H.; Girard, N.; Mok, T.; Gainor, J.; Nakagawa, K.; Wu, Y.-L.; Xu, W.; Yang, R.; Ohe, Y. Nivolumab + chemotherapy vs. chemotherapy in EGFR-mutated NSCLC after 1L or 2L EGFR-TKIs (CheckMate 722). Ann. Oncol. 2019, 30, vi126. [Google Scholar] [CrossRef]
- Riely, G.; Hui, R.; Carbone, D.; Park, K.; Carrigan, M.; Xu, X.; Dang, T.; Chih-Hsin Yang, J. P1.01-81 Phase 3 Study of Pemetrexed-Platinum with or without Pembrolizumab for TKI-Resistant/EGFR-Mutated Advanced NSCLC: KEYNOTE-789. J. Thorac. Oncol. 2018, 13, S494. [Google Scholar] [CrossRef]
- Zhou, C.; Dong, X.; Chen, G.; Wang, Z.; Wu, X.; Yao, Y.; Cheng, Y.; Pan, H.; Zhang, X.; Cui, J.; et al. IMpower151: Phase III study of atezolizumab + bevacizumab + chemotherapy in first-line metastatic nonsquamous NSCLC. In Proceedings of the 2023 IASLC World Conference on Lung Cancer, Singapore, 9–12 September 2023. [Google Scholar]
- Park, S.; Kim, T.M.; Han, J.; Lee, G.; Shim, B.Y.; Lee, Y.; Kim, S.; Kim, I.H.; Lee, S.; Kim, Y.J.; et al. Phase III, Randomized Study of Atezolizumab Plus Bevacizumab and Chemotherapy in Patients with EGFR—Or ALK -Mutated Non—Small-Cell Lung Cancer. J. Clin. Oncol. 2023, 1–11. [Google Scholar] [CrossRef]
- Attili, I.; Passaro, A.; Corvaja, C.; Trillo Aliaga, P.; Del Signore, E.; Spitaleri, G.; de Marinis, F. Immune checkpoint inhibitors in EGFR-mutant non-small cell lung cancer: A systematic review. Cancer Treat. Rev. 2023, 119, 102602. [Google Scholar] [CrossRef]
- Tomasini, P.; Barlesi, F.; Mascaux, C.; Greillier, L. Pemetrexed for advanced stage nonsquamous non-small cell lung cancer: Latest evidence about its extended use and outcomes. Ther. Adv. Med. Oncol. 2016, 8, 198–208. [Google Scholar] [CrossRef]
- Paz-Ares, L.G.; De Marinis, F.; Dediu, M.; Thomas, M.; Pujol, J.L.; Bidoli, P.; Molinier, O.; Sahoo, T.P.; Laack, E.; Reck, M.; et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 2013, 31, 2895–2902. [Google Scholar] [CrossRef]
- Han, B.H.; Yang, L.; Wang, X.; Yao, L. Di Efficacy of pemetrexed-based regimens in advanced non–small cell lung cancer patients with activating epidermal growth factor receptor mutations after tyrosine kinase inhibitor failure: A systematic review. OncoTargets Ther. 2018, 11, 2121–2129. [Google Scholar] [CrossRef]
- Emens, L.A.; Middleton, G. The interplay of immunotherapy and chemotherapy: Harnessing potential synergies. Cancer Immunol. Res. 2015, 3, 436–443. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Z.; Guo, X.; Wang, Y.; Zhou, Y.; Cai, W. Synergistic effects of nab-PTX and anti-PD-1 antibody combination against lung cancer by regulating the Pi3K/AKT pathway through the Serpinc1 gene. Front. Oncol. 2022, 12, 933646. [Google Scholar] [CrossRef]
- Jia, D.; Li, L.; Andrew, S.; Allan, D.; Li, X.; Lee, J.; Ji, G.; Yao, Z.; Gadde, S.; Figeys, D.; et al. An autocrine inflammatory forward-feedback loop after chemotherapy withdrawal facilitates the repopulation of drug-resistant breast cancer cells. Cell Death Dis. 2017, 8, e2932. [Google Scholar] [CrossRef]
- Kerr, K.M.; Bubendorf, L.; Edelman, M.J.; Marchetti, A.; Mok, T.; Novello, S.; O’Byrne, K.; Stahel, R.; Peters, S.; Felip, E.; et al. Second ESMO consensus conference on lung cancer: Pathology and molecular biomarkers for non-small-cell lung cancer. Ann. Oncol. 2014, 25, 1681–1690. [Google Scholar] [CrossRef]
- Kim, L.; Tsao, M.S. Tumour tissue sampling for lung cancer management in the era of personalized therapy: What is good enough for molecular testing? Eur. Respir. J. 2014, 44, 1011–1022. [Google Scholar] [CrossRef]
- de Alencar, V.T.L.; Figueiredo, A.B.; Corassa, M.; Gollob, K.J.; Cordeiro de Lima, V.C. Lung cancer in never smokers: Tumor immunology and challenges for immunotherapy. Front. Immunol. 2022, 13, 984349. [Google Scholar] [CrossRef]
- Lee, C.; Liao, B.-C.; Subramaniam, S.; Chiu, C.-H.; Mersiades, A.; Ho, C.-C.; Brown, C.; Lai, C.-L.; Hughes, B.G.M.; Yang, T.-Y.; et al. OA09.04 ILLUMINATE: Efficacy and Safety of Durvalumab-Tremelimumab and Chemotherapy in EGFR Mutant NSCLC Following Progression on EGFR Inhibitors. J. Thorac. Oncol. 2023, 18, S63. [Google Scholar] [CrossRef]
- Gomatou, G.; Syrigos, N.; Kotteas, E. Osimertinib Resistance: Molecular Mechanisms and Emerging Treatment Options. Cancers 2023, 15, 841. [Google Scholar] [CrossRef]
- Park, S.; Lee, Y.-G.; Park, J.H.; Lee, G.-W.; Kang, E.J.; Choi, Y.J.; Shin, S.; Han, J.-Y.; Lee, S.; Kim, Y.; et al. A phase III, open-label, randomized study of atezolizumab in combination with carboplatin + paclitaxel + bevacizumab compared with pemetrexed + cisplatin or carboplatin with stage IV non-squamous non-small cell lung cancer (NSCLC) with activating EGFR mut. J. Clin. Oncol. 2020, 38, 15. [Google Scholar] [CrossRef]
- Yang, J.C.-H.; Lee, D.H.; Lee, J.-S.; Fan, Y.; de Marinis, F.; Okamoto, I.; Inoue, T.; Rodriguez Cid, J.R.; Zhang, L.; Yang, C.-T.; et al. Pemetrexed and platinum with or without pembrolizumab for tyrosine kinase inhibitor (TKI)-resistant, EGFR -mutant, metastatic nonsquamous NSCLC: Phase 3 KEYNOTE-789 study. J. Clin. Oncol. 2023, 41, LBA9000. [Google Scholar] [CrossRef]
- Nadal, E.; Rodríguez-Abreu, D.; Simó, M.; Massutí, B.; Juan, O.; Huidobro, G.; López, R.; De Castro, J.; Estival, A.; Mosquera, J.; et al. Phase II Trial of Atezolizumab Combined with Carboplatin and Pemetrexed for Patients with Advanced Nonsquamous Non-Small-Cell Lung Cancer with Untreated Brain Metastases (Atezo-Brain, GECP17/05). J. Clin. Oncol. 2023, 41, 4478. [Google Scholar] [CrossRef]
- Oh, C.Y.; Klatt, M.G.; Bourne, C.; Dao, T.; Dacek, M.M.; Brea, E.J.; Mun, S.S.; Chang, A.Y.; Korontsvit, T.; Scheinberg, D.A. ALK and RET inhibitors promote HLA Class i antigen presentation and unmask new antigens within the tumor immunopeptidome. Cancer Immunol. Res. 2019, 7, 1984–1997. [Google Scholar] [CrossRef]
- Selenz, C.; Compes, A.; Nill, M.; Borchmann, S.; Odenthal, M.; Florin, A.; Brägelmann, J.; Büttner, R.; Meder, L.; Ullrich, R.T. EGFR Inhibition Strongly Modulates the Tumour Immune Microenvironment in EGFR-Driven Non-Small-Cell Lung Cancer. Cancers 2022, 14, 3943. [Google Scholar] [CrossRef]
- Enright, T.L.; Witt, J.S.; Burr, A.R.; Yadav, P.; Leal, T.; Baschnagel, A.M. Combined Immunotherapy and Stereotactic Radiotherapy Improves Neurologic Outcomes in Patients with Non–small-cell Lung Cancer Brain Metastases. Clin. Lung Cancer 2020, 22, 110–119. [Google Scholar] [CrossRef]
- Sun, L.; Davis, C.W.; Hwang, W.-T.; Jeffries, S.; Sulyok, L.F.; Marmarelis, M.E.; Singh, A.P.; Berman, A.T.; Feigenberg, S.J.; Levin, W.; et al. Outcomes in patients with non-small cell lung cancer with brain metastases treated with pembrolizumab-based therapy. Clin. Lung Cancer 2020, 22, 58–66. [Google Scholar] [CrossRef]
- Lee, J.O.; Kim, T.M.; Lee, S.H.; Kim, D.W.; Kim, S.; Jeon, Y.K.; Chung, D.H.; Kim, W.H.; Kim, Y.T.; Yang, S.C.; et al. Anaplastic lymphoma kinase translocation: A predictive biomarker of pemetrexed in patients with non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 1474–1480. [Google Scholar] [CrossRef]
- Camidge, D.R.; Kono, S.A.; Lu, X.; Okuyama, S.; Barón, A.E.; Oton, A.B.; Davies, A.M.; Varella-Garcia, M.; Franklin, W.; Doebele, R.C. Anaplastic lymphoma kinase gene rearrangements in non-small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. J. Thorac. Oncol. 2011, 6, 774–780. [Google Scholar] [CrossRef]
- Shaw, A.T.; Varghese, A.M.; Solomon, B.J.; Costa, D.B.; Novello, S.; Mino-Kenudson, M.; Awad, M.M.; Engelman, J.A.; Riely, G.J.; Monica, V.; et al. Pemetrexed-based chemotherapy in patients with advanced, ALK-positive non-small cell lung cancer. Ann. Oncol. 2013, 24, 59–66. [Google Scholar] [CrossRef]
- Solomon, B.J.; Mok, T.; Kim, D.-W.; Wu, Y.-L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-Line Crizotinib versus Chemotherapy in ALK -Positive Lung Cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef]
- Soria, J.C.; Tan, D.S.W.; Chiari, R.; Wu, Y.L.; Paz-Ares, L.; Wolf, J.; Geater, S.L.; Orlov, S.; Cortinovis, D.; Yu, C.J.; et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): A randomised, open-label, phase 3 study. Lancet 2017, 389, 917–929. [Google Scholar] [CrossRef]
- Lin, J.J.; Schoenfeld, A.J.; Zhu, V.W.; Yeap, B.Y.; Chin, E.; Rooney, M.; Plodkowski, A.J.; Digumarthy, S.R.; Dagogo-Jack, I.; Gainor, J.F.; et al. Efficacy of platinum/pemetrexed combination chemotherapy in ALK-positive non-small cell lung cancer refractory to second-generation ALK inhibitors. J. Thorac. Oncol. 2020, 15, 258–265. [Google Scholar] [CrossRef]

| All Patients | Brain Metastases | ALK+ | |
|---|---|---|---|
| Patient number (n) | 34 | 19 | 5 |
| Average age (range) | 59 (32–77) | 59 (32–77) | 55 (35–69) |
| Sex n (%) | |||
| Male | 15 (44) | 9 (43) | 2 (40) |
| Female | 19 (56) | 10 (47) | 3 (60) |
| Histology n (%) | |||
| Adenocarcinoma | 20 (59) | 11 (58) | 4 (80) |
| NSCLC NOS | 14 (41) | 8 (42) | 1 (20) |
| Metastatic burden | |||
| Average number of organs involved (median) | 2.7 (3) | 3 (3) | 2.8 (3) |
| Liver n (%) | 7 (21) | 4 (21) | 1 (20) |
| CNS n (%) | 19 (56) | 19 (100) | 4 (80) |
| Patients on dexamethasone n (%) | 7 (21) | 7 (37) | 0 (0) |
| PDL1 | |||
| NK n (%) | 10 (29) | 5 (26) | 2 (40) |
| <1% n (%) | 14 (41) | 8 (42) | 1 (20) |
| 1–49% n (%) | 6 (18) | 2 (11) | 1 (20) |
| ≥50% n (%) | 4 (12) | 2 (11) | 1 (20) |
| Genetic profile | |||
| EGFR | 29 (85) | 15 (79) | - |
| EGFR (del19 or L858R) n (%) | 25 (74) | 14 (74) | - |
| EGFR T790M n (%) | 8 (24) | 2 (11) | - |
| EGFR rare mutations responsive to TKI n (%) | 1 (3) | 1 (5) | - |
| EGFR exon 20 ins n (%) | 3 (9) | 1 (5) | - |
| ALK+ n (%) | 5 (15) | 4 (21) | 5 (100) |
| Previous treatment | |||
| Average TKI treatment lines (range) | 1.56 (1–4) | 1.47 (1–4) | 2.6 (2–4) |
| WBRT n (%) | 4 (12) | 4 (21) | 1 (20) |
| All Patients (n = 34) | Brain Metastases (n = 19) | ALK+ (n = 5) | ||
|---|---|---|---|---|
| ABCP treatment | ||||
| Median cycles (range) | 7 (1–35) | 7 (1–35) | 23 (2–35) | |
| Completed four cycles of chemotherapy n (%) | 27 (79) | 13 (64) | 3 (60) | |
| Radiological response (%) | Extracranial | Intracranial | ||
| Complete response | 1 (3) | 1 (5) | 1 (5) | 1 (20) |
| Partial response | 18 (53) | 8 (42) | 7 (37) | 3 (60) |
| Stable disease | 6 (18) | 4 (21) | 6 (32) | 0 |
| Disease progression | 9 (26) | 6 (32) | 5 (26) | 1 (20) |
| Clinical response | Extracranial | Intracranial | ||
| Median time to symptomatic response Days (range) | n = 11 12 (5–20) | n = 7 12.5 (4–21) | n = 1 20 | |
| 12-month PFS (%) | 32 | 48 | 80 | |
| 12-month OS (%) | 34 | 52 | 80 | |
| mPFS (months) | 6.71 | 10.75 | 6.48 | 17.06 |
| mOS (months) | 8.15 | 11.47 | 18.31 | |
| Tolerability | ||||
| Grade 3/4 events (%) | 18 (53) | 9 (47) | 3 (60) | |
| Chemo-related or Bevacizumab-related G3/4 events (%) | 9 (50) | 5 (55) | 2 (67) | |
| Immunotherapy-related G3/4 events (%) | 9 (50) | 4 (45) | 1 (33) | |
| Median time to toxicity Days (range) | 18 (0–70) | 21 (5–70) | 14 (10–16) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rathbone, M.; O’Hagan, C.; Wong, H.; Khan, A.; Cook, T.; Rose, S.; Heseltine, J.; Escriu, C. Intracranial Efficacy of Atezolizumab, Bevacizumab, Carboplatin, and Paclitaxel in Real-World Patients with Non-Small-Cell Lung Cancer and EGFR or ALK Alterations. Cancers 2024, 16, 1249. https://doi.org/10.3390/cancers16071249
Rathbone M, O’Hagan C, Wong H, Khan A, Cook T, Rose S, Heseltine J, Escriu C. Intracranial Efficacy of Atezolizumab, Bevacizumab, Carboplatin, and Paclitaxel in Real-World Patients with Non-Small-Cell Lung Cancer and EGFR or ALK Alterations. Cancers. 2024; 16(7):1249. https://doi.org/10.3390/cancers16071249
Chicago/Turabian StyleRathbone, Marcus, Conor O’Hagan, Helen Wong, Adeel Khan, Timothy Cook, Sarah Rose, Jonathan Heseltine, and Carles Escriu. 2024. "Intracranial Efficacy of Atezolizumab, Bevacizumab, Carboplatin, and Paclitaxel in Real-World Patients with Non-Small-Cell Lung Cancer and EGFR or ALK Alterations" Cancers 16, no. 7: 1249. https://doi.org/10.3390/cancers16071249
APA StyleRathbone, M., O’Hagan, C., Wong, H., Khan, A., Cook, T., Rose, S., Heseltine, J., & Escriu, C. (2024). Intracranial Efficacy of Atezolizumab, Bevacizumab, Carboplatin, and Paclitaxel in Real-World Patients with Non-Small-Cell Lung Cancer and EGFR or ALK Alterations. Cancers, 16(7), 1249. https://doi.org/10.3390/cancers16071249





