Large Granular Lymphocytic Leukemia: Clinical Features, Molecular Pathogenesis, Diagnosis and Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Clinical Manifestations of LGL Leukemia
2.1. T-LGL Leukemia
2.2. NK-LGL Leukemia
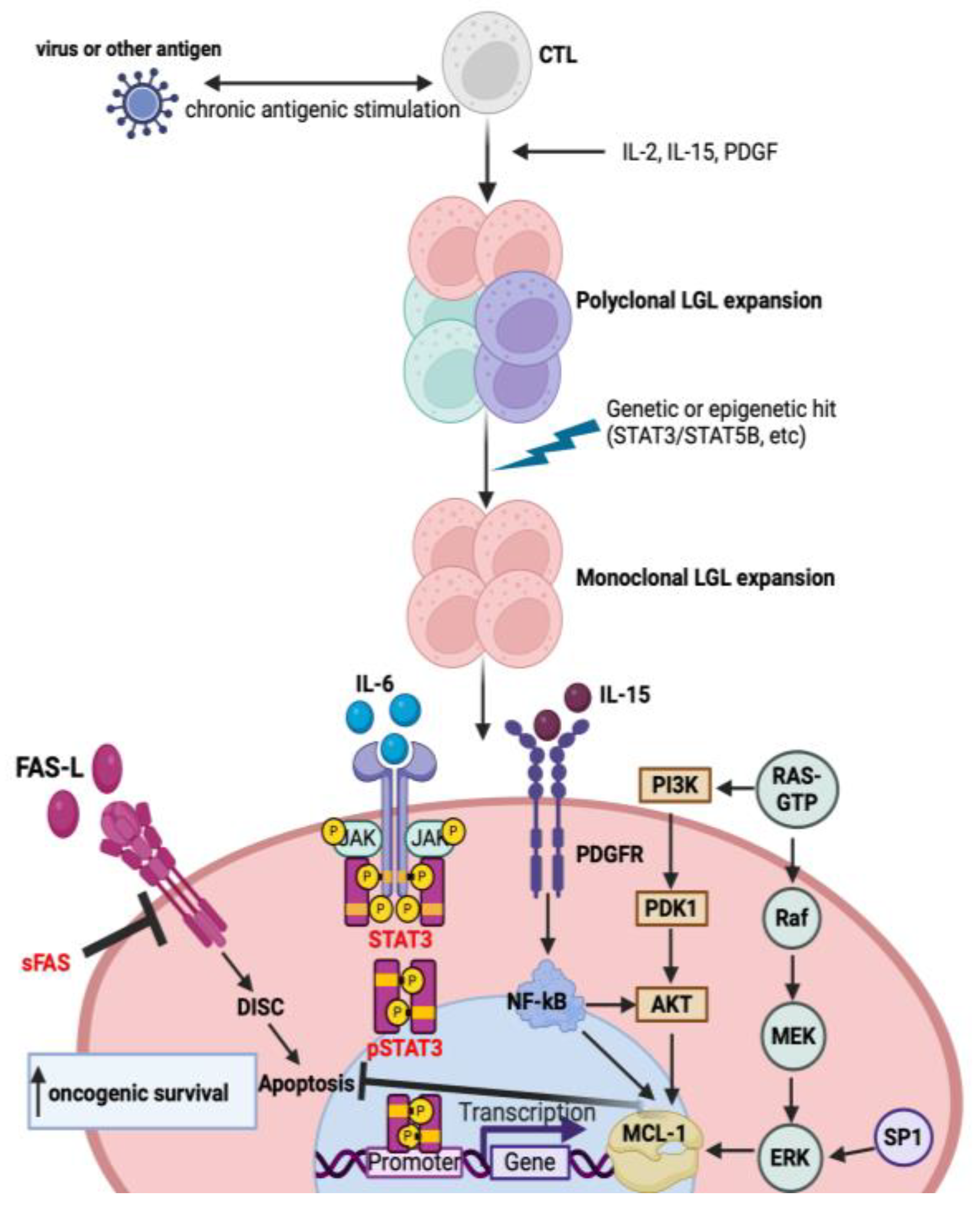
3. Pathogenesis
3.1. Cytokine Signaling Dysregulation
3.2. Molecular Pathway Dysregulation
3.3. Response to Viral Antigens
4. Associated Autoimmune and Bone Marrow Failure Disorders
4.1. LGL and Autoimmune Disorders
4.1.1. Rheumatoid Arthritis
4.1.2. Sjogren’s Syndrome
4.2. LGL and Bone Marrow Failure Disorders
4.2.1. Neutropenia
4.2.2. Pure Red Cell Aplasia
4.2.3. Autoimmune Hemolytic Anemia
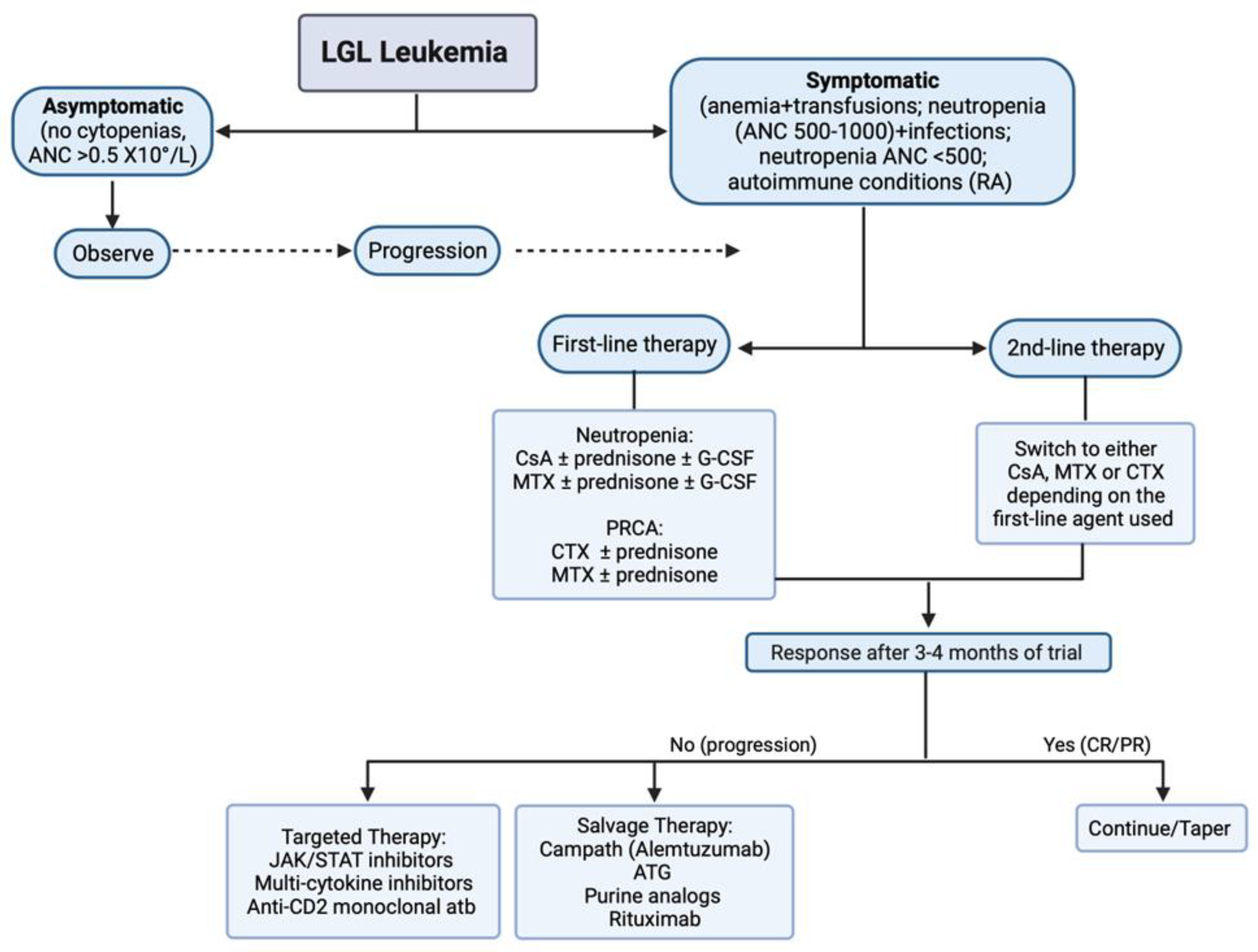
5. Diagnostic Criteria for Treatment of LGL Leukemia
6. LGL Leukemia Treatment
6.1. Salvage Therapy
6.2. Targeted Therapy
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Loughran, T.P., Jr. Clonal diseases of large granular lymphocytes. Blood 1993, 82, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lamy, T.; Loughran, T.P., Jr. Clinical features of large granular lymphocyte leukemia. Semin. Hematol. 2003, 40, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.-M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Jaffe, E.S.; Cook, J.R.; Quintanilla-Martinez, L.; Swerdlow, S.H.; Anderson, K.C.; Brousset, P.; Cerroni, L.; de Leval, L.; Dirnhofer, S.; et al. The International Consensus Classification of Mature Lymphoid Neoplasms: A report from the Clinical Advisory Committee. Blood 2022, 140, 1229–1253. [Google Scholar] [CrossRef] [PubMed]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Teramo, A.; Barilà, G.; Calabretto, G.; Ercolin, C.; Lamy, T.; Moignet, A.; Roussel, M.; Pastoret, C.; Leoncin, M.; Gattazzo, C.; et al. STAT3 mutation impacts biological and clinical features of T-LGL leukemia. Oncotarget 2017, 8, 61876–61889. [Google Scholar] [CrossRef] [PubMed]
- Oshimi, K. Clinical Features, Pathogenesis, and Treatment of Large Granular Lymphocyte Leukemias. Intern. Med. 2017, 56, 1759–1769. [Google Scholar] [CrossRef]
- Koskela, H.L.; Eldfors, S.; Ellonen, P.; van Adrichem, A.J.; Kuusanmäki, H.; Andersson, E.I.; Lagström, S.; Clemente, M.J.; Olson, T.; Jalkanen, S.E.; et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N. Engl. J. Med. 2012, 366, 1905–1913. [Google Scholar] [CrossRef]
- Jerez, A.; Clemente, M.J.; Makishima, H.; Koskela, H.; Leblanc, F.; Peng Ng, K.; Olson, T.; Przychodzen, B.; Afable, M.; Gomez-Segui, I.; et al. STAT3 mutations unify the pathogenesis of chronic lymphoproliferative disorders of NK cells and T-cell large granular lymphocyte leukemia. Blood 2012, 120, 3048–3057. [Google Scholar] [CrossRef]
- Rahul, E.; Ningombam, A.; Acharya, S.; Tanwar, P.; Ranjan, A.; Chopra, A. Large granular lymphocytic leukemia: A brief review. Am. J. Blood Res. 2022, 12, 17–32. [Google Scholar] [PubMed]
- Lamy, T.; Moignet, A.; Loughran, T.P., Jr. LGL leukemia: From pathogenesis to treatment. Blood 2017, 129, 1082–1094. [Google Scholar] [CrossRef] [PubMed]
- Cheon, H.; Dziewulska, K.H.; Moosic, K.B.; Olson, K.C.; Gru, A.A.; Feith, D.J.; Loughran, T.P., Jr. Advances in the Diagnosis and Treatment of Large Granular Lymphocytic Leukemia. Curr. Hematol. Malig. Rep. 2020, 15, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Suzumiya, J.; Nakamura, S.; Aoki, S.; Notoya, A.; Ozaki, S.; Gondo, H.; Hino, N.; Mori, H.; Sugimori, H.; et al. Aggressive natural killer-cell leukemia revisited: Large granular lymphocyte leukemia of cytotoxic NK cells. Leukemia 2004, 18, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Moignet, A.; Lamy, T. Latest Advances in the Diagnosis and Treatment of Large Granular Lymphocytic Leukemia. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.V.; Hook, C.C.; Call, T.G.; Go, R.S. A population-based study of large granular lymphocyte leukemia. Blood Cancer J. 2016, 6, e455. [Google Scholar] [CrossRef] [PubMed]
- Dinmohamed, A.G.; Brink, M.; Visser, O.; Jongen-Lavrencic, M. Population-based analyses among 184 patients diagnosed with large granular lymphocyte leukemia in the Netherlands between 2001 and 2013. Leukemia 2016, 30, 1449–1451. [Google Scholar] [CrossRef] [PubMed]
- Lamy, T.; Loughran, T.P., Jr. Current concepts: Large granular lymphocyte leukemia. Blood Rev. 1999, 13, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Loughran, T.P., Jr.; Kadin, M.E.; Starkebaum, G.; Abkowitz, J.L.; Clark, E.A.; Disteche, C.; Lum, L.G.; Slichter, S.J. Leukemia of large granular lymphocytes: Association with clonal chromosomal abnormalities and autoimmune neutropenia, thrombocytopenia, and hemolytic anemia. Ann. Intern. Med. 1985, 102, 169–175. [Google Scholar] [CrossRef]
- Loughran, T.P., Jr.; Starkebaum, G. Large granular lymphocyte leukemia. Report of 38 cases and review of the literature. Medicine 1987, 66, 397–405. [Google Scholar] [CrossRef]
- Semenzato, G.; Pandolfi, F.; Chisesi, T.; De Rossi, G.; Pizzolo, G.; Zambello, R.; Trentin, L.; Agostini, C.; Dini, E.; Vespignani, M.; et al. The lymphoproliferative disease of granular lymphocytes. A heterogeneous disorder ranging from indolent to aggressive conditions. Cancer 1987, 60, 2971–2978. [Google Scholar] [CrossRef]
- Dhodapkar, M.V.; Li, C.Y.; Lust, J.A.; Tefferi, A.; Phyliky, R.L. Clinical spectrum of clonal proliferations of T-large granular lymphocytes: A T-cell clonopathy of undetermined significance? Blood 1994, 84, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Neben, M.A.; Morice, W.G.; Tefferi, A. Clinical features in T-cell vs. natural killer-cell variants of large granular lymphocyte leukemia. Eur. J. Haematol. 2003, 71, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Osuji, N.; Matutes, E.; Tjonnfjord, G.; Grech, H.; Del Giudice, I.; Wotherspoon, A.; Swansbury, J.G.; Catovsky, D. T-cell large granular lymphocyte leukemia: A report on the treatment of 29 patients and a review of the literature. Cancer 2006, 107, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Aribi, A.; Huh, Y.; Keating, M.; O’Brien, S.; Ferrajoli, A.; Faderl, S.; Wierda, W.; Kantarjian, H.; Ravandi, F. T-cell large granular lymphocytic (T-LGL) leukemia: Experience in a single institution over 8 years. Leuk. Res. 2007, 31, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Oshimi, K. Granular lymphocyte proliferative disorders: Report of 12 cases and review of the literature. Leukemia 1988, 2, 617–627. [Google Scholar] [PubMed]
- Kawahara, S.; Sasaki, M.; Isobe, Y.; Ando, J.; Noguchi, M.; Koike, M.; Hirano, T.; Oshimi, K.; Sugimoto, K. Clinical analysis of 52 patients with granular lymphocyte proliferative disorder (GLPD) showed frequent anemia in indolent T-cell GLPD in Japan. Eur. J. Haematol. 2009, 82, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Kwong, Y.L.; Wong, K.F. Association of pure red cell aplasia with T large granular lymphocyte leukaemia. J. Clin. Pathol. 1998, 51, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Kwong, Y.L.; Au, W.Y.; Leung, A.Y.; Tse, E.W. T-cell large granular lymphocyte leukemia: An Asian perspective. Ann. Hematol. 2010, 89, 331–339. [Google Scholar] [CrossRef]
- Liu, J.H.; Wei, S.; Lamy, T.; Epling-Burnette, P.K.; Starkebaum, G.; Djeu, J.Y.; Loughran, T.P. Chronic neutropenia mediated by fas ligand. Blood 2000, 95, 3219–3222. [Google Scholar] [CrossRef]
- Burks, E.J.; Loughran, T.P., Jr. Pathogenesis of neutropenia in large granular lymphocyte leukemia and Felty syndrome. Blood Rev. 2006, 20, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Pontikoglou, C.; Kalpadakis, C.; Papadaki, H.A. Pathophysiologic mechanisms and management of neutropenia associated with large granular lymphocytic leukemia. Expert. Rev. Hematol. 2011, 4, 317–328. [Google Scholar] [CrossRef]
- Oshimi, K.; Hoshino, S.; Takahashi, M.; Akahoshi, M.; Saito, H.; Kobayashi, Y.; Hirai, H.; Takaku, F.; Yahagi, N.; Oshimi, Y.; et al. Ti (WT31)-negative, CD3-positive, large granular lymphocyte leukemia with nonspecific cytotoxicity. Blood 1988, 71, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.W.; Foon, K.A. T gamma-lymphoproliferative disease and related disorders in humans and experimental animals: A review of the clinical, cellular, and functional characteristics. Blood 1984, 64, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Handgretinger, R.; Geiselhart, A.; Moris, A.; Grau, R.; Teuffel, O.; Bethge, W.; Kanz, L.; Fisch, P. Pure red-cell aplasia associated with clonal expansion of granular lymphocytes expressing killer-cell inhibitory receptors. N. Engl. J. Med. 1999, 340, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Saunthararajah, Y.; Molldrem, J.L.; Rivera, M.; Williams, A.; Stetler-Stevenson, M.; Sorbara, L.; Young, N.S.; Barrett, J.A. Coincident myelodysplastic syndrome and T-cell large granular lymphocytic disease: Clinical and pathophysiological features. Br. J. Haematol. 2001, 112, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Karadimitris, A.; Li, K.; Notaro, R.; Araten, D.J.; Nafa, K.; Thertulien, R.; Ladanyi, M.; Stevens, A.E.; Rosenfeld, C.S.; Roberts, I.A.; et al. Association of clonal T-cell large granular lymphocyte disease and paroxysmal nocturnal haemoglobinuria (PNH): Further evidence for a pathogenetic link between T cells, aplastic anaemia and PNH. Br. J. Haematol. 2001, 115, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Huh, Y.O.; Medeiros, L.J.; Ravandi, F.; Konoplev, S.; Jorgensen, J.L.; Miranda, R.N. T-cell large granular lymphocyte leukemia associated with myelodysplastic syndrome: A clinicopathologic study of nine cases. Am. J. Clin. Pathol. 2009, 131, 347–356. [Google Scholar] [CrossRef]
- Jerez, A.; Clemente, M.J.; Makishima, H.; Rajala, H.; Gómez-Seguí, I.; Olson, T.; McGraw, K.; Przychodzen, B.; Kulasekararaj, A.; Afable, M.; et al. STAT3 mutations indicate the presence of subclinical T-cell clones in a subset of aplastic anemia and myelodysplastic syndrome patients. Blood 2013, 122, 2453–2459. [Google Scholar] [CrossRef]
- Nyland, S.B.; Krissinger, D.J.; Clemente, M.J.; Irby, R.B.; Baab, K.T.; Jarbadan, N.R.; Sokol, L.; Schaefer, E.; Liao, J.; Cuthbertson, D.; et al. Seroreactivity to LGL leukemia-specific epitopes in aplastic anemia, myelodysplastic syndrome and paroxysmal nocturnal hemoglobinuria: Results of a bone marrow failure consortium study. Leuk. Res. 2012, 36, 581–587. [Google Scholar] [CrossRef]
- Bockorny, B.; Dasanu, C.A. Autoimmune Manifestations in Large Granular Lymphocyte Leukemia. Clin. Lymphoma Myeloma Leuk. 2012, 12, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Lamy, T.; Bauer, F.A.; Liu, J.H.; Li, Y.X.; Pillemer, E.; Shahidi, H.; Gregory, S.A.; Zambello, R.; Marcolongo, R.; Semenzato, G.; et al. Clinicopathological features of aggressive large granular lymphocyte leukaemia resemble Fas ligand transgenic mice. Br. J. Haematol. 2000, 108, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Bareau, B.; Rey, J.; Hamidou, M.; Donadieu, J.; Morcet, J.; Reman, O.; Schleinitz, N.; Tournilhac, O.; Roussel, M.; Fest, T.; et al. Analysis of a French cohort of patients with large granular lymphocyte leukemia: A report on 229 cases. Haematologica 2010, 95, 1534–1541. [Google Scholar] [CrossRef]
- Harris, N.L.; Jaffe, E.S.; Diebold, J.; Flandrin, G.; Muller-Hermelink, H.K.; Vardiman, J.; Lister, T.A.; Bloomfield, C.D. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: Report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J. Clin. Oncol. 1999, 17, 3835–3849. [Google Scholar] [CrossRef] [PubMed]
- Steinway, S.N.; LeBlanc, F.; Loughran, T.P., Jr. The pathogenesis and treatment of large granular lymphocyte leukemia. Blood Rev. 2014, 28, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Gold, L.S.; Willett, W.C. The causes and prevention of cancer. Proc. Natl. Acad. Sci. USA 1995, 92, 5258–5265. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Petrus, M.; Bamford, R.; Shih, J.H.; Morris, J.C.; Janik, J.E.; Waldmann, T.A. Increased serum soluble IL-15Rα levels in T-cell large granular lymphocyte leukemia. Blood 2012, 119, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Liu, S.; Sams, G.H.; Curphey, D.P.; Santhanam, R.; Rush, L.J.; Schaefer, D.; Falkenberg, L.G.; Sullivan, L.; Jaroncyk, L.; et al. Aberrant overexpression of IL-15 initiates large granular lymphocyte leukemia through chromosomal instability and DNA hypermethylation. Cancer Cell 2012, 22, 645–655. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Liu, Y. Activation-induced cell death in T cells and autoimmunity. Cell Mol. Immunol. 2004, 1, 186–192. [Google Scholar]
- Isabelle, C.; Boles, A.; Chakravarti, N.; Porcu, P.; Brammer, J.; Mishra, A. Cytokines in the Pathogenesis of Large Granular Lymphocytic Leukemia. Front. Oncol. 2022, 12, 849917. [Google Scholar] [CrossRef]
- Gaudio, F.; Masciopinto, P.; Bellitti, E.; Musto, P.; Arcuti, E.; Battisti, O.; Cazzato, G.; Solombrino, A.; Laddaga, F.E.; Specchia, G.; et al. Molecular Features and Diagnostic Challenges in Alpha/Beta T-Cell Large Granular Lymphocyte Leukemia. Int. J. Mol. Sci. 2022, 23, 13392. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.C.; Moosic, K.B.; Jones, M.K.; Larkin, P.M.K.; Olson, T.L.; Toro, M.F.; Fox, T.E.; Feith, D.J.; Loughran, T.P., Jr. Large granular lymphocyte leukemia serum and corresponding hematological parameters reveal unique cytokine and sphingolipid biomarkers and associations with STAT3 mutations. Cancer Med. 2020, 9, 6533–6549. [Google Scholar] [CrossRef]
- Grabstein, K.H.; Eisenman, J.; Shanebeck, K.; Rauch, C.; Srinivasan, S.; Fung, V.; Beers, C.; Richardson, J.; Schoenborn, M.A.; Ahdieh, M.; et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science 1994, 264, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Shah, M.V.; Yang, J.; Nyland, S.B.; Liu, X.; Yun, J.K.; Albert, R.; Loughran, T.P., Jr. Network model of survival signaling in large granular lymphocyte leukemia. Proc. Natl. Acad. Sci. USA 2008, 105, 16308–16313. [Google Scholar] [CrossRef]
- Zambello, R.; Facco, M.; Trentin, L.; Sancetta, R.; Tassinari, C.; Perin, A.; Milani, A.; Pizzolo, G.; Rodeghiero, F.; Agostini, C.; et al. Interleukin-15 triggers the proliferation and cytotoxicity of granular lymphocytes in patients with lymphoproliferative disease of granular lymphocytes. Blood 1997, 89, 201–211. [Google Scholar] [CrossRef]
- Hodge, D.L.; Yang, J.; Buschman, M.D.; Schaughency, P.M.; Dang, H.; Bere, W.; Yang, Y.; Savan, R.; Subleski, J.J.; Yin, X.M.; et al. Interleukin-15 enhances proteasomal degradation of bid in normal lymphocytes: Implications for large granular lymphocyte leukemias. Cancer Res. 2009, 69, 3986–3994. [Google Scholar] [CrossRef]
- Miyazaki, T.; Kawahara, A.; Fujii, H.; Nakagawa, Y.; Minami, Y.; Liu, Z.J.; Oishi, I.; Silvennoinen, O.; Witthuhn, B.A.; Ihle, J.N.; et al. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science 1994, 266, 1045–1047. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.X.; Migone, T.S.; Tsang, M.; Friedmann, M.; Weatherbee, J.A.; Zhou, L.; Yamauchi, A.; Bloom, E.T.; Mietz, J.; John, S.; et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity 1995, 2, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T.A.; Tagaya, Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu. Rev. Immunol. 1999, 17, 19–49. [Google Scholar] [CrossRef]
- Mishra, A.; Sullivan, L.; Caligiuri, M.A. Molecular pathways: Interleukin-15 signaling in health and in cancer. Clin. Cancer Res. 2014, 20, 2044–2050. [Google Scholar] [CrossRef]
- Allavena, P.; Giardina, G.; Bianchi, G.; Mantovani, A. IL-15 is chemotactic for natural killer cells and stimulates their adhesion to vascular endothelium. J. Leukoc. Biol. 1997, 61, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, X.; Nyland, S.B.; Zhang, R.; Ryland, L.K.; Broeg, K.; Baab, K.T.; Jarbadan, N.R.; Irby, R.; Loughran, T.P., Jr. Platelet-derived growth factor mediates survival of leukemic large granular lymphocytes via an autocrine regulatory pathway. Blood 2010, 115, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Shvidel, L.; Duksin, C.; Tzimanis, A.; Shtalrid, M.; Klepfish, A.; Sigler, E.; Haran, M.; Eilat, E.; Berrebi, A. Cytokine release by activated T-cells in large granular lymphocytic leukemia associated with autoimmune disorders. Hematol. J. 2002, 3, 32–37. [Google Scholar] [CrossRef]
- Yang, J.; Epling-Burnette, P.K.; Painter, J.S.; Zou, J.; Bai, F.; Wei, S.; Loughran, T.P., Jr. Antigen activation and impaired Fas-induced death-inducing signaling complex formation in T-large-granular lymphocyte leukemia. Blood 2008, 111, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, J. The IL-2–IL-2 receptor pathway in health and disease: The role of the soluble IL-2 receptor. Clin. Immunol. 2020, 218, 108515. [Google Scholar] [CrossRef] [PubMed]
- Schaper, F.; Rose-John, S. Interleukin-6: Biology, signaling and strategies of blockade. Cytokine Growth Factor Rev. 2015, 26, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Teramo, A.; Gattazzo, C.; Passeri, F.; Lico, A.; Tasca, G.; Cabrelle, A.; Martini, V.; Frezzato, F.; Trimarco, V.; Ave, E.; et al. Intrinsic and extrinsic mechanisms contribute to maintain the JAK/STAT pathway aberrantly activated in T-type large granular lymphocyte leukemia. Blood 2013, 121, 3843–3854. [Google Scholar] [CrossRef]
- Kim, D.; Park, G.; Huuhtanen, J.; Ghimire, B.; Rajala, H.; Moriggl, R.; Chan, W.C.; Kankainen, M.; Myllymäki, M.; Mustjoki, S. STAT3 activation in large granular lymphocyte leukemia is associated with cytokine signaling and DNA hypermethylation. Leukemia 2021, 35, 3430–3443. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, J.S.; Rosler, K.M.; Harrison, D.A. The JAK/STAT signaling pathway. J. Cell Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef]
- Gentile, T.C.; Loughran, T.P., Jr. Interleukin-12 is a costimulatory cytokine for leukemic CD3+ large granular lymphocytes. Cell Immunol. 1995, 166, 158–161. [Google Scholar] [CrossRef]
- Papadaki, H.A.; Eliopoulos, G.D. Enhanced neutrophil extravasation may be a contributing factor in the determination of neutropenia in patients with chronic idiopathic neutropenia of adults. Eur. J. Haematol. 1998, 61, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Epling-Burnette, P.K.; Liu, J.H.; Catlett-Falcone, R.; Turkson, J.; Oshiro, M.; Kothapalli, R.; Li, Y.; Wang, J.M.; Yang-Yen, H.F.; Karras, J.; et al. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J. Clin. Investig. 2001, 107, 351–362. [Google Scholar] [CrossRef]
- Rajala, H.L.; Eldfors, S.; Kuusanmäki, H.; van Adrichem, A.J.; Olson, T.; Lagström, S.; Andersson, E.I.; Jerez, A.; Clemente, M.J.; Yan, Y.; et al. Discovery of somatic STAT5b mutations in large granular lymphocytic leukemia. Blood 2013, 121, 4541–4550. [Google Scholar] [CrossRef] [PubMed]
- Andersson, E.I.; Tanahashi, T.; Sekiguchi, N.; Gasparini, V.R.; Bortoluzzi, S.; Kawakami, T.; Matsuda, K.; Mitsui, T.; Eldfors, S.; Bortoluzzi, S.; et al. High incidence of activating STAT5B mutations in CD4-positive T-cell large granular lymphocyte leukemia. Blood 2016, 128, 2465–2468. [Google Scholar] [CrossRef]
- Kuwahara, N.; Kodaka, T.; Zushi, Y.; Sasaki, M.; Goka, T.; Maruoka, H.; Aoyama, Y.; Tsunemine, H.; Yamane, T.; Kobayashi, J.; et al. T-cell large granular lymphocytic (LGL) leukemia consists of CD4+/CD8dim and CD4−/CD8+ LGL populations in association with immune thrombocytopenia, autoimmune neutropenia, and monoclonal B-cell lymphocytosis. J. Clin. Exp. Hematop. 2019, 59, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Zambello, R.; Teramo, A.; Gattazzo, C.; Semenzato, G. Are T-LGL Leukemia and NK-Chronic Lymphoproliferative Disorder really two distinct diseases? Transl. Med. UniSa 2014, 8, 4–11. [Google Scholar]
- Lamy, T.; Liu, J.H.; Landowski, T.H.; Dalton, W.S.; Loughran, T.P., Jr. Dysregulation of CD95/CD95 ligand-apoptotic pathway in CD3+ large granular lymphocyte leukemia. Blood 1998, 92, 4771–4777. [Google Scholar] [CrossRef]
- Gelb, A.B.; van de Rijn, M.; Regula, D.P., Jr.; Cornbleet, J.P.; Kamel, O.W.; Horoupian, D.S.; Cleary, M.L.; Warnke, R.A. Epstein-Barr virus-associated natural killer-large granular lymphocyte leukemia. Hum. Pathol. 1994, 25, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.N.; Baker, B.W.; Inglis, M.J.; Nimmo, J.C.; Starling, G.C.; Deacon, E.; Rowe, M.; Beard, M.E. Epstein-Barr viral DNA in acute large granular lymphocyte (natural killer) leukemic cells. Blood 1992, 79, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- Starkebaum, G.; Loughran, T.P., Jr.; Kalyanaraman, V.S.; Kadin, M.E.; Kidd, P.G.; Singer, J.W.; Ruscetti, F.W. Serum reactivity to human T-cell leukaemia/lymphoma virus type I proteins in patients with large granular lymphocytic leukaemia. Lancet 1987, 1, 596–599. [Google Scholar] [CrossRef]
- Loughran, T.P., Jr.; Hadlock, K.G.; Perzova, R.; Gentile, T.C.; Yang, Q.; Foung, S.K.; Poiesz, B.J. Epitope mapping of HTLV envelope seroreactivity in LGL leukaemia. Br. J. Haematol. 1998, 101, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Sokol, L.; Agrawal, D.; Loughran, T.P., Jr. Characterization of HTLV envelope seroreactivity in large granular lymphocyte leukemia. Leuk. Res. 2005, 29, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Loughran, T.P., Jr.; Sherman, M.P.; Ruscetti, F.W.; Frey, S.; Coyle, T.; Montagna, R.A.; Jones, B.; Starkebaum, G.; Poiesz, B.J. Prototypical HTLV-I/II infection is rare in LGL leukemia. Leuk. Res. 1994, 18, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Pawson, R.; Schulz, T.F.; Matutes, E.; Catovsky, D. The human T-cell lymphotropic viruses types I/II are not involved in T prolymphocytic leukemia and large granular lymphocytic leukemia. Leukemia 1997, 11, 1305–1311. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Loughran, T.J.; Coyle, T.; Sherman, M.; Starkebaum, G.; Ehrlich, G.; Ruscetti, F.; Poiesz, B. Detection of human T-cell leukemia/lymphoma virus, type II, in a patient with large granular lymphocyte leukemia. Blood 1992, 80, 1116–1119. [Google Scholar] [CrossRef]
- Heneine, W.; Chan, W.C.; Lust, J.A.; Sinha, S.D.; Zaki, S.R.; Khabbaz, R.F.; Kaplan, J.E. HTLV-II infection is rare in patients with large granular lymphocyte leukemia. J. Acquir. Immune Defic. Syndr. 1994, 7, 736–737. [Google Scholar] [PubMed]
- Poullot, E.; Bouscary, D.; Guyader, D.; Ghandour, C.; Roussel, M.; Fest, T.; Houot, R.; Lamy, T. Large granular lymphocyte leukemia associated with hepatitis C virus infection and B cell lymphoma: Improvement after antiviral therapy. Leuk. Lymphoma 2013, 54, 1797–1799. [Google Scholar] [CrossRef]
- Mikhaylenko, D.S.; Nemtsova, M.V.; Bure, I.V.; Kuznetsova, E.B.; Alekseeva, E.A.; Tarasov, V.V.; Lukashev, A.N.; Beloukhova, M.I.; Deviatkin, A.A.; Zamyatnin, A.A., Jr. Genetic Polymorphisms Associated with Rheumatoid Arthritis Development and Antirheumatic Therapy Response. Int. J. Mol. Sci. 2020, 21, 4911. [Google Scholar] [CrossRef] [PubMed]
- Moosic, K.B.; Ananth, K.; Andrade, F.; Feith, D.J.; Darrah, E.; Loughran, T.P., Jr. Intersection Between Large Granular Lymphocyte Leukemia and Rheumatoid Arthritis. Front. Oncol. 2022, 12, 869205. [Google Scholar] [CrossRef]
- Stanford, S.M.; Maestre, M.F.; Campbell, A.M.; Bartok, B.; Kiosses, W.B.; Boyle, D.L.; Arnett, H.A.; Mustelin, T.; Firestein, G.S.; Bottini, N. Protein tyrosine phosphatase expression profile of rheumatoid arthritis fibroblast-like synoviocytes: A novel role of SH2 domain-containing phosphatase 2 as a modulator of invasion and survival. Arthritis Rheum. 2013, 65, 1171–1180. [Google Scholar] [CrossRef]
- Klareskog, L.; Rönnelid, J.; Saevarsdottir, S.; Padyukov, L.; Alfredsson, L. The importance of differences; On environment and its interactions with genes and immunity in the causation of rheumatoid arthritis. J. Intern. Med. 2020, 287, 514–533. [Google Scholar] [CrossRef]
- Gorodetskiy, V.R.; Sidorova, Y.V.; Kupryshina, N.A.; Vasilyev, V.I.; Probatova, N.A.; Ryzhikova, N.V.; Sudarikov, A.B. Analysis of a single-institution cohort of patients with Felty’s syndrome and T-cell large granular lymphocytic leukemia in the setting of rheumatoid arthritis. Rheumatol. Int. 2021, 41, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Romero, V.; Darrah, E.; Andrade, F. Generation of Distinct Patterns of Rheumatoid Arthritis Autoantigens by Peptidylarginine Deiminase Types 2 and 4 During Perforin-Induced Cell Damage. Arthritis Rheumatol. 2020, 72, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Gazitt, T.; Loughran, T.P., Jr. Chronic neutropenia in LGL leukemia and rheumatoid arthritis. Hematol. Am. Soc. Hematol. Educ. Program. 2017, 2017, 181–186. [Google Scholar] [CrossRef]
- Fitzgerald, J.E.; Ricalton, N.S.; Meyer, A.C.; West, S.G.; Kaplan, H.; Behrendt, C.; Kotzin, B.L. Analysis of clonal CD8+ T cell expansions in normal individuals and patients with rheumatoid arthritis. J. Immunol. 1995, 154, 3538–3547. [Google Scholar] [CrossRef]
- Schwaneck, E.C.; Renner, R.; Junker, L.; Einsele, H.; Gadeholt, O.; Geissinger, E.; Kleinert, S.; Gernert, M.; Tony, H.P.; Schmalzing, M. Prevalence and Characteristics of Persistent Clonal T Cell Large Granular Lymphocyte Expansions in Rheumatoid Arthritis: A Comprehensive Analysis of 529 Patients. Arthritis Rheumatol. 2018, 70, 1914–1922. [Google Scholar] [CrossRef]
- Gorodetskiy, V.; Vasilyev, V.; Sidorova, Y.; Biderman, B.; Kupryshina, N.; Vagida, M.; Ryzhikova, N.; Sudarikov, A. Clinical Study of the Relationship between Sjögren Syndrome and T-Cell Large Granular Lymphocytic Leukemia: Single-Center Experience. Int. J. Mol. Sci. 2022, 23, 13345. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Schattner, A.; Shvidel, L.; Berrebi, A. Characterization of T-cell large granular lymphocyte leukemia associated with Sjogren’s syndrome-an important but under-recognized association. Semin. Arthritis Rheum. 2006, 35, 306–311. [Google Scholar] [CrossRef]
- Sisto, M.; Lorusso, L.; Lisi, S. Interleukin-15 as a potential new target in Sjögren’s syndrome-associated inflammation. Pathology 2016, 48, 602–607. [Google Scholar] [CrossRef]
- Malamut, G.; El Machhour, R.; Montcuquet, N.; Martin-Lannerée, S.; Dusanter-Fourt, I.; Verkarre, V.; Mention, J.J.; Rahmi, G.; Kiyono, H.; Butz, E.A.; et al. IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. J. Clin. Investig. 2010, 120, 2131–2143. [Google Scholar] [CrossRef]
- Clemente, M.J.; Przychodzen, B.; Jerez, A.; Dienes, B.E.; Afable, M.G.; Husseinzadeh, H.; Rajala, H.L.; Wlodarski, M.W.; Mustjoki, S.; Maciejewski, J.P. Deep sequencing of the T-cell receptor repertoire in CD8+ T-large granular lymphocyte leukemia identifies signature landscapes. Blood 2013, 122, 4077–4085. [Google Scholar] [CrossRef] [PubMed]
- Calabretto, G.; Teramo, A.; Barilà, G.; Vicenzetto, C.; Gasparini, V.R.; Semenzato, G.; Zambello, R. Neutropenia and Large Granular Lymphocyte Leukemia: From Pathogenesis to Therapeutic Options. Cells 2021, 10, 2800. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.; Corcione, A.; Svahn, J.; Haupt, R.; Poggi, V.; Béka’ssy, A.N.; Scimè, R.; Pistorio, A.; Pistoia, V. TNF-α and IFN-γ are overexpressed in the bone marrow of Fanconi anemia patients and TNF-α suppresses erythropoiesis in vitro. Blood 2003, 102, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.G.; Berliner, N. T-cell large granular lymphocyte leukemia and related disorders. Oncologist 2004, 9, 247–258. [Google Scholar] [CrossRef]
- Maung, Z.T.; Norden, J.; Middleton, P.G.; Jack, F.R.; Chandler, J.E. Pure red cell aplasia: Further evidence of T cell clonal disorder. Br. J. Haematol. 1994, 87, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Go, R.S.; Li, C.Y.; Tefferi, A.; Phyliky, R.L. Acquired pure red cell aplasia associated with lymphoproliferative disease of granular T lymphocytes. Blood 2001, 98, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Fisch, P.; Handgretinger, R.; Schaefer, H.E. Pure red cell aplasia. Br. J. Haematol. 2000, 111, 1010–1022. [Google Scholar] [CrossRef]
- Lundell, R.; Hartung, L.; Hill, S.; Perkins, S.L.; Bahler, D.W. T-cell large granular lymphocyte leukemias have multiple phenotypic abnormalities involving pan-T-cell antigens and receptors for MHC molecules. Am. J. Clin. Pathol. 2005, 124, 937–946. [Google Scholar] [CrossRef]
- Sallusto, F.; Geginat, J.; Lanzavecchia, A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu. Rev. Immunol. 2004, 22, 745–763. [Google Scholar] [CrossRef]
- Fischer, L.; Hummel, M.; Burmeister, T.; Schwartz, S.; Thiel, E. Skewed expression of natural-killer (NK)-associated antigens on lymphoproliferations of large granular lymphocytes (LGL). Hematol. Oncol. 2006, 24, 78–85. [Google Scholar] [CrossRef]
- Epling-Burnette, P.K.; Painter, J.S.; Chaurasia, P.; Bai, F.; Wei, S.; Djeu, J.Y.; Loughran, T.P., Jr. Dysregulated NK receptor expression in patients with lymphoproliferative disease of granular lymphocytes. Blood 2004, 103, 3431–3439. [Google Scholar] [CrossRef]
- Scquizzato, E.; Teramo, A.; Miorin, M.; Facco, M.; Piazza, F.; Noventa, F.; Trentin, L.; Agostini, C.; Zambello, R.; Semenzato, G. Genotypic evaluation of killer immunoglobulin-like receptors in NK-type lymphoproliferative disease of granular lymphocytes. Leukemia 2007, 21, 1060–1069. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Morice, W.G.; Jevremovic, D.; Hanson, C.A. The expression of the novel cytotoxic protein granzyme M by large granular lymphocytic leukaemias of both T-cell and NK-cell lineage: An unexpected finding with implications regarding the pathobiology of these disorders. Br. J. Haematol. 2007, 137, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Pulik, M.; Lionnet, F.; Genet, P.; Petitdidier, C.; Jary, L.; Fourcade, C. CD3+ CD8+ CD56− clonal large granular lymphocyte leukaemia and HIV infection. Br. J. Haematol. 1997, 98, 444–445. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Castillo Tokumori, F.; Isenalumhe, L.; Zhang, Y.; Tandon, A.; Knepper, T.C.; Mo, Q.; Shao, H.; Zhang, L.; Sokol, L. Large granular lymphocytic leukemia—A retrospective study of 319 cases. Am. J. Hematol. 2021, 96, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Magnano, L.; Rivero, A.; Matutes, E. Large Granular Lymphocytic Leukemia: Current State of Diagnosis, Pathogenesis and Treatment. Curr. Oncol. Rep. 2022, 24, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, N.; Sawada, K.; Hirokawa, M.; Oshimi, K.; Sugimoto, K.; Matsuda, A.; Teramura, M.; Karasawa, M.; Arai, A.; Yonemura, Y.; et al. Long-term responses and outcomes following immunosuppressive therapy in large granular lymphocyte leukemia-associated pure red cell aplasia: A Nationwide Cohort Study in Japan for the PRCA Collaborative Study Group. Haematologica 2008, 93, 1555–1559. [Google Scholar] [CrossRef] [PubMed]
- Lamy, T.; Pastoret, C.; Houot, R.; Ysebaert, L.; Hunault, M.; Damaj, G.; Banos, A.; Tournilhac, O.; Choufi, B.; Marolleau, J.-P.; et al. Prospective, Multicentric Phase II Randomized Trial Comparing the Efficacy of Methotrexate or Cyclophosphamide in Large Granular Lymphocytic Leukemia: A French National Study. Report on the Interim Analysis. Blood 2019, 134, 1545. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, Q.; Hu, J.; Liu, X.; Guan, D.; Zhang, F. Clinical features and treatment outcomes in patients with T-cell large granular lymphocytic leukemia: A single-institution experience. Leuk. Res. 2020, 90, 106299. [Google Scholar] [CrossRef]
- Loughran, T.P., Jr.; Zickl, L.; Olson, T.L.; Wang, V.; Zhang, D.; Rajala, H.L.; Hasanali, Z.; Bennett, J.M.; Lazarus, H.M.; Litzow, M.R.; et al. Immunosuppressive therapy of LGL leukemia: Prospective multicenter phase II study by the Eastern Cooperative Oncology Group (E5998). Leukemia 2015, 29, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Sanikommu, S.R.; Clemente, M.J.; Chomczynski, P.; Afable, M.G., 2nd; Jerez, A.; Thota, S.; Patel, B.; Hirsch, C.; Nazha, A.; Desamito, J.; et al. Clinical features and treatment outcomes in large granular lymphocytic leukemia (LGLL). Leuk. Lymphoma 2018, 59, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Loughran, T.P.; Yao, X.; Bennett, J.M.; Litzow, M.R.; Evens, A.M.; Tallman, M.S. Results of a Prospective Multicenter Phase II Study of Initial Treatment with Methotrexate In LGL Leukemia (ECOG Protocol E5998). Blood 2010, 116, 702. [Google Scholar] [CrossRef]
- Friedman, B.; Cronstein, B. Methotrexate mechanism in treatment of rheumatoid arthritis. Jt. Bone Spine 2019, 86, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Battiwalla, M.; Melenhorst, J.; Saunthararajah, Y.; Nakamura, R.; Molldrem, J.; Young, N.S.; Barrett, A.J. HLA-DR4 predicts haematological response to cyclosporine in T-large granular lymphocyte lymphoproliferative disorders. Br. J. Haematol. 2003, 123, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, B.; Ito, S.; Feng, X.; Stephens, N.; Yunce, M.; Kajigaya, S.; Melenhorst, J.J.; Rios, O.; Scheinberg, P.; Chinian, F.; et al. Alemtuzumab in T-cell large granular lymphocytic leukaemia: Interim results from a single-arm, open-label, phase 2 study. Lancet Haematol. 2016, 3, e22–e29. [Google Scholar] [CrossRef]
- Thota, S.; Patel, B.J.; Sadaps, M.; Balasubramanian, S.; Sanikommu, S.; Hirsch, C.; Marotta, S.; Sekeres, M.A.; Risitano, A.M.; Maciejewski, J.P. Therapeutic outcomes using subcutaneous low dose alemtuzumab for acquired bone marrow failure conditions. Br. J. Haematol. 2018, 183, 133–136. [Google Scholar] [CrossRef]
- Lobbes, H.; Dervout, C.; Toussirot, E.; Felten, R.; Sibilia, J.; Wendling, D.; Gombert, B.; Ruivard, M.; Grobost, V.; Saraux, A.; et al. Rituximab for rheumatoid arthritis-associated large granular lymphocytic leukemia, a retrospective case series. Semin. Arthritis Rheum. 2020, 50, 1109–1113. [Google Scholar] [CrossRef]
- Tse, E.; Chan, J.C.; Pang, A.; Au, W.Y.; Leung, A.Y.; Lam, C.C.; Kwong, Y.L. Fludarabine, mitoxantrone and dexamethasone as first-line treatment for T-cell large granular lymphocyte leukemia. Leukemia 2007, 21, 2225–2226. [Google Scholar] [CrossRef]
- Marchand, T.; Lamy, T.; Finel, H.; Arcese, W.; Choquet, S.; Finke, J.; Huynh, A.; Irrera, G.; Karakasis, D.; Konopacki, J.; et al. Hematopoietic stem cell transplantation for T-cell large granular lymphocyte leukemia: A retrospective study of the European Society for Blood and Marrow Transplantation. Leukemia 2016, 30, 1201–1204. [Google Scholar] [CrossRef]
- Subbiah, V.; Viny, A.D.; Rosenblatt, S.; Pohlman, B.; Lichtin, A.; Maciejewski, J.P. Outcomes of splenectomy in T-cell large granular lymphocyte leukemia with splenomegaly and cytopenia. Exp. Hematol. 2008, 36, 1078–1083. [Google Scholar] [CrossRef]
- Yang, J.; LeBlanc, F.R.; Dighe, S.A.; Hamele, C.E.; Olson, T.L.; Feith, D.J.; Loughran, T.P., Jr. TRAIL mediates and sustains constitutive NF-κB activation in LGL leukemia. Blood 2018, 131, 2803–2815. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, D.; Morris, J.C.; Stetler-Stevenson, M.; Matthews, H.; Brown, M.R.; Fleisher, T.; Pittaluga, S.; Raffeld, M.; Albert, P.S.; Reitsma, D.; et al. EBV-related lymphoproliferative disease complicating therapy with the anti-CD2 monoclonal antibody, siplizumab, in patients with T-cell malignancies. Clin. Cancer Res. 2009, 15, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Frohna, P.; Tagaya, Y.; Ratnayake, A.; Doerr, N.; Basheer, A.; Al-Mawsawi, L.; Kim, W.J.; Zapata, J.; Wu, X.; Azimi, N. Results from a First-in-Human Study with Bnz-1: A Novel Peptide Inhibitor of IL-2, IL-9 and IL-15 for the Treatment of T-Cell Malignancies That Safely and Selectively Decreases Regulatory T-Cells, Natural Killer Cells, and CD8+ Central Memory T-Cells. Blood 2017, 130, 695. [Google Scholar] [CrossRef]
- Goel, N.; Needham, M.; Soler-Ferran, D.; Cotreau, M.M.; Escobar, J.; Greenberg, S. Pos1342 Depletion of Klrg1+ T Cells in a First-in-Human Clinical Trial of ABC008 in Inclusion Body Myositis (IBM). Ann. Rheum. Dis. 2022, 81, 1008–1009. [Google Scholar] [CrossRef]
- Zhang, L.; Nomura, F.; Aikawa, Y.; Kurosawa, Y.; Morishita, K.; Sudo, Y. Abstract 5586: PPMX-T003, a fully human anti-TfR1 antibody with potent efficacy against hematologic malignancies. Cancer Res. 2017, 77, 5586. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, A.J.; Ye, S.K. Highlighted STAT3 as a potential drug target for cancer therapy. BMB Rep. 2019, 52, 415–423. [Google Scholar] [CrossRef]
| NCT Number | Study Title | Intervention | Phase | Status |
|---|---|---|---|---|
| NCT04453345 | TPM Regimen (Thalidomide, Prednisone and Methotrexate) in LGLL | Thalidomide + Prednisone + Methotrexate | 2 and 3 | Recruiting |
| NCT05316116 | Siltuximab in Large Granular Lymphocytic Leukemia (LGLL) | Siltuximab | 1 | Recruiting |
| NCT05592015 | Ruxolitinib for the Treatment of T-Cell Large Granular Lymphocytic Leukemia | Ruxolitinib | 2 | Recruiting |
| NCT05532722 | ABC008 in Subjects With T-cell Large Granular Lymphocytic Leukemia (T-LGLL) | ABC008 | 1 and 2 | Recruiting |
| NCT05141682 | Oral Azacitidine for the Treatment of Relapsed or Refractory T-cell Large Granular Lymphocytic Leukemia | Oral Azacitidine | 1 and 2 | Recruiting |
| NCT05863234 | Safety Evaluation Study for Patients with Aggressive NK-cell Leukemia | PPMX-T003 | 1 and 2 | Recruiting |
| NCT05225584 | Safety, PK, PD, Clinical Activity of KT-333 in Adult Patients with Refractory Lymphoma, Large Granular Lymphocytic Leukemia, Solid Tumors | KT-333 | 1 | Recruiting |
| NCT05475925 | A Study of DR-01 in Subjects with Large Granular Lymphocytic Leukemia or Cytotoxic Lymphomas | DR-01 | 1 and 2 | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, F.; Markouli, M.; Orland, M.; Ogbue, O.; Dima, D.; Omar, N.; Mustafa Ali, M.K. Large Granular Lymphocytic Leukemia: Clinical Features, Molecular Pathogenesis, Diagnosis and Treatment. Cancers 2024, 16, 1307. https://doi.org/10.3390/cancers16071307
Ullah F, Markouli M, Orland M, Ogbue O, Dima D, Omar N, Mustafa Ali MK. Large Granular Lymphocytic Leukemia: Clinical Features, Molecular Pathogenesis, Diagnosis and Treatment. Cancers. 2024; 16(7):1307. https://doi.org/10.3390/cancers16071307
Chicago/Turabian StyleUllah, Fauzia, Mariam Markouli, Mark Orland, Olisaemeka Ogbue, Danai Dima, Najiullah Omar, and Moaath K. Mustafa Ali. 2024. "Large Granular Lymphocytic Leukemia: Clinical Features, Molecular Pathogenesis, Diagnosis and Treatment" Cancers 16, no. 7: 1307. https://doi.org/10.3390/cancers16071307
APA StyleUllah, F., Markouli, M., Orland, M., Ogbue, O., Dima, D., Omar, N., & Mustafa Ali, M. K. (2024). Large Granular Lymphocytic Leukemia: Clinical Features, Molecular Pathogenesis, Diagnosis and Treatment. Cancers, 16(7), 1307. https://doi.org/10.3390/cancers16071307






