Intrahepatic Mass-Forming Cholangiocarcinoma: Is There Additional Prognostic Value in Using Gd-EOB Enhanced MRI?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
- Histopathologically proven IMCC following surgical resection between 1 January 2011 and 31 December 2019 at the Department of Surgery, Charité—Universitätsmedizin Berlin.
- Preoperative abdominal Gd-EOB-enhanced MRI.
- Mass-forming growth pattern.
- Insufficient quality of preoperative imaging (e.g., severe motion artifacts in HBP).
- Histopathology other than IMCC (e.g., mixed HCC-CCA).
2.2. Clinical Features
2.3. Imaging
2.4. Readers
2.5. Analysis of HBP Gd-EOB Retention
2.6. Qualitative Imaging Features
2.7. Statistical Analysis
3. Results
3.1. Selection Procedure
3.2. Radiological Features
3.3. Clinical Features
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Florio, A.A.; Ferlay, J.; Znaor, A.; Ruggieri, D.; Alvarez, C.S.; Laversanne, M.; Bray, F.; McGlynn, K.A.; Petrick, J.L. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer 2020, 126, 2666–2678. [Google Scholar] [CrossRef]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Krenzien, F.; Nevermann, N.; Krombholz, A.; Benzing, C.; Haber, P.; Fehrenbach, U.; Lurje, G.; Pelzer, U.; Pratschke, J.; Schmelzle, M.; et al. Treatment of Intrahepatic Cholangiocarcinoma-A Multidisciplinary Approach. Cancers 2022, 14, 362. [Google Scholar] [CrossRef]
- Khan, S.A.; Thomas, H.C.; Davidson, B.R.; Taylor-Robinson, S.D. Cholangiocarcinoma. Lancet 2005, 366, 1303–1314. [Google Scholar] [CrossRef]
- Murakami, Y.; Uemura, K.; Sudo, T.; Hashimoto, Y.; Nakashima, A.; Kondo, N.; Sakabe, R.; Ohge, H.; Sueda, T. Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann. Surg. Oncol. 2011, 18, 651–658. [Google Scholar] [CrossRef]
- Ohtsuka, M.; Ito, H.; Kimura, F.; Shimizu, H.; Togawa, A.; Yoshidome, H.; Miyazaki, M. Results of surgical treatment for intrahepatic cholangiocarcinoma and clinicopathological factors influencing survival. Br. J. Surg. 2002, 89, 1525–1531. [Google Scholar] [CrossRef]
- Goeppert, B.; Toth, R.; Singer, S.; Albrecht, T.; Lipka, D.B.; Lutsik, P.; Brocks, D.; Baehr, M.; Muecke, O.; Assenov, Y.; et al. Integrative Analysis Defines Distinct Prognostic Subgroups of Intrahepatic Cholangiocarcinoma. Hepatology 2019, 69, 2091–2106. [Google Scholar] [CrossRef]
- Kang, Y.; Lee, J.M.; Kim, S.H.; Han, J.K.; Choi, B.I. Intrahepatic mass-forming cholangiocarcinoma: Enhancement patterns on gadoxetic acid-enhanced MR images. Radiology 2012, 264, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, Y.; Kim, H.; Yu, M.H.; Lee, E.S.; Yoon, J.H.; Joo, I.; Lee, J.M. Subtype Classification of Intrahepatic Cholangiocarcinoma Using Liver MR Imaging Features and Its Prognostic Value. Liver Cancer 2022, 11, 233–246. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Liau, J.Y.; Tsai, J.H.; Yuan, R.H.; Chang, C.N.; Lee, H.J.; Jeng, Y.M. Morphological subclassification of intrahepatic cholangiocarcinoma: Etiological, clinicopathological, and molecular features. Mod. Pathol. 2014, 27, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Kinzler, M.N.; Schulze, F.; Bankov, K.; Gretser, S.; Becker, N.; Leichner, R.; Stehle, A.; Abedin, N.; Trojan, J.; Zeuzem, S.; et al. Impact of small duct- and large duct type on survival in patients with intrahepatic cholangiocarcinoma: Results from a German tertiary center. Pathol. Res. Pract. 2022, 238, 154126. [Google Scholar] [CrossRef]
- Chung, Y.E.; Kim, M.J.; Park, Y.N.; Choi, J.Y.; Pyo, J.Y.; Kim, Y.C.; Cho, H.J.; Kim, K.A.; Choi, S.Y. Varying appearances of cholangiocarcinoma: Radiologic-pathologic correlation. Radiographics 2009, 29, 683–700. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhou, C.; Ni, X.; Huang, P.; Wu, F.; Yang, C.; Zeng, M. Preoperative subcategorization based on magnetic resonance imaging in intrahepatic cholangiocarcinoma. Cancer Imaging 2023, 23, 15. [Google Scholar] [CrossRef]
- Min, J.H.; Kim, Y.K.; Choi, S.Y.; Kang, T.W.; Lee, S.J.; Kim, J.M.; Ahn, S.; Cho, H. Intrahepatic Mass-forming Cholangiocarcinoma: Arterial Enhancement Patterns at MRI and Prognosis. Radiology 2019, 290, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.P.; Sheng, R.F.; Yang, C.; Zeng, M.S. Combined arterial and delayed enhancement patterns of MRI assist in prognostic prediction for intrahepatic mass-forming cholangiocarcinoma (IMCC). Abdom. Radiol. 2022, 47, 640–650. [Google Scholar] [CrossRef]
- Nam, J.G.; Lee, J.M.; Joo, I.; Ahn, S.J.; Park, J.Y.; Lee, K.B.; Han, J.K. Intrahepatic Mass-Forming Cholangiocarcinoma: Relationship Between Computed Tomography Characteristics and Histological Subtypes. J. Comput. Assist. Tomogr. 2018, 42, 340–349. [Google Scholar] [CrossRef]
- Fujita, N.; Asayama, Y.; Nishie, A.; Ishigami, K.; Ushijima, Y.; Takayama, Y.; Okamoto, D.; Moirta, K.; Shirabe, K.; Aishima, S.; et al. Mass-forming intrahepatic cholangiocarcinoma: Enhancement patterns in the arterial phase of dynamic hepatic CT—Correlation with clinicopathological findings. Eur. Radiol. 2017, 27, 498–506. [Google Scholar] [CrossRef]
- Yamada, S.; Morine, Y.; Imura, S.; Ikemoto, T.; Arakawa, Y.; Saito, Y.; Yoshikawa, M.; Miyazaki, K.; Shimada, M. Prognostic prediction of apparent diffusion coefficient obtained by diffusion-weighted MRI in mass-forming intrahepatic cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2020, 27, 388–395. [Google Scholar] [CrossRef]
- Koh, J.; Chung, Y.E.; Nahm, J.H.; Kim, H.Y.; Kim, K.S.; Park, Y.N.; Kim, M.J.; Choi, J.Y. Intrahepatic mass-forming cholangiocarcinoma: Prognostic value of preoperative gadoxetic acid-enhanced MRI. Eur. Radiol. 2016, 26, 407–416. [Google Scholar] [CrossRef]
- Thomaides-Brears, H.B.; Alkhouri, N.; Allende, D.; Harisinghani, M.; Noureddin, M.; Reau, N.S.; French, M.; Pantoja, C.; Mouchti, S.; Cryer, D.R.H. Incidence of Complications from Percutaneous Biopsy in Chronic Liver Disease: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2022, 67, 3366–3394. [Google Scholar] [CrossRef]
- Razumilava, N.; Gleeson, F.C.; Gores, G.J. Awareness of tract seeding with endoscopic ultrasound tissue acquisition in perihilar cholangiocarcinoma. Am. J. Gastroenterol. 2015, 110, 200. [Google Scholar] [CrossRef][Green Version]
- Fong, Z.V.; Brownlee, S.A.; Qadan, M.; Tanabe, K.K. The Clinical Management of Cholangiocarcinoma in the United States and Europe: A Comprehensive and Evidence-Based Comparison of Guidelines. Ann. Surg. Oncol. 2021, 28, 2660–2674. [Google Scholar] [CrossRef]
- Mavros, M.N.; Economopoulos, K.P.; Alexiou, V.G.; Pawlik, T.M. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014, 149, 565–574. [Google Scholar] [CrossRef]
- Auer, T.A.; Fehrenbach, U.; Grieser, C.; Penzkofer, T.; Geisel, D.; Schmelzle, M.; Müller, T.; Bläker, H.; Seehofer, D.; Denecke, T. Hepatocellular adenomas: Is there additional value in using Gd-EOB-enhanced MRI for subtype differentiation? Eur. Radiol. 2020, 30, 3497–3506. [Google Scholar] [CrossRef]
- Auer, T.A.; Walter-Rittel, T.; Geisel, D.; Schöning, W.; Schmelzle, M.; Müller, T.; Sinn, B.; Denecke, T.; Hamm, B.; Fehrenbach, U. HBP-enhancing hepatocellular adenomas and how to discriminate them from FNH in Gd-EOB MRI. BMC Med. Imaging 2021, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Chernyak, V.; Fowler, K.J.; Kamaya, A.; Kielar, A.Z.; Elsayes, K.M.; Bashir, M.R.; Kono, Y.; Do, R.K.; Mitchell, D.G.; Singal, A.G.; et al. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology 2018, 289, 816–830. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Mabilia, A.; Mazzotta, A.D.; Robin, F.; Ghallab, M.; Vibert, E.; Adam, R.; Cherqui, D.; Cunha, A.S.; Azoulay, D.; Salloum, C.; et al. R1 Vascular or Parenchymal Margins: What Is the Impact after Resection of Intrahepatic Cholangiocarcinoma? Cancers 2022, 14, 5151. [Google Scholar] [CrossRef]
- Zhang, X.F.; Xue, F.; Dong, D.H.; Weiss, M.; Popescu, I.; Marques, H.P.; Aldrighetti, L.; Maithel, S.K.; Pulitano, C.; Bauer, T.W.; et al. Number and Station of Lymph Node Metastasis After Curative-intent Resection of Intrahepatic Cholangiocarcinoma Impact Prognosis. Ann. Surg. 2021, 274, e1187–e1195. [Google Scholar] [CrossRef] [PubMed]
- Prueksapanich, P.; Piyachaturawat, P.; Aumpansub, P.; Ridtitid, W.; Chaiteerakij, R.; Rerknimitr, R. Liver Fluke-Associated Biliary Tract Cancer. Gut Liver 2018, 12, 236–245. [Google Scholar] [CrossRef]





| Imaging Feature | Definition |
|---|---|
| Size | Largest diameter in any plane. |
| Enhancement: rim APHE | The lesion gain areas of hyperintensity relative to liver parenchyma in the arterial phase, primarily in the lesion’s periphery. |
| Enhancement: non-rim APHE | As described above, but areas of relative hyperintensity are not primarily situated in the periphery. |
| Enhancement: progressive | The lesion gradually enhances through the arterial, portal venous, and delayed phases, with no hyperintensity relative to liver parenchyma in the arterial phase. |
| Enhancement: minimal | Little or no gain in signal intensity until the HBP. |
| Washout: peripheral | Reduction in lesion signal intensity relative to liver parenchyma from the arterial to portal venous phase, primarily in the lesion’s periphery. |
| Washout: non-peripheral | As described above, but signal intensity reduction is not primarily in the lesion’s periphery. |
| Shape: round | Lesion shape mostly conforms to a simple sphere or oval. |
| Shape: lobulated | Lesion shape is primarily polycyclic. |
| Margin: irregular | Margins of the lesion cannot be clearly followed in post-contrast dynamic phase imaging. |
| Margin: sharp | Margins of the lesion are clearly demarcated in post-contrast dynamic phase imaging. |
| Growth pattern: solid | Lesion appears to displace and compress surrounding liver parenchyma and structures such as blood vessels. |
| Growth pattern: infiltrative | Lesion appears to infiltrate its surroundings, with no clear transition discernible. |
| Intrahepatic metastasis: local | Daughter nodules within 3 cm of the primary lesion’s border. |
| Intrahepatic metastasis: distant | Daughter nodules further than 3 cm from the primary lesion’s border. |
| Biliary dilatation | Markedly dilated biliary ducts distal to the lesion. |
| Macrovascular invasion | Enhancing mass within the portal vein or its major branches or hepatic veins. |
| Intralesional hemorrhage | Areas of hyperintensity in precontrast T1w imaging with typical morphology for hemorrhage. |
| Cystic components | Intralesional areas of marked, homogeneous T2w hyperintensity without enhancement. |
| Diffusion restriction | Intralesional hyperintensity in diffusion-weighted imaging that persists at high B-values, with low signal intensity in the corresponding ADC map. |
| Apparent diffusion coefficient (ADC) | Measured by placing a 2D circular ROI in a hypointense area of the lesion in the corresponding ADC map. |
| T1w and T2w lesion signal intensity relative to parenchyma | Subjectively rated predominant signal intensity in precontrast T1w and T2w imaging, with and without FS, relative to liver parenchyma. Lesions with slightly but not markedly lower or higher signal intensity were rated as iso- to hypointense or iso- to hyperintense. |
| Radiological Features | Hypointense (n = 47) | Significantly-Retaining (n = 19) | p-Value |
|---|---|---|---|
| Size (mm) | 67 (55–100) | 63 (54–90) | 0.223 |
| Shape | 0.022 * | ||
| Lobulated | 100% (47/47) | 89% (17/19) | |
| Round | 0% (0/47) | 11% (2/19) | |
| Margin | 0.006 * | ||
| Irregular | 83% (39/47) | 47% (9/19) | |
| Sharp | 17% (8/47) | 53% (10/19) | |
| Growth pattern | 0.005 * | ||
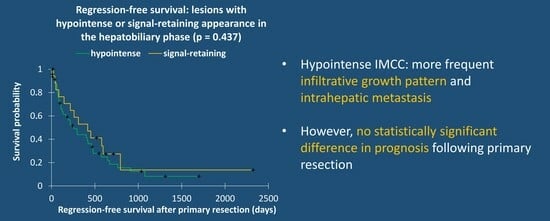
| Infiltrative | 70% (33/47) | 32% (6/19) | |
| Solid | 30% (14/47) | 68% (13/19) | |
| Local intrahepatic metastasis | 53% (25/47) | 26% (5/19) | 0.039 * |
| Distant intrahepatic metastasis | 23% (11/47) | 0% (0/19) | 0.022 * |
| Biliary dilatation | 83% (39/47) | 74% (14/19) | 0.368 |
| Macrovascular invasion | 21% (6/28) | 6% (1/18) | 0.380 |
| Intralesional hemorrhage | 2% (1/47) | 5% (1/19) | 0.490 |
| Cystic components | 23% (11/47) | 16% (3/19) | 0.741 |
| Diffusion restriction | 92% (22/24) | 89% (8/9) | 0.443 |
| Apparent diffusion coefficient | 1094 (924–1248) | 1075 (962–1181) | 0.910 |
| Enhancement pattern | 0.890 | ||
| Rim APHE | 16% (7/45) | 21% (4/19) | |
| Non-rim APHE | 18% (8/45) | 11% (2/19) | |
| Progressive enhancement | 53% (24/45) | 53% (10/19) | |
| Minimal enhancement | 13% (6/45) | 16% (3/19) | |
| Washout | 0.151 | ||
| Peripheral | 2% (1/45) | 16% (3/19) | |
| Non-peripheral | 16% (7/45) | 11% (2/19) | |
| No | 82% (34/45) | 73% (14/19) | |
| Gd-EOB retention area | N/A | ||
| 0 (0–5%) | 19% (9/47) | 0% (0/19) | |
| 1 (5–25%) | 81% (38/47) | 0% (0/19) | |
| 2 (25–50%) | 0% (0/47) | 42% (8/19) | |
| 3 (50–75%) | 0% (0/47) | 58% (11/19) | |
| 4 (75–100%) | 0% (0/47) | 0% (0/19) | |
| HBP enhancement ratio | 0.47 (0.39–0.63) | 0.70 (0.54–0.89) | 0.002 * |
| T1 signal intensity | 0.678 | ||
| Hypointense | 89% (42/47) | 84% (16/19) | |
| Iso- to hypointense | 11% (5/47) | 16% (3/19) | |
| Isointense | 0% (0/47) | 0% (0/19) | |
| Iso- to hyperintense | 0% (0/47) | 0% (0/19) | |
| Hyperintense | 0% (0/47) | 0% (0/19) | |
| T1-FS signal intensity | 0.492 | ||
| Hypointense | 85% (40/47) | 79% (15/19) | |
| Iso- to hypointense | 15% (7/47) | 21% (4/19) | |
| Isointense | 0% (0/47) | 0% (0/19) | |
| Iso- to hyperintense | 0% (0/47) | 0% (0/19) | |
| Hyperintense | 0% (0/47) | 0% (0/19) | |
| T2 signal intensity | 0.224 | ||
| Hypointense | 0% (0/47) | 0% (0/19) | |
| Iso- to hypointense | 0% (0/47) | 0% (0/19) | |
| Isointense | 0% (0/47) | 0% (0/19) | |
| Iso- to hyperintense | 49% (23/47) | 32% (6/19) | |
| Hyperintense | 51% (24/47) | 68% (13/19) | |
| T2-FS signal intensity | 0.744 | ||
| Hypointense | 0% (0/47) | 0% (0/19) | |
| Iso- to hypointense | 0% (0/47) | 0% (0/19) | |
| Isointense | 0% (0/47) | 0% (0/19) | |
| Iso- to hyperintense | 21% (10/47) | 16% (3/19) | |
| Hyperintense | 79% (37/47) | 84% (16/19) |
| Clinical Features | Hypointense (n = 47) | Significantly-Retaining (n = 19) | p-Value |
|---|---|---|---|
| Male gender | 47% (21/47) | 53% (10/19) | 0.616 |
| Age (years) | 63 (55–70) | 69 (58–77) | 0.068 |
| Laboratory values | |||
| CA19-9 (U/mL) (n = 29) | 47.8 (5.8–90.3) | 24.3 (10.0–822) | 0.966 |
| CEA (µg/L) (n = 18) | 1.6 (1.2–3.2) | 2.0 (1.2–21.2) | 0.500 |
| AFP (µg/L) (n = 20) | 4.9 (2.5–6) | 5.5 (3.1–9.3) | 0.628 |
| Total bilirubin (mg/dL) (n = 55) | 0.5 (0.3–0.8) | 0.6 (0.3–1.2) | 0.330 |
| Histological grade ° | 0.865 | ||
| 1 | 4% (2/46) | 0% (0/18) | |
| 2 | 72% (33/46) | 67% (12/18) | |
| 3 | 24% (11/46) | 33% (6/18) | |
| T-stage | 0.305 | ||
| 1 | 37% (17/46) | 37% (7/19) | |
| 2 | 48% (22/46) | 32% (6/19) | |
| 3 | 11% (5/46) | 16% (3/19) | |
| 4 | 4% (2/46) | 16% (3/19) | |
| N1 or higher | 51% (23/45) | 41% (7/17) | 0.565 |
| V1-status | 15% (7/46) | 26% (5/19) | 0.299 |
| R1-status | 24% (11/46) | 41% (7/17) | 0.322 |
| Postoperative complications | |||
| Any complication | 72% (31/43) | 88% (15/17) | 0.311 |
| Liver failure | 10% (3/31) | 15% (2/13) | 0.617 |
| Kidney failure | 7% (3/42) | 12% (2/17) | 1.000 |
| Pneumonia | 16% (7/43) | 24% (4/17) | 0.481 |
| Intra-abdominal abscess | 14% (6/43) | 29% (5/17) | 0.261 |
| Anastomotic insufficiency | 9% (4/43) | 29% (5/17) | 0.100 |
| Bile leakage requiring intervention | 19% (8/43) | 29% (5/17) | 0.486 |
| Other complications ^ | 26% (11/43) | 35% (6/17) | 0.547 |
| Postoperative hospital stay (days) | 13 (8–23) | 18 (13–49) | 1.000 |
| Postoperative ICU stay (days) | 2 (1–3) | 1 (1–5) | 0.617 |
| Recurrence-free survival | 0.410 # | ||
| Time to recurrence (days) | 204 (94–415) | 217 (62–498) | 0.830 |
| 3-month RFS | 82% (32/39) | 60% (12/20) | 0.662 |
| 6-month RFS | 71% (24/34) | 50% (11/22) | 1.000 |
| 12-month RFS | 59% (17/29) | 43% (9/21) | 0.719 |
| 18-month RFS | 45% (10/22) | 33% (6/18) | 0.240 |
| Overall survival | 0.491 # | ||
| Time to death (days) | 518 (113–809) | 464 (255–805) | 0.612 |
| 3-month OS | 97% (38/39) | 100% (14/14) | 0.662 |
| 6-month OS | 87% (34/39) | 100% (14/14) | 1.000 |
| 12-month OS | 69% (27/39) | 93% (13/14) | 0.719 |
| 18-month OS | 56% (22/39) | 71% (10/14) | 0.240 |
| Factors | Adjusted HR, RFS | p-Value | Adjusted HR, OS | p-Value | ||
|---|---|---|---|---|---|---|
| Model 1 |  |  | ||||
| HBP signal-retention | 0.84 (0.44–1.61) | 0.602 | 1.38 (0.66–2.89) | 0.397 | ||
| Hypervascular appearance | 0.70 (0.36–1.36) | 0.297 | 0.52 (0.21–1.27) | 0.151 | ||
| Model 2 | ||||||
| HBP signal-retention | 1.26 (0.62–2.53) | 0.526 | 1.64 (0.74–3.67) | 0.226 | ||
| Intrahepatic metastasis | 2.53 (1.34–4.75) | 0.004 * | 1.85 (0.86–3.96) | 0.116 | ||
| Model 3 | ||||||
| HBP signal-retention | 1.05 (0.52–2.32) | 0.891 | 3.16 (1.43–6.97) | 0.168 | ||
| N1 or higher | 2.85 (1.50–5.40) | 0.001 * | 1.73 (0.79–3.79) | 0.004 * | ||
| Model 4 | ||||||
| HBP enhancement ratio | 0.74 (0.19–2.87) | 0.667 | 1.21 (0.27–5.51) | 0.802 | ||
| Hypervascular appearance | 0.72 (0.35–1.46) | 0.356 | 0.72 (0.29–1.77) | 0.468 | ||
| Model 5 | ||||||
| HBP enhancement ratio | 0.84 (0.22–3.15) | 0.790 | 1.59 (0.36–7.04) | 0.545 | ||
| Intrahepatic metastasis | 2.31 (1.26–4.23) | 0.007 * | 1.76 (0.85–3.62) | 0.127 | ||
| Model 6 | ||||||
| HBP enhancement ratio | 1.22 (0.29–5.11) | 0.784 | 2.61 (0.54–12.5) | 0.231 | ||
| N1 or higher | 3.22 (1.64–6.32) | 0.001 * | 3.77 (1.58–8.97) | 0.003 * | ||
| 0.1 1 10 | 0.1 1 10 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halskov, S.; Krenzien, F.; Segger, L.; Geisel, D.; Hamm, B.; Pelzer, U.; Ihlow, J.; Schöning, W.; Auer, T.A.; Fehrenbach, U. Intrahepatic Mass-Forming Cholangiocarcinoma: Is There Additional Prognostic Value in Using Gd-EOB Enhanced MRI? Cancers 2024, 16, 1314. https://doi.org/10.3390/cancers16071314
Halskov S, Krenzien F, Segger L, Geisel D, Hamm B, Pelzer U, Ihlow J, Schöning W, Auer TA, Fehrenbach U. Intrahepatic Mass-Forming Cholangiocarcinoma: Is There Additional Prognostic Value in Using Gd-EOB Enhanced MRI? Cancers. 2024; 16(7):1314. https://doi.org/10.3390/cancers16071314
Chicago/Turabian StyleHalskov, Sebastian, Felix Krenzien, Laura Segger, Dominik Geisel, Bernd Hamm, Uwe Pelzer, Jana Ihlow, Wenzel Schöning, Timo Alexander Auer, and Uli Fehrenbach. 2024. "Intrahepatic Mass-Forming Cholangiocarcinoma: Is There Additional Prognostic Value in Using Gd-EOB Enhanced MRI?" Cancers 16, no. 7: 1314. https://doi.org/10.3390/cancers16071314
APA StyleHalskov, S., Krenzien, F., Segger, L., Geisel, D., Hamm, B., Pelzer, U., Ihlow, J., Schöning, W., Auer, T. A., & Fehrenbach, U. (2024). Intrahepatic Mass-Forming Cholangiocarcinoma: Is There Additional Prognostic Value in Using Gd-EOB Enhanced MRI? Cancers, 16(7), 1314. https://doi.org/10.3390/cancers16071314