In Curative Stereotactic Body Radiation Therapy for Prostate Cancer, There Is a High Possibility That 45 Gy in Five Fractions Will Not Be Tolerated without a Hydrogel Spacer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Eligibility
2.2. Treatment Planning
2.3. Radiotherapy
2.4. Study Endpoints and Statistics
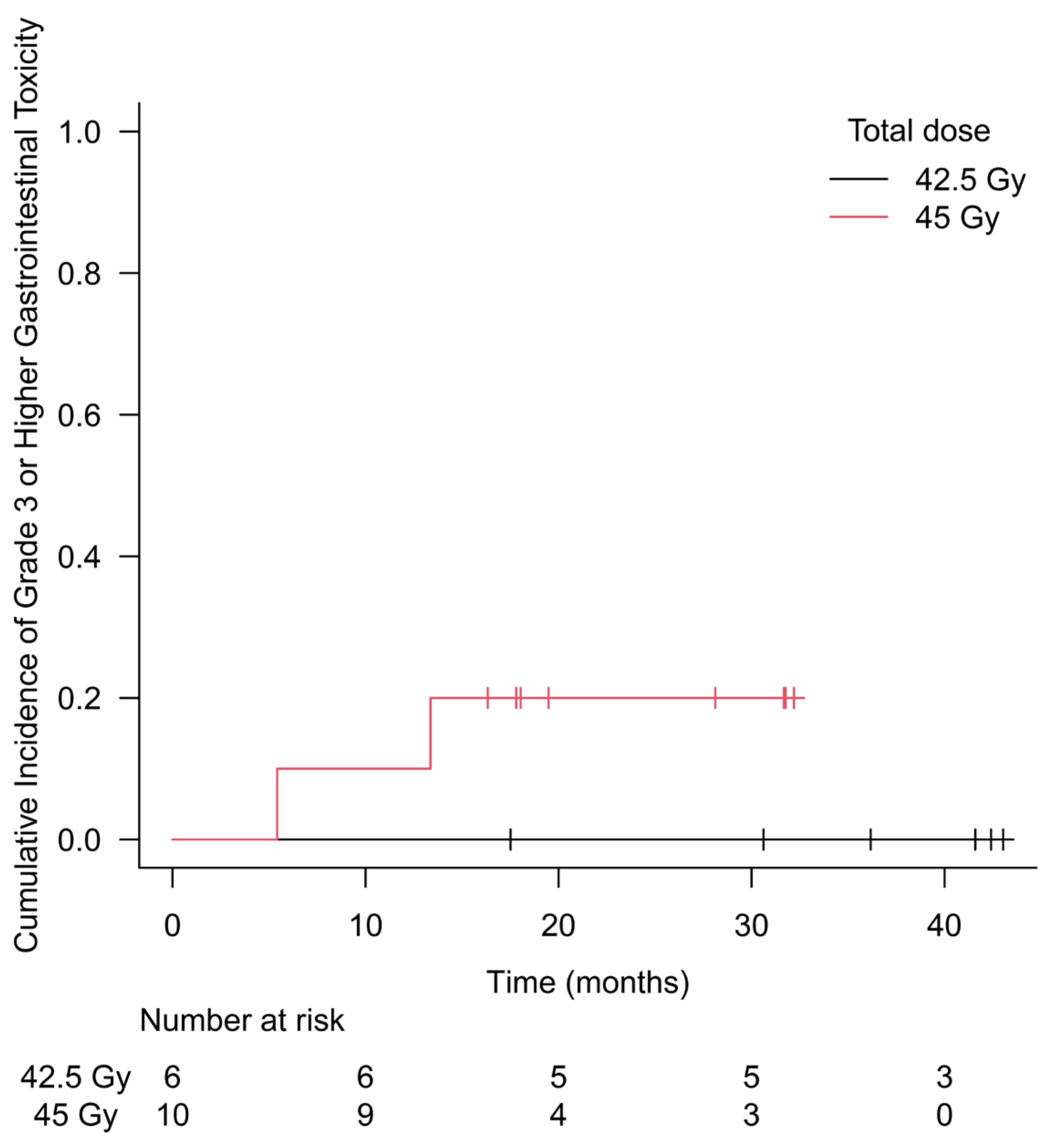
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dasu, A.; Toma-Dasu, I. Prostate alpha/beta revisited—An analysis of clinical results from 14 168 patients. Acta Oncol. 2012, 51, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Arabpour, A.; Shahbazi-Gahrouei, D. Effect of Hypofractionation on Prostate Cancer Radiotherapy. Int. J. Cancer Manag. 2017, 10, e12204. [Google Scholar] [CrossRef]
- Catton, C.N.; Lukka, H.; Gu, C.-S.; Martin, J.M.; Supiot, S.; Chung, P.W.M.; Bauman, G.S.; Bahary, J.-P.; Ahmed, S.; Cheung, P.; et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J. Clin. Oncol. 2017, 35, 1884–1890. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, K.E.; Voong, K.R.; Levy, L.B.; Allen, P.K.; Choi, S.; Schlembach, P.J.; Lee, A.K.; McGuire, S.E.; Nguyen, Q.; Pugh, T.J.; et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J. Clin. Oncol. 2018, 36, 2943–2949. [Google Scholar] [CrossRef] [PubMed]
- Cuccia, F.; Fiorentino, A.; Corrao, S.; Mortellaro, G.; Valenti, V.; Tripoli, A.; De Gregorio, G.; Serretta, V.; Verderame, F.; Ognibene, L.; et al. Moderate hypofractionated helical tomotherapy for prostate cancer in a cohort of older patients: A mono-institutional report of toxicity and clinical outcomes. Aging Clin. Exp. Res. 2019, 32, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Cuccia, F.; Mazzola, R.; Arcangeli, S.; Mortellaro, G.; Figlia, V.; Caminiti, G.; Di Paola, G.; Spera, A.; Iacoviello, G.; Alongi, F.; et al. Moderate hypofractionated helical tomotherapy for localized prostate cancer: Preliminary report of an observational prospective study. Tumori J. 2019, 105, 516–523. [Google Scholar] [CrossRef]
- King, C.R.; Freeman, D.; Kaplan, I.; Fuller, D.; Bolzicco, G.; Collins, S.; Meier, R.; Wang, J.; Kupelian, P.; Steinberg, M.; et al. Stereotactic body radiotherapy for localized prostate cancer: Pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother. Oncol. 2013, 109, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Kishan, A.U.; Dang, A.; Katz, A.J.; Mantz, C.A.; Collins, S.P.; Aghdam, N.; Chu, F.-I.; Kaplan, I.D.; Appelbaum, L.; Fuller, D.B.; et al. Long-term outcomes of stereotactic body radiotherapy for low-risk and intermediate-risk prostate cancer. JAMA Netw. Open 2019, 2, e188006. [Google Scholar] [CrossRef] [PubMed]
- Alongi, F.; Mazzola, R.; Fiorentino, A.; Corradini, S.; Aiello, D.; Figlia, V.; Gregucci, F.; Ballario, R.; Cavalleri, S.; Ruggieri, R. Phase II study of accelerated Linac-based SBRT in five consecutive fractions for localized prostate cancer. Strahlenther. Onkol. 2019, 195, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Katz, A. Stereotactic Body Radiotherapy for Low-Risk Prostate Cancer: A Ten-Year Analysis. Cureus 2017, 9, e1668. [Google Scholar] [CrossRef] [PubMed]
- De Bari, B.; Arcangeli, S.; Ciardo, D.; Mazzola, R.; Alongi, F.; Russi, E.G.; Santoni, R.; Magrini, S.M.; Jereczek-Fossa, B.A. Extreme hypofractionation for early prostate cancer: Biology meets technology. Cancer Treat. Rev. 2016, 50, 48–60. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Prostate Cancer (Version 3.2024). Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 15 March 2024).
- McBride, S.M.; Wong, D.S.; Dombrowski, J.J.; Harkins, B.; Tapella, P.; Hanscom, H.N.; Collins, S.P.; Kaplan, I.D. Hypofractionated stereotactic body radiotherapy in low-risk prostate adenocarcinoma. Cancer 2012, 118, 3681–3690. [Google Scholar] [CrossRef] [PubMed]
- Zelefsky, M.J.; Kollmeier, M.; McBride, S.; Varghese, M.; Mychalczak, B.; Gewanter, R.; Garg, M.K.; Happersett, L.; Goldman, D.A.; Pei, I.; et al. Five-year outcomes of a phase 1 dose-escalation study using stereotactic body radiosurgery for patients with low-risk and intermediate-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Kainuma, T.; Kawakami, S.; Tsumura, H.; Satoh, T.; Tabata, K.-I.; Iwamura, M.; Hayakawa, K.; Ishiyama, H. A phase I dose-escalation trial of stereotactic body radiotherapy using 4 fractions for patients with localized prostate cancer. Radiat. Oncol. 2019, 14, 158. [Google Scholar] [CrossRef]
- Boike, T.P.; Lotan, Y.; Cho, L.C.; Brindle, J.; DeRose, P.; Xie, X.-J.; Yan, J.; Foster, R.; Pistenmaa, D.; Perkins, A.; et al. Phase I Dose-Escalation Study of Stereotactic Body Radiation Therapy for Low- and Intermediate-Risk Prostate Cancer. J. Clin. Oncol. 2011, 29, 2020–2026. [Google Scholar] [CrossRef] [PubMed]
- Potters, L.; Rana, Z.; Lee, L.; Cox, B.W. Outcomes of a dose-escalated stereotactic body radiation phase 1 trial for patients with low- and intermediate-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 334–342. [Google Scholar] [CrossRef]
- Hannan, R.; Tumati, V.; Xie, X.-J.; Cho, L.C.; Kavanagh, B.D.; Brindle, J.; Raben, D.; Nanda, A.; Cooley, S.; Kim, D.N.W.; et al. Stereotactic body radiation therapy for low and intermediate risk prostate cancer—Results from a multi-institutional clinical trial. Eur. J. Cancer 2016, 59, 142–151. [Google Scholar] [CrossRef]
- Ivanova, A. Escalation, group andA +B designs for dose-finding trials. Stat. Med. 2006, 25, 3668–3678. [Google Scholar] [CrossRef]
- Roach, M., 3rd; Hanks, G.; Thames, H., Jr.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining Biochemical Failure Following Radiotherapy with or without Hormonal Therapy in Men with Clinically Localized Prostate Cancer: Recommendations of the Rtog-Astro Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Zietman, A.L.; Bae, K.; Slater, J.D.; Shipley, W.U.; Efstathiou, J.A.; Coen, J.J.; Bush, D.A.; Lunt, M.; Spiegel, D.Y.; Skowronski, R.; et al. Randomized Trial Comparing Conventional-Dose With High-Dose Conformal Radiation Therapy in Early-Stage Adenocarcinoma of the Prostate: Long-Term Results From Proton Radiation Oncology Group/American College of Radiology 95-09. J. Clin. Oncol. 2010, 28, 1106–1111. [Google Scholar] [CrossRef]
- Beckendorf, V.; Guerif, S.; Le Prisé, E.; Cosset, J.-M.; Bougnoux, A.; Chauvet, B.; Salem, N.; Chapet, O.; Bourdain, S.; Bachaud, J.-M.; et al. 70 Gy Versus 80 Gy in Localized Prostate Cancer: 5-Year Results of GETUG 06 Randomized Trial. Endocrine 2011, 80, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Creak, A.; Hall, E.; Horwich, A.; Eeles, R.; Khoo, V.; Huddart, R.; Parker, C.; Griffin, C.; Bidmead, M.; Warrington, J.; et al. Randomised pilot study of dose escalation using conformal radiotherapy in prostate cancer: Long-term follow-up. Br. J. Cancer 2013, 109, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Dearnaley, D.P.; Jovic, G.; Syndikus, I.; Khoo, V.; Cowan, R.A.; Graham, J.D.; Aird, E.G.; Bottomley, D.; Huddart, R.A.; Jose, C.C.; et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: Long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014, 15, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Heemsbergen, W.D.; Al-Mamgani, A.; Slot, A.; Dielwart, M.F.; Lebesque, J.V. Long-Term Results of the Dutch Randomized Prostate Cancer Trial: Impact of Dose-Escalation on Local, Biochemical, Clinical Failure, and Survival. Radiother. Oncol. 2014, 110, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Michalski, J.M.; Moughan, J.; Purdy, J.; Bosch, W.; Bruner, D.W.; Bahary, J.-P.; Lau, H.; Duclos, M.; Parliament, M.; Morton, G.; et al. Effect of Standard vs Dose-Escalated Radiation Therapy for Patients With Intermediate-Risk Prostate Cancer. JAMA Oncol. 2018, 4, e180039. [Google Scholar] [CrossRef] [PubMed]
- Pasalic, D.; Kuban, D.A.; Allen, P.K.; Tang, C.; Mesko, S.M.; Grant, S.R.; Augustyn, A.A.; Frank, S.J.; Choi, S.; Hoffman, K.E.; et al. Dose Escalation for Prostate Adenocarcinoma: A Long-Term Update on the Outcomes of a Phase 3, Single Institution Randomized Clinical Trial. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Kerkmeijer, L.G.W.; Groen, V.H.; Pos, F.J.; Haustermans, K.; Monninkhof, E.M.; Smeenk, R.J.; Kunze-Busch, M.; de Boer, J.C.J.; van Zijp, J.v.d.V.; van Vulpen, M.; et al. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients With Localized Prostate Cancer: Results From the FLAME Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 787–796. [Google Scholar] [CrossRef]
- Deodato, F.; Ferro, M.; Bonome, P.; Pezzulla, D.; Romano, C.; Buwenge, M.; Cilla, S.; Morganti, A.G.; Macchia, G. Stereotactic Body Radiotherapy (Sib-Vmat Technique) to Dominant Intraprostatic Lesion (Dil) for Localized Prostate Cancer: A Dose-Escalation Trial (Destroy-4). Strahlenther. Onkol. 2024, 200, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Marvaso, G.; Corrao, G.; Repetti, I.; Lorubbio, C.; Bellerba, F.; Zaffaroni, M.; Vincini, M.G.; Zerini, D.; Alessi, S.; Luzzago, S.; et al. Extreme-hypofractionated RT with concomitant boost to the DIL in PCa: A 5-year update on oncological and patient-reported outcomes for the phase II trial “GIVE ME FIVE”. World J. Urol. 2024, 42, 169. [Google Scholar] [CrossRef] [PubMed]
- Yasar, B.; Suh, Y.E.; Chapman, E.; Nicholls, L.; Henderson, D.; Jones, C.; Morrison, K.; Wells, E.; Henderson, J.; Meehan, C.; et al. Simultaneous Focal Boost with Stereotactic Radiotherapy for Localised Intermediate to High-Risk Prostate Cancer: Primary Outcomes of the Sparc Phase Ii Trial. Int. J. Radiat. Oncol. Biol. Phys. 2024. [Google Scholar] [CrossRef]
- Rogatko, A.; Schoeneck, D.; Jonas, W.; Tighiouart, M.; Khuri, F.R.; Porter, A. Translation of Innovative Designs Into Phase I Trials. J. Clin. Oncol. 2007, 25, 4982–4986. [Google Scholar] [CrossRef] [PubMed]
- Chiuzan, C.; Shtaynberger, J.; Manji, G.A.; Duong, J.K.; Schwartz, G.K.; Ivanova, A.; Lee, S.M. Dose-finding designs for trials of molecularly targeted agents and immunotherapies. J. Biopharm. Stat. 2017, 27, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Araujo, D.V.; Oliva, M.; Li, K.; Fazelzad, R.; Liu, Z.A.; Siu, L.L. Contemporary dose-escalation methods for early phase studies in the immunotherapeutics era. Eur. J. Cancer 2021, 158, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Love, S.B.; Brown, S.; Weir, C.J.; Harbron, C.; Yap, C.; Gaschler-Markefski, B.; Matcham, J.; Caffrey, L.; McKevitt, C.; Clive, S.; et al. Embracing model-based designs for dose-finding trials. Br. J. Cancer 2017, 117, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Rubinstein, L.; Arbuck, S.G.; Christian, M.C.; Freidlin, B.; Collins, J. Accelerated Titration Designs for Phase I Clinical Trials in Oncology. J. Natl. Cancer Inst. 1997, 89, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Skolnik, J.M.; Barrett, J.S.; Jayaraman, B.; Patel, D.; Adamson, P.C. Shortening the Timeline of Pediatric Phase I Trials: The Rolling Six Design. J. Clin. Oncol. 2008, 26, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Babar, M.; Katz, A.; Ciatto, M. Dosimetric and clinical outcomes of SpaceOAR in men undergoing external beam radiation therapy for localized prostate cancer: A systematic review. J. Med. Imaging Radiat. Oncol. 2021, 65, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, N.; Bahl, A.; Pinkawa, M.; Ryder, S.; Ahmadu, C.; Ross, J.; Bhattacharyya, S.; Woodward, E.; Battaglia, S.; Binns, J.; et al. Spaceoar Hydrogel Spacer for Reducing Radiation Toxicity During Radiotherapy for Prostate Cancer. A Systematic Review. Urology 2021, 156, e74–e85. [Google Scholar] [CrossRef] [PubMed]
- Velde, B.L.T.; Westhuyzen, J.; Awad, N.; Wood, M.; Shakespeare, T.P. Late toxicities of prostate cancer radiotherapy with and without hydrogel SpaceAOR insertion. J. Med. Imaging Radiat. Oncol. 2019, 63, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.A.; Tree, A.C.; Dearnaley, D.; Parker, C.C.; Prasad, V.; Roach, M.; Lawton, C.A.F. Considering benefit and risk before routinely recommending SpaceOAR. Lancet Oncol. 2021, 22, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Ogita, M.; Yamashita, H.; Nozawa, Y.; Ozaki, S.; Sawayanagi, S.; Ohta, T.; Nakagawa, K. Phase II study of stereotactic body radiotherapy with hydrogel spacer for prostate cancer: Acute toxicity and propensity score-matched comparison. Radiat. Oncol. 2021, 16, 107. [Google Scholar] [CrossRef] [PubMed]
- Ogita, M.; Yamashita, H.; Sawayanagi, S.; Takahashi, W.; Nakagawa, K. Efficacy of a hydrogel spacer in three-dimensional conformal radiation therapy for prostate cancer. Ultrasound Med. Biol. 2020, 50, 303–309. [Google Scholar] [CrossRef] [PubMed]
| Prescribed Dose, Gy | PTV | Bladder Wall | Rectum 1 | Femoral Heads | Small Bowel | Urethra | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| D95% 2, Gy | D99% 2, Gy | V20 Gy 3, cc | Max dose, Gy | V40 Gy 3, % | V25 Gy 3, % | Max Dose, Gy | V20 Gy 3, cc | Max Dose, Gy | Max Dose, Gy | |
| 42.5 | 42.5 | ≥40.375 | ≤50 | ≤46.75 | ≤25 | ≤45 | ≤30 | ≤10 | ≤29 | ≤44.625 |
| 45 | 45.0 | ≥42.750 | ≤50 | ≤49.50 | ≤25 | ≤45 | ≤30 | ≤10 | ≤29 | ≤47.250 |
| 47.5 | 47.5 | ≥45.125 | ≤50 | ≤52.25 | ≤25 | ≤45 | ≤30 | ≤10 | ≤29 | ≤49.875 |
| Characteristics | Dose | Total | |
|---|---|---|---|
| 42.5 Gy | 45 Gy | ||
| Number of patients | 6 | 10 | 16 |
| Age, years, median (range) | 72 (71–83) | 71 (63–84) | 71.5 (63–84) |
| Clinical T stage, n (%) | |||
| 1c | 0 (0%) | 0 (0%) | 0 (0%) |
| 2a | 3 (50.0%) | 4 (40.0%) | 7 (43.8%) |
| 2b | 0 (0%) | 0 (0%) | 0 (0%) |
| 2c | 1 (16.7%) | 1 (10.0%) | 2 (12.5%) |
| 3a | 2 (33.3%) | 3 (30.0%) | 5 (31.3%) |
| 3b | 0 (0%) | 2 (20.0%) | 2 (12.5%) |
| 4 | 0 (0%) | 0 (0%) | 0 (0%) |
| Grade group, n (%) | |||
| 1 | 0 (0%) | 0 (0%) | 0 (0%) |
| 2 | 1 (16.7%) | 6 (60.0%) | 7 (43.8%) |
| 3 | 2 (33.3%) | 2 (20.0%) | 4 (25.0%) |
| 4 | 0 (0%) | 0 (0%) | 0 (0%) |
| 5 | 3 (50.0%) | 2 (20.0%) | 5 (31.3%) |
| Initial PSA, ng/mL, median (range) | 11.1 (2.85–82.28) | 8.89 (3.91–44.24) | 9.34 (2.85–82.28) |
| NCCN risk, n (%) | |||
| Favorable intermediate | 1 (16.7%) | 1 (10.0%) | 2 (12.5%) |
| Unfavorable intermediate | 2 (33.3%) | 4 (40.0%) | 6 (37.5%) |
| High | 1 (16.7%) | 2 (20.0%) | 3 (18.8%) |
| Very high | 2 (33.3%) | 3 (30.0%) | 5 (31.3%) |
| ADT use, n (%) | |||
| No | 2 (33.3%) | 2 (20.0%) | 4 (25.0%) |
| Yes | 4 (66.7%) | 8 (80.0%) | 12 (75.0%) |
| Highest Grade | 42.5 Gy, n (%) | 45 Gy, n (%) | Total, n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute GU | Acute GI | Late GU | Late GI | Acute GU | Acute GI | Late GU | Late GI | Acute GU | Acute GI | Late GU | Late GI | |
| 0 | 0 (0.0%) | 3 (50.0%) | 0 (0.0%) | 2 (33.3%) | 2 (20.0%) | 2 (20.0%) | 3 (30.0%) | 2 (20.0%) | 2 (12.5%) | 5 (51.3%) | 3 (18.8%) | 4 (25.0%) |
| 1 | 4 (66.7%) | 2 (33.3%) | 4 (66.7%) | 2 (33.3%) | 5 (50.0%) | 7 (70.0%) | 2 (20.0%) | 1 (10.0%) | 9 (56.3%) | 9 (56.3%) | 6 (37.5%) | 3 (18.8%) |
| 2 | 2 (33.3%) | 1 (16.7%) | 2 (33.3%) | 2 (33.3%) | 3 (30.0%) | 1 (10.0%) | 5 (50.0%) | 5 (50.0%) | 5 (51.3%) | 2 (12.5%) | 7 (43.8%) | 7 (43.8%) |
| 3 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0% | 0 (0.0%) | 0 (0.0%) | 1 (10.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (6.3%) |
| 4 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (10.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (6.3%) |
| Patient Number | Total Dose, Gy | Highest Grade | Age, Years | Diabetes | PTV, cc | Rectal Volume 1, cc |
|---|---|---|---|---|---|---|
| 1 | 42.5 | 0 | 74 | Yes | 78.0 | 64.0 |
| 2 | 42.5 | 2 | 71 | Yes | 79.3 | 43.8 |
| 3 | 42.5 | 1 | 71 | No | 61.7 | 37.2 |
| 4 | 42.5 | 0 | 71 | No | 70.6 | 42.0 |
| 5 | 42.5 | 1 | 83 | No | 101.2 | 47.4 |
| 6 | 42.5 | 2 | 73 | No | 62.9 | 34.2 |
| 7 | 45 | 0 | 70 | No | 124.3 | 64.4 |
| 8 | 45 | 2 | 70 | No | 85.4 | 56.8 |
| 9 | 45 | 4 | 63 | No | 81.2 | 44.4 |
| 10 | 45 | 3 | 72 | No | 133.8 | 85.7 |
| 11 | 45 | 2 | 75 | No | 66.9 | 40.8 |
| 12 | 45 | 2 | 73 | No | 90.3 | 28.8 |
| 13 | 45 | 1 | 72 | No | 69.5 | 57.5 |
| 14 | 45 | 0 | 84 | No | 77.2 | 35.6 |
| 15 | 45 | 2 | 66 | No | 102.1 | 57.6 |
| 16 | 45 | 2 | 64 | No | 71.5 | 60.0 |
| Patient Number | Total Dose, Gy | Highest Grade of GI | Highest Grade of GU | PTV | Bladder Wall | Rectum 1 | Femoral Heads | Small Bowel | Urethra | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D95% 2, Gy | D99% 2, Gy | V20 Gy 3, cc | Max Dose, Gy | V40 Gy 3, % | V25 Gy 3, % | Max Dose, Gy | V20 Gy 3, cc | Max Dose, Gy | Max Dose, Gy | ||||
| 1 | 42.5 | 0 | 2 | 42.5 | 42.1 | 23.8 | 45.9 | 3.0 | 14.2 | 23.9 | 0.0 | 4.5 | 44.0 |
| 2 | 42.5 | 2 | 1 | 42.5 | 41.9 | 46.6 | 45.4 | 8.0 | 27.9 | 24.2 | 0.0 | 6.6 | 44.6 |
| 3 | 42.5 | 1 | 2 | 42.5 | 42.0 | 36.0 | 45.9 | 8.2 | 23.8 | 26.1 | 0.0 | 2.8 | 44.6 |
| 4 | 42.5 | 0 | 1 | 42.5 | 41.8 | 28.5 | 45.3 | 4.2 | 16.5 | 25.7 | 0.0 | 7.4 | 44.3 |
| 5 | 42.5 | 1 | 1 | 42.5 | 41.9 | 45.6 | 45.7 | 14.2 | 43.1 | 27.6 | 0.0 | 3.6 | 44.5 |
| 6 | 42.5 | 2 | 1 | 42.5 | 42.0 | 48.4 | 45.7 | 9.6 | 32.5 | 21.0 | 0.0 | 6.2 | 44.1 |
| 7 | 45 | 0 | 1 | 45.0 | 44.5 | 42.8 | 49.0 | 9.2 | 27.7 | 21.8 | 0.0 | 12.4 | 46.6 |
| 8 | 45 | 2 | 1 | 45.0 | 44.3 | 38.2 | 47.9 | 18.4 | 40.3 | 21.4 | 0.0 | 8.5 | 46.6 |
| 9 | 45 | 4 | 2 | 45.0 | 44.4 | 44.6 | 48.8 | 11.7 | 30.0 | 28.7 | 0.0 | 5.7 | 47.2 |
| 10 | 45 | 3 | 2 | 45.0 | 44.3 | 47.9 | 49.1 | 7.1 | 22.3 | 24.0 | 0.0 | 7.9 | 46.7 |
| 11 | 45 | 2 | 2 | 45.0 | 44.5 | 49.4 | 47.9 | 14.5 | 35.1 | 26.9 | 0.0 | 9.6 | 46.9 |
| 12 | 45 | 2 | 2 | 45.0 | 44.4 | 42.5 | 47.6 | 16.1 | 35.3 | 27.0 | 0.1 | 24.1 | 46.6 |
| 13 | 45 | 1 | 2 | 45.0 | 42.9 | 18.0 | 48.6 | 11.2 | 23.9 | 24.2 | 0.4 | 27.0 | 47.1 |
| 14 | 45 | 0 | 1 | 45.0 | 44.3 | 37.9 | 48.3 | 10.7 | 26.6 | 20.9 | 0.0 | 7.5 | 46.9 |
| 15 | 45 | 2 | 2 | 45.0 | 43.6 | 26.5 | 48.4 | 19.8 | 42.6 | 20.9 | 1.6 | 28.6 | 47.2 |
| 16 | 45 | 2 | 1 | 45.0 | 44.2 | 34.2 | 47.9 | 9.8 | 21.5 | 23.8 | 0.0 | 9.9 | 46.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawayanagi, S.; Yamashita, H.; Ogita, M.; Kawai, T.; Sato, Y.; Kume, H. In Curative Stereotactic Body Radiation Therapy for Prostate Cancer, There Is a High Possibility That 45 Gy in Five Fractions Will Not Be Tolerated without a Hydrogel Spacer. Cancers 2024, 16, 1472. https://doi.org/10.3390/cancers16081472
Sawayanagi S, Yamashita H, Ogita M, Kawai T, Sato Y, Kume H. In Curative Stereotactic Body Radiation Therapy for Prostate Cancer, There Is a High Possibility That 45 Gy in Five Fractions Will Not Be Tolerated without a Hydrogel Spacer. Cancers. 2024; 16(8):1472. https://doi.org/10.3390/cancers16081472
Chicago/Turabian StyleSawayanagi, Subaru, Hideomi Yamashita, Mami Ogita, Taketo Kawai, Yusuke Sato, and Haruki Kume. 2024. "In Curative Stereotactic Body Radiation Therapy for Prostate Cancer, There Is a High Possibility That 45 Gy in Five Fractions Will Not Be Tolerated without a Hydrogel Spacer" Cancers 16, no. 8: 1472. https://doi.org/10.3390/cancers16081472
APA StyleSawayanagi, S., Yamashita, H., Ogita, M., Kawai, T., Sato, Y., & Kume, H. (2024). In Curative Stereotactic Body Radiation Therapy for Prostate Cancer, There Is a High Possibility That 45 Gy in Five Fractions Will Not Be Tolerated without a Hydrogel Spacer. Cancers, 16(8), 1472. https://doi.org/10.3390/cancers16081472






