Prediction of Overall Disease Burden in (y)pN1 Breast Cancer Using Knowledge-Based Machine Learning Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
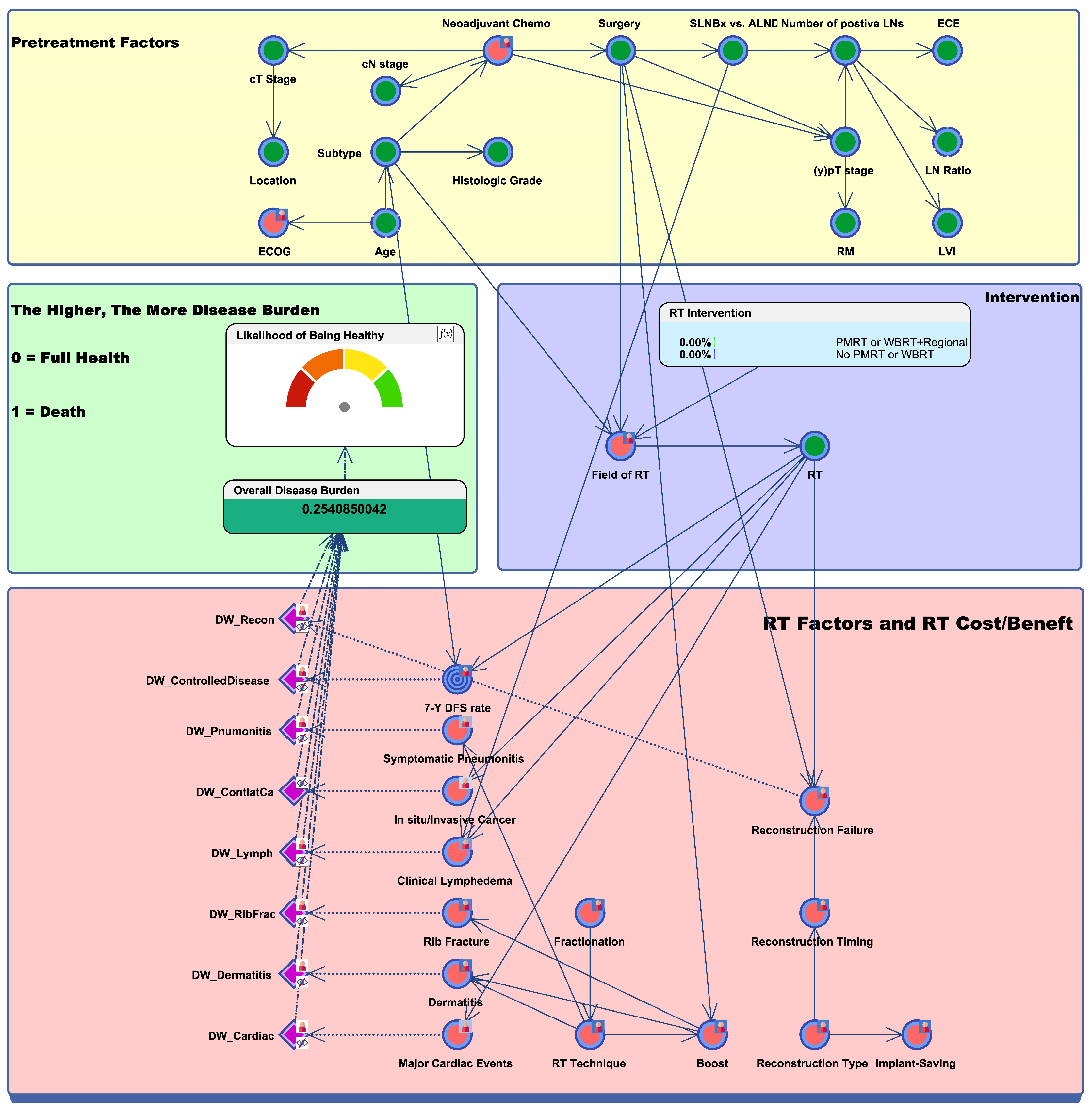
2.1. Bayesian Network Model Design and Overall Disease Burden
2.2. In Silico Prediction of Trial Results
3. Results
3.1. Outcome Inference of the Alliance A011202 Trial
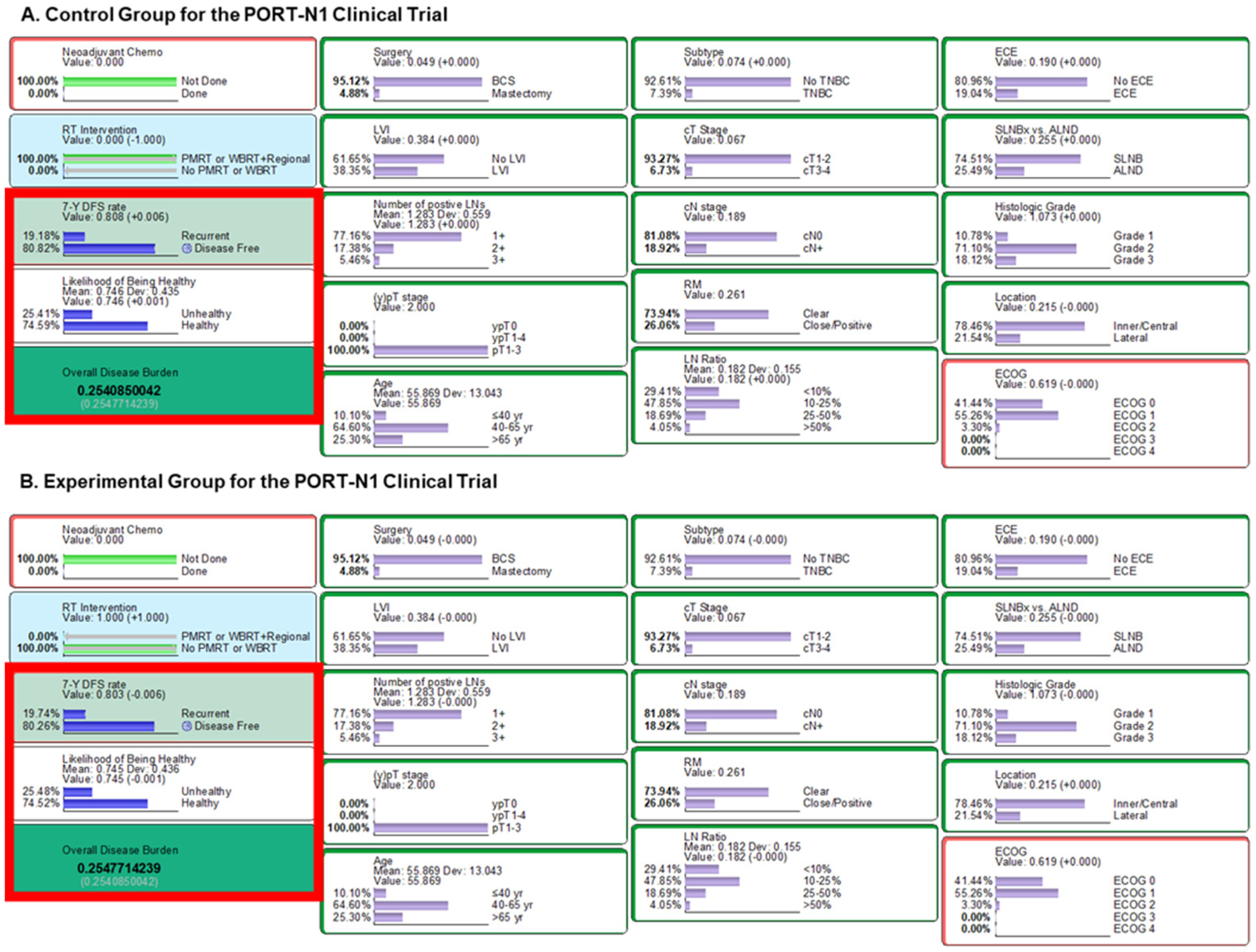
3.2. Outcome Inference of the PORT-N1 Trial
3.3. Outcome Comparison with the RAPCHEM Trial
3.4. Outcome Inference of the RT-CHARM Trial
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Breast Cancer. Natl. Compr. Cancer Netw. 2023, 4. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 5 May 2023).
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef]
- McGale, P.; Taylor, C.; Correa, C.; Cutter, D.; Duane, F.; Ewertz, M.; Wang, Y. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014, 383, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Fang, H.; Song, Y.W.; Wang, W.H.; Hu, C.; Liu, Y.P.; Jin, J.; Liu, X.-F.; Yu, Z.-H.; Ren, H.; et al. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: A randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 352–360. [Google Scholar] [CrossRef]
- Kim, D.Y.; Park, E.; Heo, C.Y.; Jin, U.S.; Kim, E.K.; Han, W.; Shin, K.H.; Kim, I.A. Influence of Hypofractionated versus Conventional Fractionated Postmastectomy Radiation Therapy in Breast Cancer Patients with Reconstruction. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 445–456. [Google Scholar] [CrossRef]
- Lee, T.H.; Chang, J.H.; Jang, B.S.; Kim, J.S.; Kim, T.H.; Park, W.; Kim, Y.B.; Kim, S.S.; Han, W.; Lee, H.-B.; et al. Protocol for the postoperative radiotherapy in N1 breast cancer patients (PORT-N1) trial, a prospective multicenter, randomized, controlled, non-inferiority trial of patients receiving breast-conserving surgery or mastectomy. BMC Cancer 2022, 22, 1179. [Google Scholar] [CrossRef]
- de Wild, S.R.; de Munck, L.; Simons, J.M.; Verloop, J.; van Dalen, T.; Elkhuizen, P.H.M.; Houben, R.M.A.; van Leeuwen, A.E.; Linn, S.C.; Pijnappel, R.M.; et al. De-escalation of radiotherapy after primary chemotherapy in cT1–2N1 breast cancer (RAPCHEM; BOOG 2010–03): 5-year follow-up results of a Dutch, prospective, registry study. Lancet Oncol. 2022, 23, 1201–1210. [Google Scholar] [CrossRef]
- Fornacon-Wood, I.; Mistry, H.; Johnson-Hart, C.; Faivre-Finn, C.; O’Connor, J.P.B.; Price, G.J. Understanding the Differences Between Bayesian and Frequentist Statistics. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 1076–1082. [Google Scholar] [CrossRef]
- Luo, Y.; El Naqa, I.; McShan, D.L.; Ray, D.; Lohse, I.; Matuszak, M.M.; Owen, D.; Jolly, S.; Lawrence, T.S.; Kong, F.-M.; et al. Unraveling biophysical interactions of radiation pneumonitis in non-small-cell lung cancer via Bayesian network analysis. Radiother. Oncol. 2017, 123, 85–92. [Google Scholar] [CrossRef]
- Lau, C.L.; Mayfield, H.J.; Sinclair, J.E.; Brown, S.J.; Waller, M.; Enjeti, A.K.; Baird, A.; Short, K.R.; Mengersen, K.; Litt, J. Risk-benefit analysis of the AstraZeneca COVID-19 vaccine in Australia using a Bayesian network modelling framework. Vaccine 2021, 39, 7429–7440. [Google Scholar] [CrossRef]
- Jang, B.S.; Chang, J.H.; Jeon, S.H.; Song, M.G.; Lee, K.H.; Im, S.A.; Kim, J.-I.; Kim, T.-Y.; Chie, E.K. Radiation Response Prediction Model Based on Integrated Clinical and Genomic Data Analysis. Cancer Res. Treat. 2022, 54, 383–395. [Google Scholar] [CrossRef]
- Mcgeachie, M.J.; Sordillo, J.E.; Gibson, T.; Weinstock, G.M.; Liu, Y.Y.; Gold, D.R.; Weiss, S.T.; Litonjua, A. Longitudinal Prediction of the Infant Gut Microbiome with Dynamic Bayesian Networks. Sci. Rep. 2016, 6, 20359. [Google Scholar] [CrossRef]
- Cai, Z.Q.; Guo, P.; Si, S.B.; Geng, Z.M.; Chen, C.; Cong, L.L. Analysis of prognostic factors for survival after surgery for gallbladder cancer based on a Bayesian network. Sci. Rep. 2017, 7, 293. [Google Scholar] [CrossRef]
- Jang, B.; Chun, S.; Choi, H.S.; Chang, J.H.; Shin, K.H.; Division for Breast Cancer, Korean Radiation Oncology Group. Estimating the Risk and Benefit of Radiation Therapy in (y)pN1 Stage Breast Cancer Patients: A Bayesian Network Model Incorporating Expert Knowledge (KROG 22-13). Comput. Methods Programs Biomed. 2024, 245, 108049. [Google Scholar] [CrossRef]
- Donker, M.; van Tienhoven, G.; Straver, M.E.; Meijnen, P.; van de Velde, C.J.H.; Mansel, R.E.; Cataliotti, L.; Westenberg, A.H.; Klinkenbijl, J.H.G.; Orzalesi, L.; et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): A randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014, 15, 1303–1310. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA 2017, 318, 918–926. [Google Scholar] [CrossRef]
- Almahariq, M.F.; Levitin, R.; Quinn, T.J.; Chen, P.Y.; Dekhne, N.; Kiran, S.; Desai, A.; Benitez, P.; Jawad, M.S.; Gustafson, G.S.; et al. Omission of Axillary Lymph Node Dissection is Associated with Inferior Survival in Breast Cancer Patients with Residual N1 Nodal Disease Following Neoadjuvant Chemotherapy. Ann. Surg. Oncol. 2021, 28, 930–940. [Google Scholar] [CrossRef]
- Ling, D.C.; Iarrobino, N.A.; Champ, C.E.; Soran, A.; Beriwal, S. Regional Recurrence Rates with or without Complete Axillary Dissection for Breast Cancer Patients with Node-Positive Disease on Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy. Adv. Radiat. Oncol. 2020, 5, 163–170. [Google Scholar] [CrossRef]
- Poortmans, P.M.; Collette, S.; Kirkove, C.; Van Limbergen, E.; Budach, V.; Struikmans, H.; Collette, L.; Fourquet, A.; Maingon, P.; Valli, M.; et al. Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. N. Engl. J. Med. 2015, 373, 317–327. [Google Scholar] [CrossRef]
- Whelan, T.J.; Olivotto, I.A.; Parulekar, W.R.; Ackerman, I.; Chua, B.H.; Nabid, A.; Vallis, K.A.; White, J.R.; Rousseau, P.; Fortin, A.; et al. Regional Nodal Irradiation in Early-Stage Breast Cancer. N. Engl. J. Med. 2015, 373, 307–316. [Google Scholar] [CrossRef]
- Kim, H.; Park, W.; Choi, D.H.; Ahn, S.J.; Kim, S.S.; Kim, E.S.; Lee, J.; Lee, K.; Kim, J.; Lee, H.-S.; et al. Abstract OT2-04-02: A phase 3 study of post-lumpectomy radiotherapy to whole breast+ regional lymph nodes vs whole breast alone for patients with pN1 breast cancer treated with taxane-based chemotherapy (KROG 1701): Trial in progress. Cancer Res. 2019, 79, OT2-04-02. [Google Scholar] [CrossRef]
- Boersma, L.J.; Verloop, J.; Voogd, A.C.; Elkhuizen, P.H.M.; Houben, R.; van Leeuwen, A.E.; Linn, S.; de Munck, L.; Pijnappel, R.; Strobbe, L.; et al. Radiotherapy after primary CHEMotherapy (RAPCHEM): Practice variation in a Dutch registration study (BOOG 2010-03). Radiother. Oncol. 2020, 145, 201–208. [Google Scholar] [CrossRef]
- Reinders, F.C.J.; Young-Afat, D.A.; Batenburg, M.C.T.; Bruekers, S.E.; van Amerongen, E.A.; Macaré van Maurik, J.F.M.; Braakenburg, A.; Zonnevylle, E.; Hoefkens, M.; Teunis, T.; et al. Higher reconstruction failure and less patient-reported satisfaction after post mastectomy radiotherapy with immediate implant-based breast reconstruction compared to immediate autologous breast reconstruction. Breast Cancer 2020, 27, 435–444. [Google Scholar] [CrossRef]
- Ricci, J.A.; Epstein, S.; Momoh, A.O.; Lin, S.J.; Singhal, D.; Lee, B.T. A meta-analysis of implant-based breast reconstruction and timing of adjuvant radiation therapy. J. Surg. Res. 2017, 218, 108–116. [Google Scholar] [CrossRef]
- Byun, H.K.; Chang, J.S.; Im, S.H.; Kirova, Y.M.; Arsene-Henry, A.; Choi, S.H.; Cho, Y.U.; Park, H.S.; Kim, J.Y.; Suh, C.-O.; et al. Risk of Lymphedema Following Contemporary Treatment for Breast Cancer: An Analysis of 7617 Consecutive Patients from a Multidisciplinary Perspective. Ann. Surg. 2021, 274, 170–178. [Google Scholar] [CrossRef]
- Tsai, R.J.; Dennis, L.K.; Lynch, C.F.; Snetselaar, L.G.; Zamba, G.K.D.; Scott-Conner, C. The risk of developing arm lymphedema among breast cancer survivors: A meta-analysis of treatment factors. Ann. Surg. Oncol. 2009, 16, 1959–1972. [Google Scholar] [CrossRef]
- Arora, P.; Boyne, D.; Slater, J.J.; Gupta, A.; Brenner, D.R.; Druzdzel, M.J. Bayesian Networks for Risk Prediction Using Real-World Data: A Tool for Precision Medicine. Value Health 2019, 22, 439–445. [Google Scholar] [CrossRef]
- Wasserman, A.; Musella, A.; Shapiro, M.; Shrager, J. Virtual Trials: Causally-validated treatment effects efficiently learned from an observational brain cancer registry. Artif. Intell. Med. 2021, 135, 102450. [Google Scholar] [CrossRef]

| RAPCHEM Trial | BN Model | |||||
|---|---|---|---|---|---|---|
| Risk Group | Definition | RT | 5-Year LRR | OBD | Likelihood of Being Healthy | 7-Year DFS |
| Intermediate | ypN1 with ALND | Whole breast/CW | 2.2% | 0.279 | 72.1% | 80.1% |
| Whole breast/CW with level I/II | N/A | 0.283 | 71.7% | 80.3% | ||
| Whole breast/CW with full regional RT | N/A | 0.286 | 71.4% | 79.9% | ||
| Intermediate or high | ypN1mi or ypN1 with SLNBx | Whole breast/CW | N/A | 0.249 | 75.1% | 80.1% |
| Whole breast/CW with level I/II | 2.2% | 0.250 | 75.0% | 80.3% | ||
| Whole breast/CW with full regional RT | 2.3% | 0.253 | 74.8% | 79.9% | ||
| Boost | Fractionation | Implant Preservation | Recon Timing | Recon Type | RT Technique | Posterior Probability P (s|H) |
|---|---|---|---|---|---|---|
| No boost | Implant-preserving RT | Immediate | 22.10% | |||
| No boost | No implant preservation | Immediate | 22.10% | |||
| Hypofractionated | Implant-preserving RT | Immediate | 22.10% | |||
| Immediate | 22.10% | |||||
| Conventional | Autologous | IMRT/VMAT/Tomo | 21.63% | |||
| No boost | Autologous | 3D-CRT/field-in-field | 21.63% | |||
| No boost | Autologous | 21.63% | ||||
| Autologous | 21.63% | |||||
| No boost | No implant preservation | 21.03% | ||||
| No implant preservation | 21.03% | |||||
| IMRT/VMAT/Tomo | 20.89% | |||||
| No boost | 20.89% | |||||
| 3D-CRT/field-in-field | 20.89% | |||||
| Conventional | 20.89% | |||||
| Boost | 20.89% | |||||
| Implant-preserving RT | 20.21% | |||||
| Implant-based | 20.21% | |||||
| Delayed | 18.29% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, S.-J.; Jang, B.-S.; Choi, H.S.; Chang, J.H.; Shin, K.H.; Division for Breast Cancer, Korean Radiation Oncology Group. Prediction of Overall Disease Burden in (y)pN1 Breast Cancer Using Knowledge-Based Machine Learning Model. Cancers 2024, 16, 1494. https://doi.org/10.3390/cancers16081494
Chun S-J, Jang B-S, Choi HS, Chang JH, Shin KH, Division for Breast Cancer, Korean Radiation Oncology Group. Prediction of Overall Disease Burden in (y)pN1 Breast Cancer Using Knowledge-Based Machine Learning Model. Cancers. 2024; 16(8):1494. https://doi.org/10.3390/cancers16081494
Chicago/Turabian StyleChun, Seok-Joo, Bum-Sup Jang, Hyeon Seok Choi, Ji Hyun Chang, Kyung Hwan Shin, and Division for Breast Cancer, Korean Radiation Oncology Group. 2024. "Prediction of Overall Disease Burden in (y)pN1 Breast Cancer Using Knowledge-Based Machine Learning Model" Cancers 16, no. 8: 1494. https://doi.org/10.3390/cancers16081494





