Simple Summary
In 2020, more than 1.9 million cases of colorectal cancer (CRC) occurred, and almost 0.9 million patients died due to CRC throughout the world. There are differences in distribution and time variations in CRC between different countries. This diversity is mainly due to differences in risk factors among populations. CRC may be prevented by primary and secondary prevention methods. Primary prevention includes avoiding risk factors, e.g., alcohol consumption, and increasing protective factors, e.g., physical activity. The secondary prevention method, called CRC screening, consists of diagnosis and treatment of precancerous lesions of the colorectum. Although a large amount of evidence is available for different aspects of CRC, controversies remain regarding the most important factors and most effective control programs for CRC in different populations. In this review, we will present the most updated evidence regarding CRC’s distribution, related factors, and preventive methods.
Abstract
Colorectal cancer (CRC) is the third most common cancer and the second most common cause of cancer mortality worldwide. There are disparities in the epidemiology of CRC across different populations, most probably due to differences in exposure to lifestyle and environmental factors related to CRC. Prevention is the most effective method for controlling CRC. Primary prevention includes determining and avoiding modifiable risk factors (e.g., alcohol consumption, smoking, and dietary factors) as well as increasing protective factors (e.g., physical activity, aspirin). Further studies, especially randomized, controlled trials, are needed to clarify the association between CRC incidence and exposure to different risk factors or protective factors. Detection and removal of precancerous colorectal lesions is also an effective strategy for controlling CRC. Multiple factors, both at the individual and community levels (e.g., patient preferences, availability of screening modalities, costs, benefits, and adverse events), should be taken into account in designing and implementing CRC screening programs. Health policymakers should consider the best decision in identifying the starting age and selection of the most effective screening strategies for the target population. This review aims to present updated evidence on the epidemiology, risk factors, and prevention of CRC.
1. Introduction
With more than 1.9 million new cases and 0.9 million deaths in 2020, colorectal cancer (CRC) was the third most common cancer and the second most common cause of cancer mortality worldwide [1,2,3]. Geographical disparities were reported in incidence and mortality rates, time trends, and the future burden of CRC across different countries and regions [1,2,3]. There were also changes in the age pattern of CRC, with increasing incidence in the young, especially in developed countries [4]. These disparities may reflect differences in exposure to the risk factors for CRC, including lifestyle and environmental factors [5,6]. Identifying and avoiding modifiable risk factors, especially lifestyle factors (e.g., alcohol consumption, smoking, obesity, unhealthy diet) as well increasing protective factors (e.g., physical activity, taking specific medications such as aspirin, healthy diet) [5,6] have a pivotal role in the primary prevention of CRC. CRC screening (secondary prevention) for the detection and removal of premalignant lesions of the colorectum is also considered an effective preventive method in CRC control programs [7,8,9]. Despite large numbers of studies being conducted on different aspects of CRC, there are still challenges regarding the most important risk factors and most effective preventive strategies for CRC in different populations. An updated, concise review of all of this information may be helpful for researchers, physicians, and policymakers. In this review, we performed a comprehensive search of the latest available literature to present the most updated evidence on the epidemiology, risk factors, and prevention of CRC.
2. Epidemiology
2.1. Mortality
According to the GLOBOCAN estimates provided by the International Agency for Research on Cancer (IARC), colorectal cancer (CRC) was the second most common cause of cancer-related death (after lung cancer) worldwide, with an estimated number of 935,173 deaths and an age-standardized mortality rate (ASMR) of 9.0 per 100,000 person-years in 2020. The highest ASMR of CRC was estimated for Europe (12.3), and the lowest one was reported for Africa (5.6) and the eastern Mediterranean region (EMRO) (5.3). The rates were almost comparable for other regions, including Oceana (9.3), Asia (8.6), Latin America (8.2), and North America (8.2) [2,10]. The GLOBOCAN estimates suggested a higher ASMR for CRC in men (11.0) than women (7.2), with 515,637 and 419,536 deaths in men and women, respectively [2,10].
2.2. Incidence
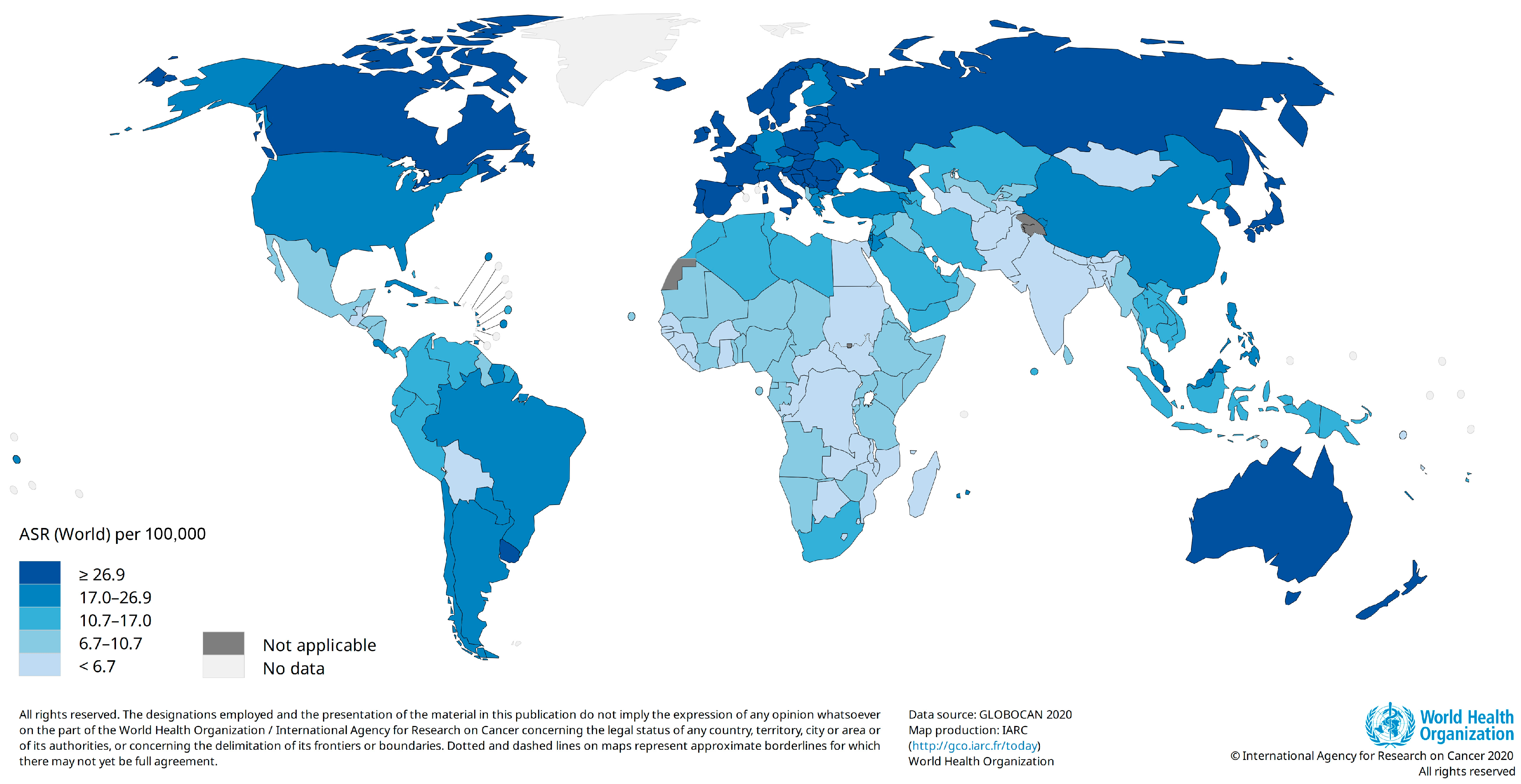
CRC was reported as the third most common cancer worldwide (after breast and lung cancers) in 2020. The number of new cases of CRC was estimated to be 1,931,590 with an age-standardized incidence rate (ASR) of 19.5 per 100,000 person-years. The highest ASRs were estimated for Europe (30.4), followed by Oceana (29.8) and North America (26.2). After Asia (17.6) and Latin America (16.6), EMRO (9.1) and Africa (8.4) had the lowest ASRs for CRC (Figure 1). Hungary (45.3) and Guinea (3.3) had the highest and lowest ASRs for CRC among the other countries. The ASRs for CRC were almost four-fold higher in high-income (30.2) countries compared with low-income (8.8) and low–middle-income (7.4) countries. Similar discrepancies were reported in the incidence rates for CRC between countries with very high human development indices (HDIs) (29.4) and high HDIs (20.4) and those with medium HDIs (6.1) and low HDIs (7.4) [2,10]. This diversity may be explained by differences in exposure to CRC risk factors. We will discuss CRC risk factors in the next sections of this paper.
Figure 1.
Estimated age-standardized incidence rate for colorectal cancer, 2020 (Data source: GLOBOCAN 2020; Map production: IARC, http://gco.iarc.fr/today, accessed on 10 October 2023).
2.3. Time Trends and Future Burden
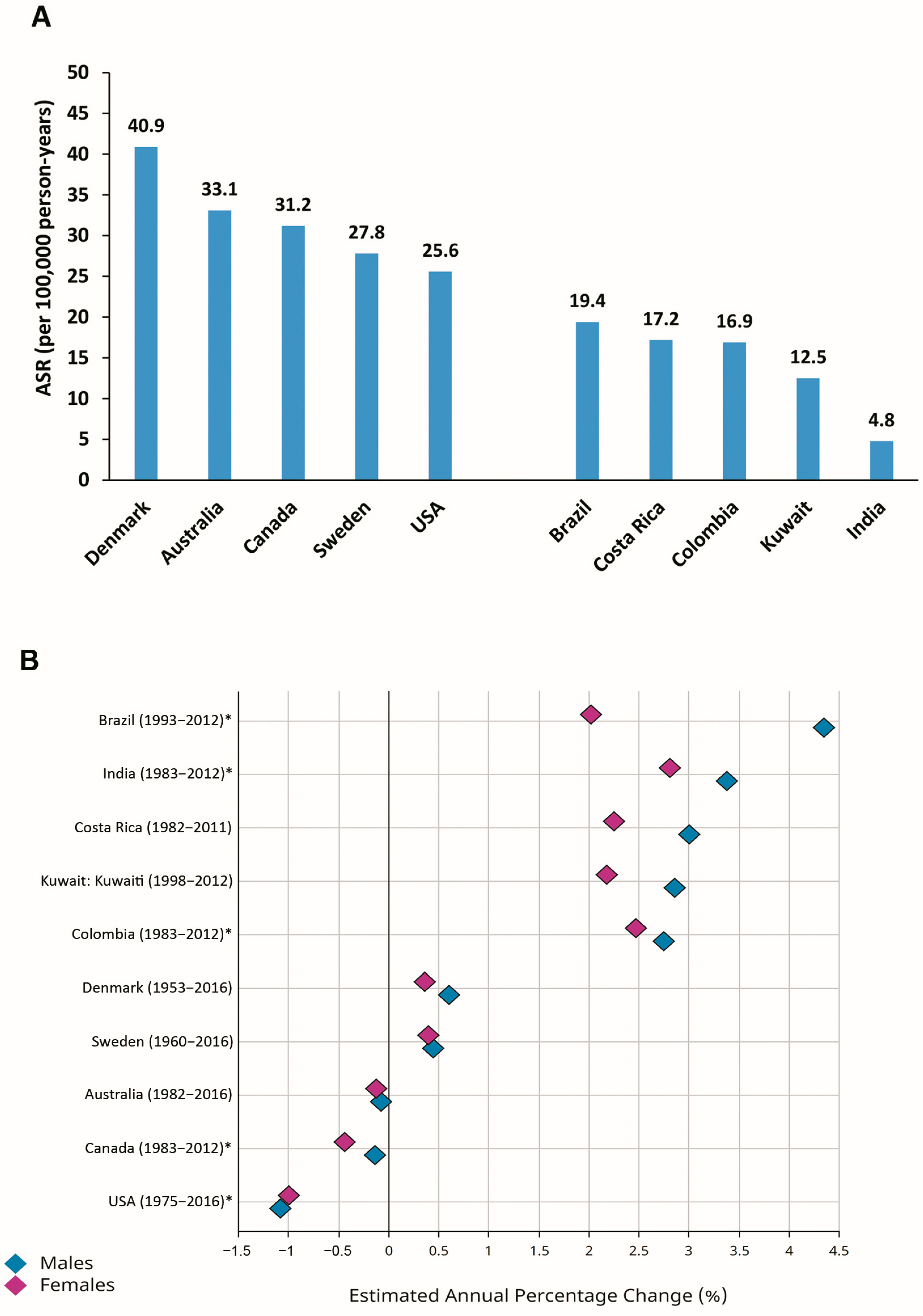
There are discrepancies in the pattern of time trends in the incidence of CRC worldwide [1,6,11,12,13]. Considering the estimated annual percentage changes (EAPCs) in the ASRs for CRC during recent decades, constant or even slightly declining trends have been reported for formerly high-risk (transitioned) countries, including Denmark, Sweden, Australia, Canada, and the USA (Figure 2). In contrast, transitioning countries with relatively low-risk of CRC, including Brazil, Costa Rica, Colombia, Kuwait, and India, have shown increasing trends in the ASRs for CRC during the last decades (Figure 2) [1,11,14].
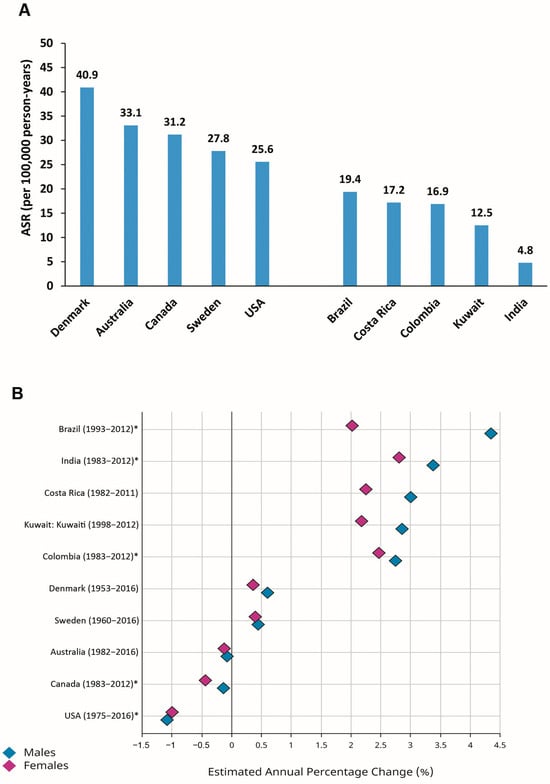
Figure 2.
Age-standardized incidence rates (ASRs) (per 100,000 person-years) in 2020 (A) and estimated annual percentage changes (EAPCs) in ASRs (B) for colorectal cancer in selected transitioned (Denmark, Australia, Canada, Sweden, USA) and transitioning (Brazil, Costa Rica, Colombia, Kuwait, India) countries (Source: GLOBOCAN 2020; Figure production, B: IARC, http://gco.iarc.fr/overtime, accessed on 23 March 2023) (* subnational data).
According to the GLOBOCAN 2020 estimates, the number of new cases of CRC will increase from 1,931,590 in 2020 to 3,154,674 in 2040, worldwide, suggesting a 63.3% increase in the number of new CRC cases. The greatest changes were estimated for currently low-risk population regions, including Africa (95.0%), EMRO (92.0%), Latin America (74.0%), and Asia (70.8%). The estimated changes in the number of new CRC cases are considerably lower in very-high-HDI countries (35.0%) compared to countries with low (10.2.6%), medium (72.1%), and high HDIs (67.9%) [3]. The increasing trends in the incidence rates for CRC in low-risk populations may be mainly explained by the increasing prevalence of risk factors, which will be discussed in detail in the next sections of this paper [6,15,16,17].
2.4. Early-Onset CRC
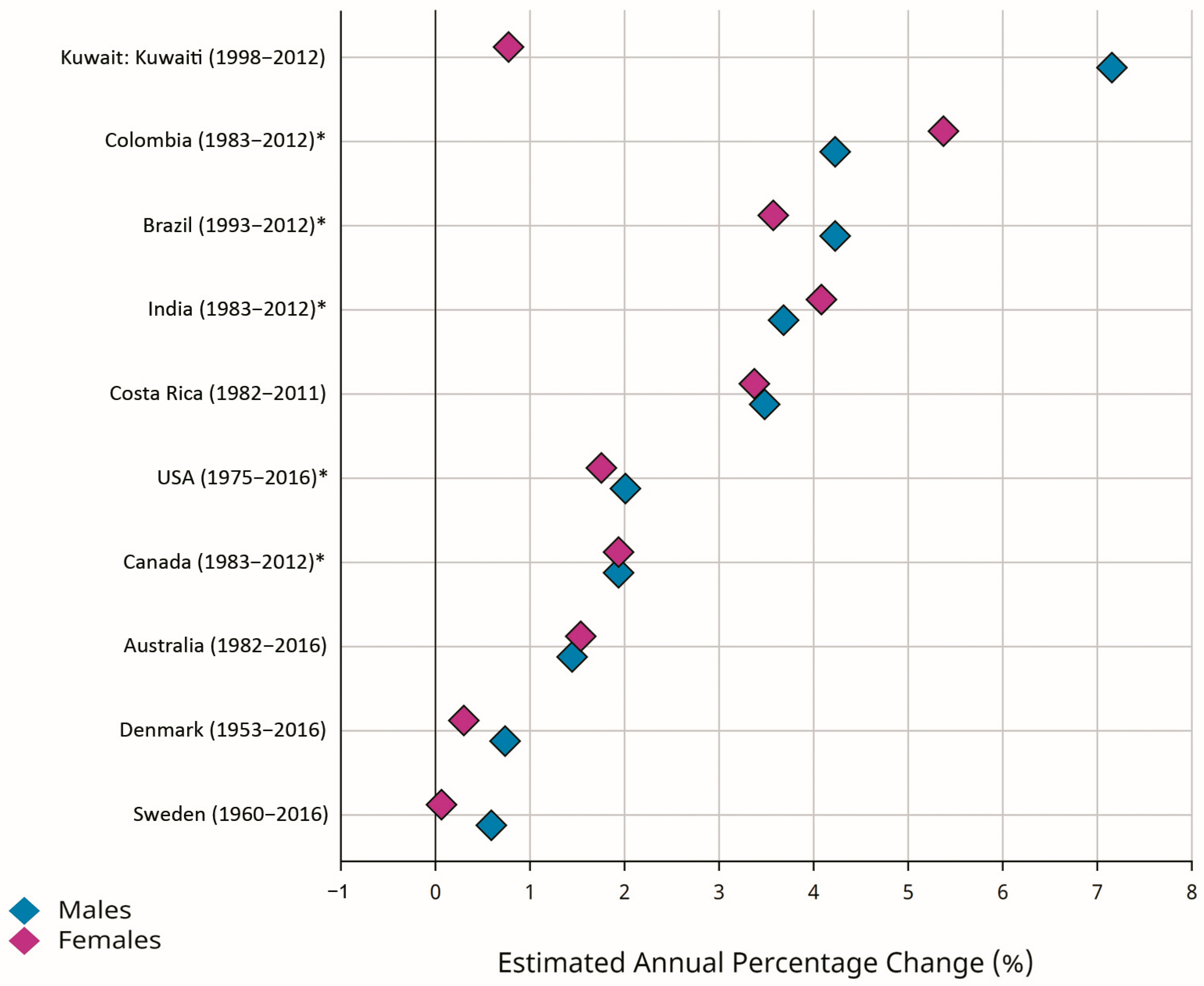
Early-onset CRC (EOCRC) occurs in individuals younger than 50 years old. According to the GLOBOCAN estimates, there were 188,069 new cases of EOCRC, with an ASR of 2.9 per 100,000 person-years worldwide. The ASRs for EOCRC were 3.0 and 2.7 per 100,000 person-years in men and women, respectively. The highest ASRs for EOCRC were estimated for North America (6.1) and Oceania (5.3), while Africa (2.0), Asia (2.6), and Latin America (2.9) were reported as low-risk areas [2,10]. Recent evidence suggested an increasing incidence rate for EOCRC in different populations, with greater changes in developing countries [1,5,18,19,20,21,22,23,24,25]. Figure 3 shows greater EAPCs for EOCRC in selected developed countries (Denmark, Australia, Canada, Sweden, USA) and developing countries (Brazil, Costa Rica, Colombia, Kuwait, India). Despite the declining trend in the incidence of CRC in the total population of the developed countries, the incidence rates for EOCRC were increasing both in the developed and developing world. The reasons for increasing trends in the incidence of EOCRC may include genetic predispositions [4,26,27,28,29,30] and exposure to environmental and lifestyle-related factors, including hyperlipidemia, obesity, alcohol consumption, metabolic syndrome, ulcerative colitis, low physical activity, low vitamin D intake, high red meat intake and high sugar-sweetened-beverage intake [28,31,32,33,34,35,36,37]. Recent evidence also suggests that changes in the types and diversity of the species of intestinal microbiota may be associated with increasing trends in the incidence of EOCRC [38,39]. Regarding the increasing trends in the incidence rates for EOCRC and the predicted future burden, it should be considered as a top priority, and further investigations are warranted to clarify its risk factors and to develop and implement the most effective control strategies in all populations worldwide.
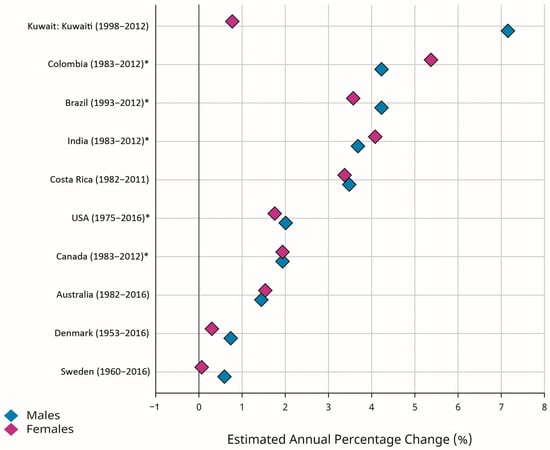
Figure 3.
Estimated annual percentage changes (EAPCs) of the age-standardized incidence rates (ASRs) for early-onset colorectal cancer (EOCRC) (age 0–49 years) in selected developed (Denmark, Australia, Canada, Sweden, USA) and developing (Brazil, Costa Rica, Colombia, Kuwait, India) countries (Source: GLOBOCAN 2020; Figure production: IARC, http://gco.iarc.fr/overtime, accessed on 23 March 2023) (* subnational data).
3. Risk Factors
CRC risk factors, including environmental and genetic factors, may be divided into modifiable and non-modifiable risk factors [40,41,42,43,44].
3.1. Modifiable Risk Factors
Modifiable risk factors for CRC may be controlled by effective risk-factor reduction measures and therefore are of special interest to policymakers for designing CRC control programs.
3.1.1. Alcohol Consumption
Various reports from different populations suggest a positive association between alcohol consumption and the risk of CRC [45,46,47,48,49,50]. Cai et al. suggested a proportional positive association between alcohol consumption and the risk of CRC, with the greatest risk in heavy drinkers (≥50 g/day of ethanol) [47]. In a pooled cohort study and Mendelian randomization analysis, Zhou et al. reported that drinking alcohol was causally associated with an increased CRC risk with an odds ratio of 1.79 (95% CI: 1.23–2.61). The findings of this study also revealed that the pathogenic effect of alcohol on CRC could be partly attributed to DNA methylation by regulating the expression of specific genes [49].
3.1.2. Smoking
The direct association between smoking and the risk of CRC was consistently reported by a large number of studies on different populations, suggesting significant dose–response effects and a reduction in CRC risk after smoking cessation [51,52,53,54,55]. By inhaling toxic chemicals in smokers, the colorectal mucosal cells are exposed to well-known carcinogens, including nitrosamines, heterocyclic amines, polycyclic aromatic hydrocarbons (PAH), and benzene. Long-term exposure to these carcinogens will result in genetic and molecular changes in colorectal cells, and, finally, the accumulation of these pro-oncogenic changes may cause the development of CRC [56,57].
3.1.3. Obesity
Reports from different studies, including large-scale cohort studies, suggest a direct association between obesity and CRC, with associations in men and women [58,59,60,61,62,63,64,65]. The association between obesity and the development of CRC may be explained by different mechanisms, mainly by the effects of pro-inflammatory cytokines (e.g., interleukin-6 and tumor necrosis factor alpha) and insulin or insulin-like growth factor on proliferation of tumor cells in obese individuals [61,63,64]. High levels of bile acids in obese individuals may increase inflammatory processes by the destruction of colorectal epithelial cells, resulting in progression to CRC [66,67].
3.1.4. Sedentary Lifestyle
The association between the risk of CRC with low physical activity or sedentary lifestyle was reported in different populations. The results of a study on data from the Asia-Pacific Cohort Studies Collaboration suggested that any physical activity was associated with 0.25 to 0.30 reduction in the hazard of CRC mortality [68]. The inverse association between physical activity and CRC incidence was suggested by several observational studies with RR reduction of 0.30 to 0.50 and similar findings in men and women [69,70,71]. The association between sedentary lifestyle and CRC risk may be partly explained by higher rates of obesity, higher plasma glucose levels, insulin resistance, and abnormal intestinal peristalsis in individuals with sedentary lifestyle [72]. Further investigations, especially clinical trials, are needed to clarify this association.
3.1.5. Unhealthy Diet (High Intake of Red and Processed Meat and Fat)
The results of a recent umbrella review of 45 meta-analyses suggested an association between dietary habits and the risk of CRC [73]. High intake of red and processed meat was consistently reported as a risk factor for CRC incidence [74,75,76]. Each 100 g/day increase in dietary red meat and each 50 g/day increase in dietary processed meats may increase the risk of CRC by 0.10–0.16 and 0.16–0.22, respectively [77,78]. Cooking meat, especially at high temperatures (e.g., grilling or barbecuing), may result in the production of various carcinogens, including heterocyclic aromatic amines and polycyclic aromatic hydrocarbons. In addition, the processing of meat (e.g., curing or smoking) may cause the formation of different carcinogens, including N-nitroso compounds and polycyclic aromatic hydrocarbons [74]. High intake of red and processed meat will expose the colorectal mucosa to these carcinogens, resulting in an increase in the risk of CRC [72]. High intake of dietary fat, especially from animal sources, was also suggested as another dietary risk factor for CRC [79,80], although another meta-analysis reported no association between dietary fat intake and the risk of CRC [81].
3.1.6. Psychological Stress
There is evidence of the direct association between different types of stress (e.g., work stress) and the risk of CRC [82,83,84,85,86]. The results of a recent meta-analysis suggested a 0.36 increase in CRC risk in those with high levels of work stress [83]. These findings were not supported by the results of other studies [87,88]. These inconsistent findings may be explained by differences in definitions and methods for the measurement of the levels of stress in different studies. Stress may cause overactivation of the hypothalamic–pituitary–adrenal axis, which in turn will result in psychological consequences, including immune system impairment, abnormal metabolic activities, and cancer [89,90]. The results of animal studies suggest associations between increased levels of norepinephrine, epinephrine, and cortisol (due to stress) and cancer initiation [91,92]. Chronic psychological stress may affect different phases of the process of tumorigenesis, including genome instability and mutation, tumor promoting, resistance to cell death, sustained proliferative signaling, induction of angiogenesis, and activation of invasion and metastasis [93]. Due to the increasing prevalence of psychological stress, as well as increasing trends in the incidence of CRC in different populations, further investigations are needed to clarify the association between stress and CRC risk.
3.2. Non-Modifiable Risk Factors
Although non-modifiable risk factors cannot be controlled, policymakers are interested in these risk factors as well. Non-modifiable risk factors may be considered for identifying high-risk individuals or populations as candidates for taking preventive interventions.
3.2.1. Age
Advancing age is a major risk factor for CRC incidence. Individuals older than 50 years are specifically at high risk, consisting of more than 90% of all CRC cases [2,10].
3.2.2. Gender
The risk of CRC is higher in men (ASR = 23.4 per 100,000) than women (16.2 per 100,000), with a male-to-female ratio of 1.4 [2,10]. The male-to-female ratio for CRC incidence is greater in high-income (1.4) than low-income countries (1.2) [2,10].
3.2.3. Genetic Predisposition
The most common forms of genetic-related (hereditary) CRC include germline mutations in the APC gene (familial adenomatous polyposis) [94,95] and germline mutations in DNA mismatch repair genes (Lynch syndrome) [96,97,98,99]. Although individuals with these germline mutations are at a much higher risk of CRC [100], they account for only five percent of CRC cases [96]. A higher proportion (16%) of inherited syndromes was reported in EOCRC cases [101,102]. Recent genome-wide association studies (GWAS) identified the association between new genetic variants and the risk of CRC, suggesting the need for further investigation to clarify their clinical implications [103,104]. According to these findings, future resequencing studies may identify rarer variants (e.g., prevalence 0.05–5%) [103].
3.2.4. Family History of CRC
A large number of studies with different designs and methodologies for various populations reported higher risk of CRC in first-degree relatives of CRC patients, with RRs of 2 to 4 [94,96]. A history of CRC [94,96,105,106] or adenomatous colonic polyps [107,108] in first-degree relatives, as important risk factors for CRC, is considered for determining high-risk groups in CRC control programs and guidelines.
3.2.5. Abdominopelvic Radiation
Receiving abdominopelvic radiation therapy in cancer survivors (e.g., those with a history of prostate cancer) was suggested as a risk factor for developing gastrointestinal cancers, especially CRC [109,110,111,112,113,114]. Some guidelines suggest CRC screening at earlier ages or at a specific intervals after cessation of radiotherapy [115].
3.2.6. Personal History of Other Diseases
Specific comorbidities were suggested as risk factors for developing CRC. Patients with inflammatory bowel disease (IBD), including ulcerative colitis or Crohn disease, are at high risk of CRC [116,117,118,119]. Higher risks of CRC were also reported in patients with other comorbidities, including cystic fibrosis [120,121], renal transplantation [122], cholecystectomy [123], coronary heart disease [124], bacterial and viral infections (e.g., human papilloma virus, Helicobacter pylori) [125,126,127,128], antibiotic use [129,130], and diabetes mellitus and insulin resistance [131,132,133]. The association between type 2 diabetes and CRC may be mainly explained by the stimulating effects of insulin (as an important growth factor) on colonic tumor cells, suggesting that pharmacological or lifestyle interventions that lower circulating insulin levels may be beneficial in preventing CRC [134].
3.2.7. Intestinal Microbiota
The gut microbiome, called the “forgotten organ” and composed of a large population of microorganisms, has a complex association with the development of CRC. Increasing evidence suggests changes in the gut microbiome and a reduction in its diversity are closely related to CRC incidence [135,136,137]. Dai et al. and Wong et al. reported the predominance of specific species in the intestinal microbiomes of CRC patients [137,138]. Recent studies provide evidence on CRC-related species of the gut microbiota [139,140]. Ma et al. performed a Mendelian randomization analysis to assess the relationship between gut microbiota and CRC [141]. They found a negative correlation between the Lachnospiraceae species and CRC risk, while the Porphyromonadaceae species, Lachnospiraceae UCG010 genus, Lachnospira genus, and Sellimonas genus had a positive relationship with the risk of CRC. Their findings suggested causal relationships between the intestinal microbiome and the risk of CRC. The results of this study revealed that dysbiosis of the intestinal microbiota may play an important role in development of CRC, suggesting their potential implications in CRC prevention [141]. Microbiome modulation was proposed as a new strategy for the prevention or treatment of CRC [136]. The relationship between gut microbiota and CRC risk may be partly explained by the role of the microbiome in metabolic activities (including the generation and regulation of bile acids, metabolism of amino acids, and carbohydrate fermentation) and the carcinogenic effects of their metabolic derivatives [142]. Further studies are warranted to clarify different aspects of the relationships between intestinal microbiota and CRC risk and their implications in CRC control programs.
3.3. High-Risk Groups for CRC
Considering the available evidence on CRC risk factors, high-risk groups for CRC include individuals with a personal history of adenomatous polyps or CRC, a family history of CRC, those with hereditary CRC syndrome (e.g., FAP, HNPCC), those with a personal history of IBD, and a history of abdominal or pelvic radiation [143,144]. Identifying the high-risk individuals/groups for CRC will help researchers and health policymakers to design targeted preventive methods and develop effective CRC control programs.
4. Prevention
Preventive methods for CRC may be classified into two main categories, namely primary prevention methods and secondary prevention methods. The primary prevention methods include avoiding CRC risk factors and increasing protective factors for CRC. The secondary prevention methods, called CRC screening, consist of methods for the diagnosis and removal of the precancerous lesions of CRC, called neoplastic colorectal polyps (e.g., colorectal adenomas).
4.1. Primary Prevention
4.1.1. Avoiding Risk Factors
Reduction in or elimination of exposure to modifiable risk factors for CRC (Section 2.1) may be associated with a reduction in CRC risk [70]. Cessation of smoking for more than 20 years was reported to be associated with a more than 50% decrease in the odds for different types of colorectal adenomas [145]. Interventions for the restriction of fat intake could reduce the risk of CRC in healthy individuals [146]. Further investigations, especially clinical trials, are warranted to clarify the effects of risk factor reduction on CRC incidence.
4.1.2. Increasing Protective Factors
There is consistent evidence suggesting that protective factors (e.g., dietary factors, physical activities, specific drugs/supplements) are associated with a decreased risk of CRC. We will review recent evidence on the association between the most important protective factors with CRC risk reduction.
Physical activity: There is substantial and consistent evidence on the protective effects of physical activity in CRC [71,147]. Different types of regular physical activity were associated with a 25–30 percent reduction in the risk of CRC [147,148,149,150,151,152]. Performing higher amounts (any amount is better than none) of aerobic physical activity with moderate to vigorous intensity during leisure time was suggested as the optimal characteristic for physical activity for the prevention of CRC [151].
Dietary factors: Different studies suggested protective effects of diets high in fruits and vegetables in CRC [153,154,155,156,157], with about a 50% decrease in the risk of CRC in individuals with the highest intake [158,159]. The risk of CRC was significantly lower in individuals with vegetarian diets compared with non-vegetarians [72,160,161]. Dairy products are another dietary factor for which CRC protective effects were consistently reported [72,73,162]. About a 10% reduction in CRC risk was reported in individuals with daily intakes of 400 g of dairy products or a daily milk intake of 200 g/day [163,164].
Dietary fiber was suggested as another protective factor from CRC. Higher intake of fiber was associated with decreased risk of CRC [72,73,165], with a 10 percent decrease in CRC risk reported for every 10 g/day increase in dietary fiber intake [15,166].
Possible protective effects were also reported (in observational studies) for other dietary factors, including fish [167], garlic [15], and coffee [168,169]. However, the findings were not consistent [157,170,171,172,173,174] and need to be confirmed in prospective interventional studies.
Chemoprevention: The preventive effects of different drugs or supplements in CRC, called chemoprevention, were documented in several studies, especially on average- and high-risk populations [175]. There is substantial consistent evidence on the protective effects of aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) in colorectal adenoma and CRC [176,177,178,179]. Several studies reported protective effects of postmenopausal hormone therapy in CRC, with more consistent findings for combined drugs (estrogen plus progestin) [180,181,182]. The protective effects of oral contraceptives were also reported in premenopausal women [183]. Findings from a systematic review of 126 articles suggested protective effects for statins in CRC [184]. Regarding the adverse events of aspirin (e.g., bleeding), especially in older individuals [185], further investigations are warranted to clarify the preventive effects of statins in CRC. Increased intake of calcium and vitamin D [186,187,188] were also proposed as protective factors for CRC, although these findings were not consistent with the results of other studies [189,190,191], suggesting the need for further solid evidence.
4.2. Secondary Prevention (CRC Screening)
The process of secondary prevention of CRC (CRC screening) consists of the diagnosis and treatment (removal) of premalignant (adenomatous) polyps of the colon and rectum. There is consistent evidence for the effects of polyp removal on reducing the risk of CRC [192,193]. The results of a recent meta-analysis suggested that different screening methods could significantly reduce CRC-specific mortality [193]. The Fecal Occult Blood Test (FOBT) and lower-GI endoscopy (sigmoidoscopy/colonoscopy) are the main methods for CRC screening. Based on the method for detecting occult blood in stool, the FOBT may be categorized into two groups, including the guaiac-based FOBT (guaiac test) and the fecal immunohistochemical (FIT)-based FOBT (FIT test).
4.2.1. Guaiac-Based FOBT (Guaiac Test)
The guaiac-based FOBT may detect both human and non-human (e.g., from dietary sources) hemoglobin. This is the major limitation of this test, resulting in a high proportion of false positives and low positive predictive values [194,195]. The high-sensitivity guaiac-based FOBT was introduced to improve the sensitivity of the guaiac-based FOBT, although the limitation of low specificity remains, suggesting that policymakers should replace this method with more accurate methods in population-based screening programs [196,197,198,199].
4.2.2. FIT-Based FOBT (FIT Test)
The FIT test identifies only intact human hemoglobin and does not detect non-human hemoglobin (e.g., from dietary sources) and also does not detect digested human hemoglobin (e.g., bleeding from upper gastrointestinal tract or respiratory tract). This advantage of the FIT test (over the guaiac test) resulted in better indices of accuracy for the FIT test in detecting colorectal neoplasia [196,197,200,201,202]. The results of a retrospective cohort study suggested high adherence to a programmatic (e.g., annual) FIT test, resulting in detection in the majority (more than 80%) of patients with CRC diagnosed within one year of testing [203]. Therefore, the FIT test is a non-invasive CRC screening method with acceptable accuracy and high adherence to programmatic testing.
4.2.3. Stool DNA Test
Stool DNA tests detect CRC-associated gene mutations in cells shed from the colorectal epithelium into stool [204,205]. Stool DNA tests were proposed as non-invasive accurate methods for detecting colorectal neoplasia [206,207,208,209], especially when combined with the FIT test (sDNA-FIT test) [210].
4.2.4. Sigmoidoscopy
Examination of the distal colon with the flexible fiber-optic sigmoidoscope (sigmoidoscopy) was suggested as an accurate and acceptable method for detecting premalignant colorectal neoplasia. Different clinical trials on sigmoidoscopy screening reported 20 to 30 percent decreases in CRC incidence and mortality, with sustainable effects over the long term, suggesting sigmoidoscopy as an effective method for CRC screening [211,212,213,214,215,216].
4.2.5. Colonoscopy
The results of different studies suggest an association between colonoscopy examination and a reduction in CRC incidence and mortality [192,216,217,218]. A recent randomized, controlled trial also reported significant effects of colonoscopy examination in reducing CRC risk (RR = 0.69) and mortality (RR = 0.50) [219]. Colonoscopy may be considered as the only CRC screening modality for specific (high-risk) populations or may be used in combination with other non-invasive modalities in general (average-risk) populations [220,221,222].
4.2.6. Computed Tomographic Colonography (CTC)
CTC includes the examination of the abdominal CT scan images of the colon and rectum, simulating the effect of a colonoscopy. The results of different studies suggest high accuracy of CTC for detecting colorectal neoplasia, especially large adenomas [223,224,225,226]. Different factors may affect the accuracy, and especially the sensitivity, of CTC, including the expertise of the radiologist, the mode of imaging (2-D or 3-D), and the characteristics of the scanner and detector. These factors should be mentioned when considering CTC for CRC screening.
4.3. Combination and Risk-Based Strategies
A combination of the FOBT and lower-GI endoscopy (sigmoidoscopy/colonoscopy) could increase the accuracy of screening programs for the detection of colorectal neoplasia, suggesting combination methods as the most effective CRC screening method [227,228]. In addition, considering risk stratification to identify high-risk individuals was suggested to increase the effectiveness of CRC screening programs. Different risk factors, including age, gender, smoking, and family history, may be considered for the calculation of risk scores [229,230,231,232]. Combining the risk score with the FIT test was suggested as an effective strategy for more accurate detection of colorectal neoplasia in asymptomatic individuals [233,234,235].
4.4. CRC Screening Guidelines
Different guidelines have been developed for CRC screening in high- and average-risk individuals. The screening methods for high-risk groups are specified according to the underlying conditions (e.g., presence of hereditary syndromes such as FAP or HNPCC, family history of CRC, etc.), but most guidelines suggest colonoscopy as the first choice for CRC screening in high-risk groups [236,237,238].
Guidelines on CRC screening in average-risk populations have been issued by different organizations [7,8,9,143,239,240]. In its 2018 guideline, the American Cancer Society (ACS) suggested starting CRC screening in individuals between 45 and 75 years old. Based on the ACS 2018 guideline, CRC screening programs may include annual FIT tests, guaiac-based FOBTs annually, stool DNA tests every 3 years, sigmoidoscopy every 5 years, CTC every 5 years, and colonoscopy every 10 years [143]. The recent (2021) U.S. Preventive Services Task Force (USPSTF) guideline also recommended CRC screening in average-risk populations aged 45–75. According to the USPSTF 2021 guideline, the following modalities are recommended for CRC screening: the guaiac-based FOBT or FIT test every year, sDNA-FIT test every 1 or 3 years, sigmoidoscopy every 5 years, CTC every 5 years, sigmoidoscopy every 10 years plus FIT test every year, and colonoscopy every 10 years [144,239]. The American College of Physicians (ACP) issued its updated CRC screening guideline in 2019. The ACP recommends CRC screening in average-risk adults between 50 and 75 years old. The screening modalities and intervals recommended by the ACP include the FIT test or guaiac-based FOBT every 2 years, sigmoidoscopy every 10 years plus FIT test every 2 years, or colonoscopy every 10 years [8,240].
All organizations emphasize that different factors both at the individual and community levels (e.g., patient preferences, availability of screening modalities, costs, benefits, and adverse events) should be taken into account in the designing and implementation of CRC screening programs. Policy makers are encouraged to pay especial attention to making the best decision in identifying the starting age and selection of the most effective screening modalities for the target population [7,8,9,143,144,239,240,241].
5. Conclusions
CRC is the third most common incident cancer and the second most common cause of cancer mortality worldwide. Individuals residing in developed countries are at higher risk of CRC, while the residents of the developing world are at lower risk for this cancer. There were declining trends in the ASR of CRC in developed countries during the last decades, while the trends in developing countries were increasing. In addition, the results of the future predictions (by 2040) show that the greatest increases in the incidence of CRC are predicted to occur in currently low-risk populations, including those in developing countries. Despite the declining trend in the incidence of CRC in the total population of the developed countries, the incidence rates of EOCRC (age below 50 years) were increasing both in the developed and developing world, suggesting the need for further investigations on its risk factors and control methods worldwide.
The most important modifiable risk factors for CRC include alcohol consumption, smoking, obesity, sedentary lifestyle, high intake of red and processed meat and fat, and psychological stress. Age, gender, genetic predisposition, family history of CRC, abdominopelvic radiation, personal history of IBD, and intestinal microbiota were proposed as non-modifiable risk factors for CRC. Policymakers may consider these risk factors for designing risk-based CRC preventive strategies. Further investigations are warranted to clarify different aspects of the association between these risk factors, especially new ones (e.g., psychological stress, intestinal microbiota), and the risk of CRC.
CRC prevention methods may be classified as “primary” and “secondary” prevention. Primary prevention includes methods for reduction in or elimination of exposure to risk factors (e.g., strategies for reducing smoking and alcohol consumption) as well as strategies for increasing protective factors (e.g., physical activity, dietary fiber, aspirin). The secondary prevention method, CRC screening, includes diagnosis and removal of colorectal adenomatous polyps. A number of methods have been proposed for CRC screening, including the FOBT (guaiac test/FIT test), the stool DNA test, lower-GI endoscopy (sigmoidoscopy/colonoscopy), and CTC. Considering a combination of screening methods (e.g., FOBT + colonoscopy) and targeting eligible individuals according to the levels of exposure to risk factors (risk-based strategy) will increase the accuracy and effectiveness of CRC screening programs. The development of CRC screening guidelines is a complex process, and different factors should be mentioned in this process, including the accuracy of screening methods (e.g., sensitivity and specificity) and the characteristics of target populations (e.g., patient preferences, availability of screening modalities, costs, benefits, and adverse events). According to the ACS guideline, average-risk individuals are suggested to start CRC screening at 45. The ACS recommended a combination of screening methods at specific intervals, including FOBT, stool DNA test, sigmoidoscopy, CTC, and colonoscopy. According to the ACP guideline, CRC screening should start at age 50. The ACP also suggest multiple screening methods at specific intervals, including the FOBT, sigmoidoscopy, and colonoscopy.
Considering effective risk reduction strategies as well as the design and successful implementation of population-specific CRC screening programs has a pivotal role in CRC control programs in each community. High-quality population-specific research is needed to select the best and most accurate and effective methods for the primary prevention and screening of CRC in target populations.
Author Contributions
G.R. and F.G.-K. wrote the manuscript. R.M. was involved in the conception, discussion, and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ervik, M.; Lam, F.; Laversanne, M.; Ferlay, J.; Bray, F. Global Cancer Observatory: Cancer Over Time. Available online: https://gco.iarc.fr/overtime (accessed on 23 March 2023).
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.fr/today (accessed on 10 October 2023).
- Ferlay, J.; Laversanne, M.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Tomorrow. Available online: https://gco.iarc.fr/tomorrow (accessed on 10 October 2023).
- Eng, C.; Hochster, H. Early-Onset Colorectal Cancer: The Mystery Remains. J. Natl. Cancer Inst. 2021, 113, 1608–1610. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Levin, T.R.; Lieberman, D.; Robertson, D.J. Colorectal cancer screening: Recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastrointest. Endosc. 2017, 86, 18–33. [Google Scholar] [CrossRef]
- Qaseem, A.; Crandall, C.J.; Mustafa, R.A.; Hicks, L.A.; Wilt, T.J.; Forciea, M.A.; Fitterman, N.; Horwitch, C.A.; Kansagara, D.; Maroto, M.; et al. Screening for Colorectal Cancer in Asymptomatic Average-Risk Adults: A Guidance Statement From the American College of Physicians. Ann. Intern. Med. 2019, 171, 643–654. [Google Scholar] [CrossRef]
- Shaukat, A.; Kahi, C.J.; Burke, C.A.; Rabeneck, L.; Sauer, B.G.; Rex, D.K. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am. J. Gastroenterol. 2021, 116, 458–479. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bray, F. Transitions in human development and the global cancer burden. In World Cancer Report; Stewart, B.W., Wild, C.P., Eds.; WHO Press: Geneve, Switzerland, 2014; Volume 34, pp. 42–55. [Google Scholar]
- Fidler, M.M.; Soerjomataram, I.; Bray, F. A global view on cancer incidence and national levels of the human development index. Int. J. Cancer 2016, 139, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e315. [Google Scholar] [CrossRef]
- GBD Colorectal Cancer Collaborators. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019, 4, 913–933. [Google Scholar] [CrossRef]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The world cancer research fund/American institute for cancer research third expert report on diet, nutrition, physical activity, and cancer: Impact and future directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef]
- Roshandel, G.; Ferlay, J.; Ghanbari-Motlagh, A.; Partovipour, E.; Salavati, F.; Aryan, K.; Mohammadi, G.; Khoshaabi, M.; Sadjadi, A.; Davanlou, M.; et al. Cancer in Iran 2008 to 2025: Recent incidence trends and short-term predictions of the future burden. Int. J. Cancer 2021, 149, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Kebria, F.; Jafari-Delouie, N.; Semnani, S.; Fazel, A.; Etemadi, A.; Norouzi, A.; Khandoozi, R.; Besharat, S.; Shokouhifar, N.; Mirkarimi, H.; et al. Colorectal cancer incidence trends in Golestan, Iran: An age-period-cohort analysis 2004–2018. Cancer Epidemiol. 2023, 86, 102415. [Google Scholar] [CrossRef]
- Brenner, D.R.; Heer, E.; Sutherland, R.L.; Ruan, Y.; Tinmouth, J.; Heitman, S.J.; Hilsden, R.J. National Trends in Colorectal Cancer Incidence Among Older and Younger Adults in Canada. JAMA Netw. Open 2019, 2, e198090. [Google Scholar] [CrossRef] [PubMed]
- Abualkhair, W.H.; Zhou, M.; Ahnen, D.; Yu, Q.; Wu, X.C.; Karlitz, J.J. Trends in Incidence of Early-Onset Colorectal Cancer in the United States Among Those Approaching Screening Age. JAMA Netw. Open 2020, 3, e1920407. [Google Scholar] [CrossRef]
- Meester, R.G.S.; Mannalithara, A.; Lansdorp-Vogelaar, I.; Ladabaum, U. Trends in Incidence and Stage at Diagnosis of Colorectal Cancer in Adults Aged 40 Through 49 Years, 1975–2015. JAMA 2019, 321, 1933–1934. [Google Scholar] [CrossRef]
- Ward, E.M.; Sherman, R.L.; Henley, S.J.; Jemal, A.; Siegel, D.A.; Feuer, E.J.; Firth, A.U.; Kohler, B.A.; Scott, S.; Ma, J.; et al. Annual Report to the Nation on the Status of Cancer, Featuring Cancer in Men and Women Age 20–49 Years. J. Natl. Cancer Inst. 2019, 111, 1279–1297. [Google Scholar] [CrossRef] [PubMed]
- Montminy, E.M.; Zhou, M.; Maniscalco, L.; Abualkhair, W.; Kim, M.K.; Siegel, R.L.; Wu, X.C.; Itzkowitz, S.H.; Karlitz, J.J. Contributions of Adenocarcinoma and Carcinoid Tumors to Early-Onset Colorectal Cancer Incidence Rates in the United States. Ann. Intern. Med. 2021, 174, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Howren, A.; Sayre, E.C.; Loree, J.M.; Gill, S.; Brown, C.J.; Raval, M.J.; Farooq, A.; De Vera, M.A. Trends in the Incidence of Young-Onset Colorectal Cancer with a Focus on Years Approaching Screening Age: A Population-Based Longitudinal Study. J. Natl. Cancer Inst. 2021, 113, 863–868. [Google Scholar] [CrossRef]
- Bailey, C.E.; Hu, C.Y.; You, Y.N.; Bednarski, B.K.; Rodriguez-Bigas, M.A.; Skibber, J.M.; Cantor, S.B.; Chang, G.J. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015, 150, 17–22. [Google Scholar] [CrossRef]
- Emile, S.H.; Horesh, N.; Freund, M.R.; Garoufalia, Z.; Gefen, R.; Silva-Alvarenga, E.; Maron, D.J.; DaSilva, G.; Wexner, S.D. Trends in the Characteristics, Treatment, and Outcomes of Rectal Adenocarcinoma in the US From 2004 to 2019: A National Cancer Database Analysis. JAMA Oncol. 2023, 9, 355–364. [Google Scholar] [CrossRef]
- Mork, M.E.; You, Y.N.; Ying, J.; Bannon, S.A.; Lynch, P.M.; Rodriguez-Bigas, M.A.; Vilar, E. High Prevalence of Hereditary Cancer Syndromes in Adolescents and Young Adults with Colorectal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3544–3549. [Google Scholar] [CrossRef]
- Chang, D.T.; Pai, R.K.; Rybicki, L.A.; Dimaio, M.A.; Limaye, M.; Jayachandran, P.; Koong, A.C.; Kunz, P.A.; Fisher, G.A.; Ford, J.M.; et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: An adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc 2012, 25, 1128–1139. [Google Scholar] [CrossRef]
- Murphy, N.; Campbell, P.T.; Gunter, M.J. Unraveling the Etiology of Early-Onset Colorectal Cancer. J. Natl. Cancer Inst. 2021, 113, 505–506. [Google Scholar] [CrossRef]
- Cercek, A.; Chatila, W.K.; Yaeger, R.; Walch, H.; Fernandes, G.D.S.; Krishnan, A.; Palmaira, L.; Maio, A.; Kemel, Y.; Srinivasan, P.; et al. A Comprehensive Comparison of Early-Onset and Average-Onset Colorectal Cancers. J. Natl. Cancer Inst. 2021, 113, 1683–1692. [Google Scholar] [CrossRef]
- Ahnen, D.J.; Wade, S.W.; Jones, W.F.; Sifri, R.; Mendoza Silveiras, J.; Greenamyer, J.; Guiffre, S.; Axilbund, J.; Spiegel, A.; You, Y.N. The increasing incidence of young-onset colorectal cancer: A call to action. Mayo Clin. Proc. 2014, 89, 216–224. [Google Scholar] [CrossRef]
- Kim, H.; Lipsyc-Sharf, M.; Zong, X.; Wang, X.; Hur, J.; Song, M.; Wang, M.; Smith-Warner, S.A.; Fuchs, C.; Ogino, S.; et al. Total Vitamin D Intake and Risks of Early-Onset Colorectal Cancer and Precursors. Gastroenterology 2021, 161, 1208–1217.e1209. [Google Scholar] [CrossRef]
- Zheng, X.; Hur, J.; Nguyen, L.H.; Liu, J.; Song, M.; Wu, K.; Smith-Warner, S.A.; Ogino, S.; Willett, W.C.; Chan, A.T.; et al. Comprehensive Assessment of Diet Quality and Risk of Precursors of Early-Onset Colorectal Cancer. J. Natl. Cancer Inst. 2021, 113, 543–552. [Google Scholar] [CrossRef]
- Hur, J.; Otegbeye, E.; Joh, H.K.; Nimptsch, K.; Ng, K.; Ogino, S.; Meyerhardt, J.A.; Chan, A.T.; Willett, W.C.; Wu, K.; et al. Sugar-sweetened beverage intake in adulthood and adolescence and risk of early-onset colorectal cancer among women. Gut 2021, 70, 2330–2336. [Google Scholar] [CrossRef] [PubMed]
- Sinicrope, F.A. Increasing Incidence of Early-Onset Colorectal Cancer. N. Engl. J. Med. 2022, 386, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, D.E.; Sutherland, R.L.; Town, S.; Chow, K.; Fan, J.; Forbes, N.; Heitman, S.J.; Hilsden, R.J.; Brenner, D.R. Risk Factors for Early-Onset Colorectal Cancer: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 1229–1240.e1225. [Google Scholar] [CrossRef]
- Hua, H.; Jiang, Q.; Sun, P.; Xu, X. Risk factors for early-onset colorectal cancer: Systematic review and meta-analysis. Front. Oncol. 2023, 13, 1132306. [Google Scholar] [CrossRef]
- Breau, G.; Ellis, U. Risk Factors Associated with Young-Onset Colorectal Adenomas and Cancer: A Systematic Review and Meta-Analysis of Observational Research. Cancer Control J. Moffitt Cancer Cent. 2020, 27, 1073274820976670. [Google Scholar] [CrossRef]
- Wu, S.; Wang, H.; Li, Y.; Xie, Y.; Huang, C.; Zhao, H.; Miyagishi, M.; Kasim, V. Transcription Factor YY1 Promotes Cell Proliferation by Directly Activating the Pentose Phosphate Pathway. Cancer Res. 2018, 78, 4549–4562. [Google Scholar] [CrossRef]
- Yang, Y.; Du, L.; Shi, D.; Kong, C.; Liu, J.; Liu, G.; Li, X.; Ma, Y. Dysbiosis of human gut microbiome in young-onset colorectal cancer. Nat. Commun. 2021, 12, 6757. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.T.; Giovannucci, E.L. Primary prevention of colorectal cancer. Gastroenterology 2010, 138, 2029–2043.e2010. [Google Scholar] [CrossRef] [PubMed]
- Malekzadeh, R.; Bishehsari, F.; Mahdavinia, M.; Ansari, R. Epidemiology and molecular genetics of colorectal cancer in iran: A review. Arch. Iran. Med. 2009, 12, 161–169. [Google Scholar] [PubMed]
- Bishehsari, F.; Mahdavinia, M.; Vacca, M.; Malekzadeh, R.; Mariani-Costantini, R. Epidemiological transition of colorectal cancer in developing countries: Environmental factors, molecular pathways, and opportunities for prevention. World J. Gastroenterol. 2014, 20, 6055–6072. [Google Scholar] [CrossRef]
- Mansour, R.; Al-Ani, A.; Al-Hussaini, M.; Abdel-Razeq, H.; Al-Ibraheem, A.; Mansour, A.H. Modifiable risk factors for cancer in the middle East and North Africa: A scoping review. BMC Public Health 2024, 24, 223. [Google Scholar] [CrossRef]
- Ionescu, V.A.; Gheorghe, G.; Bacalbasa, N.; Chiotoroiu, A.L.; Diaconu, C. Colorectal Cancer: From Risk Factors to Oncogenesis. Medicina 2023, 59, 1646. [Google Scholar] [CrossRef]
- McNabb, S.; Harrison, T.A.; Albanes, D.; Berndt, S.I.; Brenner, H.; Caan, B.J.; Campbell, P.T.; Cao, Y.; Chang-Claude, J.; Chan, A.; et al. Meta-analysis of 16 studies of the association of alcohol with colorectal cancer. Int. J. Cancer 2020, 146, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Fedirko, V.; Tramacere, I.; Bagnardi, V.; Rota, M.; Scotti, L.; Islami, F.; Negri, E.; Straif, K.; Romieu, I.; La Vecchia, C.; et al. Alcohol drinking and colorectal cancer risk: An overall and dose-response meta-analysis of published studies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011, 22, 1958–1972. [Google Scholar] [CrossRef]
- Cai, S.; Li, Y.; Ding, Y.; Chen, K.; Jin, M. Alcohol drinking and the risk of colorectal cancer death: A meta-analysis. Eur. J. Cancer Prev. 2014, 23, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Huang, J.; Wong, M.C.S. Associations of alcohol and coffee with colorectal cancer risk in East Asian populations: A Mendelian randomization study. Eur. J. Nutr. 2023, 62, 749–756. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, L.; Xiao, J.; Sun, J.; Yu, L.; Zhang, H.; Meng, X.; Yuan, S.; Timofeeva, M.; Law, P.J.; et al. Alcohol consumption, DNA methylation and colorectal cancer risk: Results from pooled cohort studies and Mendelian randomization analysis. Int. J. Cancer 2022, 151, 83–94. [Google Scholar] [CrossRef]
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef]
- Pande, M.; Lynch, P.M.; Hopper, J.L.; Jenkins, M.A.; Gallinger, S.; Haile, R.W.; LeMarchand, L.; Lindor, N.M.; Campbell, P.T.; Newcomb, P.A.; et al. Smoking and colorectal cancer in Lynch syndrome: Results from the Colon Cancer Family Registry and the University of Texas M.D. Anderson Cancer Center. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 1331–1339. [Google Scholar] [CrossRef]
- Buchanan, D.D.; Sweet, K.; Drini, M.; Jenkins, M.A.; Win, A.K.; English, D.R.; Walsh, M.D.; Clendenning, M.; McKeone, D.M.; Walters, R.J.; et al. Risk factors for colorectal cancer in patients with multiple serrated polyps: A cross-sectional case series from genetics clinics. PLoS ONE 2010, 5, e11636. [Google Scholar] [CrossRef]
- Botteri, E.; Borroni, E.; Sloan, E.K.; Bagnardi, V.; Bosetti, C.; Peveri, G.; Santucci, C.; Specchia, C.; van den Brandt, P.; Gallus, S.; et al. Smoking and Colorectal Cancer Risk, Overall and by Molecular Subtypes: A Meta-Analysis. Am. J. Gastroenterol. 2020, 115, 1940–1949. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wei, H.; Liu, W.; Coker, O.O.; Gou, H.; Liu, C.; Zhao, L.; Li, C.; Zhou, Y.; Wang, G.; et al. Cigarette smoke promotes colorectal cancer through modulation of gut microbiota and related metabolites. Gut 2022, 71, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Gram, I.T.; Park, S.Y.; Wilkens, L.R.; Haiman, C.A.; Le Marchand, L. Smoking-Related Risks of Colorectal Cancer by Anatomical Subsite and Sex. Am. J. Epidemiol. 2020, 189, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Limsui, D.; Vierkant, R.A.; Tillmans, L.S.; Wang, A.H.; Weisenberger, D.J.; Laird, P.W.; Lynch, C.F.; Anderson, K.E.; French, A.J.; Haile, R.W.; et al. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J. Natl. Cancer Inst. 2010, 102, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Chen, Y.; Wang, X.; Wang, J.; Yan, Z.; Gong, G.; Li, G.; Li, C. Meta-analysis of prospective cohort studies of cigarette smoking and the incidence of colon and rectal cancers. Eur. J. Cancer Prev. 2015, 24, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Karahalios, A.; English, D.R.; Simpson, J.A. Weight change and risk of colorectal cancer: A systematic review and meta-analysis. Am. J. Epidemiol. 2015, 181, 832–845. [Google Scholar] [CrossRef]
- Hashemi Madani, N.; Etemadi, A.; Nalini, M.; Poustchi, H.; Khajavi, A.; Mirzazade, E.; Mirfakhraei, H.; Pourshams, A.; Khoshnia, M.; Gharavi, A.; et al. Obesity and incident gastrointestinal cancers: Overall body size or central obesity measures, which factor matters? Eur. J. Cancer Prev. 2021, 30, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Bull, C.J.; Bell, J.A.; Murphy, N.; Sanderson, E.; Davey Smith, G.; Timpson, N.J.; Banbury, B.L.; Albanes, D.; Berndt, S.I.; Bézieau, S.; et al. Adiposity, metabolites, and colorectal cancer risk: Mendelian randomization study. BMC Med. 2020, 18, 396. [Google Scholar] [CrossRef] [PubMed]
- Soltani, G.; Poursheikhani, A.; Yassi, M.; Hayatbakhsh, A.; Kerachian, M.; Kerachian, M.A. Obesity, diabetes and the risk of colorectal adenoma and cancer. BMC Endocr. Disord. 2019, 19, 113. [Google Scholar] [CrossRef] [PubMed]
- Socol, C.T.; Chira, A.; Martinez-Sanchez, M.A.; Nuñez-Sanchez, M.A.; Maerescu, C.M.; Mierlita, D.; Rusu, A.V.; Ruiz-Alcaraz, A.J.; Trif, M.; Ramos-Molina, B. Leptin Signaling in Obesity and Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 4713. [Google Scholar] [CrossRef]
- Ye, P.; Xi, Y.; Huang, Z.; Xu, P. Linking Obesity with Colorectal Cancer: Epidemiology and Mechanistic Insights. Cancers 2020, 12, 1408. [Google Scholar] [CrossRef]
- Al-Zalabani, A. Preventability of Colorectal Cancer in Saudi Arabia: Fraction of Cases Attributable to Modifiable Risk Factors in 2015–2040. Int. J. Environ. Res. Public Health 2020, 17, 320. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ung, T.T.; Kim, N.H.; Jung, Y.D. Role of bile acids in colon carcinogenesis. World J. Clin. Cases 2018, 6, 577–588. [Google Scholar] [CrossRef]
- Fletcher, R.; Wang, Y.J.; Schoen, R.E.; Finn, O.J.; Yu, J.; Zhang, L. Colorectal cancer prevention: Immune modulation taking the stage. Biochim. Biophys. Acta. Rev. Cancer 2018, 1869, 138–148. [Google Scholar] [CrossRef]
- Morrison, D.S.; Parr, C.L.; Lam, T.H.; Ueshima, H.; Kim, H.C.; Jee, S.H.; Murakami, Y.; Giles, G.; Fang, X.; Barzi, F.; et al. Behavioural and metabolic risk factors for mortality from colon and rectum cancer: Analysis of data from the Asia-Pacific Cohort Studies Collaboration. Asian Pac. J. Cancer Prev. APJCP 2013, 14, 1083–1087. [Google Scholar] [CrossRef]
- Wolin, K.Y.; Yan, Y.; Colditz, G.A.; Lee, I.M. Physical activity and colon cancer prevention: A meta-analysis. Br. J. Cancer 2009, 100, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Morrow, L.; Greenwald, B. Healthy Food Choices, Physical Activity, and Screening Reduce the Risk of Colorectal Cancer. Gastroenterol. Nurs. Off. J. Soc. Gastroenterol. Nurses Assoc. 2022, 45, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; MacInnis, R.J.; English, D.R.; Karahalios, A.; Lynch, B.M. Domain-specific physical activity and sedentary behaviour in relation to colon and rectal cancer risk: A systematic review and meta-analysis. Int. J. Epidemiol. 2017, 46, 1797–1813. [Google Scholar] [CrossRef]
- Vallis, J.; Wang, P.P. The Role of Diet and Lifestyle in Colorectal Cancer Incidence and Survival. In Gastrointestinal Cancers; Morgado-Diaz, J.A., Ed.; Exon Publications: Brisbane City, Australia, 2022. [Google Scholar]
- Veettil, S.K.; Wong, T.Y.; Loo, Y.S.; Playdon, M.C.; Lai, N.M.; Giovannucci, E.L.; Chaiyakunapruk, N. Role of Diet in Colorectal Cancer Incidence: Umbrella Review of Meta-analyses of Prospective Observational Studies. JAMA Netw. Open 2021, 4, e2037341. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. In Red Meat and Processed Meat; International Agency for Research on Cancer: Lyon, France, 2018. [Google Scholar]
- World Cancer Report. Cancer Research for Cancer Prevention. Available online: https://www--iccp-portal--org.ezaccess.ir/sites/default/files/resources/IARC%20World%20Cancer%20Report%202020.pdf (accessed on 16 July 2023).
- Domingo, J.L.; Nadal, M. Carcinogenicity of consumption of red meat and processed meat: A review of scientific news since the IARC decision. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 105, 256–261. [Google Scholar] [CrossRef]
- Farvid, M.S.; Sidahmed, E.; Spence, N.D.; Mante Angua, K.; Rosner, B.A.; Barnett, J.B. Consumption of red meat and processed meat and cancer incidence: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2021, 36, 937–951. [Google Scholar] [CrossRef]
- Zhao, Z.; Feng, Q.; Yin, Z.; Shuang, J.; Bai, B.; Yu, P.; Guo, M.; Zhao, Q. Red and processed meat consumption and colorectal cancer risk: A systematic review and meta-analysis. Oncotarget 2017, 8, 83306–83314. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chinnathambi, S.; Kumar, M.; Pandian, G.N. Food Intake and Colorectal Cancer. Nutr. Cancer 2023, 75, 1710–1742. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Mohammad, N.M.A.; Jia Xin, Y.; Abdul Hamid, N.H.; Shahar, S.; Ali, R.A.R. Dietary Risk Factors and Odds of Colorectal Adenoma in Malaysia: A Case Control Study. Nutr. Cancer 2022, 74, 2757–2768. [Google Scholar] [CrossRef]
- Kim, M.; Park, K. Dietary Fat Intake and Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients 2018, 10, 1963. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, H. Do sleep quality and psychological factors link precancerous conditions of colorectal cancer? A retrospective case-control study. Expert Rev. Gastroenterol. Hepatol. 2022, 16, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Qiao, Y.; Xiang, S.; Li, W.; Gan, Y.; Chen, Y. Work stress and the risk of cancer: A meta-analysis of observational studies. Int. J. Cancer 2019, 144, 2390–2400. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, N.; Nishiyama, T.; Sawada, T.; Wang, C.; Lin, Y.; Watanabe, Y.; Tamakoshi, A.; Kikuchi, S. Perceived Stress and Colorectal Cancer Incidence: The Japan Collaborative Cohort Study. Sci. Rep. 2017, 7, 40363. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Huybrechts, I.; Michels, N. Psychosocial stress and cancer risk: A narrative review. Eur. J. Cancer Prev. 2022, 31, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Scotto, L.; Ravenda, S.; Zampino, M.G.; Pravettoni, G.; Mazzocco, K. Personality Factors in Colorectal Cancer: A Systematic Review. Front. Psychol. 2021, 12, 590320. [Google Scholar] [CrossRef]
- van Tuijl, L.A.; Basten, M.; Pan, K.Y.; Vermeulen, R.; Portengen, L.; de Graeff, A.; Dekker, J.; Geerlings, M.I.; Hoogendoorn, A.; Lamers, F.; et al. Depression, anxiety, and the risk of cancer: An individual participant data meta-analysis. Cancer 2023, 129, 3287–3299. [Google Scholar] [CrossRef]
- Hadrévi, J.; Myte, R.; Olsson, T.; Palmqvist, R.; Slunga Järvholm, L.; Van Guelpen, B. Work-Related Stress Was Not Associated with Increased Cancer Risk in a Population-Based Cohort Setting. Cancer Epidemiol. Biomark. Prev. 2022, 31, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Peng, F.; Lu, J.; He, B.; Su, Q.; Luo, H.; Deng, Z.; Jiang, T.; Su, K.; Huang, Y.; et al. Cancer and stress: NextGen strategies. Brain Behav. Immun. 2021, 93, 368–383. [Google Scholar] [CrossRef] [PubMed]
- Mravec, B.; Tibensky, M.; Horvathova, L. Stress and cancer. Part I: Mechanisms mediating the effect of stressors on cancer. J. Neuroimmunol. 2020, 346, 577311. [Google Scholar] [CrossRef] [PubMed]
- Mravec, B.; Horvathova, L.; Hunakova, L. Neurobiology of Cancer: The Role of β-Adrenergic Receptor Signaling in Various Tumor Environments. Int. J. Mol. Sci. 2020, 21, 7958. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.L.; Biggers, A.; Oddo, V.M.; Yanez, B.; Booms, E.; Sharp, L.; Naylor, K.; Wolf, P.G.; Tussing-Humphreys, L. A Perspective Review on Diet Quality, Excess Adiposity, and Chronic Psychosocial Stress and Implications for Early-Onset Colorectal Cancer. J. Nutr. 2024, 154, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, S.; Sebastián, C. Metabolic pathways regulating colorectal cancer initiation and progression. Semin. Cell Dev. Biol. 2020, 98, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shay, J.W. Multiple Roles of APC and its Therapeutic Implications in Colorectal Cancer. J. Natl. Cancer Inst. 2017, 109, djw332. [Google Scholar] [CrossRef]
- Valle, L.; de Voer, R.M.; Goldberg, Y.; Sjursen, W.; Försti, A.; Ruiz-Ponte, C.; Caldés, T.; Garré, P.; Olsen, M.F.; Nordling, M.; et al. Update on genetic predisposition to colorectal cancer and polyposis. Mol. Asp. Med. 2019, 69, 10–26. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Haanstra, J.F.; de Vos Tot Nederveen Cappel, W.H.; Gopie, J.P.; Vecht, J.; Vanhoutvin, S.A.; Cats, A.; van der Zaag-Loonen, H.J.; Langers, A.M.; Bergmann, J.H.; van de Meeberg, P.C.; et al. Quality of life after surgery for colon cancer in patients with Lynch syndrome: Partial versus subtotal colectomy. Dis. Colon Rectum 2012, 55, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Parry, S.; Win, A.K.; Parry, B.; Macrae, F.A.; Gurrin, L.C.; Church, J.M.; Baron, J.A.; Giles, G.G.; Leggett, B.A.; Winship, I.; et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: The advantage of more extensive colon surgery. Gut 2011, 60, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Yurgelun, M.B.; Kulke, M.H.; Fuchs, C.S.; Allen, B.A.; Uno, H.; Hornick, J.L.; Ukaegbu, C.I.; Brais, L.K.; McNamara, P.G.; Mayer, R.J.; et al. Cancer Susceptibility Gene Mutations in Individuals with Colorectal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, R.; Frankel, W.L.; Swanson, B.; Zhao, W.; Yilmaz, A.; Miller, K.; Bacher, J.; Bigley, C.; Nelsen, L.; Goodfellow, P.J.; et al. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients with Early-Onset Colorectal Cancer. JAMA Oncol. 2017, 3, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, C.M.; Creavin, B.; O’Connell, E.P.; Kelly, L.; O’Sullivan, M.J.; Corrigan, M.A.; Redmond, H.P. Risk of colorectal cancer associated with BRCA1 and/or BRCA2 mutation carriers: Systematic review and meta-analysis. Br. J. Surg. 2020, 107, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, Z.; Li, X.; Nyiraneza, C.; Ma, X.; Timofeeva, M.; Svinti, V.; Meng, X.; He, Y.; Bo, Y.; Morgan, S.; et al. Systematic meta-analyses, field synopsis and global assessment of the evidence of genetic association studies in colorectal cancer. Gut 2020, 69, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- Yige, L.; Dandan, Z. Progress on functional mechanisms of colorectal cancer causal SNPs in post-GWAS. Hereditas 2021, 43, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.P.; Burt, R.W.; Williams, M.S.; Haug, P.J.; Cannon-Albright, L.A. Population-based family history-specific risks for colorectal cancer: A constellation approach. Gastroenterology 2010, 138, 877–885. [Google Scholar] [CrossRef]
- Taylor, D.P.; Stoddard, G.J.; Burt, R.W.; Williams, M.S.; Mitchell, J.A.; Haug, P.J.; Cannon-Albright, L.A. How well does family history predict who will get colorectal cancer? Implications for cancer screening and counseling. Genet. Med. Off. J. Am. Coll. Med. Genet. 2011, 13, 385–391. [Google Scholar] [CrossRef]
- Imperiale, T.F.; Ransohoff, D.F. Risk for colorectal cancer in persons with a family history of adenomatous polyps: A systematic review. Ann. Intern. Med. 2012, 156, 703–709. [Google Scholar] [CrossRef]
- Song, M.; Emilsson, L.; Roelstraete, B.; Ludvigsson, J.F. Risk of colorectal cancer in first degree relatives of patients with colorectal polyps: Nationwide case-control study in Sweden. BMJ (Clin. Res. Ed.) 2021, 373, n877. [Google Scholar] [CrossRef]
- Nottage, K.; McFarlane, J.; Krasin, M.J.; Li, C.; Srivastava, D.; Robison, L.L.; Hudson, M.M. Secondary colorectal carcinoma after childhood cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 2552–2558. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.O.; Oeffinger, K.C.; Whitton, J.; Leisenring, W.; Neglia, J.; Meadows, A.; Crotty, C.; Rubin, D.T.; Diller, L.; Inskip, P.; et al. Secondary gastrointestinal cancer in childhood cancer survivors: A cohort study. Ann. Intern. Med. 2012, 156, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Desautels, D.; Czaykowski, P.; Nugent, Z.; Demers, A.A.; Mahmud, S.M.; Singh, H. Risk of colorectal cancer after the diagnosis of prostate cancer: A population-based study. Cancer 2016, 122, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.H.; Cheng, K.C.; Chao, C.M.; Lai, C.C.; Chiang, S.R.; Chen, C.M.; Liao, K.M.; Wang, J.J.; Lee, P.H.; Hung, C.M.; et al. Does radiotherapy increase the risk of colorectal cancer among prostate cancer patients? A large population-based study. J. Cancer 2020, 11, 6204–6212. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, A.J.M.; Hugen, N.; Elferink, M.A.G.; Poortmans, P.M.P.; Nagtegaal, I.D.; de Wilt, J.H.W. Increased risk for second primary rectal cancer after pelvic radiation therapy. Eur. J. Cancer 2020, 124, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, H.; Misumi, M.; Brenner, A.; Grant, E.J.; Sakata, R.; Sadakane, A.; Utada, M.; Preston, D.L.; Mabuchi, K.; Ozasa, K. Radiation risk of incident colorectal cancer by anatomical site among atomic bomb survivors: 1958–2009. Int. J. Cancer 2020, 146, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers, Version 5.0. Available online: http://www.survivorshipguidelines.org/ (accessed on 15 February 2024).
- Johansen, M.P.; Wewer, M.D.; Nordholm-Carstensen, A.; Burisch, J. Perianal Crohn’s Disease and the Development of Colorectal and Anal Cancer: A Systematic Review and Meta-analysis. J. Crohn’s Colitis 2023, 17, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Olén, O.; Erichsen, R.; Sachs, M.C.; Pedersen, L.; Halfvarson, J.; Askling, J.; Ekbom, A.; Sørensen, H.T.; Ludvigsson, J.F. Colorectal cancer in ulcerative colitis: A Scandinavian population-based cohort study. Lancet 2020, 395, 123–131. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, P.; Shao, L. Correlation of ulcerative colitis and colorectal cancer: A systematic review and meta-analysis. J. Gastrointest. Oncol. 2021, 12, 2814–2822. [Google Scholar] [CrossRef]
- Zhou, Q.; Shen, Z.F.; Wu, B.S.; Xu, C.B.; He, Z.Q.; Chen, T.; Shang, H.T.; Xie, C.F.; Huang, S.Y.; Chen, Y.G.; et al. Risk of Colorectal Cancer in Ulcerative Colitis Patients: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2019, 2019, 5363261. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Komaki, Y.; Komaki, F.; Micic, D.; Zullow, S.; Sakuraba, A. Risk of gastrointestinal cancers in patients with cystic fibrosis: A systematic review and meta-analysis. Lancet. Oncol. 2018, 19, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.; Anderson, K.; Singhania, M.; Cormier, R. Cystic Fibrosis, CFTR, and Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 2891. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Choi, M.G.; Kim, S.W.; Chung, I.S.; Yang, C.W.; Kim, Y.S.; Jung, C.K.; Lee, K.Y.; Kang, J.H. Increased incidence of colorectal malignancies in renal transplant recipients: A case control study. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2010, 10, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Li, W.; Ren, W.; Hu, D.; Song, Y. The association between cholecystectomy and the risk of colorectal cancer: An updated systematic review and meta-analysis of cohort studies. Transl. Cancer Res. 2023, 12, 1452–1465. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.O.; Jim, M.H.; Lam, K.F.; Morris, J.S.; Siu, D.C.; Tong, T.; Ng, F.H.; Wong, S.Y.; Hui, W.M.; Chan, C.K.; et al. Prevalence of colorectal neoplasm among patients with newly diagnosed coronary artery disease. JAMA 2007, 298, 1412–1419. [Google Scholar] [CrossRef]
- Liu, I.L.; Tsai, C.H.; Hsu, C.H.; Hu, J.M.; Chen, Y.C.; Tian, Y.F.; You, S.L.; Chen, C.Y.; Hsiao, C.W.; Lin, C.Y.; et al. Helicobacter pylori infection and the risk of colorectal cancer: A nationwide population-based cohort study. QJM Mon. J. Assoc. Physicians 2019, 112, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Tang, S.; Li, W.; Ng, S.C.; Chan, M.W.; Sung, J.J.; Yu, J. Hemolytic E. coli Promotes Colonic Tumorigenesis in Females. Cancer Res. 2016, 76, 2891–2900. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.; Buc, E.; Sauvanet, P.; Darcha, C.; Dubois, D.; Pereira, B.; Déchelotte, P.; Bonnet, R.; Pezet, D.; Darfeuille-Michaud, A. Colonization of the human gut by E. coli and colorectal cancer risk. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 859–867. [Google Scholar] [CrossRef]
- Baandrup, L.; Thomsen, L.T.; Olesen, T.B.; Andersen, K.K.; Norrild, B.; Kjaer, S.K. The prevalence of human papillomavirus in colorectal adenomas and adenocarcinomas: A systematic review and meta-analysis. Eur. J. Cancer 2014, 50, 1446–1461. [Google Scholar] [CrossRef]
- Lu, S.S.M.; Mohammed, Z.; Häggström, C.; Myte, R.; Lindquist, E.; Gylfe, Å.; Van Guelpen, B.; Harlid, S. Antibiotics Use and Subsequent Risk of Colorectal Cancer: A Swedish Nationwide Population-Based Study. J. Natl. Cancer Inst. 2022, 114, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Simin, J.; Fornes, R.; Liu, Q.; Olsen, R.S.; Callens, S.; Engstrand, L.; Brusselaers, N. Antibiotic use and risk of colorectal cancer: A systematic review and dose-response meta-analysis. Br. J. Cancer 2020, 123, 1825–1832. [Google Scholar] [CrossRef]
- Goto, A.; Yamaji, T.; Sawada, N.; Momozawa, Y.; Kamatani, Y.; Kubo, M.; Shimazu, T.; Inoue, M.; Noda, M.; Tsugane, S.; et al. Diabetes and cancer risk: A Mendelian randomization study. Int. J. Cancer 2020, 146, 712–719. [Google Scholar] [CrossRef]
- Kim, D.S.; Scherer, P.E. Obesity, Diabetes, and Increased Cancer Progression. Diabetes Metab. J. 2021, 45, 799–812. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, W.; Song, M.; Smith-Warner, S.A.; Yang, J.; Li, Y.; Ma, W.; Hu, Y.; Ogino, S.; Hu, F.B.; et al. Type 2 diabetes and risk of colorectal cancer in two large U.S. prospective cohorts. Br. J. Cancer 2018, 119, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.; Song, M.; Papadimitriou, N.; Carreras-Torres, R.; Langenberg, C.; Martin, R.M.; Tsilidis, K.K.; Barroso, I.; Chen, J.; Frayling, T.M.; et al. Associations Between Glycemic Traits and Colorectal Cancer: A Mendelian Randomization Analysis. J. Natl. Cancer Inst. 2022, 114, 740–752. [Google Scholar] [CrossRef]
- Cheng, Y.; Ling, Z.; Li, L. The Intestinal Microbiota and Colorectal Cancer. Front. Immunol. 2020, 11, 615056. [Google Scholar] [CrossRef]
- Rebersek, M. Gut microbiome and its role in colorectal cancer. BMC Cancer 2021, 21, 1325. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 690–704. [Google Scholar] [CrossRef]
- Dai, Z.; Coker, O.O.; Nakatsu, G.; Wu, W.K.K.; Zhao, L.; Chen, Z.; Chan, F.K.L.; Kristiansen, K.; Sung, J.J.Y.; Wong, S.H.; et al. Multi-cohort analysis of colorectal cancer metagenome identified altered bacteria across populations and universal bacterial markers. Microbiome 2018, 6, 70. [Google Scholar] [CrossRef]
- Wirbel, J.; Pyl, P.T.; Kartal, E.; Zych, K.; Kashani, A.; Milanese, A.; Fleck, J.S.; Voigt, A.Y.; Palleja, A.; Ponnudurai, R.; et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med. 2019, 25, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Debelius, J.W.; Engstrand, L.; Matussek, A.; Brusselaers, N.; Morton, J.T.; Stenmarker, M.; Olsen, R.S. The Local Tumor Microbiome Is Associated with Survival in Late-Stage Colorectal Cancer Patients. Microbiol. Spectr. 2023, 11, e0506622. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zheng, Z.; Li, J.; He, Y.; Kang, W.; Ye, X. Association between the gut microbiota, inflammatory factors, and colorectal cancer: Evidence from Mendelian randomization analysis. Front. Microbiol. 2024, 15, 1309111. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ma, W.; Wang, Y.; Wang, Y.; Sun, X.; Zheng, Q. Gut microbiome-metabolites axis: A friend or foe to colorectal cancer progression. Biomed. Pharmacother. 2024, 173, 116410. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.D.; Fontham, E.T.H.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.-C.T.; et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA A Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Force, U.P.S.T. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 1965–1977. [Google Scholar] [CrossRef]
- Shrubsole, M.J.; Wu, H.; Ness, R.M.; Shyr, Y.; Smalley, W.E.; Zheng, W. Alcohol drinking, cigarette smoking, and risk of colorectal adenomatous and hyperplastic polyps. Am. J. Epidemiol. 2008, 167, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Ocvirk, S.; Wilson, A.S.; Appolonia, C.N.; Thomas, T.K.; O’Keefe, S.J.D. Fiber, Fat, and Colorectal Cancer: New Insight into Modifiable Dietary Risk Factors. Curr. Gastroenterol. Rep. 2019, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical Activity in Cancer Prevention and Survival: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1252–1261. [Google Scholar] [CrossRef]
- Matthews, C.E.; Moore, S.C.; Arem, H.; Cook, M.B.; Trabert, B.; Håkansson, N.; Larsson, S.C.; Wolk, A.; Gapstur, S.M.; Lynch, B.M.; et al. Amount and Intensity of Leisure-Time Physical Activity and Lower Cancer Risk. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 686–697. [Google Scholar] [CrossRef]
- Mazzilli, K.M.; Matthews, C.E.; Salerno, E.A.; Moore, S.C. Weight Training and Risk of 10 Common Types of Cancer. Med. Sci. Sports Exerc. 2019, 51, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Kyu, H.H.; Bachman, V.F.; Alexander, L.T.; Mumford, J.E.; Afshin, A.; Estep, K.; Veerman, J.L.; Delwiche, K.; Iannarone, M.L.; Moyer, M.L.; et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: Systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ (Clin. Res. Ed.) 2016, 354, i3857. [Google Scholar] [CrossRef] [PubMed]
- Orange, S.T. What is the optimal type and dose of physical activity for colorectal cancer prevention? Best Pract. Res. Clin. Gastroenterol. 2023, 66, 101841. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Ishikawa, H.; Sakai, T.; Ayabe, M.; Wakabayashi, K.; Mutoh, M.; Matsuura, N. Effect of physical fitness on colorectal tumor development in patients with familial adenomatous polyposis. Medicine 2019, 98, e17076. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Kim, Y.; Je, Y. Dairy Consumption and Risks of Colorectal Cancer Incidence and Mortality: A Meta-analysis of Prospective Cohort Studies. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2309–2322. [Google Scholar] [CrossRef] [PubMed]
- Puzzono, M.; Mannucci, A.; Grannò, S.; Zuppardo, R.A.; Galli, A.; Danese, S.; Cavestro, G.M. The Role of Diet and Lifestyle in Early-Onset Colorectal Cancer: A Systematic Review. Cancers 2021, 13, 5933. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Je, Y. Nuts and legumes consumption and risk of colorectal cancer: A systematic review and meta-analysis. Eur. J. Epidemiol. 2022, 37, 569–585. [Google Scholar] [CrossRef]
- Carroll, K.L.; Frugé, A.D.; Heslin, M.J.; Lipke, E.A.; Greene, M.W. Diet as a Risk Factor for Early-Onset Colorectal Adenoma and Carcinoma: A Systematic Review. Front. Nutr. 2022, 9, 896330. [Google Scholar] [CrossRef] [PubMed]
- Alegria-Lertxundi, I.; Bujanda, L.; Arroyo-Izaga, M. Role of Dairy Foods, Fish, White Meat, and Eggs in the Prevention of Colorectal Cancer: A Systematic Review of Observational Studies in 2018–2022. Nutrients 2022, 14, 3430. [Google Scholar] [CrossRef]
- Sansbury, L.B.; Wanke, K.; Albert, P.S.; Kahle, L.; Schatzkin, A.; Lanza, E. The effect of strict adherence to a high-fiber, high-fruit and -vegetable, and low-fat eating pattern on adenoma recurrence. Am. J. Epidemiol. 2009, 170, 576–584. [Google Scholar] [CrossRef]
- Lee, J.E.; Chan, A.T. Fruit, vegetables, and folate: Cultivating the evidence for cancer prevention. Gastroenterology 2011, 141, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Bella, F.; Sciacca, S.; Galvano, F.; Grosso, G. Vegetarianism and breast, colorectal and prostate cancer risk: An overview and meta-analysis of cohort studies. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2017, 30, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Orlich, M.J.; Singh, P.N.; Sabaté, J.; Fan, J.; Sveen, L.; Bennett, H.; Knutsen, S.F.; Beeson, W.L.; Jaceldo-Siegl, K.; Butler, T.L.; et al. Vegetarian dietary patterns and the risk of colorectal cancers. JAMA Intern. Med. 2015, 175, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Laure Preterre, A.; Iqbal, K.; Bechthold, A.; De Henauw, S.; Michels, N.; Devleesschauwer, B.; et al. Food groups and risk of colorectal cancer. Int. J. Cancer 2018, 142, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.R.; Abar, L.; Chan, D.S.M.; Vingeliene, S.; Polemiti, E.; Stevens, C.; Greenwood, D.; Norat, T. Foods and beverages and colorectal cancer risk: A systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Barrubés, L.; Babio, N.; Becerra-Tomás, N.; Rosique-Esteban, N.; Salas-Salvadó, J. Association Between Dairy Product Consumption and Colorectal Cancer Risk in Adults: A Systematic Review and Meta-Analysis of Epidemiologic Studies. Adv. Nutr. 2019, 10, S190–S211. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, A.T.; Coleman, H.G.; Huang, W.Y.; Kitahara, C.M.; Cantwell, M.M.; Berndt, S.I. Dietary fiber intake and risk of colorectal cancer and incident and recurrent adenoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am. J. Clin. Nutr. 2015, 102, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Chan, D.S.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ (Clin. Res. Ed.) 2011, 343, d6617. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Feng, B.; Li, K.; Zhu, X.; Liang, S.; Liu, X.; Han, S.; Wang, B.; Wu, K.; Miao, D.; et al. Fish consumption and colorectal cancer risk in humans: A systematic review and meta-analysis. Am. J. Med. 2012, 125, 551–559.e555. [Google Scholar] [CrossRef]
- Schmit, S.L.; Rennert, H.S.; Rennert, G.; Gruber, S.B. Coffee Consumption and the Risk of Colorectal Cancer. Cancer Epidemiol. Biomark. Prev. 2016, 25, 634–639. [Google Scholar] [CrossRef]
- Nakagawa-Senda, H.; Ito, H.; Hosono, S.; Oze, I.; Tanaka, H.; Matsuo, K. Coffee consumption and the risk of colorectal cancer by anatomical subsite in Japan: Results from the HERPACC studies. Int. J. Cancer 2017, 141, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Chapelle, N.; Martel, M.; Toes-Zoutendijk, E.; Barkun, A.N.; Bardou, M. Recent advances in clinical practice: Colorectal cancer chemoprevention in the average-risk population. Gut 2020, 69, 2244–2255. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Lee, I.M.; Manson, J.E.; Buring, J.E.; Dushkes, R.; Gordon, D.; Walter, J.; Wu, K.; Chan, A.T.; Ogino, S.; et al. Effect of Supplementation with Marine ω-3 Fatty Acid on Risk of Colorectal Adenomas and Serrated Polyps in the US General Population: A Prespecified Ancillary Study of a Randomized Clinical Trial. JAMA Oncol. 2020, 6, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Sartini, M.; Bragazzi, N.L.; Spagnolo, A.M.; Schinca, E.; Ottria, G.; Dupont, C.; Cristina, M.L. Coffee Consumption and Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients 2019, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Micek, A.; Gniadek, A.; Kawalec, P.; Brzostek, T. Coffee consumption and colorectal cancer risk: A dose-response meta-analysis on prospective cohort studies. Int. J. Food Sci. Nutr. 2019, 70, 986–1006. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.M. Coffee Consumption and Colon Cancer Risk: A Meta-Epidemiological Study of Asian Cohort Studies. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Macrae, F.A.; Goldberg, M. Colorectal Cancer: Epidemiology, Risk Factors and Protective Factors; UptoDate: Waltham, MA, USA, 2023. [Google Scholar]
- Drew, D.A.; Schuck, M.M.; Magicheva-Gupta, M.V.; Stewart, K.O.; Gilpin, K.K.; Miller, P.; Parziale, M.P.; Pond, E.N.; Takacsi-Nagy, O.; Zerjav, D.C.; et al. Effect of Low-dose and Standard-dose Aspirin on PGE(2) Biosynthesis Among Individuals with Colorectal Adenomas: A Randomized Clinical Trial. Cancer Prev. Res. 2020, 13, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Uribe, L.; Wu, W.; Gelincik, O.; Bommi, P.V.; Francisco-Cruz, A.; Solis, L.M.; Lynch, P.M.; Lim, R.; Stoffel, E.M.; Kanth, P.; et al. Naproxen chemoprevention promotes immune activation in Lynch syndrome colorectal mucosa. Gut 2021, 70, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Mutoh, M.; Sato, Y.; Doyama, H.; Tajika, M.; Tanaka, S.; Horimatsu, T.; Takeuchi, Y.; Kashida, H.; Tashiro, J.; et al. Chemoprevention with low-dose aspirin, mesalazine, or both in patients with familial adenomatous polyposis without previous colectomy (J-FAPP Study IV): A multicentre, double-blind, randomised, two-by-two factorial design trial. Lancet Gastroenterol. Hepatol. 2021, 6, 474–481. [Google Scholar] [CrossRef]
- Ishikawa, H. Aspirin for Chemoprevention of Colorectal Adenoma and Cancer. Gan Kagaku Ryoho. Cancer Chemother. 2021, 48, 1425–1428. [Google Scholar]
- Lin, K.J.; Cheung, W.Y.; Lai, J.Y.; Giovannucci, E.L. The effect of estrogen vs. combined estrogen-progestogen therapy on the risk of colorectal cancer. Int. J. Cancer 2012, 130, 419–430. [Google Scholar] [CrossRef]
- Green, J.; Czanner, G.; Reeves, G.; Watson, J.; Wise, L.; Roddam, A.; Beral, V. Menopausal hormone therapy and risk of gastrointestinal cancer: Nested case-control study within a prospective cohort, and meta-analysis. Int. J. Cancer 2012, 130, 2387–2396. [Google Scholar] [CrossRef] [PubMed]
- Gartlehner, G.; Patel, S.V.; Reddy, S.; Rains, C.; Schwimmer, M.; Kahwati, L. Hormone Therapy for the Primary Prevention of Chronic Conditions in Postmenopausal Persons: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2022, 328, 1747–1765. [Google Scholar] [CrossRef]
- Iversen, L.; Sivasubramaniam, S.; Lee, A.J.; Fielding, S.; Hannaford, P.C. Lifetime cancer risk and combined oral contraceptives: The Royal College of General Practitioners’ Oral Contraception Study. Am. J. Obstet. Gynecol. 2017, 216, 580.e581–580.e589. [Google Scholar] [CrossRef]
- Dobrzycka, M.; Spychalski, P.; Łachiński, A.J.; Kobiela, P.; Jędrusik, P.; Kobiela, J. Statins and Colorectal Cancer—A Systematic Review. Exp. Clin. Endocrinol. Diabetes 2020, 128, 255–262. [Google Scholar] [CrossRef]
- Guirguis-Blake, J.M.; Evans, C.V.; Perdue, L.A.; Bean, S.I.; Senger, C.A. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. In Aspirin Use to Prevent Cardiovascular Disease and Colorectal Cancer: An Evidence Update for the U.S. Preventive Services Task Force; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2022. [Google Scholar]
- Zhang, X.; Keum, N.; Wu, K.; Smith-Warner, S.A.; Ogino, S.; Chan, A.T.; Fuchs, C.S.; Giovannucci, E.L. Calcium intake and colorectal cancer risk: Results from the nurses’ health study and health professionals follow-up study. Int. J. Cancer 2016, 139, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Zoltick, E.S.; Weinstein, S.J.; Fedirko, V.; Wang, M.; Cook, N.R.; Eliassen, A.H.; Zeleniuch-Jacquotte, A.; Agnoli, C.; Albanes, D.; et al. Circulating Vitamin D and Colorectal Cancer Risk: An International Pooling Project of 17 Cohorts. J. Natl. Cancer Inst. 2019, 111, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Xue, M.; Zhao, Y.; Han, Y.; Zhang, S.; Zhang, J.; Li, C.; Xu, J. Low circulating 25-hydroxyvitamin D level is associated with increased colorectal cancer mortality: A systematic review and dose-response meta-analysis. Biosci. Rep. 2020, 40, BSR20201008. [Google Scholar] [CrossRef] [PubMed]
- Crockett, S.D.; Barry, E.L.; Mott, L.A.; Ahnen, D.J.; Robertson, D.J.; Anderson, J.C.; Wallace, K.; Burke, C.A.; Bresalier, R.S.; Figueiredo, J.C.; et al. Calcium and vitamin D supplementation and increased risk of serrated polyps: Results from a randomised clinical trial. Gut 2019, 68, 475–486. [Google Scholar] [CrossRef]
- Lappe, J.; Watson, P.; Travers-Gustafson, D.; Recker, R.; Garland, C.; Gorham, E.; Baggerly, K.; McDonnell, S.L. Effect of Vitamin D and Calcium Supplementation on Cancer Incidence in Older Women: A Randomized Clinical Trial. JAMA 2017, 317, 1234–1243. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Brenner, H.; Chang-Claude, J.; Seiler, C.M.; Rickert, A.; Hoffmeister, M. Protection from colorectal cancer after colonoscopy: A population-based, case-control study. Ann. Intern. Med. 2011, 154, 22–30. [Google Scholar] [CrossRef]
- Zheng, S.; Schrijvers, J.J.A.; Greuter, M.J.W.; Kats-Ugurlu, G.; Lu, W.; de Bock, G.H. Effectiveness of Colorectal Cancer (CRC) Screening on All-Cause and CRC-Specific Mortality Reduction: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 1948. [Google Scholar] [CrossRef]
- Hewitson, P.; Glasziou, P.; Watson, E.; Towler, B.; Irwig, L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): An update. Am. J. Gastroenterol. 2008, 103, 1541–1549. [Google Scholar] [CrossRef]
- Hewitson, P.; Glasziou, P.; Irwig, L.; Towler, B.; Watson, E. Screening for colorectal cancer using the faecal occult blood test, Hemoccult. Cochrane Database Syst. Rev. 2007, 2007, Cd001216. [Google Scholar] [CrossRef]
- Meklin, J.; SyrjÄnen, K.; Eskelinen, M. Fecal Occult Blood Tests in Colorectal Cancer Screening: Systematic Review and Meta-analysis of Traditional and New-generation Fecal Immunochemical Tests. Anticancer Res. 2020, 40, 3591–3604. [Google Scholar] [CrossRef]
- Meklin, J.; Syrjänen, K.; Eskelinen, M. Colorectal Cancer Screening with Traditional and New-generation Fecal Immunochemical Tests: A Critical Review of Fecal Occult Blood Tests. Anticancer Res. 2020, 40, 575–581. [Google Scholar] [CrossRef]
- Young, G.P.; Symonds, E.L.; Allison, J.E.; Cole, S.R.; Fraser, C.G.; Halloran, S.P.; Kuipers, E.J.; Seaman, H.E. Advances in Fecal Occult Blood Tests: The FIT revolution. Dig. Dis. Sci. 2015, 60, 609–622. [Google Scholar] [CrossRef]
- Tinmouth, J.; Lansdorp-Vogelaar, I.; Allison, J.E. Faecal immunochemical tests versus guaiac faecal occult blood tests: What clinicians and colorectal cancer screening programme organisers need to know. Gut 2015, 64, 1327–1337. [Google Scholar] [CrossRef]
- Imperiale, T.F.; Gruber, R.N.; Stump, T.E.; Emmett, T.W.; Monahan, P.O. Performance Characteristics of Fecal Immunochemical Tests for Colorectal Cancer and Advanced Adenomatous Polyps: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2019, 170, 319–329. [Google Scholar] [CrossRef]
- Grobbee, E.J.; Wisse, P.H.A.; Schreuders, E.H.; van Roon, A.; van Dam, L.; Zauber, A.G.; Lansdorp-Vogelaar, I.; Bramer, W.; Berhane, S.; Deeks, J.J.; et al. Guaiac-based faecal occult blood tests versus faecal immunochemical tests for colorectal cancer screening in average-risk individuals. Cochrane Database Syst. Rev. 2022, 6, Cd009276. [Google Scholar] [CrossRef]
- Salimzadeh, H.; Sauvaget, C.; Delavari, A.; Sadeghi, A.; Amani, M.; Salimzadeh, S.; Karimi, A.; Ghanbari Motlagh, A.; Lucas, E.; Basu, P.; et al. Colorectal Cancer Screening Pilot Project in Tehran-Iran, a Feasibility Study. Arch. Iran. Med. 2023, 26, 138–146. [Google Scholar] [CrossRef]
- Jensen, C.D.; Corley, D.A.; Quinn, V.P.; Doubeni, C.A.; Zauber, A.G.; Lee, J.K.; Zhao, W.K.; Marks, A.R.; Schottinger, J.E.; Ghai, N.R.; et al. Fecal Immunochemical Test Program Performance Over 4 Rounds of Annual Screening: A Retrospective Cohort Study. Ann. Intern. Med. 2016, 164, 456–463. [Google Scholar] [CrossRef]
- Janz, T.; Lu, K.; Povlow, M.R.; Urso, B. A Review of Colorectal Cancer Detection Modalities, Stool DNA, and Fecal Immunochemistry Testing in Adults Over the Age of 50. Cureus 2016, 8, e931. [Google Scholar] [CrossRef]
- Pickhardt, P.J. Emerging stool-based and blood-based non-invasive DNA tests for colorectal cancer screening: The importance of cancer prevention in addition to cancer detection. Abdom. Radiol. 2016, 41, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- Stürzlinger, H.; Conrads-Frank, A.; Eisenmann, A.; Invansits, S.; Jahn, B.; Janzic, A.; Jelenc, M.; Kostnapfel, T.; Mencej Bedrac, S.; Mühlberger, N.; et al. Stool DNA testing for early detection of colorectal cancer: Systematic review using the HTA Core Model(®) for Rapid Relative Effectiveness Assessment. Ger. Med. Sci. GMS E-J. 2023, 21, Doc06. [Google Scholar] [CrossRef]
- Kisiel, J.B.; Eckmann, J.D.; Limburg, P.J. Multitarget Stool DNA for Average Risk Colorectal Cancer Screening: Major Achievements and Future Directions. Gastrointest. Endosc. Clin. N. Am. 2020, 30, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, J.D.; Ebner, D.W.; Kisiel, J.B. Multi-Target Stool DNA Testing for Colorectal Cancer Screening: Emerging Learning on Real-world Performance. Curr. Treat. Options Gastroenterol. 2020, 18, 109–119. [Google Scholar] [CrossRef]
- Carethers, J.M. Fecal DNA Testing for Colorectal Cancer Screening. Annu. Rev. Med. 2020, 71, 59–69. [Google Scholar] [CrossRef]
- Imperiale, T.F.; Ransohoff, D.F.; Itzkowitz, S.H.; Levin, T.R.; Lavin, P.; Lidgard, G.P.; Ahlquist, D.A.; Berger, B.M. Multitarget stool DNA testing for colorectal-cancer screening. N. Engl. J. Med. 2014, 370, 1287–1297. [Google Scholar] [CrossRef]
- Juul, F.E.; Cross, A.J.; Schoen, R.E.; Senore, C.; Pinsky, P.; Miller, E.; Segnan, N.; Wooldrage, K.; Wieszczy-Szczepanik, P.; Armaroli, P.; et al. 15-Year Benefits of Sigmoidoscopy Screening on Colorectal Cancer Incidence and Mortality: A Pooled Analysis of Randomized Trials. Ann. Intern. Med. 2022, 175, 1525–1533. [Google Scholar] [CrossRef]
- Holme, Ø.; Løberg, M.; Kalager, M.; Bretthauer, M.; Hernán, M.A.; Aas, E.; Eide, T.J.; Skovlund, E.; Lekven, J.; Schneede, J.; et al. Long-Term Effectiveness of Sigmoidoscopy Screening on Colorectal Cancer Incidence and Mortality in Women and Men: A Randomized Trial. Ann. Intern. Med. 2018, 168, 775–782. [Google Scholar] [CrossRef]
- Miller, E.A.; Pinsky, P.F.; Schoen, R.E.; Prorok, P.C.; Church, T.R. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: Long-term follow-up of the randomised US PLCO cancer screening trial. Lancet Gastroenterol. Hepatol. 2019, 4, 101–110. [Google Scholar] [CrossRef]
- Swartz, A.W.; Eberth, J.M.; Josey, M.J.; Strayer, S.M. Reanalysis of All-Cause Mortality in the U.S. Preventive Services Task Force 2016 Evidence Report on Colorectal Cancer Screening. Ann. Intern. Med. 2017, 167, 602–603. [Google Scholar] [CrossRef]
- Tinmouth, J.; Vella, E.T.; Baxter, N.N.; Dubé, C.; Gould, M.; Hey, A.; Ismaila, N.; McCurdy, B.R.; Paszat, L. Colorectal Cancer Screening in Average Risk Populations: Evidence Summary. Can. J. Gastroenterol. Hepatol. 2016, 2016, 2878149. [Google Scholar] [CrossRef]
- Shroff, J.; Thosani, N.; Batra, S.; Singh, H.; Guha, S. Reduced incidence and mortality from colorectal cancer with flexible-sigmoidoscopy screening: A meta-analysis. World J. Gastroenterol. 2014, 20, 18466–18476. [Google Scholar] [CrossRef]
- Doubeni, C.A.; Weinmann, S.; Adams, K.; Kamineni, A.; Buist, D.S.; Ash, A.S.; Rutter, C.M.; Doria-Rose, V.P.; Corley, D.A.; Greenlee, R.T.; et al. Screening colonoscopy and risk for incident late-stage colorectal cancer diagnosis in average-risk adults: A nested case-control study. Ann. Intern. Med. 2013, 158, 312–320. [Google Scholar] [CrossRef]
- Nishihara, R.; Wu, K.; Lochhead, P.; Morikawa, T.; Liao, X.; Qian, Z.R.; Inamura, K.; Kim, S.A.; Kuchiba, A.; Yamauchi, M.; et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N. Engl. J. Med. 2013, 369, 1095–1105. [Google Scholar] [CrossRef]
- Bretthauer, M.; Løberg, M.; Wieszczy, P.; Kalager, M.; Emilsson, L.; Garborg, K.; Rupinski, M.; Dekker, E.; Spaander, M.; Bugajski, M.; et al. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N. Engl. J. Med. 2022, 387, 1547–1556. [Google Scholar] [CrossRef]
- Ali, O.; Gupta, S.; Brain, K.; Lifford, K.J.; Paranjothy, S.; Dolwani, S. Acceptability of alternative technologies compared with faecal immunochemical test and/or colonoscopy in colorectal cancer screening: A systematic review. J. Med. Screen. 2023, 30, 14–27. [Google Scholar] [CrossRef]
- Gimeno-García, A.Z.; Quintero, E. Role of colonoscopy in colorectal cancer screening: Available evidence. Best Pract. Res. Clin. Gastroenterol. 2023, 66, 101838. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.J.; Rex, D.K.; Ciani, O.; Drummond, M.F. Colonoscopy vs the Fecal Immunochemical Test: Which is Best? Gastroenterology 2024, S0016-5085, 00164-1. [Google Scholar] [CrossRef] [PubMed]
- Zalis, M.E.; Blake, M.A.; Cai, W.; Hahn, P.F.; Halpern, E.F.; Kazam, I.G.; Keroack, M.; Magee, C.; Näppi, J.J.; Perez-Johnston, R.; et al. Diagnostic accuracy of laxative-free computed tomographic colonography for detection of adenomatous polyps in asymptomatic adults: A prospective evaluation. Ann. Intern. Med. 2012, 156, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Sali, L.; Grazzini, G.; Mascalchi, M. CT colonography: Role in FOBT-based screening programs for colorectal cancer. Clin. J. Gastroenterol. 2017, 10, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, P.J.; Correale, L.; Delsanto, S.; Regge, D.; Hassan, C. CT Colonography Performance for the Detection of Polyps and Cancer in Adults ≥ 65 Years Old: Systematic Review and Meta-Analysis. AJR. Am. J. Roentgenol. 2018, 211, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Spada, C.; Hassan, C.; Bellini, D.; Burling, D.; Cappello, G.; Carretero, C.; Dekker, E.; Eliakim, R.; de Haan, M.; Kaminski, M.F.; et al. Imaging alternatives to colonoscopy: CT colonography and colon capsule. European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastrointestinal and Abdominal Radiology (ESGAR) Guideline—Update 2020. Eur. Radiol. 2021, 31, 2967–2982. [Google Scholar] [CrossRef] [PubMed]
- Kato, J.; Morikawa, T.; Kuriyama, M.; Yamaji, Y.; Wada, R.; Mitsushima, T.; Yamamoto, K. Combination of sigmoidoscopy and a fecal immunochemical test to detect proximal colon neoplasia. Clin. Gastroenterol. Hepatol. 2009, 7, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Young, B.R.; Gwede, C.K.; Thomas, B.; Vázquez-Otero, C.; Ewing, A.; Best, A.L.; Aguado Loi, C.X.; Martinez-Tyson, D.; Schneider, T.; Meade, C.D.; et al. A Systematic Review of U.S.-Based Colorectal Cancer Screening Uptake Intervention Systematic Reviews: Available Evidence and Lessons Learned for Research and Practice. Front. Public Health 2019, 7, 145. [Google Scholar] [CrossRef]
- Chiu, H.M.; Ching, J.Y.; Wu, K.C.; Rerknimitr, R.; Li, J.; Wu, D.C.; Goh, K.L.; Matsuda, T.; Kim, H.S.; Leong, R.; et al. A Risk-Scoring System Combined with a Fecal Immunochemical Test Is Effective in Screening High-Risk Subjects for Early Colonoscopy to Detect Advanced Colorectal Neoplasms. Gastroenterology 2016, 150, 617–625.e613. [Google Scholar] [CrossRef]
- Imperiale, T.F.; Monahan, P.O.; Stump, T.E.; Glowinski, E.A.; Ransohoff, D.F. Derivation and Validation of a Scoring System to Stratify Risk for Advanced Colorectal Neoplasia in Asymptomatic Adults: A Cross-sectional Study. Ann. Intern. Med. 2015, 163, 339–346. [Google Scholar] [CrossRef]
- Javadinasab, H.; Daroudi, R.; Salimzadeh, H.; Delavari, A.; Vezvaie, P.; Malekzadeh, R. Cost-effectiveness of Screening Colonoscopy in Iranian High Risk Population. Arch. Iran. Med. 2017, 20, 564–571. [Google Scholar] [PubMed]
- Salimzadeh, H.; Bishehsari, F.; Amani, M.; Ansari, R.; Sotoudeh, M.; Delavari, A.; Malekzadeh, R. Advanced colonic neoplasia in the first degree relatives of colon cancer patients: A colonoscopy-based study. Int. J. Cancer 2016, 139, 2243–2251. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, K.G.; Ho, K.Y.; Chiu, H.M.; Zhu, F.; Ching, J.Y.; Wu, D.C.; Matsuda, T.; Byeon, J.S.; Lee, S.K.; Goh, K.L.; et al. The Asia-Pacific Colorectal Screening score: A validated tool that stratifies risk for colorectal advanced neoplasia in asymptomatic Asian subjects. Gut 2011, 60, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Aniwan, S.; Rerknimitr, R.; Kongkam, P.; Wisedopas, N.; Ponuthai, Y.; Chaithongrat, S.; Kullavanijaya, P. A combination of clinical risk stratification and fecal immunochemical test results to prioritize colonoscopy screening in asymptomatic participants. Gastrointest. Endosc. 2015, 81, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wang, S.; Liu, Z.; Liu, Y.; Wang, Y.; Han, Y.; Gao, W.; Liu, X.; Li, H.; Zhang, Q.; et al. A risk scoring system for advanced colorectal neoplasia in high-risk participants to improve current colorectal cancer screening in Tianjin, China. BMC Gastroenterol. 2022, 22, 466. [Google Scholar] [CrossRef] [PubMed]
- Leddin, D.; Lieberman, D.A.; Tse, F.; Barkun, A.N.; Abou-Setta, A.M.; Marshall, J.K.; Samadder, N.J.; Singh, H.; Telford, J.J.; Tinmouth, J.; et al. Clinical Practice Guideline on Screening for Colorectal Cancer in Individuals with a Family History of Nonhereditary Colorectal Cancer or Adenoma: The Canadian Association of Gastroenterology Banff Consensus. Gastroenterology 2018, 155, 1325–1347.e1323. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A.N.; Lieberman, D.; Leontiadis, G.I.; Tse, F.; Barkun, A.N.; Abou-Setta, A.; Marshall, J.K.; Samadder, J.; Singh, H.; Telford, J.J.; et al. Colorectal cancer screening for patients with a family history of colorectal cancer or adenomas. Can. Fam. Physician Med. De Fam. Can. 2019, 65, 784–789. [Google Scholar]
- Su, Y.; Zhan, H.R.; Sun, X.; Chen, R.; Sun, L.; Hao, J.J.; Zhang, X.P.; Tian, Y.; Chen, R. Global guidelines of colorectal cancer screening in high-risk population with family history of colorectal cancer: A systematic review. Zhonghua Liu Xing Bing Xue Za Zhi 2022, 43, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Perdue, L.A.; Henrikson, N.B.; Bean, S.I.; Blasi, P.R. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. In Screening for Colorectal Cancer: An Evidence Update for the U.S. Preventive Services Task Force; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2021. [Google Scholar]
- Qaseem, A.; Harrod, C.S.; Crandall, C.J.; Wilt, T.J.; Balk, E.M.; Cooney, T.G.; Cross, J.T., Jr.; Fitterman, N.; Maroto, M.; Obley, A.J.; et al. Screening for Colorectal Cancer in Asymptomatic Average-Risk Adults: A Guidance Statement from the American College of Physicians (Version 2). Ann. Intern. Med. 2023, 176, 1092–1100. [Google Scholar] [CrossRef]
- American Cancer Society. Screening for colon and rectal cancer in average-risk adults. CA A Cancer J. Clin. 2018, 68, 282–283. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).