Unsaturated Fatty Acid Synthesis Is Associated with Worse Survival and Is Differentially Regulated by MYCN and Tumor Suppressor microRNAs in Neuroblastoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
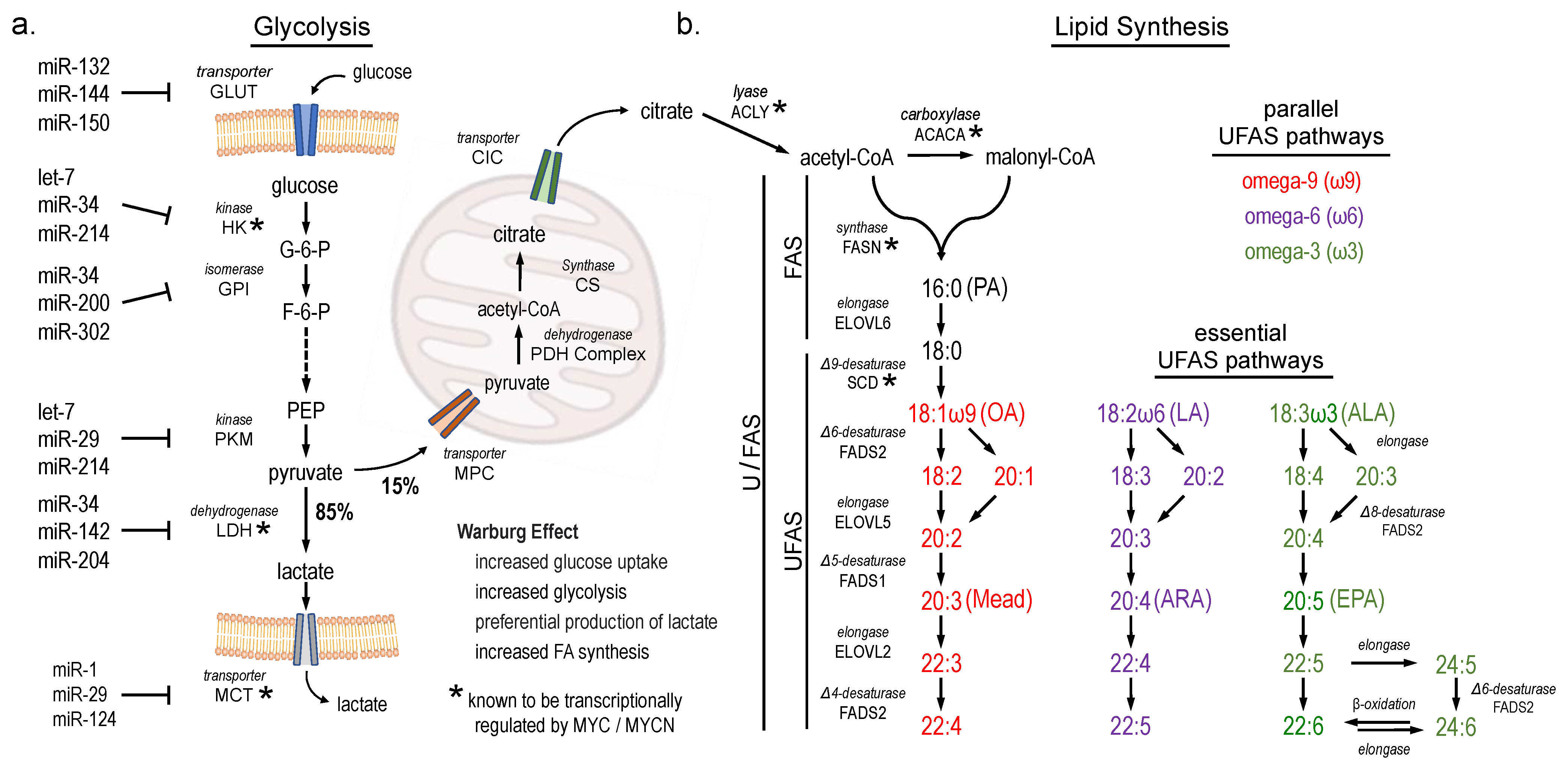
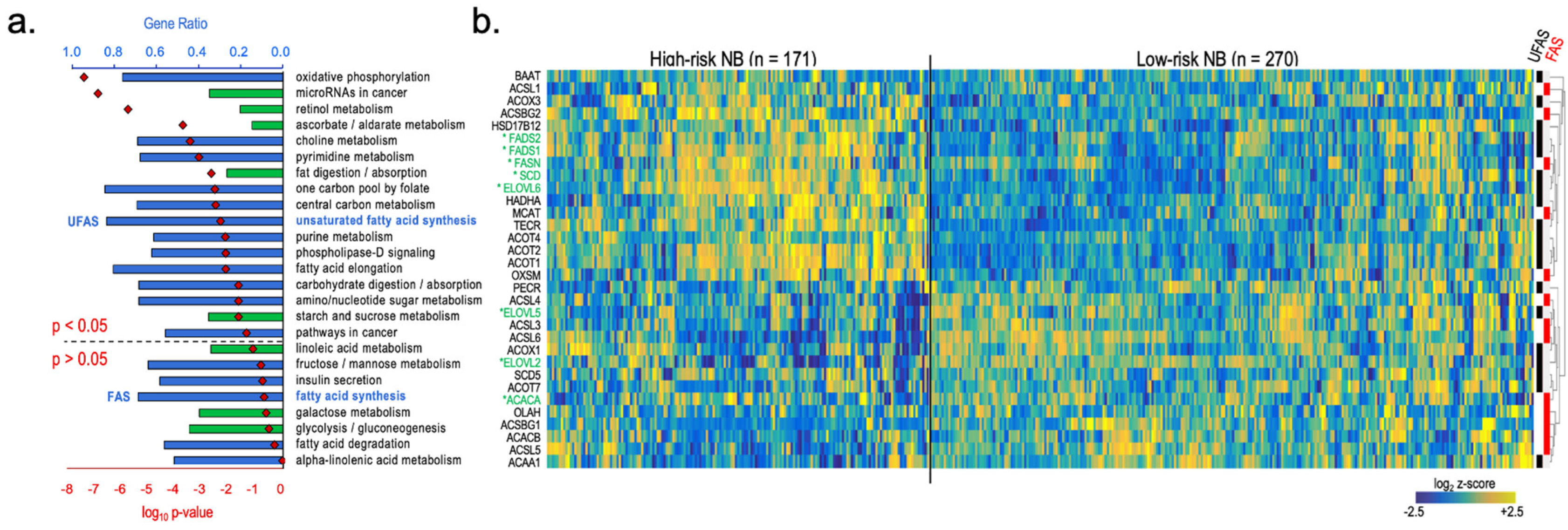
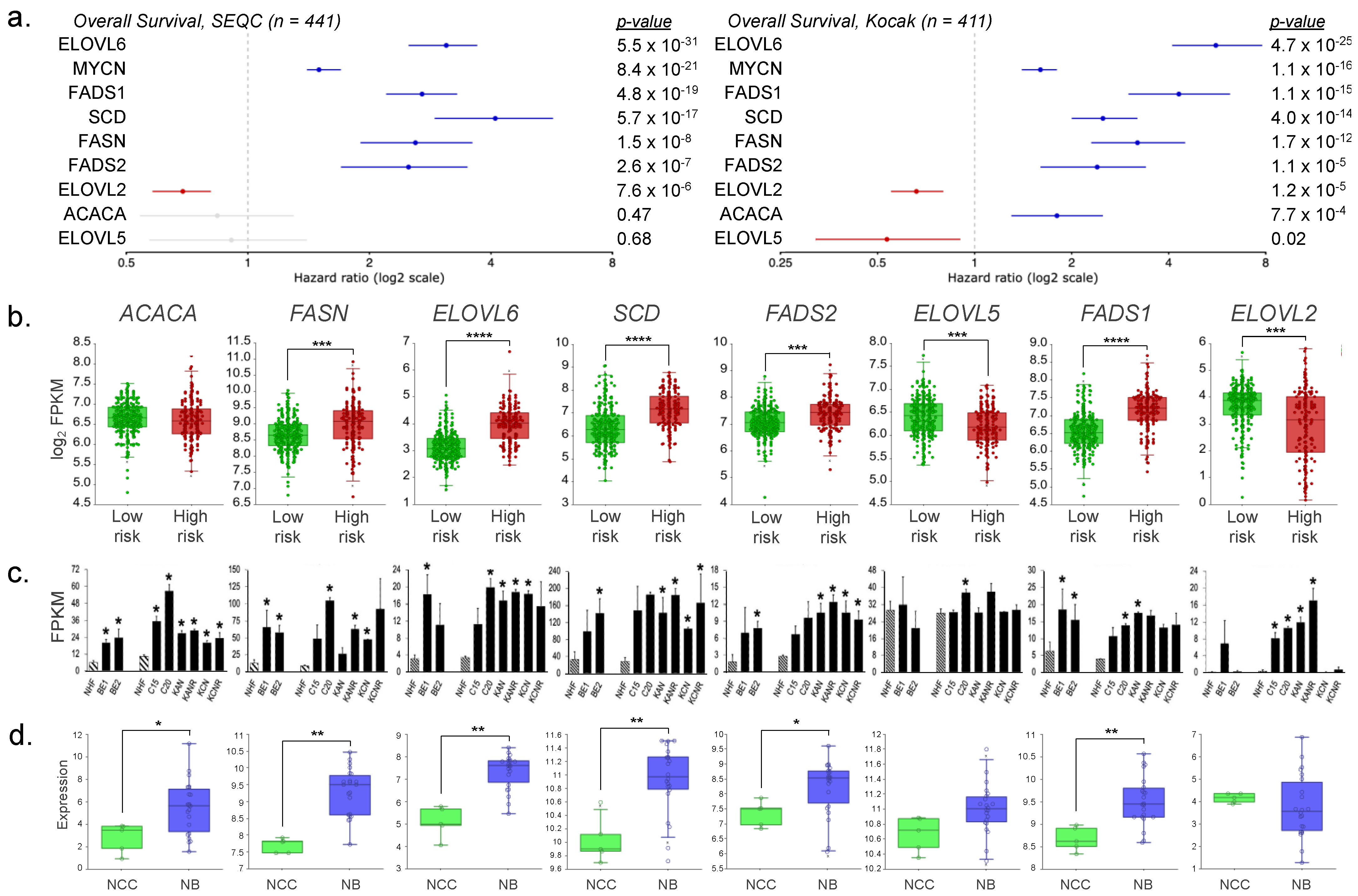
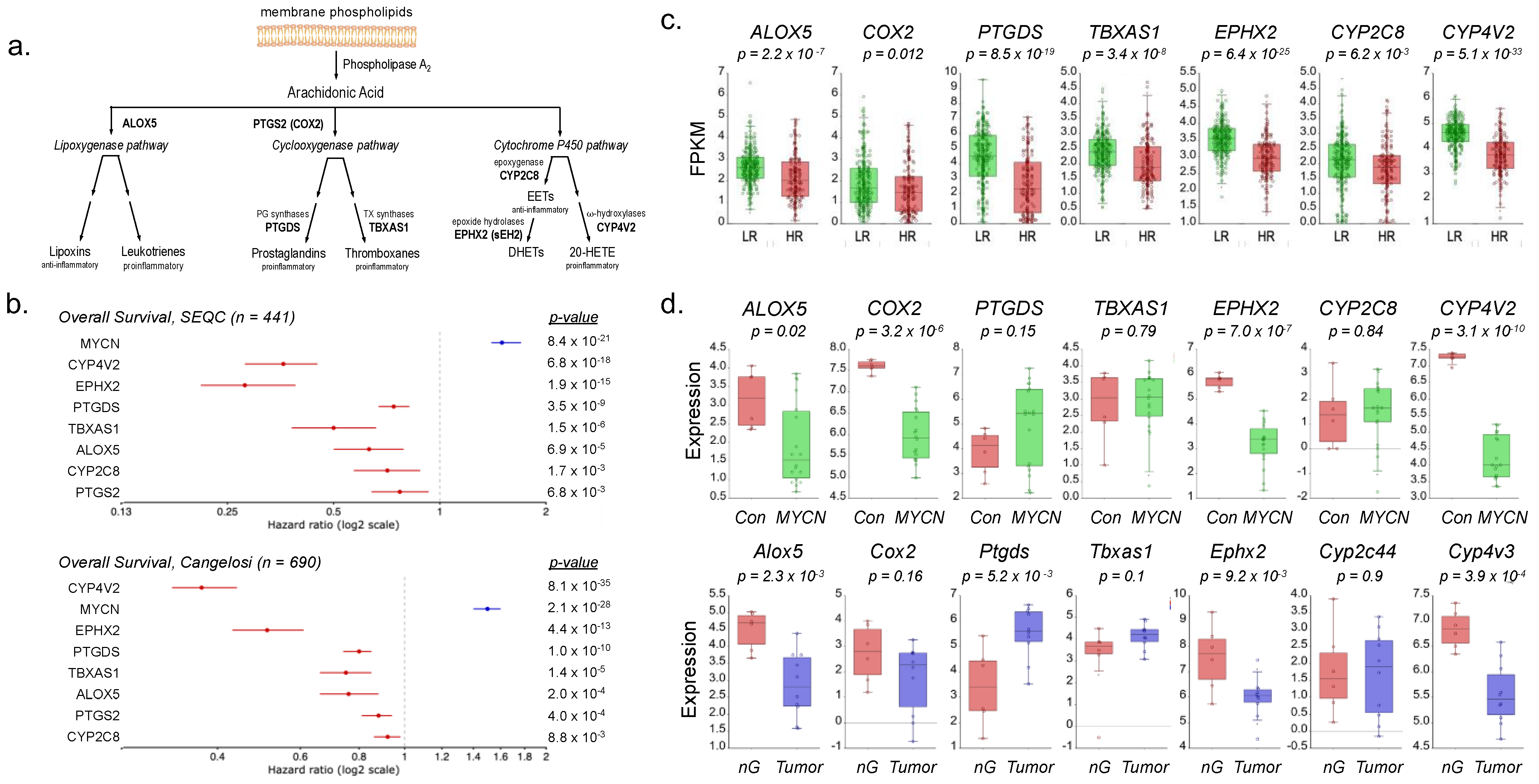
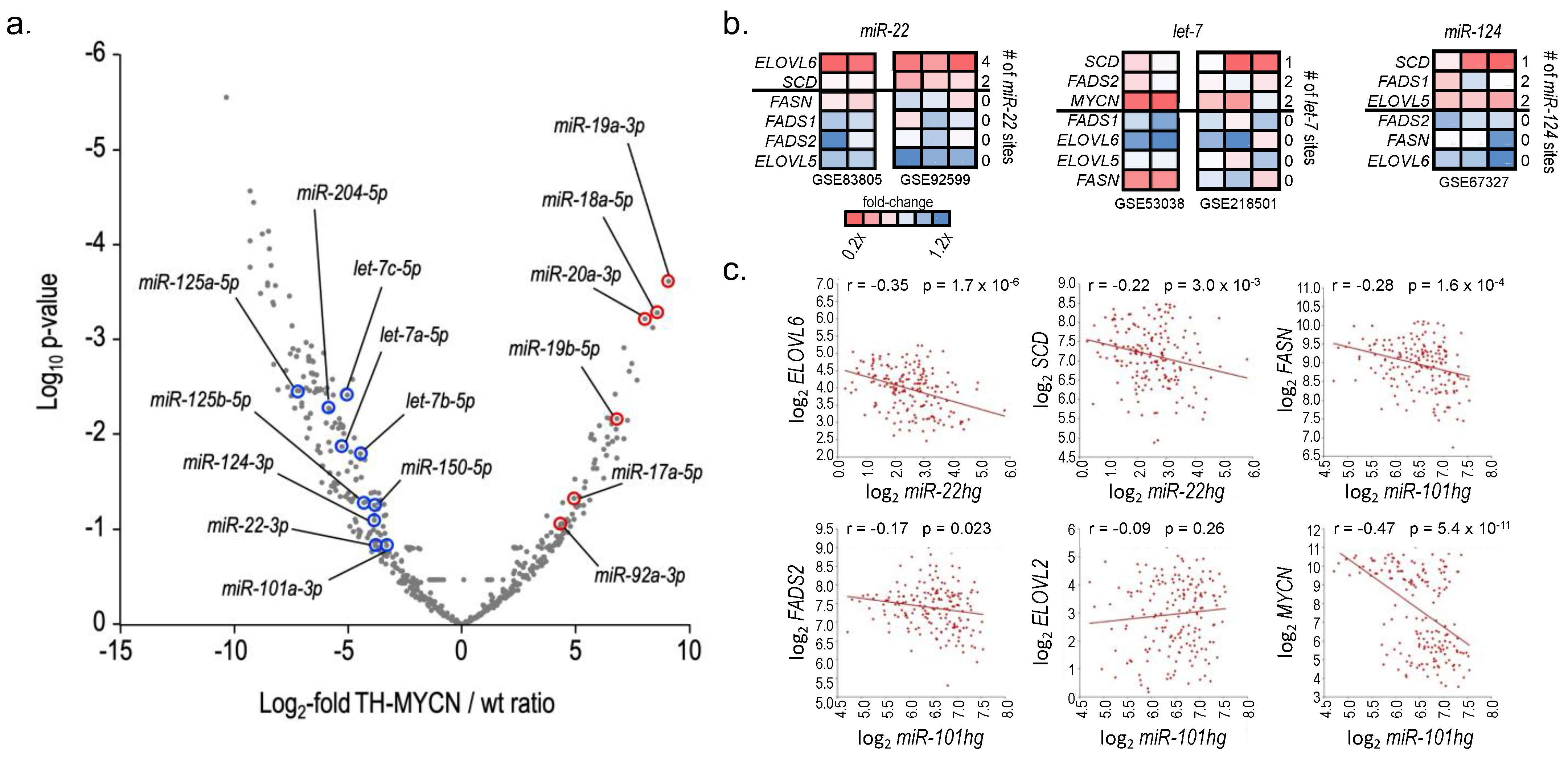
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALA | alpha-linolenic acid (18:3ω3) |
| ARA | arachidonic acid (20:4ω6) |
| DHA | docosahexaenoic acid (22:6ω3) |
| DTrA | docosatrienoic acid (22:3ω9) |
| EFAD | essential fatty acid deficiency |
| FAS | de novo fatty acid synthesis |
| FAS genes: ACACA, FASN, ELOVL6 | |
| FPKM | fragments per kilobase per million mapped fragments |
| GC | gas chromatography |
| HUFA | highly unsaturated fatty acids; three or more double bonds |
| HR | high risk (neuroblastoma) |
| LA | linoleic acid (18:2ω6) |
| LR | low risk (neuroblastoma) |
| miRNAs | microRNAs |
| MNA | MYCN amplified (neuroblastoma) |
| MNon | MYCN non-amplified (neuroblastoma) |
| MYC | proto-oncogenic transcription factor |
| MYCN | proto-oncogenic transcription factor |
| NB | neuroblastoma |
| NCC | neural crest cells |
| NHF | normal human fibroblasts |
| OA | oleic acid (18:1ω9) |
| ROS | reactive oxygen species |
| TSmiRs | tumor suppressor microRNAs |
| UFAS | unsaturated fatty acid synthesis |
| UFAS genes: SCD, FADS2, ELOVL5, FADS1, ELOVL2 | |
| U/FAS | both fatty acid synthesis (FAS) and unsaturated fatty acid synthesis (UFAS) |
| U/FAS genes: ACACA, FASN, ELOVL6, SCD, FADS2, ELOVL5, FADS1, ELOVL2 | |
| ω3 | omega-3 |
| ω6 | omega-6 |
| ω9 | omega-9 |
| 3′ UTR | 3′ untranslated region |
References
- Johnsen, J.I.; Dyberg, C.; Wickström, M. Neuroblastoma—A Neural Crest Derived Embryonal Malignancy. Front. Mol. Neurosci. 2019, 12, 9. [Google Scholar] [CrossRef]
- Matthay, K.K.; George, R.E.; Yu, A.L. Promising therapeutic targets in neuroblastoma. Clin. Cancer Res. 2012, 18, 2740–2753. [Google Scholar] [CrossRef]
- Maris, J.M. Recent advances in neuroblastoma. N. Engl. J. Med. 2010, 362, 2202–2211. [Google Scholar] [CrossRef]
- Maris, J.M.; Hogarty, M.D.; Bagatell, R.; Cohn, S.L. Neuroblastoma. Lancet 2007, 369, 2106–2120. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Weiss, W.A. Neuroblastoma and MYCN. Cold Spring Harb Perspect. Med. 2013, 3, a014415. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.W.; Liu, Y.; He, S.; Durbin, A.D.; Abraham, B.J.; Easton, J.; Shao, Y.; Xu, B.; Zhu, S.; Zhang, X. c-MYC Drives a Subset of High-Risk Pediatric Neuroblastomas and Is Activated through Mechanisms Including Enhancer Hijacking and Focal Enhancer Amplification. Cancer Discov. 2018, 8, 320–335. [Google Scholar] [CrossRef]
- Kulathunga, N.; Kohno, S.; Linn, P.; Nishimoto, Y.; Horike, S.I.; Zaraiskii, M.I.; Kumar, S.; Muranaka, H.; Takahashi, C. Peripubertal high-fat diet promotes c-Myc stabilization in mammary gland epithelium. Cancer Sci. 2020, 111, 2336–2348. [Google Scholar] [CrossRef] [PubMed]
- Labbe, D.P.; Zadra, G.; Yang, M.; Reyes, J.M.; Lin, C.Y.; Cacciatore, S.; Ebot, E.M.; Creech, A.L.; Giunchi, F.; Fiorentino, M.; et al. High-fat diet fuels prostate cancer progression by rewiring the metabolome and amplifying the MYC program. Nat. Commun. 2019, 10, 4358. [Google Scholar] [CrossRef]
- Gouw, A.M.; Margulis, K.; Liu, N.S.; Raman, S.J.; Mancuso, A.; Toal, G.G.; Tong, L.; Mosley, A.; Hsieh, A.L.; Sullivan, D.K.; et al. The MYC Oncogene Cooperates with Sterol-Regulated Element-Binding Protein to Regulate Lipogenesis Essential for Neoplastic Growth. Cell Metab. 2019, 30, 556–572.e5. [Google Scholar] [CrossRef]
- Ruiz-Perez, M.V.; Sainero-Alcolado, L.; Oliynyk, G.; Matuschek, I.; Balboni, N.; Ubhayasekera, S.; Snaebjornsson, M.T.; Makowski, K.; Aaltonen, K.; Bexell, D.; et al. Inhibition of fatty acid synthesis induces differentiation and reduces tumor burden in childhood neuroblastoma. iScience 2021, 24, 102128. [Google Scholar] [CrossRef]
- Tao, L.; Mohammad, M.A.; Milazzo, G.; Moreno-Smith, M.; Patel, T.D.; Zorman, B.; Badachhape, A.; Hernandez, B.E.; Wolf, A.B.; Zeng, Z.; et al. MYCN-driven fatty acid uptake is a metabolic vulnerability in neuroblastoma. Nat. Commun. 2022, 13, 3728. [Google Scholar] [CrossRef] [PubMed]
- Zirath, H.; Frenzel, A.; Oliynyk, G.; Segerstrom, L.; Westermark, U.K.; Larsson, K.; Munksgaard Persson, M.; Hultenby, K.; Lehtio, J.; Einvik, C.; et al. MYC inhibition induces metabolic changes leading to accumulation of lipid droplets in tumor cells. Proc. Natl. Acad. Sci. USA 2013, 110, 10258–10263. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, C.; Sun, L.; Huang, D.; Li, T.; He, X.; Wu, G.; Yang, Z.; Zhong, X.; Song, L.; et al. Lin28/let-7 axis regulates aerobic glycolysis and cancer progression via PDK1. Nat. Commun. 2014, 5, 5212. [Google Scholar] [CrossRef]
- Lino, C.A.; de Oliveira-Silva, T.; Lunardon, G.; Balbino-Silva, C.; Lima, V.M.; Huang, Z.P.; Donato, J., Jr.; Takano, A.P.C.; Barreto-Chaves, M.L.; Wang, D.Z.; et al. Ablation of miRNA-22 protects against obesity-induced adipocyte senescence and ameliorates metabolic disorders in middle-aged mice. Mech. Ageing Dev. 2023, 210, 111775. [Google Scholar] [CrossRef] [PubMed]
- Davalos, A.; Goedeke, L.; Smibert, P.; Ramirez, C.M.; Warrier, N.P.; Andreo, U.; Cirera-Salinas, D.; Rayner, K.; Suresh, U.; Pastor-Pareja, J.C.; et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 9232–9237. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Mehla, K.; Hollingsworth, M.A.; Johnson, K.R. Regulation of Aerobic Glycolysis by microRNAs in Cancer. Mol. Cell. Pharmacol. 2011, 3, 125–134. [Google Scholar]
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef] [PubMed]
- Svoronos, A.A.; Engelman, D.M.; Slack, F.J. OncomiR or Tumor Suppressor? The Duplicity of MicroRNAs in Cancer. Cancer Res. 2016, 76, 3666–3670. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.T.; Tsanov, K.M.; Pearson, D.S.; Roels, F.; Spina, C.S.; Ebright, R.; Seligson, M.; de Soysa, Y.; Cahan, P.; Theissen, J.; et al. Multiple mechanisms disrupt the let-7 microRNA family in neuroblastoma. Nature 2016, 535, 246–251. [Google Scholar] [CrossRef]
- Guo, J.; Dong, Q.; Fang, Z.; Chen, X.; Lu, H.; Wang, K.; Yin, Y.; Cai, X.; Zhao, N.; Chen, J.; et al. Identification of miRNAs that are associated with tumor metastasis in neuroblastoma. Cancer Biol. Ther. 2010, 9, 446–452. [Google Scholar] [CrossRef]
- olenaar, J.J.; Domingo-Fernandez, R.; Ebus, M.E.; Lindner, S.; Koster, J.; Drabek, K.; Mestdagh, P.; van Sluis, P.; Valentijn, L.J.; van Nes, J.; et al. LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression. Nat. Genet. 2012, 44, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Stallings, R.L. MicroRNA involvement in the pathogenesis of neuroblastoma: Potential for microRNA mediated therapeutics. Curr. Pharm. Des. 2009, 15, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Ding, M.; Zhang, H.; Xu, X.; Tang, J. Molecular mechanisms and clinical applications of miR-22 in regulating malignant progression in human cancer (Review). Int. J. Oncol. 2017, 50, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Buechner, J.; Tomte, E.; Haug, B.H.; Henriksen, J.R.; Lokke, C.; Flaegstad, T.; Einvik, C. Tumour-suppressor microRNAs let-7 and mir-101 target the proto-oncogene MYCN and inhibit cell proliferation in MYCN-amplified neuroblastoma. Br. J. Cancer 2011, 105, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Kosti, A.; Du, L.; Shivram, H.; Qiao, M.; Burns, S.; Garcia, J.G.; Pertsemlidis, A.; Iyer, V.R.; Kokovay, E.; Penalva, L.O.F. ELF4 Is a Target of miR-124 and Promotes Neuroblastoma Proliferation and Undifferentiated State. Mol. Cancer Res. 2020, 18, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yang, Y.; Zhang, P.; Wang, F.; Ma, Y.; Qin, H.; Wang, Y. miR-150 functions as a tumour suppressor in human colorectal cancer by targeting c-Myb. J. Cell. Mol. Med. 2014, 18, 2125–2134. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.; Tivnan, A.; Fay, J.; Bryan, K.; Meehan, M.; Creevey, L.; Lynch, J.; Bray, I.M.; O’Meara, A.; Tracey, L.; et al. MicroRNA-204 increases sensitivity of neuroblastoma cells to cisplatin and is associated with a favourable clinical outcome. Br. J. Cancer 2012, 107, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.T.; Mohrhauer, H. A Hypothesis Involving Competitive Inhibitions in the Metabolism of Polyunsaturated Fatty Acids. Acta Chem. Scandivanica 1963, 17, S84–S90. [Google Scholar] [CrossRef]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef]
- Kang, K.S.; Wang, P.; Yamabe, N.; Fukui, M.; Jay, T.; Zhu, B.T. Docosahexaenoic acid induces apoptosis in MCF-7 cells in vitro and in vivo via reactive oxygen species formation and caspase 8 activation. PLoS ONE 2010, 5, e10296. [Google Scholar] [CrossRef]
- D’Eliseo, D.; Di Renzo, L.; Santoni, A.; Velotti, F. Docosahexaenoic acid (DHA) promotes immunogenic apoptosis in human multiple myeloma cells, induces autophagy and inhibits STAT3 in both tumor and dendritic cells. Genes Cancer 2017, 8, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Song, E.A.; Kim, H. Docosahexaenoic Acid Induces Oxidative DNA Damage and Apoptosis, and Enhances the Chemosensitivity of Cancer Cells. Int. J. Mol. Sci. 2016, 17, 1257. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.C.; Bootman, M.D.; Scott, J.D. Second Messengers. Cold Spring Harb. Perspect. Biol. 2016, 8, a005926. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.R.; Butler, L.M.; Hoy, A.J. The diversity and breadth of cancer cell fatty acid metabolism. Cancer Metab. 2021, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Horrobin, D.F. The reversibility of cancer: The relevance of cyclic AMP, calcium, essential fatty acids and prostaglandin E1. Med. Hypotheses 1980, 6, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, N.; Lupien, L.; Kuemmerle, N.R.B.; Kinlaw, W.B.; Swinnen, J.V.; Smans, K. Lipogenesis and lipolysis: The pathways exploited by the cancer cells to acquire fatty acids. Prog. Lipid Res. 2013, 52, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Röhrig, F.; Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 2016, 16, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, J. The expanded role of fatty acid metabolism in cancer: New aspects and targets. Precis. Clin. Med. 2019, 2, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.W.; Wu, C.T.; Chen, D.R.; Yeh, C.Y.; Lin, C. High levels of arachidonic acid and peroxisome proliferator-activated receptor-alpha in breast cancer tissues are associated with promoting cancer cell proliferation. J. Nutr. Biochem. 2013, 24, 274–281. [Google Scholar] [CrossRef]
- Nakazawa, I.; Mead, J.F.; Yonemoto, R.H. In vitro activity of the fatty acyl desaturases of human cancerous and noncancerous tissues. Lipids 1976, 11, 79–82. [Google Scholar] [CrossRef]
- Okazaki, N.; Araki, E. Eicosatrienoic acid omega9 in serum lipids of patients with hepatocellular carcinoma. Clin. Chim. Acta 1974, 53, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Park, H.G.; Zhang, J.Y.; Foster, C.; Sudilovsky, D.; Schwed, D.A.; Mecenas, J.; Devapatla, S.; Lawrence, P.; Kothapalli, K.S.D.; Brenna, J.T. A rare eicosanoid precursor analogue, sciadonic acid (5Z,11Z,14Z-20:3), detected in vivo in hormone positive breast cancer tissue. Prostaglandins Leukot Essent Fat. Acids 2018, 134, 1–6. [Google Scholar] [CrossRef] [PubMed]
- ark, H.G.; Kothapalli, K.S.D.; Park, W.J.; DeAllie, C.; Liu, L.; Liang, A.; Lawrence, P.; Brenna, J.T. Palmitic acid (16:0) competes with omega-6 linoleic and omega-3 ɑ-linolenic acids for FADS2 mediated Δ6-desaturation. Biochim. Biophys. Acta 2016, 1861, 91–97. [Google Scholar]
- Wang, Z.; Park, H.G.; Wang, D.H.; Kitano, R.; Kothapalli, K.S.D.; Brenna, J.T. Fatty acid desaturase 2 (FADS2) but not FADS1 desaturates branched chain and odd chain saturated fatty acids. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158572. [Google Scholar] [CrossRef]
- Park, H.G.; Engel, M.G.; Vogt-Lowell, K.; Lawrence, P.; Kothapalli, K.S.; Brenna, J.T. The role of fatty acid desaturase (FADS) genes in oleic acid metabolism: FADS1 Δ7 desaturates 11-20:1 to 7,11-20:2. Prostaglandins Leukot Essent Fat. Acids 2018, 128, 21–25. [Google Scholar] [CrossRef]
- Park, H.G.; Park, W.J.; Kothapalli, K.S.D.; Brenna, J.T. The fatty acid desaturase 2 (FADS2) gene product catalyzes Δ4 desaturation to yield n-3 docosahexaenoic acid and n-6 docosapentaenoic acid in human cells. FASEB J. 2015, 29, 3911–3919. [Google Scholar] [CrossRef] [PubMed]
- Garcés, R.; Mancha, M. One-step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues. Anal. Biochem. 1993, 211, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.H.; Wang, Z.; Chen, R.; Brenna, J.T. Characterization and Semiquantitative Analysis of Novel Ultratrace C10-24 Monounsaturated Fatty Acid in Bovine Milkfat by Solvent-Mediated Covalent Adduct Chemical Ionization (CACI) MS/MS. J. Agric. Food Chem. 2020, 68, 7482–7489. [Google Scholar] [CrossRef] [PubMed]
- Van Pelt, C.K.; Brenna, J.T. Acetonitrile chemical ionization tandem mass spectrometry to locate double bonds in polyunsaturated fatty acid methyl esters. Anal. Chem. 1999, 71, 1981–1989. [Google Scholar] [CrossRef]
- McGeary, S.E.; Lin, K.S.; Shi, C.Y.; Pham, T.M.; Bisaria, N.; Kelley, G.M.; Bartel, D.P. The biochemical basis of microRNA targeting efficacy. Science 2019, 366, eaav1741. [Google Scholar] [CrossRef]
- Su, Z.; Fang, H.; Hong, H.; Shi, L.; Zhang, W.; Zhang, W.; Zhang, Y.; Dong, Z.; Lancashire, L.J.; Bessarabova, M.; et al. An investigation of biomarkers derived from legacy microarray data for their utility in the RNA-seq era. Genome Biol. 2014, 15, 523. [Google Scholar] [CrossRef] [PubMed]
- R2: Genomics Analysis and Visualization Platform. R2: Genomics Analysis and Visualization Platform. Available online: http://r2.amc.nl (accessed on 19 April 2024).
- van Groningen, T.; Koster, J.; Valentijn, L.J.; Zwijnenburg, D.A.; Akogul, N.; Hasselt, N.E.; Broekmans, M.; Haneveld, F.; Nowakowska, N.E.; Bras, J.; et al. Neuroblastoma is composed of two super-enhancer-associated differentiation states. Nat. Genet. 2017, 49, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- de Pontual, L.; Zaghloul, N.A.; Thomas, S.; Davis, E.E.; McGaughey, D.M.; Dollfus, H.; Baumann, C.; Bessling, S.L.; Babarit, C.; Pelet, A.; et al. Epistasis between RET and BBS mutations modulates enteric innervation and causes syndromic Hirschsprung disease. Proc. Natl. Acad. Sci. USA 2009, 106, 13921–13926. [Google Scholar] [CrossRef] [PubMed]
- Łastowska, M.; Viprey, V.; Santibanez-Koref, M.; Wappler, I.; Peters, H.; Cullinane, C.; Roberts, P.; Hall, A.G.; Tweddle, D.A.; Pearson, A.D.; et al. Identification of candidate genes involved in neuroblastoma progression by combining genomic and expression microarrays with survival data. Oncogene 2007, 26, 7432–7444. [Google Scholar] [CrossRef] [PubMed]
- Fix, A.; Lucchesi, C.; Ribeiro, A.; Lequin, D.; Pierron, G.; Schleiermacher, G.; Delattre, O.; Janoueix-Lerosey, I. Characterization of amplicons in neuroblastoma: High-resolution mapping using DNA microarrays, relationship with outcome, and identification of overexpressed genes. Genes Chromosomes Cancer 2008, 47, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, J.J.; Koster, J.; Zwijnenburg, D.A.; van Sluis, P.; Valentijn, L.J.; van der Ploeg, I.; Hamdi, M.; van Nes, J.; Westerman, B.A.; van Arkel, J.; et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature 2012, 483, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Kocak, H.; Ackermann, S.; Hero, B.; Kahlert, Y.; Oberthuer, A.; Juraeva, D.; Roels, F.; Theissen, J.; Westermann, F.; Deubzer, H.; et al. Hox-C9 activates the intrinsic pathway of apoptosis and is associated with spontaneous regression in neuroblastoma. Cell Death Dis. 2013, 4, e586. [Google Scholar] [CrossRef] [PubMed]
- Bersani, F.; Lingua, M.F.; Morena, D.; Foglizzo, V.; Miretti, S.; Lanzetti, L.; Carra, G.; Morotti, A.; Ala, U.; Provero, P.; et al. Deep Sequencing Reveals a Novel miR-22 Regulatory Network with Therapeutic Potential in Rhabdomyosarcoma. Cancer Res. 2016, 76, 6095–6106. [Google Scholar] [CrossRef] [PubMed]
- Inwood, S.; Buehler, E.; Betenbaugh, M.; Lal, M.; Shiloach, J. Identifying HIPK1 as Target of miR-22-3p Enhancing Recombinant Protein Production from HEK 293 Cell by Using Microarray and HTP siRNA Screen. Biotechnol. J. 2018, 13, 1700342. [Google Scholar] [CrossRef]
- Viswanathan, S.R.; Daley, G.Q.; Gregory, R.I. Selective blockade of microRNA processing by Lin28. Science 2008, 320, 97–100. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, H.; Han, K.; Kim, S.C.; Choi, Y.; Park, S.W.; Bak, G.; Lee, Y.; Choi, J.K.; Kim, T.K.; et al. SET7/9 methylation of the pluripotency factor LIN28A is a nucleolar localization mechanism that blocks let-7 biogenesis in human ESCs. Cell Stem Cell 2014, 15, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Shields, B.B.; Pecot, C.V.; Gao, H.; McMillan, E.; Potts, M.; Nagel, C.; Purinton, S.; Wang, Y.; Ivan, C.; Kim, H.S.; et al. A genome-scale screen reveals context-dependent ovarian cancer sensitivity to miRNA overexpression. Mol. Syst. Biol. 2015, 11, 842. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.C.; Zeitels, L.R.; Hwang, H.W.; Chivukula, R.R.; Wentzel, E.A.; Dews, M.; Jung, J.; Gao, P.; Dang, C.V.; Beer, M.A.; et al. Lin-28B transactivation is necessary for Myc-mediated let-7 repression and proliferation. Proc. Natl. Acad. Sci. USA 2009, 106, 3384–3389. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.T. The ratio of trienoic: Tetraenoic acids in tissue lipids as a measure of essential fatty acid requirement. J. Nutr. 1960, 70, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, D.H.; Guenter, P.; Griebel, D.; Abrams, S.A.; Bark, S.; Baker, M.; Berry, K.L.; Bistrian, B.R.; Brenna, J.T.; Bonnot, D.; et al. Proceedings From FDA/A.S.P.E.N. Public Workshop: Clinical Trial Design for Intravenous Fat Emulsion Products, October 29, 2013. JPEN J. Parenter. Enter. Nutr. 2015, 39, 768–786. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Anderson, M.J. Pure linoleate deficiency in the rat: Influence on growth, accumulation of n-6 polyunsaturates, and [1-14C]linoleate oxidation. J. Lipid Res. 1997, 38, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Bandino, A.; Geerts, D.; Koster, J.; Bachmann, A.S. Deoxyhypusine synthase (DHPS) inhibitor GC7 induces p21/Rb-mediated inhibition of tumor cell growth and DHPS expression correlates with poor prognosis in neuroblastoma patients. Cell Oncol. 2014, 37, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Wang, Y.; Skibbe, J.R.; Hu, C.; Dong, L.; Ferchen, K.; Su, R.; Li, C.; Huang, H.; Weng, H.; Huang, H.; et al. ALOX5 exhibits anti-tumor and drug-sensitizing effects in MLL-rearranged leukemia. Sci. Rep. 2017, 7, 1853. [Google Scholar] [CrossRef]
- Puissant, A.; Frumm, S.M.; Alexe, G.; Bassil, C.F.; Qi, J.; Chanthery, Y.H.; Nekritz, E.A.; Zeid, R.; Gustafson, W.C.; Greninger, P.; et al. Targeting MYCN in neuroblastoma by BET bromodomain inhibition. Cancer Discov. 2013, 3, 308–323. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Pedroza-Torres, A.; Romero-Cordoba, S.L.; Justo-Garrido, M.; Salido-Guadarrama, I.; Rodriguez-Bautista, R.; Montano, S.; Muniz-Mendoza, R.; Arriaga-Canon, C.; Fragoso-Ontiveros, V.; Alvarez-Gomez, R.M.; et al. MicroRNAs in Tumor Cell Metabolism: Roles and Therapeutic Opportunities. Front. Oncol. 2019, 9, 1404. [Google Scholar] [CrossRef] [PubMed]
- Aravindan, N.; Subramanian, K.; Somasundaram, D.B.; Herman, T.S.; Aravindan, S. MicroRNAs in neuroblastoma tumorigenesis, therapy resistance, and disease evolution. Cancer Drug Resist. 2019, 2, 1086–1105. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Lin, Z.Y.; Tong, Q.S. The roles of microRNAs in neuroblastoma. World J. Pediatr. 2014, 10, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Imam, J.S.; Plyler, J.R.; Bansal, H.; Prajapati, S.; Bansal, S.; Rebeles, J.; Chen, H.I.; Chang, Y.F.; Panneerdoss, S.; Zoghi, B.; et al. Genomic loss of tumor suppressor miRNA-204 promotes cancer cell migration and invasion by activating AKT/mTOR/Rac1 signaling and actin reorganization. PLoS ONE 2012, 7, e52397. [Google Scholar] [CrossRef] [PubMed]
- Beckers, A.; Van Peer, G.; Carter, D.R.; Mets, E.; Althoff, K.; Cheung, B.B.; Schulte, J.H.; Mestdagh, P.; Vandesompele, J.; Marshall, G.M.; et al. MYCN-targeting miRNAs are predominantly downregulated during MYCN-driven neuroblastoma tumor formation. Oncotarget 2015, 6, 5204–5216. [Google Scholar] [CrossRef] [PubMed]
- Misiak, D.; Hagemann, S.; Bell, J.L.; Busch, B.; Lederer, M.; Bley, N.; Schulte, J.H.; Huttelmaier, S. The MicroRNA Landscape of MYCN-Amplified Neuroblastoma. Front. Oncol. 2021, 11, 647737. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.Y.; Xiao, C. MicroRNA Mechanisms of Action: What have We Learned from Mice? Front. Genet. 2015, 6, 328. [Google Scholar] [CrossRef] [PubMed]
- Baskerville, S.; Bartel, D.P. Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA 2005, 11, 241–247. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.; Su, X. Emerging impact of the long noncoding RNA MIR22HG on proliferation and apoptosis in multiple human cancers. J. Exp. Clin. Cancer Res. 2020, 39, 271. [Google Scholar] [CrossRef]
- Heyd, V.L.; Eynard, A.R. Effects of eicosatrienoic acid (20:3 n-9, Mead’s acid) on some promalignant-related properties of three human cancer cell lines. Prostaglandins Other Lipid Mediat. 2003, 71, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Eynard, A.R.; Jiang, W.G.; Mansel, R.E. Eicosatrienoic acid (20:3 n-9) inhibits the expression of E-cadherin and desmoglein in human squamous cell carcinoma in vitro. Prostaglandins Leukot Essent Fat. Acids 1998, 59, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, K.S.D.; Park, H.G.; Kothapalli, N.S.L.; Brenna, J.T. FADS2 function at the major cancer hotspot 11q13 locus alters fatty acid metabolism in cancer. Prog. Lipid Res. 2023, 92, 101242. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T.; Kothapalli, K.S.D. New understandings of the pathway of long-chain polyunsaturated fatty acid biosynthesis. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Park, W.J.; Kothapalli, K.S.D.; Lawrence, P.; Tyburczy, C.; Brenna, J.T. An alternate pathway to long-chain polyunsaturates: The FADS2 gene product Delta8-desaturates 20:2n-6 and 20:3n-3. J. Lipid Res. 2009, 50, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.K.; Gibson, R.A.; Cook-Johnson, R.J.; Cleland, L.G.; James, M.J. Elongase reactions as control points in long-chain polyunsaturated fatty acid synthesis. PLoS ONE 2011, 6, e29662. [Google Scholar] [CrossRef] [PubMed]
- West, L.; Yin, Y.; Pierce, S.R.; Fang, Z.; Fan, Y.; Sun, W.; Tucker, K.; Staley, A.; Zhou, C.; Bae-Jump, V. Docosahexaenoic acid (DHA), an omega-3 fatty acid, inhibits tumor growth and metastatic potential of ovarian cancer. Am. J. Cancer Res. 2020, 10, 4450–4463. [Google Scholar]
- Yin, Y.; Sui, C.; Meng, F.; Ma, P.; Jiang, Y. The omega-3 polyunsaturated fatty acid docosahexaenoic acid inhibits proliferation and progression of non-small cell lung cancer cells through the reactive oxygen species-mediated inactivation of the PI3K/Akt pathway. Lipids Health Dis. 2017, 16, 87. [Google Scholar] [CrossRef]
- Shchepinov, M.S. Polyunsaturated Fatty Acid Deuteration against Neurodegeneration. Trends Pharmacol. Sci. 2020, 41, 236–248. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target Ther. 2021, 6, 263. [Google Scholar]
- Xiao, L.; Yeung, H.; Haber, M.; Norris, M.D.; Somers, K. Immunometabolism: A ‘Hot’ Switch for ‘Cold’ Pediatric Solid Tumors. Trends Cancer 2021, 7, 751–777. [Google Scholar] [CrossRef] [PubMed]
- Wolpaw, A.J.; Grossmann, L.D.; Dessau, J.L.; Dong, M.M.; Aaron, B.J.; Brafford, P.A.; Volgina, D.; Pascual-Pasto, G.; Rodriguez-Garcia, A.; Uzun, Y.; et al. Epigenetic state determines inflammatory sensing in neuroblastoma. Proc. Natl. Acad. Sci. USA 2022, 119, e2102358119. [Google Scholar] [CrossRef]
- Bae, S.; Kim, M.K.; Kim, H.S.; Moon, Y.A. Arachidonic acid induces ER stress and apoptosis in HT-29 human colon cancer cells. Anim. Cells Syst. 2020, 24, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guo, X.; Chen, C.; Li, J. The prognostic value of arachidonic acid metabolism in breast cancer by integrated bioinformatics. Lipids Health Dis. 2022, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Liao, P.; Wang, W.; Wang, W.; Kryczek, I.; Li, X.; Bian, Y.; Sell, A.; Wei, S.; Grove, S.; Johnson, J.K.; et al. CD8(+) T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. CD8+ T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell 2022, 40, 365–378.e6. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheeter, D.A.; Garza, S.; Park, H.G.; Benhamou, L.-R.E.; Badi, N.R.; Espinosa, E.C.; Kothapalli, K.S.D.; Brenna, J.T.; Powers, J.T. Unsaturated Fatty Acid Synthesis Is Associated with Worse Survival and Is Differentially Regulated by MYCN and Tumor Suppressor microRNAs in Neuroblastoma. Cancers 2024, 16, 1590. https://doi.org/10.3390/cancers16081590
Sheeter DA, Garza S, Park HG, Benhamou L-RE, Badi NR, Espinosa EC, Kothapalli KSD, Brenna JT, Powers JT. Unsaturated Fatty Acid Synthesis Is Associated with Worse Survival and Is Differentially Regulated by MYCN and Tumor Suppressor microRNAs in Neuroblastoma. Cancers. 2024; 16(8):1590. https://doi.org/10.3390/cancers16081590
Chicago/Turabian StyleSheeter, Dennis A., Secilia Garza, Hui Gyu Park, Lorraine-Rana E. Benhamou, Niharika R. Badi, Erika C. Espinosa, Kumar S. D. Kothapalli, J. Thomas Brenna, and John T. Powers. 2024. "Unsaturated Fatty Acid Synthesis Is Associated with Worse Survival and Is Differentially Regulated by MYCN and Tumor Suppressor microRNAs in Neuroblastoma" Cancers 16, no. 8: 1590. https://doi.org/10.3390/cancers16081590
APA StyleSheeter, D. A., Garza, S., Park, H. G., Benhamou, L.-R. E., Badi, N. R., Espinosa, E. C., Kothapalli, K. S. D., Brenna, J. T., & Powers, J. T. (2024). Unsaturated Fatty Acid Synthesis Is Associated with Worse Survival and Is Differentially Regulated by MYCN and Tumor Suppressor microRNAs in Neuroblastoma. Cancers, 16(8), 1590. https://doi.org/10.3390/cancers16081590





