Simple Summary
FGFR3::TACC3 fusion is a driver, potentially targetable, alteration detected in approximately 4% of diffuse gliomas. Diffuse gliomas with FGFR3::TACC3 fusion (F3T3 gliomas) and high-grade histological features harbor molecular stigmata and the DNA methylation profile of glioblastoma, though they are associated with slightly longer patient survival. Histologically low-grade F3T3 gliomas are molecularly heterogeneous and likely comprise three epigenetic groups. One includes tumors, exclusive to adults, displaying genetic and epigenetic features of glioblastoma and potentially representing precursors of high-grade gliomas. The second group lacks the molecular features of glioblastoma and has an epigenetic profile similar to that of dysembryoplastic neuroepithelial tumors. Finally, tumors in the third group are epigenetically close to gangliogliomas. Owing to their genetic and epigenetic heterogeneity, F3T3 gliomas do not represent a distinct nosological entity. Further research is needed to clarify the prognosis, refine the grading, and determine the optimal treatment approaches for these tumors.
Abstract
FGFR3::TACC3 fusion is a driver, potentially targetable, genetic alteration identified in approximately 4% of high-grade diffuse gliomas and rare cases with low-grade histology. Herein, we review the genetic and epigenetic features of these tumors and highlight the challenges in their classification and grading. Diffuse gliomas with FGFR3::TACC3 fusion display unique histopathological and molecular features, including an oligodendroglioma-like appearance, calcifications, and CD34 extravascular immunoreactivity. High-grade tumors exhibit molecular alterations and a DNA methylation profile typical of glioblastoma, suggesting that they may represent a subtype clinically characterized by a slightly better prognosis. Tumors with low-grade morphology are genetically and epigenetically heterogeneous. Some, exclusive to adults, have molecular alterations typical of glioblastoma, although most do not match any methylation classes, using version 12.5 of the Heidelberg classifier. Another group, which mostly affects children or adolescents, lacks the molecular features of glioblastoma and has a DNA methylation profile similar to that of low-grade glioneuronal tumors. In conclusion, diffuse gliomas with FGFR3::TACC3 fusion do not constitute a distinct nosological entity, owing to their genetic and epigenetic diversity. Further studies are warranted to clarify the biological aggressiveness of tumors with low-grade histology to refine the grading and determine the optimal treatment strategy.
1. Introduction
Gliomas account for approximately 26% of all central nervous system (CNS) tumors [1]. They are currently classified according to the fifth edition of the World Health Organization (WHO) classification of CNS tumors, which was published in 2021 (WHO 2021) [2]. Prior to the WHO 2021 classification, the diagnosis of gliomas was largely reliant on tumor histology, leading to considerable interobserver variability. However, tumors with similar morphology can exhibit different molecular features, which are more closely associated with biological and clinical aggressiveness than histopathology. Therefore, the WHO 2021 classification incorporates both molecular and histological characteristics as diagnostic criteria for gliomas, enabling a more comprehensive diagnostic approach.
According to the WHO 2021 classification, gliomas are distinguished into diffuse, which have an infiltrative growth pattern, and circumscribed, which harbor an expansive growth pattern [2]. They are graded into four grades of malignancy, with grade 1 being the most indolent and grade 4 being the most aggressive [2]. Diffuse gliomas are further categorized into “adult-type”, “pediatric-type” low-grade, and “pediatric-type” high-grade. The former mainly affect adults, whereas “pediatric-type” diffuse gliomas are more common in children and young adults. However, both types can affect patients of any age group. Based on the mutational status of IDH1/2 and the presence of chromosome 1p and 19q deletion, adult-type diffuse gliomas are classified as astrocytoma IDH-mutant (grades 2, 3, or 4), oligodendroglioma IDH-mutant and 1p/19q codeleted (grades 2 or 3), and glioblastoma (GBM) IDH-wildtype (grade 4) [2]. The latter has the worst prognosis, with a median survival of only 8 months [1]. It is defined as a diffuse astrocytic tumor, which lacks mutations in IDH1/2 and in H3, and shows one or more of the following histological or genetic features: microvascular proliferation, necrosis, TERT promoter (pTERT) mutation, EGFR gene amplification, and +7/−10 chromosome copy number changes [3]. Nevertheless, the GBM IDH-wild type represents a heterogeneous group of tumors, sharing the absence of mutations in IDH1/2 and H3, which can be further dissected.
Pediatric-type low-grade diffuse gliomas comprise four different types: diffuse astrocytoma MYB or MYBL1-altered (grade 1), angiocentric glioma (grade 1), diffuse low-grade glioma with Mitogen-Activated Protein Kinase (MAPK) pathway alterations (no definitive grade assigned by WHO 2021), and polymorphous low-grade neuroepithelial tumor of the young (PLNTY) (grade 1) [2,4]. Finally, pediatric-type high-grade diffuse gliomas include diffuse midline glioma H3 K27-altered (grade 4), diffuse hemispheric glioma H3 G34-mutant (grade 4), diffuse pediatric-type high-grade glioma H3 and IDH-wildtype (grade 4), and infant-type hemispheric glioma (no definite grade assigned by the WHO 2021) [2].
The Fibroblast Growth Factor Receptor (FGFR) family comprises five distinct members, including four membrane-bound tyrosine kinase receptors (RTKs), FGFR1, FGFR2, FGFR3, and FGFR4, and a kinase-lacking coreceptor, FGFR5 or FGFRL1 [5]. They can bind to various ligands (FGF, Cadherins, Nectins, Neuroplastin, NCAMs, L1-CAM, Neurexins, Ig-LON, FLRTs, Integrins) through their extracellular membrane domains [6]. FGFR gene alterations, including amplification, mutations, and rearrangements, have been detected in several types of tumors, with a reported frequency ranging between 7 and 9.2% [7,8,9]. Rearrangements represent approximately 10% of all FGFR alterations in cancer, with transforming acidic coiled coil containing the protein 3 (TACC3) being the most frequent fusion partner [9]. The latter encodes a centrosomal protein with coiled-coil domain, which is involved in mitosis [10]. Notably, FGFR3::TACC3 fusion has been found in approximately 4% of diffuse gliomas with morphological features consistent with GBM IDH-wildtype and in 4% of diffuse gliomas with low-grade histology [11,12]. Diffuse gliomas with FGFR3::TACC3 fusion (F3T3 gliomas) may potentially be treated with FGFR inhibitors [13]. Therefore, their identification may be relevant for therapeutic purposes.
In this review, we summarize the histopathological and molecular features of F3T3 gliomas, the current challenges in the diagnosis and classification of these tumors, and methods for detecting FGFR3::TACC3 fusion in tumor tissue.
2. FGFR3::TACC3 Fusion
FGFR3 and TACC3 are located 48 kb apart on human chromosome 4p16 [14]. The FGFR3::TACC3 fusion gene results from tandem duplication and inversion of a 70 kb region on 4p16.3 [15], with at least fourteen possible rearrangements between FGFR3 and TACC3, depending on different breakpoints in the two genes. The most frequent is the FGFR3ex17-TACCex11 rearrangement, followed by FGFR3ex17-TACC3ex10 and FGFR3ex17-TACC3ex8 [16]. Despite the high variation among the breaking points of the FGFR3-TACC3 fusion protein, the intracellular tyrosine kinase domain of FGFR3 is fused upstream of the coil-coiled domain at the C-terminus of TACC3 in all cases [17]. The FGFR3-TACC3 fusion protein has a driver role in glioma oncogenesis. Indeed, astrocytes transfected with this protein can grow in anchorage-independent conditions and form gliomas if injected into immunodeficient mice [14]. Although the mechanisms by which the FGFR3-TACC3 protein induces gliomagenesis have not been fully clarified, in vitro studies have shown that this protein displays constitutive, ligand-independent phosphorylation of the tyrosine kinase domain in FGFR3 and induces aneuploidy [14]. Indeed, owing to an arc-shaped configuration, the FGFR3-TACC3 protein encases the metaphase spindle poles with an asymmetry towards one of the poles during mitosis, thus generating aneuploidy [14]. The oncogenic potential of this fusion seems also related to the activation of oxidative phosphorylation and mitochondrial metabolism [18].
As it is an oncogenic driver, is clonal, and is stable over recurrence, the FGFR3-TACC3 protein represents an attractive therapeutic target for gliomas. However, trials testing the efficacy of FGFR inhibitors in patients with F3T3 gliomas have demonstrated only moderate efficacy in comparison to the remarkable results obtained in other cancers harboring the FGFR3::TACC3 fusion [13]. A potential reason for this could be the difficulty in these drugs in permeating the blood–brain barrier [13].
Given that FGFR3 and TACC3 are located in close proximity, on 4p 16.3 [14], detection of the fusion using Fluorescent In Situ Hybridization (FISH) assay is not feasible [19]. The gold standard for detecting this fusion is either targeted RNA-sequencing or whole transcriptome sequencing. Additionally, RT-PCR on either frozen or formalin-fixed and paraffin-embedded samples, as well as next-generation sequencing covering the intronic regions of the two genes involved in the fusion, can be used [16,20,21,22]. In a study, next-generation sequencing demonstrated higher sensitivity in identifying FGFR3::TACC3 fusions in gliomas than RT-PCR performed on frozen tissue [23].
Assessment of FGFR3 immunohistochemical expression represents a useful screening tool for the identification of F3T3 gliomas with FGFR3::TACC3 fusion [20,24]. Indeed, tumors harboring this genetic alteration overexpress FGFR3 owing to the loss of a specific sequence in the 3′-untranslated region of FGFR3, which is essential for gene regulation by miR-99 [21]. According to published studies, the immunohistochemical overexpression of FGFR3 predicts FGFR3::TACC3 fusion with a sensitivity of 92–100% and a specificity of 86–100% [16,20,23].
3. Histopathological Features of Diffuse Gliomas with FGFR3::TACC3 Fusion
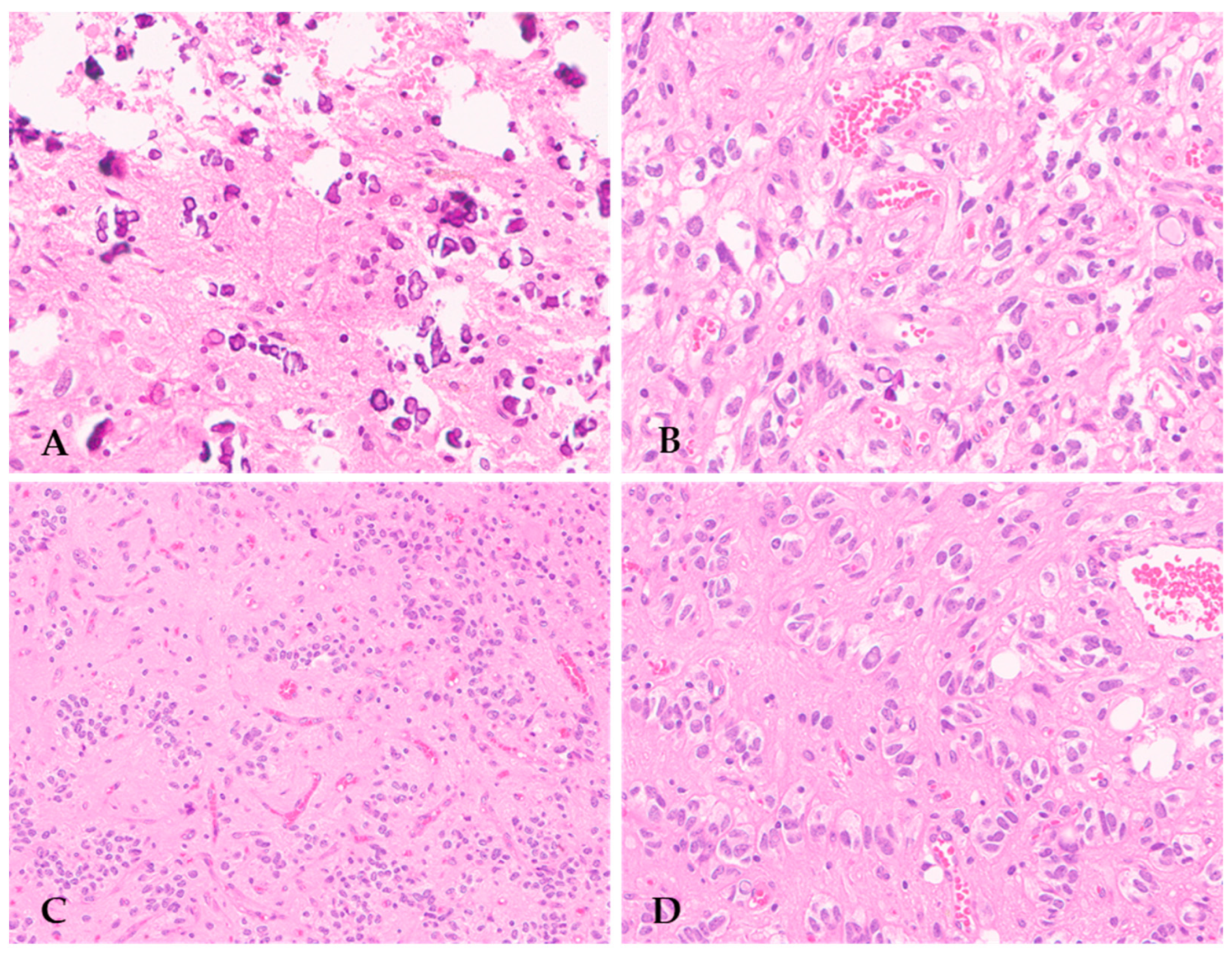
The majority of F3T3 gliomas display high-grade histopathological features, including brisk mitotic activity, microvascular proliferation, and/or necrosis, consistent with GBM IDH-wildtype morphology [20]. More rarely, they lack these histological features of malignancy and appear as low-grade diffuse gliomas [20]. However, both low- and high-grade F3T3 gliomas show distinctive recurrent histopathological features, which are useful clues for their identification in routine practice. In a cohort of 30 cases, Bielle et al. first noticed that F3T3 gliomas displayed recurring morphological features, consisting of calcifications, an endocrinoid vascular pattern formed by thin and ramified vessels, oligodendroglial-like tumor cells with ovoid and uniform nuclei and a clear peri-nuclear halo, nuclear palisading, and desmoplasia (Figure 1) [20].
Figure 1.
Recurring peculiar morphological features of F3T3 gliomas, including numerous calcifications (A) (original magnification, ×200), oligodendroglial-like cells with clear perinuclear halo (B) (original magnification, ×200), perivascular arrangement resembling pseudorosettes of ependymoma (C) (original magnification, ×100), and nuclear palisading (D) (original magnification, ×200).
These authors also highlighted that a proportion of F3T3 gliomas may show a peri-vascular arrangement of tumor cells with interposed anuclear zones, simulating the pseudorosettes of ependymoma [20] (Figure 1C). Subsequent studies have confirmed the presence of these recurrent histopathological features in other cohorts of F3T3 gliomas, strengthening the association between morphology and genetics [12,25,26,27,28]. Notably, high-grade F3T3 gliomas had a significantly lower mitotic rate and a higher vascular density than GBM IDH-wildtype devoid of the FGFR3::TACC3 fusion [20].
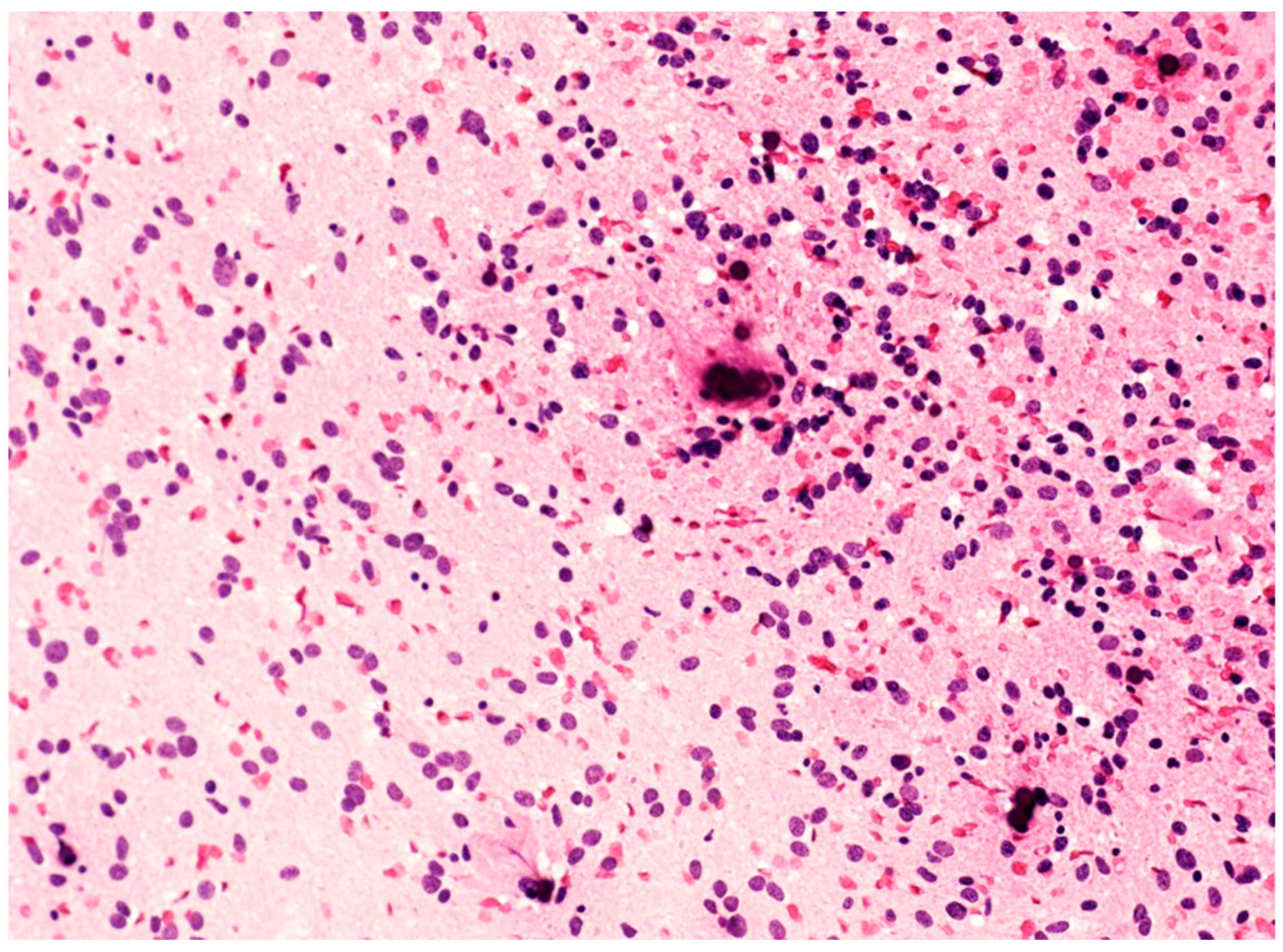
The peculiar histopathological aspects of F3T3 gliomas can also be observed in cytological smears during intra-operative examination (unpublished data). In this setting, a diffuse glioma showing numerous calcifications and relatively monotonous cells with an oligodendroglial-like appearance and ovoid nuclei (Figure 2) should prompt consideration of the possibility of F3T3 glioma.
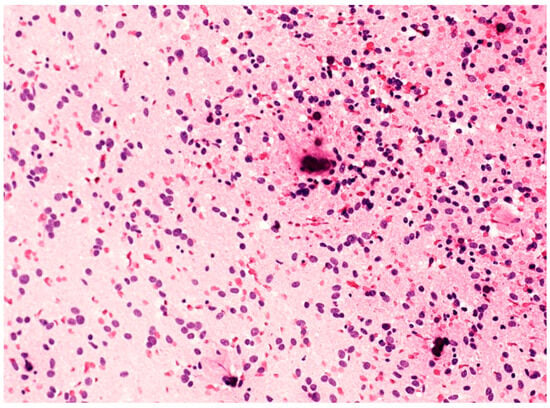
Figure 2.
Cytological smear of an F3T3 glioma, showing monotonous oligodendroglial-like cells with roundish/ovoid nuclei and calcifications (original magnification, ×200).
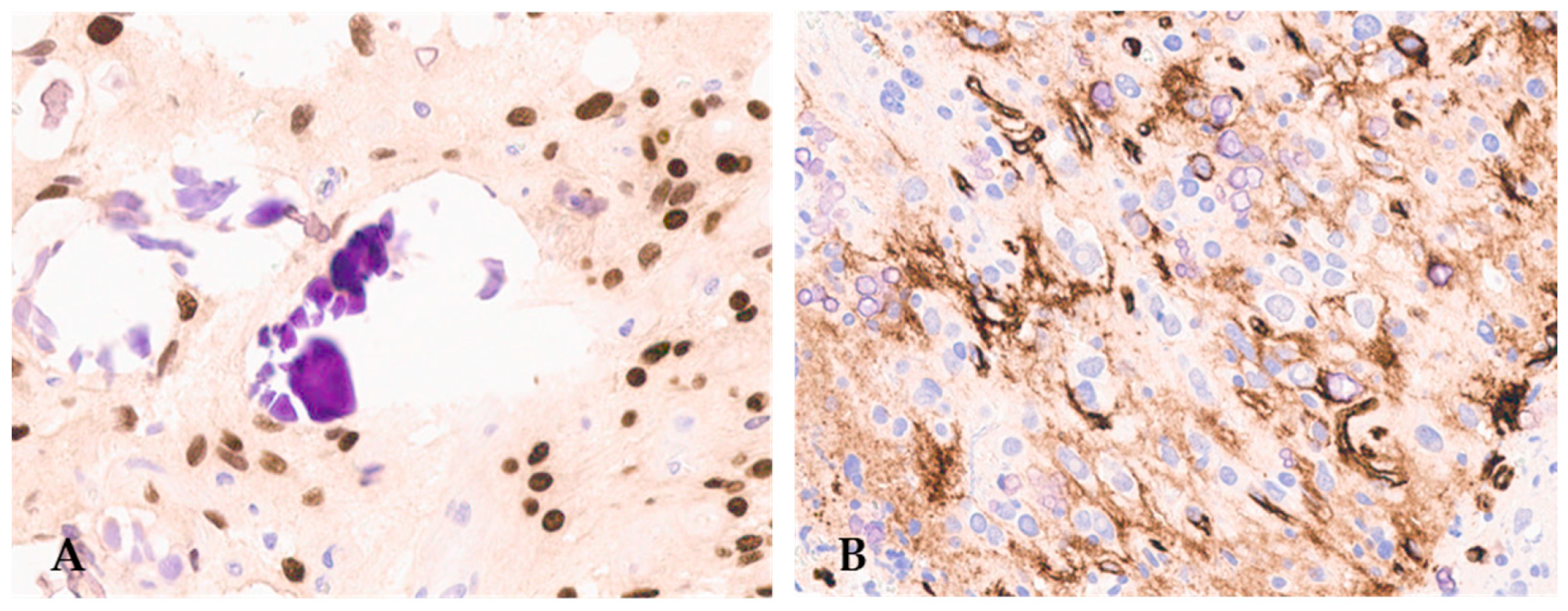
F3T3 gliomas also have peculiar immunohistochemical characteristics. In addition to GFAP and OLIG2 positivity in the tumor cells (Figure 3), as expected for a diffuse glioma, more than 50% of cases exhibit CD34 immunostaining in ramified cells or along the cell membrane (Figure 3) [12,20,27].
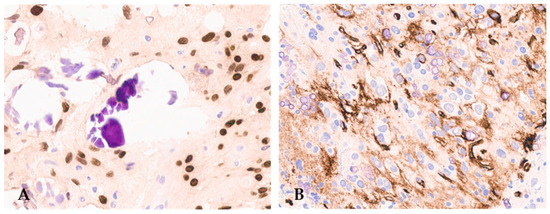
Figure 3.
F3T3 diffuse glioma is positive for OLIG2 (A) (original magnification, ×200) and displays extra-vascular immuno-reactivity for CD34 (B) (original magnification, ×200).
ATRX expression is typically retained, and P53 is positive in less than 10% of tumor cells [20], in accordance with the absence of mutations in ATRX or TP53 in these tumors. Immunostaining for IDH1 p. R132H is consistently negative [20]. As mentioned above, strong immunoexpression of FGFR3 was reported in the majority of F3T3 gliomas and mainly in areas showing the typical recurrent morphological features, suggesting that neoplastic cells in these zones could have the highest activation of FGFR3 downstream signaling [20].
4. Molecular Features of Diffuse Gliomas with High-Grade Histology and FGFR3::TACC3 Fusion
F3T3 gliomas typically lack IDH1/2 mutations and 1p/19q codeletion [11,16,20,27], which is important for their differential diagnosis towards oligodendrogliomas. These tumors are also persistently H3-wildtype and lack BRAF p. V600E mutation [11,16,20].
Similar to GBM IDH-wildtype, over 80% of histologically high-grade F3T3 gliomas harbor concurrent gains of chromosome 7 and loss of chromosome 10 (+7/−10) and/or pTERT mutation [28]. Moreover, more than 50% of these tumors feature CDKN2A/B homozygous deletion [11]. Nonetheless, in contrast to GBM IDH-wildtype, EGFR amplification is rare in F3T3 gliomas. Among 197 cases across four different cohorts, only 11 (5.6%) had EGFR amplification [11,16,20,28], whereas this genetic alteration was detected in approximately 43% of GBMs of IDH-wildtype [11,16]. Additional molecular distinguishing features of GBM IDH-wildtype are significantly higher frequencies of CDK4 and MDM2 amplification in high-grade F3T3 gliomas (22% vs. 7% for CDK4 amplification; 20% vs. 4% for MDM2 amplification) [16], whereas MGMT promoter methylation is similarly frequent in the two tumors [16,28].
PDGFRA, MET, and KIT amplification do not appear to be part of the molecular portrait of high-grade F3T3 gliomas, as they were absent in a cohort of 36 cases [11].
Over the last ten years, there has been accumulating evidence that different tumors exhibit distinct DNA methylation profiles, depending on their cell of origin and the genetic alterations that they acquired during initiation and progression [29]. Therefore, DNA methylation profiling is currently used for CNS tumor classification and for identification of novel tumor types [29]. In practice, the DNA methylation profile of a neoplastic sample is compared with data from over 2800 tumors collected in the Brain Tumor Classifier of Heidelberg University (website: www.molecularneuropathology.org). The classifier algorithm is based on the random forest algorithm, and the main output is a classification score that indicates similarity to one of the included CNS tumor methylation classes [29]. A score from 0 to 1 is generated for every class, and a score above the cut-off of 0.90 indicates a match with the methylation class. Over the years, the Heidelberg classifier has been periodically updated, giving rise to different versions, with the most recent being version 12.8.
Of 66 F3T3 gliomas with high-grade histology, which were profiled for DNA methylation in three different studies, 54 matched the methylation class glioblastoma “IDH-wildtype” and 12 did not match any classes, using version 11b4 of the Heidelberg classifier [11,12,28]. A comparison between 34 high-grade F3T3 gliomas and 100 GBM IDH-wildtype cases lacking FGF3::TACC3 fusion revealed that the former were more likely to be assigned the mesenchymal or RTK II subclass than the latter [11].
Notably, two different studies have highlighted the inconsistency of the methylation class for some F3T3 gliomas using different versions of the Heidelberg Classifier [12,28]. In the cohort studied by Wu et al. [28], six F3T3 gliomas matched the methylation class GBM IDH-wildtype in version 11b4 of the classifier. However, only one matched the same methylation class in version 12.5 of the classifier, whereas the other five did not match any methylation classes but achieved the highest score for GBM mesenchymal subclass in four cases and ganglioglioma in one [28]. In a separate cohort reported by Metais et al., four histologically high-grade F3T3 gliomas were classified as GBM IDH-wildtype in version 11b4 of the classifier. Two of these cases did not match any classes in version 12.5, and one of these had the highest score for ganglioglioma [12]. Although most histologically high-grade F3T3 gliomas do not match any methylation class in version 12.5 of the classifier, it should be noted that on t-distributed stochastic neighbor embedding (t-SNE), they tend to form a homogeneous cluster near the GBM mesenchymal subtype [12], which underlines the genetic and epigenetic similarity with GBM.
5. Molecular Features of Diffuse Gliomas with Low-Grade Histology and FGFR3::TACC3 Fusion
About 3.5% of histologically low-grade diffuse gliomas harbor FGFR3::TACC3 fusion [24], with approximately forty-nine cases reported to date across various studies [12,20,23,27,28,30,31,32,33,34,35,36,37] (Table 1).

Table 1.
Molecular features of diffuse glioma cases with FGFR3::TACC3 fusion reported to date.
We observed an additional case in a 68-year old woman (case 30 in Table 1). Considering 43 cases with available information on patient age, 11 were diagnosed in children or adolescents (<18 years) (Table 1).
Histologically low-grade F3T3 gliomas display genetic and epigenetic heterogeneity.
Molecular stigmata of GBM IDH-wildtype were observed in 69% of cases (28/41); 51% (21/41) harbored +7/−10, and 62.5% (25/40) showed pTERT mutations. EGFR amplification was present in only two of 41 cases (4.8%) (cases 37 and 45 in Table 1), both of which had pTERT mutation and +7/−10. Notably, the molecular features of GBM IDH-wildtype were found only in tumors from adult patients, whereas no tumors from patients under the age of 18 harbored these genetic alterations (p < 0.0001).
DNA methylation profiling was available in 24 of 28 cases with molecular alterations typical of GBM (Table 1). Using version 11b4 of the classifier, fourteen cases matched the methylation class GBM IDH-wildtype, whereas ten did not match any methylation class. Notably, when using version 12.5 of the classifier, no cases had a match with any classes, with the exception of one case that had no match in 11b4 and matched ganglioglioma in version 12.5 (case 23 in Table 1). However, this latter case had an adverse clinical course, and the patient had disease progression at 28 months and died after 66 months [12].
DNA methylation profiling was available for seven histologically low-grade F3T3 gliomas that lacked molecular stigmata of GBM (cases 21, 22, 24, 26, 27, 28, and 29 in Table 1). All these cases were from patients below 18 years of age, with the exception of one case from a 29-year-old man (case 27 in Table 1). Two cases, in a 12-year-old and a 6-year-old patient, matched ganglioglioma or disembryoplastic neuroepithelial tumor (DNET), using either version 1b4 or 12.5 of the classifier (cases 22 and 28 in Table 1) [12]. Four cases did not match any methylation classes in both versions of the classifier (cases 21, 24, 26, 29); however, they reached the highest score for a glioneuronal tumor methylation class [12]. The remaining case (case 25 in Table 1) did not match any classes in version 11b4 of the classifier but matched ganglioglioma in version 12.5 [12].
Although most of the 15 histologically low-grade F3T3 gliomas analyzed by Metais et al. [12] did not match any methylation class in version 12.5 of the classifier, t-SNE DNA methylation profiling data analysis showed that they were distributed in three clusters [12]. One was formed of tumors, exclusive to adults, that displayed molecular features of GBM IDH-wildtype and were near the GBM mesenchymal subtype methylation class in the t-SNE plot (cases 16–20 in Table 1) [12]. These tumors may be precursors of histologically high-grade F3T3 gliomas. The second cluster (cases 21–25) comprised tumors, either in adults or in children, that either had or lacked molecular alterations of GBM IDH-wildtype and were near ganglioglioma in the t-SNE plot [12]. Finally, the third cluster included four tumors that, expect for one case, were diagnosed in children, lacked molecular stigmata of GBM and were near DNET in the t-SNE plot [12].
It should be noted that F3T3 gliomas lacking pTERT mutations, those having other FGFR3::TACC3 fusions besides FGFR3 (ex17)::TACC3 (ex11), or those resected from patients younger than 40 years exhibit a significantly better prognosis [12].
6. Histological Differential Diagnosis of F3T3 Gliomas
Due to their morphological characteristics, F3T3 gliomas may be histologically mistaken for other tumors. Oligodendroglioma is one of the primary differential diagnoses, as it also displays a diffuse growth pattern, clear cells, a thin capillary network, and microcalcifications [25]. However, oligodendroglioma is defined by the co-occurrence of mutations in the IDH1 or IDH2 genes and 1p/19q codeletion, which are absent in F3T3 gliomas. Therefore, in diffuse gliomas displaying tumor cells with roundish/ovoid nuclei and clear cytoplasm, examining IDH mutations is essential to eliminate oligodendroglioma as a potential diagnosis. Notably, the FGFG3::TACC3 fusion was not found in any oligodendrogliomas IDH-mutant and 1p/19q codeleted, suggesting that this genetic alteration is exclusive to gliomas of astrocytic lineage [39].
Ganglioglioma may also be included in the differential diagnosis of F3T3 gliomas, as it shares characteristics such as desmoplasia, CD34 extra-vascular immunostaining, and microcalcifications [40]. This grade 1 glioneuronal tumor primarily occurs in the temporal lobe and is molecularly characterized by alterations in the MAPK pathway [40]. A crucial diagnostic feature of ganglioglioma is the presence of neoplastic ganglionic cells that lack NeuN immunoreactivity, distinguishing it from F3T3 diffuse gliomas. As previously mentioned, some histologically low-grade F3T3 gliomas display a DNA methylation profile matching the methylation class of ganglioglioma, as determined using version 12.5 of the Heidelberg classifier [12]. Furthermore, t-distributed stochastic neighbor embedding DNA-methylation profiling data analysis revealed a close proximity between at least some F3T3 gliomas and the ganglioglioma methylation class [12], suggesting epigenetic similarity between the two tumors. Although ganglioglioma may harbor mutations or fusions of FGFR genes, FGFR3::TACC3 fusion has not been observed in any tumor with histological features of ganglioglioma [12].
The most challenging differential diagnosis for histologically low-grade F3T3 glioma involves PLNTY. This is a grade 1, IDH-wildtype, diffuse glioma arising in young individuals with seizures. It displays frequent oligodendroglioma-like components, calcifications, CD34 immunoreactivity, and a unique DNA methylation profile [41]. The WHO 2021 classification specifies the essential diagnostic criteria for PLNTY, which include the presence of the BRAF p. V600E mutation, FGFR2 or FGFR3 fusions, or other MAPK abnormalities [41]. The similarity in morphology and the inclusion of FGFR3 fusions as a diagnostic criterion have likely led to confusion and overlap between PLNTY and F3T3 gliomas. However, to date, no histologically low-grade F3T3 gliomas profiled for DNA methylation have matched the methylation class of PLNTY [12,28]. In the original series of PLNTYs described by Huse et al., the only case harboring FGF3::TACC3 fusion was not subjected to DNA methylation profiling [33]. These findings suggest that FGFR3::TACC3 fusion is not a part of the molecular portrait of PLNTY and that PLNTYs reported to have FGFR3::TACC3 fusion were likely misdiagnosed, because none of these cases was analyzed for DNA methylation profile to confirm a match to this tumor methylation class [30,31,37,42]. Furthermore, one case reported as PLNTY and harboring FGFR3::TACC3 fusion displayed a clinical course atypical for PLNTY and underwent malignant transformation [30], reinforcing the notion that PLNTY and histologically low-grade F3T3 gliomas are distinct tumor types.
7. Conclusions
F3T3 gliomas do not constitute a distinct nosological tumor type according to WHO 2021 classification, due to their genetic and epigenetic heterogeneity.
Some of these tumors possess high-grade histopathological features similar to those of GBM and exhibit a DNA methylation profile consistent with GBM. However, they are characterized by a significantly better prognosis, suggesting that they may represent a distinct subtype of GBM.
The classification of histologically low-grade F3T3 gliomas is more complex, due to their higher genetic and epigenetic heterogeneity, which presents significant challenges in determining whether and in what circumstances they should be classified as low grade or high grade. A subset of histologically low-grade F3T3 gliomas, exclusive to adults, exhibits molecular features of GBM, including +7/−10 and/or pTERT mutation, and may represent precursors of high-grade F3T3 gliomas. DNA methylation profiling did not prove useful in classifying these tumors, as the majority failed to match any methylation classes, and one case matching ganglioglioma methylation class displayed an unfavorable clinical course. Furthermore, follow-up data for these cases are limited, which makes it difficult to establish whether these tumors should be classified as GBMs based on their molecular profile. However, t-SNE DNA methylation analysis showed that most of these tumors form a cluster near the GBM mesenchymal methylation class, suggesting genetic and epigenetic similarities between the two.
Another group of histologically low-grade F3T3 gliomas, almost exclusive to pediatric patients, lacks molecular alterations of GBM. Some of these cases match methylation classes of glioneuronal tumors, suggesting a similarity. It is currently unclear what the most appropriate diagnosis for these neoplasias is, but diffuse glioma with MAPK pathway alterations may be a possible option.
In summary, the majority of the existing literature pertains to high-grade F3T3 gliomas, with limited information available for low-grade cases. Further research is required to better understand the correlation between the molecular features, or age of onset, and the clinical aggressiveness of these tumors, with the aim of refining the grading system and determining the most appropriate treatment approach.
Author Contributions
Conceptualization, V.B.; methodology, D.M., E.M. and V.B.; data curation, E.M. and D.M.; writing—review and editing, V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of University and Research, PRIN 2022, “Radiological, molecular and histological study of glioblastoma IDH wild-type characterized by RB1 alteration: towards the identification of a new variant potentially responsive to immunotherapy”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016–2020. Neuro Oncol. 2023, 25, iv1–iv99. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Giannini, C.; Perry, A.; Reifenberger, G.; Ng, H.K.; Soffietti, R.; Suvà, M.; Sarkar, C.; Wick, W.; Aldape, K.; et al. Glioblastoma, IDH-wildtype. In Central Nervous System Tumours, 5th ed.; Brat, D.J., von Deimling, A., Eds.; IARC Press: Lyon, France, 2021. [Google Scholar]
- Whitfield, B.T.; Huse, J.T. Classification of adult-type diffuse gliomas: Impact of the World Health Organization 2021 update. Brain Pathol. 2022, 32, e13062. [Google Scholar] [CrossRef] [PubMed]
- Harmer, N.J.; Ilag, L.L.; Mulloy, B.; Pellegrini, L.; Robinson, C.V.; Blundell, T.L. Towards a resolution of the stoichiometry of the fibroblast growth factor (FGF)-FGF receptor-heparin complex. J. Mol. Biol. 2004, 339, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Latko, M.; Czyrek, A.; Porębska, N.; Kucińska, M.; Otlewski, J.; Zakrzewska, M.; Opaliński, Ł. Cross-Talk between Fibroblast Growth Factor Receptors and Other Cell Surface Proteins. Cells 2019, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Carneiro, B.A.; Taxter, T.; Tavora, F.A.; Kalyan, A.; Pai, S.A.; Chae, Y.K.; Giles, F.J. FGFR3-TACC3 fusion in solid tumors: Mini review. Oncotarget 2016, 7, 55924–55938. [Google Scholar] [CrossRef] [PubMed]
- Helsten, T.; Elkin, S.; Arthur, E.; Tomson, B.N.; Carter, J.; Kurzrock, R. The FGFR Landscape in Cancer: Analysis of 4,853 Tumors by Next-Generation Sequencing. Clin. Cancer Res. 2016, 22, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Yang, J.; Wang, Y.; Xu, J.; Wang, X.; Du, F.; Hu, X.; Guo, H.; Song, C.; Tao, R.; et al. Comprehensive identification of FGFR1-4 alterations in 5 557 Chinese patients with solid tumors by next-generation sequencing. Am. J. Cancer Res. 2021, 11, 3893–3906. [Google Scholar] [PubMed]
- Gergely, F.; Kidd, D.; Jeffers, K.; Wakefield, J.G.; Raff, J.W. D-TACC: A novel centrosomal protein required for normal spindle function in the early Drosophila embryo. EMBO J. 2000, 19, 241–252. [Google Scholar] [CrossRef]
- Mata, D.A.; Benhamida, J.K.; Lin, A.L.; Vanderbilt, C.M.; Yang, S.R.; Villafania, L.B.; Ferguson, D.C.; Jonsson, P.; Miller, A.M.; Tabar, V.; et al. Genetic and epigenetic landscape of IDH-wildtype glioblastomas with FGFR3-TACC3 fusions. Acta Neuropathol. Commun. 2020, 8, 186. [Google Scholar] [CrossRef]
- Métais, A.; Tauziède-Espariat, A.; Garcia, J.; Appay, R.; Uro-Coste, E.; Meyronet, D.; Maurage, C.A.; Vandenbos, F.; Rigau, V.; Chiforeanu, D.C.; et al. Clinico-pathological and epigenetic heterogeneity of diffuse gliomas with FGFR3::TACC3 fusion. Acta Neuropathol. Commun. 2023, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Sansone, G.; Santonocito, O.S.; Mazzanti, C.M.; Sanson, M.; Di Stefano, A.L. Diffuse Gliomas with FGFR3-TACC3 Fusions: Oncogenic Mechanisms, Hallmarks, and Therapeutic Perspectives. Cancers 2023, 15, 5555. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Chan, J.M.; Zoppoli, P.; Niola, F.; Sullivan, R.; Castano, A.; Liu, E.M.; Reichel, J.; Porrati, P.; Pellegatta, S.; et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science 2012, 337, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.C.; Engels, M.; Annala, M.; Zhang, W. Emergence of FGFR family gene fusions as therapeutic targets in a wide spectrum of solid tumours. J. Pathol. 2014, 232, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.L.; Picca, A.; Saragoussi, E.; Bielle, F.; Ducray, F.; Villa, C.; Eoli, M.; Paterra, R.; Bellu, L.; Mathon, B.; et al. Clinical, molecular, and radiomic profile of gliomas with FGFR3-TACC3 fusions. Neuro Oncol. 2020, 22, 1614–1624. [Google Scholar] [CrossRef]
- Lasorella, A.; Sanson, M.; Iavarone, A. FGFR-TACC gene fusions in human glioma. Neuro Oncol. 2017, 19, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Frattini, V.; Pagnotta, S.M.; Tala; Fan, J.J.; Russo, M.V.; Lee, S.B.; Garofano, L.; Zhang, J.; Shi, P.; Lewis, G.; et al. A metabolic function of FGFR3-TACC3 gene fusions in cancer. Nature 2018, 553, 222–227. [Google Scholar] [CrossRef]
- Kurobe, M.; Kojima, T.; Nishimura, K.; Kandori, S.; Kawahara, T.; Yoshino, T.; Ueno, S.; Iizumi, Y.; Mitsuzuka, K.; Arai, Y.; et al. Development of RNA-FISH Assay for Detection of Oncogenic FGFR3-TACC3 Fusion Genes in FFPE Samples. PLoS ONE 2016, 11, e0165109. [Google Scholar] [CrossRef]
- Bielle, F.; Di Stefano, A.L.; Meyronet, D.; Picca, A.; Villa, C.; Bernier, M.; Schmitt, Y.; Giry, M.; Rousseau, A.; Figarella-Branger, D.; et al. Diffuse gliomas with FGFR3-TACC3 fusion have characteristic histopathological and molecular features. Brain Pathol. 2018, 28, 674–683. [Google Scholar] [CrossRef]
- Parker, B.C.; Annala, M.J.; Cogdell, D.E.; Granberg, K.J.; Sun, Y.; Ji, P.; Li, X.; Gumin, J.; Zheng, H.; Hu, L.; et al. The tumorigenic FGFR3-TACC3 gene fusion escapes miR-99a regulation in glioblastoma. J. Clin. Investig. 2013, 123, 855–865. [Google Scholar] [CrossRef]
- Priesterbach-Ackley, L.P.; van Kuik, J.; Tops, B.B.J.; Lasorella, A.; Iavarone, A.; van Hecke, W.; Robe, P.A.; Wesseling, P.; de Leng, W.W.J. RT-PCR assay to detect FGFR3::TACC3 fusions in formalin-fixed, paraffin-embedded glioblastoma samples. Neurooncol. Pract. 2024, 11, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Schittenhelm, J.; Ziegler, L.; Sperveslage, J.; Mittelbronn, M.; Capper, D.; Burghardt, I.; Poso, A.; Biskup, S.; Skardelly, M.; Tabatabai, G. FGFR3 overexpression is a useful detection tool for FGFR3 fusions and sequence variations in glioma. Neurooncol. Pract. 2021, 8, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.L.; Fucci, A.; Frattini, V.; Labussiere, M.; Mokhtari, K.; Zoppoli, P.; Marie, Y.; Bruno, A.; Boisselier, B.; Giry, M.; et al. Detection, Characterization, and Inhibition of FGFR-TACC Fusions in IDH Wild-type Glioma. Clin. Cancer Res. 2015, 21, 3307–3317. [Google Scholar] [CrossRef]
- Broggi, G.; Piombino, E.; Altieri, R.; Romano, C.; Certo, F.; Barbagallo, G.M.V.; Vigneri, P.; Condorelli, D.; Colarossi, L.; Colarossi, C.; et al. Glioblastoma, IDH-Wild Type With FGFR3-TACC3 Fusion: When Morphology May Reliably Predict the Molecular Profile of a Tumor. A Case Report and Literature Review. Front. Neurol. 2022, 13, 823015. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.; Davies, K.D.; Kleinschmidt-DeMasters, B.K. Can adult IDH-wildtype glioblastomas with FGFR3:TACC3 fusions be reliably predicted by histological features? Clin. Neuropathol. 2021, 40, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Martelli, F.M.; Marastoni, E.; Barresi, V. A diffuse glioma with oligodendroglial-like cells and extensive calcifications. Brain Pathol. 2024, 34, e13187. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Lopes Abath Neto, O.; Bale, T.A.; Benhamida, J.; Mata, D.; Turakulov, R.; Abdullaev, Z.; Marker, D.; Ketchum, C.; Chung, H.J.; et al. DNA methylation analysis of glioblastomas harboring FGFR3-TACC3 fusions identifies a methylation subclass with better patient survival. Acta Neuropathol. 2022, 144, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Capper, D.; Stichel, D.; Sahm, F.; Jones, D.T.W.; Schrimpf, D.; Sill, M.; Schmid, S.; Hovestadt, V.; Reuss, D.E.; Koelsche, C.; et al. Practical implementation of DNA methylation and copy-number-based CNS tumor diagnostics: The Heidelberg experience. Acta Neuropathol. 2018, 136, 181–210. [Google Scholar] [CrossRef] [PubMed]
- Bale, T.A.; Sait, S.F.; Benhamida, J.; Ptashkin, R.; Haque, S.; Villafania, L.; Sill, M.; Sadowska, J.; Akhtar, R.B.; Liechty, B.; et al. Malignant transformation of a polymorphous low grade neuroepithelial tumor of the young (PLNTY). Acta Neuropathol. 2021, 141, 123–125. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, T.; Guo, X.; Zhang, F.; Fan, M.; Jin, H.; Liu, D. Polymorphous low-grade neuroepithelial tumor of the young: Case report and review focus on the radiological features and genetic alterations. BMC Neurol. 2020, 20, 123. [Google Scholar] [CrossRef]
- Granberg, K.J.; Annala, M.; Lehtinen, B.; Kesseli, J.; Haapasalo, J.; Ruusuvuori, P.; Yli-Harja, O.; Visakorpi, T.; Haapasalo, H.; Nykter, M.; et al. Strong FGFR3 staining is a marker for FGFR3 fusions in diffuse gliomas. Neuro Oncol. 2017, 19, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Huse, J.T.; Snuderl, M.; Jones, D.T.; Brathwaite, C.D.; Altman, N.; Lavi, E.; Saffery, R.; Sexton-Oates, A.; Blumcke, I.; Capper, D.; et al. Polymorphous low-grade neuroepithelial tumor of the young (PLNTY): An epileptogenic neoplasm with oligodendroglioma-like components, aberrant CD34 expression, and genetic alterations involving the MAP kinase pathway. Acta Neuropathol. 2017, 133, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Severson, E.; Gay, L.; Vergilio, J.A.; Elvin, J.; Suh, J.; Daniel, S.; Covert, M.; Frampton, G.M.; Hsu, S.; et al. Comprehensive Genomic Profiling of 282 Pediatric Low- and High-Grade Gliomas Reveals Genomic Drivers, Tumor Mutational Burden, and Hypermutation Signatures. Oncologist 2017, 22, 1478–1490. [Google Scholar] [CrossRef] [PubMed]
- Lazo De La Vega, L.; Comeau, H.; Sallan, S.; Al-Ibraheemi, A.; Gupta, H.; Li, Y.Y.; Tsai, H.K.; Kang, W.; Ward, A.; Church, A.J.; et al. Rare FGFR Oncogenic Alterations in Sequenced Pediatric Solid and Brain Tumors Suggest FGFR Is a Relevant Molecular Target in Childhood Cancer. JCO Precis. Oncol. 2022, 6, e2200390. [Google Scholar] [CrossRef] [PubMed]
- Qaddoumi, I.; Orisme, W.; Wen, J.; Santiago, T.; Gupta, K.; Dalton, J.D.; Tang, B.; Haupfear, K.; Punchihewa, C.; Easton, J.; et al. Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol. 2016, 131, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Riva, G.; Cima, L.; Villanova, M.; Ghimenton, C.; Sina, S.; Riccioni, L.; Munari, G.; Fassan, M.; Giangaspero, F.; Eccher, A. Low-grade neuroepithelial tumor: Unusual presentation in an adult without history of seizures. Neuropathology 2018, 38, 557–560. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.F.; Athukuri, P.; Anand, A.; Gopakumar, S.; Jalali, A.; Patel, A.J.; Rao, G.; Goodman, J.C.; Lu, H.C.; Mandel, J.J. Varied histomorphology and clinical outcomes of FGFR3-TACC3 fusion gliomas. Neurosurg. Focus 2022, 53, E16. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.D.; Zhou, S.; Huse, J.T.; de Groot, J.F.; Xiu, J.; Subramaniam, D.S.; Mehta, S.; Gatalica, Z.; Swensen, J.; Sanai, N.; et al. Targetable Gene Fusions Associate With the IDH Wild-Type Astrocytic Lineage in Adult Gliomas. J. Neuropathol. Exp. Neurol. 2018, 77, 437–442. [Google Scholar] [CrossRef]
- Solomon, D.A.; Varlet, P.; Blumcke, I.; Capper, D.; Gupta, H. Ganglioglioma. In WHO Classification of Tumours, Central Nervous System Tumours, 5th ed.; von Deimling, A., Figarella-Branger, D., Eds.; IARC Press: Lyon, France, 2021. [Google Scholar]
- Rosenblum, M.K.; Ellison, D.W.; Huse, J.T.; Blumcke, I. Polymorphous low-grade neuroepithelial tumour of the young. In WHO Classification of Tumours, Central Nervous System Tumours, 5th ed.; Reifenberger, G., Perry, A., Eds.; IARC Press: Lyon, France, 2021. [Google Scholar]
- Golub, D.; Lynch, D.G.; Pan, P.C.; Liechty, B.; Slocum, C.; Bale, T.; Pisapia, D.J.; Juthani, R. Polymorphous low-grade neuroepithelial tumor of the young with FGFR3-TACC3 fusion mimicking high-grade glioma: Case report and series of high-grade correlates. Front. Oncol. 2023, 13, 1307591. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).