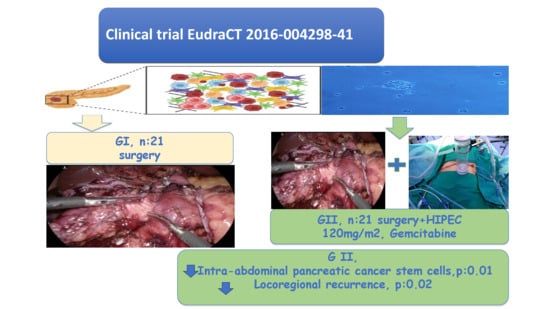
Safety and Effectiveness of Perioperative Hyperthermic Intraperitoneal Chemotherapy with Gemcitabine in Patients with Resected Pancreatic Ductal Adenocarcinoma: Clinical Trial EudraCT 2016-004298-41
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Population
2.2. Exclusion Criteria
2.3. Data Collection
2.4. Surgical Procedure
2.5. HIPEC
2.6. Isolation of PaCSCs
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.; Vaquero, E.; Maurel, J.; Antoni Bombí, J.; De Juan, C.; Feliu, J.; Fernández Cruz, L.; Ginés, A.; Girela, E.; Rodríguez, R.; et al. Recommendations for diagnosis, staging and treatment of pancreatic cancer (part II). Med. Clin. 2010, 134, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Hermann, P.C.; Huber, S.L.; Herrler, T.; Aicher, A.; Ellwart, J.W.; Guba, M.; Bruns, C.J.; Heeschen, C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007, 1, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Hezel, A.F.; Kimmelman, A.C.; Stanger, B.Z.; Bardeesy, N.; Depinho, R.A. Genetics and biology of pancreatic ductal adenocarcinoma. Genes. Dev. 2006, 20, 1218–1249. [Google Scholar] [CrossRef] [PubMed]
- Muniraj, T.; Jamidar, P.A.; Aslanian, H.R. Pancreatic cancer: A comprehensive review and update. Dis. Mon. 2013, 59, 368–402. [Google Scholar] [CrossRef] [PubMed]
- Ansari, D.; Chen, B.C.; Dong, L.; Zhou, M.T.; Andersson, R. Pancreatic cancer: Translational research aspects and clinical implications. World J. Gastroenterol. 2012, 18, 1417–1424. [Google Scholar] [CrossRef]
- Bardeesy, N.; De Pinho, R.A. Pancreatic cancer biology and genetics. Nat. Rev. Cancer 2002, 2, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Hermann, P.C.; Sainz, B. Pancreatic cancer stem cells: A state or an entity? Semin. Cancer Biol. 2018, 53, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ischenko, I.; Seeliger, H.; Kleespies, A.; Angele, M.K.; Eichhorn, M.E.; Jauch, K.W.; Bruns, C.J. Pancreatic cancer stem cells: New understanding of tumorigenesis, clinical implications. Lagenbecks Arch. Surg. 2010, 395, 1–10. [Google Scholar] [CrossRef]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer stem cells–perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Fojo, T.; Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Z. Origin of Cancer Stem Cells: The Role of Self-Renewal and Differentiation. Ann. Surg. Oncol. 2008, 15, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Reguart, N.; He, B.; Taron, M.; You, L.; Jablons, D.M.; Rosell, R. The role of Wnt signaling in cancer and stem cells. Future Oncol. 2005, 1, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Taipale, J.; Beachy, P.A. The Hedgehog and Wnt signaling pathways in cancer. Nature 2001, 411, 349–354. [Google Scholar] [CrossRef]
- Li, C.; Heidt, D.G.; Dalerba, P.; Burant, C.F.; Zhang, L.; Adsay, V.; Wicha, M.; Clarke, M.F.; Simeone, D.M. Identification of pancreatic cancer stem cells. Cancer Res. 2007, 67, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, T.; Ohuchida, K.; Mizumoto, K.; Cui, L.; Ikenaga, N.; Sato, N.; Tanaka, M. Enhanced Cell Migration and Invasion of CD133 Pancreatic Cancer Cells Cocultured With Pancreatic Stromal Cells. Cancer 2010, 116, 3357–3368. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.C.; Wang, H.P.; Huang, H.Y.; Wu, M.S.; Chiang, H.; Tien, Y.W.; Lin, Y.L.; Lin, J.T. CXCR4 Expression Predicts Early Liver Recurrence and Poor Survival After Resection of Pancreatic Adenocarcinoma. Clin. Trans. Gastroenterol. 2012, 3, e22. [Google Scholar] [CrossRef]
- Marchesi, F.; Monti, P.; Leone, B.E.; Zerbi, A.; Vecchi, A.; Piemonti, L.; Mantovani, A.; Allavena, P. Increased survival, proliferation, and migration in metastatic human pancreatic tumor cells expressing functional CXCR4. Cancer Res. 2004, 64, 8420–8427. [Google Scholar] [CrossRef]
- Glehen, O.; Mohamed, F.; Gilly, F.N. Peritoneal carcinomatosis from digestive tract cancer: New management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 2004, 5, 219–228. [Google Scholar] [CrossRef]
- Chua, T.C.; Yan, T.D.; Saxena, A.; Morris, D.L. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: A systematic review of morbidity and mortality. Ann. Surg. 2009, 249, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Roviello, F.; Caruso, S.; Marrelli, D.; Pedrazzani, C.; Neri, A.; De Stefano, A.; Pinto, E. Treatment of peritoneal carcinomatosis with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: State of the art and future developments. Surg. Oncol. 2011, 20, e38–e54. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Surgical management of peritoneal carcinosis: Diagnosis, prevention and treatment. Langenbecks Arch. Chir. 1988, 373, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Pelz, J.O.W.; Doerfer, J.; Dimmler, A.; Hohenberger, W.; Meyer, T. Histological response of peritoneal carcinomatosis after hyperthermic intraperitoneal chemoperfusion (HIPEC) in experimental investigations. BMC Cancer 2006, 6, 162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pestieau, S.R.; Schnake, K.J.; Stuart, O.A.; Sugarbaker, P.H. Impact of carrier solutions on pharmacokinetics of intraperitoneal chemotherapy. Cancer Chemother. Pharmacol. 2001, 47, 269–276. [Google Scholar] [PubMed]
- Morgan, R.J.; Synold, T.W.; Xi, B.; Lim, D.; Shibata, S.; Margolin, K.; Schwarz, R.E.; Leong, L.; Somlo, G.; Twardowski, P.; et al. Phase I Trial of intraperitoneal gemcitabine in the treatment of advanced malignancies primarily confined to the peritoneal cavity. Clin. Cancer Res. 2007, 13, 1232–1237. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gamblin, T.K.; Egorin, M.J.; Zuhowski, E.G.; Lagattuta, T.F.; Herscher, L.L.; Russo, A.; Libutti, S.K.; Alexander, H.R.; Dedrick, R.L.; Bartlett, D.L. Intraperitoneal gemcitabine pharmacokinetics: A pilot and pharmacokinetic study in patients with advanced adenocarcinoma of the pancreas. Cancer Chemother. Pharmacol. 2008, 62, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.A.; Moore, M.J.; Andersen, J.; Green, M.R.; Rothenberg, M.L.; Modiano, M.R.; Cripps, M.C.; Portenoy, R.K.; Storniolo, A.M.; Tarassoff, P.; et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. 1997, 15, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Ridwelski, K.; Meyer, F.; Hribaschek, A.; Kasper, U.; Lippert, H. Intraoperative and early postoperative chemotherapy into the abdominal cavity using gemcitabine may prevent postoperative occurrence of peritoneal carcinomatosis. J. Surg. Oncol. 2002, 79, 10–16. [Google Scholar] [CrossRef]
- Tentes, A.; Pallas, N.; Karamveri, C.; Kyziridis, D.; Hristakis, C. Cytoreduction and HIPEC for peritoneal carcinomatosis of pancreatic cancer. J. BUON 2018, 23, 482–483. [Google Scholar]
- Evan, T.; Wang, V.M.-Y.; Behrens, A. The roles of intratumour heterogeneity inthe biology and treatment of pancreatic ductal adenocarcinoma. Oncogene 2022, 41, 4686–4695. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H.; Stwart, O.A. Intraperitoneal gemcitabine chemotherapy is safe for patients with resected pancreatic cancer:final clinical and pharmacologic data from a phase II protocol and recommended future directions. J. Gastrointest. Oncol. 2021, 12, 99–109. [Google Scholar] [CrossRef]
- Glockzin, G.; Ghali, N.; Lang, S.A.; Schlitt, H.J.; Piso, P. Results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal cancer. J. Surg. Oncol. 2009, 100, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Kamath, A.; Yoo, D.; Stuart, O.A.; Bijelic, L.; Sugarbaker, P.H. Rationale for an intraperitoneal gemcitabine chemotherapy treatment for patients with resected pancreatic cancer. Recent. Pat. Anticancer. Drug Discov. 2009, 4, 174–179. [Google Scholar] [CrossRef]
- Sugarbaker, P.H.; Stuart, O.A.; Bijelic, L. Intraperitoneal gemcitabine chemotherapy treatment for patients with resected pancreatic cancer: Rationale and report of early data. Int. J. Surg. Oncol. 2011, 2011, 161862. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tentes, A.A.; Kyziridis, D.; Kakolyris, S.; Pallas, N.; Zorbas, G.; Korakianitis, O.; Mavroudis, C.; Courcoutsakis, N.; Prasopoulos, P. Preliminary results of hyperthermic intraperitoneal intraoperative chemotherapy as an adjuvant in resectable pancreatic cancer. Gastroenterol. Res. Pract. 2012, 2012, 506571. [Google Scholar] [CrossRef] [PubMed]
- Tentes, A.A.; Stamou, K.; Pallas, N.; Karamveri, C.; Kyziridis, D.; Hristakis, C. The effect of hyperthermic intraoperative chemotherapy (HIPEC) as an adjuvant in patients with resectable pancreatic cancer. Int. J. Hyperth. 2016, 32, 895–899. [Google Scholar] [CrossRef]
- García-Santos, E.P.; Padilla-Valverde, D.; Villarejo-Campos, P.; Murillo-Lázaro, C.; Fernández-Grande, E.; Palomino-Muñoz, T.; Rodríguez-Martínez, M.; Amo-Salas, M.; Nuñez-Guerrero, P.; Sánchez-García, S.; et al. The utility of hyperthermic intra-abdominal chemotherapy with gemcitabine for the inhibition of tumor progression in an experimental model of pancreatic peritoneal carcinomatosis, in relation to their behavior with pancreatic cancer stem cells CD133+ CXCR4. Pancreatology 2016, 16, 632–639. [Google Scholar] [CrossRef]
- Sánchez García, S.; Padilla Valverde, D.; Villarejo Campos, P.; Martín-Fernández, J.; García-Rojo, M.; Rodríguez-Martínez, M. Experimental development of an intra-abdominal chemohyperthermia model using a closed abdomen technique and a PRS-1.0 Combat® CO2 Recirculation System. Surgery 2014, 155, 719–725. [Google Scholar] [CrossRef]
- Sánchez García, S.; Villarejo Campos, P.; Padilla Valverde, D.; Amo-Salas, M.; Martín-Fernández, J. Intraperitoneal Chemotherapy Hyperthermia (HIPEC) for Peritoneal Carcinomatosis of Ovarian Cancer Origin by Fluid and CO2 Recirculation Using the Closed-Abdomen Technique (Combat PRS-1.0): A Clinical Pilot Study. Int. J. Hyperth. 2016, 32, 488–498. [Google Scholar] [CrossRef]
- Padilla-Valverde, D.; Sanchez-Garcia, S.; García-Santos, E.; Marcote-Ibañez, C.; Molina-Robles, M.; Martín-Fernández, J.; Villarejo-Campos, P. Usefulness of thermographic analysis to control temperature homogeneity in the development and implementation of a closed recirculating CO2 chemohyperthermia model. Int. J. Hyperth. 2016, 30, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pascual-Ramírez, J.; Sánchez-García, S.; González -Ruiz de la Herrán, F.; Villarejo Campos, P.; López de la Manzanara Cano, C.; Haya Palazuelo, J.; Padilla Valverde, D.; Martín Fernández, J. Security and efficiency of a closed-system, turbulent-flow circuit for hyperthermic intraperitoneal chemotherapy after cytoreductive ovarian surgery: Perioperative outputs. Arch. Gynecol. Obstet. 2014, 290, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-García, S.; Padilla-Valverde, D.; Villarejo-Campos, P.; García-Santos, E.P.; Martín-Fernández, J. Hyperthermic chemotherapy intra-abdominal laparoscopic approach: Development of a laparoscopic model using CO2 recirculation system and clinical translation in peritoneal carcinomatosis. Int. J. Hyperth. 2017, 33, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Valverde, D.; Garcia-Santos, E.; Sanchez, S.; Manzanares, C.; Rodriguez, M.; González, L.; Ambrós, A.; Cano, J.M.; Serrano, L.; Bodoque, R.; et al. Safety of perioperative hyperthermic intraperitoneal chemotherapy with gemcitabine in patients with resected pancreatic adenocarcinoma: A pilot study of the clinical trial EudraCT 2016-00429841. J. Gastrointest. Oncol. 2021, 12, S80–S90. [Google Scholar] [CrossRef] [PubMed]
- Yurtas, C.; Horvath, P.; Fischer, I.; Meisner, C.; Nadalin, S.; Königsrainer, I.; Königsrainer, A.; Beckert, S.; Löffler, M.W. A prospective, Phase I/II, Open-Label. Pilot. Trial to asses the safety of hyperthermic intraperitoneal chemotherapy after oncological resection of pancreatic adenocarcinoma. Ann. Surg. Oncol. 2021, 28, 9086–9095. [Google Scholar]
- Larentzakis, A.; Anagnostou, E.; Georgiou, K.; Vrakopoulou, G.Z.; Zografos, C.G.; Zografos, G.C.; Toutouzas, K.G. Place of Hyperthermic intraperitoneal chemotherapy in the armament against pancreatic adenocarcinoma: A survival, mortality and morbidity systematic review. Oncol. Lett. 2021, 21, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Filis, P.; Kanelloupoulou, A.; Gogadis, A.; Filis, N.; Kamposioras, K.; Kapoulitsa, F.; Mauri, D. Hyperthermic intraperitoneal chemotherapy for management of gastrointestinal and biliary tract malignancies:a systematic review and meta-analysis of randomized trials. Ann. Gastroenterol. 2023, 36, 87–96. [Google Scholar] [PubMed]
- Frasini, S.; Calabretto, F.; Granieri, S.; Fugazzola, P.; Viganò, J.; Fazzini, N.; Ansaloni, L.; Cobianchi, L. Intraperitoneal chemotherapy in the management of pancreatic adenocarcinoma: A systematic review and meta-analysis. EJSO 2022, 48, 1911–1921. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata, T.; Matsuda, Y.; Yoshimura, H.; Sasaki, N.; Ishiwata, S.; Ishikawa, N.; Takubo, K.; Arai, T.; Aida, J. Pancreatic cancer stem cells: Features and detection methods. Pathol. Oncol. Res. 2018, 24, 797–805. [Google Scholar] [CrossRef]
- Thomas, R.M.; Kim, J.; Revelo-Penafiel, M.P.; Angel, R.; Dawson, D.W.; Lowy, A.M. The chemokine receptor CXCR4 is expressed in pancreatic intraepithelial neoplasia. Gut 2008, 57, 1555–1560. [Google Scholar] [CrossRef]
- Philipp-Abbrederis, K.; Herrmann, K.; Knop, S.; Schottelius, M.; Eiber, M.; Lückerath, K.; Pietschmann, E.; Habringer, S.; Gerngroß, C.; Franke, K.; et al. In vivo molecular imaging of chemokine receptor CXCR4 expression in patients with advanced multiple myeloma. EMBO Mol. Med. 2015, 7, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Florek, M.; Haase, M.; Marzesco, A.M.; Freund, D.; Ehninger, G.; Huttner, W.B.; Corbeil, D. Prominin-1/CD133, a neural and hematopoietic stem cell marker, is expressed in adult human differentiated cells and certain types of kidney cancer. Cell Tissue Res. 2005, 319, 15–26. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Group II (n = 21) | Group I (n = 21) | p-Value |
|---|---|---|---|
| Age (years, M ± SEM) | 64 ± 1.7 | 69.3 ± 2 | 0.024 * |
| Overall survival (months, median) | 17.1 (0.6–61.9) | 18 (0.8–61.8) | 0.899 |
| Disease-free survival (months, median) | 14 (2–66) | 10 (2–66) | 0.888 |
| Operative time (minutes, median) | 350 (280–420) | 300 (120–360) | 0.002 * |
| Hospital stay (days, median) | 11 (7–28) | 17 (4–69) | 0.302 |
| PaCSCs (CXCR4 + CD133 + EpCAM) | 19.7 ± 8.2 | 13.8 ± 7.1 | 0.919 |
| Sex | n (%) | n (%) | |
| Male | 6 (29) | 13 (62) | 0.03 * |
| Female | 15 (71) | 8 (38) | |
| Symptoms | |||
| HTA | 10 (48) | 9 (43) | 0.757 |
| DM | 9 (43) | 7 (33) | 0.525 |
| DL | 10 (48) | 6 (29) | 0.204 |
| Jaundice | 17 (81) | 12 (57) | 0.095 |
| Abdomnal pain | 8 (38) | 7 (33) | 0.747 |
| Constitutional syndrome | 5 (24) | 5 (24) | 0.999 |
| Resections | |||
| TP without splenectomy | 1 (5) | 4 (19) | 0.502 |
| TP with splenectomy | 9 (43) | 5 (24) | |
| CDP | 9 (43) | 8 (38) | |
| STP | 1 (5) | 1 (5) | |
| DP | 0 | 1 (5) | |
| CCP | 1 (5) | 2 (10) | |
| Adjuvant treatment | |||
| No | 9 (43) | 5 (24) | 0.190 |
| Yes | 12 (57) | 16 (76) | |
| Chemotherapy regimen | |||
| No | 9 (43) | 5 (24) | 0.215 |
| Gem | 3 (14) | 8 (38) | |
| FOL | 2 (10) | 4 (19) | |
| Gem + Nab-P | 4 (19) | 2 (10) | |
| CAP | 2 (10) | 0 (0) | |
| Gem + CAP | 1 (5) | 2 (10) | |
| Pancreatitis complications | |||
| Delayed gastric emptying | 2 (10) | 2 (10) | 0.999 |
| Pancreatic fistula (B,C) | 0 | 1 (5) | 0.757 |
| Hemorrhage | 0 | 2 (10) | 0.525 |
| Clavien-Dindo | |||
| I | 4 (19) | 3 (14) | 0.804 |
| II | 5 (24) | 3 (14) | |
| III | 2 (10) | 3 (14) | |
| IV | 0 | 1 (5) | |
| V | 1 (5) | 1 (5) | |
| Differentiation | |||
| Good | 4 (19) | 3 (14) | 0.08 |
| Moderate | 7 (33) | 14 (67) | |
| Poor | 10 (48) | 4 (19) | |
| Invasion | |||
| Neurologic invasion | 15 (71) | 15 (71) | 0.999 |
| Vascular invasion | 7 (33) | 8 (38) | 0.747 |
| Lymphatic Invasion | 7 (33) | 11 (52) | 0.212 |
| TNM | |||
| Ia | 2 (10) | 5 (24) | 0.419 |
| Ib | 6 (29) | 3 (14) | |
| IIa | 1 (5) | 2 (10) | |
| IIb | 6 (29) | 8 (38) | |
| III | 6 (29) | 3 (14) | |
| Locoregional recurrence | 2 (10) | 11 (52) | 0.004 * |
| Distant recurrence | |||
| No | 12 (57) | 14 (62) | 0.757 |
| Liver | 7 (33) | 8 (38) | |
| Lung | 2 (10) | 0 (0) | |
| Mortality (>30 days) | 13 (62) | 13 (62) | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padilla-Valverde, D.; Bodoque-Villar, R.; García-Santos, E.; Sanchez, S.; Manzanares-Campillo, C.; Rodriguez, M.; González, L.; Ambrós, A.; Cano, J.M.; Padilla-Marcote, M.; et al. Safety and Effectiveness of Perioperative Hyperthermic Intraperitoneal Chemotherapy with Gemcitabine in Patients with Resected Pancreatic Ductal Adenocarcinoma: Clinical Trial EudraCT 2016-004298-41. Cancers 2024, 16, 1718. https://doi.org/10.3390/cancers16091718
Padilla-Valverde D, Bodoque-Villar R, García-Santos E, Sanchez S, Manzanares-Campillo C, Rodriguez M, González L, Ambrós A, Cano JM, Padilla-Marcote M, et al. Safety and Effectiveness of Perioperative Hyperthermic Intraperitoneal Chemotherapy with Gemcitabine in Patients with Resected Pancreatic Ductal Adenocarcinoma: Clinical Trial EudraCT 2016-004298-41. Cancers. 2024; 16(9):1718. https://doi.org/10.3390/cancers16091718
Chicago/Turabian StylePadilla-Valverde, David, Raquel Bodoque-Villar, Esther García-Santos, Susana Sanchez, Carmen Manzanares-Campillo, Marta Rodriguez, Lucia González, Alfonso Ambrós, Juana M. Cano, Maria Padilla-Marcote, and et al. 2024. "Safety and Effectiveness of Perioperative Hyperthermic Intraperitoneal Chemotherapy with Gemcitabine in Patients with Resected Pancreatic Ductal Adenocarcinoma: Clinical Trial EudraCT 2016-004298-41" Cancers 16, no. 9: 1718. https://doi.org/10.3390/cancers16091718
APA StylePadilla-Valverde, D., Bodoque-Villar, R., García-Santos, E., Sanchez, S., Manzanares-Campillo, C., Rodriguez, M., González, L., Ambrós, A., Cano, J. M., Padilla-Marcote, M., Redondo-Calvo, J., Martin, J., & Serrano-Oviedo, L. (2024). Safety and Effectiveness of Perioperative Hyperthermic Intraperitoneal Chemotherapy with Gemcitabine in Patients with Resected Pancreatic Ductal Adenocarcinoma: Clinical Trial EudraCT 2016-004298-41. Cancers, 16(9), 1718. https://doi.org/10.3390/cancers16091718