Susceptibility of Melanoma Cells to Targeted Therapy Correlates with Protection by Blood Neutrophils
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort and Healthy Donors
2.2. Isolation of Neutrophils
2.3. Comparing Neutrophil Isolation Methods
2.4. Purity and Phenotyping of Neutrophils
2.5. ELISA
2.6. Multiplex Analysis
2.7. Cell Lines
2.8. Protection Assays
2.9. Cell Cycle Analysis
2.10. Lysis of Neutrophils and Inactivation of Contents
2.11. Time-Dependent Protection of Melanoma Cells
2.12. Cytospins and HE Staining
2.13. 3D Spheroid Assay
2.14. Target Blocking Experiment
2.15. Visualization and Interference with NETs
2.16. Statistical Analysis
3. Results
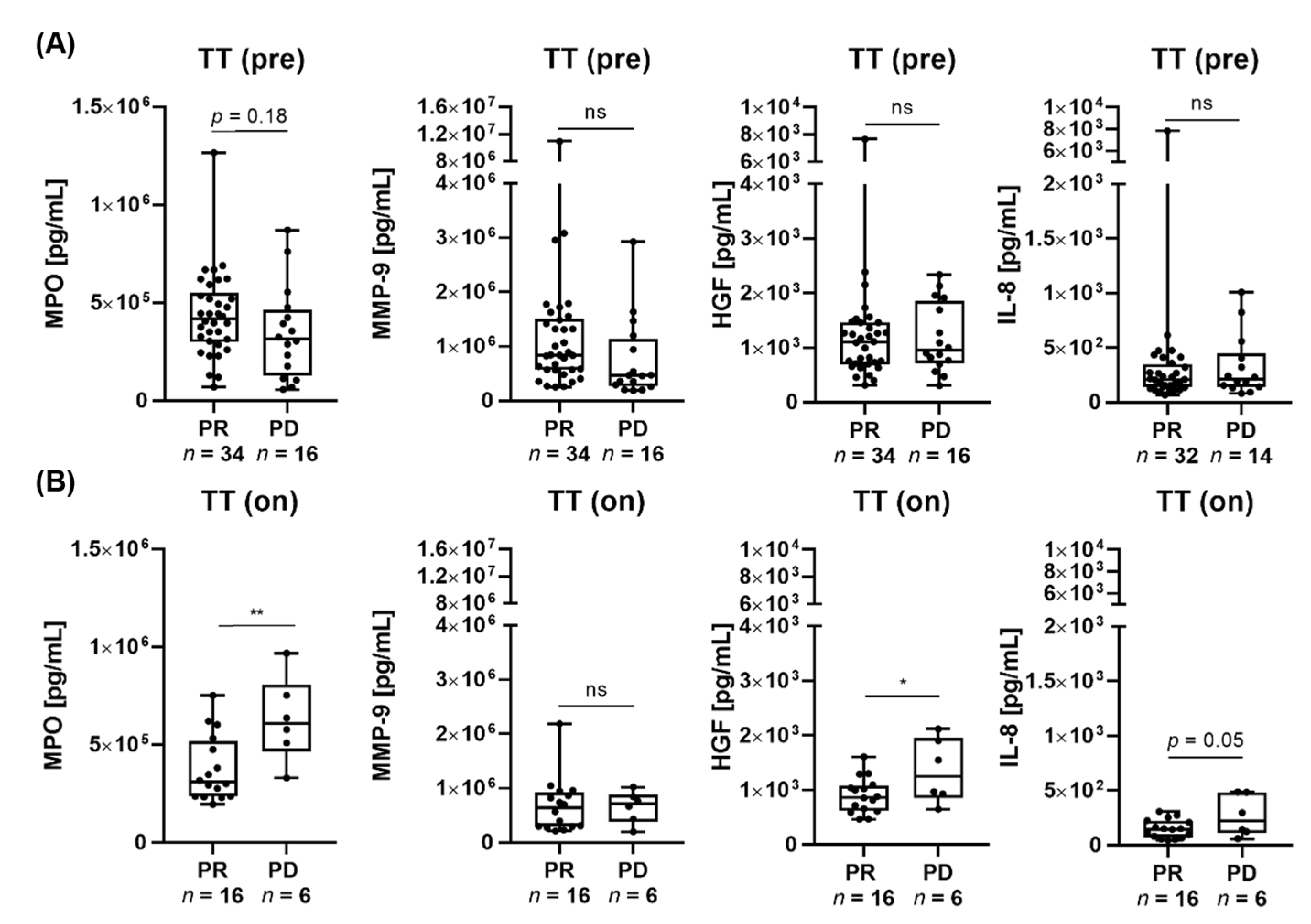
3.1. Low MPO and HGF Serum Levels Are Associated with Response to Targeted Therapy and Immunotherapy in Patients with Advanced Melanoma
3.2. Peripheral Neutrophils from Stage IV Melanoma Patients Prior to Drug Administration Show Lower CD16 Expression Compared to Healthy Donors
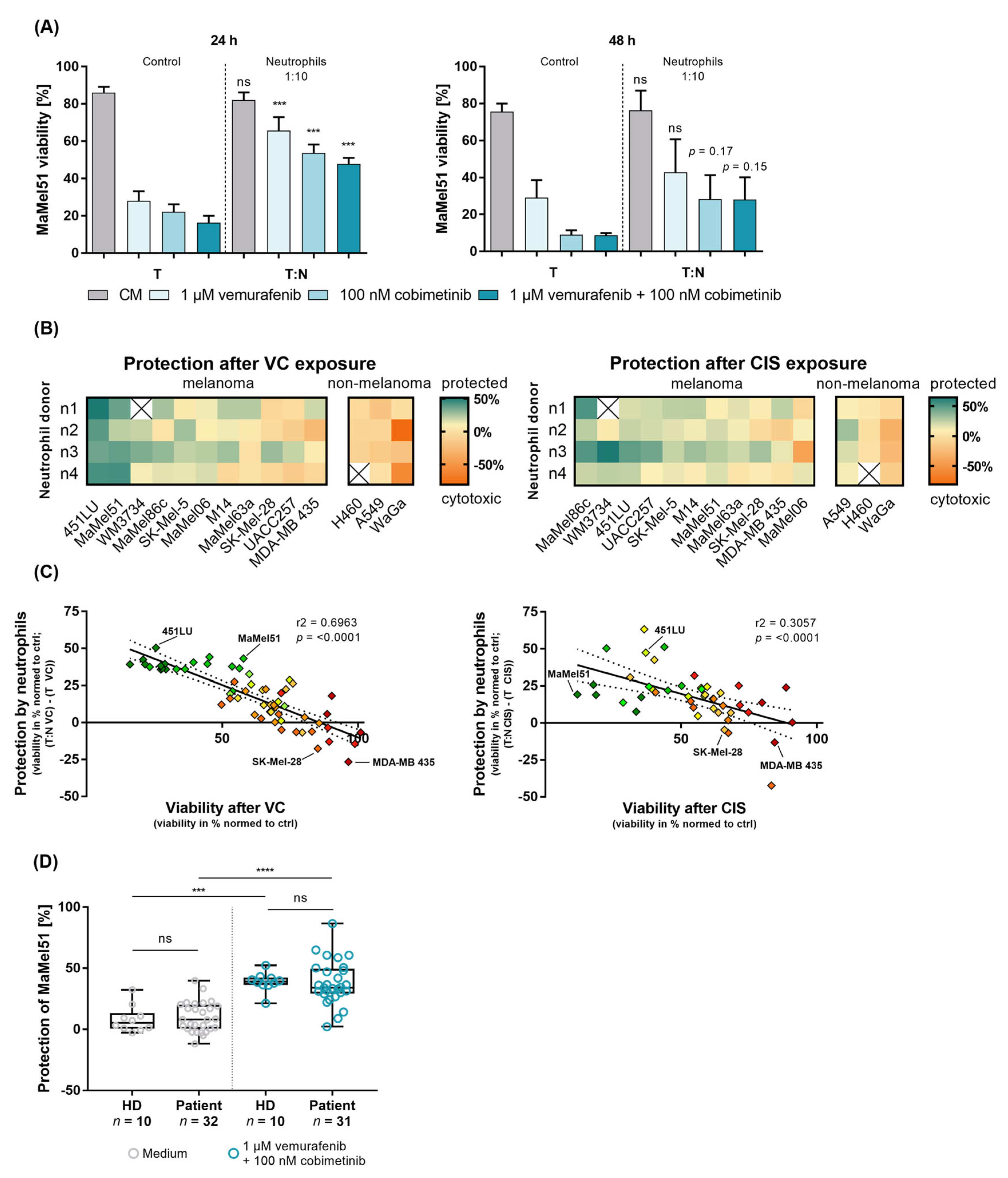
3.3. Peripheral Blood Neutrophils from Patients and Healthy Donors Similarly Prevent BRAF-/MEK-Inhibition-Induced Apoptosis of Melanoma Cells In Vitro
3.4. Melanoma Cells and Neutrophils Engage in a Reciprocal Relationship In Vitro
3.5. Neutrophils Protect Melanoma Cells from BRAF-/MEK-Inhibition-Induced Cell Cycle Arrest
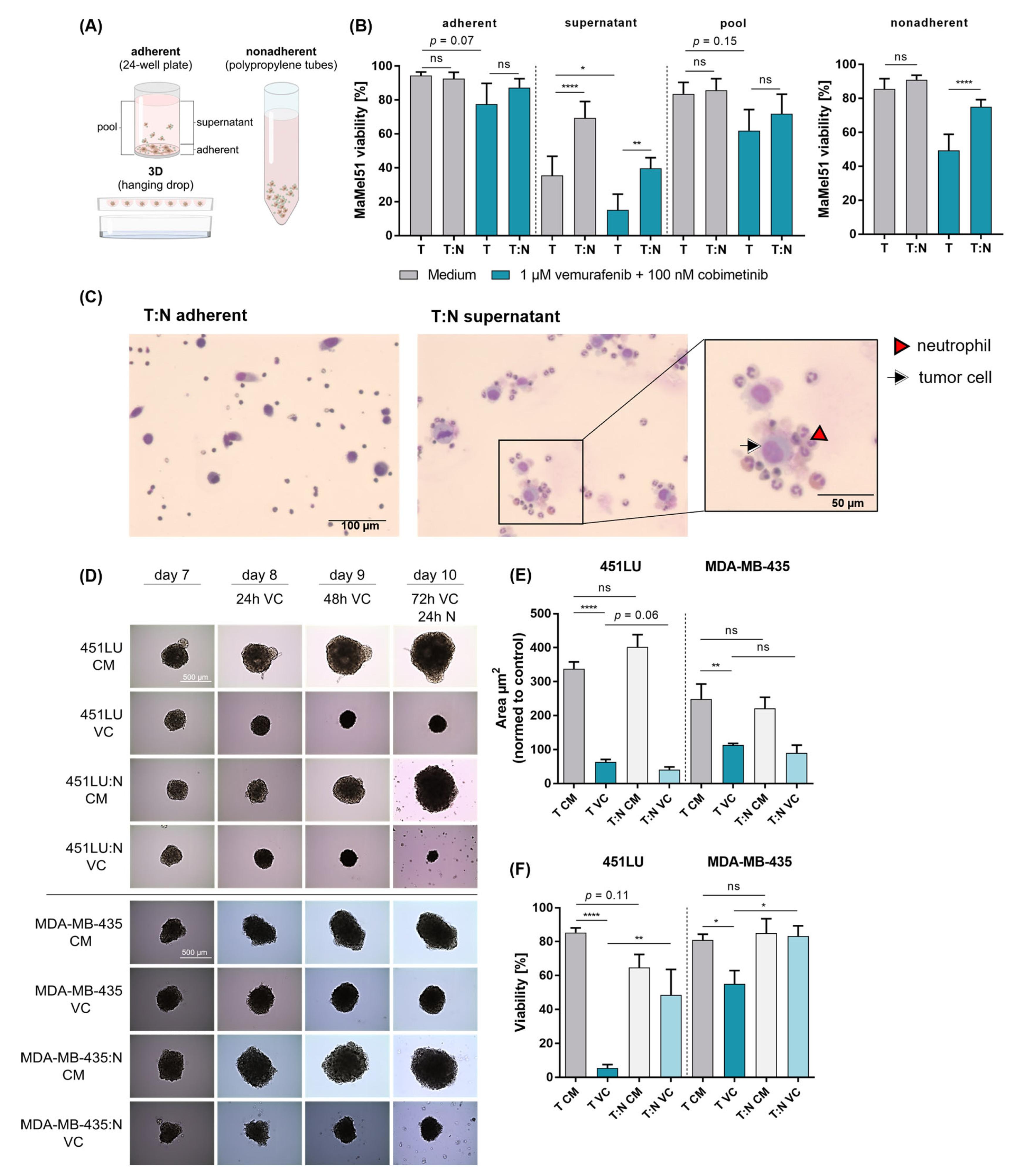
3.6. Cell–Cell Contact Dictates the Active Process of Neutrophil-Mediated Protection of Melanoma in the Context of Dual BRAF/MEK Inhibition
3.7. Neutrophil-Induced Protection of Melanoma Cells Is Observed in Nonadherent and 3D Cultures
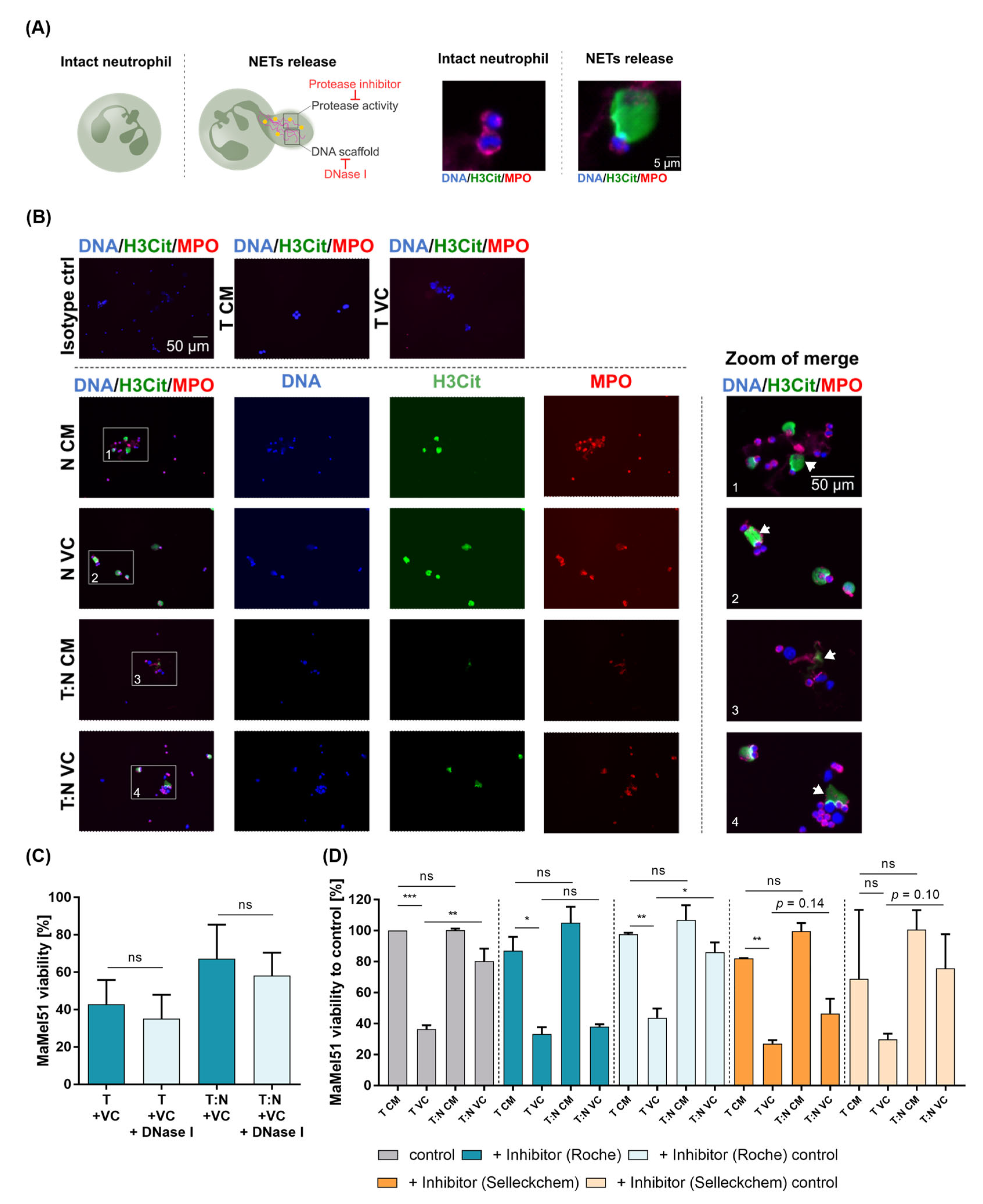
3.8. Neutrophil Extracellular Traps form in Melanoma Cell–Neutrophil Cocultures and Protease Inhibitors Prevent Protection of Melanoma Cells by Neutrophils
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiong, S.; Dong, L.; Cheng, L. Neutrophils in cancer carcinogenesis and metastasis. J. Hematol. Oncol. 2021, 14, 173. [Google Scholar] [CrossRef] [PubMed]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014, 9, 181–218. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Seruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocana, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef]
- Wu, L.; Saxena, S.; Singh, R.K. Neutrophils in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1224, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Furumaya, C.; Martinez-Sanz, P.; Bouti, P.; Kuijpers, T.W.; Matlung, H.L. Plasticity in Pro- and Anti-tumor Activity of Neutrophils: Shifting the Balance. Front. Immunol. 2020, 11, 2100. [Google Scholar] [CrossRef]
- Guthrie, G.J.; Charles, K.A.; Roxburgh, C.S.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef]
- Iriondo, O.; Yu, M. Unexpected Friendship: Neutrophils Help Tumor Cells En Route to Metastasis. Dev. Cell 2019, 49, 308–310. [Google Scholar] [CrossRef]
- Sprouse, M.L.; Welte, T.; Boral, D.; Liu, H.N.; Yin, W.; Vishnoi, M.; Goswami-Sewell, D.; Li, L.; Pei, G.; Jia, P.; et al. PMN-MDSCs Enhance CTC Metastatic Properties through Reciprocal Interactions via ROS/Notch/Nodal Signaling. Int. J. Mol. Sci. 2019, 20, 1916. [Google Scholar] [CrossRef]
- Ma, J.; Kuzman, J.; Ray, A.; Lawson, B.O.; Khong, B.; Xuan, S.; Hahn, A.W.; Khong, H.T. Neutrophil-to-lymphocyte Ratio (NLR) as a predictor for recurrence in patients with stage III melanoma. Sci. Rep. 2018, 8, 4044. [Google Scholar] [CrossRef]
- Shaul, M.E.; Fridlender, Z.G. Cancer-related circulating and tumor-associated neutrophils—Subtypes, sources and function. FEBS J. 2018, 285, 4316–4342. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.T.; Miner, T.J.; Vezeridis, M.P. Is the neutrophil-to-lymphocyte ratio a useful prognostic indicator in melanoma patients? Melanoma Manag. 2020, 7, MMT47. [Google Scholar] [CrossRef]
- Ferrucci, P.F.; Gandini, S.; Battaglia, A.; Alfieri, S.; Di Giacomo, A.M.; Giannarelli, D.; Cappellini, G.C.; De Galitiis, F.; Marchetti, P.; Amato, G.; et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br. J. Cancer 2015, 112, 1904–1910. [Google Scholar] [CrossRef]
- He, G.; Zhang, H.; Zhou, J.; Wang, B.; Chen, Y.; Kong, Y.; Xie, X.; Wang, X.; Fei, R.; Wei, L.; et al. Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2015, 34, 141. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.O.; Schmidt, H.; Moller, H.J.; Donskov, F.; Hoyer, M.; Sjoegren, P.; Christensen, I.J.; Steiniche, T. Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer 2012, 118, 2476–2485. [Google Scholar] [CrossRef]
- Sconocchia, G.; Zlobec, I.; Lugli, A.; Calabrese, D.; Iezzi, G.; Karamitopoulou, E.; Patsouris, E.S.; Peros, G.; Horcic, M.; Tornillo, L.; et al. Tumor infiltration by FcgammaRIII (CD16)+ myeloid cells is associated with improved survival in patients with colorectal carcinoma. Int. J. Cancer 2011, 128, 2663–2672. [Google Scholar] [CrossRef]
- Slattery, M.J.; Dong, C. Neutrophils influence melanoma adhesion and migration under flow conditions. Int. J. Cancer 2003, 106, 713–722. [Google Scholar] [CrossRef]
- Coussens, L.M.; Tinkle, C.L.; Hanahan, D.; Werb, Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 2000, 103, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Houghton, A.M.; Rzymkiewicz, D.M.; Ji, H.; Gregory, A.D.; Egea, E.E.; Metz, H.E.; Stolz, D.B.; Land, S.R.; Marconcini, L.A.; Kliment, C.R.; et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat. Med. 2010, 16, 219–223. [Google Scholar] [CrossRef]
- Jensen, H.K.; Donskov, F.; Marcussen, N.; Nordsmark, M.; Lundbeck, F.; von der Maase, H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J. Clin. Oncol. 2009, 27, 4709–4717. [Google Scholar] [CrossRef]
- Nozawa, H.; Chiu, C.; Hanahan, D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc. Natl. Acad. Sci. 2006, 103, 12493–12498. [Google Scholar] [CrossRef] [PubMed]
- Salemi, R.; Falzone, L.; Madonna, G.; Polesel, J.; Cina, D.; Mallardo, D.; Ascierto, P.A.; Libra, M.; Candido, S. MMP-9 as a Candidate Marker of Response to BRAF Inhibitors in Melanoma Patients With BRAF(V600E) Mutation Detected in Circulating-Free DNA. Front. Pharmacol. 2018, 9, 856. [Google Scholar] [CrossRef]
- Peng, H.H.; Liang, S.; Henderson, A.J.; Dong, C. Regulation of interleukin-8 expression in melanoma-stimulated neutrophil inflammatory response. Exp. Cell Res. 2007, 313, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, M.; Fontana, F.; Marzagalli, M.; Gagliano, N.; Sommariva, M.; Limonta, P. Melanoma Stem Cells Educate Neutrophils to Support Cancer Progression. Cancers 2022, 14, 3391. [Google Scholar] [CrossRef] [PubMed]
- Neubert, E.; Meyer, D.; Rocca, F.; Gunay, G.; Kwaczala-Tessmann, A.; Grandke, J.; Senger-Sander, S.; Geisler, C.; Egner, A.; Schon, M.P.; et al. Chromatin swelling drives neutrophil extracellular trap release. Nat. Commun. 2018, 9, 3767. [Google Scholar] [CrossRef] [PubMed]
- Albrengues, J.; Shields, M.A.; Ng, D.; Park, C.G.; Ambrico, A.; Poindexter, M.E.; Upadhyay, P.; Uyeminami, D.L.; Pommier, A.; Kuttner, V.; et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 2018, 361, eaao4227. [Google Scholar] [CrossRef] [PubMed]
- Weide, L.M.; Schedel, F.; Weishaupt, C. Neutrophil Extracellular Traps Correlate with Tumor Necrosis and Size in Human Malignant Melanoma Metastases. Biology 2023, 12, 822. [Google Scholar] [CrossRef] [PubMed]
- Hu-Lieskovan, S.; Mok, S.; Homet Moreno, B.; Tsoi, J.; Robert, L.; Goedert, L.; Pinheiro, E.M.; Koya, R.C.; Graeber, T.G.; Comin-Anduix, B.; et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci. Transl. Med. 2015, 7, 279ra241. [Google Scholar] [CrossRef]
- Peiffer, L.; Farahpour, F.; Sriram, A.; Spassova, I.; Hoffmann, D.; Kubat, L.; Stoitzner, P.; Gambichler, T.; Sucker, A.; Ugurel, S.; et al. BRAF and MEK inhibition in melanoma patients enables reprogramming of tumor infiltrating lymphocytes. Cancer Immunol. Immunother. 2021, 70, 1635–1647. [Google Scholar] [CrossRef]
- Ribas, A.; Lawrence, D.; Atkinson, V.; Agarwal, S.; Miller, W.H., Jr.; Carlino, M.S.; Fisher, R.; Long, G.V.; Hodi, F.S.; Tsoi, J.; et al. Combined BRAF and MEK inhibition with PD-1 blockade immunotherapy in BRAF-mutant melanoma. Nat. Med. 2019, 25, 936–940. [Google Scholar] [CrossRef]
- Benarafa, C. Tumor-induced inflammation alters neutrophil phenotype and disease progression. Breast Cancer Res. 2015, 17, 135. [Google Scholar] [CrossRef] [PubMed]
- Mousset, A.; Lecorgne, E.; Bourget, I.; Lopez, P.; Jenovai, K.; Cherfils-Vicini, J.; Dominici, C.; Rios, G.; Girard-Riboulleau, C.; Liu, B.; et al. Neutrophil extracellular traps formed during chemotherapy confer treatment resistance via TGF-beta activation. Cancer Cell 2023, 41, 757–775 e710. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Krijgsman, O.; Tsoi, J.; Robert, L.; Hugo, W.; Song, C.; Kong, X.; Possik, P.A.; Cornelissen-Steijger, P.D.; Geukes Foppen, M.H.; et al. Low MITF/AXL ratio predicts early resistance to multiple targeted drugs in melanoma. Nat. Commun. 2014, 5, 5712. [Google Scholar] [CrossRef] [PubMed]
- Riesenberg, S.; Groetchen, A.; Siddaway, R.; Bald, T.; Reinhardt, J.; Smorra, D.; Kohlmeyer, J.; Renn, M.; Phung, B.; Aymans, P.; et al. MITF and c-Jun antagonism interconnects melanoma dedifferentiation with pro-inflammatory cytokine responsiveness and myeloid cell recruitment. Nat. Commun. 2015, 6, 8755. [Google Scholar] [CrossRef] [PubMed]
- Wendlinger, S.; Wohlfarth, J.; Kreft, S.; Siedel, C.; Kilian, T.; Dischinger, U.; Heppt, M.V.; Wistuba-Hamprecht, K.; Meier, F.; Goebeler, M.; et al. Blood Eosinophils Are Associated with Efficacy of Targeted Therapy in Patients with Advanced Melanoma. Cancers 2022, 14, 2294. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Ugurel, S.; Thirumaran, R.K.; Bloethner, S.; Gast, A.; Sucker, A.; Mueller-Berghaus, J.; Rittgen, W.; Hemminki, K.; Becker, J.C.; Kumar, R.; et al. B-RAF and N-RAS mutations are preserved during short time in vitro propagation and differentially impact prognosis. PLoS ONE 2007, 2, e236. [Google Scholar] [CrossRef]
- Houben, R.; Hesbacher, S.; Sarma, B.; Schulte, C.; Sarosi, E.M.; Popp, S.; Adam, C.; Kervarrec, T.; Schrama, D. Inhibition of T-antigen expression promoting glycogen synthase kinase 3 impairs merkel cell carcinoma cell growth. Cancer Lett. 2022, 524, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Mattes, J.; Hulett, M.; Xie, W.; Hogan, S.; Rothenberg, M.E.; Foster, P.; Parish, C. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: An eotaxin and STAT6-dependent process. J. Exp. Med. 2003, 197, 387–393. [Google Scholar] [CrossRef]
- Rudnicki, M.; McBurney, M.; Robertson, E. Teratocarcinomas and Embryonic Stem Cells: A Practical Approach; IRL Press: Oxford, UK, 1987; pp. 19–49. [Google Scholar]
- Kosnopfel, C.; Wendlinger, S.; Niessner, H.; Siewert, J.; Sinnberg, T.; Hofmann, A.; Wohlfarth, J.; Schrama, D.; Berthold, M.; Siedel, C.; et al. Inhibition of p90 ribosomal S6 kinases disrupts melanoma cell growth and immune evasion. J. Exp. Clin. Cancer Res. 2023, 42, 175. [Google Scholar] [CrossRef]
- Brinkmann, V.; Laube, B.; Abu Abed, U.; Goosmann, C.; Zychlinsky, A. Neutrophil extracellular traps: How to generate and visualize them. J. Vis. Exp. 2010, 36, e1724. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Shi, L.; Wang, B.; Yang, J.; Xiao, Z.; Du, P.; Wang, Q.; Yang, W. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: A systematic review and meta-analysis of 66 cohort studies. Cancer Treat. Rev. 2017, 58, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Xue, F.; Takahara, T.; Kudo, H.; Yata, Y.; Zhang, W.; Sugiyama, T. Matrix metalloproteinase-9 contributes to the mobilization of bone marrow cells in the injured liver. Cell Transplant 2012, 21, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.W.; Gammon, S.T.; Yang, P.; Ma, W.; Wang, J.; Piwnica-Worms, D. Inhibition of myeloperoxidase enhances immune checkpoint therapy for melanoma. J. Immunother. Cancer 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Sanmamed, M.F.; Perez-Gracia, J.L.; Schalper, K.A.; Fusco, J.P.; Gonzalez, A.; Rodriguez-Ruiz, M.E.; Onate, C.; Perez, G.; Alfaro, C.; Martin-Algarra, S.; et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1988–1995. [Google Scholar] [CrossRef]
- Bustos, S.O.; da Silva Pereira, G.J.; de Freitas Saito, R.; Gil, C.D.; Zanatta, D.B.; Smaili, S.S.; Chammas, R. Galectin-3 sensitized melanoma cell lines to vemurafenib (PLX4032) induced cell death through prevention of autophagy. Oncotarget 2018, 9, 14567–14579. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huang, Y.; Huang, L.; Xu, Y.; Wang, Z.; Li, H.; Zhang, T.; Zhong, M.; Gao, W.Q.; Zhang, Y. CD16 expression on neutrophils predicts treatment efficacy of capecitabine in colorectal cancer patients. BMC Immunol. 2020, 21, 46. [Google Scholar] [CrossRef] [PubMed]
- Millrud, C.R.; Kagedal, A.; Kumlien Georen, S.; Winqvist, O.; Uddman, R.; Razavi, R.; Munck-Wikland, E.; Cardell, L.O. NET-producing CD16(high) CD62L(dim) neutrophils migrate to tumor sites and predict improved survival in patients with HNSCC. Int. J. Cancer 2017, 140, 2557–2567. [Google Scholar] [CrossRef]
- Liu, S.; Wu, W.; Du, Y.; Yin, H.; Chen, Q.; Yu, W.; Wang, W.; Yu, J.; Liu, L.; Lou, W.; et al. The evolution and heterogeneity of neutrophils in cancers: Origins, subsets, functions, orchestrations and clinical applications. Mol. Cancer 2023, 22, 148. [Google Scholar] [CrossRef]
- Shah, K.P.; Song, H.; Ye, F.; Johnson, D.B. Prognostic Clinical and Radiographic Biomarkers for BRAF-Targeted Therapy in Advanced Melanoma. Oncologist 2021, 26, e333–e335. [Google Scholar] [CrossRef]
- Borst, A.; Haferkamp, S.; Grimm, J.; Rosch, M.; Zhu, G.; Guo, S.; Li, C.; Gao, T.; Meierjohann, S.; Schrama, D.; et al. BIK is involved in BRAF/MEK inhibitor induced apoptosis in melanoma cell lines. Cancer Lett. 2017, 404, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Haferkamp, S.; Borst, A.; Adam, C.; Becker, T.M.; Motschenbacher, S.; Windhovel, S.; Hufnagel, A.L.; Houben, R.; Meierjohann, S. Vemurafenib induces senescence features in melanoma cells. J. Investig. Dermatol. 2013, 133, 1601–1609. [Google Scholar] [CrossRef]
- Entman, M.L.; Youker, K.; Shoji, T.; Kukielka, G.; Shappell, S.B.; Taylor, A.A.; Smith, C.W. Neutrophil induced oxidative injury of cardiac myocytes. A compartmented system requiring CD11b/CD18-ICAM-1 adherence. J. Clin. Investig. 1992, 90, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.H.; Feng, L.; Su, X.; Brassard, A.; Dhoparee-Doomah, I.; Ferri, L.E.; Spicer, J.D.; Cools-Lartigue, J.J. Neutrophil Extracellular Traps in Cancer Therapy Resistance. Cancers 2022, 14, 1359. [Google Scholar] [CrossRef] [PubMed]
- Falzone, L.; Salemi, R.; Travali, S.; Scalisi, A.; McCubrey, J.A.; Candido, S.; Libra, M. MMP-9 overexpression is associated with intragenic hypermethylation of MMP9 gene in melanoma. Aging 2016, 8, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Vaisanen, A.H.; Kallioinen, M.; Turpeenniemi-Hujanen, T. Comparison of the prognostic value of matrix metalloproteinases 2 and 9 in cutaneous melanoma. Hum. Pathol. 2008, 39, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.A.; Takebe, N.; Hinoue, T.; Hoadley, K.A.; Cardenas, M.F.; Hamilton, A.M.; Laird, P.W.; Wang, L.; Johnson, A.; Dewal, N.; et al. Molecular Features of Cancers Exhibiting Exceptional Responses to Treatment. Cancer Cell 2021, 39, 38–53 e37. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M. Serum albumin: A pharmacokinetic marker for optimizing treatment outcome of immune checkpoint blockade. J. Immunother. Cancer 2022, 10, e005670. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.R.; Doig, T.; Anderson, N.; Brenn, T.; Doherty, V.; Xu, Y.; Bartlett, J.M.; Smyth, J.F.; Melton, D.W. Association of galectin-3 expression with melanoma progression and prognosis. Eur. J. Cancer 2012, 48, 865–874. [Google Scholar] [CrossRef]
- Mabbitt, J.; Holyer, I.D.; Roper, J.A.; Nilsson, U.J.; Zetterberg, F.R.; Vuong, L.; Mackinnon, A.C.; Pedersen, A.; Slack, R.J. Resistance to anti-PD-1/anti-PD-L1: Galectin-3 inhibition with GB1211 reverses galectin-3-induced blockade of pembrolizumab and atezolizumab binding to PD-1/PD-L1. Front. Immunol. 2023, 14, 1250559. [Google Scholar] [CrossRef]
- Wu, X.; Giobbie-Hurder, A.; Connolly, E.M.; Li, J.; Liao, X.; Severgnini, M.; Zhou, J.; Rodig, S.; Hodi, F.S. Anti-CTLA-4 based therapy elicits humoral immunity to galectin-3 in patients with metastatic melanoma. Oncoimmunology 2018, 7, e1440930. [Google Scholar] [CrossRef] [PubMed]
- Yeap, W.H.; Wong, K.L.; Shimasaki, N.; Teo, E.C.; Quek, J.K.; Yong, H.X.; Diong, C.P.; Bertoletti, A.; Linn, Y.C.; Wong, S.C. CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes. Sci. Rep. 2016, 6, 34310. [Google Scholar] [CrossRef] [PubMed]
- Maleki, E.H.; Bahrami, A.R.; Matin, M.M. Cancer cell cycle heterogeneity as a critical determinant of therapeutic resistance. Genes Dis. 2024, 11, 189–204. [Google Scholar] [CrossRef]
- Jacquemin, V.; Antoine, M.; Dom, G.; Detours, V.; Maenhaut, C.; Dumont, J.E. Dynamic Cancer Cell Heterogeneity: Diagnostic and Therapeutic Implications. Cancers 2022, 14, 280. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes-Bastos, D.; Frony, A.C.; Barja-Fidalgo, C.; Moraes, J.A. Melanoma-derived extracellular vesicles skew neutrophils into a pro-tumor phenotype. J. Leukoc. Biol. 2022, 111, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Modestino, L.; Cristinziano, L.; Trocchia, M.; Ventrici, A.; Capone, M.; Madonna, G.; Loffredo, S.; Ferrara, A.L.; Romanelli, M.; Simeone, E.; et al. Melanoma-derived soluble mediators modulate neutrophil biological properties and the release of neutrophil extracellular traps. Cancer Immunol. Immunother. 2023, 72, 3363–3376. [Google Scholar] [CrossRef]
- Neagu, M.; Constantin, C.; Dumitrascu, G.R.; Lupu, A.R.; Caruntu, C.; Boda, D.; Zurac, S. Inflammation markers in cutaneous melanoma—Edgy biomarkers for prognosis. Discoveries 2015, 3, e38. [Google Scholar] [CrossRef]
- Basu, S.; Hodgson, G.; Katz, M.; Dunn, A.R. Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood 2002, 100, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Mayadas, T.N.; Cullere, X. Neutrophil beta2 integrins: Moderators of life or death decisions. Trends Immunol. 2005, 26, 388–395. [Google Scholar] [CrossRef]
- Savill, J.; Haslett, C.; Hellewell, P.; Williams, T. Fate of Neutrophils; Academic: London, UK, 1994. [Google Scholar]
- Engblom, C.; Pfirschke, C.; Zilionis, R.; Da Silva Martins, J.; Bos, S.A.; Courties, G.; Rickelt, S.; Severe, N.; Baryawno, N.; Faget, J.; et al. Osteoblasts remotely supply lung tumors with cancer-promoting SiglecF(high) neutrophils. Science 2017, 358, eaal5081. [Google Scholar] [CrossRef]
- Wu, L.; Saxena, S.; Goel, P.; Prajapati, D.R.; Wang, C.; Singh, R.K. Breast Cancer Cell-Neutrophil Interactions Enhance Neutrophil Survival and Pro-Tumorigenic Activities. Cancers 2020, 12, 2884. [Google Scholar] [CrossRef]
- Gonzalez Gonzalez, M.; Cichon, I.; Scislowska-Czarnecka, A.; Kolaczkowska, E. Challenges in 3D culturing of neutrophils: Assessment of cell viability. J. Immunol. Methods 2018, 457, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Oliver, T.G. Partners in Crime: Neutrophil-CTC Collusion in Metastasis. Trends Immunol. 2019, 40, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Kiniwa, Y.; Nakamura, K.; Mikoshiba, A.; Ashida, A.; Akiyama, Y.; Morimoto, A.; Okuyama, R. Usefulness of monitoring circulating tumor cells as a therapeutic biomarker in melanoma with BRAF mutation. BMC Cancer 2021, 21, 287. [Google Scholar] [CrossRef]
- Hattar, K.; Franz, K.; Ludwig, M.; Sibelius, U.; Wilhelm, J.; Lohmeyer, J.; Savai, R.; Subtil, F.S.; Dahlem, G.; Eul, B.; et al. Interactions between neutrophils and non-small cell lung cancer cells: Enhancement of tumor proliferation and inflammatory mediator synthesis. Cancer Immunol. Immunother. 2014, 63, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Strell, C.; Lang, K.; Niggemann, B.; Zaenker, K.S.; Entschladen, F. Neutrophil granulocytes promote the migratory activity of MDA-MB-468 human breast carcinoma cells via ICAM-1. Exp. Cell Res. 2010, 316, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Rawat, K.; Syeda, S.; Shrivastava, A. Neutrophil-derived granule cargoes: Paving the way for tumor growth and progression. Cancer Metastasis Rev. 2021, 40, 221–244. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Aldabbous, L.; Abdul-Salam, V.; McKinnon, T.; Duluc, L.; Pepke-Zaba, J.; Southwood, M.; Ainscough, A.J.; Hadinnapola, C.; Wilkins, M.R.; Toshner, M.; et al. Neutrophil Extracellular Traps Promote Angiogenesis: Evidence From Vascular Pathology in Pulmonary Hypertension. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2078–2087. [Google Scholar] [CrossRef]
- Cools-Lartigue, J.; Spicer, J.; McDonald, B.; Gowing, S.; Chow, S.; Giannias, B.; Bourdeau, F.; Kubes, P.; Ferri, L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Investig. 2013, 123, 3446–3458. [Google Scholar] [CrossRef] [PubMed]

| Variables | Donor | % | ||
|---|---|---|---|---|
| Individual donors | 60 | 100 | ||
| Patient | Stage | I/II 3 | 3 | 5.07 |
| IV 4 | 40 | 66.6 | ||
| Healthy donor | 17 | 28.3 |
| Variable | Patients | ||
|---|---|---|---|
| Age | Median (range) | 70 years (36–91) | % |
| Individual patients | 145 | 100 | |
| Sex | male | 83 | 57.2 |
| female | 39 | 26.9 | |
| unknown 2 | 23 | 15.9 | |
| Stage | III | 14 | 9.7 |
| IV | 131 | 90.3 | |
| M-category | M1a | 12 | 8.3 |
| M1b | 37 | 25.5 | |
| M1c | 67 | 46.2 | |
| M1d | 11 | 7.6 | |
| First-line therapy | yes | 134 | 92.4 |
| no | 11 | 7.6 | |
| Therapy after study inclusion | anti-PD-1 | 60 | 41.4 |
| anti-PD-1 + anti-CTLA-4 | 32 | 22.1 | |
| BRAFi + MEKi 3,4 | 48 | 33.1 | |
| ImmunoCobiVem or others | 5 | 3.4 | |
| LDH | >1× ULN 5 | 62 | 42.8 |
| <1× ULN | 83 | 57.2 |
| Cell Line | Accession | Disease | Site of Derivation | BRAF | TERT | TP53 |
|---|---|---|---|---|---|---|
| 451LU; xenograft: WM164 | CVCL_6357 | cutaneous melanoma | metastatic; arm; skin metastatic; established from lung of a nude mouse. | V600E; heterozygous | n.a. | mut |
| MaMel51 | CVCL_A186 | melanoma | metastatic; lymph node | V600E | mut | n.a. |
| WM3734 | CVCL_6800 | melanoma | metastatic; brain | V600E; heterozygous | n.a. | n.a. |
| MaMel86c | CVCL_C7TP | cutaneous nodular melanoma | metastatic; lymph node | V600E | n.a. | mut |
| Sk-Mel-5 | CVCL_0527 | cutaneous melanoma | metastatic; axillary lymph node | V600E | mut | wt |
| MaMel06 | CVCL_A119 | cutaneous nodular melanoma | metastatic; lymph node | V600E | mut | n.a. |
| M14 | CVCL_1395 | amelanotic melanoma | metastatic; right buttock; hypodermis; subcutaneous | V600E; heterozygous | n.a. | mut |
| MaMel63a | CVCL_A198 | melanoma | metastatic; hypodermis; skin/ cutaneous/subcutaneous | V600E | mut | n.a. |
| Sk-Mel-28 | CVCL_0526 | cutaneous melanoma | in situ, skin; melanocyte of skin | V600E; homozygous | mut | mut |
| UACC257 | CVCL_1779 | melanoma | unknown | V600E; heterozygous | mut | wt |
| MDA-MB-435 derivative: M14 | CVCL_0417 | amelanotic melanoma | metastatic; right buttock; hypodermis; subcutaneous | V600E; heterozygous | n.a. | mut |
| H460 | CVCL_0459 | lung large cell carcinoma | metastatic; pleural effusion | n.a. | n.a. | wt |
| A549 | CVCL_0023 | lung adenocarcinoma | in situ; lung | n.a. | n.a. | wt |
| WaGa | CVCL_E998 | Merkel cell carcinoma, cutaneous neuroendocrine carcinoma | metastatic; ascites | n.a. | n.a. | n.a. |
| Treatment with VC | Treatment with CIS | ||
|---|---|---|---|
| Cell Lines | Protection in % (Mean of n3–4) | Cell Lines | Protection in % (Mean of n3–4) |
| 451LU | +42.0% | MaMel86c | +37.2% |
| MaMel51 | +34.7% | WM3734 | +30.9% (n3) |
| WM3734 | +21.0% (n3) | 451LU | +27.9% |
| MaMel86c | +17.4% | UACC257 | +22.9% |
| Sk-Mel-5 | +16.3% | Sk-Mel-5 | +19.4% |
| MaMel06 | +14.4% | M14 | +19.1% |
| M14 | +13.6% | MaMel51 | +17.6% |
| MaMel63a | +6.9% | MaMel63a | +12.7% |
| Sk-Mel-28 | +2.5% | Sk-Mel-28 | +10.0% |
| UACC257 | −2.8% | MDA-MB-435 | +6.2% |
| MDA-MB-435 | −2.4% | MaMel06 | −5.9% |
| H460 | −8.1% (n3) | A549 | +17.4% |
| A549 | −11.2% | H460 | +0.9% |
| WaGa | −40.6% | WaGa | −19.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wendlinger, S.; Wohlfarth, J.; Siedel, C.; Kreft, S.; Kilian, T.; Junker, S.; Schmid, L.; Sinnberg, T.; Dischinger, U.; Heppt, M.V.; et al. Susceptibility of Melanoma Cells to Targeted Therapy Correlates with Protection by Blood Neutrophils. Cancers 2024, 16, 1767. https://doi.org/10.3390/cancers16091767
Wendlinger S, Wohlfarth J, Siedel C, Kreft S, Kilian T, Junker S, Schmid L, Sinnberg T, Dischinger U, Heppt MV, et al. Susceptibility of Melanoma Cells to Targeted Therapy Correlates with Protection by Blood Neutrophils. Cancers. 2024; 16(9):1767. https://doi.org/10.3390/cancers16091767
Chicago/Turabian StyleWendlinger, Simone, Jonas Wohlfarth, Claudia Siedel, Sophia Kreft, Teresa Kilian, Sarah Junker, Luisa Schmid, Tobias Sinnberg, Ulrich Dischinger, Markus V. Heppt, and et al. 2024. "Susceptibility of Melanoma Cells to Targeted Therapy Correlates with Protection by Blood Neutrophils" Cancers 16, no. 9: 1767. https://doi.org/10.3390/cancers16091767
APA StyleWendlinger, S., Wohlfarth, J., Siedel, C., Kreft, S., Kilian, T., Junker, S., Schmid, L., Sinnberg, T., Dischinger, U., Heppt, M. V., Wistuba-Hamprecht, K., Meier, F., Erpenbeck, L., Neubert, E., Goebeler, M., Gesierich, A., Schrama, D., Kosnopfel, C., & Schilling, B. (2024). Susceptibility of Melanoma Cells to Targeted Therapy Correlates with Protection by Blood Neutrophils. Cancers, 16(9), 1767. https://doi.org/10.3390/cancers16091767






