Hypnosis-Assisted Awake Craniotomy for Eloquent Brain Tumors: Advantages and Pitfalls
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preoperative Anesthetic Management
2.2. Intraoperative Anesthetic and Surgical Procedures
2.3. Specificities of HAAC Technique
2.4. Specificities of MAC Technique
2.5. Postoperative Management, Neuropsychological Tests, and Periprocedural Questionnaires
- -
- -
- a Visual Analog Scale (VAS) was used to quantify stress, comfort, and pain preoperatively, during surgery, and at the end of the procedure;
- -
- the Impact of Events Scale-Revised (IES-R) [39], the Peritraumatic Distress Inventory (PDI) [40], and the Peritraumatic Dissociation Experiences Questionnaire (PDEQ) [41] were used 3 to 7 days after the procedure to retrospectively assess the psychological impact and level of dissociation at the time of surgery;
- -
- a series of 19 questions designed by our team to assess the level of satisfaction with surgical, anesthetic, and neuropsychological management, identify painful memories during the surgical procedure, test orientation in time and space, and establish whether patients would undergo a second surgery under similar conditions (Supplementary file S1).
2.6. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hervey-Jumper, S.L.; Berger, M.S. Technical nuances of awake brain tumor surgery and the role of maximum safe resection. J. Neurosurg. Sci. 2015, 59, 351–360. [Google Scholar]
- Hervey-Jumper, S.L.; Berger, M.S. Maximizing safe resection of low- and high-grade glioma. J. Neurooncol. 2016, 130, 269–282. [Google Scholar] [PubMed]
- Gulati, S.; Jakola, A.S.; Nerland, U.S.; Weber, C.; Solheim, O. The risk of getting worse: Surgically acquired deficits, perioperative complications, and functional outcomes after primary resection of glioblastoma. World Neurosurg. 2011, 76, 572–579. [Google Scholar] [PubMed]
- Klein, M.; Duffau, H.; De Witt Hamer, P.C. Cognition and resective surgery for diffuse infiltrative glioma: An overview. J. Neurooncol. 2012, 108, 309–318. [Google Scholar] [PubMed]
- Del Bene, M.; Perin, A.; Casali, C.; Legnani, F.; Saladino, A.; Mattei, L.; Vetrano, I.G.; Saini, M.; DiMeco, F.; Prada, F. Advanced Ultrasound Imaging in Glioma Surgery: Beyond Gray-Scale B-mode. Front. Oncol. 2018, 8, 576. [Google Scholar] [CrossRef] [PubMed]
- Moiraghi, A.; Prada, F.; Delaidelli, A.; Guatta, R.; May, A.; Bartoli, A.; Saini, M.; Perin, A.; Walchli, T.; Momjian, S.; et al. Navigated Intraoperative 2-Dimensional Ultrasound in High-Grade Glioma Surgery: Impact on Extent of Resection and Patient Outcome. Oper. Neurosurg. 2020, 18, 363–373. [Google Scholar]
- Prada, F.; Ciocca, R.; Corradino, N.; Gionso, M.; Raspagliesi, L.; Vetrano, I.G.; Doniselli, F.; Del Bene, M.; DiMeco, F. Multiparametric Intraoperative Ultrasound in Oncological Neurosurgery: A Pictorial Essay. Front. Neurosci. 2022, 16, 881661. [Google Scholar]
- Verburg, N.; de Witt Hamer, P.C. State-of-the-art imaging for glioma surgery. Neurosurg. Rev. 2021, 44, 1331–1343. [Google Scholar] [PubMed]
- Krivosheya, D.; Prabhu, S.S. Combining Functional Studies with Intraoperative MRI in Glioma Surgery. Neurosurg. Clin. N. Am. 2017, 28, 487–497. [Google Scholar]
- Leroy, H.A.; Delmaire, C.; Le Rhun, E.; Drumez, E.; Lejeune, J.P.; Reyns, N. High-field intraoperative MRI and glioma surgery: Results after the first 100 consecutive patients. Acta Neurochir. 2019, 161, 1467–1474. [Google Scholar]
- Azad, T.D.; Duffau, H. Limitations of functional neuroimaging for patient selection and surgical planning in glioma surgery. Neurosurg. Focus 2020, 48, E12. [Google Scholar] [PubMed]
- Duffau, H. New Philosophy, Clinical Pearls, and Methods for Intraoperative Cognition Mapping and Monitoring “a la carte” in Brain Tumor Patients. Neurosurgery 2021, 88, 919–930. [Google Scholar] [PubMed]
- Duffau, H. Oncological and functional neurosurgery: Perspectives for the decade regarding diffuse gliomas. Rev. Neurol. 2023, 179, 437–448. [Google Scholar] [PubMed]
- Szelenyi, A.; Bello, L.; Duffau, H.; Fava, E.; Feigl, G.C.; Galanda, M.; Neuloh, G.; Signorelli, F.; Sala, F. Workgroup for Intraoperative Management in Low-Grade Glioma Surgery within the European Low-Grade Glioma N: Intraoperative electrical stimulation in awake craniotomy: Methodological aspects of current practice. Neurosurg. Focus 2010, 28, E7. [Google Scholar] [PubMed]
- Rossi, M.; Conti Nibali, M.; Vigano, L.; Puglisi, G.; Howells, H.; Gay, L.; Sciortino, T.; Leonetti, A.; Riva, M.; Fornia, L.; et al. Resection of tumors within the primary motor cortex using high-frequency stimulation: Oncological and functional efficiency of this versatile approach based on clinical conditions. J. Neurosurg. 2019, 1–13. [Google Scholar] [PubMed]
- Sarubbo, S.; Tate, M.; De Benedictis, A.; Merler, S.; Moritz-Gasser, S.; Herbet, G.; Duffau, H. Mapping critical cortical hubs and white matter pathways by direct electrical stimulation: An original functional atlas of the human brain. Neuroimage 2020, 205, 116237. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Puglisi, G.; Conti Nibali, M.; Vigano, L.; Sciortino, T.; Gay, L.; Leonetti, A.; Zito, P.; Riva, M.; Bello, L. Asleep or awake motor mapping for resection of perirolandic glioma in the nondominant hemisphere? Development and validation of a multimodal score to tailor the surgical strategy. J. Neurosurg. 2022, 136, 16–29. [Google Scholar] [PubMed]
- De Witt Hamer, P.C.; Robles, S.G.; Zwinderman, A.H.; Duffau, H.; Berger, M.S. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: A meta-analysis. J. Clin. Oncol. 2012, 30, 2559–2565. [Google Scholar] [PubMed]
- Sanai, N.; Mirzadeh, Z.; Berger, M.S. Functional outcome after language mapping for glioma resection. N. Engl. J. Med. 2008, 358, 18–27. [Google Scholar] [CrossRef]
- Sacko, O.; Lauwers-Cances, V.; Brauge, D.; Sesay, M.; Brenner, A.; Roux, F.E. Awake craniotomy vs surgery under general anesthesia for resection of supratentorial lesions. Neurosurgery 2011, 68, 1192–1198; discussion 1198–1199. [Google Scholar]
- Moiraghi, A.; Roux, A.; Peeters, S.; Pelletier, J.B.; Baroud, M.; Trancart, B.; Oppenheim, C.; Lechapt, E.; Benevello, C.; Parraga, E.; et al. Feasibility, Safety and Impact on Overall Survival of Awake Resection for Newly Diagnosed Supratentorial IDH-Wildtype Glioblastomas in Adults. Cancers 2021, 13, 2911. [Google Scholar] [PubMed]
- Eseonu, C.I.; ReFaey, K.; Garcia, O.; John, A.; Quinones-Hinojosa, A.; Tripathi, P. Awake Craniotomy Anesthesia: A Comparison of the Monitored Anesthesia Care and Asleep-Awake-Asleep Techniques. World Neurosurg. 2017, 104, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Beez, T.; Boge, K.; Wager, M.; Whittle, I.; Fontaine, D.; Spena, G.; Braun, S.; Szelenyi, A.; Bello, L.; Duffau, H.; et al. Tolerance of awake surgery for glioma: A prospective European Low Grade Glioma Network multicenter study. Acta Neurochir. 2013, 155, 1301–1308. [Google Scholar] [PubMed]
- Wahab, S.S.; Grundy, P.L.; Weidmann, C. Patient experience and satisfaction with awake craniotomy for brain tumours. Br. J. Neurosurg. 2011, 25, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, T.A.; Bala, A.; Piwowarska, J.; Podgorska, A.; Olejnik, A.; Koczyk, K.; Marchel, A. Monitored Anesthesia Care Protocol for Awake Craniotomy and Patient’s Perspective on the Procedure. World Neurosurg. 2023, 170, e151–e158. [Google Scholar] [CrossRef] [PubMed]
- Whittle, I.R.; Midgley, S.; Georges, H.; Pringle, A.M.; Taylor, R. Patient perceptions of "awake" brain tumour surgery. Acta Neurochir. 2005, 147, 275–277; discussion 277. [Google Scholar] [PubMed]
- Milian, M.; Luerding, R.; Ploppa, A.; Decker, K.; Psaras, T.; Tatagiba, M.; Gharabaghi, A.; Feigl, G.C. “Imagine your neighbor mows the lawn”: A pilot study of psychological sequelae due to awake craniotomy: Clinical article. J. Neurosurg. 2013, 118, 1288–1295. [Google Scholar] [PubMed]
- Starowicz-Filip, A.; Prochwicz, K.; Myszka, A.; Krzyzewski, R.; Stachura, K.; Chrobak, A.A.; Rajtar-Zembaty, A.M.; Betkowska-Korpala, B.; Kwinta, B. Subjective experience, cognitive functioning and trauma level of patients undergoing awake craniotomy due to brain tumor-Preliminary study. Appl. Neuropsychol. Adult 2022, 29, 983–992. [Google Scholar] [CrossRef]
- Hejrati, N.; Spieler, D.; Samuel, R.; Regli, L.; Weyerbrock, A.; Surbeck, W. Conscious Experience and Psychological Consequences of Awake Craniotomy. World Neurosurg. 2019, 129, e381–e386. [Google Scholar]
- Hansen, E.; Brawanski, A. “Awake-awake” or “conscious sedation” for awake craniotomies? Acta Neurochir. 2014, 156, 1495. [Google Scholar]
- Zemmoura, I.; Fournier, E.; El-Hage, W.; Jolly, V.; Destrieux, C.; Velut, S. Hypnosis for Awake Surgery of Low-grade Gliomas: Description of the Method and Psychological Assessment. Neurosurgery 2016, 78, 53–61. [Google Scholar] [PubMed]
- Frati, A.; Pesce, A.; Palmieri, M.; Iasanzaniro, M.; Familiari, P.; Angelini, A.; Salvati, M.; Rocco, M.; Raco, A. Hypnosis-Aided Awake Surgery for the Management of Intrinsic Brain Tumors versus Standard Awake-Asleep-Awake Protocol: A Preliminary, Promising Experience. World Neurosurg. 2019, 121, e882–e891. [Google Scholar] [CrossRef]
- Hansen, E.; Bejenke, C. Negative and positive suggestions in anaesthesia: Improved communication with anxious surgical patients. Anaesthesist 2010, 59, 199–202, 196–204, 199–208. [Google Scholar]
- Hansen, E.; Seemann, M.; Zech, N.; Doenitz, C.; Luerding, R.; Brawanski, A. Awake craniotomies without any sedation: The awake-awake-awake technique. Acta Neurochir. 2013, 155, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Raabe, A.; Beck, J.; Schucht, P.; Seidel, K. Continuous dynamic mapping of the corticospinal tract during surgery of motor eloquent brain tumors: Evaluation of a new method. J. Neurosurg. 2014, 120, 1015–1024. [Google Scholar]
- McAuliffe, N.; Nicholson, S.; Rigamonti, A.; Hare, G.M.T.; Cusimano, M.; Garavaglia, M.; Pshonyak, I.; Das, S. Awake craniotomy using dexmedetomidine and scalp blocks: A retrospective cohort study. Can. J. Anaesth. 2018, 65, 1129–1137. [Google Scholar]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta. Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.S.; Marmar, C.R. The Impact of Event Scale—Revised. In Assessing Psychological Trauma and PTSD; JWTM: Guilford, UK, 1996; pp. 399–411. [Google Scholar]
- Brunet, A.; Weiss, D.S.; Metzler, T.J.; Best, S.R.; Neylan, T.C.; Rogers, C.; Fagan, J.; Marmar, C.R. The Peritraumatic Distress Inventory: A proposed measure of PTSD criterion A2. Am. J. Psychiatry 2001, 158, 1480–1485. [Google Scholar] [CrossRef]
- Birmes, P.; Brunet, A.; Benoit, M.; Defer, S.; Hatton, L.; Sztulman, H.; Schmitt, L. Validation of the Peritraumatic Dissociative Experiences Questionnaire self-report version in two samples of French-speaking individuals exposed to trauma. Eur. Psychiatry 2005, 20, 145–151. [Google Scholar]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [PubMed]
- Ferracci, F.X.; Duffau, H. Improving surgical outcome for gliomas with intraoperative mapping. Expert Rev. Neurother. 2018, 18, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Natalini, D.; Ganau, M.; Rosenkranz, R.; Petrinic, T.; Fitzgibbon, K.; Antonelli, M.; Prisco, L. Comparison of the Asleep-Awake-Asleep Technique and Monitored Anesthesia Care During Awake Craniotomy: A Systematic Review and Meta-analysis. J. Neurosurg. Anesthesiol. 2022, 34, e1–e13. [Google Scholar] [PubMed]
- Frost, E.A.; Booij, L.H. Anesthesia in the patient for awake craniotomy. Curr. Opin. Anaesthesiol. 2007, 20, 331–335. [Google Scholar] [PubMed]
- Wajer, I.; Kal, J.; Robe, P.A.; van Zandvoort, M.J.E.; Ruis, C. Awake craniotomy does not lead to increased psychological complaints. Acta Neurochir. 2023, 165, 2505–2512. [Google Scholar] [PubMed]
- Milian, M.; Tatagiba, M.; Feigl, G.C. Patient response to awake craniotomy—A summary overview. Acta Neurochir. 2014, 156, 1063–1070. [Google Scholar] [PubMed]
- Pesce, A.; Palmieri, M.; Cofano, F.; Iasanzaniro, M.; Angelini, A.; D’Andrea, G.; Monticelli, M.; Zeppa, P.; Santonio, F.V.; Zenga, F.; et al. Standard awake surgery versus hypnosis aided awake surgery for the management of high grade gliomas: A non-randomized cohort comparison controlled trial. J. Clin. Neurosci. 2020, 77, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Weerink, M.A.S.; Struys, M.; Hannivoort, L.N.; Barends, C.R.M.; Absalom, A.R.; Colin, P. Clinical Pharmacokinetics and Pharmacodynamics of Dexmedetomidine. Clin. Pharmacokinet. 2017, 56, 893–913. [Google Scholar] [PubMed]
- Holler, M.; Koranyi, S.; Strauss, B.; Rosendahl, J. Efficacy of Hypnosis in Adults Undergoing Surgical Procedures: A meta-analytic update. Clin. Psychol. Rev. 2021, 85, 102001. [Google Scholar]
- Zaccarini, S.; Fernandez, A.; Wolff, A.; Magnusson, L.; Rehberg-Klug, B.; Grape, S.; Schoettker, P.; Berna, C. Hypnosis in the operating room: Are anesthesiology teams interested and well-informed? BMC Anesthesiol. 2023, 23, 287. [Google Scholar] [CrossRef]


| Variable | HAAC Cohort (14 Patients) | MAC Cohort (8 Patients) | p-Value |
|---|---|---|---|
| Mean age +/− SD | 42.7 years +/− 9.3 | 49.9 years +/− 11.1 | 0.12 |
| Women | 7 (50%) | 4 (50%) | 1 |
Tumor localization:
Left hemisphere | 8 1 1 1 2 1 12 | 2 1 3 2 0 0 8 | 0.17 1 0.11 0.53 0.51 1 0.51 |
Diagnosis:
| 7 2 3 2 | 2 1 5 0 | 0.38 1 0.08 0.51 |
Recurrent tumor
| 4 (28.6%) 1/4 | 3 (37.5%) 3/3 | 0.67 0.14 |
| Median KPS +/− IQR | 100 +/− 10 | 90 +/− 10 | 0.6 |
| Complete resection | 9 (64.3%) | 4 (50%) | 0.66 |
| Tumor volume +/− SD | 38.7 cm3 +/− 40.2 | 60.6 cm3 +/− 68.3 | 0.43 |
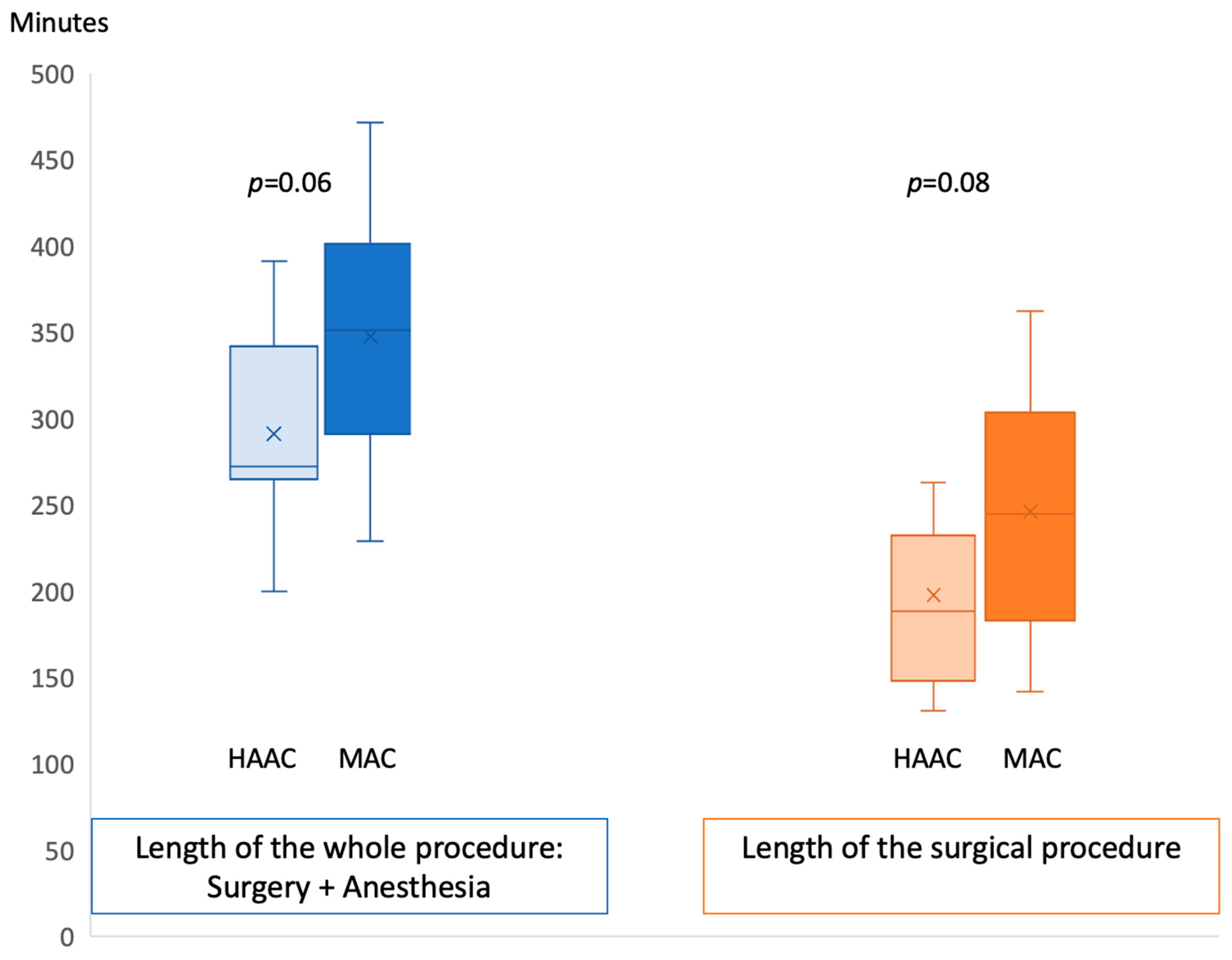
| Mean length of the surgical procedure (with anesthesia time) +/− SD | 291 min, +/− 59 | 352 min, +/− 75 | 0.06 |
| Surgery time +/− SD | 196 min +/− 46.2 | 246 min +/− 74.6 | 0.08 |
| Anesthesia time +/− SD | 43 min +/− 32.5 | 57.7 min +/− 7.8 | 0.25 |
Intraoperative events:
| 0 2 (14.3%) | 0 0 | 1 0.25 |
| Variable | HAAC Cohort | MAC Cohort | p-Value |
|---|---|---|---|
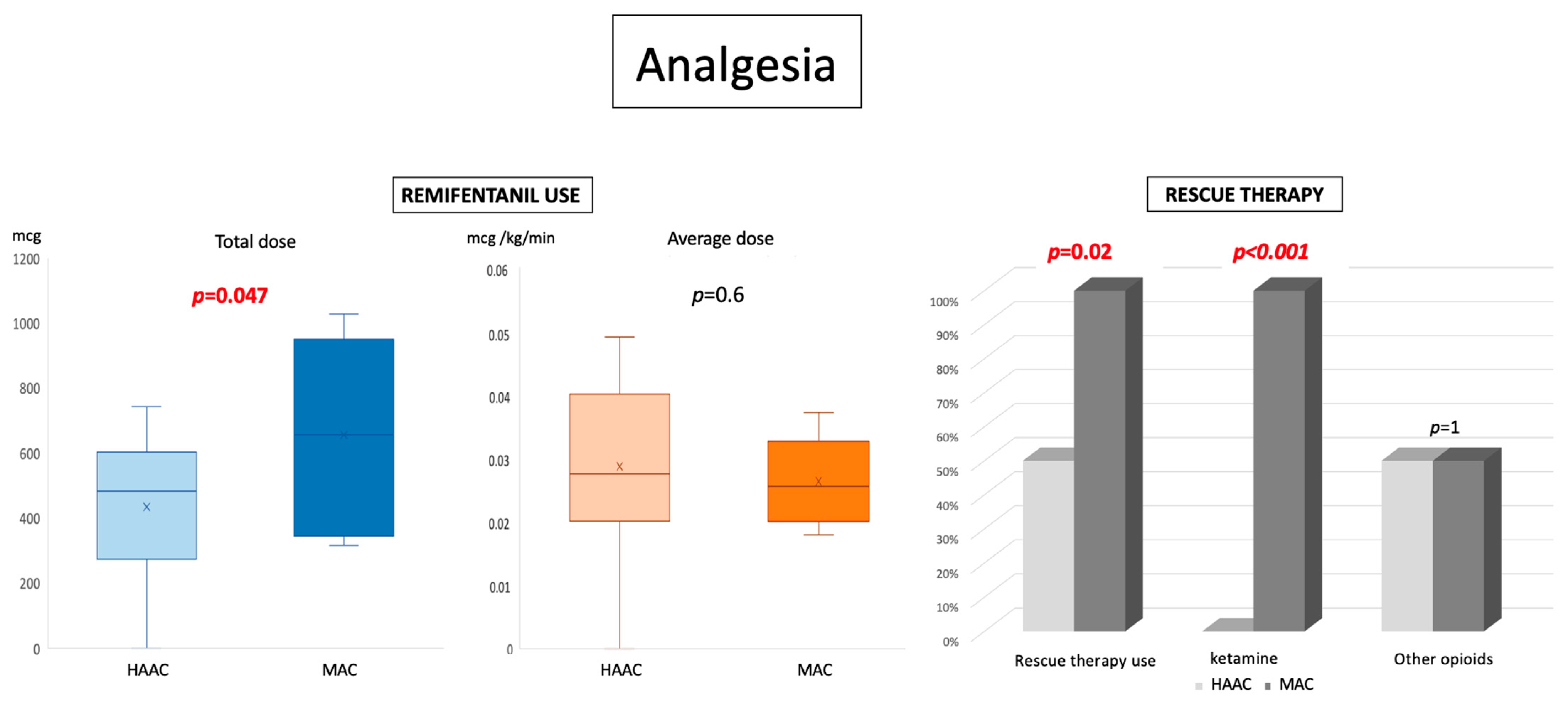
Analgesia
| 436 mcg, +/− 205 0.029 mcg/kg/min +/− 0.013 210 min, +/− 88 7/14 0/14 7/14 | 659 mcg, +/− 289 0.026 mcg/kg/min +/− 0.006 288min, +/− 89 8/8 8/8 4/8 | 0.047 0.63 0.059 0.02 <0.00001 1 |
Hypnotics
| 4/14 96.2 mcg, +/− 85.5 1.3 mcg/kg; +/− 1.2 7/14 93 mcg, +/− 53 0.71 mcg/kg/h, +/− 0.3 | 8/8 1238 mcg, +/− 346 14.8 mcg/kg, +/− 5.3 4/8 128 mcg, +/− 54 0.31 mcg/kg/h, +/− 0.1 | 0.002 0.00008 <0.0001 1 0.32 0.025 |
| Noradrenaline | 0/14 | 4/8 | 0.0017 |
| Variable | HAAC Cohort | MAC Cohort | p-Value |
|---|---|---|---|
| Preoperative depression confirmed at HADS Preoperative anxiety confirmed at HADS | 2/11 (18%) 7/11 (63.6%) | 0/7 (0%) 2/7 (28.6%) | 0.52 0.33 |
| High perceived stress preoperatively (PSS) Mean VAS for preoperative stress +/− SD Mean VAS for perioperative stress +/− SD Mean VAS for postoperative stress +/− SD | 0/10 (0%) 5.8 +/− 2.8 5.4 +/− 3.01 3.54 +/− 2.44 | 1/6 (17%) 3 +/− 2.83 2.7 +/− 2.8 5.2 +/− 1.72 | 0.37 0.09 0.09 0.15 |
| Mean perioperative pain (mean +/− SD) Maximal perioperative pain (mean +/− SD) | 3.8 +/− 2.32 7.25 +/− 2.8 | 0.9 +/− 1.52 2.7 +/− 2.86 | 0.03 0.009 |
| Mean perioperative discomfort (mean +/− SD) | 4.9 +/− 3.2 | 3.6 +/− 3.8 | 0.5 |
| Posttraumatic events distress: Pathological IES-R Pathological PDI Pathological PDEQ | 3/10 (30%) 1/10 (10%) 6/10 (60%) | 0/7 (0%) 2/8 (25%) 4/8 (50%) | 0.2 0.5 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cossu, G.; Vandenbulcke, A.; Zaccarini, S.; Gaudet, J.G.; Hottinger, A.F.; Rimorini, N.; Potie, A.; Beaud, V.; Guerra-Lopez, U.; Daniel, R.T.; et al. Hypnosis-Assisted Awake Craniotomy for Eloquent Brain Tumors: Advantages and Pitfalls. Cancers 2024, 16, 1784. https://doi.org/10.3390/cancers16091784
Cossu G, Vandenbulcke A, Zaccarini S, Gaudet JG, Hottinger AF, Rimorini N, Potie A, Beaud V, Guerra-Lopez U, Daniel RT, et al. Hypnosis-Assisted Awake Craniotomy for Eloquent Brain Tumors: Advantages and Pitfalls. Cancers. 2024; 16(9):1784. https://doi.org/10.3390/cancers16091784
Chicago/Turabian StyleCossu, Giulia, Alberto Vandenbulcke, Sonia Zaccarini, John G. Gaudet, Andreas F. Hottinger, Nina Rimorini, Arnaud Potie, Valerie Beaud, Ursula Guerra-Lopez, Roy T. Daniel, and et al. 2024. "Hypnosis-Assisted Awake Craniotomy for Eloquent Brain Tumors: Advantages and Pitfalls" Cancers 16, no. 9: 1784. https://doi.org/10.3390/cancers16091784
APA StyleCossu, G., Vandenbulcke, A., Zaccarini, S., Gaudet, J. G., Hottinger, A. F., Rimorini, N., Potie, A., Beaud, V., Guerra-Lopez, U., Daniel, R. T., Berna, C., & Messerer, M. (2024). Hypnosis-Assisted Awake Craniotomy for Eloquent Brain Tumors: Advantages and Pitfalls. Cancers, 16(9), 1784. https://doi.org/10.3390/cancers16091784






