Simple Summary
Mycosis fungoides (MF) is a primary T-cell lymphoma that manifests on the skin. Using the American Association for Cancer Research (AACR) Project Genomics, Evidence, Neoplasia, Information, Exchange (GENIE), a publicly accessible genomic repository, this study explores the mutational profile of MF in a large, diverse patient cohort. This research uncovers the most common somatic mutations in FAT1, KMT2D, TP53, JAK3, and SETBP1, which disrupt key pathways controlling cell adhesion, chromatin structure, p53 activity, and cytokine signaling. These alterations provide insight into disease mechanisms and reveal potential diagnostic, prognostic, and therapeutic targets. Significant co-mutation patterns suggest cooperation between signaling and epigenetic pathways, while the identification of mutation enrichments across racial groups provides new insight into potential genetic and environmental influences on disease biology. These findings highlight the potential for the development of biomarker- and patient-targeted therapeutic strategies in MF.
Abstract
Background: Mycosis fungoides (MF) is a rare cutaneous T-cell lymphoma (CTCL) that presents clinically on the skin as patches, plaques, or tumors. MF often mimics benign inflammatory conditions which leads to difficult and delayed diagnosis, worsening prognosis despite available treatment options. This study seeks to improve diagnosis and identify potential therapeutic targets by better characterizing MF’s genetic landscape using the AACR Project GENIE dataset. Methods: Retrospective analysis of MF cases was conducted using the AACR Project GENIE database accessed from cBioPortal (v17.0-public) on 5 June 2025. Data analysis included identifying recurrent somatic mutations, assessing patterns of mutation co-occurrence and mutual exclusivity using non-parametric tests with Benjamini–Hochberg False Discovery Rate (FDR) correction, and examining enrichment of specific mutations based on sex and race, with significance of p < 0.05. Results: Recurrent alterations included FAT1 (28.2%), KMT2D (19.2%), TP53 (13.5%), JAK3 (11.5%), and SETBP1 (11.5%), highlighting the role of Wnt signaling, epigenetic dysregulation, the p53 pathway, and JAK/STAT signaling in MF pathogenesis. Mutations with significant co-occurrence and enrichment in White, Black, and Asian populations were identified. Conclusions: The findings of this study provide a comprehensive understanding of MF’s molecular profile. The discovery of commonly mutated pathways (Wnt, p53, JAK/STAT, and epigenetic regulators) suggests potential targets for the development of future therapies. Furthermore, the enrichment of certain mutations based on race and patterns of alteration co-occurrence offer possibilities for patient-tailored treatment approaches.
1. Introduction
Mycosis fungoides (MF) is the most common subtype of cutaneous T-cell lymphoma (CTCL), accounting for approximately 50% of cases [1]. MF is classified as a primary cutaneous lymphoma arising from skin-homing CD4⁺ T cells, characterized histopathologically by epidermotropism of atypical helper T lymphocytes [2,3,4]. MF progresses in stages from erythematous patches to plaques and tumors, with advanced stages spreading to lymph nodes, blood, and visceral organs, significantly decreasing survival [3,5].
MF is a rare malignancy with an estimated annual incidence of 6.4 cases per million people [6]. It primarily affects individuals over age 50 and is more common in men, with a male-to-female ratio of approximately 2:1 [7,8]. While more common in African Americans and Hispanics, MF remains most prevalent among Caucasians due to demographic size [9,10]. Risk factors include chronic smoking, obesity, eczema, and occupational exposures such as farming, woodworking, and painting [10,11].
Diagnosing MF remains a clinical challenge, particularly in early stages when it mimics benign inflammatory dermatoses. Skin biopsy is the gold standard [3] and is supported by ancillary tests such as T-cell receptor gene rearrangement, flow cytometry, and immunohistochemical markers including TOX, CCR4, and CLA [12,13]. Disease staging follows the TNMB system, which is crucial in guiding treatment decisions [14]. Early-stage MF (IA–IIA) is typically managed with skin-directed therapies like topical corticosteroids, phototherapy, and localized radiation, while systemic agents, retinoids, interferon-α, HDAC inhibitors, and monoclonal antibodies, are reserved for advanced stages (IIB–IV) [15,16]. Despite available treatments, prognosis remains poor, with a 10-year survival of 42% in tumor-stage MF and a 5-year survival of 27% in advanced CTCL [5,17].
Over the past decade, genomic studies have identified recurrent alterations in tumor suppressor genes (TP53, FAT1, KMT2D, TET2) and signaling molecules (JAK3, STAT3) [5,17,18]. Epigenetic dysregulation also plays a key role in MF, with frequent mutations in chromatin modifiers (SETD2, DNMT3A) and methylation regulators (TET2, SOCS1) [1,13]. These findings implicate disruptions in DNA repair, apoptosis, and cytokine signaling in MF pathogenesis. However, small sample sizes, inconsistent methodologies, limited cohort diversity [5,17,18], low tumor cell content in lesional skin, and diagnostic overlap with Sézary syndrome [19] hinder past efforts, leaving MF’s genomic landscape incomplete and underscoring the need for deeper molecular profiling.
This study seeks to address the under-characterization of MF by evaluating its mutational landscape using a large, diverse cohort derived from a publicly accessible genomic repository. By identifying recurrent mutations and disrupted pathways, our findings aim to expand the molecular framework of MF to improve diagnostic precision, risk stratification, and guide more effective treatment strategies.
2. Materials and Methods
This study was deemed exempt from institutional review board oversight by Creighton University (Phoenix, AZ, USA) because it involved analysis of de-identified, publicly available data from the AACR Project GENIE database. Clinical and genomic information from 2017 onward was accessed through the cBioPortal platform (version 17.0-public) on 5 June 2025. The AACR GENIE database compiles genomic sequencing data from 19 international cancer centers, reflecting a range of sequencing methodologies. Genomic sequencing in the AACR GENIE database is conducted using unbiased whole-genome/exome sequencing or targeted panels with up to 555 genes. In this dataset, all samples were sequenced using targeted next-generation sequencing (NGS) panels, with the majority processed using DFCI-ONCOPANEL-3 (32%), MSK-IMPACT-HEME-468 (20%), and MSK-IMPACT-HEME-400 (14%). No samples in this cohort were sequenced using whole-exome sequencing (WES) or whole-genome sequencing (WGS). Sequencing depth typically exceeded 500×, consistent with expectations for targeted panels. Regarding sample composition, approximately 65% of specimens were derived from primary tumors and 100% of cases were tumor-only samples. Matched tumor-normal information was not explicitly annotated, limiting the ability to exclude germline variants across the cohort.
Although each participating institution uses its own internal pipeline for mutation calling and annotation, all data were standardized according to GENIE harmonization protocols via Genome NEXUS. This includes commonly used tools such as GATK for variant detection and ANNOVAR for annotation, though specific software and versions vary by institution. It is important to note that differences in bioinformatic pipelines may exist both across and within contributing institutions. While therapeutic response and clinical outcome data are available for certain cancer types in the database, treatment details were not documented for MF.
Our patient cohort included individuals diagnosed with MF, selected from a broader dataset of mature T-cell and natural killer (NK) cell neoplasms. Tumor samples were categorized as either primary (from the original tumor location) or metastatic (from secondary sites). To assess differences in alteration frequencies per gene between primary and metastatic groups, a chi-squared test was applied according to the prevalence of mutations in each group. The dataset contained genetic information, histologic classification, and patient demographics, including sex, age, and race. Although targeted sequencing panels varied across institutions, most captured commonly mutated cancer-related genes like FAT1, KMT2D, and TP53. Genes without established clinical relevance were typically excluded from panel designs, and mutations classified as synonymous were omitted. Structural variants and copy number alterations (CNAs) were not analyzed. Patients with incomplete data were excluded from this study as well. Accounting for factors like panel size helps mitigate platform variability and improves comparability across different sequencing assays.
All analyses were carried out using R/R Studio (R Foundation for Statistical Computing, Boston, MA, USA), with a p-value < 0.05 considered statistically significant. For continuous variables, results are expressed as mean values ± standard deviations (SD). Categorical data are described by absolute counts and percentages. The chi-squared test was used to explore associations between categorical variables. To compare continuous variables between two independent groups, data distribution was first assessed. Normally distributed variables were compared using a two-sided Student’s t-test, while non-normally distributed variables were analyzed with the Mann–Whitney U test. To account for the possibility of false positives arising from multiple hypothesis testing, adjustments were applied using the Benjamini–Hochberg false discovery rate (FDR) correction (q < 0.05) for all relevant analyses, including the racial enrichment and co-occurrence tests. Confidence intervals for co-occurrence proportions were calculated using the Clopper–Pearson (exact) method for binomial data, which provides conservative but widely accepted estimates, particularly appropriate for small sample sizes.
Mutation data were obtained from the AACR GENIE harmonized MAF (mutation annotation format) files, which ensure consistent variant annotations, such as standardized gene names and protein changes, across institutions. For this analysis, only nonsynonymous alterations, including missense, truncating, in frame, and splice-site mutations, were retained. Variants were further filtered based on a minimum variant allele frequency (VAF) threshold of 5% and sequencing depth of at least 100×.
3. Results
3.1. Mycosis Fungoides Patient Demographics
Given the relatively small number of MF cases in available genomic datasets, adult, pediatric, primary, and metastatic tumor specimens were grouped together for all analyses. Details regarding patient demographics can be found in Table 1. A total of 156 tumor samples from 147 individuals were considered. Of these patients, 83 (56.5%) were male and 61 (41.5%) were female. Analysis of ethnicity revealed that 129 individuals (87.8%) were non-Hispanic/non-Spanish, 7 (4.8%) were Hispanic or Spanish, and ethnicity was not reported for 11 (7.5%) patients. Racial categorization showed that 111 (75.5%) patients were White, 16 (10.9%) were Black, 9 (6.1%) were Asian, 3 (2.0%) identified with other racial backgrounds, and 7 (4.8%) had unreported racial data. Among the samples, 102 (65.4%) were derived from primary lesions, 11 (7.1%) from metastatic sites, and 31 (19.9%) lacked site annotation.

Table 1.
Mycosis fungoides patient demographics (N = 147).
3.2. Recurrent Somatic Mutations in MF
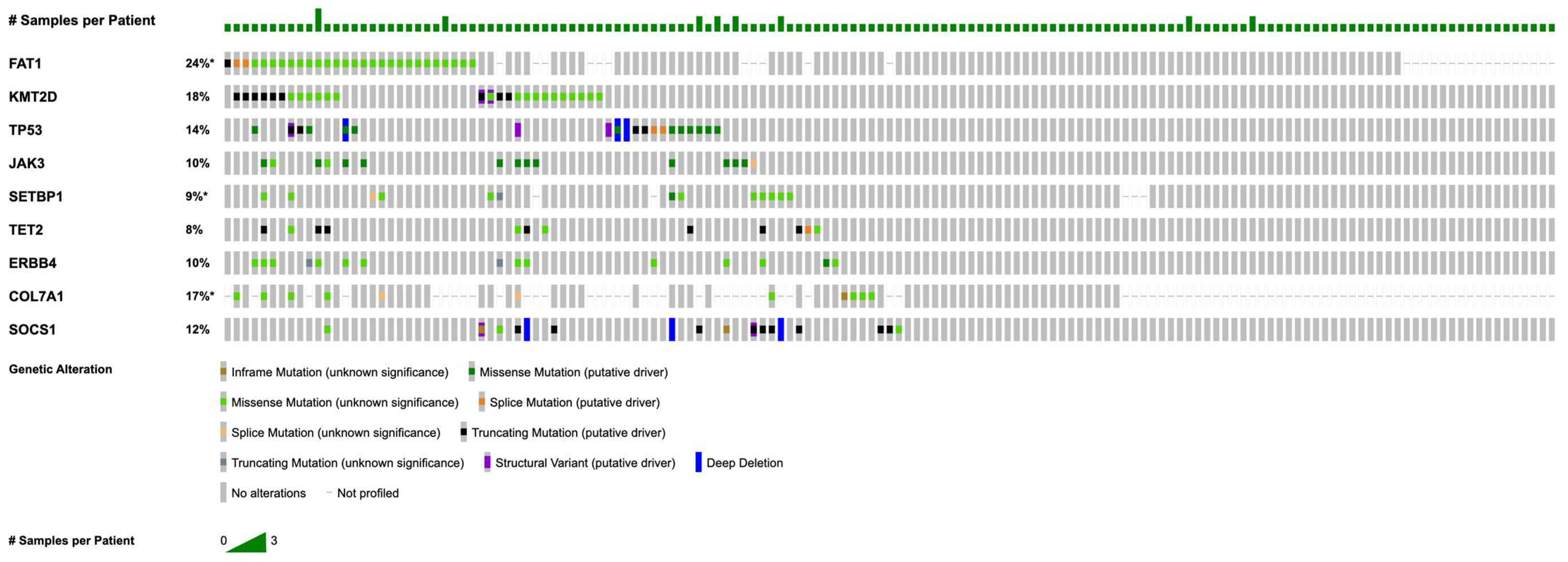
Figure 1 illustrates the most commonly detected somatic alterations within this MF cohort, with detailed frequencies provided in Table 2. The most prevalent mutations were in FAT1 (n = 44; 28.2%), followed by recurrent alterations in KMT2D (n = 30; 19.2%), TP53 (n = 21; 13.5%), JAK3 (n = 18; 11.5%), SETBP1 (n = 18; 11.5%), TET2 (n = 17; 10.9%), ERBB4 (n = 17; 10.9%), COL7A1 (n = 17; 10.9%), and SOCS1 (n = 14; 9.0%).

Figure 1.
OncoPrint of recurrent mutations in mycosis fungoides (for genes with n ≥ 5, coverage ≥ 100×, VAF ≥ 5%). Asterisk (*) denotes incomplete sample profiling for FAT1, SETBP1, and COL7A1. Note that percentages in the OncoPrint may differ slightly from those in Table 2, as the figure displays only samples profiled for all listed genes to create a complete visual matrix.

Table 2.
Mutation frequency in mycosis fungoides.
3.3. Mutation Profiles of Frequently Altered Genes
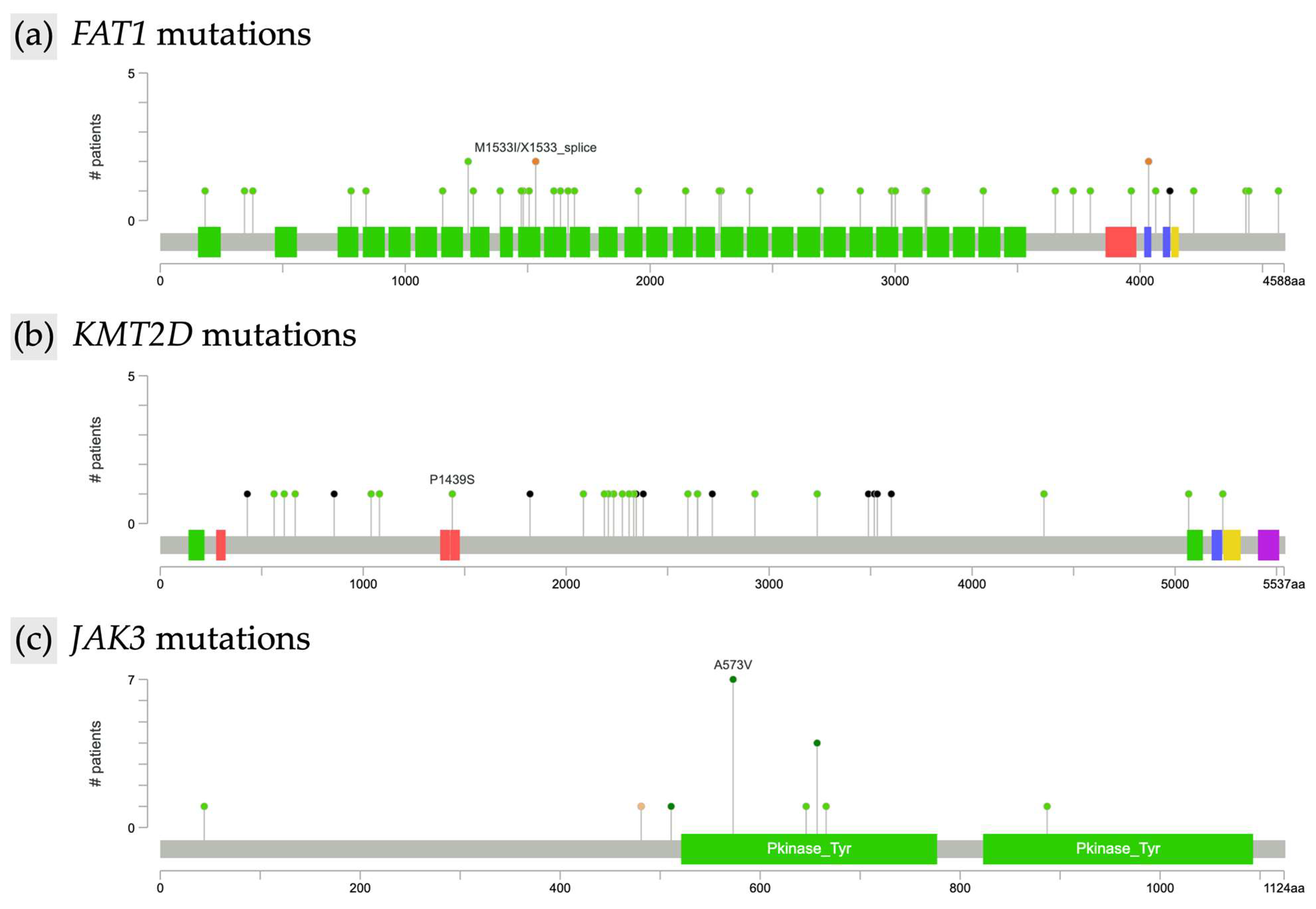
Further analysis characterized the types of mutations within the most frequently altered genes. In FAT1 (n = 44), 41 mutations were missense and three were of other types, all resulting in unique protein changes. FAT1 alterations were scattered across the gene without evidence of recurrent hotspots, as illustrated by the lollipop plot (Figure 2a). In KMT2D (n = 30), identified mutations included 14 missense and 6 nonsense alterations, with all KMT2D mutations leading to unique protein changes. As with FAT1, KMT2D mutations were diffusely distributed along the gene and lacked focal clustering (Figure 2b). TP53 mutations (n = 21) included 15 missense alterations, and all TP53 mutations were unique at the protein level. JAK3 mutations (n = 18) were predominantly missense (17 missense, 1 other type). Notably, recurrent hotspot mutations were identified in JAK3, including eight instances of the A573V alteration and four distinct changes affecting amino acid position R657 (R657Q, R657W, R657G) (Figure 2c). Among SETBP1 mutations (n = 18), 16 were missense; all SETBP1 mutations resulted in unique protein changes. TET2 alterations (n = 17) included 7 missense and 6 nonsense mutations, and all TET2 mutations led to unique protein changes. ERBB4 mutations (n = 17) were primarily missense (n = 15), with two nonsense mutations also identified; all ERBB4 alterations resulted in unique protein changes. Finally, of the COL7A1 mutations (n = 17), 13 were missense, and all COL7A1 mutations were unique at the protein level.
Figure 2.
Lollipop plot of recurrent mutations in mycosis fungoides. (a) FAT1, (b) KMT2D, and (c) JAK3.
3.4. Genetic Differences by Sex and Race
When evaluated by sex, no statistically significant gene enrichments were identified after false discovery rate (FDR) correction. However, race-specific enrichments were observed following FDR correction (q < 0.05). Among Asian patients, alterations in ATP8B1, SHH, SYNE1, OGG1, MDM4, SPINK4, and XPO1 were found exclusively within this group and were significantly enriched (each n = 1; p < 0.001). PRPF40B mutations were detected in both Asian and White patients but were significantly enriched in Asian patients (n = 1; p < 0.001). In Black patients, EME1 alterations were observed exclusively and showed significant enrichment (n = 1; p < 0.001), whereas ADGRA2 mutations were significantly enriched and occurred only in White patients (n = 1; p < 0.001). Racial variations in mutation profiles are summarized in Table 3.

Table 3.
Somatic mutation enrichment by race.
3.5. Co-Occurrence and Mutual Exclusivity of Mutations
Among frequently altered genes, notable co-mutation trends emerged. JAK3 mutations significantly co-occurred with ERBB4 (n = 10/21; p < 0.001), KMT2D (n = 9/34; p < 0.001), SOCS1 (n = 7/25; p < 0.001), FAT1 (n = 7/34; p = 0.004), and TET2 (n = 6/22; p < 0.001). KMT2D alterations were significantly associated with mutations in FAT1 (n = 13/39; p < 0.001), ERBB4 (n = 9/17; p < 0.001), and TET2 (n = 8/33; p < 0.001). ERBB4 mutations also showed frequent co-occurrence with FAT1 (n = 8/36; p = 0.005) and TET2 (n = 6/23; p < 0.001). Lastly, SOCS1 alterations showed co-occurrence with SETBP1 (n = 6/24; p < 0.001) and TET2 (n = 5/25; p = 0.007). These findings are detailed in Table 4. No gene pairs demonstrated statistically significant mutual exclusivity (p > 0.100 for all comparisons).

Table 4.
Significant co-occurring mutations in mycosis fungoides.
4. Discussion
4.1. Genomic Landscape and Demographic Trends
Utilizing the AACR Project GENIE dataset, this study defines the mutational profile of mycosis fungoides (MF). Comprehensive analysis identified the most frequently mutated genes and revealed significant co-occurrence patterns, along with race-specific enrichments of low-frequency alterations. These findings provide new insights into the molecular complexity of MF and highlight the role of disrupted signaling, epigenetic regulation, and immune pathways in its pathogenesis.
Our cohort was predominantly male (56.5%) and White (75.5%), consistent with established MF demographics, which report a higher incidence in middle-aged men, with Caucasians being the most frequently affected group due to their larger representation in the general population [6,8,10]. Following FDR correction, no statistically significant sex-based mutational differences were observed; however, several race-specific mutations emerged. Asian patients exhibited enrichment of ATP8B1, SHH, SYNE1, OGG1, MDM4, SPINK4, XPO1, and PRPF40B, whereas EME1 was significantly enriched in Black patients, and ADGRA2 in White patients. These novel associations suggest underlying genetic or environmental influences that merit further exploration, especially given the disproportionate burden of early-onset and more aggressive disease among African American and Hispanic populations [6,8,9,10]. Nonetheless, these race-specific enrichments must be interpreted with caution. The statistical power of this sub-analysis is severely limited by the small number of patients in the Asian (n = 9) and Black (n = 16) cohorts, making the results susceptible to statistical instability. These associations, while statistically significant after FDR correction, are based on single-event mutations and require validation in larger, more diverse cohorts before any definitive conclusions can be drawn.
4.2. Commonly Mutated Genes and Pathways
Considerable genetic heterogeneity was exhibited by our MF cohort, with recurrent mutations observed in genes regulating cell adhesion, epigenetic modification, cytokine signaling, and DNA repair. The most frequently mutated genes were FAT1 (28.2%), KMT2D (19.2%), TP53 (13.5%), JAK3 (11.5%), SETBP1 (11.5%), TET2 (10.9%), ERBB4 (10.9%), COL7A1 (10.9%), and SOCS1 (9.0%). These findings align with prior studies reporting FAT1, KMT2D, TP53, JAK3, TET2, and SOCS1 as key contributors to MF biology [1,5,13,17,18]. Notably, ERBB4 and COL7A1 are not emphasized in MF literature despite their relatively high prevalence in our cohort and may warrant further exploration as underrecognized contributors to MF.
The most frequently mutated genes in our dataset affect a range of biological processes. TP53 is a central regulator of DNA damage response and apoptosis, while KMT2D, TET2, and SETBP1 are epigenetic modifiers that control chromatin structure and transcription [5,20]. JAK3 and SOCS1 are key components of the JAK/STAT cytokine signaling pathway, regulating cell proliferation and survival [13,17]. FAT1 modulates Wnt signaling and cell adhesion, whereas ERBB4 activates receptor tyrosine kinase pathways that promote cell growth [21,22]. Although the role of COL7A1 in MF remains unclear, it plays an essential role in dermal-epidermal junction integrity by encoding collagen VII. This suggests its alteration may reflect broader changes in skin-specific extracellular matrix biology [23].
While JAK3 demonstrated a focal hotspot at A573V, most mutations, including those in FAT1 and KMT2D, were widely dispersed across their coding regions without evidence of consistent clustering. This diffuse distribution pattern is characteristic of tumor suppressor and chromatin-modifying genes, where loss-of-function arises from diverse disruptive variants rather than single-site activating mutations. The absence of clearly defined driver events in FAT1 and KMT2D aligns with the broader genomic heterogeneity observed in mycosis fungoides, which has been described as lacking a unifying mutational signature [5,19]. Nevertheless, the recurrent involvement of pathways, including p53, epigenetic modifiers, JAK signaling, and Wnt signaling, supports the development of targeted therapeutics.
It is important to recognize that our findings should be interpreted in the context of the broader MF genomic literature, which reveals some variability. For instance, a recent comprehensive study by Fléchon et al. using WES identified recurrent JUNB alterations (13%) as a key driver in MF, a finding not prominent in our cohort. Conversely, our study identified FAT1 as the most frequently mutated gene (28.2%), an alteration not emphasized in their analysis. These discrepancies likely stem from methodological differences: the targeted sequencing panels used in the GENIE database may not be designed to capture the complex structural variants or non-coding mutations affecting JUNB, while the higher prevalence of FAT1 in our cohort may reflect the specific gene inclusion on these panels or differences in cohort characteristics [24].
4.3. p53 Pathway
In our cohort, TP53 mutations were present in 13.5% of cases, aligning with previous literature reporting this mutation in 24% of tumor-stage MF cases [5]. p53 is a tumor suppressor that plays a critical role in genomic surveillance by triggering cell cycle arrest or apoptosis in response to DNA damage, preventing the survival of genetically damaged cells. Loss of TP53 function disrupts G1/S checkpoint control, allowing abnormal cells to evade apoptosis, continue dividing, and accumulate additional mutations. These findings support a model in which TP53 inactivation contributes to genomic instability and the proliferation of pathogenic lymphocytes classically seen in MF [25].
This alteration has been associated with advanced disease, poor prognosis, and chemotherapy resistance in MF and related CTCLs, underscoring its significance in disease progression [5,20]. Its presence, therefore, indicates a more aggressive disease phenotype and may influence the consideration of more intensive therapeutic strategies. Additionally, TP53 mutations may help guide treatment selection towards therapies that induce apoptosis of neoplastic T cells through p53-independent pathways, such as psoralen plus ultraviolet A (PUVA) therapy, which is commonly used in the treatment of early-stage disease and remission maintenance [26].
4.4. Epigenetic Modification
Epigenetic modification is the regulation of gene expression through DNA methylation and chromatin remodeling. Dysregulation of these processes has been recognized as a hallmark of MF [1]. A recent study reported mutations in epigenetic regulators in 45.78% of MF cases, with 37.85% involving genes related to DNA methylation [20]. Similarly, our cohort demonstrated frequent mutations in regulators, including KMT2D (19.2%), TET2 (10.9%), and SETBP1 (11.5%).
KMT2D encodes a histone methyltransferase that facilitates H3K4 methylation and gene activation. Loss-of-function mutations in KMT2D are frequently reported in MF and lead to altered gene expression and tumor suppressor loss. These mutations are associated with poor prognosis in peripheral T-cell lymphomas (PTCLs), with evidence suggesting KMT2D mutations independently predict worse overall survival [1,17,20]. Therapeutically targeting KMT2D holds promise, as emerging preclinical data suggests KDM5 inhibitors may restore H3K4 methylation and suppress tumor growth in KMT2D-mutant lymphomas [27].
TET2 contributes to DNA demethylation and is essential for regulating gene expression and immune cell differentiation. Mutations lead to global DNA hypermethylation, altered gene expression, and contribute to lymphomagenesis and disease progression [28]. In the broader context of T-cell lymphomas, TET2 mutations were identified in 53% of PTCLs and were associated with adverse clinical features, including advanced stage and poor prognosis [20].
SETBP1 mutations have yet to be discussed in the context of MF. However, studies on gain-of-function mutations in myeloid malignancies describe its role in promoting transcriptional dysregulation by upregulating genes associated with development and proliferation, enhancing oncogenic gene expression [29]. Together, these epigenetic alterations reflect an ongoing loss of transcriptional regulation and contribute to the immune dysregulation and cellular plasticity observed in MF.
4.5. JAK/STAT Signaling
Aberrant activation of the JAK/STAT signaling axis has emerged as a central mechanism in MF pathogenesis. In our analysis, JAK3 mutations occurred in 11.5% of samples with a hotspot alteration of A573V, a point mutation previously reported in MF with a variant allele frequency of 41.67%. This specific point mutation has been previously shown to be a gain-of-function alteration that activates IL-2-mediated signaling, promoting malignant T-cell proliferation and survival, thus representing a key oncogenic driver in a subset of MF patients [5]. Our identification of JAK3 mutations is consistent with its established role in MF pathogenesis; however, reported frequencies vary across studies, with 8.3% in MF tumor-stage samples in McGirt et al. and 33.3% in MF patients in Bastidas Torres et al., underscoring the influence of sequencing platforms and cohort selection on the reported genomic landscape [5,17].
Mutations in SOCS1 (9.0%), a negative feedback regulator of JAK signaling, further support dysregulation of this pathway in MF. Previous studies have shown that co-alterations of JAK3 and SOCS1 amplify downstream STAT signaling and enhance tumor cell survival [13,17]. The presence of both activating mutations and loss of tumor suppressors points to a convergent mechanism of sustained cytokine signaling in MF.
Targeting the JAK/STAT pathway has emerged as a promising therapeutic strategy in MF. Cerdulatinib, a reversible ATP-competitive inhibitor of JAK1, JAK2, and JAK3, demonstrated an overall response rate (ORR) of 35% in a Phase II clinical trial for PTCL [NCT01994382]. Notably, MF patients exhibited the highest response, with an ORR of 45% and complete response of 9%. These findings support cerdulatinib as a well-tolerated and effective treatment for MF, particularly in cases harboring JAK3 or SOCS1 alterations [30,31]. Similarly, McGirt et al. demonstrated that three CTCL cell lines were sensitive to the JAK3 inhibitor tofacitinib. Hut-78, harboring an activating JAK3 mutation, was most responsive, though HH and MyLa cells also showed sensitivity to JAK3 inhibition, indicating pathway dependence for growth and survival [5]. In our cohort, activating JAK3 mutations were found in 11.5% of patients, reinforcing the importance of genomic screening to identify MF patients who may benefit from JAK inhibitors as a targeted, mechanism-based treatment strategy.
4.6. Wnt Signaling
FAT1 was the most commonly mutated gene in our cohort (28.2%), highlighting its important role in MF pathogenesis. This finding aligns with previous studies reporting FAT1 alterations in 39% of MF and PTCL cases, where they have been associated with more aggressive disease and poorer overall survival. Loss-of-function mutations in FAT1 result in aberrant Wnt activation, increased proliferation, and tumor progression. Furthermore, these alterations may compromise epithelial integrity, enhancing cellular motility and invasiveness in MF [18].
4.7. Co-Occurrence and Mutual Exclusivity Patterns
Our study identified significant co-occurrence among several genes in MF, including JAK3, KMT2D, ERBB4, FAT1, TET2, SOCS1, and SETBP1, with no evidence of mutually exclusive gene pairs. These co-mutational patterns suggest that MF pathogenesis involves the cooperative disruption of multiple oncogenic pathways rather than isolated single-gene effects.
Prior studies have reported that JAK3 mutations often appear alongside alterations in epigenetic regulators such as KMT2D and TET2, supporting a model of synergistic interactions between cytokine signaling and chromatin remodeling [5]. Additionally, experimental data indicate that concurrent JAK3 activation and SOCS1 loss can amplify downstream STAT signaling, reinforcing the biological plausibility of this co-mutational axis in promoting T-cell proliferation and survival [13]. Separate reports have also emphasized the convergence of alterations affecting histone-modifying enzymes, transcription factors, and signaling molecules, though formal co-occurrence testing was not conducted [17]. Together, these observations are consistent with our findings and suggest that genetic cooperation, particularly between immune signaling and epigenetic dysfunction, is a fundamental feature of MF.
4.8. Limitations
This study has several limitations that should be considered when interpreting the findings. First, the cohort size for MF, although relatively large for a rare cancer, remains limited in statistical power for certain subgroup analyses, particularly those stratified by race or histologic variant. As a result, some findings should be interpreted cautiously and validated in larger, demographically diverse datasets. Second, the cross-sectional nature of the dataset and absence of longitudinal sampling prevent assessment of how mutations evolve during disease progression. This limits our ability to distinguish early driver events from later-occurring passenger mutations. Third, inconsistencies in assay sensitivity, reporting, or gene coverage across platforms could introduce technical bias in mutation frequency estimates. The GENIE consortium aggregates data from multiple centers using different sequencing platforms and pipelines. This heterogeneity means that not all genes were assessed with the same depth or consistency across the entire cohort. For example, a gene included on a large panel at one institution may be absent from a smaller panel at another, potentially leading to an underestimation of its true mutation frequency in the overall cohort. While the GENIE harmonization process mitigates some of these issues, residual batch effects and platform-specific biases cannot be fully excluded. Fourth, the AACR Project GENIE database lacks transcriptomic data, which limits the ability to assess gene expression changes and downstream pathway activation. This is particularly relevant in MF, where overexpression of genes can occur even in the absence of mutations. The lack of transcriptomic and microRNA data also prevents investigation into non-mutational mechanisms of dysregulation, such as altered microRNA expression, which may serve as useful diagnostic or prognostic markers. Fifth, detailed clinical data such as disease stage, risk stratification scores, overall or progression-free survival are not available in the public GENIE dataset for this cohort, precluding any assessment of the prognostic significance of the observed mutations. Sixth, the absence of treatment data in GENIE restricts the ability to evaluate how mutational profiles relate to therapy response or resistance. Without information on therapies received, histologic subtype, or clinical course, we cannot assess how specific genetic alterations may influence treatment outcomes or confound genomic comparisons between primary and relapsed disease. Seventh, while GENIE attempts to exclude redundant samples, the inclusion of multiple tumor specimens from the same patient cannot be entirely ruled out, potentially skewing mutation frequencies. Eighth, all MF cases are analyzed as a single group, without subclassification by disease stage or variant. This aggregation limits the capacity to explore potential subtype-specific mutation patterns or their clinical implications. Ninth, methylation data is not included, preventing analysis of how DNA methylation impacts gene regulation, tumor progression, or treatment response. Tenth, a major limitation of this study is the use of tumor-only sequencing data, which precludes the definitive exclusion of germline variants. Although we applied standard filtering criteria and the GENIE data harmonization pipeline includes filters for common polymorphisms, rare pathogenic germline variants may still be present in the dataset. This could potentially lead to an overestimation of the frequency of certain somatic mutations. Consequently, some of the identified alterations, particularly those with a high variant allele frequency approaching 50% or 100%, could be of germline origin. Therefore, these findings, especially for genes known to harbor pathogenic germline variants, should be interpreted with caution until validated with matched tumor-normal sequencing.
Despite these limitations, this analysis contributes meaningful insights into the molecular profile of MF, highlighting key pathways involved in its pathogenesis and offering potential avenues for future research and therapeutic development.
5. Conclusions
This study enhances the current understanding of MF’s mutational profile. Our findings validate the frequent involvement of FAT1, TP53, JAK3, and epigenetic regulators in MF biology, while identifying novel co-mutation and racial enrichment patterns. These findings underscore the need for studies clarifying the impact of these mutations and for clinical trials evaluating pathway-specific therapies and tailored treatment strategies. Future research should prioritize multi-omic integration, longitudinal sampling, and inclusive cohort design to support the development of precision-guided therapies in MF.
Author Contributions
Conceptualization, B.H. and P.T.S.; methodology, B.H.; validation, G.S.S. and B.H.; formal analysis, G.S.S. and B.H.; data curation, G.S.S. and B.H.; writing—original draft preparation, G.S.S. and B.H.; writing—review and editing, G.S.S., B.H., P.T.S., and A.T.; visualization, G.S.S. and B.H.; supervision, P.T.S. and A.T.; project administration, P.T.S. and A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study because the AACR Project GENIE is an open-access cancer genomics resource that includes de-identified patient information. This significantly reduces potential harm to individuals and removes the requirement for obtaining consent from each participant.
Informed Consent Statement
Patient consent was waived because this study relied solely on de-identified data obtained from the publicly accessible AACR Project GENIE database, which does not require individual informed consent.
Data Availability Statement
The data presented in this study are available from the AACR GENIE Database at https://genie.cbioportal.org/ (accessed on 5 June 2025).
Acknowledgments
The authors declare that they have no acknowledgments.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ATP | Adenosine Triphosphate |
| ADGRA2 | A Disintegrin and G Protein–Coupled Receptor A2 |
| AACR | American Association for Cancer Research |
| ANNOVAR | Annotate Variation |
| CNAs | Chromosomal Copy Number Alterations |
| CLA | Circulating Leukocyte Antigen |
| CD4+ | Cluster of Differentiation 4 Positive |
| CTCL | Cutaneous T-Cell Lymphoma |
| DFCI-ONCOPANEL-3 | Dana-Farber Cancer Institute OncoPanel 3 |
| DNA | Deoxyribonucleic Acid |
| DNMT3A | DNA Methyltransferase 3 Alpha |
| EME1 | EME1 Homolog, DNA Repair Endonuclease Subunit |
| ERBB4 | Epidermal Growth Factor Receptor 4 |
| XPO1 | Exportin 1 |
| FDR | False Discovery Rate Correction |
| FAT1 | FAT Atypical Cadherin 1 |
| GATK | Genome Analysis Toolkit |
| GENIE | Genomics, Evidence, Neoplasia, Information, Exchange |
| NEXUS | Genome Nexus |
| HDAC inhibitors | Histone Deacetylase Inhibitors |
| KDM5 | Histone Lysine Demethylase 5 |
| IL-2 | Interleukin-2 |
| JAK1 | Janus Kinase 1 |
| JAK2 | Janus Kinase 2 |
| JAK3 | Janus Kinase 3 |
| KMT2D | Lysine Methyltransferase 2D |
| MSK | Memorial Sloan Kettering |
| MSK-IMPACT-HEME-400 | Memorial Sloan Kettering Integrated Mutation Profiling of Actionable Cancer Targets Hematologic Panel with 400 genes |
| with 468 genes | MSK-IMPACT-HEME-468 |
| microRNA | MicroRNA |
| MDM4 | Mouse Double Minute 4 Homolog |
| MAF | Mutation Annotation Format |
| MF | Mycosis Fungoides |
| NK | Natural Killer |
| NGS | Next-Generation Sequencing |
| ORR | Objective Response Rate |
| OGG1 | 8-Oxoguanine DNA Glycosylase |
| PTCLs | Peripheral T-Cell Lymphomas |
| P53 | p53 Tumor Suppressor |
| PRPF40B | Pre-mRNA-Processing Factor 40 Homolog B |
| SETBP1 | SET Binding Protein 1 |
| SETD2 | SET Domain Containing 2 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| SHH | Sonic Hedgehog Signaling Molecule |
| SPINK4 | Spink Serine Peptidase Inhibitor Kazal Type 4 |
| SD | Standard Deviation |
| SOCS1 | Suppressor of Cytokine Signaling 1 |
| SYNE1 | Synaptic Nuclear Envelope Protein |
| TOX | Transcription Factor TOX |
| TP53 | Tumor Protein p53 |
| VAF | Variant Allele Frequency |
| WES | Whole-Exome Sequencing |
| WGS | Whole-Genome Sequencing |
| Wnt | Wnt Signaling Pathway |
References
- Elenitoba-Johnson, K.; Wilcox, R. A new molecular paradigm in mycosis fungoides and Sézary syndrome. Semin. Diagn. Pathol. 2017, 34, 15–21. [Google Scholar] [CrossRef]
- Cerroni, L. Mycosis fungoides-clinical and histopathologic features, differential diagnosis, and treatment. Semin. Cutan. Med. Surg. 2018, 37, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Miyashiro, D.; Sanches, J.A. Mycosis fungoides and Sézary syndrome: Clinical presentation, diagnosis, staging, and therapeutic management. Front. Oncol. 2023, 13, 1141108. [Google Scholar] [CrossRef]
- Willemze, R.; Cerroni, L.; Kempf, W.; Berti, E.; Facchetti, F.; Swerdlow, S.H.; Jaffe, E.S. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood 2019, 133, 1703–1714. [Google Scholar] [CrossRef] [PubMed]
- Mcgirt, L.Y.; Jia, P.; Baerenwald, D.A.; Duszynski, R.J.; Dahlman, K.B.; Zic, J.A.; Zwerner, J.P.; Hucks, D.; Dave, U.; Zhao, Z.; et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood 2015, 126, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.B.; Goyal, S.; Niyogusaba, T.; O’leary, C.; Ayers, A.; Tarabadkar, E.S.; Khan, M.K.; Lechowicz, M.J. Clinical Presentation and Outcome Differences Between Black Patients and Patients of Other Races and Ethnicities with Mycosis Fungoides and Sézary Syndrome. JAMA Dermatol. 2022, 158, 1293–1299. [Google Scholar] [CrossRef]
- Morales Suárez-Varela, M.M.; Llopis González, A.; Marquina Vila, A.; Bell, J. Mycosis fungoides: Review of epidemiological observations. Dermatology 2000, 201, 21–28. [Google Scholar] [CrossRef]
- Sun, G.; Berthelot, C.; Li, Y.; Glass, D.A.; George, D.; Pandya, A.; Kurzrock, R.; Duvic, M. Poor prognosis in non-Caucasian patients with early-onset mycosis fungoides. J. Am. Acad. Dermatol. 2008, 60, 231. [Google Scholar] [CrossRef]
- Huang, A.H.; Kwatra, S.G.; Khanna, R.; Semenov, Y.R.; Okoye, G.A.; Sweren, R.J. Racial Disparities in the Clinical Presentation and Prognosis of Patients with Mycosis Fungoides. J. Natl. Med. Assoc. 2019, 111, 633–639. [Google Scholar] [CrossRef]
- Su, C.; Nguyen, K.A.; Bai, H.X.; Cao, Y.; Tao, Y.; Xiao, R.; Karakousis, G.; Zhang, P.J.; Zhang, G. Racial disparity in mycosis fungoides: An analysis of 4495 cases from the US National Cancer Database. J. Am. Acad. Dermatol. 2017, 77, 497. [Google Scholar] [CrossRef]
- Aschebrook-Kilfoy, B.; Cocco, P.; La Vecchia, C.; Chang, E.T.; Vajdic, C.M.; Kadin, M.E.; Spinelli, J.J.; Morton, L.M.; Kane, E.V.; Sampson, J.N.; et al. Medical History, Lifestyle, Family History, and Occupational Risk Factors for Mycosis Fungoides and Sezary Syndrome: The InterLymph Non-Hodgkin Lymphoma Subtypes Project. JNCI Monogr. 2014, 2014, 98–105. [Google Scholar] [CrossRef] [PubMed]
- García-Díaz, N.; Piris, M.Á.; Ortiz-Romero, P.L.; Vaqué, J.P. Mycosis Fungoides and Sézary Syndrome: An Integrative Review of the Pathophysiology, Molecular Drivers, and Targeted Therapy. Cancers 2021, 13, 1931. [Google Scholar] [CrossRef] [PubMed]
- Walia, R.; Yeung, C.C.S. An Update on Molecular Biology of Cutaneous T Cell Lymphoma. Front. Oncol. 2020, 9, 1558. [Google Scholar] [CrossRef]
- Hristov, A.C.; Tejasvi, T.; Wilcox, R.A. Mycosis fungoides and Sézary syndrome: 2019 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2019, 94, 1027–1041. [Google Scholar] [CrossRef]
- Stuver, R.; Geller, S. Advances in the treatment of mycoses fungoides and Sézary syndrome: A narrative update in skin-directed therapies and immune-based treatments. Front. Immunol. 2023, 14, 1284045. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, S.; Hoppe, R.; Prince, H.M. How I treat mycosis fungoides and Sézary syndrome. Blood 2016, 127, 3142–3153. [Google Scholar] [CrossRef]
- Bastidas Torres, A.N.; Cats, D.; Mei, H.; Szuhai, K.; Willemze, R.; Vermeer, M.H.; Tensen, C.P. Genomic analysis reveals recurrent deletion of JAK-STAT signaling inhibitors HNRNPK and SOCS1 in mycosis fungoides. Genes Chromosomes Cancer 2018, 57, 653. [Google Scholar] [CrossRef]
- Laginestra, M.A. Whole exome sequencing reveals mutations in FAT1 tumor suppressor gene clinically impacting on peripheral T-cell lymphoma not otherwise specified. Mod. Pathol. 2020, 33, 179. [Google Scholar] [CrossRef]
- Park, J.; Daniels, J.; Wartewig, T.; Ringbloom, K.G.; Estela Martinez-Escala, M.; Choi, S.; Thomas, J.J.; Doukas, P.G.; Yang, J.; Snowden, C.; et al. Integrated genomic analyses of cutaneous T-cell lymphomas reveal the molecular bases for disease heterogeneity. Blood 2021, 138, 1225–1236. [Google Scholar] [CrossRef]
- Wang, L. TP53 and KMT2D mutations associated with worse prognosis in peripheral T-cell lymphomas. Cancer Med. 2024, 13, e70027. [Google Scholar] [CrossRef]
- Lucas, L.M.; Dwivedi, V.; Senfeld, J.I.; Cullum, R.L.; Mill, C.P.; Piazza, J.T.; Bryant, I.N.; Cook, L.J.; Miller, S.T.; Lott, J.H.; et al. The Yin and Yang of ERBB4: Tumor Suppressor and Oncoprotein. Pharmacol. Rev. 2022, 74, 18. [Google Scholar] [CrossRef]
- Morris, L.G.T.; Kaufman, A.M.; Gong, Y.; Ramaswami, D.; Walsh, L.A.; Turcan, Ş.; Eng, S.; Kannan, K.; Zou, Y.; Peng, L.; et al. Recurrent somatic mutation of FAT1 in multiple human cancers leads to aberrant Wnt activation. Nat. Genet. 2013, 45, 253. [Google Scholar] [CrossRef]
- Nyström, A.; Velati, D.; Mittapalli, V.R.; Fritsch, A.; Kern, J.S.; Bruckner-Tuderman, L. Collagen VII plays a dual role in wound healing. J. Clin. Investig. 2013, 123, 3498–3509. [Google Scholar] [CrossRef] [PubMed]
- Fléchon, L.; Arib, I.; Dutta, A.K.; Hasan Bou Issa, L.; Sklavenitis-Pistofidis, R.; Tilmont, R.; Stewart, C.; Dubois, R.; Poulain, S.; Copin, M.; et al. Genomic profiling of mycosis fungoides identifies patients at high risk of disease progression. Blood Adv. 2024, 8, 3109. [Google Scholar] [CrossRef]
- Aydin, F.; Levent, Y.; Nilgun, S.; Pancar, Y.E.; Yasar, T.A. Implications of bax, fas, and p53 in the pathogenesis of early-stage mycosis fungoides and alterations in expression following photochemotherapy. Indian J. Dermatol. 2011, 56, 501–504. [Google Scholar] [CrossRef]
- Vieyra-Garcia, P.; Fink-Puches, R.; Porkert, S.; Lang, R.; Pöchlauer, S.; Ratzinger, G.; Tanew, A.; Selhofer, S.; Paul-Gunther, S.; Hofer, A.; et al. Evaluation of Low-Dose, Low-Frequency Oral Psoralen–UV-A Treatment With or Without Maintenance on Early-Stage Mycosis Fungoides. JAMA Dermatol. 2019, 155, 538–547. [Google Scholar] [CrossRef]
- Heward, J.; Koniali, L.; Avola, A.D.; Close, K.; Yeomans, A.; Philpott, M.; Dunford, J.; Rahim, T.; Al Seraihi, A.F.; Wang, J.; et al. KDM5 inhibition offers a novel therapeutic strategy for the treatment of KMT2D mutant lymphomas. Blood 2021, 138, 370–381. [Google Scholar] [CrossRef]
- Carty, S.A. Biological insights into the role of TET2 in T cell lymphomas. Front. Oncol. 2023, 13, 1199108. [Google Scholar] [CrossRef] [PubMed]
- Makishima, H. Somatic SETBP1 mutations in myeloid neoplasms. Int. J. Hematol. 2017, 105, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.M.; Feldman, T.A.; Hess, B.T.; Khodadoust, M.S.; Kim, Y.H.; Munoz, J.; Patel, M.R.; Phillips, T.J.; Smith, S.D.; Smith, S.M.; et al. A Phase 2 Study of the Dual SYK/JAK Inhibitor Cerdulatinib Demonstrates Good Tolerability and Clinical Response in Relapsed/Refractory Peripheral T-Cell Lymphoma and Cutaneous T-Cell Lymphoma. Blood 2019, 134 (Suppl. S1), 466. [Google Scholar] [CrossRef]
- Vahabi, S.M.; Bahramian, S.; Esmaeili, F.; Danaei, B.; Kalantari, Y.; Fazeli, P.; Sadeghi, S.; Hajizadeh, N.; Assaf, C.; Etesami, I. JAK Inhibitors in Cutaneous T-Cell Lymphoma: Friend or Foe? A Systematic Review of the Published Literature. Cancers 2024, 16, 861. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).