Regulatory T Cells in Invasive Breast Cancer: Prognosis, Mechanisms and Therapy
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Clinical and Molecular Heterogeneity of Invasive Breast Cancer
1.2. The Breast Tumor Immune Microenvironment
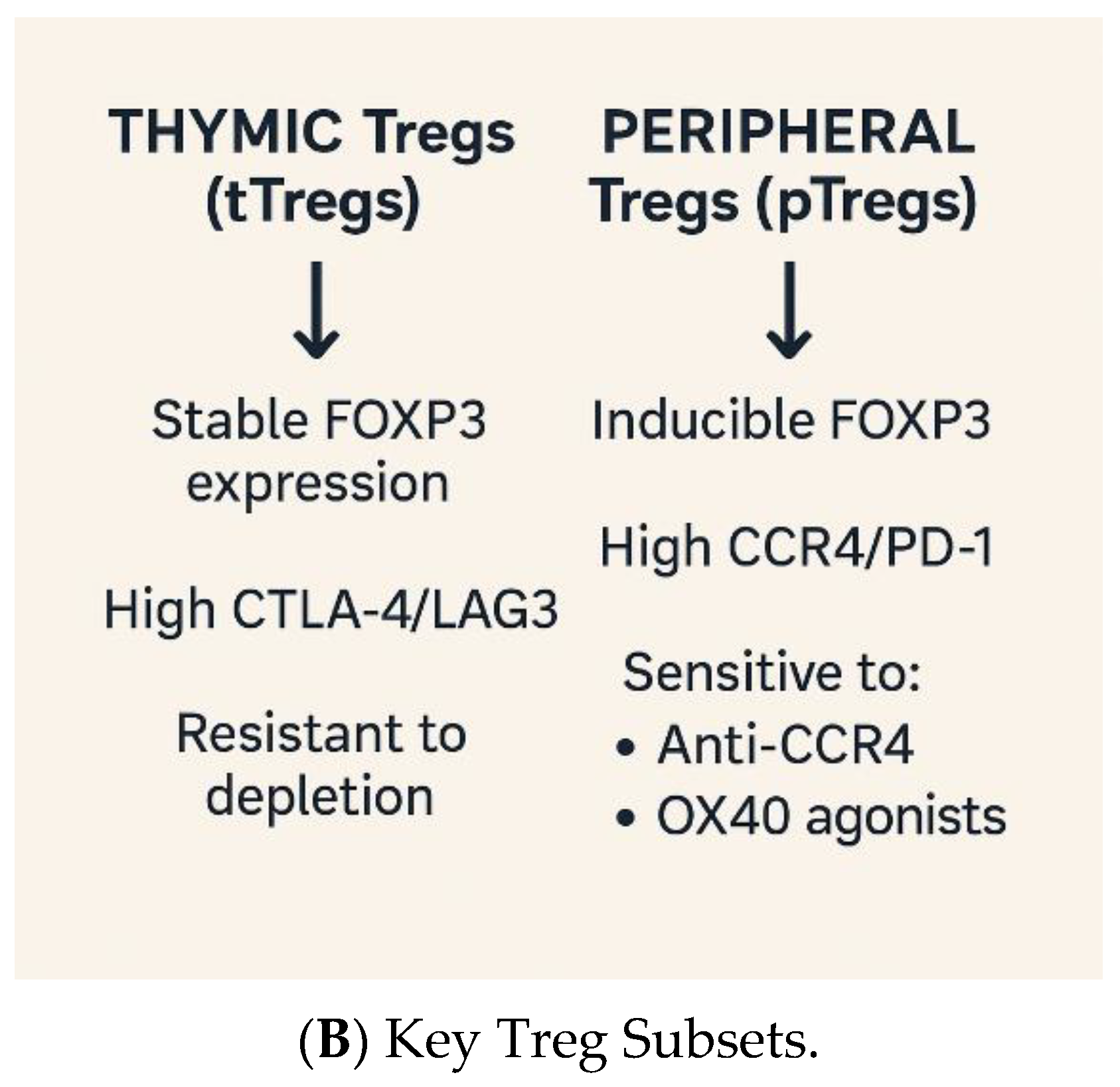
1.3. Regulatory T Cells: Guardians of Tolerance, Enablers of Evasion
1.4. Rationale for Targeting Tregs
2. Tregs in Breast Cancer Biology
2.1. Recruitment and Accumulation of Tregs in the Tumor Microenvironment
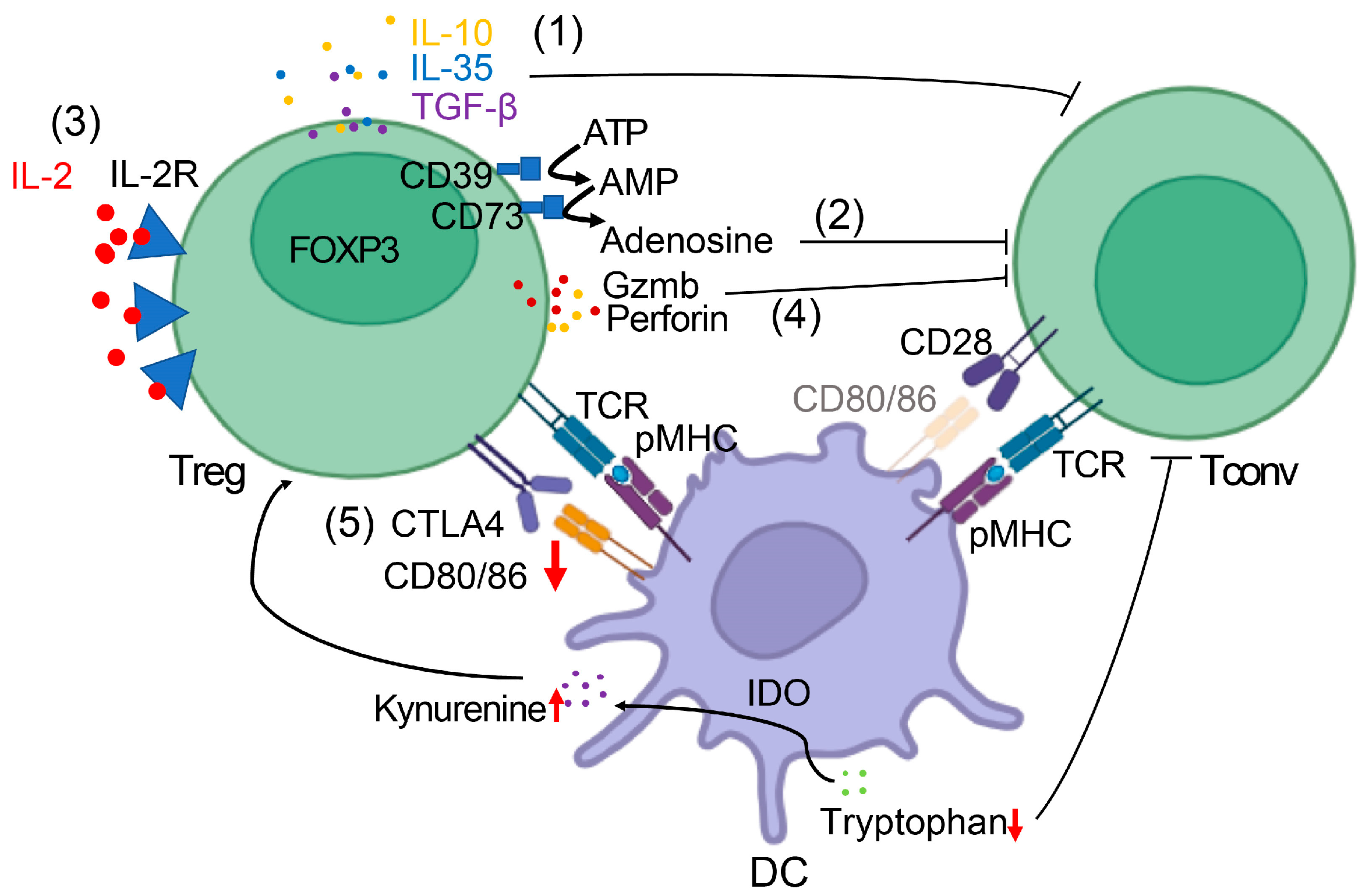
2.2. Mechanisms of Suppression
2.3. Pro-Tumorigenic Functions Beyond Immune Suppression
3. Tregs as Prognostic Biomarkers in Breast Cancer
3.1. Clinical Evidence Linking Treg Abundance to Disease Outcomes
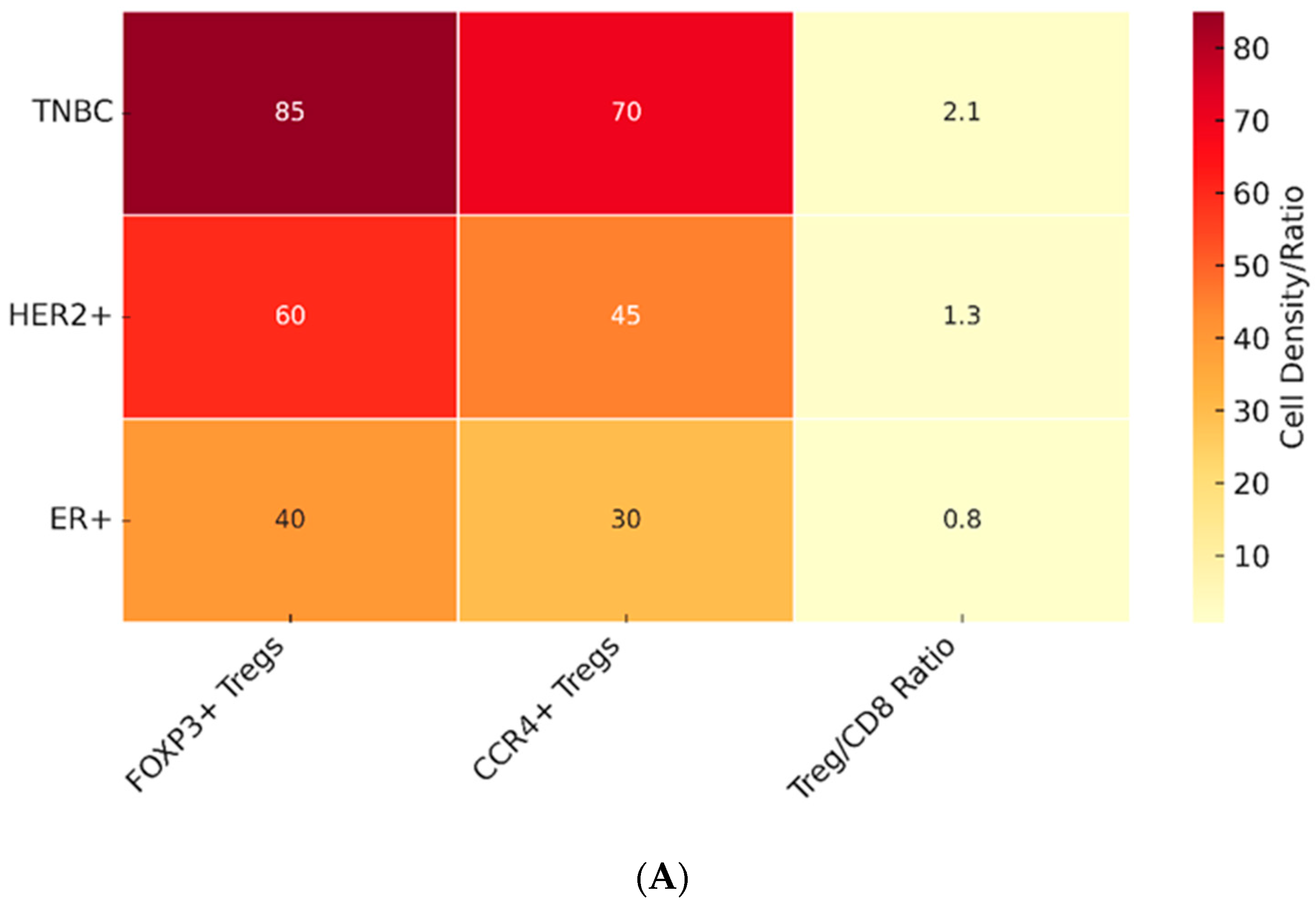
3.2. Tregs in Different Breast Cancer Subtypes
3.3. The Treg Paradox: Context-Dependent Roles in Prognosis
3.4. Methodological Considerations in Treg Assessment
3.5. Composite Biomarkers for Improved Prognostication
4. Tregs and Therapeutic Responses
4.1. Conventional Therapies and Treg Modulation
4.2. Immunotherapy and Treg-Targeted Strategies
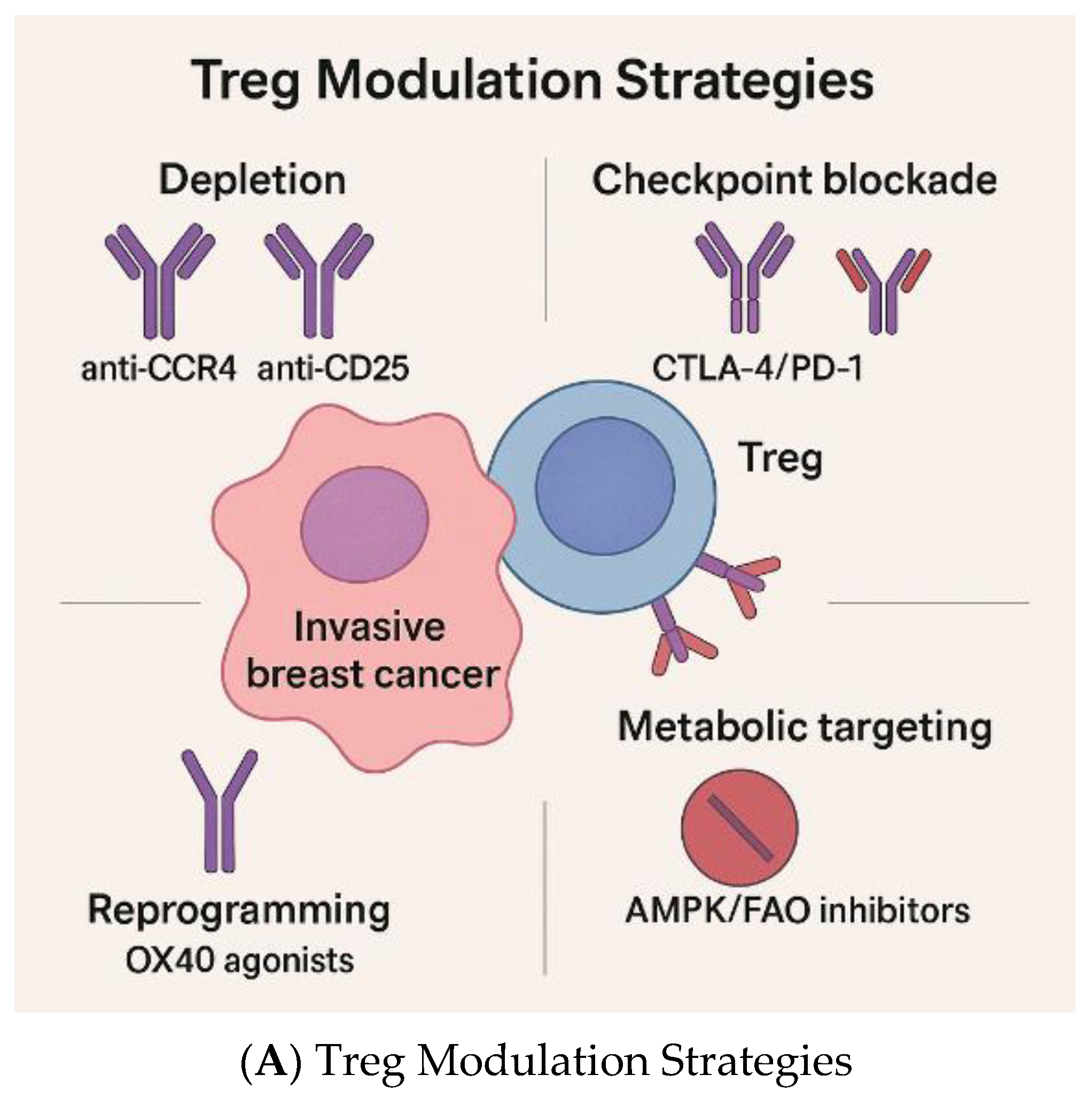
4.3. Emerging Treg-Specific Therapeutic Approaches
4.4. Targeting Tregs in Clinical Trials
4.5. Challenges in Treg-Targeted Therapy
4.6. The Next Steps
5. Future Directions
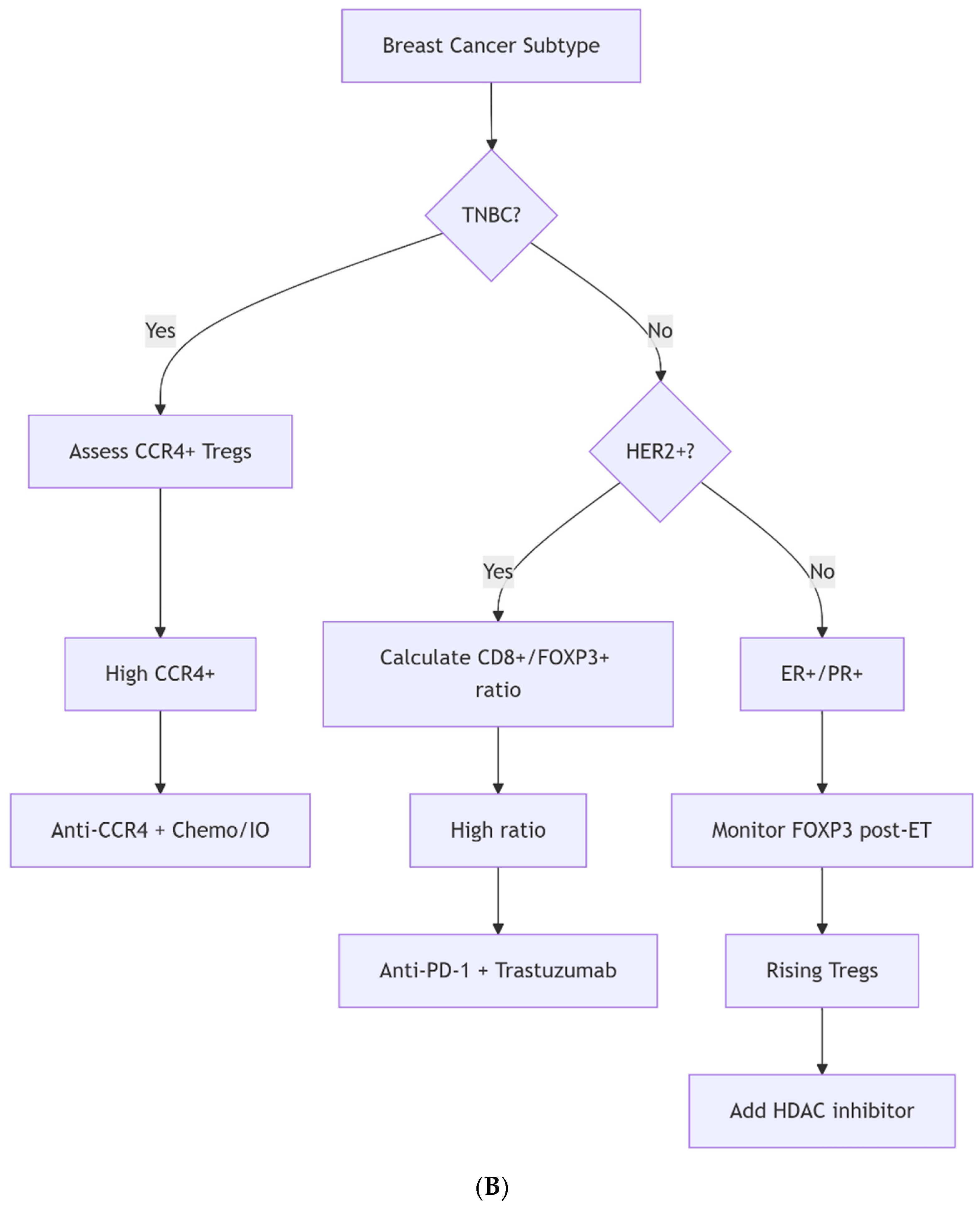
5.1. Precision Modulation and Biomarker-Guided Approaches
5.2. Rational Combinatorial Strategies
5.3. Advanced Preclinical Models and Translational Platforms
5.4. Implementation in Clinical Trials
5.5. Artificial Intelligence (AI)—Driven Approaches
6. Limitations
7. Clinical Implications
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Glossary
| Glossary of Key Terms |
| Adenosine Pathway (CD39/CD73) An enzymatic cascade on Tregs that converts ATP into immunosuppressive adenosine. |
| Angiogenesis Formation of new blood vessels is promoted by Treg-derived VEGF and MMPs, supporting tumor growth and metastasis. |
| CCL22/CCR4 Axis Chemokine–receptor signaling pathway that recruits CCR4⁺ Tregs into the tumor microenvironment. |
| CD25 (IL-2 receptor α-chain) A High-affinity IL-2 receptor subunit constitutively expressed on Tregs; allows IL-2 consumption and effector T-cell starvation. |
| CD36 A fatty acid transporter upregulated in intratumoral Tregs, supporting metabolic adaptation and suppressive functions. |
| CD8+ T Cells Cytotoxic lymphocytes that kill cancer cells directly via perforin and granzyme release. |
| Checkpoint Inhibitors Immunotherapies targeting PD-1/PD-L1 or CTLA-4 that restore effector T cell function. |
| Composite Biomarkers Integrated immune metrics (e.g., CD8+/FOXP3+ ratio and immune context scores) predict outcomes more accurately than Tregs alone. |
| CTLA-4 Inhibitory checkpoint receptor on Tregs; competes with CD28 for CD80/CD86 binding, reducing T-cell activation. |
| Denileukin Diftitox Fusion protein combining IL-2 and diphtheria toxin; depletes CD25+ Tregs but may also affect activated effector T cells. |
| Epithelial–Mesenchymal Transition (EMT) A biological process in which epithelial tumor cells acquire invasive mesenchymal traits; promoted by TGF-β from Tregs. |
| FOXP3 Master transcription factor defining Treg lineage and function. |
| Granzyme/Perforin Cytotoxic proteins secreted by Tregs to directly kill effector immune cells. |
| IDO (Indoleamine 2,3-dioxygenase) This enzyme depletes tryptophan and produces immunosuppressive metabolites that promote Treg activity. |
| Immune Contexture Score Composite measure of immune infiltrates, proliferation, and checkpoint expression that refines prognosis. |
| LAG-3, TIM-3, TIGIT Inhibitory checkpoint receptors on Tregs and effector T cells contribute to this suppression. |
| Metronomic Chemotherapy Low-dose, continuous chemotherapy (e.g., cyclophosphamide) that selectively reduces Tregs while sparing effector T cells. |
| Mogamulizumab Monoclonal antibody targeting CCR4; selectively depletes CCR4⁺ Tregs. |
| PGE2 (Prostaglandin E2) Lipid mediators produced in tumors that induce FOXP3 expression and enhance Treg activity. |
| PD-1/PD-L1 Checkpoint receptor–ligand pair suppressing T cell activity and sustaining Treg function in tumors. |
| Regulatory T Cells (Tregs) CD4+CD25+FOXP3+ lymphocytes are essential for immune tolerance but are co-opted by tumors to suppress immunity. |
| Tertiary Lymphoid Structures (TLS) Organized immune aggregates in tumors are often associated with favorable outcomes. |
| TILs (Tumor-infiltrating lymphocytes) Immune cells found in tumors, including cytotoxic T cells and Tregs. |
| VEGF (Vascular Endothelial Growth Factor) Potent angiogenic factor secreted by Tregs that promotes tumor vascularization. |
References
- Tan, P.H.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Lazar, A.J.; Morris, E.A.; Sahin, A.; Salgado, R.; et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology 2020, 77, 181–185. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thurlimann, B.; Senn, H.J.; Panel members. Strategies for subtypes—Dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Cronin, K.A.; Kurian, A.W.; Andridge, R. Differences in Breast Cancer Survival by Molecular Subtypes in the United States. Cancer Epidemiol. Biomark. Prev. 2018, 27, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; De Angelis, C.; Licata, L.; Gianni, L. Treatment landscape of triple-negative breast cancer—Expanded options, evolving needs. Nat. Rev. Clin. Oncol. 2022, 19, 91–113. [Google Scholar] [CrossRef]
- Yates, L.R.; Knappskog, S.; Wedge, D.; Farmery, J.H.R.; Gonzalez, S.; Martincorena, I.; Alexandrov, L.B.; Van Loo, P.; Haugland, H.K.; Lilleng, P.K.; et al. Genomic Evolution of Breast Cancer Metastasis and Relapse. Cancer Cell 2017, 32, 169–184.E7. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Loi, S.; Drubay, D.; Adams, S.; Pruneri, G.; Francis, P.A.; Lacroix-Triki, M.; Joensuu, H.; Dieci, M.V.; Badve, S.; Demaria, S.; et al. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J. Clin. Oncol. 2019, 37, 559–569. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Desmedt, C.; Haibe-Kains, B.; Wirapati, P.; Buyse, M.; Larsimont, D.; Bontempi, G.; Delorenzi, M.; Piccart, M.; Sotiriou, C. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin. Cancer Res. 2008, 14, 5158–5165. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Larsson, L.; Stenbeck, L.; Salmen, F.; Ehinger, A.; Wu, S.Z.; Al-Eryani, G.; Roden, D.; Swarbrick, A.; Borg, A.; et al. Spatial deconvolution of HER2-positive breast cancer delineates tumor-associated cell type interactions. Nat. Commun. 2021, 12, 6012. [Google Scholar] [CrossRef]
- Wu, S.Z.; Al-Eryani, G.; Roden, D.L.; Junankar, S.; Harvey, K.; Andersson, A.; Thennavan, A.; Wang, C.; Torpy, J.R.; Bartonicek, N.; et al. A single-cell and spatially resolved atlas of human breast cancers. Nat. Genet. 2021, 53, 1334–1347. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Vignali, D.A.; Collison, L.W.; Workman, C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008, 8, 523–532. [Google Scholar] [CrossRef]
- Wing, K.; Onishi, Y.; Prieto-Martin, P.; Yamaguchi, T.; Miyara, M.; Fehervari, Z.; Nomura, T.; Sakaguchi, S. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008, 322, 271–275. [Google Scholar] [CrossRef]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef]
- Pandiyan, P.; Zheng, L.; Ishihara, S.; Reed, J.; Lenardo, M.J. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat. Immunol. 2007, 8, 1353–1362. [Google Scholar] [CrossRef]
- Fallarino, F.; Grohmann, U.; Hwang, K.W.; Orabona, C.; Vacca, C.; Bianchi, R.; Belladonna, M.L.; Fioretti, M.C.; Alegre, M.L.; Puccetti, P. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 2003, 4, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Deaglio, S.; Dwyer, K.M.; Gao, W.; Friedman, D.; Usheva, A.; Erat, A.; Chen, J.F.; Enjyoji, K.; Linden, J.; Oukka, M.; et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007, 204, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Cai, S.F.; Fehniger, T.A.; Song, J.; Collins, L.I.; Piwnica-Worms, D.R.; Ley, T.J. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity 2007, 27, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Clambey, E.T.; McNamee, E.N.; Westrich, J.A.; Glover, L.E.; Campbell, E.L.; Jedlicka, P.; de Zoeten, E.F.; Cambier, J.C.; Stenmark, K.R.; Colgan, S.P.; et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc. Natl. Acad. Sci. USA 2012, 109, E2784–E2793. [Google Scholar] [CrossRef]
- Bates, G.J.; Fox, S.B.; Han, C.; Leek, R.D.; Garcia, J.F.; Harris, A.L.; Banham, A.H. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J. Clin. Oncol. 2006, 24, 5373–5380. [Google Scholar] [CrossRef]
- Liu, S.; Foulkes, W.D.; Leung, S.; Gao, D.; Lau, S.; Kos, Z.; Nielsen, T.O. Prognostic significance of FOXP3+ tumor-infiltrating lymphocytes in breast cancer depends on estrogen receptor and human epidermal growth factor receptor-2 expression status and concurrent cytotoxic T-cell infiltration. Breast Cancer Res. 2014, 16, 432. [Google Scholar] [CrossRef]
- Tanaka, H.; Tanaka, J.; Kjaergaard, J.; Shu, S. Depletion of CD4+ CD25+ regulatory cells augments the generation of specific immune T cells in tumor-draining lymph nodes. J. Immunother. 2002, 25, 207–217. [Google Scholar] [CrossRef]
- Simpson, T.R.; Li, F.; Montalvo-Ortiz, W.; Sepulveda, M.A.; Bergerhoff, K.; Arce, F.; Roddie, C.; Henry, J.Y.; Yagita, H.; Wolchok, J.D.; et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med. 2013, 210, 1695–1710. [Google Scholar] [CrossRef]
- Zhang, A.; Fan, T.; Liu, Y.; Yu, G.; Li, C.; Jiang, Z. Regulatory T cells in immune checkpoint blockade antitumor therapy. Mol. Cancer 2024, 23, 251. [Google Scholar] [CrossRef]
- Rech, A.J.; Mick, R.; Martin, S.; Recio, A.; Aqui, N.A.; Powell, D.J., Jr.; Colligon, T.A.; Trosko, J.A.; Leinbach, L.I.; Pletcher, C.H.; et al. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci. Transl. Med. 2012, 4, 134ra162. [Google Scholar] [CrossRef]
- Kurose, K.; Ohue, Y.; Wada, H.; Iida, S.; Ishida, T.; Kojima, T.; Doi, T.; Suzuki, S.; Isobe, M.; Funakoshi, T.; et al. Phase Ia Study of FoxP3+ CD4 Treg Depletion by Infusion of a Humanized Anti-CCR4 Antibody, KW-0761, in Cancer Patients. Clin. Cancer Res. 2015, 21, 4327–4336. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Rasmussen, J.P.; Rudensky, A.Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007, 8, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, J.M.; Pagliano, O.; Fourcade, J.; Sun, Z.; Wang, H.; Sander, C.; Kirkwood, J.M.; Chen, T.H.; Maurer, M.; Korman, A.J.; et al. TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. J. Clin. Investig. 2015, 125, 2046–2058. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhao, L.; Yang, F.; Yang, Y.; Zhang, H.; Du, K.; Tian, X.; Fan, R.; Si, G.; Wang, K.; et al. A CD25xTIGIT bispecific antibody induces anti-tumor activity through selective intratumoral Treg cell depletion. Mol. Ther. 2024, 32, 4075–4094. [Google Scholar] [CrossRef]
- Chen, Q.; Shen, M.; Yan, M.; Han, X.; Mu, S.; Li, Y.; Li, L.; Wang, Y.; Li, S.; Li, T.; et al. Targeting tumor-infiltrating CCR8(+) regulatory T cells induces antitumor immunity through functional restoration of CD4(+) T(convs) and CD8(+) T cells in colorectal cancer. J. Transl. Med. 2024, 22, 709. [Google Scholar] [CrossRef]
- Van, D.H.; Dombrecht, B.; Kiss, M.; Roose, H.; Allen, E.; Van, O.E.; Kancheva, D.; Martens, L.; Murgaski, A.; Bardet, P.M.R.; et al. Therapeutic depletion of CCR8+ tumor-infiltrating regulatory T cells elicits antitumor immunity and synergizes with anti-PD-1 therapy. J Immunother Cancer. 2021, 9, e001749. [Google Scholar]
- Sarkar, T.; Dhar, S.; Sa, G. Tumor-infiltrating T-regulatory cells adapt to altered metabolism to promote tumor-immune escape. Curr. Res. Immunol. 2021, 2, 132–141. [Google Scholar] [CrossRef]
- Russo, V.; Protti, M.P. Tumor-derived factors affecting immune cells. Cytokine Growth Factor. Rev. 2017, 36, 79–87. [Google Scholar] [CrossRef]
- Wei, S.; Kryczek, I.; Zou, W. Regulatory T-cell compartmentalization and trafficking. Blood 2006, 108, 426–431. [Google Scholar] [CrossRef]
- Yan, M.; Jene, N.; Byrne, D.; Millar, E.K.; O’Toole, S.A.; McNeil, C.M.; Bates, G.J.; Harris, A.L.; Banham, A.H.; Sutherland, R.L.; et al. Recruitment of regulatory T cells is correlated with hypoxia-induced CXCR4 expression, and is associated with poor prognosis in basal-like breast cancers. Breast Cancer Res. 2011, 13, R47. [Google Scholar] [CrossRef]
- Finetti, F.; Travelli, C.; Ercoli, J.; Colombo, G.; Buoso, E.; Trabalzini, L. Prostaglandin E2 and Cancer: Insight into Tumor Progression and Immunity. Biology 2020, 9, 434. [Google Scholar] [CrossRef]
- Sawant, D.V.; Yano, H.; Chikina, M.; Zhang, Q.; Liao, M.; Liu, C.; Callahan, D.J.; Sun, Z.; Sun, T.; Tabib, T.; et al. Adaptive plasticity of IL-10(+) and IL-35(+) T(reg) cells cooperatively promotes tumor T cell exhaustion. Nat. Immunol. 2019, 20, 724–735. [Google Scholar] [CrossRef]
- Hurwitz, A.A.; Watkins, S.K. Immune suppression in the tumor microenvironment: A role for dendritic cell-mediated tolerization of T cells. Cancer Immunol. Immunother. 2012, 61, 289–293. [Google Scholar] [CrossRef]
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer 2020, 19, 116. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, J.; Liu, S.; Lv, Y.; Zhang, R.; Zhou, X.; Zhang, Y.; Weng, S.; Xu, H.; Ba, Y.; et al. Infiltrating treg reprogramming in the tumor immune microenvironment and its optimization for immunotherapy. Biomark. Res. 2024, 12, 97. [Google Scholar] [CrossRef]
- Schiering, C.; Krausgruber, T.; Chomka, A.; Frohlich, A.; Adelmann, K.; Wohlfert, E.A.; Pott, J.; Griseri, T.; Bollrath, J.; Hegazy, A.N.; et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 2014, 513, 564–568. [Google Scholar] [CrossRef]
- Laine, A.; Labiad, O.; Hernandez-Vargas, H.; This, S.; Sanlaville, A.; Leon, S.; Dalle, S.; Sheppard, D.; Travis, M.A.; Paidassi, H.; et al. Regulatory T cells promote cancer immune-escape through integrin alphavbeta8-mediated TGF-beta activation. Nat. Commun. 2021, 12, 6228. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, L.; Cai, W.; Li, L.; Zhang, R.; Huang, W.; Cao, Y. Unveiling the role of TGF-beta signaling pathway in breast cancer prognosis and immunotherapy. Front. Oncol. 2024, 14, 1488137. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, A.; Samstein, R.M.; Treuting, P.; Liang, Y.; Pils, M.C.; Heinrich, J.M.; Jack, R.S.; Wunderlich, F.T.; Bruning, J.C.; Muller, W.; et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 2011, 34, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Collison, L.W.; Workman, C.J.; Kuo, T.T.; Boyd, K.; Wang, Y.; Vignali, K.M.; Cross, R.; Sehy, D.; Blumberg, R.S.; Vignali, D.A. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 2007, 450, 566–569. [Google Scholar] [CrossRef]
- Collison, L.W.; Chaturvedi, V.; Henderson, A.L.; Giacomin, P.R.; Guy, C.; Bankoti, J.; Finkelstein, D.; Forbes, K.; Workman, C.J.; Brown, S.A.; et al. IL-35-mediated induction of a potent regulatory T cell population. Nat. Immunol. 2010, 11, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Tekguc, M.; Wing, J.B.; Osaki, M.; Long, J.; Sakaguchi, S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2023739118. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, H.; Yang, X.; Hu, H.; Liu, P.; Liu, H. Crosstalk between dendritic cells and regulatory T cells: Protective effect and therapeutic potential in multiple sclerosis. Front. Immunol. 2022, 13, 970508. [Google Scholar] [CrossRef] [PubMed]
- Gautron, A.S.; Dominguez-Villar, M.; de Marcken, M.; Hafler, D.A. Enhanced suppressor function of TIM-3+ FoxP3+ regulatory T cells. Eur. J. Immunol. 2014, 44, 2703–2711. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, Y.; Zhang, J.P.; Liang, J.; Li, L.; Zheng, L. Tim-3 expression defines regulatory T cells in human tumors. PLoS ONE 2013, 8, e58006. [Google Scholar] [CrossRef]
- Kim, M.J.; Ha, S.J. Differential Role of PD-1 Expressed by Various Immune and Tumor Cells in the Tumor Immune Microenvironment: Expression, Function, Therapeutic Efficacy, and Resistance to Cancer Immunotherapy. Front. Cell Dev. Biol. 2021, 9, 767466. [Google Scholar] [CrossRef]
- Qureshi, O.S.; Zheng, Y.; Nakamura, K.; Attridge, K.; Manzotti, C.; Schmidt, E.M.; Baker, J.; Jeffery, L.E.; Kaur, S.; Briggs, Z.; et al. Trans-endocytosis of CD80 and CD86: A molecular basis for the cell-extrinsic function of CTLA-4. Science 2011, 332, 600–603. [Google Scholar] [CrossRef]
- Yan, J.; Chen, D.; Ye, Z.; Zhu, X.; Li, X.; Jiao, H.; Duan, M.; Zhang, C.; Cheng, J.; Xu, L.; et al. Molecular mechanisms and therapeutic significance of Tryptophan Metabolism and signaling in cancer. Mol. Cancer 2024, 23, 241. [Google Scholar] [CrossRef]
- Mezrich, J.D.; Fechner, J.H.; Zhang, X.; Johnson, B.P.; Burlingham, W.J.; Bradfield, C.A. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010, 185, 3190–3198. [Google Scholar] [CrossRef]
- Munn, D.H.; Mellor, A.L. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J. Clin. Investig. 2007, 117, 1147–1154. [Google Scholar] [CrossRef]
- Soliman, H.; Rawal, B.; Fulp, J.; Lee, J.H.; Lopez, A.; Bui, M.M.; Khalil, F.; Antonia, S.; Yfantis, H.G.; Lee, D.H.; et al. Analysis of indoleamine 2-3 dioxygenase (IDO1) expression in breast cancer tissue by immunohistochemistry. Cancer Immunol. Immunother. 2013, 62, 829–837. [Google Scholar] [CrossRef]
- Huppert, L.A.; Green, M.D.; Kim, L.; Chow, C.; Leyfman, Y.; Daud, A.I.; Lee, J.C. Tissue-specific Tregs in cancer metastasis: Opportunities for precision immunotherapy. Cell Mol. Immunol. 2022, 19, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Toker, A.; Liu, Z.Q.; Ohashi, P.S. Turning the Tide Against Regulatory T Cells. Front. Oncol. 2019, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Samanta, A.; Song, X.; Iacono, K.T.; Bembas, K.; Tao, R.; Basu, S.; Riley, J.L.; Hancock, W.W.; Shen, Y.; et al. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc. Natl. Acad. Sci. USA 2007, 104, 4571–4576. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.A.; Tucker-Heard, G.; Perdue, N.R.; Killebrew, J.R.; Urdahl, K.B.; Campbell, D.J. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 2009, 10, 595–602. [Google Scholar] [CrossRef]
- Zagorulya, M.; Yim, L.; Morgan, D.M.; Edwards, A.; Torres-Mejia, E.; Momin, N.; McCreery, C.V.; Zamora, I.L.; Horton, B.L.; Fox, J.G.; et al. Tissue-specific abundance of interferon-gamma drives regulatory T cells to restrain DC1-mediated priming of cytotoxic T cells against lung cancer. Immunity 2023, 56, 386–405.E10. [Google Scholar] [CrossRef]
- Elkoshi, Z. On the Prognostic Power of Tumor-Infiltrating Lymphocytes—A Critical Commentary. Front. Immunol. 2022, 13, 892543. [Google Scholar] [CrossRef]
- Quintero-Fabian, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argaez, V.; Lara-Riegos, J.; Ramirez-Camacho, M.A.; Alvarez-Sanchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Al-Ostoot, F.H.; Salah, S.; Khamees, H.A.; Khanum, S.A. Tumor angiogenesis: Current challenges and therapeutic opportunities. Cancer Treat. Res. Commun. 2021, 28, 100422. [Google Scholar] [CrossRef]
- Rundhaug, J.E. Matrix metalloproteinases and angiogenesis. J. Cell Mol. Med. 2005, 9, 267–285. [Google Scholar] [CrossRef]
- Hua, H.; Li, M.; Luo, T.; Yin, Y.; Jiang, Y. Matrix metalloproteinases in tumorigenesis: An evolving paradigm. Cell Mol. Life Sci. 2011, 68, 3853–3868. [Google Scholar] [CrossRef] [PubMed]
- Masood, R.; Cai, J.; Zheng, T.; Smith, D.L.; Hinton, D.R.; Gill, P.S. Vascular endothelial growth factor (VEGF) is an autocrine growth factor for VEGF receptor-positive human tumors. Blood 2001, 98, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Massague, J. TGFbeta in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Xu, Y.; Lin, C. Heterogeneity and subtypes of CD4(+) regulatory T cells: Implications for tumor therapy. Front. Immunol. 2023, 14, 1291796. [Google Scholar] [CrossRef]
- Shan, F.; Somasundaram, A.; Bruno, T.C.; Workman, C.J.; Vignali, D.A.A. Therapeutic targeting of regulatory T cells in cancer. Trends Cancer 2022, 8, 944–961. [Google Scholar] [CrossRef]
- Bates, S.E.; Rosing, D.R.; Fojo, T.; Piekarz, R.L. Challenges of evaluating the cardiac effects of anticancer agents. Clin. Cancer Res. 2006, 12, 3871–3874. [Google Scholar] [CrossRef]
- West, N.R.; Kost, S.E.; Martin, S.D.; Milne, K.; Deleeuw, R.J.; Nelson, B.H.; Watson, P.H. Tumour-infiltrating FOXP3(+) lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. Br. J. Cancer 2013, 108, 155–162. [Google Scholar] [CrossRef]
- Loi, S.; Sirtaine, N.; Piette, F.; Salgado, R.; Viale, G.; Van Eenoo, F.; Rouas, G.; Francis, P.; Crown, J.P.; Hitre, E.; et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J. Clin. Oncol. 2013, 31, 860–867. [Google Scholar] [CrossRef]
- Hayashi, K.; Nogawa, D.; Kobayashi, M.; Asakawa, A.; Ohata, Y.; Kitagawa, S.; Kubota, K.; Takahashi, H.; Yamada, M.; Oda, G.; et al. Quantitative high-throughput analysis of tumor infiltrating lymphocytes in breast cancer. Front. Oncol. 2022, 12, 901591. [Google Scholar] [CrossRef]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 2018, 33, 463–479.E10. [Google Scholar] [CrossRef]
- Shin, S.J.; Park, I.; Go, H.; Ko, J.; Lee, Y.; Kim, J.H.; Ahn, S.G.; Jeong, J.; Bae, S.J.; Cha, Y.J. Immune environment of high-TIL breast cancer: Triple negative and hormone receptor positive HER2 negative. NPJ Breast Cancer 2024, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Denkert, C.; Campbell, C.; Savas, P.; Nuciforo, P.; Aura, C.; de Azambuja, E.; Eidtmann, H.; Ellis, C.E.; Baselga, J.; et al. Tumor-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol. 2015, 1, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Giobbie-Hurder, A.; Gombos, A.; Bachelot, T.; Hui, R.; Curigliano, G.; Campone, M.; Biganzoli, L.; Bonnefoi, H.; Jerusalem, G.; et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): A single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. 2019, 20, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Esteva, F.J.; Beresford, M.; Saura, C.; De Laurentiis, M.; Kim, S.B.; Im, S.A.; Wang, Y.; Salgado, R.; Mani, A.; et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): A phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 2020, 21, 1283–1295. [Google Scholar] [CrossRef]
- Ali, H.R.; Provenzano, E.; Dawson, S.J.; Blows, F.M.; Liu, B.; Shah, M.; Earl, H.M.; Poole, C.J.; Hiller, L.; Dunn, J.A.; et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann. Oncol. 2014, 25, 1536–1543. [Google Scholar] [CrossRef]
- Smith, I.; Robertson, J.; Kilburn, L.; Wilcox, M.; Evans, A.; Holcombe, C.; Horgan, K.; Kirwan, C.; Mallon, E.; Sibbering, M.; et al. Long-term outcome and prognostic value of Ki67 after perioperative endocrine therapy in postmenopausal women with hormone-sensitive early breast cancer (POETIC): An open-label, multicentre, parallel-group, randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1443–1454. [Google Scholar] [CrossRef]
- Stanton, S.E.; Adams, S.; Disis, M.L. Variation in the Incidence and Magnitude of Tumor-Infiltrating Lymphocytes in Breast Cancer Subtypes: A Systematic Review. JAMA Oncol. 2016, 2, 1354–1360. [Google Scholar] [CrossRef]
- Magnuson, A.M.; Kiner, E.; Ergun, A.; Park, J.S.; Asinovski, N.; Ortiz-Lopez, A.; Kilcoyne, A.; Paoluzzi-Tomada, E.; Weissleder, R.; Mathis, D.; et al. Identification and validation of a tumor-infiltrating Treg transcriptional signature conserved across species and tumor types. Proc. Natl. Acad. Sci. USA 2018, 115, E10672–E10681. [Google Scholar] [CrossRef]
- De Simone, M.; Arrigoni, A.; Rossetti, G.; Gruarin, P.; Ranzani, V.; Politano, C.; Bonnal, R.J.P.; Provasi, E.; Sarnicola, M.L.; Panzeri, I.; et al. Transcriptional Landscape of Human Tissue Lymphocytes Unveils Uniqueness of Tumor-Infiltrating T Regulatory Cells. Immunity 2016, 45, 1135–1147. [Google Scholar] [CrossRef]
- Hu, G.; Cheng, P.; Pan, J.; Wang, S.; Ding, Q.; Jiang, Z.; Cheng, L.; Shao, X.; Huang, L.; Huang, J. An IL6-Adenosine Positive Feedback Loop between CD73(+) gammadeltaTregs and CAFs Promotes Tumor Progression in Human Breast Cancer. Cancer Immunol. Res. 2020, 8, 1273–1286. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, G.; Cai, Y. Regulatory T cell-associated gene signature correlates with prognostic risk and immune infiltration in patients with breast cancer. Transl. Cancer Res. 2024, 13, 6766–6781. [Google Scholar] [CrossRef]
- Antonioli, L.; Blandizzi, C.; Pacher, P.; Hasko, G. Immunity, inflammation and cancer: A leading role for adenosine. Nat. Rev. Cancer 2013, 13, 842–857. [Google Scholar] [CrossRef]
- Shang, B.; Liu, Y.; Jiang, S.J.; Liu, Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 15179. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.; Zhang, Z.; Lai, Y.; Chen, Z.; Huang, J. Worse outcome in breast cancer with higher tumor-infiltrating FOXP3+ Tregs: A systematic review and meta-analysis. BMC Cancer 2016, 16, 687. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shao, N.; Aierken, N.; Xie, C.; Ye, R.; Qian, X.; Hu, Z.; Zhang, J.; Lin, Y. Prognostic value of tumor-infiltrating Foxp3+ regulatory T cells in patients with breast cancer: A meta-analysis. J. Cancer 2017, 8, 4098–4105. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Y.; Lu, F.; Zhao, X.; Nie, Z.; He, B. The prognostic values of FOXP3(+) tumor-infiltrating T cells in breast cancer: A systematic review and meta-analysis. Clin. Transl. Oncol. 2023, 25, 1830–1843. [Google Scholar] [CrossRef]
- Goda, N.; Nakashima, C.; Nagamine, I.; Otagaki, S. The Effect of Intratumoral Interrelation among FOXP3+ Regulatory T Cells on Treatment Response and Survival in Triple-Negative Breast Cancer. Cancers 2022, 14, 2138. [Google Scholar] [CrossRef]
- Koletsa, T.; Kotoula, V.; Koliou, G.A.; Manousou, K.; Chrisafi, S.; Zagouri, F.; Sotiropoulou, M.; Pentheroudakis, G.; Papoudou-Bai, A.; Christodoulou, C.; et al. Prognostic impact of stromal and intratumoral CD3, CD8 and FOXP3 in adjuvantly treated breast cancer: Do they add information over stromal tumor-infiltrating lymphocyte density? Cancer Immunol. Immunother. 2020, 69, 1549–1564. [Google Scholar] [CrossRef]
- Nishikawa, H.; Koyama, S. Mechanisms of regulatory T cell infiltration in tumors: Implications for innovative immune precision therapies. J. Immunother. Cancer 2021, 9, e002591. [Google Scholar] [CrossRef]
- Xu, L.; Saunders, K.; Huang, S.P.; Knutsdottir, H.; Martinez-Algarin, K.; Terrazas, I.; Chen, K.; McArthur, H.M.; Maues, J.; Hodgdon, C.; et al. A comprehensive single-cell breast tumor atlas defines epithelial and immune heterogeneity and interactions predicting anti-PD-1 therapy response. Cell Rep. Med. 2024, 5, 101511. [Google Scholar] [CrossRef]
- Wang, X.; Venet, D.; Lifrange, F.; Larsimont, D.; Rediti, M.; Stenbeck, L.; Dupont, F.; Rouas, G.; Garcia, A.J.; Craciun, L.; et al. Spatial transcriptomics reveals substantial heterogeneity in triple-negative breast cancer with potential clinical implications. Nat. Commun. 2024, 15, 10232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gong, S.; Liu, X. Spatial transcriptomics: A new frontier in accurate localization of breast cancer diagnosis and treatment. Front. Immunol. 2024, 15, 1483595. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Kan, C.; Sun, M.; Yang, F.; Wong, M.; Wang, S.; Zheng, H. Mapping Breast Cancer Microenvironment Through Single-Cell Omics. Front. Immunol. 2022, 13, 868813. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method From the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma In Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv. Anat. Pathol. 2017, 24, 235–251. [Google Scholar] [CrossRef]
- Parra, E.R.; Ferrufino-Schmidt, M.C.; Tamegnon, A.; Zhang, J.; Solis, L.; Jiang, M.; Ibarguen, H.; Haymaker, C.; Lee, J.J.; Bernatchez, C.; et al. Immuno-profiling and cellular spatial analysis using five immune oncology multiplex immunofluorescence panels for paraffin tumor tissue. Sci. Rep. 2021, 11, 8511. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pages, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Li, J.Y.; Duan, X.F.; Wang, L.P.; Xu, Y.J.; Huang, L.; Zhang, T.F.; Liu, J.Y.; Li, F.; Zhang, Z.; Yue, D.L.; et al. Selective depletion of regulatory T cell subsets by docetaxel treatment in patients with nonsmall cell lung cancer. J. Immunol. Res. 2014, 2014, 286170. [Google Scholar] [CrossRef]
- Park, J.Y.; Jang, M.J.; Chung, Y.H.; Kim, K.Y.; Kim, S.S.; Lee, W.B.; You, S.; Choi, Y.S.; Hur, D.Y.; Kim, D. Doxorubicin enhances CD4(+) T-cell immune responses by inducing expression of CD40 ligand and 4-1BB. Int. Immunopharmacol. 2009, 9, 1530–1539. [Google Scholar] [CrossRef]
- Ge, Y.; Domschke, C.; Stoiber, N.; Schott, S.; Heil, J.; Rom, J.; Blumenstein, M.; Thum, J.; Sohn, C.; Schneeweiss, A.; et al. Metronomic cyclophosphamide treatment in metastasized breast cancer patients: Immunological effects and clinical outcome. Cancer Immunol. Immunother. 2012, 61, 353–362. [Google Scholar] [CrossRef]
- Kim, R.; Kin, T. Current and Future Therapies for Immunogenic Cell Death and Related Molecules to Potentially Cure Primary Breast Cancer. Cancers 2021, 13, 4756. [Google Scholar] [CrossRef]
- Sanchez-Margalet, V.; Barco-Sanchez, A.; Vilarino-Garcia, T.; Jimenez-Cortegana, C.; Perez-Perez, A.; Henao-Carrasco, F.; Virizuela-Echaburu, J.A.; Nogales-Fernandez, E.; Alamo-de la Gala, M.C.; Lobo-Acosta, M.A.; et al. Circulating regulatory T cells from breast cancer patients in response to neoadjuvant chemotherapy. Transl. Cancer Res. 2019, 8, 59–65. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Deng, Y.; Yu, X.; Wang, H.; Li, Z. Research Progress on the Role of Regulatory T Cell in Tumor Microenvironment in the Treatment of Breast Cancer. Front. Oncol. 2021, 11, 766248. [Google Scholar] [CrossRef]
- Dodagatta-Marri, E.; Meyer, D.S.; Reeves, M.Q.; Paniagua, R.; To, M.D.; Binnewies, M.; Broz, M.L.; Mori, H.; Wu, D.; Adoumie, M.; et al. alpha-PD-1 therapy elevates Treg/Th balance and increases tumor cell pSmad3 that are both targeted by alpha-TGFbeta antibody to promote durable rejection and immunity in squamous cell carcinomas. J. Immunother. Cancer 2019, 7, 62. [Google Scholar] [CrossRef]
- Lax, B.M.; Palmeri, J.R.; Lutz, E.A.; Sheen, A.; Stinson, J.A.; Duhamel, L.; Santollani, L.; Kennedy, A.; Rothschilds, A.M.; Spranger, S.; et al. Both intratumoral regulatory T cell depletion and CTLA-4 antagonism are required for maximum efficacy of anti-CTLA-4 antibodies. Proc. Natl. Acad. Sci. USA 2023, 120, e2300895120. [Google Scholar] [CrossRef] [PubMed]
- Kidani, Y.; Nogami, W.; Yasumizu, Y.; Kawashima, A.; Tanaka, A.; Sonoda, Y.; Tona, Y.; Nashiki, K.; Matsumoto, R.; Hagiwara, M.; et al. CCR8-targeted specific depletion of clonally expanded Treg cells in tumor tissues evokes potent tumor immunity with long-lasting memory. Proc. Natl. Acad. Sci. USA 2022, 119, e2114282119. [Google Scholar] [CrossRef] [PubMed]
- Plitas, G.; Konopacki, C.; Wu, K.; Bos, P.D.; Morrow, M.; Putintseva, E.V.; Chudakov, D.M.; Rudensky, A.Y. Regulatory T Cells Exhibit Distinct Features in Human Breast Cancer. Immunity 2016, 45, 1122–1134. [Google Scholar] [CrossRef]
- Weaver, J.D.; Stack, E.C.; Bugge, J.A.; Hu, C.; McGrath, L.; Mueller, A.; Wong, M.; Klebanov, B.; Rahman, T.; Kaufman, R.; et al. Differential expression of CCR8 in tumors versus normal tissue allows specific depletion of tumor-infiltrating T regulatory cells by GS-1811, a novel Fc-optimized anti-CCR8 antibody. Oncoimmunology 2022, 11, 2141007. [Google Scholar] [CrossRef]
- Sun, Z.; Ren, Z.; Yang, K.; Liu, Z.; Cao, S.; Deng, S.; Xu, L.; Liang, Y.; Guo, J.; Bian, Y.; et al. A next-generation tumor-targeting IL-2 preferentially promotes tumor-infiltrating CD8(+) T-cell response and effective tumor control. Nat. Commun. 2019, 10, 3874. [Google Scholar] [CrossRef]
- Merchant, R.; Galligan, C.; Munegowda, M.A.; Pearce, L.B.; Lloyd, P.; Smith, P.; Merchant, F.; To, M.D. Fine-tuned long-acting interleukin-2 superkine potentiates durable immune responses in mice and non-human primate. J. Immunother. Cancer 2022, 10, e003155. [Google Scholar] [CrossRef]
- Moynihan, K.D.; Kumar, M.P.; Sultan, H.; Pappas, D.C.; Park, T.; Chin, S.M.; Bessette, P.; Lan, R.Y.; Nguyen, H.C.; Mathewson, N.D.; et al. IL2 Targeted to CD8+ T Cells Promotes Robust Effector T-cell Responses and Potent Antitumor Immunity. Cancer Discov. 2024, 14, 1206–1225. [Google Scholar] [CrossRef] [PubMed]
- Camirand, G.; Lakkis, F.G. Tipping the balance toward transplantation tolerance: In vivo therapy using a mutated IL-2. J. Clin. Investig. 2024, 134, e178570. [Google Scholar] [CrossRef] [PubMed]
- Glisson, B.S.; Leidner, R.S.; Ferris, R.L.; Powderly, J.; Rizvi, N.A.; Keam, B.; Schneider, R.; Goel, S.; Ohr, J.P.; Burton, J.; et al. Safety and Clinical Activity of MEDI0562, a Humanized OX40 Agonist Monoclonal Antibody, in Adult Patients with Advanced Solid Tumors. Clin. Cancer Res. 2020, 26, 5358–5367. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Burris, H.A.; de Miguel Luken, M.J.; Pishvaian, M.J.; Bang, Y.J.; Gordon, M.; Awada, A.; Camidge, D.R.; Hodi, F.S.; McArthur, G.A.; et al. First-In-Human Phase I Study of the OX40 Agonist MOXR0916 in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2022, 28, 3452–3463. [Google Scholar] [CrossRef]
- Remer, M.; Al-Shamkhani, A.; Glennie, M.; Johnson, P. Mogamulizumab and the treatment of CCR4-positive T-cell lymphomas. Immunotherapy 2014, 6, 1187–1206. [Google Scholar] [CrossRef]
- Coiffier, B.; Federico, M.; Caballero, D.; Dearden, C.; Morschhauser, F.; Jager, U.; Trumper, L.; Zucca, E.; Gomes da Silva, M.; Pettengell, R.; et al. Therapeutic options in relapsed or refractory peripheral T-cell lymphoma. Cancer Treat. Rev. 2014, 40, 1080–1088. [Google Scholar] [CrossRef]
- Litzinger, M.T.; Fernando, R.; Curiel, T.J.; Grosenbach, D.W.; Schlom, J.; Palena, C. IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood 2007, 110, 3192–3201. [Google Scholar] [CrossRef]
- Jacobs, J.F.; Punt, C.J.; Lesterhuis, W.J.; Sutmuller, R.P.; Brouwer, H.M.; Scharenborg, N.M.; Klasen, I.S.; Hilbrands, L.B.; Figdor, C.G.; de Vries, I.J.; et al. Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: A phase I/II study in metastatic melanoma patients. Clin. Cancer Res. 2010, 16, 5067–5078. [Google Scholar] [CrossRef]
- Labrijn, A.F.; Janmaat, M.L.; Reichert, J.M.; Parren, P. Bispecific antibodies: A mechanistic review of the pipeline. Nat. Rev. Drug Discov. 2019, 18, 585–608. [Google Scholar] [CrossRef]
- Chen, H.; Luan, X.; Paholak, H.J.; Burnett, J.P.; Stevers, N.O.; Sansanaphongpricha, K.; He, M.; Chang, A.E.; Li, Q.; Sun, D. Depleting tumor-associated Tregs via nanoparticle-mediated hyperthermia to enhance anti-CTLA-4 immunotherapy. Nanomedicine 2020, 15, 77–92. [Google Scholar] [CrossRef]
- Li, Z.; Deng, Y.; Sun, H.; Tan, C.; Li, H.; Mo, F.; Wang, Y.; Li, J.; Zhou, Z.; Sun, M. Redox modulation with a perfluorocarbon nanoparticle to reverse Treg-mediated immunosuppression and enhance anti-tumor immunity. J. Control. Release 2023, 358, 579–590. [Google Scholar] [CrossRef]
- Lu, Q.; Kou, D.; Lou, S.; Ashrafizadeh, M.; Aref, A.R.; Canadas, I.; Tian, Y.; Niu, X.; Wang, Y.; Torabian, P.; et al. Nanoparticles in tumor microenvironment remodeling and cancer immunotherapy. J. Hematol. Oncol. 2024, 17, 16. [Google Scholar] [CrossRef]
- Moon, T.J.; Ta, H.M.; Bhalotia, A.; Paulsen, K.E.; Hutchinson, D.W.; Arkema, G.M.; Choi, A.S.; Haynie, M.G.; Ogunnaike, L.; Dever, M.; et al. Nanoparticles targeting immune checkpoint protein VISTA induce potent antitumor immunity. J. Immunother. Cancer 2024, 12, e008977. [Google Scholar] [CrossRef] [PubMed]
- Kachikwu, E.L.; Iwamoto, K.S.; Liao, Y.P.; DeMarco, J.J.; Agazaryan, N.; Economou, J.S.; McBride, W.H.; Schaue, D. Radiation enhances regulatory T cell representation. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Klug, F.; Prakash, H.; Huber, P.E.; Seibel, T.; Bender, N.; Halama, N.; Pfirschke, C.; Voss, R.H.; Timke, C.; Umansky, L.; et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013, 24, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Ghiringhelli, F.; Menard, C.; Puig, P.E.; Ladoire, S.; Roux, S.; Martin, F.; Solary, E.; Le Cesne, A.; Zitvogel, L.; Chauffert, B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 2007, 56, 641–648. [Google Scholar] [CrossRef]
- Lutsiak, M.E.; Semnani, R.T.; De Pascalis, R.; Kashmiri, S.V.; Schlom, J.; Sabzevari, H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 2005, 105, 2862–2868. [Google Scholar] [CrossRef]
- Noordam, L.; Kaijen, M.E.H.; Bezemer, K.; Cornelissen, R.; Maat, L.; Hoogsteden, H.C.; Aerts, J.; Hendriks, R.W.; Hegmans, J.; Vroman, H. Low-dose cyclophosphamide depletes circulating naive and activated regulatory T cells in malignant pleural mesothelioma patients synergistically treated with dendritic cell-based immunotherapy. Oncoimmunology 2018, 7, e1474318. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, S.; Lv, S.; Liu, X.; Li, W.; Song, Y.; Rong, D.; Zheng, P.; Huang, H.; Zheng, H. Combined oral low-dose cyclophosphamide endocrine therapy may improve clinical response among patients with metastatic breast cancer via Tregs in TLSs. Sci. Rep. 2024, 14, 13432. [Google Scholar] [CrossRef]
- Bendell, J.; LoRusso, P.; Overman, M.; Noonan, A.M.; Kim, D.W.; Strickler, J.H.; Kim, S.W.; Clarke, S.; George, T.J.; Grimison, P.S.; et al. First-in-human study of oleclumab, a potent, selective anti-CD73 monoclonal antibody, alone or in combination with durvalumab in patients with advanced solid tumors. Cancer Immunol. Immunother. 2023, 72, 2443–2458. [Google Scholar] [CrossRef]
- Ingram, J.R.; Blomberg, O.S.; Rashidian, M.; Ali, L.; Garforth, S.; Fedorov, E.; Fedorov, A.A.; Bonanno, J.B.; Le Gall, C.; Crowley, S.; et al. Anti-CTLA-4 therapy requires an Fc domain for efficacy. Proc. Natl. Acad. Sci. USA 2018, 115, 3912–3917. [Google Scholar] [CrossRef] [PubMed]
- Solomon, I.; Amann, M.; Goubier, A.; Arce Vargas, F.; Zervas, D.; Qing, C.; Henry, J.Y.; Ghorani, E.; Akarca, A.U.; Marafioti, T.; et al. CD25-T(reg)-depleting antibodies preserving IL-2 signaling on effector T cells enhance effector activation and antitumor immunity. Nat. Cancer 2020, 1, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Willingham, S.B.; Ho, P.Y.; Hotson, A.; Hill, C.; Piccione, E.C.; Hsieh, J.; Liu, L.; Buggy, J.J.; McCaffery, I.; Miller, R.A. A2AR Antagonism with CPI-444 Induces Antitumor Responses and Augments Efficacy to Anti-PD-(L)1 and Anti-CTLA-4 in Preclinical Models. Cancer Immunol. Res. 2018, 6, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Dummer, R.; Hamid, O.; Gajewski, T.F.; Caglevic, C.; Dalle, S.; Arance, A.; Carlino, M.S.; Grob, J.J.; Kim, T.M.; et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): A phase 3, randomised, double-blind study. Lancet Oncol. 2019, 20, 1083–1097. [Google Scholar] [CrossRef]
- Davar, D.; Zappasodi, R.; Wang, H.; Naik, G.S.; Sato, T.; Bauer, T.; Bajor, D.; Rixe, O.; Newman, W.; Qi, J.; et al. Phase IB Study of GITR Agonist Antibody TRX518 Singly and in Combination with Gemcitabine, Pembrolizumab, or Nivolumab in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2022, 28, 3990–4002. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E., III; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Arce Vargas, F.; Furness, A.J.S.; Litchfield, K.; Joshi, K.; Rosenthal, R.; Ghorani, E.; Solomon, I.; Lesko, M.H.; Ruef, N.; Roddie, C.; et al. Fc Effector Function Contributes to the Activity of Human Anti-CTLA-4 Antibodies. Cancer Cell 2018, 33, 649–663.E4. [Google Scholar] [CrossRef]
- Gambardella, V.; Ong, M.; Rodriguez-Ruiz, M.E.; Machiels, J.P.; Sanmamed, M.F.; Galvao, V.; Spreafico, A.; Renouf, D.J.; Luen, S.J.; Galot, R.; et al. Safety and Antitumor Activity of a Novel aCD25 Treg Depleter RG6292 as a Single Agent and in Combination with Atezolizumab in Patients with Solid Tumors. Cancer Res. Commun. 2025, 5, 422–432. [Google Scholar] [CrossRef]
- Pousse, L.; Korfi, K.; Medeiros, B.C.; Berrera, M.; Kumpesa, N.; Eckmann, J.; Hutter, I.K.; Griesser, V.; Karanikas, V.; Klein, C.; et al. CD25 targeting with the afucosylated human IgG1 antibody RG6292 eliminates regulatory T cells and CD25+ blasts in acute myeloid leukemia. Front. Oncol. 2023, 13, 1150149. [Google Scholar] [CrossRef]
- Nagira, Y.; Nagira, M.; Nagai, R.; Nogami, W.; Hirata, M.; Ueyama, A.; Yoshida, T.; Yoshikawa, M.; Shinonome, S.; Yoshida, H.; et al. S-531011, a Novel Anti-Human CCR8 Antibody, Induces Potent Antitumor Responses through Depletion of Tumor-Infiltrating CCR8-Expressing Regulatory T Cells. Mol. Cancer Ther. 2023, 22, 1063–1072. [Google Scholar] [CrossRef]
- Seifert, M.; Benmebarek, M.R.; Briukhovetska, D.; Markl, F.; Dorr, J.; Cadilha, B.L.; Jobst, J.; Stock, S.; Andreu-Sanz, D.; Lorenzini, T.; et al. Impact of the selective A2(A)R and A2(B)R dual antagonist AB928/etrumadenant on CAR T cell function. Br. J. Cancer 2022, 127, 2175–2185. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Autio, K.; Golan, T.; Dobrenkov, K.; Chartash, E.; Chen, Q.; Wnek, R.; Long, G.V. Phase I Study of MK-4166, an Anti-human Glucocorticoid-Induced TNF Receptor Antibody, Alone or with Pembrolizumab in Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Loibl, S.; Noske, A.; Roller, M.; Muller, B.M.; Komor, M.; Budczies, J.; Darb-Esfahani, S.; Kronenwett, R.; Hanusch, C.; et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 2010, 28, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Kashiwagi, S.; Goto, W.; Kurata, K.; Noda, S.; Takashima, T.; Onoda, N.; Tanaka, S.; Ohsawa, M.; Hirakawa, K. Tumour-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting treatment responses to neoadjuvant chemotherapy of aggressive breast cancer. Br. J. Surg. 2016, 103, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, M.; Sasano, H.; Tamaki, K.; Hirakawa, H.; Takahashi, Y.; Nakagawa, S.; Watanabe, G.; Tada, H.; Suzuki, A.; Ohuchi, N.; et al. Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: A retrospective multicenter study. Breast Cancer Res. 2015, 17, 124. [Google Scholar] [CrossRef]
- Loibl, S.; O’Shaughnessy, J.; Untch, M.; Sikov, W.M.; Rugo, H.S.; McKee, M.D.; Huober, J.; Golshan, M.; von Minckwitz, G.; Maag, D.; et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): A randomised, phase 3 trial. Lancet Oncol. 2018, 19, 497–509. [Google Scholar] [CrossRef]
- Campbell, M.J.; Wolf, D.M.; Yau, C.; Brown-Swigart, L.; Wulfkuhle, J.; Gallagher, I.R.; Zhu, Z.; Bolen, J.; Vandenberg, S.; Hoyt, C.; et al. Multi-platform biomarkers of response to an immune checkpoint inhibitor in the neoadjuvant I-SPY 2 trial for early-stage breast cancer. Cell Rep. Med. 2024, 5, 101799. [Google Scholar] [CrossRef]
- Goswami, T.K.; Singh, M.; Dhawan, M.; Mitra, S.; Emran, T.B.; Rabaan, A.A.; Mutair, A.A.; Alawi, Z.A.; Alhumaid, S.; Dhama, K. Regulatory T cells (Tregs) and their therapeutic potential against autoimmune disorders—Advances and challenges. Hum. Vaccin. Immunother. 2022, 18, 2035117. [Google Scholar] [CrossRef]
- Holmgaard, R.B.; Zamarin, D.; Li, Y.; Gasmi, B.; Munn, D.H.; Allison, J.P.; Merghoub, T.; Wolchok, J.D. Tumor-Expressed IDO Recruits and Activates MDSCs in a Treg-Dependent Manner. Cell Rep. 2015, 13, 412–424. [Google Scholar] [CrossRef]
- Azizi, E.; Carr, A.J.; Plitas, G.; Cornish, A.E.; Konopacki, C.; Prabhakaran, S.; Nainys, J.; Wu, K.; Kiseliovas, V.; Setty, M.; et al. Single-Cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment. Cell 2018, 174, 1293–1308.E36. [Google Scholar] [CrossRef]
- Najjar, R. Redefining Radiology: A Review of Artificial Intelligence Integration in Medical Imaging. Diagnostics 2023, 13, 2760. [Google Scholar] [CrossRef]
- Alum, E.U. AI-driven biomarker discovery: Enhancing precision in cancer diagnosis and prognosis. Discov. Oncol. 2025, 16, 313. [Google Scholar] [CrossRef]
- Gaur, K.; Jagtap, M.M. Role of Artificial Intelligence and Machine Learning in Prediction, Diagnosis, and Prognosis of Cancer. Cureus 2022, 14, e31008. [Google Scholar] [CrossRef]
- You, Y.; Lai, X.; Pan, Y.; Zheng, H.; Vera, J.; Liu, S.; Deng, S.; Zhang, L. Artificial intelligence in cancer target identification and drug discovery. Signal Transduct. Target. Ther. 2022, 7, 156. [Google Scholar] [CrossRef]
| Signature/Module | Representative Genes | Evidence in Breast Cancer/Outcome Link | Key References |
|---|---|---|---|
| Breast tumor-infiltrating Treg (TI-Treg) marker set (CCR8-centered) | CCR8, FOXP3, IL2RA (CD25), CTLA4, ICOS, TIGIT; tissue/TI-Treg markers LAYN, MAGEH1 | Highly activated TI-Tregs in breast tumors selectively upregulate CCR8; co-expression of LAYN/MAGEH1/CCR8 correlates with poor prognosis. | [88,89]. |
| Pan-cancer TI-Treg (TITR) signature | CCR8, IL1R2, LAYN, MAGEH1, CTLA4, ICOS, TNFRSF1B (TNFR2) | Conserved TI-Treg program identified across human tumors, validated computationally and functionally, with breast cancer cohorts included in cross-tumor analysis. | [88,89]. |
| Inhibitory-receptor (checkpoint) module (“Immunosuppressive”) | CTLA4, LAG3, HAVCR2 (TIM-3), TIGIT, ICOS; often co-expressed with CCR8 | Human TI-Tregs upregulate multiple checkpoints, including TIM-3 and LAG-3, as part of the activated tumor-Treg phenotype; linked to suppression in breast cancer and other solid tumors. | [54,55]. |
| Adenosinergic (“Metabolic-Treg”) module | ENTPD1 (CD39), NT5E (CD73), ADORA2A | CD39/CD73+ Tregs generate adenosine, suppressing anti-tumor immunity. In breast cancer, CD73+ γδ Tregs and CD39/CD73-high Tregs correlate with poor prognosis. | [29,92]. |
| Breast cancer Treg-associated prognostic signatures (data-driven) | Study-defined (e.g., 6-gene Treg-associated prognostic signature) | Prognostic signatures derived from TCGA and other BC cohorts link Treg biology to survival and therapy sensitivity. | [91]. |
| Mechanism/Strategy | Example Agent(s) | How It Relates to Tregs | Representative Evidence (Preclinical/Clinical) | Key References |
|---|---|---|---|---|
| CTLA-4 blockade with Fc-effector activity | Ipilimumab; Fc-optimized anti-CTLA-4 variants | Preferential intratumoral Treg depletion via FcγR-mediated effector functions; contributes to efficacy | Mouse and humanized models show Treg depletion augments anti-tumor immunity; clinical correlative data support Fc-dependence | [28,141,147]. |
| CD25 (IL-2Rα)-targeted Treg depletion (non-IL-2-blocking) | RG6292/vopikitug (afucosylated anti-CD25) | Selective Treg depletion while preserving IL-2 signaling on Teff cells | Potent Treg depletion and synergy with ICB in preclinical models; early clinical reports show on-target Treg reduction | [142,148,149]. |
| CCR8-directed depletion of tumor-resident Tregs | BMS-986340, DKY709, BAY 3375968 | CCR8 is enriched on intratumoral Tregs; antibodies aim to deplete these cells | Preclinical: CCR8 mAbs deplete Tregs and boost CD8 responses; Clinical: first-in-human CCR8 mAb BMS-986340 ongoing (NCT04895709) | [150].; NCT04895709 (BMS-986340). |
| CD73 (ecto-5′-nucleotidase) blockade | Oleclumab (MEDI9447) | Lowers adenosine production that sustains Tregs and suppresses Teff cells | First-in-human safety/pharmacodynamic data; combinations under study in solid tumors | [140]. |
| Adenosine receptor antagonists (A2A/A2B) | Ciforadenant (CPI-444); Etrumadenant (AB928) | Block adenosine signaling that promotes Treg function and inhibits effector T cells | Preclinical CPI-444 restores T-cell function and synergizes with ICB; etrumadenant shows acceptable PK/PD and early clinical safety | [143,151].; Seitz L et al., Invest New Drugs 2019 (AB928 phase-1 HV). |
| IDO1 inhibition (tryptophan–kynurenine axis) | Epacadostat | Aims to limit tolerogenic DC and Treg-supportive metabolism | Phase III ECHO-301/KEYNOTE-252 (melanoma) negative for efficacy with pembrolizumab; concept under reevaluation | [144]. |
| GITR agonism | TRX518; MK-4166; BMS-986156 | Can attenuate Treg suppressive function and costimulate Teff | Early-phase trials show pharmacodynamic effects (Treg reduction/activation markers) with modest single-agent activity; combinations under study | [145,152]. |
| TGF-β pathway blockade/trap-PD-L1 fusion | Galunisertib; Bintrafusp alfa | TGF-β supports immune exclusion and Treg-dominant TMEs; blockade may relieve suppression | Urothelial cancer study linked TGF-β signaling with T-cell exclusion and resistance to PD-L1; multiple combo trials in solid tumors | [146]. |
| NCT ID | Intervention | Target | Phase | Status | Notes/Outcomes |
|---|---|---|---|---|---|
| NCT04895709 | BMS-986340 | CCR8 | I/II | Recruiting | Selective depletion of intratumoral Tregs; biomarker analyses ongoing. |
| NCT04158583/NCT04642365 | RG6292 (vopikitug) | CD25 | I | Terminated | Demonstrated both peripheral and intratumoral Treg depletion; however, clinical efficacy was limited in overcoming resistance. |
| NCT02281409 | Mogamulizumab (KW-0761) | CCR4 | I/II | Completed | Achieved Treg reduction; safety acceptable; limited breast cancer-specific benefit observed. |
| NCT03719326 | Etrumadenant (AB928) ± Pembrolizumab | A2A/A2B | I/Ib | Completed | Adenosine pathway blockade reduced Treg-mediated suppression; combination approach under further evaluation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, A.; Ayoub, S.; Zhang, H.; Wu, Y.; Rau, M.; Ma, X. Regulatory T Cells in Invasive Breast Cancer: Prognosis, Mechanisms and Therapy. Cancers 2025, 17, 3172. https://doi.org/10.3390/cancers17193172
Xu A, Ayoub S, Zhang H, Wu Y, Rau M, Ma X. Regulatory T Cells in Invasive Breast Cancer: Prognosis, Mechanisms and Therapy. Cancers. 2025; 17(19):3172. https://doi.org/10.3390/cancers17193172
Chicago/Turabian StyleXu, Aizhang, Sama Ayoub, Haijun Zhang, Yuhang Wu, Marcellino Rau, and Xiaojing Ma. 2025. "Regulatory T Cells in Invasive Breast Cancer: Prognosis, Mechanisms and Therapy" Cancers 17, no. 19: 3172. https://doi.org/10.3390/cancers17193172
APA StyleXu, A., Ayoub, S., Zhang, H., Wu, Y., Rau, M., & Ma, X. (2025). Regulatory T Cells in Invasive Breast Cancer: Prognosis, Mechanisms and Therapy. Cancers, 17(19), 3172. https://doi.org/10.3390/cancers17193172






