New Promising Steroidal Aromatase Inhibitors with Multi-Target Action on Estrogen and Androgen Receptors for Breast Cancer Treatment
Simple Summary
Abstract
1. Introduction
2. Material and Methods
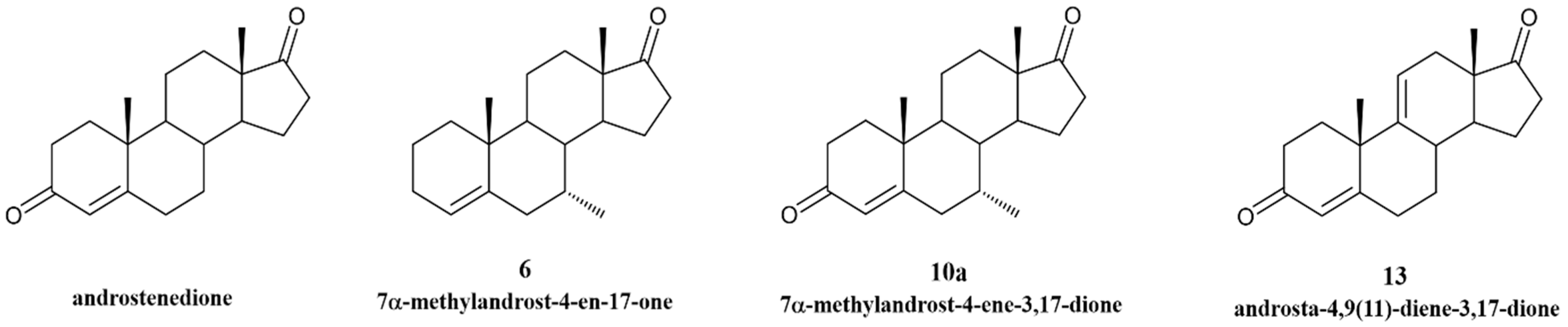
2.1. New Steroidal Aromatase Inhibitors Under Study
2.2. Cell Cultures
2.3. Studies of Cell Viability
2.4. Analysis of Cell Cycle Progression by Flow Cytometry
2.5. Analysis of Apoptotic Cell Death
2.6. Western Blot Assay
2.7. RNA Extraction and qPCR
2.8. ER and AR Transactivation Assays
2.9. Statistical Analysis
3. Results
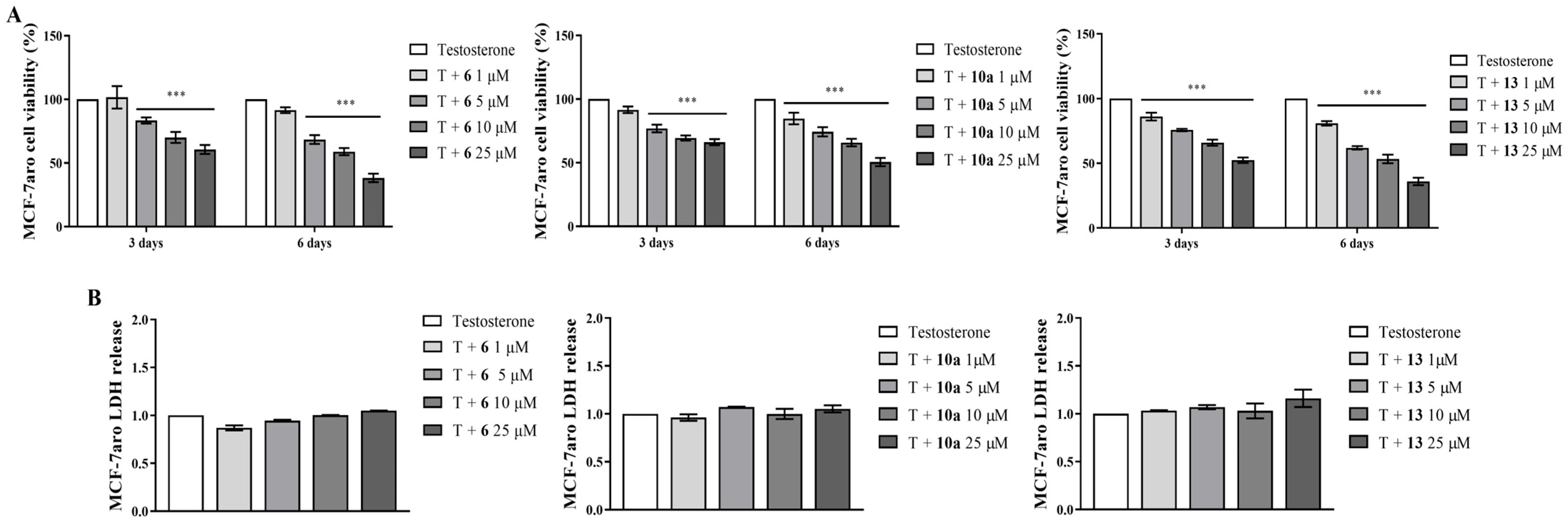
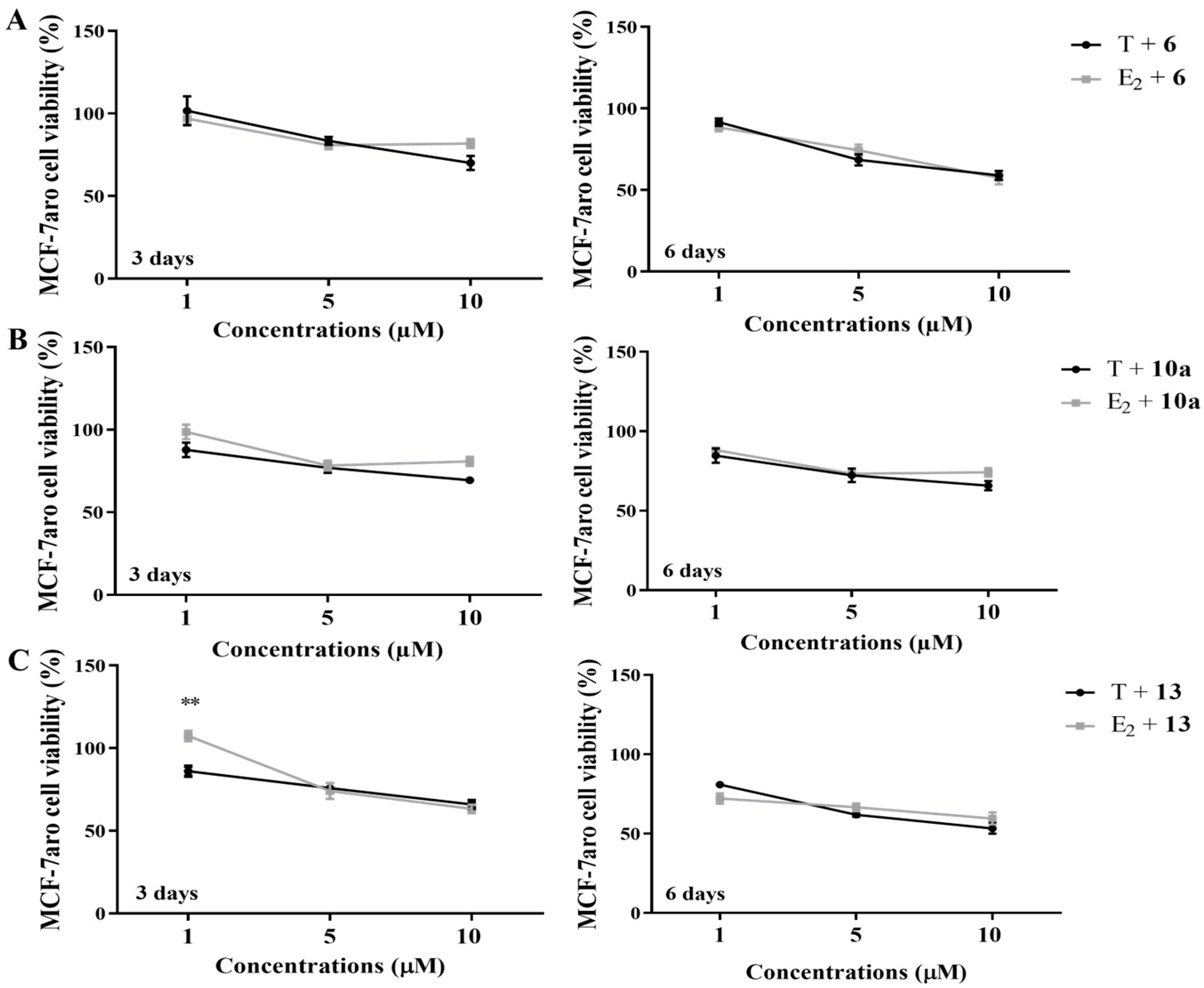
3.1. Effects on Viability of Non-Tumoral Cell Lines and of Sensitive ER+ Breast Cancer Cells
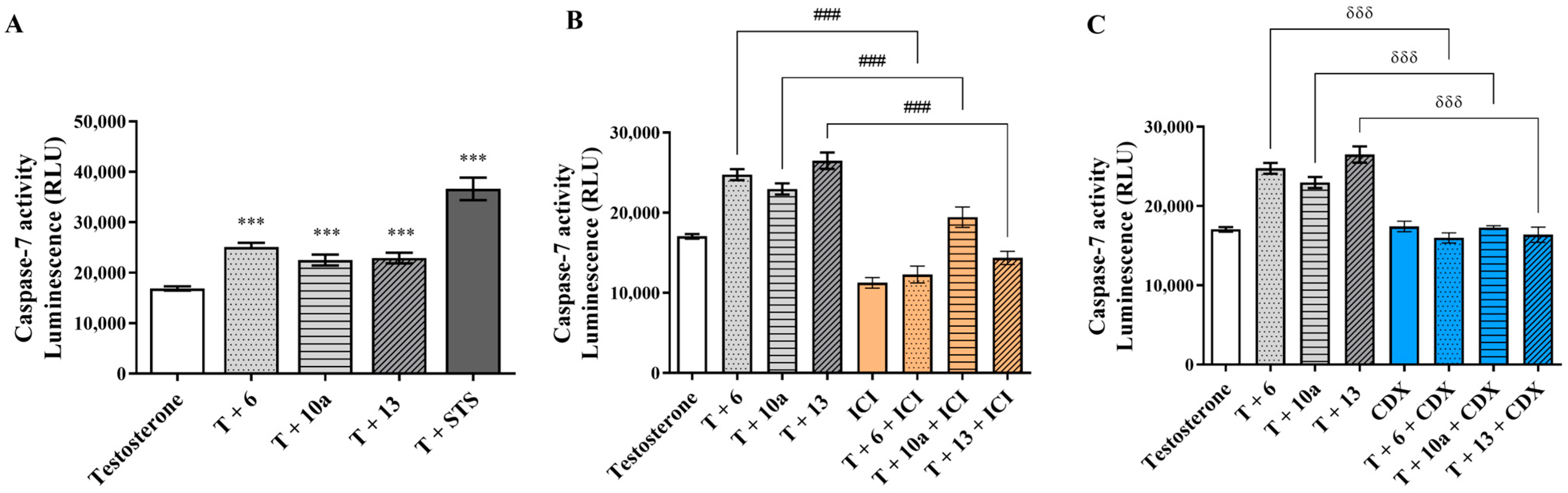
3.2. Behavior of AIs on Proliferation and on Cell Death of Sensitive Breast Cancer Cells
3.3. The Underlying Molecular Targets of the New AIs in Sensitive Breast Cancer Cells
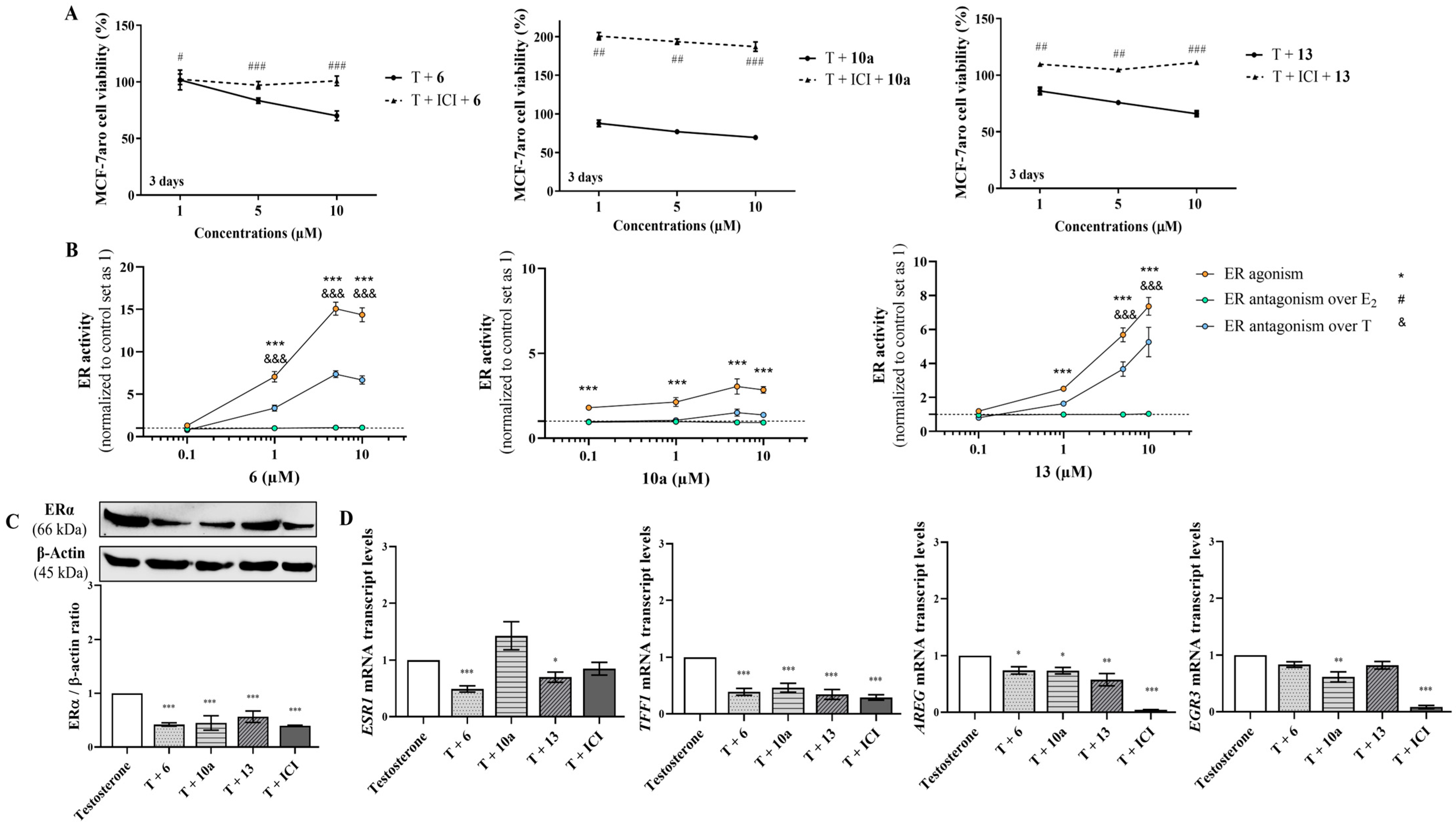
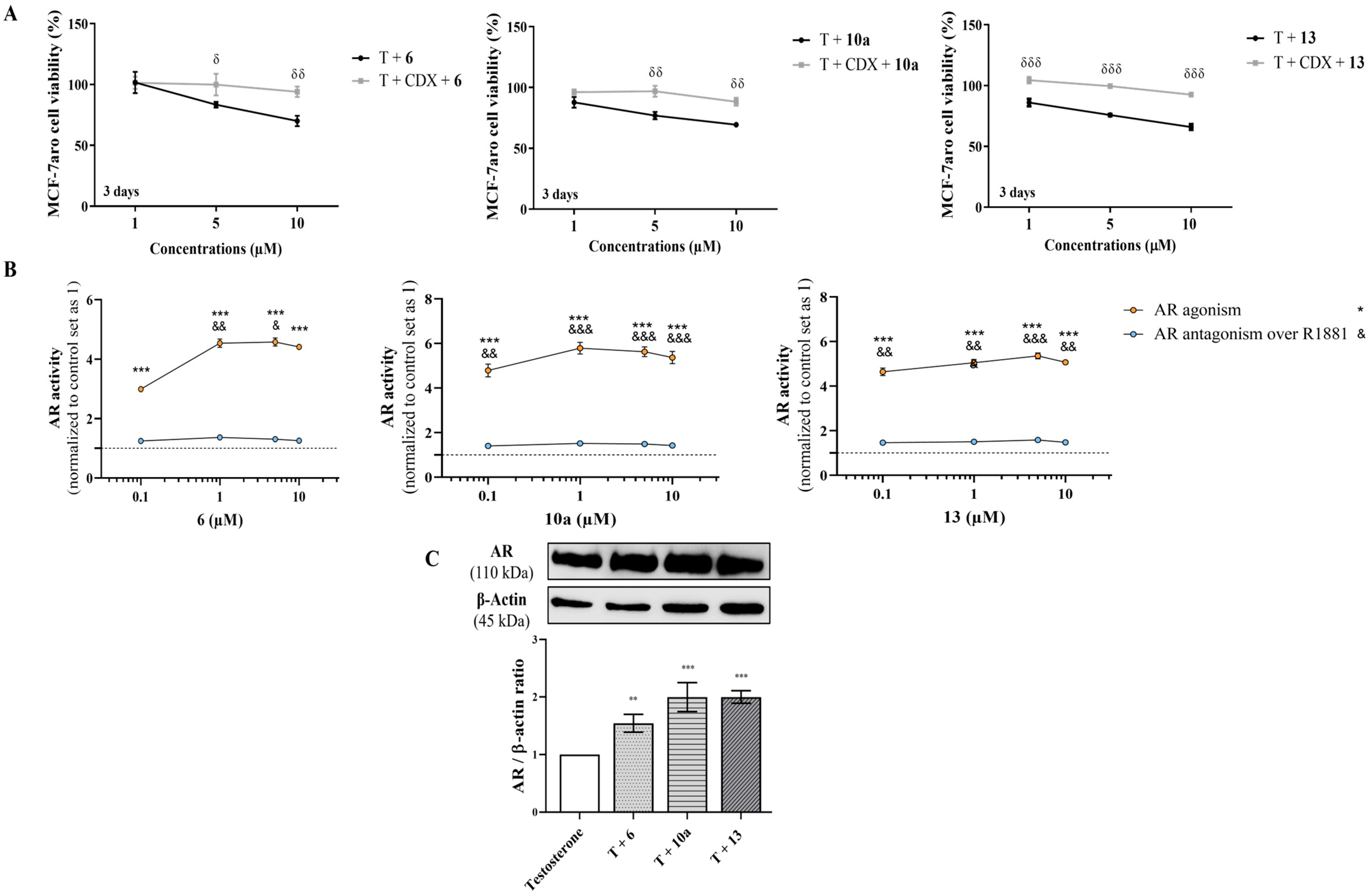
3.4. Effects on Resistant ER+ Breast Cancer Cells
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almeida, C.F.; Oliveira, A.; Ramos, M.J.; Fernandes, P.A.; Teixeira, N.; Amaral, C. CristinaAmaral, Estrogen receptor-positive (ER+) breast cancer treatment: Are multi-target compounds the next promising approach? Biochem. Pharmacol. 2020, 177, 113989. [Google Scholar]
- Augusto, T.; Correia-da-Silva, G.; Rodrigues, C.M.P.; Teixeira, N.; Amaral, C. Acquired-resistance to aromatase inhibitors: Where we stand! Endocr. Relat. Cancer 2018, 25, R283–R301. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Almeida, C.; Correia-da-Silva, G.; Teixeira, N.; Amaral, C. Influence of tumor microenvironment on the different breast cancer subtypes and applied therapies. Biochem. Pharmacol. 2024, 223, 116178. [Google Scholar] [CrossRef] [PubMed]
- Orrantia-Borunda, E.; Anchondo-Nunez, P.; Acuna-Aguilar, L.E.; Gomez-Valles, F.O.; Ramirez-Valdespino, C.A. Subtypes of Breast Cancer. In Breast Cancer; Mayrovitz, H.N., Ed.; Exon Publications: Brisbane, Australia, 2022; Chapter 3. [Google Scholar]
- Sleightholm, R.; Neilsen, B.K.; Elkhatib, S.; Flores, L.; Dukkipati, S.; Zhao, R.; Choudhury, S.; Gardner, B.; Carmichael, J.; Smith, L.; et al. Percentage of Hormone Receptor Positivity in Breast Cancer Provides Prognostic Value: A Single-Institute Study. J. Clin. Med. Res. 2021, 13, 9–19. [Google Scholar] [CrossRef]
- Razavi, P.; Chang, M.T.; Xu, G.; Bandlamudi, C.; Ross, D.S.; Vasan, N.; Cai, Y.; Bielski, C.M.; Donoghue, M.T.A.; Jonsson, P.; et al. The Genomic Landscape of Endocrine-Resistant Advanced Breast Cancers. Cancer Cell 2018, 34, 427–438.e6. [Google Scholar] [CrossRef]
- Piezzo, M.; Chiodini, P.; Riemma, M.; Cocco, S.; Caputo, R.; Cianniello, D.; Di Gioia, G.; Di Lauro, V.; Rella, F.D.; Fusco, G.; et al. Progression-Free Survival and Overall Survival of CDK 4/6 Inhibitors Plus Endocrine Therapy in Metastatic Breast Cancer: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 6400. [Google Scholar] [CrossRef]
- Ma, J.; Chan, J.J.; Toh, C.H.; Yap, Y.S. Emerging systemic therapy options beyond CDK4/6 inhibitors for hormone receptor-positive HER2-negative advanced breast cancer. NPJ Breast Cancer 2023, 9, 74. [Google Scholar] [CrossRef]
- Apostolidou, K.; Zografos, E.; Papatheodoridi, M.A.; Fiste, O.; Dimopoulos, M.A.; Zagouri, F. Oral SERDs alone or in combination with CDK 4/6 inhibitors in breast cancer: Current perspectives and clinical trials. Breast 2024, 75, 103729. [Google Scholar] [CrossRef]
- Corti, C.; De Angelis, C.; Bianchini, G.; Malorni, L.; Giuliano, M.; Hamilton, E.; Jeselsohn, R.; Jhaveri, K.; Curigliano, G.; Criscitiello, C. Novel endocrine therapies: What is next in estrogen receptor positive, HER2 negative breast cancer? Cancer Treat. Rev. 2023, 117, 102569. [Google Scholar] [CrossRef]
- Papadimitriou, M.C.; Pazaiti, A.; Iliakopoulos, K.; Markouli, M.; Michalaki, V.; Papadimitriou, C.A. Resistance to CDK4/6 inhibition: Mechanisms and strategies to overcome a therapeutic problem in the treatment of hormone receptor-positive metastatic breast cancer. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119346. [Google Scholar] [CrossRef]
- Wander, S.A.; Cohen, O.; Gong, X.; Johnson, G.N.; Buendia-Buendia, J.E.; Lloyd, M.R.; Kim, D.; Luo, F.; Mao, P.; Helvie, K.; et al. The Genomic Landscape of Intrinsic and Acquired Resistance to Cyclin-Dependent Kinase 4/6 Inhibitors in Patients with Hormone Receptor-Positive Metastatic Breast Cancer. Cancer Discov. 2020, 10, 1174–1193. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.H.; Downton, T.; Freelander, A.; Hurwitz, J.; Caldon, C.E.; Lim, E. CDK4/6 inhibitor resistance in estrogen receptor positive breast cancer, a 2023 perspective. Front. Cell Dev. Biol. 2023, 11, 1148792. [Google Scholar] [CrossRef]
- Neupane, N.; Bawek, S.; Gurusinghe, S.; Ghaffary, E.M.; Mirmosayyeb, O.; Thapa, S.; Falkson, C.; O’Regan, R.; Dhakal, A. Oral SERD, a Novel Endocrine Therapy for Estrogen Receptor-Positive Breast Cancer. Cancers 2024, 16, 619. [Google Scholar] [CrossRef] [PubMed]
- Munson, B.P.; Chen, M.; Bogosian, A.; Kreisberg, J.F.; Licon, K.; Abagyan, R.; Kuenzi, B.M.; Ideker, T. De novo generation of multi-target compounds using deep generative chemistry. Nat. Commun. 2024, 15, 3636. [Google Scholar] [CrossRef] [PubMed]
- Anighoro, A.; Bajorath, J.; Rastelli, G. Polypharmacology: Challenges and opportunities in drug discovery. J. Med. Chem. 2014, 57, 7874–7887. [Google Scholar] [CrossRef]
- Antolin, A.A.; Workman, P.; Mestres, J.; Al-Lazikani, B. Polypharmacology in Precision Oncology: Current Applications and Future Prospects. Curr. Pharm. Des. 2016, 22, 6935–6945. [Google Scholar] [CrossRef]
- Knight, Z.A.; Lin, H.; Shokat, K.M. Targeting the cancer kinome through polypharmacology. Nat. Rev. Cancer 2010, 10, 130–137. [Google Scholar] [CrossRef]
- Kabir, A.; Muth, A. Polypharmacology: The science of multi-targeting molecules. Pharmacol. Res. 2022, 176, 106055. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Popovic-Nikolic, M.R.; Nikolic, K.; Uliassi, E.; Bolognesi, M.L. A perspective on multi-target drug discovery and design for complex diseases. Clin. Transl. Med. 2018, 7, 3. [Google Scholar] [CrossRef]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERalpha) and beta (ERbeta): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef]
- Safarpour, D.; Pakneshan, S.; Tavassoli, F.A. Androgen receptor (AR) expression in 400 breast carcinomas: Is routine AR assessment justified? Am. J. Cancer Res. 2014, 4, 353–368. [Google Scholar] [PubMed]
- Park, S.; Koo, J.S.; Kim, M.S.; Park, H.S.; Lee, J.S.; Kim, S.I.; Park, B.W.; Lee, K.S. Androgen receptor expression is significantly associated with better outcomes in estrogen receptor-positive breast cancers. Ann. Oncol. 2011, 22, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.; Cinausero, M.; Iacono, D.; Pelizzari, G.; Bonotto, M.; Vitale, M.G.; Gerratana, L.; Puglisi, F. Androgen receptor in estrogen receptor positive breast cancer: Beyond expression. Cancer Treat. Rev. 2017, 61, 15–22. [Google Scholar] [CrossRef]
- Amaral, C.; Augusto, T.V.; Almada, M.; Cunha, S.C.; Correia-da-Silva, G.; Teixeira, N. The potential clinical benefit of targeting androgen receptor (AR) in estrogen-receptor positive breast cancer cells treated with Exemestane. Biochim. Biophys. Acta Mol. Basis. Dis. 2020, 1866, 165661. [Google Scholar] [CrossRef]
- Chia, K.; O’Brien, M.; Brown, M.; Lim, E. Targeting the androgen receptor in breast cancer. Curr. Oncol. Rep. 2015, 17, 4. [Google Scholar] [CrossRef]
- Narayanan, R.; Dalton, J.T. Androgen Receptor: A Complex Therapeutic Target for Breast Cancer. Cancers 2016, 8, 108. [Google Scholar] [CrossRef]
- Amaral, C.; Correia-da-Silva, G.; Almeida, C.F.; Valente, M.J.; Varela, C.; Tavares-da-Silva, E.; Vinggaard, A.M.; Teixeira, N.; Roleira, F.M.F. An Exemestane Derivative, Oxymestane-D1, as a New Multi-Target Steroidal Aromatase Inhibitor for Estrogen Receptor-Positive (ER(+)) Breast Cancer: Effects on Sensitive and Resistant Cell Lines. Molecules 2023, 28, 789. [Google Scholar] [CrossRef]
- Zhao, L.M.; Jin, H.S.; Liu, J.; Skaar, T.C.; Ipe, J.; Lv, W.; Flockhart, D.A.; Cushman, M. A new Suzuki synthesis of triphenylethylenes that inhibit aromatase and bind to estrogen receptors alpha and beta. Bioorg. Med. Chem. 2016, 24, 5400–5409. [Google Scholar] [CrossRef][Green Version]
- Almeida, C.F.; Teixeira, N.; Oliveira, A.; Augusto, T.V.; Correia-da-Silva, G.; Ramos, M.J.; Fernandes, P.A.; Amaral, C. Discovery of a multi-target compound for estrogen receptor-positive (ER(+)) breast cancer: Involvement of aromatase and ERs. Biochimie 2021, 181, 65–76. [Google Scholar] [CrossRef]
- Almeida, C.F.; Teixeira, N.; Valente, M.J.; Vinggaard, A.M.; Correia-da-Silva, G.; Amaral, C. Cannabidiol as a Promising Adjuvant Therapy for Estrogen Receptor-Positive Breast Tumors: Unveiling Its Benefits with Aromatase Inhibitors. Cancers 2023, 15, 2517. [Google Scholar] [CrossRef]
- Amaral, C.; Trouille, F.M.; Almeida, C.F.; Correia-da-Silva, G.; Teixeira, N. Unveiling the mechanism of action behind the anti-cancer properties of cannabinoids in ER(+) breast cancer cells: Impact on aromatase and steroid receptors. J. Steroid Biochem. Mol. Biol. 2021, 210, 105876. [Google Scholar] [CrossRef] [PubMed]
- Amaral, C.; Varela, C.L.; Mauricio, J.; Sobral, A.F.; Costa, S.C.; Roleira, F.M.F.; Tavares-da-Silva, E.J.; Correia-da-Silva, G.; Teixeira, N. Anti-tumor efficacy of new 7alpha-substituted androstanes as aromatase inhibitors in hormone-sensitive and resistant breast cancer cells. J. Steroid Biochem. Mol. Biol. 2017, 171, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Augusto, T.V.; Amaral, C.; Varela, C.L.; Bernardo, F.; da Silva, E.T.; Roleira, F.F.M.; Costa, S.; Teixeira, N.; Correia-da-Silva, G. Effects of new C6-substituted steroidal aromatase inhibitors in hormone-sensitive breast cancer cells: Cell death mechanisms and modulation of estrogen and androgen receptors. J. Steroid Biochem. Mol. Biol. 2019, 195, 105486. [Google Scholar] [CrossRef] [PubMed]
- Amaral, C.; Varela, C.; Azevedo, M.; da Silva, E.T.; Roleira, F.M.; Chen, S.; Correia-da-Silva, G.; Teixeira, N. Effects of steroidal aromatase inhibitors on sensitive and resistant breast cancer cells: Aromatase inhibition and autophagy. J. Steroid Biochem. Mol. Biol. 2013, 135, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Liu, J.; Lu, D.; Flockhart, D.A.; Cushman, M. Synthesis of mixed (E,Z)-, (E)-, and (Z)-norendoxifen with dual aromatase inhibitory and estrogen receptor modulatory activities. J. Med. Chem. 2013, 56, 4611–4618. [Google Scholar] [CrossRef]
- Lv, W.; Liu, J.; Skaar, T.C.; Flockhart, D.A.; Cushman, M. Design and synthesis of norendoxifen analogues with dual aromatase inhibitory and estrogen receptor modulatory activities. J. Med. Chem. 2015, 58, 2623–2648. [Google Scholar] [CrossRef]
- Lv, W.; Liu, J.; Skaar, T.C.; O’Neill, E.; Yu, G.; Flockhart, D.A.; Cushman, M. Synthesis of Triphenylethylene Bisphenols as Aromatase Inhibitors That Also Modulate Estrogen Receptors. J. Med. Chem. 2016, 59, 157–170. [Google Scholar] [CrossRef]
- Varela, C.L.; Amaral, C.; Correia-da-Silva, G.; Costa, S.C.; Carvalho, R.A.; Costa, G.; Alcaro, S.; Teixeira, N.A.; Tavares-da-Silva, E.J.; Roleira, F.M. Exploring new chemical functionalities to improve aromatase inhibition of steroids. Bioorg. Med. Chem. 2016, 24, 2823–2831. [Google Scholar] [CrossRef]
- Roleira, F.M.F.; Varela, C.; Amaral, C.; Costa, S.C.; Correia-da-Silva, G.; Moraca, F.; Costa, G.; Alcaro, S.; Teixeira, N.A.A.; Tavares da Silva, E.J. C-6alpha- vs C-7alpha-Substituted Steroidal Aromatase Inhibitors: Which Is Better? Synthesis, Biochemical Evaluation, Docking Studies, and Structure-Activity Relationships. J. Med. Chem. 2019, 62, 3636–3657. [Google Scholar] [CrossRef]
- Almeida, C.F.; Amaral, C.; Augusto, T.V.; Correia-da-Silva, G.; Marques de Andrade, C.; Torqueti, M.R.; Teixeira, N. The anti-cancer potential of crotoxin in estrogen receptor-positive breast cancer: Its effects and mechanism of action. Toxicon 2021, 200, 69–77. [Google Scholar] [CrossRef]
- Zhou, D.J.; Pompon, D.; Chen, S.A. Stable expression of human aromatase complementary DNA in mammalian cells: A useful system for aromatase inhibitor screening. Cancer Res. 1990, 50, 6949–6954. [Google Scholar] [PubMed]
- Sun, X.Z.; Zhou, D.; Chen, S. Autocrine and paracrine actions of breast tumor aromatase. A three-dimensional cell culture study involving aromatase transfected MCF-7 and T-47D cells. J. Steroid Biochem. Mol. Biol. 1997, 63, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Karlsberg, K.; Kijima, I.; Yuan, Y.C.; Smith, D.; Ye, J.; Chen, S. Letrozole-, anastrozole-, and tamoxifen-responsive genes in MCF-7aro cells: A microarray approach. Mol. Cancer Res. 2005, 3, 203–218. [Google Scholar] [CrossRef]
- Masri, S.; Phung, S.; Wang, X.; Wu, X.; Yuan, Y.C.; Wagman, L.; Chen, S. Genome-wide analysis of aromatase inhibitor-resistant, tamoxifen-resistant, and long-term estrogen-deprived cells reveals a role for estrogen receptor. Cancer Res. 2008, 68, 4910–4918. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Chen, S. The development, application and limitations of breast cancer cell lines to study tamoxifen and aromatase inhibitor resistance. J. Steroid Biochem. Mol. Biol. 2012, 131, 83–92. [Google Scholar] [CrossRef]
- Amaral, C.; Borges, M.; Melo, S.; da Silva, E.T.; Correia-da-Silva, G.; Teixeira, N. Apoptosis and autophagy in breast cancer cells following exemestane treatment. PLoS ONE 2012, 7, e42398. [Google Scholar] [CrossRef]
- Augusto, T.V.; Amaral, C.; Almeida, C.F.; Teixeira, N.; Correia-da-Silva, G. Differential biological effects of aromatase inhibitors: Apoptosis, autophagy, senescence and modulation of the hormonal status in breast cancer cells. Mol. Cell Endocrinol. 2021, 537, 111426. [Google Scholar] [CrossRef]
- Masri, S.; Phung, S.; Wang, X.; Chen, S. Molecular characterization of aromatase inhibitor-resistant, tamoxifen-resistant and LTEDaro cell lines. J. Steroid Biochem. Mol. Biol. 2010, 118, 277–282. [Google Scholar] [CrossRef]
- Zilli, M.; Grassadonia, A.; Tinari, N.; Di Giacobbe, A.; Gildetti, S.; Giampietro, J.; Natoli, C.; Iacobelli, S. Molecular mechanisms of endocrine resistance and their implication in the therapy of breast cancer. Biochim. Biophys. Acta 2009, 1795, 62–81. [Google Scholar] [CrossRef]
- Chen, S.; Masri, S.; Hong, Y.; Wang, X.; Phung, S.; Yuan, Y.C.; Wu, X. New experimental models for aromatase inhibitor resistance. J. Steroid Biochem. Mol. Biol. 2007, 106, 8–15. [Google Scholar] [CrossRef][Green Version]
- Chen, S. An “omics” approach to determine the mechanisms of acquired aromatase inhibitor resistance. OMICS 2011, 15, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Augusto, T.V.; Amaral, C.; Wang, Y.; Chen, S.; Almeida, C.F.; Teixeira, N.; Correia-da-Silva, G. Effects of PI3K inhibition in AI-resistant breast cancer cell lines: Autophagy, apoptosis, and cell cycle progression. Breast Cancer Res. Treat. 2021, 190, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Amaral, C.; Varela, C.; Borges, M.; Tavares da Silva, E.; Roleira, F.M.; Correia-da-Silva, G.; Teixeira, N. Steroidal aromatase inhibitors inhibit growth of hormone-dependent breast cancer cells by inducing cell cycle arrest and apoptosis. Apoptosis 2013, 18, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, H.; Nishio, K.; Fukumoto, H.; Tomonari, A.; Suzuki, T.; Saijo, N. Alteration of caspase-3 (CPP32/Yama/apopain) in wild-type MCF-7, breast cancer cells. Oncol. Rep. 1999, 6, 33–37. [Google Scholar] [CrossRef]
- Yoshitake, R.; Mori, H.; Ha, D.; Wu, X.; Wang, J.; Wang, X.; Saeki, K.; Chang, G.; Shim, H.J.; Chan, Y.; et al. Molecular features of luminal breast cancer defined through spatial and single-cell transcriptomics. Clin. Transl. Med. 2024, 14, e1548. [Google Scholar] [CrossRef]
- Wang, Y.; Tzeng, Y.T.; Chang, G.; Wang, X.; Chen, S. Amphiregulin retains ERalpha expression in acquired aromatase inhibitor resistant breast cancer cells. Endocr. Relat. Cancer 2020, 27, 671–683. [Google Scholar] [CrossRef]
- Inoue, A.; Omoto, Y.; Yamaguchi, Y.; Kiyama, R.; Hayashi, S.I. Transcription factor EGR3 is involved in the estrogen-signaling pathway in breast cancer cells. J. Mol. Endocrinol. 2004, 32, 649–661. [Google Scholar] [CrossRef]
- Almeida, C.F.; Palmeira, A.; Valente, M.J.; Correia-da-Silva, G.; Vinggaard, A.M.; Sousa, M.E.; Teixeira, N.; Amaral, C. Molecular Targets of Minor Cannabinoids in Breast Cancer: In Silico and In Vitro Studies. Pharmaceuticals 2024, 17, 1245. [Google Scholar] [CrossRef]
- OECD. Test No. 458: Stably Transfected Human Androgen Receptor Transcriptional Activation Assay for Detection of Androgenic Agonist and Antagonist Activity of Chemicals; OECD Publishing: Paris, France, 2020. [Google Scholar]
- OECD. Test No. 455: Performance-Based Test Guideline for Stably Transfected Transactivation In Vitro Assays to Detect Estrogen Receptor Agonists and Antagonists; OECD Publishing: Paris, France, 2021. [Google Scholar]
- Min, H.Y.; Lee, H.Y. Molecular targeted therapy for anticancer treatment. Exp. Mol. Med. 2022, 54, 1670–1694. [Google Scholar] [CrossRef]
- Amaral, C.; Lopes, A.; Varela, C.L.; da Silva, E.T.; Roleira, F.M.; Correia-da-Silva, G.; Teixeira, N. Exemestane metabolites suppress growth of estrogen receptor-positive breast cancer cells by inducing apoptosis and autophagy: A comparative study with Exemestane. Int. J. Biochem. Cell Biol. 2015, 69, 183–195. [Google Scholar] [CrossRef]
- Varela, C.L.; Amaral, C.; Tavares da Silva, E.; Lopes, A.; Correia-da-Silva, G.; Carvalho, R.A.; Costa, S.C.; Roleira, F.M.; Teixeira, N. Exemestane metabolites: Synthesis, stereochemical elucidation, biochemical activity and anti-proliferative effects in a hormone-dependent breast cancer cell line. Eur. J. Med. Chem. 2014, 87, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Thiantanawat, A.; Long, B.J.; Brodie, A.M. Signaling pathways of apoptosis activated by aromatase inhibitors and antiestrogens. Cancer Res. 2003, 63, 8037–8050. [Google Scholar] [PubMed]
- Patel, H.K.; Bihani, T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol. Ther. 2018, 186, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Nardone, A.; Weir, H.; Delpuech, O.; Brown, H.; De Angelis, C.; Cataldo, M.L.; Fu, X.; Shea, M.J.; Mitchell, T.; Veeraraghavan, J.; et al. The oral selective oestrogen receptor degrader (SERD) AZD9496 is comparable to fulvestrant in antagonising ER and circumventing endocrine resistance. Br. J. Cancer 2019, 120, 331–339. [Google Scholar] [CrossRef]
- Tsai, J.M.; Aguirre, J.D.; Li, Y.D.; Brown, J.; Focht, V.; Kater, L.; Kempf, G.; Sandoval, B.; Schmitt, S.; Rutter, J.C.; et al. UBR5 forms ligand-dependent complexes on chromatin to regulate nuclear hormone receptor stability. Mol. Cell 2023, 83, 2753–2767.e10. [Google Scholar] [CrossRef]


| Target Gene | Primer Sequences (5′–3′) | Ta/°C | |
|---|---|---|---|
| Sense | Anti-Sense | ||
| TUBA1A | CTGGAGCACTCTGATTGT | ATAAGGCGGTTAAGGTTAGT | 55 |
| ACTB | TGCCATCCTAAAAGCCACCC | AGACCAAAAGCCTTCATACATCTC | 55 |
| ESR1 | CCTGATCATGGAGGGTCAAA | TGGGCTTACTGACCAACCTG | 55 |
| TFF1 | GTGGTTTTCCTGGTGTCACG | AGGATAGAAGCACCAGGGGA | 55 |
| EGR3 | GACTCCCCTTCCAACTGGTG | GGATACATGGCCTCCACGTC | 56 |
| AREG | TGTCGCTCTTGATACTCGGC | ATGGTTCACGCTTCCCAGAG | 56 |
| G0/G1 | S | G2/M | |
|---|---|---|---|
| Testosterone | 73.77 ± 0.74 | 7.84 ± 0.29 | 16.00 ± 0.73 |
| T + 6 | 82.19 ± 0.53 *** | 2.67 ± 0.17 *** | 14.26 ± 0.41 * |
| T + 10a | 85.92 ± 1.23 *** | 2.00 ± 0.19 *** | 11.97 ± 0.85 *** |
| T + 13 | 84.50 ± 0.67 *** | 2.16 ± 0.22 *** | 12.16 ± 0.33 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, C.; Almeida, C.F.; Valente, M.J.; Varela, C.L.; Costa, S.C.; Roleira, F.M.F.; Tavares-da-Silva, E.; Vinggaard, A.M.; Teixeira, N.; Correia-da-Silva, G. New Promising Steroidal Aromatase Inhibitors with Multi-Target Action on Estrogen and Androgen Receptors for Breast Cancer Treatment. Cancers 2025, 17, 165. https://doi.org/10.3390/cancers17020165
Amaral C, Almeida CF, Valente MJ, Varela CL, Costa SC, Roleira FMF, Tavares-da-Silva E, Vinggaard AM, Teixeira N, Correia-da-Silva G. New Promising Steroidal Aromatase Inhibitors with Multi-Target Action on Estrogen and Androgen Receptors for Breast Cancer Treatment. Cancers. 2025; 17(2):165. https://doi.org/10.3390/cancers17020165
Chicago/Turabian StyleAmaral, Cristina, Cristina F. Almeida, Maria João Valente, Carla L. Varela, Saul C. Costa, Fernanda M. F. Roleira, Elisiário Tavares-da-Silva, Anne Marie Vinggaard, Natércia Teixeira, and Georgina Correia-da-Silva. 2025. "New Promising Steroidal Aromatase Inhibitors with Multi-Target Action on Estrogen and Androgen Receptors for Breast Cancer Treatment" Cancers 17, no. 2: 165. https://doi.org/10.3390/cancers17020165
APA StyleAmaral, C., Almeida, C. F., Valente, M. J., Varela, C. L., Costa, S. C., Roleira, F. M. F., Tavares-da-Silva, E., Vinggaard, A. M., Teixeira, N., & Correia-da-Silva, G. (2025). New Promising Steroidal Aromatase Inhibitors with Multi-Target Action on Estrogen and Androgen Receptors for Breast Cancer Treatment. Cancers, 17(2), 165. https://doi.org/10.3390/cancers17020165






