FLT3: A 35-Year Voyage from Discovery to the Next Generation of Targeted Therapy in AML
Simple Summary
Abstract
1. Biology of FLT3
1.1. FLT3 in Hematopoietic Stem Cells (HSCs)
1.2. FLT3 in Immunity
1.3. FLT3 Signaling
2. FLT3 in Hematological Malignancies
2.1. FLT3 Mutations in AML
2.1.1. Frequency and Types
2.1.2. Clinical Presentation
2.1.3. Prognostic Impact and Co-Mutations
2.1.4. Historical Treatment Context
2.2. FLT3 Mutations in APL
2.3. FLT3 in Acute Lymphoblastic Leukemia
3. Development of FLT3 Inhibitors in AML
3.1. First-Generation FLT3 Inhibitors
3.1.1. Lessons Learned from the Use Sorafenib
3.1.2. Upfront Approval: Midostaurin
3.2. Second-Generation FLT3 Inibitors
3.2.1. Relapsed/Refractory AML: Gilteritinib
3.2.2. Upfront Approval: Quizartinib
3.2.3. Crenolanib: The Next Generation of Type I FLT3 Inhibition
3.3. Use of FLT3 Inhibitors in Maintenance
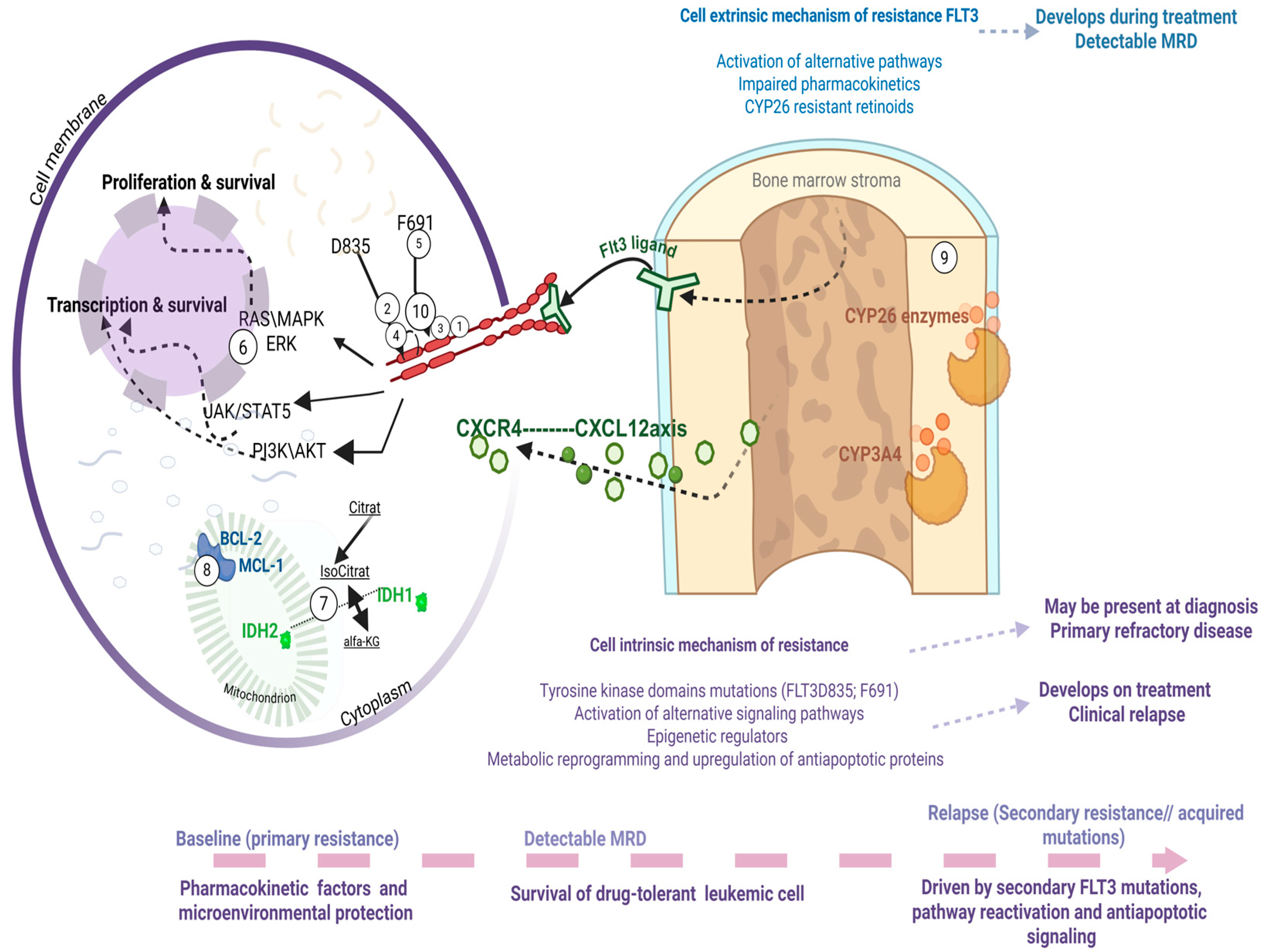
4. Mechanisms of Resistance to FLT3 Inhibitors
4.1. Cell Intrinsic: Acquisition of Additional Mutations
4.1.1. Tyrosine Kinase Domains Mutations
4.1.2. Activation of Alternative Signaling Pathways
4.1.3. Epigenetic Regulators
4.1.4. Metabolic Reprogramming and Upregulation of Antiapoptotic Proteins
4.2. Cell Extrinsic: Activation of Alternative Pathways; Impaired Pharmacokinetics; Retinoids
4.2.1. General Mechanisms: Cytokine Signaling and Pharmacokinetics
4.2.2. Differentiation, FLT3 Expression, and Retinoid Metabolism
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kiyoi, H.; Kawashima, N.; Ishikawa, Y. FLT3 mutations in acute myeloid leukemia: Therapeutic paradigm beyond inhibitor development. Cancer Sci. 2020, 111, 312–322. [Google Scholar] [CrossRef]
- Gilliland, D.G.; Griffin, J.D. The roles of FLT3 in hematopoiesis and leukemia. Blood 2002, 100, 1532–1542. [Google Scholar] [CrossRef]
- Cueto, F.J.; Sancho, D. The Flt3L/Flt3 Axis in Dendritic Cell Biology and Cancer Immunotherapy. Cancers 2021, 13, 1525. [Google Scholar] [CrossRef] [PubMed]
- Shurin, M.R.; Esche, C.; Lotze, M.T. FLT3: Receptor and ligand. Biology and potential clinical application. Cytokine Growth Factor. Rev. 1998, 9, 37–48. [Google Scholar] [CrossRef]
- McKenna, H.J.; Stocking, K.L.; Miller, R.E.; Brasel, K.; De Smedt, T.; Maraskovsky, E.; Maliszewski, C.R.; Lynch, D.H.; Smith, J.; Pulendran, B.; et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood 2000, 95, 3489–3497. [Google Scholar] [CrossRef] [PubMed]
- Onai, N.; Obata-Onai, A.; Tussiwand, R.; Lanzavecchia, A.; Manz, M.G. Activation of the Flt3 signal transduction cascade rescues and enhances type I interferon-producing and dendritic cell development. J. Exp. Med. 2006, 203, 227–238. [Google Scholar] [CrossRef]
- Saunders, D.; Lucas, K.; Ismaili, J.; Wu, L.; Maraskovsky, E.; Dunn, A.; Shortman, K. Dendritic cell development in culture from thymic precursor cells in the absence of granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1996, 184, 2185–2196. [Google Scholar] [CrossRef]
- Schlaweck, S.; Radcke, A.; Kampmann, S.; Becker, B.V.; Brossart, P.; Heine, A. The Immunomodulatory Effect of Different FLT3 Inhibitors on Dendritic Cells. Cancers 2024, 16, 3719. [Google Scholar] [CrossRef]
- Ramos, M.I.; Tak, P.P.; Lebre, M.C. Fms-like tyrosine kinase 3 ligand-dependent dendritic cells in autoimmune inflammation. Autoimmun. Rev. 2014, 13, 117–124. [Google Scholar] [CrossRef]
- Laouar, Y.; Welte, T.; Fu, X.Y.; Flavell, R.A. STAT3 is required for Flt3L-dependent dendritic cell differentiation. Immunity 2003, 19, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Kazi, J.U.; Rönnstrand, L. FMS-like Tyrosine Kinase 3/FLT3: From Basic Science to Clinical Implications. Physiol. Rev. 2019, 99, 1433–1466. [Google Scholar] [CrossRef]
- Takahashi, S. Downstream molecular pathways of FLT3 in the pathogenesis of acute myeloid leukemia: Biology and therapeutic implications. J. Hematol. Oncol. 2011, 4, 13. [Google Scholar] [CrossRef]
- Vela-Ojeda, J.; Cardenas, P.V.; Garcia-Ruiz Esparza, M.A.; Montiel Cervantes, L.A.; Chavez, J.G.; Caballero, A.H.; Majluf-Cruz, A.; Vega-Lopez, A.; Reyes-Maldonado, E. FLT3-ITD and CD135 Over-Expression are Frequent Findings of Poor Survival in Adult Patients with Acute Leukemias. Arch. Med. Res. 2021, 52, 217–223. [Google Scholar] [CrossRef]
- Kennedy, V.E.; Smith, C.C. FLT3 Mutations in Acute Myeloid Leukemia: Key Concepts and Emerging Controversies. Front. Oncol. 2020, 10, 612880. [Google Scholar] [CrossRef]
- Piloto, O.; Wright, M.; Brown, P.; Kim, K.T.; Levis, M.; Small, D. Prolonged exposure to FLT3 inhibitors leads to resistance via activation of parallel signaling pathways. Blood 2007, 109, 1643–1652. [Google Scholar] [CrossRef]
- Patel, J.P.; Gonen, M.; Figueroa, M.E.; Fernandez, H.; Sun, Z.; Racevskis, J.; Van Vlierberghe, P.; Dolgalev, I.; Thomas, S.; Aminova, O.; et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N. Engl. J. Med. 2012, 366, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Schlenk, R.F.; Russell, N.H.; Levis, M.J. Targeting FLT3 mutations in AML: Review of current knowledge and evidence. Leukemia 2019, 33, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Dohner, K.; Thiede, C.; Jahn, N.; Panina, E.; Gambietz, A.; Larson, R.A.; Prior, T.W.; Marcucci, G.; Jones, D.; Krauter, J.; et al. Impact of NPM1/FLT3-ITD genotypes defined by the 2017 European LeukemiaNet in patients with acute myeloid leukemia. Blood 2020, 135, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Ghiaur, G.; Levis, M. Mechanisms of Resistance to FLT3 Inhibitors and the Role of the Bone Marrow Microenvironment. Hematol. Oncol. Clin. N. Am. 2017, 31, 681–692. [Google Scholar] [CrossRef]
- Dohner, H.; Wei, A.H.; Roboz, G.J.; Montesinos, P.; Thol, F.R.; Ravandi, F.; Dombret, H.; Porkka, K.; Sandhu, I.; Skikne, B.; et al. Prognostic impact of NPM1 and FLT3 mutations in patients with AML in first remission treated with oral azacitidine. Blood 2022, 140, 1674–1685. [Google Scholar] [CrossRef]
- Perl, A.E. Approaching a therapeutic inflection point for FLT3-mutated AML. Blood 2025, 145, 2834–2839. [Google Scholar] [CrossRef]
- Engen, C.; Hellesoy, M.; Grob, T.; Al Hinai, A.; Brendehaug, A.; Wergeland, L.; Bedringaas, S.L.; Hovland, R.; Valk, P.J.M.; Gjertsen, B.T. FLT3-ITD mutations in acute myeloid leukaemia—Molecular characteristics, distribution and numerical variation. Mol. Oncol. 2021, 15, 2300–2317. [Google Scholar] [CrossRef]
- Pratz, K.W.; Levis, M. How I treat FLT3-mutated AML. Blood 2017, 129, 565–571. [Google Scholar] [CrossRef]
- Fedorov, K.; Maiti, A.; Konopleva, M. Targeting FLT3 Mutation in Acute Myeloid Leukemia: Current Strategies and Future Directions. Cancers 2023, 15, 2312. [Google Scholar] [CrossRef]
- Beitinjaneh, A.; Jang, S.; Roukoz, H.; Majhail, N.S. Prognostic significance of FLT3 internal tandem duplication and tyrosine kinase domain mutations in acute promyelocytic leukemia: A systematic review. Leuk. Res. 2010, 34, 831–836. [Google Scholar] [CrossRef]
- Gale, R.E.; Hills, R.; Pizzey, A.R.; Kottaridis, P.D.; Swirsky, D.; Gilkes, A.F.; Nugent, E.; Mills, K.I.; Wheatley, K.; Solomon, E.; et al. Relationship between FLT3 mutation status, biologic characteristics, and response to targeted therapy in acute promyelocytic leukemia. Blood 2005, 106, 3768–3776. [Google Scholar] [CrossRef] [PubMed]
- Picharski, G.L.; Andrade, D.P.; Fabro, A.; Lenzi, L.; Tonin, F.S.; Ribeiro, R.C.; Figueiredo, B.C. The Impact of Flt3 Gene Mutations in Acute Promyelocytic Leukemia: A Meta-Analysis. Cancers 2019, 11, 1311. [Google Scholar] [CrossRef] [PubMed]
- Li, A.Y.; Kashanian, S.M.; Hambley, B.C.; Zacholski, K.; Duong, V.H.; El Chaer, F.; Holtzman, N.G.; Gojo, I.; Webster, J.A.; Norsworthy, K.J.; et al. FLT3-ITD Allelic Burden and Acute Promyelocytic Leukemia Risk Stratification. Biology 2021, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.A.; Mabon, M.E.; Silverman, L.B.; Li, A.; Gribben, J.G.; Fox, E.A.; Sallan, S.E.; Korsmeyer, S.J. FLT3 mutations in childhood acute lymphoblastic leukemia. Blood 2004, 103, 3544–3546. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Wang, F.; Wang, M.; Liu, H.; Chen, X.; Cao, P.; Ma, X.; Teng, W.; Zhang, X.; et al. The mutational spectrum of FLT3 gene in acute lymphoblastic leukemia is different from acute myeloid leukemia. Cancer Gene Ther. 2020, 27, 81–88. [Google Scholar] [CrossRef]
- Roberts, K.G.; Li, Y.; Payne-Turner, D.; Harvey, R.C.; Yang, Y.L.; Pei, D.; McCastlain, K.; Ding, L.; Lu, C.; Song, G.; et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N. Engl. J. Med. 2014, 371, 1005–1015. [Google Scholar] [CrossRef]
- Armstrong, S.A.; Staunton, J.E.; Silverman, L.B.; Pieters, R.; den Boer, M.L.; Minden, M.D.; Sallan, S.E.; Lander, E.S.; Golub, T.R.; Korsmeyer, S.J. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat. Genet. 2002, 30, 41–47. [Google Scholar] [CrossRef]
- Biojone, E.R.; Guido, B.C.; Cavalcante, L.L.M.; Santos Júnior, A.d.C.M.d.; Pontes, R.M.d.; Furtado, F.M.; Córdoba, J.C.; Magalhães, I.M.Q.; de Oliveira, D.M.; Camargo, R. Prevalence of FLT3 gene mutation and its expression in Brazilian pediatric B-ALL patients: Clinical implications. Front. Pediatr. 2024, 12, 1505060. [Google Scholar] [CrossRef]
- Brown, P.; Levis, M.; Shurtleff, S.; Campana, D.; Downing, J.; Small, D. FLT3 inhibition selectively kills childhood acute lymphoblastic leukemia cells with high levels of FLT3 expression. Blood 2005, 105, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Chillon, M.C.; Gomez-Casares, M.T.; Lopez-Jorge, C.E.; Rodriguez-Medina, C.; Molines, A.; Sarasquete, M.E.; Alcoceba, M.; Miguel, J.D.; Bueno, C.; Montes, R.; et al. Prognostic significance of FLT3 mutational status and expression levels in MLL-AF4+ and MLL-germline acute lymphoblastic leukemia. Leukemia 2012, 26, 2360–2366. [Google Scholar] [CrossRef]
- Yang, M.; Safavi, S.; Woodward, E.L.; Duployez, N.; Olsson-Arvidsson, L.; Ungerback, J.; Sigvardsson, M.; Zaliova, M.; Zuna, J.; Fioretos, T.; et al. 13q12.2 deletions in acute lymphoblastic leukemia lead to upregulation of FLT3 through enhancer hijacking. Blood 2020, 136, 946–956. [Google Scholar] [CrossRef]
- Yang, X.; Sexauer, A.; Levis, M. Bone marrow stroma-mediated resistance to FLT3 inhibitors in FLT3-ITD AML is mediated by persistent activation of extracellular regulated kinase. Br. J. Haematol. 2014, 164, 61–72. [Google Scholar] [CrossRef]
- Woods, A.C.; Norsworthy, K.J. Differentiation Syndrome in Acute Leukemia: APL and Beyond. Cancers 2023, 15, 4767. [Google Scholar] [CrossRef] [PubMed]
- Levis, M.; Brown, P.; Smith, B.D.; Stine, A.; Pham, R.; Stone, R.; Deangelo, D.; Galinsky, I.; Giles, F.; Estey, E.; et al. Plasma inhibitory activity (PIA): A pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood 2006, 108, 3477–3483. [Google Scholar] [CrossRef]
- Fallahi, P.; Ferrari, S.M.; Santini, F.; Corrado, A.; Materazzi, G.; Ulisse, S.; Miccoli, P.; Antonelli, A. Sorafenib and thyroid cancer. BioDrugs 2013, 27, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Lierman, E.; Lahortiga, I.; Van Miegroet, H.; Mentens, N.; Marynen, P.; Cools, J. The ability of sorafenib to inhibit oncogenic PDGFRbeta and FLT3 mutants and overcome resistance to other small molecule inhibitors. Haematologica 2007, 92, 27–34. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef]
- Borthakur, G.; Kantarjian, H.; Ravandi, F.; Zhang, W.; Konopleva, M.; Wright, J.J.; Faderl, S.; Verstovsek, S.; Mathews, S.; Andreeff, M.; et al. Phase I study of sorafenib in patients with refractory or relapsed acute leukemias. Haematologica 2011, 96, 62–68. [Google Scholar] [CrossRef]
- Ravandi, F.; Alattar, M.L.; Grunwald, M.R.; Rudek, M.A.; Rajkhowa, T.; Richie, M.A.; Pierce, S.; Daver, N.; Garcia-Manero, G.; Faderl, S.; et al. Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood 2013, 121, 4655–4662. [Google Scholar] [CrossRef]
- Ohanian, M.; Garcia-Manero, G.; Levis, M.; Jabbour, E.; Daver, N.; Borthakur, G.; Kadia, T.; Pierce, S.; Burger, J.; Richie, M.A.; et al. Sorafenib Combined with 5-azacytidine in Older Patients with Untreated FLT3-ITD Mutated Acute Myeloid Leukemia. Am. J. Hematol. 2018, 93, 1136–1141. [Google Scholar] [CrossRef]
- Rollig, C.; Serve, H.; Huttmann, A.; Noppeney, R.; Muller-Tidow, C.; Krug, U.; Baldus, C.D.; Brandts, C.H.; Kunzmann, V.; Einsele, H.; et al. Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): A multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2015, 16, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Loo, S.; Roberts, A.W.; Anstee, N.S.; Kennedy, G.A.; He, S.; Schwarer, A.P.; Enjeti, A.K.; D’Rozario, J.; Marlton, P.; Bilmon, I.A.; et al. Sorafenib plus intensive chemotherapy in newly diagnosed FLT3-ITD AML: A randomized, placebo-controlled study by the ALLG. Blood 2023, 142, 1960–1971. [Google Scholar] [CrossRef] [PubMed]
- Motyckova, G.; Stone, R.M. Development of Midostaurin as a Tyrosine Kinase Inhibitor. In Targeted Therapy of Acute Myeloid Leukemia; Andreeff, M., Ed.; Springer: New York, NY, USA, 2015; pp. 201–214. [Google Scholar]
- Zhao, J.C.; Agarwal, S.; Ahmad, H.; Amin, K.; Bewersdorf, J.P.; Zeidan, A.M. A review of FLT3 inhibitors in acute myeloid leukemia. Blood Rev. 2022, 52, 100905. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.M.; DeAngelo, D.J.; Klimek, V.; Galinsky, I.; Estey, E.; Nimer, S.D.; Grandin, W.; Lebwohl, D.; Wang, Y.; Cohen, P.; et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood 2005, 105, 54–60. [Google Scholar] [CrossRef]
- Fischer, T.; Stone, R.M.; Deangelo, D.J.; Galinsky, I.; Estey, E.; Lanza, C.; Fox, E.; Ehninger, G.; Feldman, E.J.; Schiller, G.J.; et al. Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J. Clin. Oncol. 2010, 28, 4339–4345. [Google Scholar] [CrossRef]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Dohner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef]
- Strati, P.; Kantarjian, H.; Ravandi, F.; Nazha, A.; Borthakur, G.; Daver, N.; Kadia, T.; Estrov, Z.; Garcia-Manero, G.; Konopleva, M.; et al. Phase I/II trial of the combination of midostaurin (PKC412) and 5-azacytidine for patients with acute myeloid leukemia and myelodysplastic syndrome. Am. J. Hematol. 2015, 90, 276–281. [Google Scholar] [CrossRef]
- Mori, M.; Kaneko, N.; Ueno, Y.; Yamada, M.; Tanaka, R.; Saito, R.; Shimada, I.; Mori, K.; Kuromitsu, S. Gilteritinib, a FLT3/AXL inhibitor, shows antileukemic activity in mouse models of FLT3 mutated acute myeloid leukemia. Investig. New Drugs 2017, 35, 556–565. [Google Scholar] [CrossRef]
- Smith, C.C.; Lasater, E.A.; Lin, K.C.; Wang, Q.; McCreery, M.Q.; Stewart, W.K.; Damon, L.E.; Perl, A.E.; Jeschke, G.R.; Sugita, M.; et al. Crenolanib is a selective type I pan-FLT3 inhibitor. Proc. Natl. Acad. Sci. USA 2014, 111, 5319–5324. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.; Hernandez, D.; Rajkhowa, T.; Smith, S.C.; Raman, J.R.; Nguyen, B.; Small, D.; Levis, M. Preclinical studies of gilteritinib, a next-generation FLT3 inhibitor. Blood 2017, 129, 257–260. [Google Scholar] [CrossRef]
- Galanis, A.; Ma, H.; Rajkhowa, T.; Ramachandran, A.; Small, D.; Cortes, J.; Levis, M. Crenolanib is a potent inhibitor of FLT3 with activity against resistance-conferring point mutants. Blood 2014, 123, 94–100. [Google Scholar] [CrossRef]
- Smith, C.C.; Lin, K.; Stecula, A.; Sali, A.; Shah, N.P. FLT3 D835 mutations confer differential resistance to type II FLT3 inhibitors. Leukemia 2015, 29, 2390–2392. [Google Scholar] [CrossRef]
- Perl, A.E.; Altman, J.K.; Cortes, J.; Smith, C.; Litzow, M.; Baer, M.R.; Claxton, D.; Erba, H.P.; Gill, S.; Goldberg, S.; et al. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: A multicentre, first-in-human, open-label, phase 1-2 study. Lancet Oncol. 2017, 18, 1061–1075. [Google Scholar] [CrossRef]
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C.; et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N. Engl. J. Med. 2019, 381, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- Pratz, K.W.; Cherry, M.; Altman, J.K.; Cooper, B.W.; Podoltsev, N.A.; Cruz, J.C.; Lin, T.L.; Schiller, G.J.; Jurcic, J.G.; Asch, A.; et al. Gilteritinib in Combination with Induction and Consolidation Chemotherapy and as Maintenance Therapy: A Phase IB Study in Patients with Newly Diagnosed AML. J. Clin. Oncol. 2023, 41, 4236–4246. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.S.; Montesinos, P.; Minden, M.D.; Lee, J.H.; Heuser, M.; Naoe, T.; Chou, W.C.; Laribi, K.; Esteve, J.; Altman, J.K.; et al. Phase 3 trial of gilteritinib plus azacitidine vs. azacitidine for newly diagnosed FLT3mut+ AML ineligible for intensive chemotherapy. Blood 2022, 140, 1845–1857. [Google Scholar] [CrossRef]
- Short, N.J.; Daver, N.; Dinardo, C.D.; Kadia, T.; Nasr, L.F.; Macaron, W.; Yilmaz, M.; Borthakur, G.; Montalban-Bravo, G.; Garcia-Manero, G.; et al. Azacitidine, Venetoclax, and Gilteritinib in Newly Diagnosed and Relapsed or Refractory FLT3-Mutated AML. J. Clin. Oncol. 2024, 42, 1499–1508. [Google Scholar] [CrossRef]
- Maiti, A.; DiNardo, C.D.; Daver, N.G.; Rausch, C.R.; Ravandi, F.; Kadia, T.M.; Pemmaraju, N.; Borthakur, G.; Bose, P.; Issa, G.C.; et al. Triplet therapy with venetoclax, FLT3 inhibitor and decitabine for FLT3-mutated acute myeloid leukemia. Blood Cancer J. 2021, 11, 25. [Google Scholar] [CrossRef]
- Zarrinkar, P.P.; Gunawardane, R.N.; Cramer, M.D.; Gardner, M.F.; Brigham, D.; Belli, B.; Karaman, M.W.; Pratz, K.W.; Pallares, G.; Chao, Q.; et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood 2009, 114, 2984–2992. [Google Scholar] [CrossRef]
- Cortes, J.E.; Kantarjian, H.; Foran, J.M.; Ghirdaladze, D.; Zodelava, M.; Borthakur, G.; Gammon, G.; Trone, D.; Armstrong, R.C.; James, J.; et al. Phase I study of quizartinib administered daily to patients with relapsed or refractory acute myeloid leukemia irrespective of FMS-like tyrosine kinase 3-internal tandem duplication status. J. Clin. Oncol. 2013, 31, 3681–3687. [Google Scholar] [CrossRef]
- Moore, A.S.; Faisal, A.; Gonzalez de Castro, D.; Bavetsias, V.; Sun, C.; Atrash, B.; Valenti, M.; de Haven Brandon, A.; Avery, S.; Mair, D.; et al. Selective FLT3 inhibition of FLT3-ITD+ acute myeloid leukaemia resulting in secondary D835Y mutation: A model for emerging clinical resistance patterns. Leukemia 2012, 26, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Perl, A.E.; Dohner, H.; Kantarjian, H.; Martinelli, G.; Kovacsovics, T.; Rousselot, P.; Steffen, B.; Dombret, H.; Estey, E.; et al. Quizartinib, an FLT3 inhibitor, as monotherapy in patients with relapsed or refractory acute myeloid leukaemia: An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2018, 19, 889–903. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Tallman, M.S.; Schiller, G.J.; Trone, D.; Gammon, G.; Goldberg, S.L.; Perl, A.E.; Marie, J.P.; Martinelli, G.; Kantarjian, H.M.; et al. Phase 2b study of 2 dosing regimens of quizartinib monotherapy in FLT3-ITD-mutated, relapsed or refractory AML. Blood 2018, 132, 598–607. [Google Scholar] [CrossRef]
- Cortes, J.E.; Khaled, S.; Martinelli, G.; Perl, A.E.; Ganguly, S.; Russell, N.; Kramer, A.; Dombret, H.; Hogge, D.; Jonas, B.A.; et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): A multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Erba, H.P.; Montesinos, P.; Kim, H.J.; Patkowska, E.; Vrhovac, R.; Zak, P.; Wang, P.N.; Mitov, T.; Hanyok, J.; Kamel, Y.M.; et al. Quizartinib plus chemotherapy in newly diagnosed patients with FLT3-internal-tandem-duplication-positive acute myeloid leukaemia (QuANTUM-First): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1571–1583. [Google Scholar] [CrossRef]
- Swaminathan, M.; Kantarjian, H.M.; Levis, M.; Guerra, V.; Borthakur, G.; Alvarado, Y.; DiNardo, C.D.; Kadia, T.; Garcia-Manero, G.; Ohanian, M.; et al. A phase I/II study of the combination of quizartinib with azacitidine or low-dose cytarabine for the treatment of patients with acute myeloid leukemia and myelodysplastic syndrome. Haematologica 2021, 106, 2121–2130. [Google Scholar] [CrossRef]
- Yilmaz, M.; Kantarjian, H.M.; Muftuoglu, M.; Kadia, T.M.; Konopleva, M.; Borthakur, G.; Dinardo, C.D.; Pemmaraju, N.; Short, N.J.; Alvarado, Y.; et al. Quizartinib with decitabine and venetoclax (triplet) is highly active in patients with FLT3-ITD mutated acute myeloid leukemia (AML). J. Clin. Oncol. 2021, 39, e19019. [Google Scholar] [CrossRef]
- Cortes, J. Quizartinib: A potent and selective FLT3 inhibitor for the treatment of patients with FLT3-ITD–positive AML. J. Hematol. Oncol. 2024, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- Panetta, J.C.; Baker, S.D.; Kantarjian, H.M.; Stewart, C.; Pond, B.; Macaraeg, M.; Makinde, T.; Vali, S.; Daver, N.; Ramachandran, A.; et al. Population Pharmacokinetics of Crenolanib, a Type I FLT3 Inhibitor, in Patients with Relapsed/Refractory AML. Blood 2015, 126, 3695. [Google Scholar] [CrossRef]
- Zhang, H.; Savage, S.; Schultz, A.R.; Bottomly, D.; White, L.; Segerdell, E.; Wilmot, B.; McWeeney, S.K.; Eide, C.A.; Nechiporuk, T.; et al. Clinical resistance to crenolanib in acute myeloid leukemia due to diverse molecular mechanisms. Nat. Commun. 2019, 10, 244. [Google Scholar] [CrossRef]
- Randhawa, J.K.; Kantarjian, H.M.; Borthakur, G.; Thompson, P.A.; Konopleva, M.V.; Daver, N.G.; Pemmaraju, N.; Jabbour, E.J.; Kadia, T.M.; Estrov, Z.E.; et al. Results of a Phase II Study of Crenolanib in Relapsed/Refractory Acute Myeloid Leukemia Patients (Pts) with Activating FLT3 Mutations. Blood 2014, 124, 389. [Google Scholar] [CrossRef]
- Wang, E.S.; Goldberg, A.D.; Tallman, M.; Walter, R.B.; Karanes, C.; Sandhu, K.; Vigil, C.E.; Collins, R.; Jain, V.; Stone, R.M. Crenolanib and Intensive Chemotherapy in Adults with Newly Diagnosed FLT3-Mutated AML. J. Clin. Oncol. 2024, 42, 1776–1787. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.E.; Larson, R.A.; Podoltsev, N.A.; Strickland, S.; Wang, E.S.; Atallah, E.; Schiller, G.J.; Martinelli, G.; Neubauer, A.; Sierra, J.; et al. Follow-up of patients with R/R FLT3-mutation-positive AML treated with gilteritinib in the phase 3 ADMIRAL trial. Blood 2022, 139, 3366–3375. [Google Scholar] [CrossRef]
- Xuan, L.; Wang, Y.; Yang, K.; Shao, R.; Huang, F.; Fan, Z.; Chi, P.; Xu, Y.; Xu, N.; Deng, L.; et al. Sorafenib maintenance after allogeneic haemopoietic stem-cell transplantation in patients with FLT3-ITD acute myeloid leukaemia: Long-term follow-up of an open-label, multicentre, randomised, phase 3 trial. Lancet Haematol. 2023, 10, e600–e611. [Google Scholar] [CrossRef]
- Burchert, A.; Bug, G.; Fritz, L.V.; Finke, J.; Stelljes, M.; Rollig, C.; Wollmer, E.; Wasch, R.; Bornhauser, M.; Berg, T.; et al. Sorafenib Maintenance After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia with FLT3-Internal Tandem Duplication Mutation (SORMAIN). J. Clin. Oncol. 2020, 38, 2993–3002. [Google Scholar] [CrossRef]
- Xuan, L.; Wang, Y.; Huang, F.; Fan, Z.; Xu, Y.; Sun, J.; Xu, N.; Deng, L.; Li, X.; Liang, X.; et al. Sorafenib maintenance in patients with FLT3-ITD acute myeloid leukaemia undergoing allogeneic haematopoietic stem-cell transplantation: An open-label, multicentre, randomised phase 3 trial. Lancet Oncol. 2020, 21, 1201–1212. [Google Scholar] [CrossRef]
- Maziarz, R.T.; Levis, M.; Patnaik, M.M.; Scott, B.L.; Mohan, S.R.; Deol, A.; Rowley, S.D.; Kim, D.D.H.; Hernandez, D.; Rajkhowa, T.; et al. Midostaurin after allogeneic stem cell transplant in patients with FLT3-internal tandem duplication-positive acute myeloid leukemia. Bone Marrow Transpl. 2021, 56, 1180–1189. [Google Scholar] [CrossRef]
- Levis, M.J.; Hamadani, M.; Logan, B.; Jones, R.J.; Singh, A.K.; Litzow, M.; Wingard, J.R.; Papadopoulos, E.B.; Perl, A.E.; Soiffer, R.J.; et al. Gilteritinib as Post-Transplant Maintenance for AML with Internal Tandem Duplication Mutation of FLT3. J. Clin. Oncol. 2024, 42, 1766–1775. [Google Scholar] [CrossRef]
- Levis, M.J.; Hamadani, M.; Logan, B.; Jones, R.J.; Singh, A.K.; Litzow, M.R.; Wingard, J.R.; Papadopoulos, E.B.; Perl, A.E.; Soiffer, R.J.; et al. Measurable residual disease and posttransplantation gilteritinib maintenance for patients with FLT3-ITD–mutated AML. Blood 2025, 145, 2138–2148. [Google Scholar] [CrossRef]
- Levis, M.J.; Hamadani, M.; Logan, B.R.; Jones, R.J.; Singh, A.K.; Litzow, M.R.; Wingard, J.R.; Papadopoulos, E.B.; Perl, A.E.; Soiffer, R.J.; et al. Impact of transplant conditioning, NPM1 mutations, and measurable residual disease in FLT3-ITD acute myeloid leukemia. Blood Adv. 2025, 9, 5123–5133. [Google Scholar] [CrossRef]
- Pollard, J.A.; Alonzo, T.A.; Gerbing, R.; Brown, P.; Fox, E.; Choi, J.; Fisher, B.; Hirsch, B.; Kahwash, S.; Getz, K.; et al. Sorafenib in Combination with Standard Chemotherapy for Children with High Allelic Ratio FLT3/ITD+ Acute Myeloid Leukemia: A Report From the Children’s Oncology Group Protocol AAML1031. J. Clin. Oncol. 2022, 40, 2023–2035. [Google Scholar] [CrossRef]
- Cooper, T.M.; Alonzo, T.A.; Tasian, S.K.; Kutny, M.A.; Hitzler, J.; Pollard, J.A.; Aplenc, R.; Meshinchi, S.; Kolb, E.A. Children’s Oncology Group’s 2023 blueprint for research: Myeloid neoplasms. Pediatr Blood Cancer 2023, 70 (Suppl. S6), e30584. [Google Scholar] [CrossRef]
- Kennedy, V.E.; Smith, C.C. FLT3 targeting in the modern era: From clonal selection to combination therapies. Int. J. Hematol. 2024, 120, 528–540. [Google Scholar] [CrossRef]
- Karbowski, C.; Goldstein, R.; Frank, B.; Kim, K.; Li, C.M.; Homann, O.; Hensley, K.; Brooks, B.; Wang, X.; Yan, Q.; et al. Nonclinical Safety Assessment of AMG 553, an Investigational Chimeric Antigen Receptor T-Cell Therapy for the Treatment of Acute Myeloid Leukemia. Toxicol. Sci. 2020, 177, 94–107. [Google Scholar] [CrossRef]
- Popescu, B.; Jones, M.F.; Piao, M.; Tran, E.; Koh, A.; Lomeli, I.; Peretz, C.A.C.; Murad, N.; Abelson, S.; Morales, C.; et al. Multi-selective RAS(ON) Inhibition Targets Oncogenic RAS Mutations and Overcomes RAS/MAPK-Mediated Resistance to FLT3 and BCL2 Inhibitors in Acute Myeloid Leukemia. bioRxiv, 2025; preprint. [Google Scholar] [CrossRef]
- Stein, E.M.; Derkach, A.; Melamed, G.; Block, A.; Kim, J.; Georgieva, G.; Thompson, V.; Levine, R.L.; Intlekofer, A.M.; Abdel-Wahab, O.; et al. A Phase 1b Multi-Center Study of the FLT3 Inhibitor Gilteritinib in Combination with the IDH1 Inhibitor Ivosidenib or the IDH2 Inhibitor Enasidenib for Patients with Relapsed or Refractory Acute Myeloid Leukemia Who Have Co-Occurring FLT3/IDH1 or FLT3/IDH2 Mutations. Blood 2023, 142, 5945. [Google Scholar] [CrossRef]
- Singh Mali, R.; Zhang, Q.; DeFilippis, R.A.; Cavazos, A.; Kuruvilla, V.M.; Raman, J.; Mody, V.; Choo, E.F.; Dail, M.; Shah, N.P.; et al. Venetoclax combines synergistically with FLT3 inhibition to effectively target leukemic cells in FLT3-ITD+ acute myeloid leukemia models. Haematologica 2021, 106, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Perl, A.E.; Maly, J.; Levis, M.; Ritchie, E.; Litzow, M.; McCloskey, J.; Smith, C.C.; Schiller, G.; Bradley, T.; et al. Venetoclax Plus Gilteritinib for FLT3-Mutated Relapsed/Refractory Acute Myeloid Leukemia. J. Clin. Oncol. 2022, 40, 4048–4059. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.J.; Sugita, M.; Koblish, H.; Perl, A.E.; Carroll, M. Hematopoietic cytokines mediate resistance to targeted therapy in FLT3-ITD acute myeloid leukemia. Blood Adv. 2019, 3, 1061–1072. [Google Scholar] [CrossRef]
- Levis, M.; Perl, A.; Schiller, G.; Fathi, A.T.; Roboz, G.; Wang, E.S.; Altman, J.; Rajkhowa, T.; Ando, M.; Suzuki, T.; et al. A phase 1 study of the irreversible FLT3 inhibitor FF-10101 in relapsed or refractory acute myeloid leukemia. Blood Adv. 2024, 8, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Yamaura, T.; Nakatani, T.; Uda, K.; Ogura, H.; Shin, W.; Kurokawa, N.; Saito, K.; Fujikawa, N.; Date, T.; Takasaki, M.; et al. A novel irreversible FLT3 inhibitor, FF-10101, shows excellent efficacy against AML cells with FLT3 mutations. Blood 2018, 131, 426–438. [Google Scholar] [CrossRef]
- Chang, Y.T.; Hernandez, D.; Alonso, S.; Gao, M.; Su, M.; Ghiaur, G.; Levis, M.J.; Jones, R.J. Role of CYP3A4 in bone marrow microenvironment-mediated protection of FLT3/ITD AML from tyrosine kinase inhibitors. Blood Adv. 2019, 3, 908–916. [Google Scholar] [CrossRef]
- Ma, H.S.; Greenblatt, S.M.; Shirley, C.M.; Duffield, A.S.; Bruner, J.K.; Li, L.; Nguyen, B.; Jung, E.; Aplan, P.D.; Ghiaur, G.; et al. All-trans retinoic acid synergizes with FLT3 inhibition to eliminate FLT3/ITD+ leukemia stem cells in vitro and in vivo. Blood 2016, 127, 2867–2878. [Google Scholar] [CrossRef]
- Ghiaur, G.; Yegnasubramanian, S.; Perkins, B.; Gucwa, J.L.; Gerber, J.M.; Jones, R.J. Regulation of human hematopoietic stem cell self-renewal by the microenvironment’s control of retinoic acid signaling. Proc. Natl. Acad. Sci. USA 2013, 110, 16121–16126. [Google Scholar] [CrossRef]
- Su, M.; Alonso, S.; Jones, J.W.; Yu, J.; Kane, M.A.; Jones, R.J.; Ghiaur, G. All-Trans Retinoic Acid Activity in Acute Myeloid Leukemia: Role of Cytochrome P450 Enzyme Expression by the Microenvironment. PLoS ONE 2015, 10, e0127790. [Google Scholar] [CrossRef]
- Kegyes, D.; Teodorescu, P.; Supeanu, T.; Nagai, Y.; Zhong, G.; Mathews, V.; Isoherranen, N.; Ghiaur, G. Arsenic trioxide for reprogramming the bone marrow microenvironment to eliminate acute myeloid leukemia blasts. Hemasphere 2025, 9, e70191. [Google Scholar] [CrossRef] [PubMed]

| Disease | Frequency of FLT3 Alterations | Predominant Mutation Type(s) | Common Co-Mutations | Clinical Associations | Prognostic Impact | Current Role of FLT3 Inhibitors |
|---|---|---|---|---|---|---|
| AML | ~30% | ITD (20–30%) (15% in pediatric population) TKD (5–10%) | NPM1 DNMT3A | Hyperleukocytosis, CNS involvement | VAF & ITD length matter Co-mutations shift risk | Approved and standard of care in some settings |
| APL | 30–40% | ITD, TKD | - | DIC Microgranular variant | Mitigated by ATRA + ATO High VAF/long ITD may still worsen | None established. Salvage therapy in relapsed APL (case reports and Ghiaur personal communication |
| ALL | 2–22% (higher in certain subgroups: 18% of MLLr 28% of hyperdiploid) | ITD, TKD overexpression | MLLr ZNF384r | Poor prognosis in MLL-AF4+ High relapse rates | Overexpression adverse in certain subtypes | Investigational (notably sensitive to FLT3 inhibition) |
| FLT3 Inhibitor | Type & Binding Class | FLT3 Targets | Other Kinase Targets | Key Clinical Trials | Major Limitations | FDA-Approved Indication |
|---|---|---|---|---|---|---|
| Midostaurin | Type I (multikinase inhibitor) | FLT3-ITD, FLT3-TKD | PKC KIT PDGFR VEGFR | RATIFY (CALGB 10603, frontline FLT3-mut AML +7+3) RADIUS (post-allo-HCT maintenance) | Multikinase off-targets modest single-agent activity | Approved (2017) for newly diagnosed FLT3-mut AML in combination with standard chemotherapy |
| Gilteritinib | Type I (selective FLT3 inhibitor) | FLT3-ITD, FLT3-TKD (D835) | AXL ALK LTK | ADMIRAL (R/R AML vs. salvage chemo) MORPHO (post-allo-HCT maintenance) frontline and triplet studies | QTc prolongation; differentiation syndrome; resistance via RAS/MAPK activation | Approved (2018) for relapsed/refractory FLT3-mutated AML |
| Quizartinib | Type II (selective FLT3 inhibitor) | FLT3-ITD (inactive vs. D835) | c-KIT (moderate) | QuANTUM-R (R/R FLT3-ITD AML) QuANTUM-First (frontline FLT3-ITD AML + maintenance) | Ineffective against TKD mutations QTc prolongation cytopenias | Approved (2023) for newly diagnosed FLT3-ITD AML in combination with intensive chemotherapy |
| Crenolanib | Type I (highly selective FLT3 inhibitor) | FLT3-ITD, FLT3-TKD (incl. D835, F691L) | Minimal off-target (weak PDGFR/KIT) | Phase II (R/R AML) ongoing Phase III (NCT03258931) vs. midostaurin (frontline) | GI and hepatic toxicity not yet approved | Not approved (under phase III investigation for FLT3-mut AML) |
| Sorafenib | Type II (multikinase inhibitor) | FLT3-ITD (limited TKD activity) | RAF VEGFR PDGFR KIT | Phase I/II (R/R AML) SORAML (frontline AML, ±FLT3 mut) ALLG AMLM16 | Broad off-target activity Cytopenias Limited OS benefit QTc prolongation | Not approved for AML (approved for renal, hepatic, thyroid carcinoma) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stancioaica, M.-C.; Coriu, D.; Ghiaur, G. FLT3: A 35-Year Voyage from Discovery to the Next Generation of Targeted Therapy in AML. Cancers 2025, 17, 3415. https://doi.org/10.3390/cancers17213415
Stancioaica M-C, Coriu D, Ghiaur G. FLT3: A 35-Year Voyage from Discovery to the Next Generation of Targeted Therapy in AML. Cancers. 2025; 17(21):3415. https://doi.org/10.3390/cancers17213415
Chicago/Turabian StyleStancioaica, Maria-Camelia, Daniel Coriu, and Gabriel Ghiaur. 2025. "FLT3: A 35-Year Voyage from Discovery to the Next Generation of Targeted Therapy in AML" Cancers 17, no. 21: 3415. https://doi.org/10.3390/cancers17213415
APA StyleStancioaica, M.-C., Coriu, D., & Ghiaur, G. (2025). FLT3: A 35-Year Voyage from Discovery to the Next Generation of Targeted Therapy in AML. Cancers, 17(21), 3415. https://doi.org/10.3390/cancers17213415






