Evaluating Therapeutic Efficacy of the Vascular Disrupting Agent OXi8007 Against Kidney Cancer in Mice †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.1.1. Cell Toxicity: Renca Sulforhodamine B (SRB) Assay
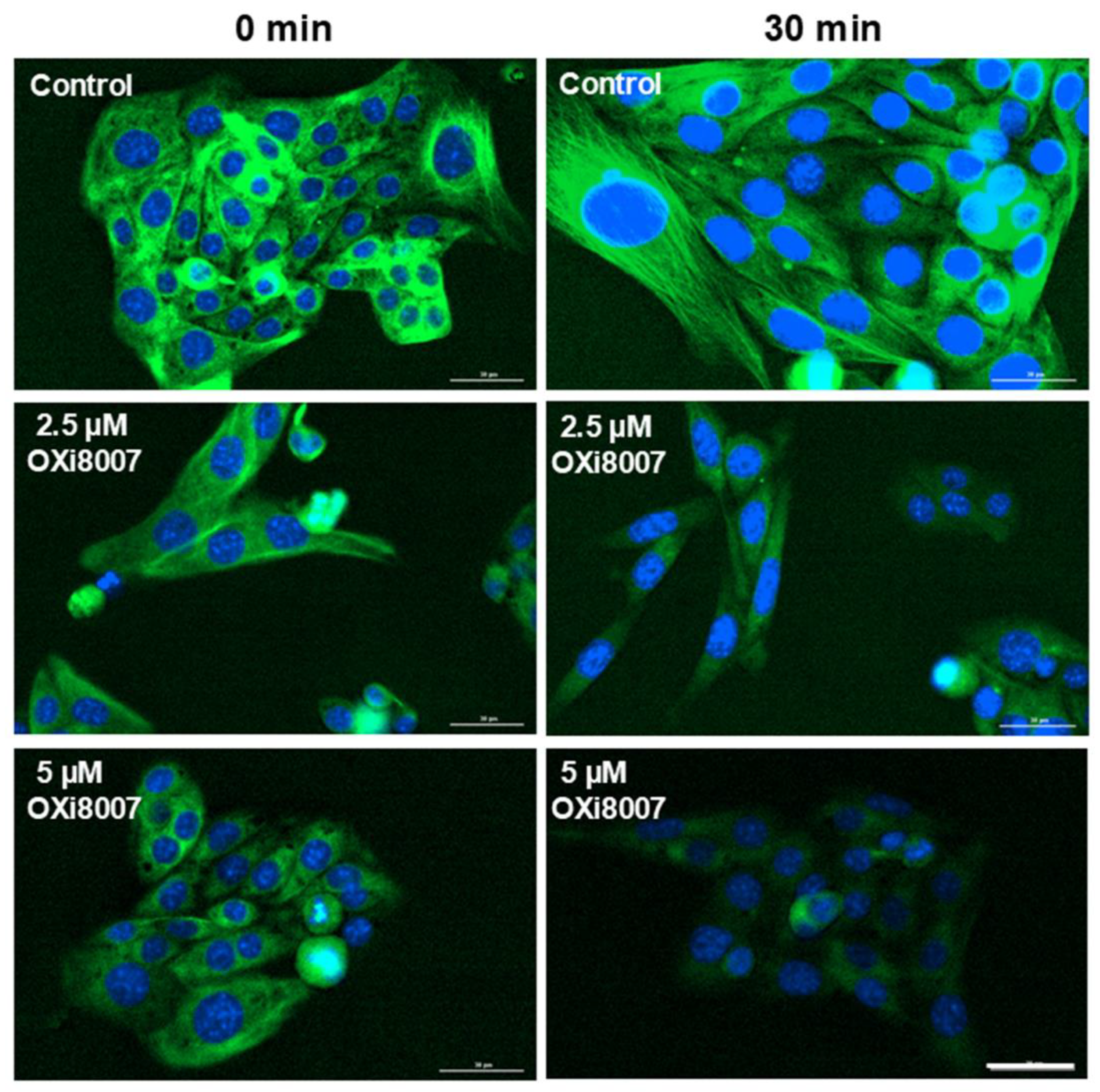
2.1.2. Microtubule Disruption in Renca Cells Treated with OXi8007
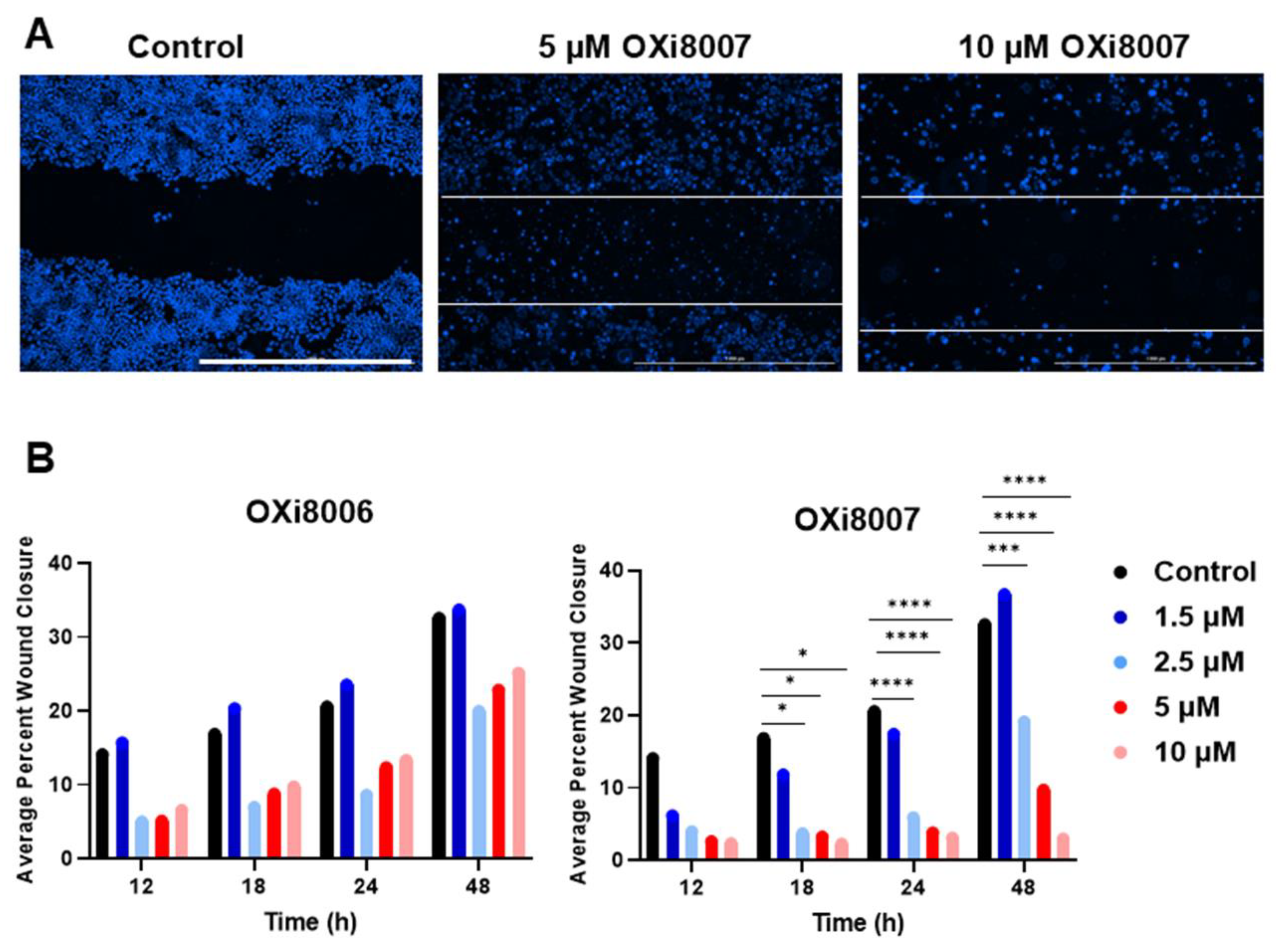
2.1.3. Wound (Scratch) Assay of OXi8006 and OXi8007
2.2. Mouse Models and Imaging Methods
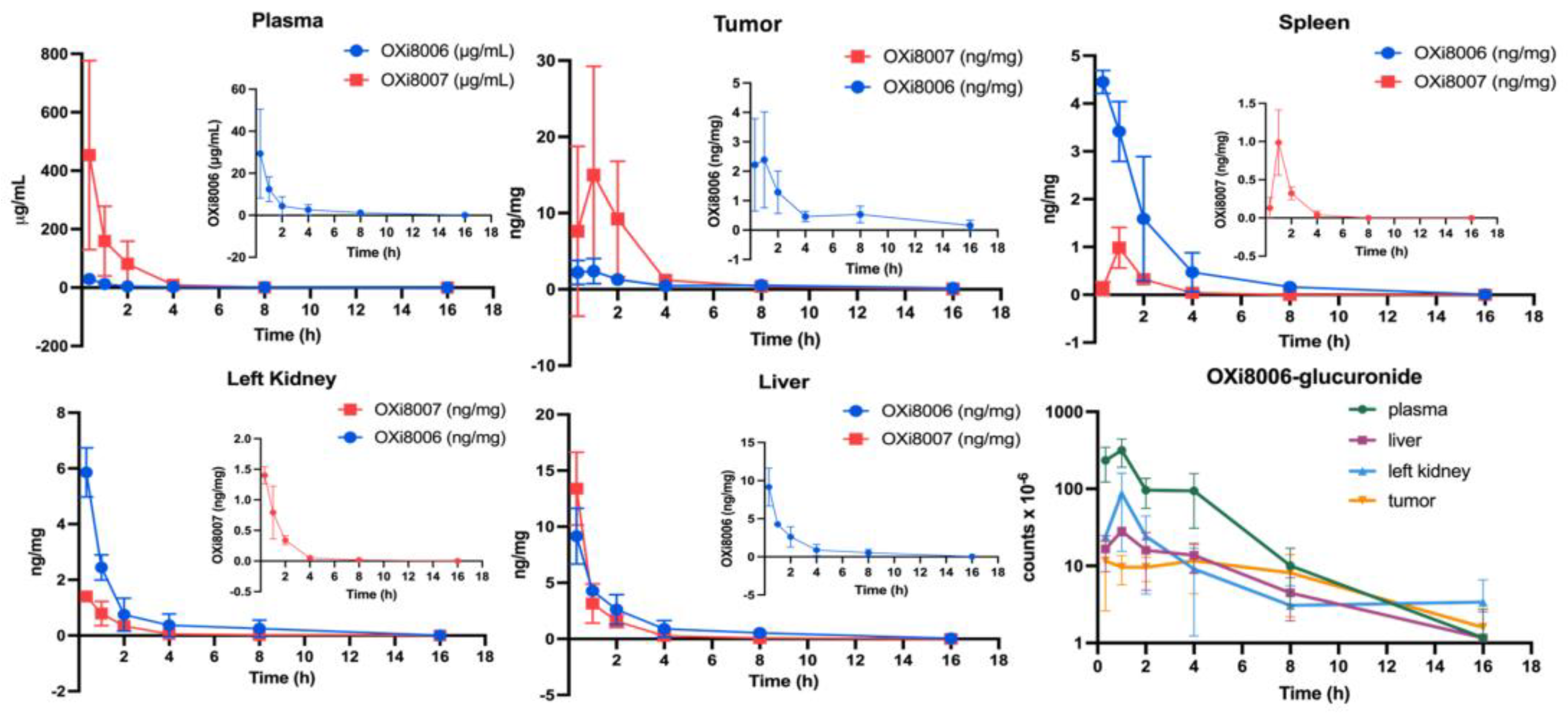
2.2.1. Pharmacokinetic Studies of OXi8007 in Renca Tumor-Bearing BALB/c Mice
2.2.2. Blood Pressure Assessment
2.2.3. Bioluminescence Imaging (BLI)
2.2.4. Multispectral Optoacoustic Tomography (MSOT)
2.3. Treatment Response to OXi8007
2.3.1. Acute Response to OXi8007
2.3.2. Drug Combination Therapy
3. Results
3.1. OXi8007 Mechanism and Cell Studies
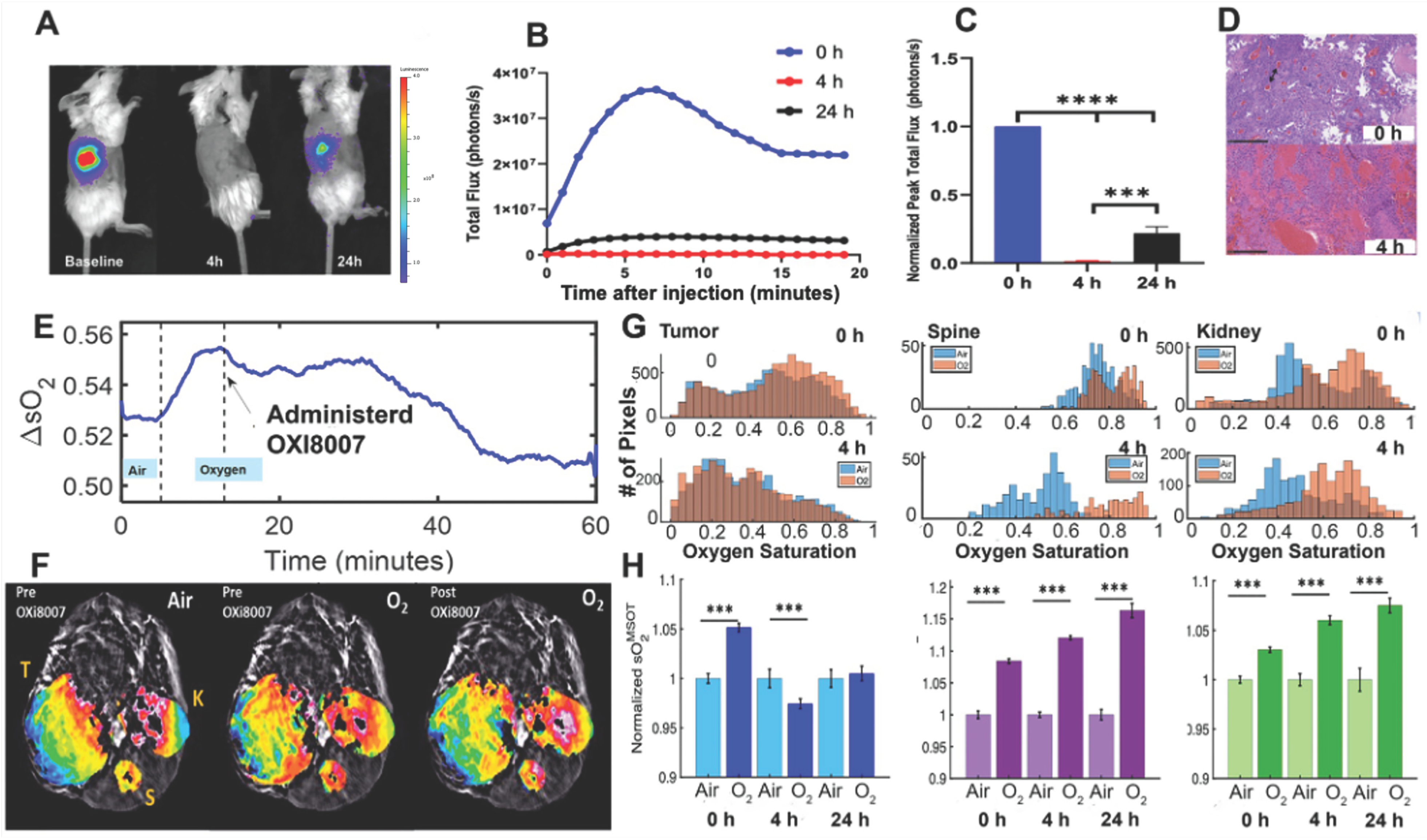
3.1.1. Pharmacokinetic Studies of OXi8007 in Renca-Luc Tumors Bearing BALB/c Mice
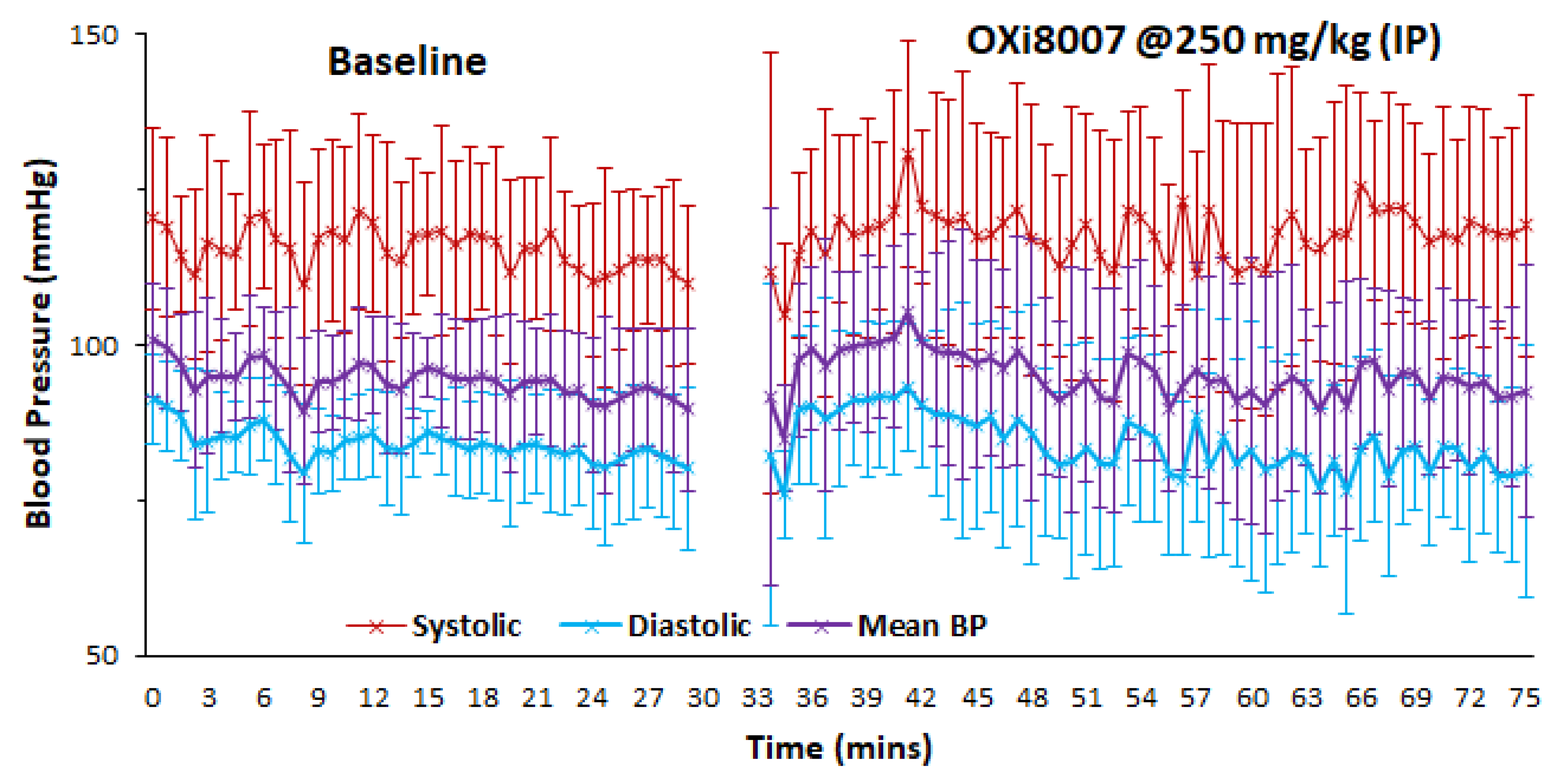
3.1.2. Blood Pressure Fluctuation upon Administrating OXi8007
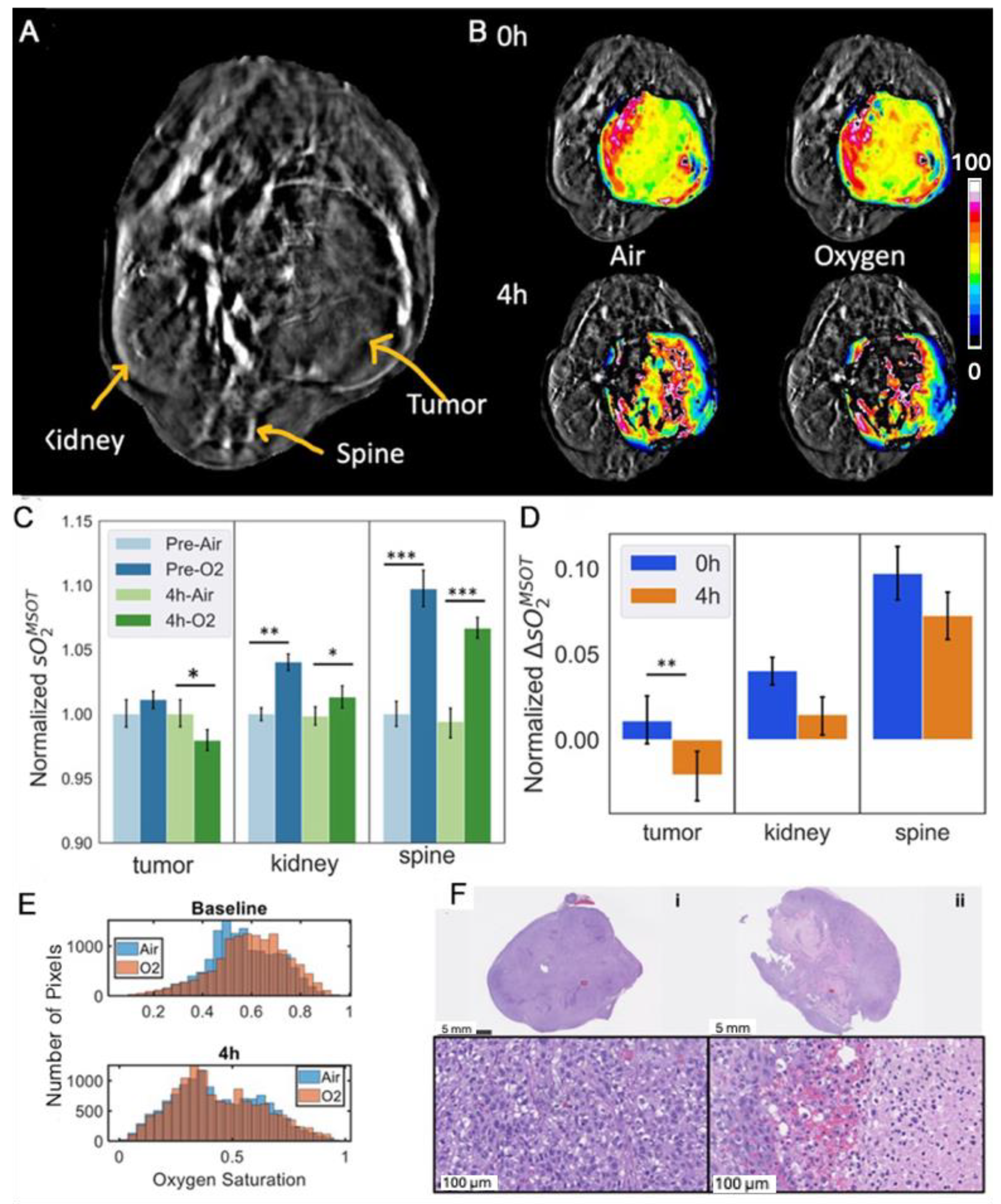
3.2. Acute Renca-Luc Tumor Response to OXi8007
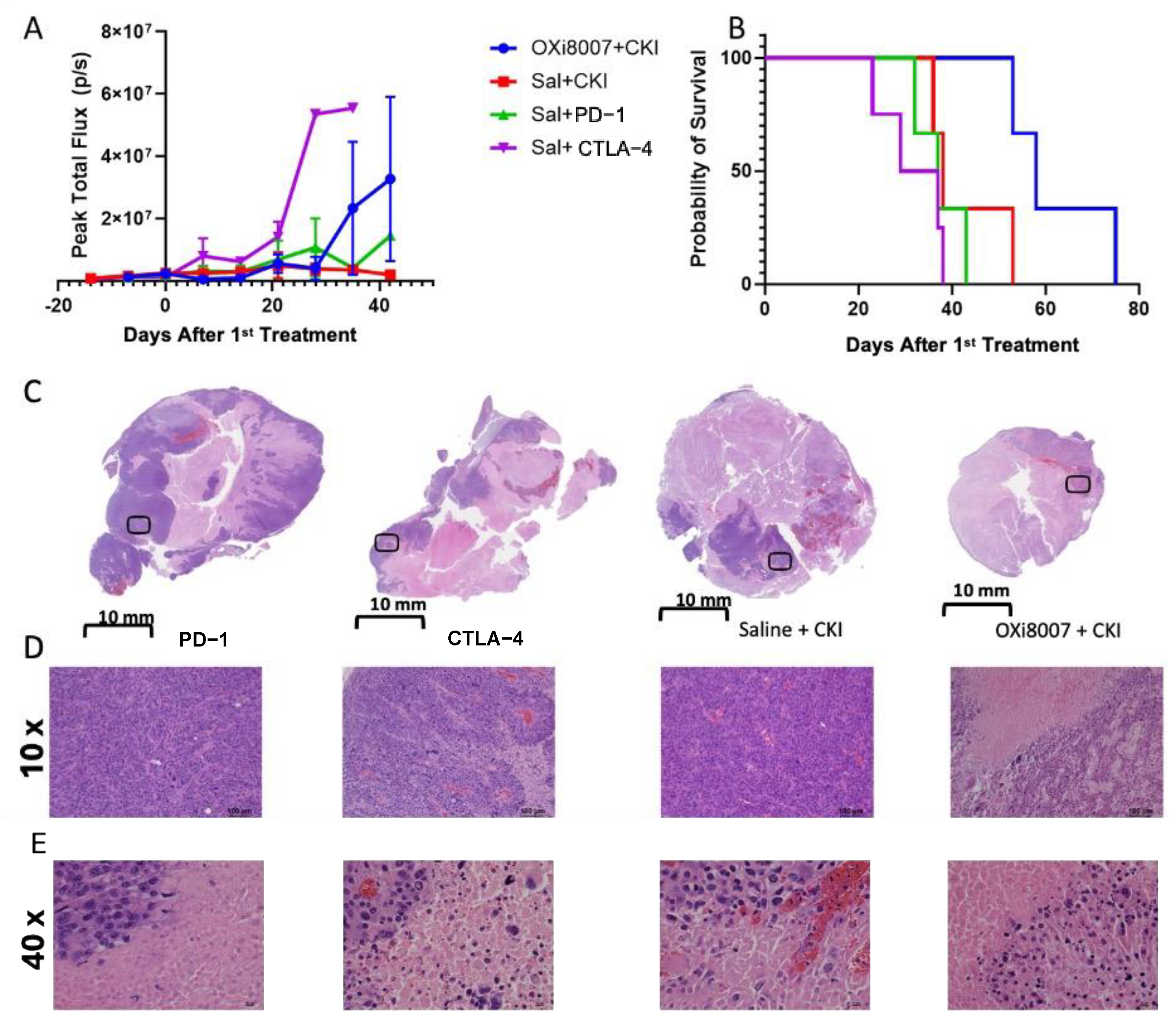
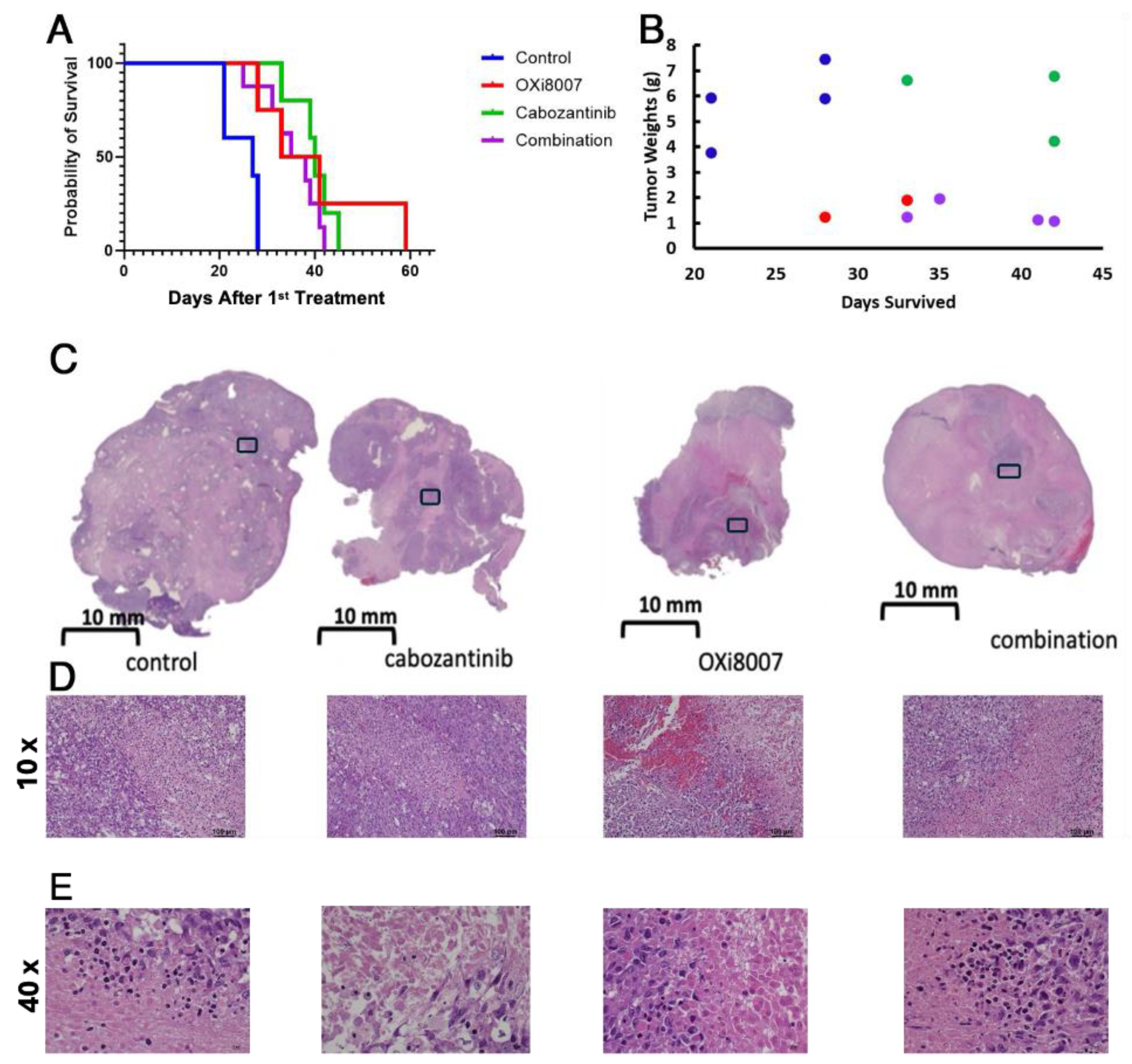
3.3. Combination Therapy
3.4. Effects of OXi8007 on Patient-Derived XP258 Tumors
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| AUC | Area under the curve |
| BLI | Bioluminescence imaging |
| CKI | Checkpoint inhibitor |
| H&E | Hematoxylin and eosin |
| MSOT | Multispectral optoacoustic tomography |
| RCC | Renal cell carcinoma |
| VDA | Vascular-disrupting agent |
References
- Choueiri, T.K.; Escudier, B.; Powles, T.; Tannir, N.M.; Mainwaring, P.N.; Rini, B.I.; Hammers, H.J.; Donskov, F.; Roth, B.J.; Peltola, K.; et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): Final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016, 17, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Key Statistics About Kidney Cancer. Available online: https://www.cancer.org/cancer/types/kidney-cancer/about/key-statistics.html (accessed on 4 September 2024).
- Mason, R.P.; Zhao, D.W.; Liu, L.; Trawick, M.L.; Pinney, K.G. A perspective on vascular disrupting agents that interact with tubulin: Preclinical tumor imaging and biological assessment. Integr. Biol.-UK 2011, 3, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Siemann, D.W. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by Tumor-Vascular Disrupting Agents. Cancer Treat. Rev. 2011, 37, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Denekamp, J. Vascular Attack as a Therapeutic Strategy for Cancer. Cancer Metast Rev. 1990, 9, 267–282. [Google Scholar] [CrossRef]
- Huang, X.; Molema, G.; King, S.; Watkins, L.; Edington, T.S.; Thorpe, P.E. Tumor infarction in mice by anti-body directed targeting of tissue factor to tumor vasculature. Science 1997, 275, 547–550. [Google Scholar] [CrossRef]
- Thorpe, P.E. Vascular targeting agents as cancer therapeutics. Clin. Cancer Res. 2004, 10, 415–427. [Google Scholar] [CrossRef]
- Siemann, D.W.; Chaplin, D.J.; Horsman, M.R. Realizing the Potential of Vascular Targeted Therapy: The Rationale for Combining Vascular Disrupting Agents and Anti-Angiogenic Agents to Treat Cancer. Cancer Investig. 2017, 35, 519–534. [Google Scholar] [CrossRef]
- Sherbet, G.V. Suppression of angiogenesis and tumour progression by combretastatin and derivatives. Cancer Lett. 2017, 403, 289–295. [Google Scholar] [CrossRef]
- McLoughlin, E.C.; O’Boyle, N.M. Colchicine-Binding Site Inhibitors from Chemistry to Clinic: A Review. Pharmaceuticals 2020, 13, 8. [Google Scholar] [CrossRef]
- Waddell, S.; Zhao, G.; Liu, Z.; Chen, H.; Zhang, W.; Wang, Y.; Miller, D.D.; Yue, J.; Li, W. VERU-111, an Orally Available Tubulin Inhibitor, Suppresses Ovarian Tumor Growth and Metastasis. J. Pharmacol. Exp. Ther. 2024, 392, 100006. [Google Scholar] [CrossRef]
- Bothwell, K.D.; Folaron, M.; Seshadri, M. Preclinical Activity of the Vascular Disrupting Agent OXi4503 against Head and Neck Cancer. Cancers 2016, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Wordeman, L.; Vicente, J.J. Microtubule Targeting Agents in Disease: Classic Drugs, Novel Roles. Cancers 2021, 13, 5650. [Google Scholar] [CrossRef] [PubMed]
- Petroni, G.; Buqué, A.; Coussens, L.M.; Galluzzi, L. Targeting oncogene and non-oncogene addiction to inflame the tumour microenvironment. Nat. Rev. Drug Discov. 2022, 21, 440–462. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; O’Kelly, D.; Schuetze, R.; Carlson, G.; Zhou, H.; Trawick, M.L.; Pinney, K.G.; Mason, R.P. Non-Invasive Evaluation of Acute Effects of Tubulin Binding Agents: A Review of Imaging Vascular Disruption in Tumors. Molecules 2021, 26, 2551. [Google Scholar] [CrossRef]
- Nepali, K.; Ojha, R.; Lee, H.Y.; Liou, J.P. Early investigational tubulin inhibitors as novel cancer therapeutics. Expert. Opin. Investig. Drugs 2016, 25, 917–936. [Google Scholar] [CrossRef]
- Siemann, D.W.; Shi, W.Y. Dual targeting of tumor vasculature: Combining avastin and vascular disrupting agents (CA4P or OXi4503). Anticancer Res. 2008, 28, 2027–2031. [Google Scholar]
- Inglis, D.J.; Lavranos, T.C.; Beaumont, D.M.; Leske, A.F.; Brown, C.K.; Hall, A.J.; Kremmidiotis, G. The vascular disrupting agent BNC105 potentiates the efficacy of VEGF and mTOR inhibitors in renal and breast cancer. Cancer Biol. Ther. 2014, 15, 1552–1560. [Google Scholar] [CrossRef]
- Siemann, D.W.; Rojiani, A.M. Antitumor efficacy of conventional anticancer drugs is enhanced by the vascular targeting agent ZD6126. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 1512–1517. [Google Scholar] [CrossRef]
- Ellis, L.; Shah, P.; Hammers, H.; Lehet, K.; Sotomayor, P.; Azabdaftari, G.; Seshadri, M.; Pili, R. Vascular disruption in combination with mTOR inhibition in renal cell carcinoma. Mol. Cancer Ther. 2012, 11, 383–392. [Google Scholar] [CrossRef]
- Nguyen, L.; Fifis, T.; Christophi, C. Vascular disruptive agent OXi4503 and anti-angiogenic agent Sunitinib combination treatment prolong survival of mice with CRC liver metastasis. BMC Cancer 2016, 16, 533. [Google Scholar] [CrossRef]
- Hadimani, M.B.; MacDonough, M.T.; Ghatak, A.; Strecker, T.E.; Lopez, R.; Sriram, M.; Nguyen, B.L.; Hall, J.J.; Kessler, R.J.; Shirali, A.R.; et al. Synthesis of a 2-Aryl-3-aroyl Indole Salt (OXi8007) Resembling Combretastatin A-4 with Application as a Vascular Disrupting Agent. J. Nat. Prod. 2013, 76, 1668–1678. [Google Scholar] [CrossRef]
- Ren, W.; Deng, Y.L.; Ward, J.D.; Vairin, R.; Bai, R.L.; Wanniarachchi, H.I.; Hamal, K.B.; Tankoano, P.E.; Tamminga, C.S.; Bueno, L.M.A.; et al. Synthesis and biological evaluation of structurally diverse 6-aryl-3-aroy-l-indole analogues as inhibitors of tubulin polymerization. Eur. J. Med. Chem. 2024, 263, 115794. [Google Scholar] [CrossRef] [PubMed]
- Vairin, R.; Tamminga, C.; Shi, Z.; Borchardt, C.; Jambulapati, J.; Bai, R.; Wanniarachchi, H.; Bueno, L.; Hamel, E.; Mason, R.P.; et al. Design, Synthesis and Biological Evaluation of 2-Phenyl Indole Analogues of OXi8006 as Colchicine Site Inhibitors of Tubulin Polymerization and Vascular Disrupting Agents. Bioorg. Med. Chem. 2025, 118, 117981. [Google Scholar] [CrossRef] [PubMed]
- Strecker, T.E.; Odutola, S.O.; Lopez, R.; Cooper, M.S.; Tidmore, J.K.; Charlton-Sevcik, A.K.; Li, L.; MacDonough, M.T.; Hadimani, M.B.; Ghatak, A.; et al. The vascular disrupting activity of OXi8006 in endothelial cells and its phosphate prodrug OXi8007 in breast tumor xenografts. Cancer Lett. 2015, 369, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hallac, R.R.; Lopez, R.; Denney, R.; MacDonough, M.T.; Li, L.; Liu, L.; Graves, E.E.; Trawick, M.L.; Pinney, K.G.; et al. Evaluation of tumor ischemia in response to an indole-based vascular disrupting agent using BLI and (19)F MRI. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 143–153. [Google Scholar]
- Siemann, D.W.; Chaplin, D.J.; Walicke, P.A. A review and update of the current status of the vasculature-disabling agent combretastatin-A4 phosphate (CA4P). Expert Opin. Investig. Drugs 2009, 18, 189–197. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Do, D.V.; Sepah, Y.J.; Shah, S.M.; Van Anden, E.; Hafiz, G.; Donahue, J.K.; Rivers, R.; Balkissoon, J.; Handa, J.T.; et al. Vascular disrupting agent for neovascular age related macular degeneration: A pilot study of the safety and efficacy of intravenous combretastatin A-4 phosphate. BMC Pharmacol. Toxicol. 2013, 14, 7. [Google Scholar] [CrossRef]
- Abma, E.; Daminet, S.; Smets, P.; Ni, Y.; de Rooster, H. Combretastatin A4-phosphate and its potential in veterinary oncology: A review. Vet. Comp. Oncol. 2017, 15, 184–193. [Google Scholar] [CrossRef]
- Serkova, N.J.; Glunde, K.; Haney, C.R.; Farhoud, M.; De Lille, A.; Redente, E.F.; Simberg, D.; Westerly, D.C.; Griffin, L.; Mason, R.P. Preclinical Applications of Multi-Platform Imaging in Animal Models of Cancer. Cancer Res. 2021, 81, 1189–1200. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, L.; Hahn, E.W.; Mason, R.P. Tumor physiological response to combretastatin A4 phosphate assessed by MRI. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 872–880. [Google Scholar] [CrossRef]
- Alhasan, M.K.; Liu, L.; Lewis, M.A.; Magnusson, J.; Mason, R.P. Comparison of Optical and Power Doppler Ultrasound Imaging for Non-Invasive Evaluation of Arsenic Trioxide as a Vascular Disrupting Agent in Tumors. PLoS ONE 2012, 7, e46106. [Google Scholar] [CrossRef] [PubMed]
- Colliez, F.; Fruytier, A.C.; Magat, J.; Neveu, M.A.; Cani, P.D.; Gallez, B.; Jordan, B.F. Monitoring Combretastatin A4-induced tumor hypoxia and hemodynamic changes using endogenous MR contrast and DCE-MRI. Magn. Reson. Med. 2016, 75, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.P.; McIntyre, D.J.; Checkley, D.; Tessier, J.J.; Howe, F.A.; Griffiths, J.R.; Ashton, S.E.; Ryan, A.J.; Blakey, D.C.; Waterton, J.C. Tumour dose response to the antivascular agent ZD6126 assessed by magnetic resonance imaging. Br. J. Cancer 2003, 88, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Beauregard, D.A.; Pedley, R.B.; Hill, S.A.; Brindle, K.M. Differential sensitivity of two adenocarcinoma xenografts to the anti-vascular drugs combretastatin A4 phosphate and 5,6-dimethylxanthenone-4-acetic acid, assessed using MRI and MRS. NMR Biomed. 2002, 15, 99–105. [Google Scholar] [CrossRef]
- Siim, B.G.; Laux, W.T.; Rutland, M.D.; Palmer, B.N.; Wilson, W.R. Scintigraphic Imaging of the Hypoxia Marker 99mTechnetium-labeled 2,2′-(1,4-Diaminobutane)bis(2-methyl-3-butanone) Dioxime (99mTc-labeled HL-91; Prognox): Noninvasive Detection of Tumor Response to the Antivascular Agent 5,6-Dimethylxanthenone-4-acetic Acid. Cancer Res. 2000, 60, 4582–4588. [Google Scholar]
- Zhao, D.; Richer, E.; Antich, P.P.; Mason, R.P. Antivascular effects of combretastatin A4 phosphate in breast cancer xenograft assessed using dynamic bioluminescence imaging (BLI) and confirmed by magnetic resonance imaging (MRI). FASEB J. 2008, 22, 2445–2451. [Google Scholar] [CrossRef]
- McNally, L.R.; Mezera, M.; Morgan, D.E.; Frederick, P.J.; Yang, E.S.; Eltoum, I.E.; Grizzle, W.E. Current and Emerging Clinical Applications of Multispectral Optoacoustic Tomography (MSOT) in Oncology. Clin. Cancer Res. 2016, 22, 3432–3439. [Google Scholar] [CrossRef]
- O’Kelly, D.; Guo, Y.; Mason, R.P. Evaluating online filtering algorithms to enhance dynamic multispectral optoacoustic tomography. Photoacoustics 2020, 19, 100184. [Google Scholar] [CrossRef]
- Wanniarachchi, H.I.; Bueno, L.M.A.; Rayas, R.; Hamal, K.B.; Pavlich, C.I.; Trawick, M.L.; Pinney, K.G.; Liu, L.; Mason, R.P. Acute Vasculature Disruption in Orthotopic Kidney Tumors Based on Vascular Disrupting Agent (OXi8007) Examined by Multispectral Optoacoustic Tomography (MSOT). In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2024, San Francisco, CA, USA, 28–31 January 2024. [Google Scholar]
- Rich, L.J.; Seshadri, M. Photoacoustic imaging of vascular hemodynamics: Validation with blood oxygenation level-dependent MR imaging. Radiology 2015, 275, 110–118. [Google Scholar] [CrossRef]
- Tomaszewski, M.R.; Gehrung, M.; Joseph, J.; Quiros-Gonzalez, I.; Disselhorst, J.A.; Bohndiek, S.E. Oxygen-Enhanced and Dynamic Contrast-Enhanced Optoacoustic Tomography Provide Surrogate Biomarkers of Tumor Vascular Function, Hypoxia, and Necrosis. Cancer Res. 2018, 78, 5980–5991. [Google Scholar] [CrossRef]
- Balasundaram, G.; Ding, L.; Li, X.T.; Attia, A.B.E.; Dean-Ben, X.L.; Ho, C.J.H.; Chandrasekharan, P.; Tay, H.C.; Lim, H.Q.; Ong, C.B.; et al. Noninvasive Anatomical and Functional Imaging of Orthotopic Glioblastoma Development and Therapy using Multispectral Optoacoustic Tomography. Transl. Oncol. 2018, 11, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, H.; Gerberich, J.L.; Odutola, S.O.; Charlton-Sevcik, A.K.; Li, M.; Tanpure, R.P.; Tidmore, J.K.; Trawick, M.L.; Pinney, K.G.; et al. Imaging-Guided Evaluation of the Novel Small-Molecule Benzosuberene Tubulin-Binding Agent KGP265 as a Potential Therapeutic Agent for Cancer Treatment. Cancers 2021, 13, 4769. [Google Scholar] [CrossRef] [PubMed]
- Grassi, P.; Verzoni, E.; Ratta, R.; Mennitto, A.; de Braud, F.; Procopio, G. Cabozantinib in the treatment of advanced renal cell carcinoma: Design, development, and potential place in the therapy. Drug Des. Devel Ther. 2016, 10, 2167–2172. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, Y.; Gi, M.; Yamano, S.; Tachibana, H.; Okuno, T.; Tamada, S.; Nakatani, T.; Wanibuchi, H. Anti-PD-L1 treatment enhances antitumor effect of everolimus in a mouse model of renal cell carcinoma. Cancer Sci. 2016, 107, 1736–1744. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Aren Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthelemy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Hammers, H. Immunotherapy in kidney cancer: The past, present, and future. Curr. Opin. Urol. 2016, 26, 543–547. [Google Scholar] [CrossRef]
- Motzer, R.J.; Rini, B.I.; McDermott, D.F.; Redman, B.G.; Kuzel, T.M.; Harrison, M.R.; Vaishampayan, U.N.; Drabkin, H.A.; George, S.; Logan, T.F.; et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J. Clin. Oncol. 2015, 33, 1430–1437. [Google Scholar] [CrossRef]
- Ding, J.; Wang, C.; Chang, X. Establishment of a bioluminescent Renca cell line for renal carcinoma research. Int. Urol. Nephrol. 2018, 50, 55–61. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, Z.; Yang, H.; Li, X.; Wang, Q.; Wang, L.; Li, S. Enhance the anti-renca carcinoma effect of a DNA vaccine targeting G250 gene by co-expression with cytotoxic T-lymphocyte associated antigen-4(CTLA-4). Biomed. Pharmacother. 2017, 90, 147–152. [Google Scholar] [CrossRef]
- Makhov, P.; Joshi, S.; Ghatalia, P.; Kutikov, A.; Uzzo, R.G.; Kolenko, V.M. Resistance to Systemic Therapies in Clear Cell Renal Cell Carcinoma: Mechanisms and Management Strategies. Mol. Cancer Ther. 2018, 17, 1355–1364. [Google Scholar] [CrossRef]
- Chen, W.; Hill, H.; Christie, A.; Kim, M.S.; Holloman, E.; Pavia-Jimenez, A.; Homayoun, F.; Ma, Y.; Patel, N.; Yell, P.; et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 2016, 539, 112–117. [Google Scholar] [CrossRef]
- Elias, R.; Tcheuyap, V.T.; Kaushik, A.K.; Singla, N.; Gao, M.; Reig Torras, O.; Christie, A.; Mulgaonkar, A.; Woolford, L.; Stevens, C.; et al. A renal cell carcinoma tumorgraft platform to advance precision medicine. Cell Rep. 2021, 37, 110055. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Schuetze, R.; Gerberich, J.L.; Lopez, R.; Odutola, S.O.; Tanpure, R.P.; Charlton-Sevcik, A.K.; Tidmore, J.K.; Taylor, E.A.S.; Kapur, P.; et al. Demonstrating Tumor Vascular Disrupting Activity of the Small-Molecule Dihydronaphthalene Tubulin-Binding Agent OXi6196 as a Potential Therapeutic for Cancer Treatment. Cancers 2022, 14, 4208. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Siles, R.; Ackley, J.F.; Hadimani, M.B.; Hall, J.J.; Mugabe, B.E.; Guddneppanavar, R.; Monk, K.A.; Chapuis, J.-C.; Pettit, G.R.; Chaplin, D.J.; et al. Combretastatin Dinitrogen-Substituted Stilbene Analogues as Tubulin-Binding and Vascular-Disrupting Agents. J. Nat. Prod. 2008, 71, 313–320. [Google Scholar] [CrossRef]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a High-Flux Anticancer Drug Screen Using a Diverse Panel of Cultured Human Tumor Cell Lines. JNCI J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef]
- Pavía-Jiménez, A.; Tcheuyap, V.T.; Brugarolas, J. Establishing a human renal cell carcinoma tumorgraft platform for preclinical drug testing. Nat. Protoc. 2014, 9, 1848–1859. [Google Scholar] [CrossRef]
- Liu, L.; Beck, H.; Wang, X.; Hsieh, H.P.; Mason, R.P.; Liu, X. Tubulin-destabilizing agent BPR0L075 induces vascular-disruption in human breast cancer mammary fat pad xenografts. PLoS ONE 2012, 7, e43314. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Liu, L.; Mason, R.P.; Gimi, B. Dynamic bioluminescence and fluorescence imaging of the effects of the antivascular agent Combretastatin-A4P (CA4P) on brain tumor xenografts. Cancer Lett. 2015, 356, 462–469. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Q.; Wang, Y.; Huang, J.; Fang, Y.; Wu, W.; Wu, W.; Wu, F.; Yu, X.; Sun, Y. A high-throughput and simultaneous determination of combretastatin A-4 phosphate and its metabolites in human plasma using HPLC–MS/MS: Application to a clinical pharmacokinetic study. Biomed. Chromatogr. 2021, 35, e5204. [Google Scholar] [CrossRef] [PubMed]
- Rustin, G.J.; Galbraith, S.M.; Anderson, H.; Stratford, M.; Folkes, L.K.; Sena, L.; Gumbrell, L.; Price, P.M. Phase I clinical trial of weekly combretastatin A4 phosphate: Clinical and pharmacokinetic results. J. Clin. Oncol. 2003, 21, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Busk, M.; Bohn, A.B.; Skals, M.; Wang, T.; Horsman, M.R. Combretastatin-induced hypertension and the consequences for its combination with other therapies. Vasc. Pharmacol. 2011, 54, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, D.J.; Peters, C.E.; Horsman, M.R.; Trotter, M.J. Drug induced perturbations in tumor blood flow: Therapeutic potential and possible limitations. Radiother. Oncol. 1991, 20 (Suppl. 1), 93–101. [Google Scholar] [CrossRef]
- Okunieff, P.; Walsh, C.S.; Vaupel, P.; Kallinowski, F.; Hitzig, B.M.; Neuringer, L.J.; Suit, H.D. Effects of hydralazine on in vivo tumor energy metabolism, hematopoietic radiation sensitivity, and cardiovascular parameters. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 1145–1148. [Google Scholar] [CrossRef]
- Dalal, S.; Burchill, S.A. Preclinical evaluation of vascular-disrupting agents in Ewing’s sarcoma family of tumours. Eur. J. Cancer 2009, 45, 713–722. [Google Scholar] [CrossRef]
- Pinney, K.; Wang, F.; Hadimani, M.; Mejia, M. Indole-Containing Compounds with Anti-Tubulin Vascular Targeting Activity. U.S. Patent 2007/0082872 A1, 12 April 2007. [Google Scholar]
- Liu, H.; Sun, S.; Wang, G.; Lu, M.; Zhang, X.; Wei, X.; Gao, X.; Huang, C.; Li, Z.; Zheng, J.; et al. Tyrosine Kinase Inhibitor Cabozantinib Inhibits Murine Renal Cancer by Activating Innate and Adaptive Immunity. Front. Oncol. 2021, 11, 663517. [Google Scholar] [CrossRef]
- Lee, H.W.; Seo, H.S.; Yeom, S.-Y.; Kim, S.-N.; Kim, C.R.; Park, D.-H.; Park, W.; Choy, Y.B.; Park, C.G.; Seo, S.I. Cabozantinib-Loaded PLGA Nanoparticles: A Potential Adjuvant Strategy for Surgically Resected High-Risk Non-Metastatic Renal Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 12634. [Google Scholar] [CrossRef]
- Zhao, H.; Nolley, R.; Chan, A.M.W.; Rankin, E.B.; Peehl, D.M. Cabozantinib inhibits tumor growth and metastasis of a patient-derived xenograft model of papillary renal cell carcinoma with MET mutation. Cancer Biol. Ther. 2017, 18, 863–871. [Google Scholar] [CrossRef]
- Kwilas, A.R.; Ardiani, A.; Donahue, R.N.; Aftab, D.T.; Hodge, J.W. Dual effects of a targeted small-molecule inhibitor (cabozantinib) on immune-mediated killing of tumor cells and immune tumor microenvironment permissiveness when combined with a cancer vaccine. J. Transl. Med. 2014, 12, 294. [Google Scholar] [CrossRef]
- Garon, E.B.; Neidhart, J.D.; Gabrail, N.Y.; de Oliveira, M.R.; Balkissoon, J.; Kabbinavar, F. A randomized Phase II trial of the tumor vascular disrupting agent CA4P (fosbretabulin tromethamine) with carboplatin, paclitaxel, and bevacizumab in advanced nonsquamous non-small-cell lung cancer. Onco Targets Ther. 2016, 9, 7275–7283. [Google Scholar] [CrossRef]
- Blay, J.Y.; Pápai, Z.; Tolcher, A.W.; Italiano, A.; Cupissol, D.; López-Pousa, A.; Chawla, S.P.; Bompas, E.; Babovic, N.; Penel, N.; et al. Ombrabulin plus cisplatin versus placebo plus cisplatin in patients with advanced soft-tissue sarcomas after failure of anthracycline and ifosfamide chemotherapy: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015, 16, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Modulation Of The Tumour Microenvironment Using Either Vascular Disrupting Agents or STAT3 Inhibition in Order to Synergise With PD1 Inhibition in Microsatellite Stable, Refractory Colorectal Cancer. 2018. Available online: https://clinconnect.io/trials/NCT03647839 (accessed on 20 February 2025).
- Hill, S.A.; Chaplin, D.J.; Lewis, G.; Tozer, G.M. Schedule dependence of combretastatin A4 phosphate in transplanted and spontaneous tumour models. Int. J. Cancer 2002, 102, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wu, L.; Qiu, L. Water-Soluble Combretastatin A4 Phosphate Orally Delivered via Composite Nanoparticles With Improved Inhibition Effect Toward S180 Tumors. J. Pharm. Sci. 2017, 106, 3076–3083. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, L.; Li, X.F. Targeting Hypoxia: Hypoxia-Activated Prodrugs in Cancer Therapy. Front. Oncol. 2021, 11, 700407. [Google Scholar] [CrossRef] [PubMed]
- Legigan, T.; Clarhaut, J.; Tranoy-Opalinski, I.; Monvoisin, A.; Renoux, B.; Thomas, M.; Le Pape, A.; Lerondel, S.; Papot, S. The First Generation of β-Galactosidase-Responsive Prodrugs Designed for the Selective Treatment of Solid Tumors in Prodrug Monotherapy. Angew. Chem. Int. Ed. 2012, 51, 11606–11610. [Google Scholar] [CrossRef]
- Chaplin, D.J.; Hill, S.A. The development of combretastatin A4 phosphate as a vascular targeting agent. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 1491–1496. [Google Scholar] [CrossRef]
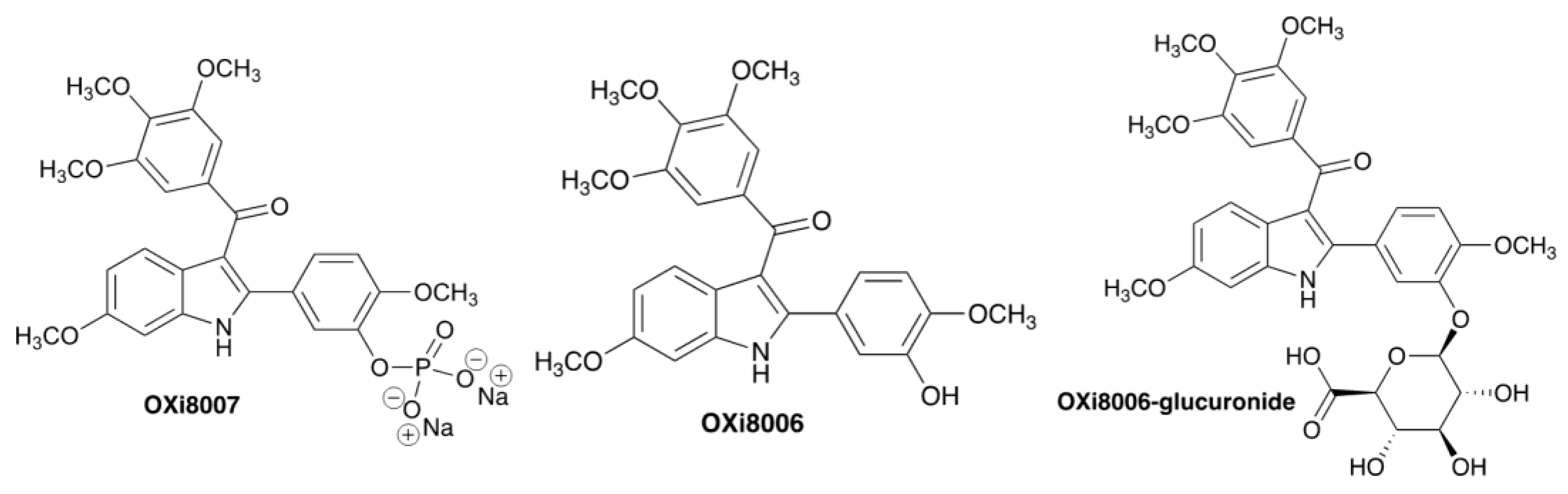

| Tumor Cells | Compound | IC50 (µM) |
|---|---|---|
| Renca | CA4 | 0.048 ± 0.002 |
| OXi8006 | 1.575 ± 0.153 | |
| OXi8007 | 1.450 ± 0.001 | |
| Renca-luc | CA4 | 0.109 ± 0.023 |
| OXi8006 | 1.142 ± 0.097 | |
| OXi8007 | 1.562 ± 0.048 |
| OXi8007 | T1/2 (h) | Tmax (min) | Cmax (ng/mg) | AUC (h × ng/ mg) |
|---|---|---|---|---|
| plasma | 0.82 | 20 | 453 # | 511 # |
| tumor and right kidney | 2 | 60 | 14.99 | 36.31 |
| left kidney | 1.18 | 20 | 1.4 | 2.05 |
| liver | 1.1 | 20 | 13.4 | 12.67 |
| brain | 0.51 | 60 | 0.07 | 0.11 |
| lungs | 0.7 | 20 | 1.61 | 2.3 |
| heart | 0.61 | 20 | 1.62 | 1.32 |
| spleen | 0.65 | 60 | 0.99 | 1.41 |
| OXi8006 | T1/2 (h) | Tmax (min) | Cmax (ng/mg) | AUC (h × ng/mg) |
|---|---|---|---|---|
| plasma | 1.99 | 20 | 29 # | 47 # |
| tumor and right kidney | 4.23 | 60 | 2.39 | 10.24 |
| left kidney | 1.73 | 20 | 5.86 | 8.72 |
| liver | 2.25 | 20 | 9.16 | 18.19 |
| brain | 1.71 | 20 | 0.22 | 0.54 |
| lungs | 2.56 | 20 | 4.18 | 9.45 |
| heart | 2.02 | 20 | 5.31 | 9.04 |
| spleen | 1.72 | 20 | 4.45 | 9.88 |
| OXi8006 Glucuronide | T1/2 (h) | Tmax (h) | Cmax (counts × 10−6) + | AUC (h × counts × 10−6) + |
|---|---|---|---|---|
| plasma | 1.95 | 1 | 1266 | 3490 |
| tumor | 5.87 | 4 | 11.65 | 118.22 |
| left kidney | 3.75 | 1 | 87.2 | 180.6 |
| liver | 3.37 | 1 | 28.23 | 128.7 |
| brain | 1.15 | 1 | 0.95 | 4.13 |
| lungs | 2.05 | 1 | 20.87 | 69.33 |
| heart | 1.61 | 1 | 9.8 | 24.52 |
| spleen | 2.2 | 1 | 6.33 | 18.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanniarachchi, H.I.; Schuetze, R.; Deng, Y.; Hamal, K.B.; Pavlich, C.I.; Tankoano, P.E.O.; Tamminga, C.; Hammers, H.; Kapur, P.; Bueno, L.M.A.; et al. Evaluating Therapeutic Efficacy of the Vascular Disrupting Agent OXi8007 Against Kidney Cancer in Mice. Cancers 2025, 17, 771. https://doi.org/10.3390/cancers17050771
Wanniarachchi HI, Schuetze R, Deng Y, Hamal KB, Pavlich CI, Tankoano PEO, Tamminga C, Hammers H, Kapur P, Bueno LMA, et al. Evaluating Therapeutic Efficacy of the Vascular Disrupting Agent OXi8007 Against Kidney Cancer in Mice. Cancers. 2025; 17(5):771. https://doi.org/10.3390/cancers17050771
Chicago/Turabian StyleWanniarachchi, Hashini I., Regan Schuetze, Yuling Deng, Khagendra B. Hamal, Cyprian I. Pavlich, Pouguiniseli E. O. Tankoano, Caleb Tamminga, Hans Hammers, Payal Kapur, Lorena M. A. Bueno, and et al. 2025. "Evaluating Therapeutic Efficacy of the Vascular Disrupting Agent OXi8007 Against Kidney Cancer in Mice" Cancers 17, no. 5: 771. https://doi.org/10.3390/cancers17050771
APA StyleWanniarachchi, H. I., Schuetze, R., Deng, Y., Hamal, K. B., Pavlich, C. I., Tankoano, P. E. O., Tamminga, C., Hammers, H., Kapur, P., Bueno, L. M. A., Rayas, R., Wang, T., Liu, L., Trawick, M. L., Pinney, K. G., & Mason, R. P. (2025). Evaluating Therapeutic Efficacy of the Vascular Disrupting Agent OXi8007 Against Kidney Cancer in Mice. Cancers, 17(5), 771. https://doi.org/10.3390/cancers17050771








