Current Landscape and Future Directions in Cancer Immunotherapy: Therapies, Trials, and Challenges
Simple Summary
Abstract
1. Introduction
2. Targeted Antibodies
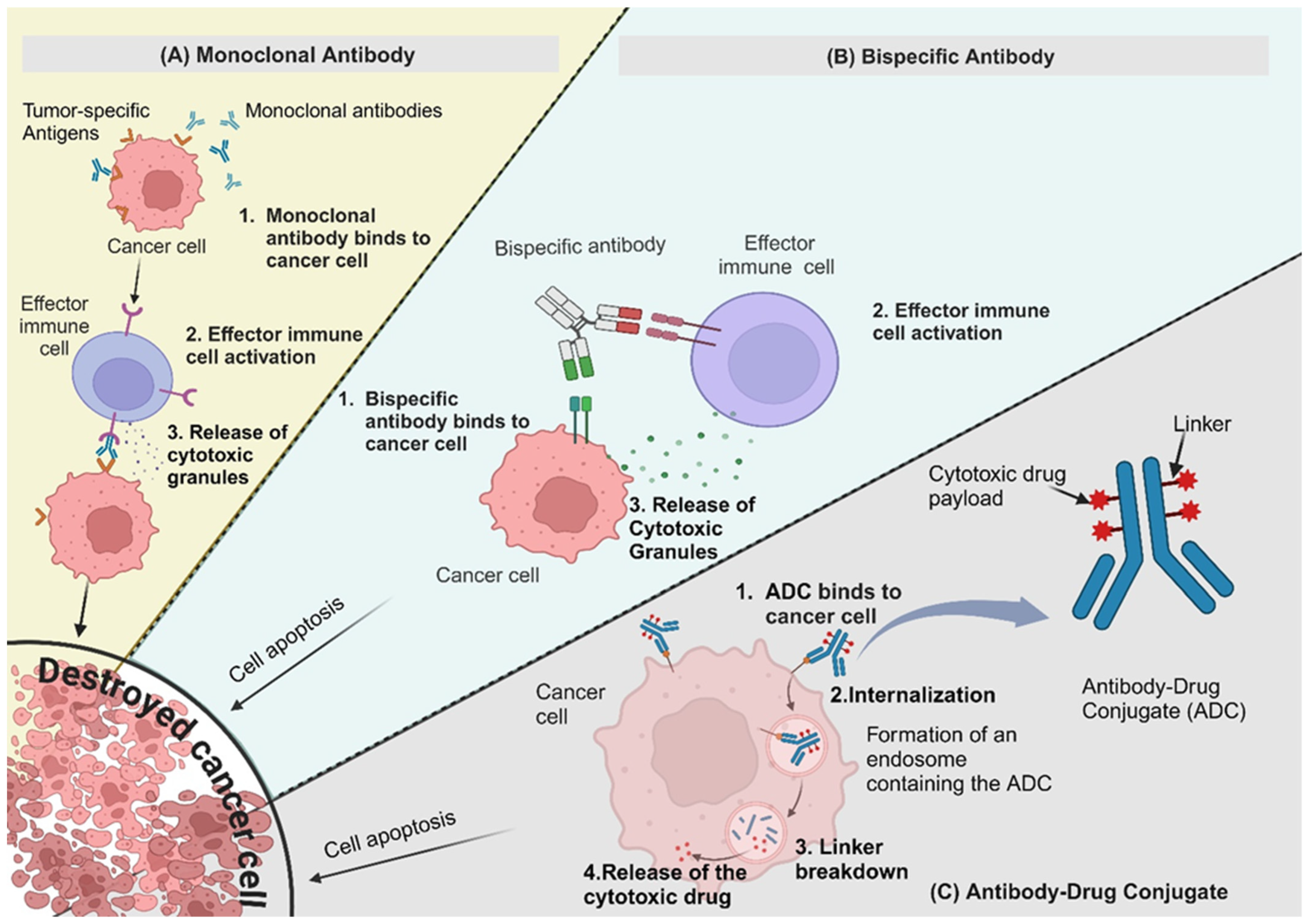
2.1. Monoclonal Antibodies (mAbs)
2.2. Bispecific Antibodies (BsAbs)
2.3. Antibody-Drug Conjugates (ADCs)
2.4. Antibody–Oligonucleotide Conjugates (AOCs)
3. Immune Checkpoint Inhibitors (ICIs)
Nanocarrier-Based Delivery of Immune Checkpoint Inhibitors
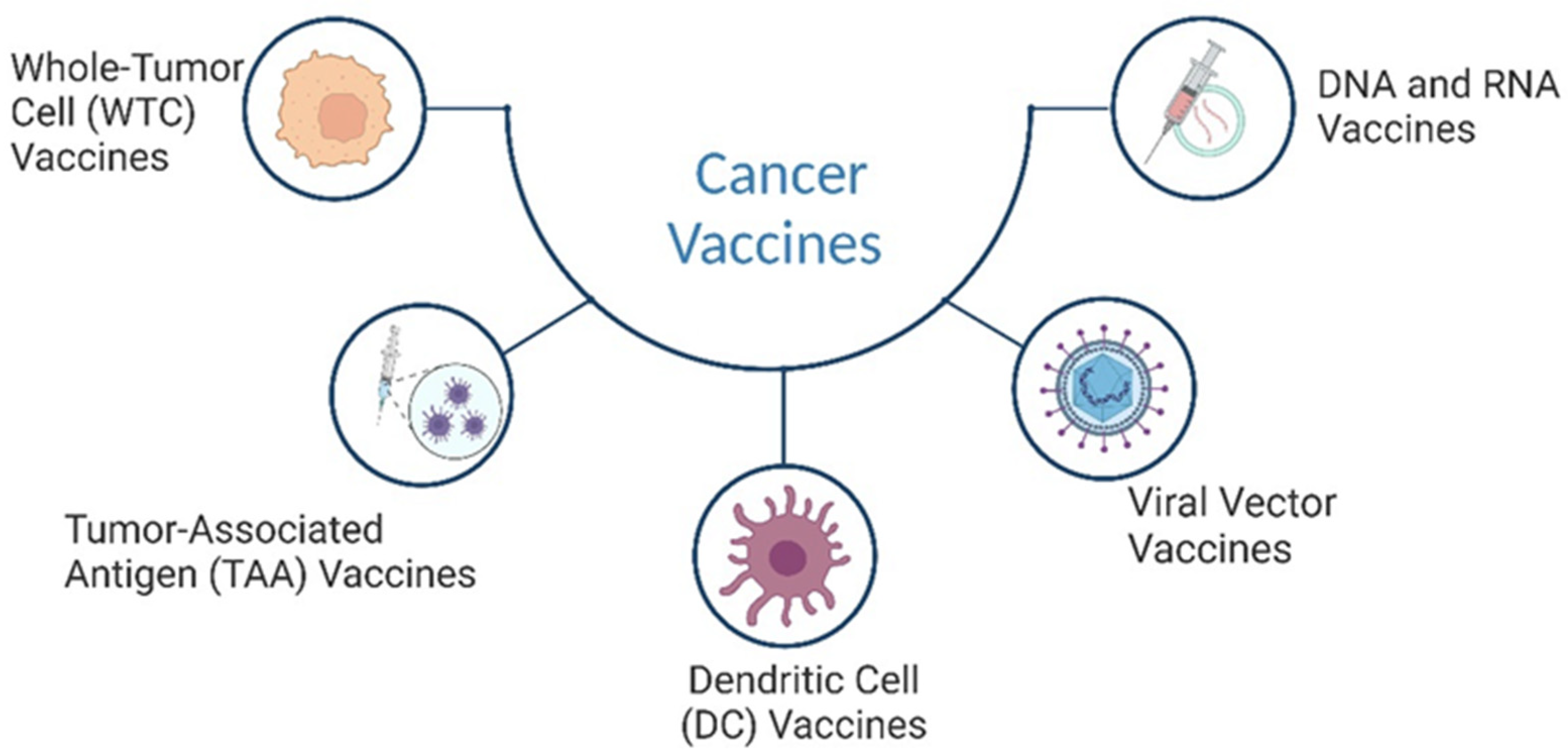
4. Cancer Vaccines
Nanocarrier-Based Approaches for Cancer Vaccine Delivery
5. Cytokines
6. Oncolytic Viruses
7. Adoptive Cell Therapy
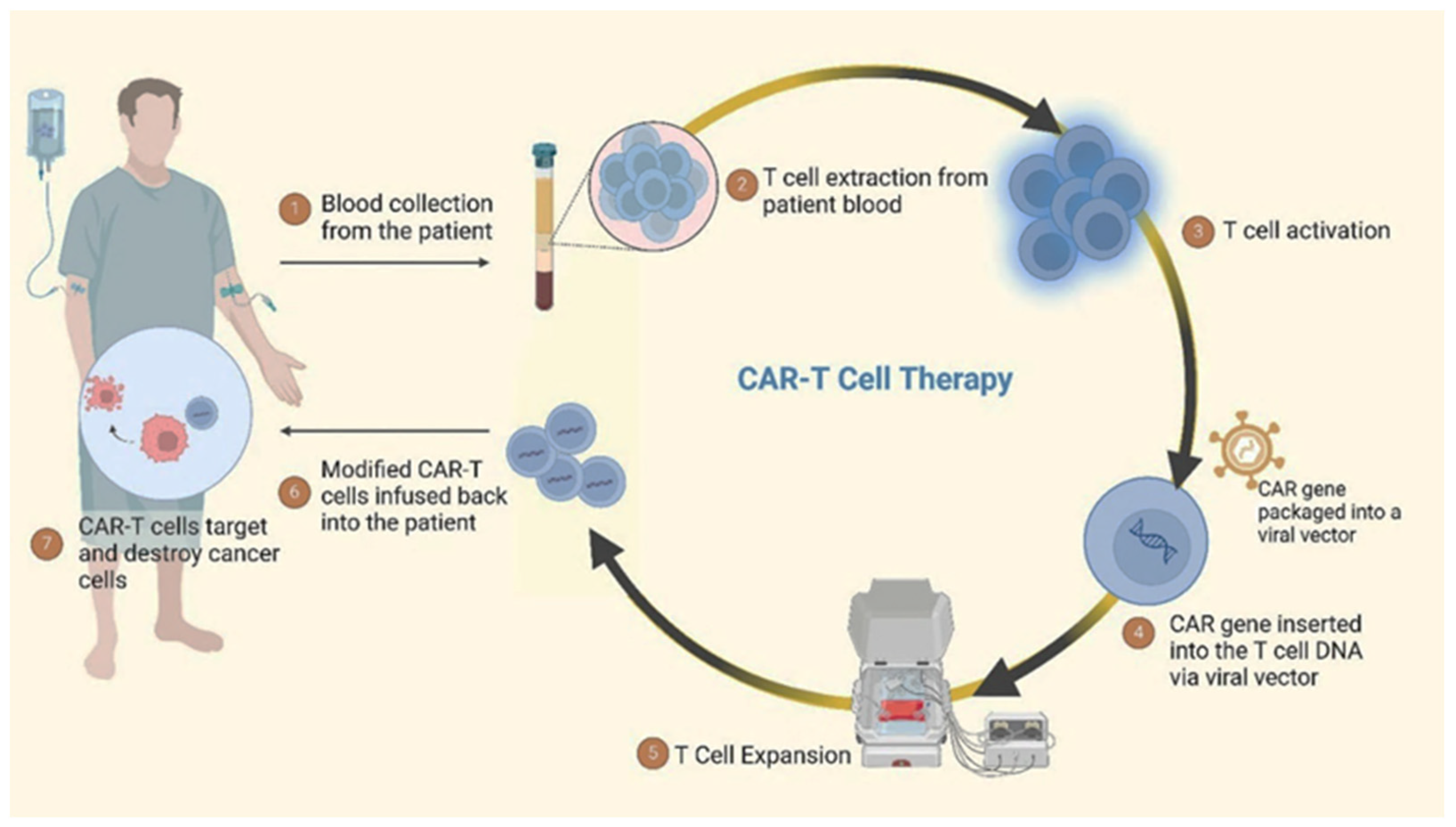
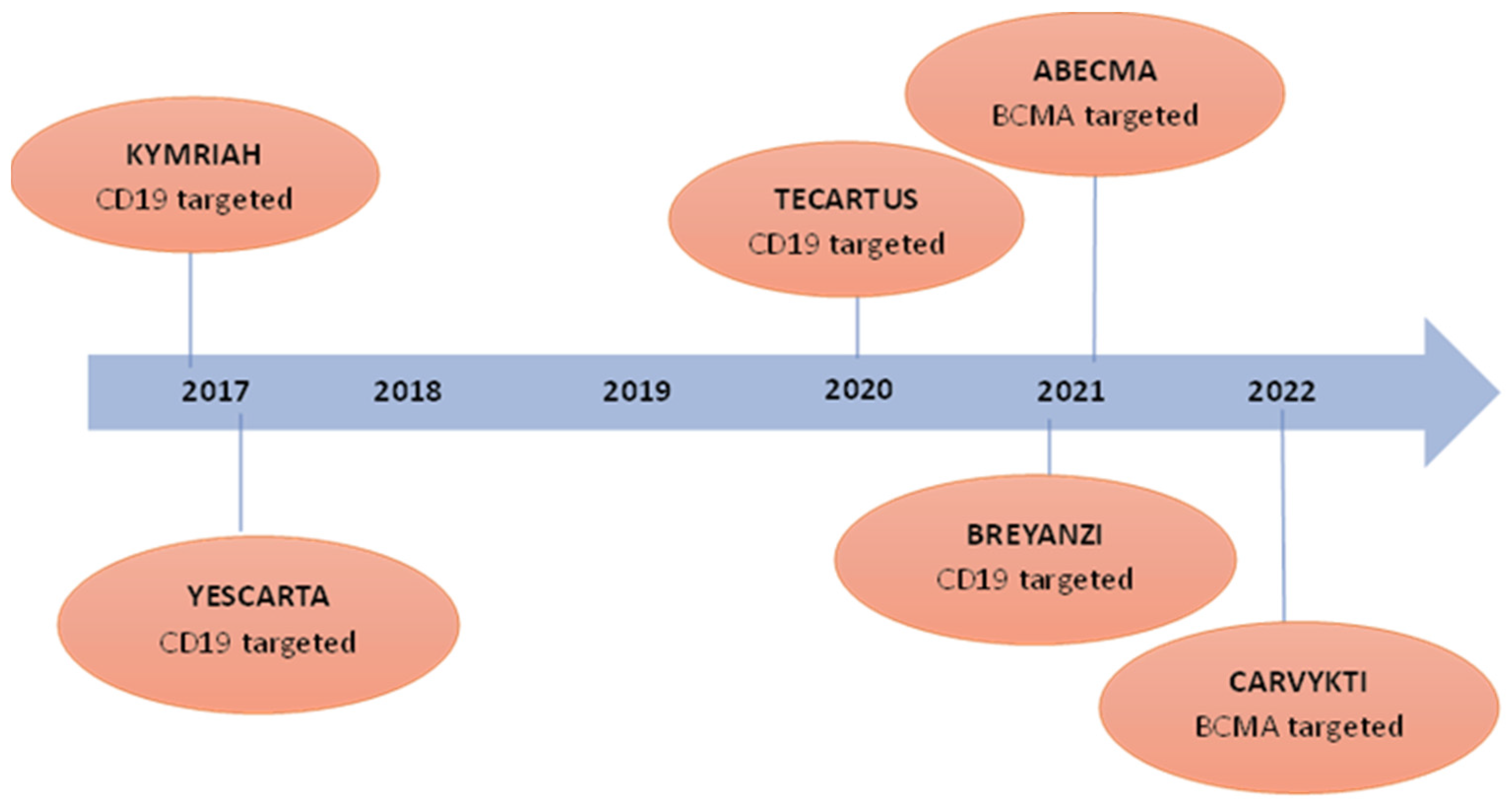
7.1. Chimeric Antigen Receptor T (CAR-T) Cell Therapy
7.2. Engineered Natural Killer (NK) Cells
8. Advances in Gene Therapy
8.1. Integration of Nanotechnology
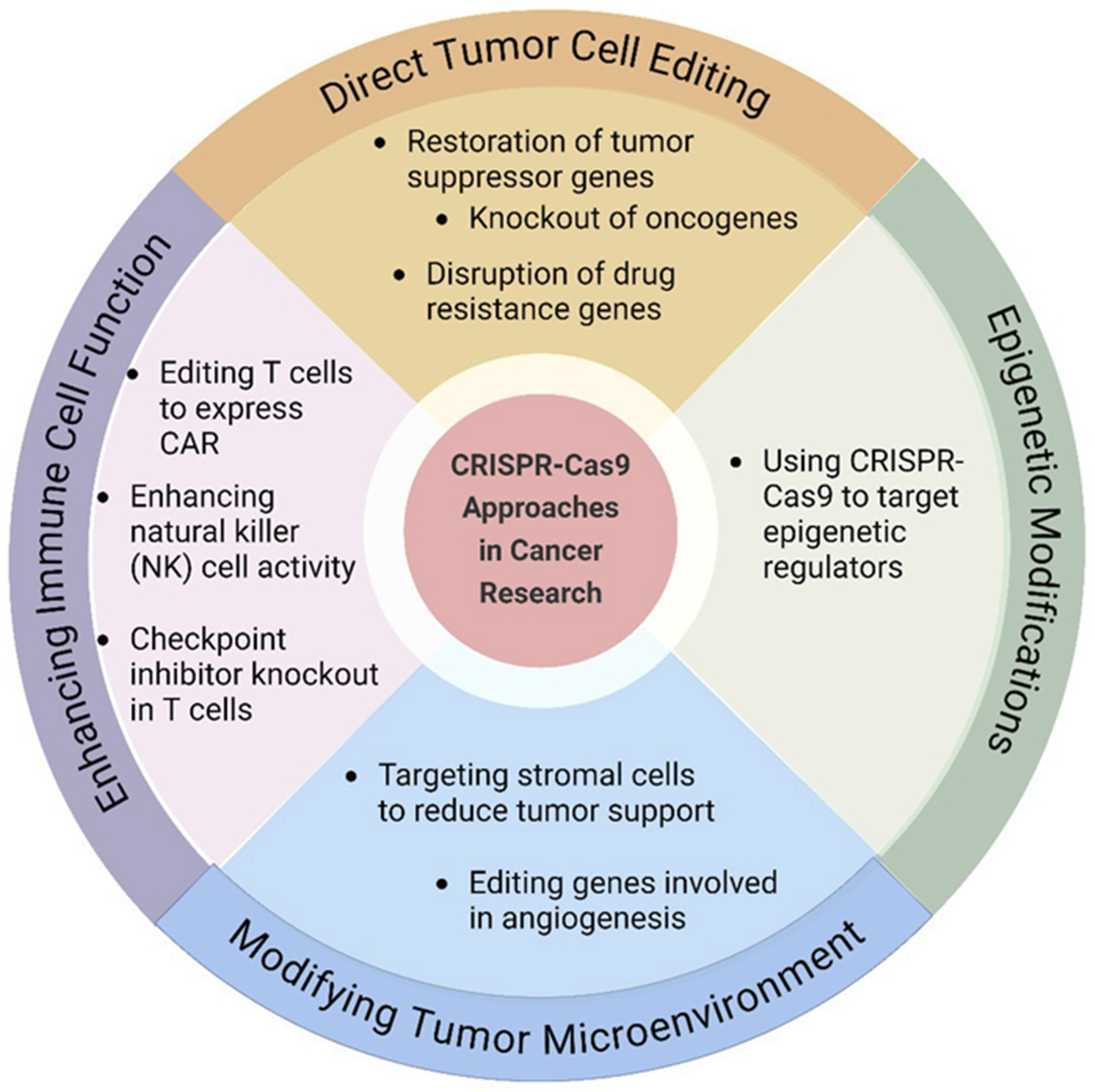
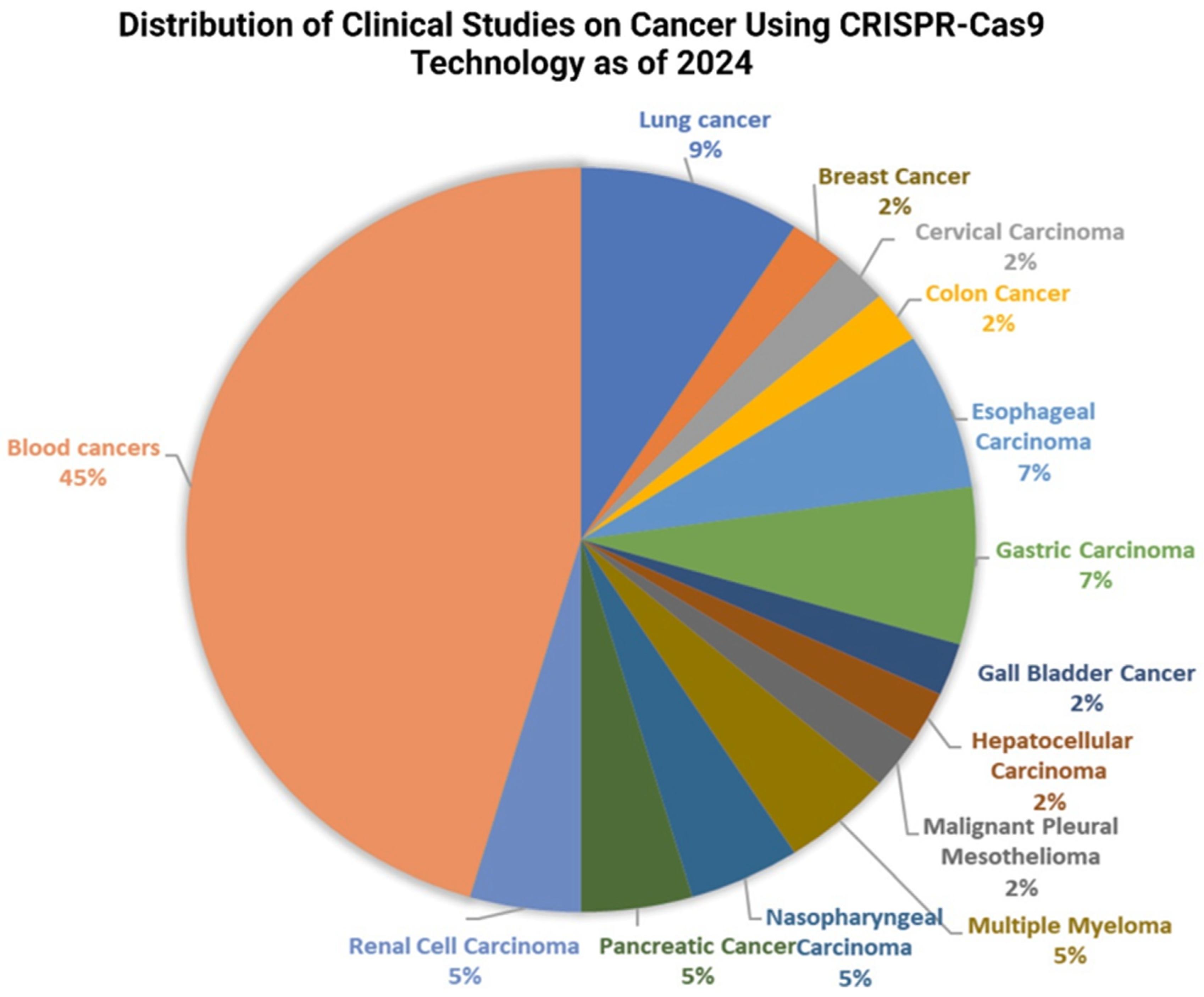
8.2. CRISPR-Cas9 Genome Editing
9. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Deo, S.V.S.; Sharma, J.; Kumar, S. GLOBOCAN 2020 Report on Global Cancer Burden: Challenges and Opportunities for Surgical Oncologists. Ann. Surg. Oncol. 2022, 29, 6497–6500. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhao, H.; Wang, R.; Chen, Y.; Ouyang, X.; Li, W.; Sun, Y.; Peng, A. Cancer epigenetics: From laboratory studies and clinical trials to precision medicine. Cell Death Discov. 2024, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; DeGiovanni, P.-J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Sharma, N.; Sahi, S.V. Advances in Cancer Therapeutics: Conventional Thermal Therapy to Nanotechnology-Based Photothermal Therapy. Pharmaceutics 2021, 13, 1174. [Google Scholar] [CrossRef]
- Wu, X.; Deng, Z.; Zhao, Q. Immunotherapy Improves Disease Prognosis by Affecting the Tumor Microenvironment: A Bibliometric Study. Front. Immunol. 2022, 13, 967076. [Google Scholar] [CrossRef]
- Amin, S.; Baine, M.J.; Meza, J.L.; Lin, C. The Impact of Neoadjuvant and Adjuvant Immunotherapy on the Survival of Pancreatic Cancer Patients: A Retrospective Analysis. BMC Cancer 2020, 20, 538. [Google Scholar] [CrossRef]
- Varney, V.; Hamid, Q.; Gaga, M.; Sun, Y.; Jacobson, M.R.; Frew, A.J.; Kay, A.B.; Durham, S.R. Influence of Grass Pollen Immunotherapy on Cellular Infiltration and Cytokine mRNA Expression During Allergen-Induced Late-Phase Cutaneous Responses. J. Clin. Investig. 1993, 92, 644–651. [Google Scholar] [CrossRef]
- Shindo, G.; Endo, T.; Onda, M.; Goto, S.; Miyamoto, Y.; Kaneko, T. Is the CD4/CD8 Ratio an Effective Indicator for Clinical Estimation of Adoptive Immunotherapy for Cancer Treatment? J. Cancer Ther. 2013, 4, 1382. [Google Scholar] [CrossRef][Green Version]
- Chen, L.; He, Y.; Zhu, J.; Zhao, S.; Qi, S.; Chen, X.; Zhang, H.; Ni, Z.; Zhou, Y.; Chen, G.; et al. The roles and mechanism of m(6)A RNA methylation regulators in cancer immunity. Biomed. Pharmacother. = Biomed. Pharmacother. 2023, 163, 114839. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Jiang, T.; Wang, J. Role of LMO7 in cancer (Review). Oncol. Rep. 2024, 52, 117. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Li, D.; Peng, Y.; Wang, S.; Hu, S.; Liu, M.; Ding, J.; Zhou, W. Metal organic framework coated MnO(2) nanosheets delivering doxorubicin and self-activated DNAzyme for chemo-gene combinatorial treatment of cancer. Int. J. Pharm. 2020, 585, 119513. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Nishina, Y.; Bianco, A. A Glutathione Responsive Nanoplatform Made of Reduced Graphene Oxide and MnO2 Nanoparticles for Photothermal and Chemodynamic Combined Therapy. Carbon 2021, 178, 783–791. [Google Scholar] [CrossRef]
- Borchmann, S.; Selenz, C.; Lohmann, M.; Ludwig, H.; Gassa, A.; Brägelmann, J.; Lohneis, P.; Meder, L.; Mattlener, J.; Breid, S.; et al. Tripartite Antigen-Agnostic Combination Immunotherapy Cures Established Poorly Immunogenic Tumors. J. Immunother. Cancer 2022, 10, e004781. [Google Scholar] [CrossRef]
- Choi, B.; Kim, D.H. Multifunctional Nanocarriers-Mediated Synergistic Combination of Immune Checkpoint Inhibitor Cancer Immunotherapy and Interventional Oncology Therapy. Adv. Nanobiomed Res. 2021, 1, 2100010. [Google Scholar] [CrossRef]
- Büttner, N.; Schmidt, N.M.; Thimme, R. Perspectives of Immunotherapy in Hepatocellular Carcinoma (HCC). Z. Gastroenterol. 2016, 54, 1334–1342. [Google Scholar] [CrossRef]
- Capaccione, K.M.; Doubrovin, M.; Braumuller, B.; Leibowitz, D.; Bhatt, N.; Momen-Heravi, F.; Molotkov, A.; Kissner, M.; Goldner, K.; Soffing, M.; et al. Evaluating the Combined Anticancer Response of Checkpoint Inhibitor Immunotherapy and FAP-Targeted Molecular Radiotherapy in Murine Models of Melanoma and Lung Cancer. Cancers 2022, 14, 4575. [Google Scholar] [CrossRef] [PubMed]
- Kather, J.N.; Berghoff, A.S.; Ferber, D.; Suarez-Carmona, M.; Reyes-Aldasoro, C.C.; Valous, N.A.; Rojas-Moraleda, R.; Jäger, D.; Halama, N. Large-Scale Database Mining Reveals Hidden Trends and Future Directions for Cancer Immunotherapy. Oncoimmunology 2018, 7, e1444412. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, B.S.; Xie, B.; Moussa, E.M.; Iyer, L.K.; Chandrasekhar, S.; Panchal, J.P.; Topp, E.M. Structure of Monoclonal Antibodies. In Biobetters: Protein Engineering to Approach the Curative; Rosenberg, A., Demeule, B., Eds.; Springer: New York, NY, USA, 2015; pp. 81–89. [Google Scholar]
- Zahavi, D.; Weiner, L. Monoclonal antibodies in cancer therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Higel, F.; Seidl, A.; Sörgel, F.; Frieß, W. N-Glycosylation Heterogeneity and the Influence on Structure, Function and Pharmacokinetics of Monoclonal Antibodies and Fc Fusion Proteins. Eur. J. Pharm. Biopharm. 2016, 100, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Mimura, Y.; Katoh, T.; Saldova, R.; O’Flaherty, R.; Izumi, T.; Mimura-Kimura, Y.; Utsunomiya, T.; Mizukami, Y.; Yamamoto, K.; Matsumoto, T.; et al. Glycosylation Engineering of Therapeutic IgG Antibodies: Challenges for the Safety, Functionality and Efficacy. Protein Cell 2017, 9, 47–62. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Kervinen, J.; Müller, E.; Tanaka, T.; Muranaka, K. Analytical Characterization of Monoclonal Antibodies with Novel Fc Receptor-Based Chromatography Technique. In Monoclonal Antibodies; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Kosuge, H.; Nagatoishi, S.; Kiyoshi, M.; Ishii-Watabe, A.; Terao, Y.; Ide, T.; Tsumoto, K. Biophysical Characterization of the Contribution of the Fab Region to the IgG-FcγRIIIa Interaction. Biochemistry 2022, 62, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Near, R.I.; Zhong, X.; Gao, W. Cross-Species Higher Sensitivities of FcγRIIIA/FcγRIV to Afucosylated IgG for Enhanced ADCC. Antib. Ther. 2021, 4, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Kosuge, H.; Nagatoishi, S.; Kiyoshi, M.; Ishii-Watabe, A.; Tanaka, T.; Terao, Y.; Oe, S.; Ide, T.; Tsumoto, K. Highly Sensitive HPLC Analysis and Biophysical Characterization of N-glycans of IgG-Fc Domain in Comparison Between CHO and 293 Cells Using FcγRIIIa Ligand. Biotechnology Progress 2020, 36, e3016. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Fang, W.; Yang, Y.; Huang, Y.; Zou, W.; Wang, Z.; Ding, M.; Peng, Y.; Xiao, S.; et al. SI-B001 plus chemotherapy in patients with locally advanced or metastatic EGFR/ALK wild-type non-small cell lung cancer: A phase II, multicenter, open-label study. J. Clin. Oncol. 2023, 41, 9025. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Zhou, T.; Chen, G.; Huang, Y.; Liu, F.; Liu, Z.; Qu, S.; Lei, Y.; Chen, X.; et al. A phase Ib study of SHR-1701, a bifunctional fusion protein targeting PD-L1 and TGF-β, in patients with recurrent or metastatic nasopharyngeal carcinoma (RM-NPC). J. Clin. Oncol. 2022, 40, 6024. [Google Scholar] [CrossRef]
- Mu, S.; Liang, Z.; Wang, Y.; Chu, W.; Chen, Y.L.; Wang, Q.; Wang, G.; Wang, C. PD-L1/TIGIT bispecific antibody showed survival advantage in animal model. Clin. Transl. Med. 2022, 12, e754. [Google Scholar] [CrossRef]
- Lesokhin, A.M.; Tomasson, M.H.; Arnulf, B.; Bahlis, N.J.; Miles Prince, H.; Niesvizky, R.; Rodrίguez-Otero, P.; Martinez-Lopez, J.; Koehne, G.; Touzeau, C.; et al. Elranatamab in relapsed or refractory multiple myeloma: Phase 2 MagnetisMM-3 trial results. Nat. Med. 2023, 29, 2259–2267. [Google Scholar] [CrossRef] [PubMed]
- Valente, D.; Mauriac, C.; Schmidt, T.; Focken, I.; Beninga, J.; Mackness, B.C.; Qiu, H.; Vicat, P.; Kandira, A.; Radošević, K.; et al. Pharmacokinetics of Novel Fc-Engineered Monoclonal and Multispecific Antibodies in Cynomolgus Monkeys and Humanized FcRn Transgenic Mouse Models. MAbs 2020, 12, 1829337. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Dadey, D.Y.A.; Rau, M.J.; Fitzpatrick, J.; Shah, H.K.; Saikia, M.; Townsend, R.; Thotala, D.; Hallahan, D.E.; Kapoor, V. Blocking the Functional Domain of TIP1 by Antibodies Sensitizes Cancer to Radiation Therapy. Biomed. Pharmacother. 2023, 166, 115341. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Galisteo, A.; Compte, M.; Álvarez-Vallina, L.; Sanz, L. When Three Is Not a Crowd: Trispecific Antibodies for Enhanced Cancer Immunotherapy. Theranostics 2023, 13, 1028. [Google Scholar] [CrossRef]
- Ravi, G.; Costa, L.J. Bispecific T-cell Engagers for Treatment of Multiple Myeloma. Am. J. Hematol. 2023, 98, S13–S21. [Google Scholar] [CrossRef]
- Singh, K.; Hotchkiss, K.; Mohan, A.; Reedy, J.L.; Sampson, J.H.; Khasraw, M. For Whom the T Cells Troll? Bispecific T-Cell Engagers in Glioblastoma. J. Immunother. Cancer 2021, 9, e003679. [Google Scholar] [CrossRef]
- Syed, Y.Y. Sacituzumab Govitecan: First Approval. Drugs 2020, 80, 1019–1025. [Google Scholar] [CrossRef]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.-O.; Callander, N.; Lendvai, N.; Sborov, D. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020, 21, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Bang, Y.; Iwasa, S.; Sugimoto, N.; Ryu, M.; Sakai, D.; Chung, H.; Kawakami, H.; Yabusaki, H.; Lee, J. 1422MO Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER2-low, advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma: Results of the exploratory cohorts in the phase II, multicenter, open-label DESTINY-Gastric01 study. Ann. Oncol. 2020, 31, S899–S900. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Chae, Y.S.; Lee, K.S. Trastuzumab deruxtecan (T-DXd) versus treatment of physician’s choice (TPC) in patients (pts) with HER2-low unresectable and/or metastatic breast cancer (mBC): Results of DESTINY-Breast04, a randomized, phase 3 study. Am. Soc. Clin. Oncol. Am. Soc. Clin. Oncol. 2024, 31, 858–868. [Google Scholar]
- Lu, G.; Nishio, N.; Berg, N.S.v.d.; Martin, B.A.; Fakurnejad, S.; Keulen, S.v.; Colevas, A.D.; Thurber, G.M.; Rosenthal, E.L. Co-Administered Antibody Improves Penetration of Antibody–dye Conjugate Into Human Cancers with Implications for Antibody–drug Conjugates. Nat. Commun. 2020, 11, 5667. [Google Scholar] [CrossRef] [PubMed]
- Chaundler, C.S.P.; Fu, R.; Wang, N.; Lou, H.; Almeida, G.S.; Hadi, L.M.; Aboagye, E.O.; Ghaem-Maghami, S. Kinetics and Efficacy of Antibody Drug Conjugates in 3D Tumour Models. bioRxiv 2023. [Google Scholar] [CrossRef]
- Lewis, C.D.; Singh, A.K.; Hsu, F.F.; Thotala, D.; Hallahan, D.E.; Kapoor, V. Targeting a Radiosensitizing Antibody–Drug Conjugate to a Radiation-Inducible Antigen. Clin. Cancer Res. 2021, 27, 3224–3233. [Google Scholar] [CrossRef]
- Dugal-Tessier, J.; Thirumalairajan, S.; Jain, N. Antibody-Oligonucleotide Conjugates: A Twist to Antibody-Drug Conjugates. J. Clin. Med. 2021, 10, 838. [Google Scholar] [CrossRef] [PubMed]
- Äärelä, A.; Räsänen, K.; Holm, P.; Salo, H.M.; Virta, P. Synthesis of Site-Specific Antibody–[60]Fullerene–Oligonucleotide Conjugates for Cellular Targeting. ACS Appl. Bio Mater. 2023, 6, 3189–3198. [Google Scholar] [CrossRef]
- Malecová, B.; Burke, R.S.; Cochran, M.A.; Hood, M.D.; Johns, R.; Kovach, P.R.; Doppalapudi, V.R.; Erdoğan, G.; Arias, J.; Darimont, B.; et al. Targeted Tissue Delivery of RNA Therapeutics Using Antibody–oligonucleotide Conjugates (AOCs). Nucleic Acids Res. 2023, 51, 5901–5910. [Google Scholar] [CrossRef]
- Bae, J.; Song, Y. Engineering a Cell-Penetrating Hyperstable Antibody scFv(Ras)—An Extraordinary Approach to Cancer Therapeutics. Synth. Syst. Biotechnol. 2021, 6, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Vafaei, R.; Khaki, Z.; Salehi, M.; Jalili, N.; Esmailinejad, M.R.; Muhammadnajad, A.; Nassiri, S.M.; Vajhi, A.; Kalbolandi, S.M.; Mirzaei, R.; et al. Development of a MET-targeted Single-Chain Antibody Fragment as an Anti-Oncogene Targeted Therapy for Breast Cancer. Investig. New Drugs 2023, 41, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Baurand, P.-E.; Balland, J.; Reynas, C.; Ramseyer, M.; Vivier, D.; Bellaye, P.-S.; Collin, B.; Paul, C.; Denat, F.; Asgarov, K.; et al. Development of Anti-Lrrc15 Small Fragments for Imaging Purposes Using a Phage-Display ScFv Approach. Int. J. Mol. Sci. 2022, 23, 12677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Pei, P.; Wang, Y.; Guo, Q.; Luo, S.-Z.; Chen, L. A Single-chain Variable Fragment-anticancer Lytic Peptide (scFv-ACLP) Fusion Protein for Targeted Cancer Treatment. Chem. Biol. Drug Des. 2023, 101, 1406–1415. [Google Scholar] [CrossRef]
- Cancer Research UK. Checkpoint Inhibitors. Available online: https://www.cancerresearchuk.org/about-cancer/treatment/immunotherapy/types/checkpoint-inhibitors?_gl=1*fwmws8*_gcl_au*MTI1MTQxODMxMC4xNzAyNDc0MzI5*_ga*MTIwOTg1NzM3OS4xNjk5NjIzNTc0*_ga_58736Z2GNN*MTcwMzM1NDk1NC4zNy4xLjE3MDMzNTQ5NzUuMC4wLjA.&_ga=2.186033770.2146793522.1703354954-1209857379.1699623574 (accessed on 20 October 2024).
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A moving target in immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, K.; Meti, N.; Miller, W.H., Jr.; Hudson, M. Adverse events associated with immune checkpoint inhibitor treatment for cancer. CMAJ Can. Med. Assoc. J. = J. De L’association Medicale Can. 2019, 191, E40–E46. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Ruffo, E.; Wu, R.C.; Bruno, T.C.; Workman, C.J.; Vignali, D.A.A. Lymphocyte-activation gene 3 (LAG3): The next immune checkpoint receptor. Semin. Immunol. 2019, 42, 101305. [Google Scholar] [CrossRef] [PubMed]
- Bolm, L.; Petruch, N.; Sivakumar, S.; Annels, N.E.; Frampton, A.E. Gene of the month: T-cell immunoreceptor with immunoglobulin and ITIM domains (TIGIT). J. Clin. Pathol. 2022, 75, 217–221. [Google Scholar] [CrossRef]
- Hosseinkhani, N.; Derakhshani, A.; Shadbad, M.A.; Argentiero, A.; Racanelli, V.; Kazemi, T.; Mokhtarzadeh, A.; Brunetti, O.; Silvestris, N.; Baradaran, B. The Role of V-Domain Ig Suppressor of T Cell Activation (VISTA) in Cancer Therapy: Lessons Learned and the Road Ahead. Front Immunol 2021, 12, 676181. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Si, Q.; Qi, R.; Liu, W.; Li, M.; Guo, M.; Wei, L.; Yao, Z. Indoleamine 2,3-Dioxygenase 1: A Promising Therapeutic Target in Malignant Tumor. Front Immunol 2021, 12, 800630. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Kato, S.; Nesline, M.K.; Conroy, J.M.; DePietro, P.; Pabla, S.; Kurzrock, R. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat. Rev. 2022, 110, 102461. [Google Scholar] [CrossRef] [PubMed]
- Paschou, S.A.; Stefanaki, K.; Psaltopoulou, T.; Liontos, M.; Koutsoukos, K.; Zagouri, F.; Lambrinoudaki, I.; Dimopoulos, M.A. How we treat endocrine complications of immune checkpoint inhibitors. ESMO Open 2021, 6, 100011. [Google Scholar] [CrossRef]
- Yin, Q.; Wu, L.; Han, L.; Zheng, X.; Tong, R.; Li, L.; Bai, L.; Bian, Y. Immune-related adverse events of immune checkpoint inhibitors: A review. Front. Immunol. 2023, 14, 1167975. [Google Scholar] [CrossRef] [PubMed]
- Vaddepally, R.; Doddamani, R.; Sodavarapu, S.; Madam, N.R.; Katkar, R.; Kutadi, A.P.; Mathew, N.; Garje, R.; Chandra, A.B. Review of Immune-Related Adverse Events (irAEs) in Non-Small-Cell Lung Cancer (NSCLC)-Their Incidence, Management, Multiorgan irAEs, and Rechallenge. Biomedicines 2022, 10, 790. [Google Scholar] [CrossRef]
- Strauss, J.; Madan, R.A.; Gulley, J.L. Considerations for the Combination of Anticancer Vaccines and Immune Checkpoint Inhibitors. Expert Opin. Biol. Ther. 2016, 16, 895–901. [Google Scholar] [CrossRef]
- Kyi, C.; Postow, M.A. Immune Checkpoint Inhibitor Combinations in Solid Tumors: Opportunities and Challenges. Immunotherapy 2016, 8, 821–837. [Google Scholar] [CrossRef]
- Fuereder, T. Resistance to Immune Checkpoint Inhibitors. Next Steps and Combinational Approaches. memo-Mag. Eur. Med. Oncol. 2019, 12, 123–127. [Google Scholar] [CrossRef]
- Debien, V.; Caluwé, A.D.; Wang, X.; Piccart-Gebhart, M.; Tuohy, V.K.; Romano, E.; Buisseret, L. Immunotherapy in Breast Cancer: An Overview of Current Strategies and Perspectives. NPJ Breast Cancer 2023, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Llombart–Cussac, A.; André, F.; Harbeck, N.; Schmid, P.; Cescon, D.W.; Ahn, J.S.; Nanda, R.; Bardia, A.; Li, F.; et al. KEYLYNK-009: A Phase II/III, Open-Label, Randomized Study of Pembrolizumab (Pembro) Plus Olaparib vs Pembro Plus Chemotherapy After Induction with First-Line Pembro Plus Chemotherapy in Patients with Locally Recurrent Inoperable or Metastatic Triple-Negative Breast Cancer (TNBC). J. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Yi, M.; Jiao, D.; Qin, S.; Chu, Q.; Wu, K.; Li, A.P. Synergistic Effect of Immune Checkpoint Blockade and Anti-Angiogenesis in Cancer Treatment. Mol. Cancer 2019, 18, 60. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.H.; Lee, C.-H.; Makker, V.; Rasco, D.W.; Dutcus, C.E.; Wu, J.; Stepan, D.E.; Shumaker, R.; Motzer, R.J. Phase IB/II Trial of Lenvatinib Plus Pembrolizumab in Patients with Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. J. Clin. Oncol. 2020, 38, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Natarajan, S.K.; Nandi, D.; Kulkarni, A. Dual Inhibitors-Loaded Nanotherapeutics That Target Kinase Signaling Pathways Synergize with Immune Checkpoint Inhibitor. Cell. Mol. Bioeng. 2019, 12, 357–373. [Google Scholar] [CrossRef]
- Park, T.H.; Bae, S.-H.; Bong, S.M.; Ryu, S.E.; Jang, H.; Lee, B.I. Crystal Structure of the Kinase Domain of MerTK in Complex with AZD7762 Provides Clues for Structure-Based Drug Development. Int. J. Mol. Sci. 2020, 21, 7878. [Google Scholar] [CrossRef] [PubMed]
- Mygland, L.; Tveita, A.; Strand, M.F.; Solberg, N.; Olsen, P.; Lund, K.; Waaler, J.; Lycke, M.; Dybing, E.; Nygaard, V.; et al. Tankyrase Inhibition Sensitizes Melanoma to PD-1 Immune Checkpoint Blockade in Syngeneic Mouse Models. Commun. Biol. 2020, 3, 196. [Google Scholar] [CrossRef]
- Isshiki, T.; Isobe, K.; Tochigi, N.; Sunakawa, M.; Nakamura, Y.; Shibuya, K.; Sakamoto, S.; Takai, Y.; Homma, S. Successful Use of Pembrolizumab to Treat Refractory Thymic Carcinoma with High PD-L1 Expression. Case Rep. Oncol. 2018, 11, 688–692. [Google Scholar] [CrossRef]
- Du, Z.; Abedalthagafi, M.; Aizer, A.A.; McHenry, A.R.; Sun, H.H.; Bray, M.A.; Viramontes, O.; Machaidze, R.; Brastianos, P.K.; Reardon, D.A.; et al. Increased expression of the immune modulatory molecule PD-L1 (CD274) in anaplastic meningioma. Oncotarget 2015, 6, 4704–4716. [Google Scholar] [CrossRef]
- Weissferdt, A.; Fujimoto, J.; Kalhor, N.; Rodríguez, J.; Bassett, R.L.; Wistuba, I.I.; Moran, C.A. Expression of PD-1 and PD-L1 in Thymic Epithelial Neoplasms. Mod. Pathol. 2017, 30, 826–833. [Google Scholar] [CrossRef]
- Jakopović, M.; Bitar, L.; Seiwerth, F.; Marušić, A.; Krpina, K.; Samaržija, M. Immunotherapy for Thymoma. J. Thorac. Dis. 2020, 12, 7635. [Google Scholar] [CrossRef] [PubMed]
- Hirata, A.; Saraya, T.; Kobayashi, F.; Noda, A.; Aso, K.; Sakuma, S.; Kurokawa, N.; Inoue, M.; Mikura, S.; Oda, M.; et al. Immune-Related Adverse Events with Immune Checkpoint Inhibitors. Medicine 2021, 100, e25275. [Google Scholar] [CrossRef]
- Kawkgi, O.M.E.; Li, D.; Kotwal, A.; Wermers, R.A. Hypoparathyroidism: An Uncommon Complication Associated with Immune Checkpoint Inhibitor Therapy. Mayo Clin. Proc. Innov. Qual. Outcomes 2020, 4, 821–825. [Google Scholar] [CrossRef]
- Chen, E.M.; Quijano, A.; Seo, Y.-M.; Jackson, C.B.; Josowitz, A.; Noorbakhsh, S.; Merlettini, A.; Sundaram, R.K.; Focarete, M.L.; Jiang, Z.; et al. Biodegradable PEG-poly(ω-pentadecalactone-co-p-dioxanone) Nanoparticles for Enhanced and Sustained Drug Delivery to Treat Brain Tumors. Biomaterials 2018, 178, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.; Andaloussi, A.E.; Elmasry, K.; Handoussa, A.E.; Azab, M.S.; Elsawey, A.; Al-Hendy, A.; Ismail, N. PDL-1 Blockade Prevents T Cell Exhaustion, Inhibits Autophagy, and Promotes Clearance of Leishmania Donovani. Infect. Immun. 2018, 86, 10-1128. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, J.; Huang, Y.; Lu, B.; Li, S. Improved Cancer Immunochemotherapy via Optimal Co-Delivery of Chemotherapeutic and Immunomodulatory Agents. Mol. Pharm. 2018, 15, 5162–5173. [Google Scholar] [CrossRef] [PubMed]
- Nomizo, T.; Ozasa, H.; Tsuji, T.; Funazo, T.; Yasuda, Y.; Yoshida, H.; Yagi, Y.; Sakamori, Y.; Nagai, H.; Hirai, T.; et al. Clinical Impact of Single Nucleotide Polymorphism in PD-L1 on Response to Nivolumab for Advanced Non-Small-Cell Lung Cancer Patients. Sci. Rep. 2017, 7, 45124. [Google Scholar] [CrossRef]
- Bellmunt, J.; Wit, R.d.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.Á.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Mahoney, M.R.; Tine, B.A.V.; Atkins, J.N.; Milhem, M.; Jahagirdar, B.; Antonescu, C.R.; Horvath, E.; Tap, W.D.; Schwartz, G.K.; et al. Nivolumab with or Without Ipilimumab Treatment for Metastatic Sarcoma (Alliance A091401): Two Open-Label, Non-Comparative, Randomised, Phase 2 Trials. Lancet Oncol. 2018, 19, 416–426. [Google Scholar] [CrossRef]
- Abdel-Wahab, N.; Shah, M.; Suarez-Almazor, M.E. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. PLoS ONE 2016, 11, e0160221. [Google Scholar] [CrossRef] [PubMed]
- Kittai, A.; Oldham, H.; Cetnar, J.; Taylor, M.K. Immune Checkpoint Inhibitors in Organ Transplant Patients. J. Immunother. 2017, 40, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Nayereh, K.G.; Khadem, G. Preventive and Therapeutic Vaccines against Human Papillomaviruses Associated Cervical Cancers. Iran. J. Basic Med. Sci. 2012, 15, 585–601. [Google Scholar]
- Ayatollahi, H.; Homaei-shandiz, F.; Kooshyar, M.M.; Tabatabaee-Yazdi, S.A.; Mehrjerdian, M.; Jafarian, A.H.; Sadeghian, M.H.; Keramati, M.R.; Ghasemian-Moghadam, H.R.; Sheikhi, M. Human papilloma virus 16/18 genotypes in patients with squamous cell carcinoma of cervix in northeast Iran. Niger. Med. J. J. Niger. Med. Assoc. 2014, 55, 495–498. [Google Scholar]
- Sharma, R.; Yolcu, E.S.; Shirwan, H. SA-4-1BBL as a Novel Adjuvant for the Development of Therapeutic Cancer Vaccines. Expert Rev. Vaccines 2014, 13, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, T.; Kitagawa, K. Antitumor Effect of Oral Cancer Vaccine with Bifidobacterium delivering WT1 Protein to Gut Immune System Is Superior to WT1 Peptide Vaccine. Hum. Vaccines Immunother. 2018, 14, 159–162. [Google Scholar] [CrossRef]
- Ransom, J.H.; Pelle, B.; Hubers, H.A.J.M.; Keynton, L.M.; Hanna, M.G.; Pomato, N. Identification of Colon-tumor-associated Antigens by T-cell Lines Derived from Tumor-infiltrating Lymphocytes and Peripheral-blood Lymphocytes from Patients Immunized with an Autologous Tumor-cell/Bacillus Calmette-Guérin Vaccine. Int. J. Cancer 1993, 54, 734–740. [Google Scholar] [CrossRef]
- de Gruijl, T.D.; van den Eertwegh, A.J.M.; Pinedo, H.M.; Scheper, R.J. Whole-cell cancer vaccination: From autologous to allogeneic tumor- and dendritic cell-based vaccines. Cancer Immunol. Immunother. 2008, 57, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wang, Y.; Pang, M.; Zhang, J. STAT3-blocked Whole-Cell Hepatoma Vaccine Induces Cellular and Humoral Immune Response Against HCC. J. Exp. Clin. Cancer Res. 2017, 36, 156. [Google Scholar] [CrossRef]
- Olin, M.R.; Low, W.C.; McKenna, D.H.; Haines, S.J.; Dahlheimer, T.; Nascene, D.; Gustafson, M.P.; Dietz, A.B.; Clark, H.B.; Chen, W.; et al. Vaccination with Dendritic Cells Loaded with Allogeneic Brain Tumor Cells for Recurrent Malignant Brain Tumors Induces a CD4+IL17+ Response. J. Immunother. Cancer 2014, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, J.M.; Levy, R. Dendritic cell vaccines for cancer immunotherapy. Annu. Rev. Med. 1999, 50, 507–529. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Peng, J.; Liu, P.; Wu, Q. The Efficacy of Dendritic Cell Vaccine for Newly Diagnosed Glioblastoma: A Meta-Analysis of Randomized Controlled Studies. Clin. Neuropharmacol. 2021, 44, 216–221. [Google Scholar] [CrossRef]
- Bai, R.; Chen, N.; Li, L.; Du, N.; Bai, L.; Lv, Z.; Tian, H.; Cui, J. Mechanisms of Cancer Resistance to Immunotherapy. Front. Oncol. 2020, 10, 1290. [Google Scholar] [CrossRef] [PubMed]
- Leitner, W.W.; Ying, H.; Restifo, N.P. DNA and RNA-based vaccines: Principles, progress and prospects. Vaccine 1999, 18, 765–777. [Google Scholar] [CrossRef]
- Grimmett, E.; Al-Share, B.; Alkassab, M.B.; Zhou, R.W.; Desai, A.; Rahim, M.M.A.; Woldie, I. Cancer vaccines: Past, present and future; a review article. Discov. Oncol. 2022, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, H.; Jehng, T.; Li, Y.; Chen, Z.; Lee, K.-D.; Shen, H.-T.; Jones, L.; Huang, X.F.; Chen, S.-Y. A Novel Anti-Pd-L1 Vaccine for Cancer Immunotherapy and Immunoprevention. Cancers 2019, 11, 1909. [Google Scholar] [CrossRef]
- Tomić, S.; Petrović, A.; Puač, N.; Škoro, N.; Bekić, M.; Petrović, Z.L.; Čolić, M. Plasma-Activated Medium Potentiates the Immunogenicity of Tumor Cell Lysates for Dendritic Cell-Based Cancer Vaccines. Cancers 2021, 13, 1626. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer vaccines as promising immuno-therapeutics: Platforms and current progress. J. Hematol. Oncol. 2022, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Erhart, F.; Hackl, M.; Hahne, H.; Buchroithner, J.; Meng, C.; Klingenbrunner, S.; Reitermaier, R.; Fischhuber, K.; Skalicky, S.; Berger, W.; et al. Combined Proteomics/miRNomics of Dendritic Cell Immunotherapy-Treated Glioblastoma Patients as a Screening for Survival-Associated Factors. NPJ Vaccines 2020, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Cappuccini, F.; Bryant, R.J.; Pollock, E.; Carter, L.M.; Verrill, C.; Hollidge, J.; Poulton, I.; Baker, M.; Mitton, C.H.; Baines, A.; et al. Safety and Immunogenicity of Novel 5T4 Viral Vectored Vaccination Regimens in Early Stage Prostate Cancer: A Phase I Clinical Trial. J. Immunother. Cancer 2020, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.; Mo, F.; Zhang, S.; Lu, L.; Han, N.; Liu, L.; Qiu, M.; Li, H.; Han, W.; Ma, D.; et al. Combination Treatment of Radiofrequency Ablation and Peptide Neoantigen Vaccination: Promising Modality for Future Cancer Immunotherapy. Front. Immunol. 2022, 13, 1000681. [Google Scholar] [CrossRef]
- Toh, U.; Sakurai, S.; Saku, S.; Takao, Y.; Okabe, M.; Iwakuma, N.; Shichijo, S.; Yamada, A.; Itoh, K.; Akagi, Y. Early Phase II Study of Mixed 19-peptide Vaccine Monotherapy for Refractory Triple-negative Breast Cancer. Cancer Sci. 2020, 111, 2760–2769. [Google Scholar] [CrossRef] [PubMed]
- Makhoul, I.; Ibrahim, S.M.; Jousheghany, F.; Siegel, E.; Rogers, L.J.; Lee, J.J.; Piña-Oviedo, S.; Post, G.R.; Beck, J.T.; Kieber-Emmons, T.; et al. P10s-Padre Vaccine Combined with Neoadjuvant Chemotherapy in ER-positive Breast Cancer Patients Induces Humoral and Cellular Immune Responses. Oncotarget 2021, 12, 2252. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Mo, F.; Shou, J.; Wang, H.; Luo, K.; Zhang, S.; Han, N.; Li, H.; Ye, S.; Zhou, Z. A pan-cancer clinical study of personalized neoantigen vaccine monotherapy in treating patients with various types of advanced solid tumors. Clin. Cancer Res. 2020, 26, 4511–4520. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Han, N.; Jiang, J.; Xu, Y.; Ma, D.; Lu, L.; Guo, X.; Qiu, M.; Huang, Q. A neoantigen-based peptide vaccine for patients with advanced pancreatic cancer refractory to standard treatment. Front. Immunol. 2021, 12, 691605. [Google Scholar] [CrossRef]
- van de Loosdrecht, A.; van Elssen, J.; Gjertsen, B.T.; Wagner, E.M.; Holderried, T.; Platzbecker, U.; Cloos, J.J.; Rovers, J. Conversion from MRD positive to negative status in AML patients in CR1 after treatment with an allogeneic leukemia-derived dendritic cell vaccine. Blood 2020, 136, 13–14. [Google Scholar] [CrossRef]
- Sahin, U.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R.A.; Vormehr, M.; Gold, M.; Maurus, D.; Schwarck-Kokarakis, D.; Kuhn, A.N.; Omokoko, T. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 2020, 585, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Tsoras, A.N.; Champion, J.A. Cross-Linked Peptide Nanoclusters for Delivery of Oncofetal Antigen as a Cancer Vaccine. Bioconjugate Chem. 2018, 29, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Mu, W.; Pang, X.; Liu, Y.; Zhang, N.; Song, Y.; Garg, S. Cascade Cytosol Delivery of Dual-Sensitive Micelle-Tailored Vaccine for Enhancing Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2018, 10, 37797–37811. [Google Scholar] [CrossRef]
- Sunoqrot, S.; Abdel Gaber, S.A.; Abujaber, R.; Al-Majawleh, M.; Talhouni, S. Lipid- And Polymer-Based Nanocarrier Platforms for Cancer Vaccine Delivery. ACS Appl. Bio Mater. 2024, 7, 4998–5019. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, S.; Wang, X.; Zhu, G. Nanovaccines for Cancer Immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2019, 11, e1559. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, X.; Yue, Y.; Zhang, K.; Cheng, K.; Feng, Q.; Ma, N.; Liang, J.; Zhang, T.; Zhang, L.; et al. Rapid Surface Display of mRNA Antigens by Bacteria-Derived Outer Membrane Vesicles for a Personalized Tumor Vaccine. Adv. Mater. 2022, 34, 2109984. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, H.; Wang, Z.; Bian, E.; Zhao, B. The Role and Application of Small Extracellular Vesicles in Glioma. Cancer Cell Int. 2024, 24, 229. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Fichter, M.; Kuhn, G.; Brückner, M.; Kappel, C.; Schunke, J.; Klaus, T.; Grabbe, S.; Landfester, K.; Mailänder, V. Achieving Dendritic Cell Subset-Specific Targeting in Vivo by Site-Directed Conjugation of Targeting Antibodies to Nanocarriers. Nano Today 2022, 43, 101375. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Wang, J.; Wang, H. Advances in Cancer Nanovaccines: Harnessing Nanotechnology for Broadening Cancer Immune Response. Chemmedchem 2023, 18, e202200673. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Ma, P.; Luo, X.; Cheng, H.; Wang, Z.; Ye, E.; Loh, X.J.; Wu, Y.; Liu, Y. Stimuli-Responsive Polymeric Nanovaccines Toward Next-Generation Immunotherapy. Acs Nano 2023, 17, 9826–9849. [Google Scholar] [CrossRef]
- Guan, H.; Singh, N.P.; Singh, U.P.; Nagarkatti, P.S.; Nagarkatti, M. Resveratrol prevents endothelial cells injury in high-dose interleukin-2 therapy against melanoma. PLoS ONE 2012, 7, e35650. [Google Scholar] [CrossRef]
- Hanzly, M.; Aboumohamed, A.; Yarlagadda, N.; Creighton, T.; Digiorgio, L.; Fredrick, A.; Rao, G.; Mehedint, D.; George, S.; Attwood, K. High-dose interleukin-2 therapy for metastatic renal cell carcinoma: A contemporary experience. Urology 2014, 83, 1129–1134. [Google Scholar] [CrossRef]
- Conlon, K.C.; Miljkovic, M.D.; Waldmann, T.A. Cytokines in the treatment of cancer. J. Interferon Cytokine Res. 2019, 39, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Liu, L. Interferon Alpha Induces the Apoptosis of Cervical Cancer HeLa Cells by Activating Both the Intrinsic Mitochondrial Pathway and ER Stress-Induced Pathway. Int. J. Mol. Sci. 2016, 17, 1832. [Google Scholar] [CrossRef]
- Shah, N.R.; Declouette, B.; Ansari-Gilani, K.; Alhomoud, M.S.; Hoimes, C.; Ramaiya, N.H.; Güler, E. High-dose interleukin-2 therapy related adverse events and implications on imaging. Diagn. Interv. Radiol. 2021, 27, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, Q.; Jiang, J.; Zhang, J.; Song, X.; Cui, J.; Ye, Y.; Wang, Z.; Zhang, X.; Ren, X. Randomized, Multicenter, Open-Label Trial of Autologous Cytokine-Induced Killer Cell Immunotherapy Plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer: NCT01631357. Signal Transduct. Target. Ther. 2020, 5, 244. [Google Scholar] [CrossRef] [PubMed]
- Crake, R.; Strother, M.; Phillips, E.; Doogue, M.; Zhang, M.; Frampton, C.; Robinson, B.A.; Currie, M.J. Influence of Serum Inflammatory Cytokines on Cytochrome P450 Drug Metabolising Activity During Breast Cancer Chemotherapy: A Patient Feasibility Study. Sci. Rep. 2021, 11, 5648. [Google Scholar] [CrossRef] [PubMed]
- Safi, S.; Yamauchi, Y.; Hoffmann, H.; Weichert, W.; Jost, P.J.; Winter, H.; Muley, T.; Beckhove, P. Circulating Interleukin-4 Is Associated with a Systemic T Cell Response Against Tumor-Associated Antigens in Treatment-Naïve Patients with Resectable Non-Small-Cell Lung Cancer. Cancers 2020, 12, 3496. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.J.; Peng, K.-W.; Bell, J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012, 30, 658–670. [Google Scholar] [CrossRef]
- Masemann, D.; Boergeling, Y.; Ludwig, S. Employing RNA viruses to fight cancer: Novel insights into oncolytic virotherapy. Biol. Chem. 2017, 398, 891–909. [Google Scholar] [CrossRef] [PubMed]
- Conry, R.M.; Westbrook, B.; McKee, S.; Norwood, T.G. Talimogene laherparepvec: First in class oncolytic virotherapy. Hum. Vaccines Immunother. 2018, 14, 839–846. [Google Scholar] [CrossRef]
- Chesney, J.A.; Ribas, A.; Long, G.V.; Kirkwood, J.M.; Dummer, R.; Puzanov, I.; Hoeller, C.; Gajewski, T.F.; Gutzmer, R.; Rutkowski, P. Randomized, double-blind, placebo-controlled, global phase III trial of talimogene laherparepvec combined with pembrolizumab for advanced melanoma. J. Clin. Oncol. 2023, 41, 528. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Hoeller, C.; Gruter, I.P.; Michielin, O. Combining talimogene laherparepvec with immunotherapies in melanoma and other solid tumors. Cancer Immunol. Immunother. 2017, 66, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.E. Teserpaturev/G47Δ: First Approval. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2022, 36, 667–672. [Google Scholar] [CrossRef]
- Lu, W.; Zheng, S.; Li, X.F.; Huang, J.J.; Zheng, X.; Li, Z. Intra-tumor injection of H101, a recombinant adenovirus, in combination with chemotherapy in patients with advanced cancers: A pilot phase II clinical trial. World J. Gastroenterol. 2004, 10, 3634–3638. [Google Scholar] [CrossRef]
- Liang, M. Oncorine, the World First Oncolytic Virus Medicine and its Update in China. Curr. Cancer Drug Targets 2017, 18, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Chowaniec, H.; Ślubowska, A.; Mroczek, M.; Borowczyk, M.; Braszka, M.; Dworacki, G.; Dobosz, P.; Wichtowski, M. New hopes for the breast cancer treatment: Perspectives on the oncolytic virus therapy. Front. Immunol. 2024, 15, 1375433. [Google Scholar] [CrossRef]
- Moehler, M.; Heo, J.; Lee, H.C.; Tak, W.Y.; Chao, Y.; Paik, S.W.; Yim, H.J.; Byun, K.S.; Baron, A.; Ungerechts, G.; et al. Vaccinia-based oncolytic immunotherapy Pexastimogene Devacirepvec in patients with advanced hepatocellular carcinoma after sorafenib failure: A randomized multicenter Phase IIb trial (TRAVERSE). Oncoimmunology 2019, 8, 1615817. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, R.; Tran, H.; Selvaggi, G.; Hagerman, A.; Thompson, B.; Coffey, M. The oncolytic virus, pelareorep, as a novel anticancer agent: A review. Investig. New Drugs 2015, 33, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Sun, B.Z.; Xue, T.; Li, R.; Lin, C.; Gao, Y.; Sun, L.; Yang, Z.; Wang, D. Advances in CAR T-Cell Therapy in Bile Duct, Pancreatic, and Gastric Cancers. Front. Immunol. 2022, 13, 1025608. [Google Scholar] [CrossRef]
- Gao, Z. CAR-T Cell Therapy: Existing Treatments and Improvements. Highlights Sci. Eng. Technol. 2023, 36, 436–444. [Google Scholar] [CrossRef]
- Snook, A.E.; Patel, R.; Ghilardi, G.; Nimmagadda, A.; Yang, S.; Qian, D.; Porazzi, P.; Mazanet, R.; Schuster, S.J.; Barta, S.K.; et al. Senza5 TM CART5: An Autologous CD5-Deleted Anti-CD5 CART Product with Enhanced Anti-T-Cell Lymphoma Activity. Blood 2023, 142, 3462. [Google Scholar] [CrossRef]
- Lemoine, J.; Ruella, M.; Houot, R. Overcoming Intrinsic Resistance of Cancer Cells to CAR T-Cell Killing. Clin. Cancer Res. 2021, 27, 6298–6306. [Google Scholar] [CrossRef] [PubMed]
- Ponterio, E.; De Maria, R.; Haas, T.L. Identification of Targets to Redirect CAR T Cells in Glioblastoma and Colorectal Cancer: An Arduous Venture. Front. Immunol. 2020, 11, 565631. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M.E. CAR-T cell therapy: Current advances and future research possibilities. J. Sci. Res. Med. Biol. Sci. 2021, 2, 86–116. [Google Scholar] [CrossRef]
- Mohanty, R.; Chowdhury, C.R.; Arega, S.; Sen, P.; Ganguly, P.; Ganguly, N. CAR T cell therapy: A new era for cancer treatment. Oncol. Rep. 2019, 42, 2183–2195. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, O.; Keeler, A.M.; Flotte, T.R. CAR T-cell therapy: Progress and prospects. Hum. Gene Ther. Methods 2017, 28, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Labanieh, L.; Majzner, R.G.; Mackall, C.L. Programming CAR-T Cells to Kill Cancer. Nat. Biomed. Eng. 2018, 2, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, J.; Zhou, G.; Liu, T.-M.; Dai, Y.; Nie, G.; Zhao, Q. Remodeling of Tumor Microenvironment by Tumor-Targeting Nanozymes Enhances Immune Activation of CAR T Cells for Combination Therapy. Small 2021, 17, 2102624. [Google Scholar] [CrossRef] [PubMed]
- Donnadieu, E.; Dupré, L.; Pinho, L.G.; Cotta-de-Almeida, V. Surmounting the obstacles that impede effective CAR T cell trafficking to solid tumors. J. Leucoc. Biol. 2020, 108, 1067–1079. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Yuan, X.; Wang, W.; Wang, Y. Chimeric Antigen Receptor-Engineered NK Cells: New Weapons of Cancer Immunotherapy with Great Potential. Exp. Hematol. Oncol. 2022, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, I.; Solomou, E.E. A Panorama of Immune Fighters Armored with CARs in Acute Myeloid Leukemia. Cancers 2023, 15, 3054. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Han, D.; Liang, Y.; Ham, J.D.; Romee, R.; Chen, J. CAR-NK Cells: A Promising Cellular Immunotherapy for Cancer. Ebiomedicine 2020, 59, 102975. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wen, Q.; Zhang, X. CAR-NK Cell Therapy for Hematological malignancies: Recent Updates from ASH 2022. J. Hematol. Oncol. 2023, 16, 35. [Google Scholar] [CrossRef]
- Yu, S.J.; Ma, C.; Heinrich, B.; Brown, Z.J.; Sandhu, M.; Zhang, Q.; Fu, Q.; Agdashian, D.; Rosato, U.; Korangy, F.; et al. Targeting the Crosstalk Between Cytokine-Induced Killer Cells and Myeloid-Derived Suppressor Cells in Hepatocellular Carcinoma. J. Hepatol. 2019, 70, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Pietà, A.D.; Cappuzzello, E.; Palmerini, P.; Ventura, A.; Visentin, A.; Astori, G.; Chieregato, K.; Mozzo, V.; Perbellini, O.; Tisi, M.C.; et al. Innovative Therapeutic Strategy for B-Cell Malignancies That Combines Obinutuzumab and Cytokine-Induced Killer Cells. J. Immunother. Cancer 2021, 9, e002475. [Google Scholar] [CrossRef] [PubMed]
- Garofano, F.; Sharma, A.; Abken, H.; Gonzalez-Carmona, M.A.; Schmidt-Wolf, I.G.H. A Low Dose of Pure Cannabidiol Is Sufficient to Stimulate the Cytotoxic Function of CIK Cells Without Exerting the Downstream Mediators in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2022, 23, 3783. [Google Scholar] [CrossRef] [PubMed]
- Dehno, M.N.; Li, Y.; Weiher, H.; Schmidt-Wolf, I.G.H. Increase in Efficacy of Checkpoint Inhibition by Cytokine-Induced-Killer Cells as a Combination Immunotherapy for Renal Cancer. Int. J. Mol. Sci. 2020, 21, 3078. [Google Scholar] [CrossRef]
- Li, X.; Zhou, H.; Huang, W.; Xue-juan, W.; Meng, M.; Hou, Z.; Liao, L.; Tang, W.; Xie, Y.; Wang, R.; et al. Retrospective Analysis of the Efficacy of Adjuvant Cytokine-induced Killer Cell Immunotherapy Combined with Chemotherapy in Colorectal Cancer Patients After Surgery. Clin. Transl. Immunol. 2022, 11, e1368. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, I.; Castiglia, S.; Adamini, A.; Mandese, A.; Sismondi, F.; Rustichelli, D.; Leone, M.; Pinnetta, G.; Giordanengo, L.; Mareschi, K.; et al. Validation of the Media Fill Method for Cytokine-Induced Killer Cells Manufacturing Process. Transl. Med. Commun. 2023, 8, 16. [Google Scholar] [CrossRef]
- Castiglia, S.; Adamini, A.; Rustichelli, D.; Castello, L.; Mareschi, K.; Pinnetta, G.; Leone, M.; Mandese, A.; Ferrero, I.; Mesiano, G. Cytokines Induced Killer Cells Produced in Good Manufacturing Practices Conditions: Identification of the Most Advantageous and Safest Expansion Method in Terms of Viability, Cellular Growth and Identity. J. Transl. Med. 2018, 16, 237. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Menezes, M.E.; Bhatia, S.; Wang, X.Y.; Emdad, L.; Sarkar, D.; Fisher, P.B. Gene therapies for cancer: Strategies, challenges and successes. J. Cell. Physiol. 2015, 230, 259–271. [Google Scholar] [CrossRef]
- Aggarwal, A.; Mittal, R. Gene Therapy: Recent Advancement and Challenges. Asian J. Biochem. Genet. Mol. Biol. 2022, 10, 1–7. [Google Scholar] [CrossRef]
- Linton, S.S.; Sherwood, S.G.; Drews, K.; Kester, M. Targeting Cancer Cells in the Tumor Microenvironment: Opportunities and Challenges in Combinatorial Nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2016, 8, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Male, D.; Gromnicova, R. Nanocarriers for Delivery of Oligonucleotides to the CNS. Int. J. Mol. Sci. 2022, 23, 760. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jin, N.; Wang, Q.; Liu, T.; Liu, K.; Li, Y.; Bai, Y.; Chen, X. MiRNA Delivery System Based on Stimuli-Responsive Gold Nanoparticle Aggregates for Multimodal Tumor Therapy. ACS Appl. Bio Mater. 2019, 2, 2833–2839. [Google Scholar] [CrossRef]
- Zhang, M.; Shao, S.; Yue, H.; Wang, X.; Zhang, W.; Chen, F.; Zheng, L.; Xing, J.; Qin, Y. High Stability Au NPs: From Design to Application in Nanomedicine. Int. J. Nanomed. 2021, 16, 6067–6094. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Yun, Y.H.; Park, K. Smart Nanoparticles for Drug Delivery: Boundaries and Opportunities. Chem. Eng. Sci. 2015, 125, 158–164. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Z.; Tian, H.; Chen, X. Production and clinical development of nanoparticles for gene delivery. Mol. Ther.-Methods Clin. Dev. 2016, 3, 16023. [Google Scholar] [CrossRef] [PubMed]
- Amreddy, N.; Babu, A.; Muralidharan, R.; Munshi, A.; Ramesh, R. Polymeric Nanoparticle-Mediated Gene Delivery for Lung Cancer Treatment. In Polymeric Gene Delivery Systems; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Guerrero-Cázares, H.; Tzeng, S.Y.; Young, N.P.; Abutaleb, A.O.; Quiñones-Hinojosa, A.; Green, J.J. Biodegradable polymeric nanoparticles show high efficacy and specificity at DNA delivery to human glioblastoma in vitro and in vivo. ACS Nano 2014, 8, 5141–5153. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Tao, Z.; Zhu, B.; Zhang, W.; Liu, C.; Chen, S.; Song, M. Targeted Delivery of Drugs and Genes Using Polymer Nanocarriers for Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 9118. [Google Scholar] [CrossRef]
- Girigoswami, A.; Girigoswami, K. Potential Applications of Nanoparticles in Improving the Outcome of Lung Cancer Treatment. Genes 2023, 14, 1370. [Google Scholar] [CrossRef] [PubMed]
- Kunz, M.; Brandl, M.; Bhattacharya, A.; Nobereit-Siegel, L.; Ewe, A.; Weirauch, U.; Hering, D.; Reinert, A.; Guzman, J.; Weigelt, K.; et al. Nanoparticle-Complexed antimiRs for Inhibiting Tumor Growth and Metastasis in Prostate Carcinoma and Melanoma. J. Nanobiotechnology 2020, 18, 173. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.J.; Bugno, J.; Lee, S.R.; Hong, S. Dendrimer-based Nanocarriers: A Versatile Platform for Drug Delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1409. [Google Scholar] [CrossRef] [PubMed]
- Gorzkiewicz, M.; Kopeć, O.; Janaszewska, A.; Konopka, M.; Pedziwiatr-Werbicka, E.; Tarasenko, I.I.; Bezrodnyi, V.V.; Neelov, I.M.; Klajnert-Maculewicz, B. Poly(lysine) Dendrimers Form Complexes with siRNA and Provide Its Efficient Uptake by Myeloid Cells: Model Studies for Therapeutic Nucleic Acid Delivery. Int. J. Mol. Sci. 2020, 21, 3138. [Google Scholar] [CrossRef]
- Laurini, E.; Aulić, S.; Fermeglia, M.; Pricl, S. Evolution from Covalent to Self-Assembled PAMAM-Based Dendrimers as Nanovectors for siRNA Delivery in Cancer by Coupled in Silico-Experimental Studies. Part I: Covalent siRNA Nanocarriers. Pharmaceutics 2019, 11, 351. [Google Scholar] [CrossRef] [PubMed]
- Kanu, G.A.; Parambath, J.B.M.; Odeh, R.O.A.; Mohamed, A.A. Gold Nanoparticle-Mediated Gene Therapy. Cancers 2022, 14, 5366. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Cao, X.; Cun, X.; Hu, G.; Zhou, Y.; Zhang, Y.; Lu, L.; He, Q.; Gao, H. Matrix metalloproteinase-sensitive size-shrinkable nanoparticles for deep tumor penetration and pH triggered doxorubicin release. Biomaterials 2015, 60, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Mitra, M.; Kandalam, M.; Rangasamy, J.; Shankar, B.; Maheswari, U.K.; Swaminathan, S.; Krishnakumar, S. Novel epithelial cell adhesion molecule antibody conjugated polyethyleneimine-capped gold nanoparticles for enhanced and targeted small interfering RNA delivery to retinoblastoma cells. Mol. Vis. 2013, 19, 1029–1038. [Google Scholar]
- Yang, Z.; Liu, T.; Xie, Y.; Sun, Z.; Liu, H.; Lin, J.; Liu, C.; Mao, Z.-W.; Nie, S. Chitosan layered gold nanorods as synergistic therapeutics for photothermal ablation and gene silencing in triple-negative breast cancer. Acta Biomater. 2015, 25, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, A.; Hao, X.; Guo, R.; Shi, X.; Cao, X. PEGylated dendrimer-entrapped gold nanoparticles with low immunogenicity for targeted gene delivery. RSC Adv. 2018, 8, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Q.; Li, J.; Yang, M.; Zhao, N.; Xu, F.J. Rattle-Structured Rough Nanocapsules with in-Situ-Formed Gold Nanorod Cores for Complementary Gene/Chemo/Photothermal Therapy. ACS Nano 2018, 12, 5646–5656. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, S.; Yuan, J.; Xu, X.; Li, H.; Lv, Z.; Yu, W.; Duan, S.; Hu, Y. Reverse Multidrug Resistance in Human HepG2/ADR by Anti-miR-21 Combined with Hyperthermia Mediated by Functionalized Gold Nanocages. Mol. Pharm. 2018, 15, 3767–3776. [Google Scholar] [CrossRef]
- Lihuang, L.; Qiuyan, G.; Yanxiu, L.; Mindan, L.; Jun, Y.; Yunlong, G.; Qiang, Z.; Benqiang, S.; Xiumin, W.; Liang-cheng, L.; et al. Targeted combination therapy for glioblastoma by co-delivery of doxorubicin, YAP-siRNA and gold nanorods. J. Mater. Sci. Technol. 2021, 63, 81–90. [Google Scholar] [CrossRef]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon nanotubes: Smart drug/gene delivery carriers. Int. J. Nanomed. 2021, 16, 1681–1706. [Google Scholar] [CrossRef]
- Ahmed, M.; Jiang, X.; Deng, Z.; Narain, R. Cationic Glyco-Functionalized Single-Walled Carbon Nanotubes as Efficient Gene Delivery Vehicles. Bioconjugate Chem. 2009, 20, 2017–2022. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-Rius, A.; Pablo, A.D.; Ramos-Pérez, V.; Borrós, S. Tailoring Carbon Nanotubes Surface for Gene Delivery Applications. Plasma Process. Polym. 2014, 11, 704–713. [Google Scholar] [CrossRef]
- Nia, A.H.; Behnam, B.; Taghavi, S.; Oroojalian, F.; Eshghi, H.; Shier, W.T.; Abnous, K.; Ramezani, M. Evaluation of chemical modification effects on DNA plasmid transfection efficiency of single-walled carbon nanotube–succinate–polyethylenimine conjugates as non-viral gene carriers. MedChemComm 2017, 8, 364–375. [Google Scholar]
- Taghavi, S.; Nia, A.H.; Abnous, K.; Ramezani, M. Polyethylenimine-functionalized carbon nanotubes tagged with AS1411 aptamer for combination gene and drug delivery into human gastric cancer cells. Int. J. Pharm. 2017, 516, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Kam, N.W.S.; Liu, Z.; Dai, H. Functionalization of Carbon Nanotubes via Cleavable Disulfide Bonds for Efficient Intracellular Delivery of siRNA and Potent Gene Silencing. J. Am. Chem. Soc. 2005, 127, 12492–12493. [Google Scholar] [CrossRef]
- Podesta, J.E.; Al-Jamal, K.T.; Herrero, M.A.; Tian, B.; Ali-Boucetta, H.; Hegde, V.; Bianco, A.; Prato, M.; Kostarelos, K. Antitumor Activity and Prolonged Survival by Carbon-Nanotube-Mediated Therapeutic siRNA Silencing in a Human Lung Xenograft Model. Small 2009, 5, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hao, Y.; Wang, N.; Wang, L.; Jia, S.; Wang, Y.; Yang, L.; Zhang, Y.; Zhang, Z. DOTAP Functionalizing Single-Walled Carbon Nanotubes as Non-Viral Vectors for Efficient Intracellular siRNA Delivery. Drug Deliv. 2016, 23, 830–838. [Google Scholar] [CrossRef]
- Guo, C.; Al-Jamal, W.T.; Toma, F.M.; Bianco, A.; Prato, M.; Al-Jamal, K.T.; Kostarelos, K. Design of Cationic Multiwalled Carbon Nanotubes as Efficient siRNA Vectors for Lung Cancer Xenograft Eradication. Bioconjugate Chem. 2015, 26, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Huang, H.-Y.; Chen, L.-Q.; Du, H.-H.; Cui, J.-H.; Zhang, L.W.; Lee, B.-J.; Cao, Q.-R. Enhanced lysosomal escape of pH-responsive polyethylenimine–betaine functionalized carbon nanotube for the codelivery of survivin small interfering RNA and doxorubicin. ACS Appl. Mater. Interfaces 2019, 11, 9763–9776. [Google Scholar] [CrossRef]
- Sanz, V.; Coley, H.M.; Silva, S.R.P.; McFadden, J. Protamine and Chloroquine Enhance Gene Delivery and Expression Mediated by RNA-Wrapped Single Walled Carbon Nanotubes. J. Nanosci. Nanotechnol. 2012, 12, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Kaboudin, B.; Saghatchi, F.; Kazemi, F.; Akbari-Birgani, S. A novel magnetic carbon nanotubes functionalized with pyridine groups: Synthesis, characterization and their application as an efficient carrier for plasmid DNA and aptamer. ChemistrySelect 2018, 3, 6743–6749. [Google Scholar] [CrossRef]
- Ünal, Ö.; Akkoç, Y.; Koçak, M.; Nalbat, E.; Dogan-Ekici, A.I.; Acar, H.Y.; Gözüaçık, D. Treatment of Breast Cancer with Autophagy Inhibitory microRNAs Carried by AGO2-conjugated Nanoparticles. J. Nanobiotechnol. 2020, 18, 65. [Google Scholar] [CrossRef]
- Horvath, P.; Barrangou, R. CRISPR/Cas, the immune system of bacteria and archaea. Science 2010, 327, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Uz, M.; Altınkaya, S.A.; Mallapragada, S.K. Stimuli Responsive Polymer-Based Strategies for Polynucleotide Delivery. J. Mater. Res. 2017, 32, 2930–2953. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Mirgayazova, R.; Khadiullina, R.; Chasov, V.; Mingaleeva, R.; Miftakhova, R.; Rizvanov, A.A.; Bulatov, E. Therapeutic Editing of the TP53 Gene: Is CRISPR/Cas9 an Option? Genes 2020, 11, 704. [Google Scholar] [CrossRef]
- Rahimi, S.; Roushandeh, A.M.; Ebrahimi, A.; Samadani, A.A.; Kuwahara, Y.; Roudkenar, M.H. CRISPR/Cas9-mediated knockout of Lcn2 effectively enhanced CDDP-induced apoptosis and reduced cell migration capacity of PC3 cells. Life Sci. 2019, 231, 116586. [Google Scholar] [CrossRef]
- Lee, H.K.; Lim, H.M.; Park, S.H.; Nam, M.J. Knockout of Hepatocyte Growth Factor by CRISPR/Cas9 System Induces Apoptosis in Hepatocellular Carcinoma Cells. J. Pers. Med. 2021, 11, 983. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Qin, R.-Y. Mechanism and its regulation of tumor-induced angiogenesis. World J. Gastroenterol. WJG 2003, 9, 1144. [Google Scholar] [CrossRef] [PubMed]
- Yiu, G.; Tieu, E.; Nguyen, A.T.; Wong, B.; Smit-McBride, Z. Genomic disruption of VEGF-A expression in human retinal pigment epithelial cells using CRISPR-Cas9 endonuclease. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5490–5497. [Google Scholar] [CrossRef] [PubMed]
- Ameri, H.; Murat, C.; Arbabi, A.; Jiang, W.; Janga, S.R.; Qin, P.Z. Reduced Expression of VEGF-A in Human Retinal Pigment Epithelial Cells and Human Muller Cells Following CRISPR-Cas9 Ribonucleoprotein-Mediated Gene Disruption. Transl. Vis. Sci. Technol. 2020, 9, 23. [Google Scholar] [CrossRef]
- Liu, Q.; Fan, D.; Adah, D.; Wu, Z.; Liu, R.; Yan, Q.T.; Zhang, Y.; Du, Z.Y.; Wang, D.; Li, Y. CRISPR/Cas9-mediated hypoxia inducible factor-1α knockout enhances the antitumor effect of transarterial embolization in hepatocellular carcinoma. Oncol. Rep. 2018, 40, 2547–2557. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xie, H.; Liu, Y.; Xia, C.; Cun, X.; Long, Y.; Chen, X.; Deng, M.; Guo, R.; Zhang, Z.; et al. Knockdown of hypoxia-inducible factor-1 alpha by tumor targeted delivery of CRISPR/Cas9 system suppressed the metastasis of pancreatic cancer. J. Control. Release 2019, 304, 204–215. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Talwar, C.S.; Park, K.-H.; Ahn, W.-C.; Lee, S.-J.; Go, S.-R.; Cho, J.H.; Kim, D.Y.; Kim, Y.-S.; Cho, S. Design of hypoxia responsive CRISPR-Cas9 for target gene regulation. Sci. Rep. 2023, 13, 16763. [Google Scholar] [CrossRef]
- Nüssing, S.; House, I.G.; Kearney, C.J.; Chen, A.X.Y.; Vervoort, S.J.; Beavis, P.A.; Oliaro, J.; Johnstone, R.W.; Trapani, J.A.; Parish, I.A. Efficient CRISPR/Cas9 Gene Editing in Uncultured Naive Mouse T Cells for in Vivo Studies. J. Immunol. 2020, 204, 2308–2315. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kozhaya, L.; Taştan, C.; Placek, L.; Dogan, M.; Horne, M.; Abblett, R.L.; Karhan, E.; Vaeth, M.; Feske, S.; et al. Functional Interrogation of Primary Human T Cells via CRISPR Genetic Editing. J. Immunol. 2018, 201, 1586–1598. [Google Scholar] [CrossRef]
- Choi, B.D.; Yu, X.; Castaño, A.P.; Darr, H.; Henderson, D.; Bouffard, A.A.; Larson, R.C.; Scarfò, I.; Bailey, S.R.; Gerhard, G.M.; et al. CRISPR-Cas9 Disruption of PD-1 Enhances Activity of Universal EGFRvIII CAR T Cells in a Preclinical Model of Human Glioblastoma. J. Immunother. Cancer 2019, 7, 304. [Google Scholar] [CrossRef]
- Wang, Z.; Li, N.; Feng, K.; Chen, M.; Zhang, Y.; Liu, Y.; Yang, Q.; Nie, J.; Tang, N.; Zhang, X.; et al. Phase I Study of CAR-T Cells with PD-1 and TCR Disruption in Mesothelin-Positive Solid Tumors. Cell. Mol. Immunol. 2021, 18, 2188–2198. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, Y.; Zhang, M.; Ge, W.; Li, Y.; Yang, L.; Wei, G.; Han, L.; Wang, H.; Yu, S. CRISPR/Cas9-engineered universal CD19/CD22 dual-targeted CAR-T cell therapy for relapsed/refractory B-cell acute lymphoblastic leukemia. Clin. Cancer Res. 2021, 27, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Song, J.; Zhang, J.; Zhang, H.; Sun, B.; Sun, J. Nano-Immunotherapy for Each Stage of Cancer Cellular Immunity: Which, Why, and What? Theranostics 2021, 11, 7471–7487. [Google Scholar] [CrossRef]
- Lu, Y.; Zeng, T.; Zhang, H.; Yang, L.; Zhu, X.; Liu, H.; Sun, B.; Ji, C.; Li, T.; Huang, L.; et al. Nano-Immunotherapy for Lung Cancer. Nano Transmed. 2023, 2, e9130018. [Google Scholar] [CrossRef]
- Li, X. Emerging Role of Nanotechnology in Cancer Immunotherapy. Highlights Sci. Eng. Technol. 2023, 36, 1347–1355. [Google Scholar] [CrossRef]
- Xu, T.; Liu, Z.; Huang, L.; Jing, J.; Liu, X. Modulating the Tumor Immune Microenvironment with Nanoparticles: A Sword for Improving the Efficiency of Ovarian Cancer Immunotherapy. Front. Immunol. 2022, 13, 1057850. [Google Scholar] [CrossRef]
- Musetti, S.; Huang, L. Nanoparticle-Mediated Remodeling of the Tumor Microenvironment to Enhance Immunotherapy. Acs Nano 2018, 12, 11740–11755. [Google Scholar] [CrossRef] [PubMed]
- Raza, F.; Zafar, H.; Zhang, S.; Kamal, Z.; Su, J.; Yuan, W.; Qiu, M. Recent Advances in Cell Membrane-Derived Biomimetic Nanotechnology for Cancer Immunotherapy. Adv. Healthc. Mater. 2021, 10, 2002081. [Google Scholar] [CrossRef]
- Jin, C.; Wang, K.; Oppong-Gyebi, A.; Hu, J. Application of Nanotechnology in Cancer Diagnosis and Therapy—A Mini-Review. Int. J. Med. Sci. 2020, 17, 2964–2973. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Mongia, P.; Sahu, S.K.; Ram, A. Nanocarriers Based Anticancer Drugs: Current Scenario and Future Perceptions. Curr. Drug Targets 2016, 17, 206–228. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Deng, P.; Wang, X.; Peng, C.; Peng, L. Structural characteristics and immunomodulatory effects of polysaccharides extracted from plant seeds: A review. Trends Food Sci. Technol. 2024, 153, 104747. [Google Scholar] [CrossRef]
- Ni, K.; Luo, T.; Nash, G.T.; Lin, W. Nanoscale Metal–Organic Frameworks for Cancer Immunotherapy. Acc. Chem. Res. 2020, 53, 1739–1748. [Google Scholar] [CrossRef]
- Lei, L.; Dong, Z.; Yang, F.; Zhang, X. Metal–Organic Nanomaterials for Tumor Metabolic Blockade and Image to Increase Tumor Therapy. ACS Appl. Mater. Interfaces 2024, 16, 57995–58005. [Google Scholar] [CrossRef]
- Rossi, G.R.; Sun, J.; Lin, C.Y.; Wong, J.K.M.; Alim, L.; Lam, P.Y.; Khosrotehrani, K.; Wolvetang, E.; Cheetham, S.W.; Derrick, E.B.; et al. A Scalable, Spin-free Approach to Generate Enhanced Induced Pluripotent Stem Cell–derived natural Killer Cells For cancer Immunotherapy. Immunol. Cell Biol. 2024, 102, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, M. Cancer Immunotherapy in Routine Cost-effective Cancer Care? Embo Mol. Med. 2018, 10, e9660. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, M.M.; Stamatis, C.; Lakelin, M.; Farid, S.S.; Titchener-Hooker, N.J.; Shah, N. Autologous CAR T-Cell Therapies Supply Chain: Challenges and Opportunities? Cancer Gene Ther. 2020, 27, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Kondo, J.; Ekawa, T.; Endo, H.; Yamazaki, K.; Tanaka, N.; Kukita, Y.; Okuyama, H.; Okami, J.; Imamura, F.; Ohue, M.; et al. High-throughput Screening in Colorectal Cancer Tissue-originated Spheroids. Cancer Sci. 2018, 110, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H. mRNA Vaccines in Cancer Treatment; Academic Press: Cambridge, MA, USA, 2024; Volume 1. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.; Weissman, D. mRNA Vaccines—A New Era in Vaccinology. Nat. Rev. Cancer 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Kennedy, L.B.; Salama, A.K.S. A Review of Cancer Immunotherapy Toxicity. Ca A Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O.; Brogdon, C.F.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing Toxicities Associated with Immune Checkpoint Inhibitors: Consensus Recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kong, D.; Wang, C.; Chen, J.; Li, J.; Liu, Z.; Li, X.; Wang, Z.; Ge, Y.; Wang, X. A Systematic Review and Meta-Analysis of Immune-Related Adverse Events of Anti-Pd-1 Drugs in Randomized Controlled Trials. Technol. Cancer Res. Treat. 2020, 19, 1533033820967454. [Google Scholar] [CrossRef]
- Choi, J.; Lee, S.Y. Clinical Characteristics and Treatment of Immune-Related Adverse Events of Immune Checkpoint Inhibitors. Immune Netw. 2020, 20, e9. [Google Scholar] [CrossRef] [PubMed]
- Gümüşay, Ö.; Callan, J.F.; Rugo, H.S. Immunotherapy Toxicity: Identification and Management. Breast Cancer Res. Treat. 2022, 192, 1–17. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, Y.; Wang, D.; Zhang, Z. Treatment-Related Adverse Events as Surrogate to Response Rate to Immune Checkpoint Blockade. Medicine 2020, 99, e22153. [Google Scholar] [CrossRef]

| Monoclonal Antibody (mAb) | mAb Type | Target | Approval Year | Approved Cancers |
|---|---|---|---|---|
| Rituximab (Rituxan/MabThera) | Chimeric mAb | CD20 | 1997 | B non-Hodgkin’s lymphoma (NHL), Chronic lymphocytic leukaemia (CLL) |
| Trastuzumab (Herceptin) | Humanised mAb | HER2 | 1998 | Breast cancer, Gastric cancer |
| Bevacizumab (Avastin) | Humanised mAb | VEGF | 2004 | Metastatic colorectal cancer, brain tumours, non-small-cell lung cancer (NSCLC), renal cell carcinoma |
| Cetuximab (Erbitux) | Chimeric mAb | EGFR | 2004 | Non-small-cell lung cancer (NSCLC), advanced squamous cell carcinoma of the head and neck |
| Panitumumab (Vectibix) | Fully human mAb | EGFR | 2006 | Metastatic colorectal cancer |
| Pertuzumab (Perjeta) | Humanised mAb | HER2 | 2012 | Breast cancer |
| Ramucirumab (Cyramza) | Fully human mAb | VEGFR-2 | 2014 | Advanced or metastatic gastric cancer or gastro-oesophageal junction adenocarcinoma |
| Daratumumab (Darzalex) | Humanised mAb | CD38 | 2015 | Multiple myeloma |
| Checkpoint Target | Checkpoint Inhibitor | Approval Year | Approved Cancers |
|---|---|---|---|
| PD-1 | Pembrolizumab (Keytruda) | 2014 | Melanoma, non-small-cell lung cancer (NSCLC), head and neck squamous cell carcinoma (HNSCC), urothelial carcinoma Classical Hodgkin lymphoma, microsatellite instability-high (MSI-H) or mismatch repair-deficient solid tumours |
| Nivolumab (Opdivo) | 2014 | Melanoma, non-small cell lung cancer (NSCLC), melanoma renal cell carcinoma, classical Hodgkin lymphoma, urothelial carcinoma, head, and neck squamous cell carcinoma | |
| Cemiplimab (Libtayo) | 2018 | Metastatic cutaneous squamous cell carcinoma (SCC) Advanced cutaneous squamous cell carcinoma (cSCC) | |
| PD-L1 | Atezolizumab (Tecentriq) | 2016 | Urothelial carcinoma, non-small-cell lung cancer (NSCLC), bladder cancer, hepatocellular carcinoma |
| Avelumab (Bavencio) | 2017 | <etastatic Merkel cell carcinoma (mMCC) Urothelial carcinoma | |
| Durvalumab (Imfinzi) | 2017 | Non-small-cell lung cancer (NSCLC), urothelial carcinoma | |
| CTLA-4 | Ipilimumab (Yervoy) | 2011 | Metastatic melanoma |
| Tremelimumab (Imjudo) | 2022 | Unresectable hepatocellular carcinoma Metastatic non-small cell lung cancer |
| Vaccine | Target Antigen | Use | Approval Year | Cancer Type |
|---|---|---|---|---|
| Hepatitis B | Hepatitis B virus (HBV) surface antigen (HBsAg) | Preventative | 1981 | Hepatocellular carcinoma caused by chronic HBV infection |
| Bacillus Calmette-Guerin (BCG) | Non-pathogenic Mycobaterium bovis | Therapeutic | 1990 | High-risk non-muscle-invasive bladder cancer (NMIBC) |
| Cervarix (discontinued) | L1 protein of Human papilloma virus (HPV) types 16 and 18 | Preventative | 2009 | HPV-associated cervical, oropharyngeal, anal, penile, and vulvovaginal cancers |
| Sipuleucel-T (Provenge) | Prostate acid phosphatase (PAP) protein | Therapeutic | 2010 | Castration-resistant prostatic cancer |
| Gardasil-9 | L1 protein of HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58 | Preventative | 2014 | HPV-associated cervical, oropharyngeal, anal, penile, and vulvovaginal cancers |
| NCT Number | Immunotherapy | Oncological Indication(s) | Phase of Trial | Outcome |
|---|---|---|---|---|
| NCT04191135 | KEYLYNK-009 | Triple-Negative Breast Neoplasms | Phase II | Maintenance therapy with pembrolizumab plus Olaparib showed comparable outcomes to pembrolizumab plus chemotherapy in patients with locally recurrent inoperable or metastatic TNBC. |
| NCT03815942 | ChAdOx1-MVA 5T4 vaccine | Advanced Prostate Cancer/Advanced Metastatic Prostate Cancer | Phase I/II | An excellent safety profile elicited robust T-cell responses both in circulation and within the prostate gland, supporting its evaluation in efficacy trials. |
| NCT02229084 | P10s-PADRE | HR+/HER2 Early Stage Breast Cancer | Phase I/II | Found to be safe and immunogenic when administered alongside standard chemotherapy. |
| NCT03662815 | iNeo-Vac-P01 | Advanced Pancreatic Cancer Patient | Phase I | Feasible and safe, showing promising antitumor efficacy in patients with advanced solid tumours. |
| NCT03697707 | DCP-001 | Acute Myeloid Leukaemia in Remission | Phase II | Demonstrated potential in treating AML, warranting further investigation. |
| NCT02410733 | FixVac (BNT111) | Melanoma | Phase I | Suggest the general utility of non-mutant shared tumour antigens as targets for cancer vaccination. |
| NCT01631357 | Autologous cytokine-induced killer (CIK) cell immunotherapy combined with chemotherapy | Non-Small-Cell Lung Cancer/Squamous Cell Carcinoma | Phase II/III | The combination therapy improved chemotherapy efficacy and demonstrated a favourable safety profile. |
| NCT02272855 | HF10—a HSV-1 oncolytic immunotherapy | Unresectable or Metastatic melanoma | Phase II | Demonstrated a favorable benefit/risk profile and induced immune-cell infiltration, showing promising antitumor activity. |
| NCT03252808 | HF10 in combination with chemotherapy | Unresectable Pancreatic Cancer | Phase I | The safety and optimal dosing regimen of HF10 alongside chemotherapy |
| NCT06136910 | Oncorine (H101) combined with Tislelizumab and chemotherapy | Advanced Non-Small-Cell Lung Cancer/Advanced Non-Small Cell Lung Cancer (NSCLC) | Phase I/II | Evaluating the efficacy and safety of the combination therapy. |
| NCT01598129 | ONCOS-102 with low-dose cyclophosphamide | Refractory Solid Tumors | Phase I | Aimed to determine the optimal dose, safety, and preliminary efficacy of the combination therapy |
| NCT03206073 | Pexa-Vec (JX-594) in combination with tremelimumab and durvalumab | Colorectal Cancer/Metastatic Colorectal cancer | Phase I/II | Investigating the safety and efficacy of the combination therapy in patients refractory to standard therapies. |
| NCT01455389 | DOTAP: Chol-TUSC2 (FUS1) Gene Therapy in Combination with Erlotinib | Non-Small-Cell Lung Cancer | Phase I/II | Demonstrated safety and feasibility of systemic gene therapy using LNPs for delivering the TUSC2 tumor suppressor gene, with evidence of gene expression in tumor biopsies |
| NCT03739931 | mRNA-2752—a Lipid Nanoparticle Encapsulating mRNAs Encoding Human OX40L, IL-23, and IL-36γ | Triple Negative Breast Cancer, HNSCC, Non-Hodgkins, Urothelial Cancer, Immune Checkpoint Refractory Melanoma, and NSCLC Lymphoma | Phase I | mRNA-2752 was well-tolerated and showed preliminary evidence of antitumor activity, both as monotherapy and in combination with durvalumab |
| NCT01591356 | EphA2 siRNA | Solid Tumors | Phase I | The study concluded that the liposomal siRNA formulation was well-tolerated, with potential therapeutic effects. |
| NCT00938574 | Atu027—a liposomal siRNA formulation targeting protein kinase N3 (PKN3) | Advanced Solid Tumors | Phase I | The study demonstrated that Atu027 was safe and showed preliminary evidence of anti-tumor activity. |
| NCT03289455 | AUTO3-PA1—a CAR T cell treatment targeting CD19 and CD22 | B Acute Lymphoblastic Leukaemia/Recurrent Childhood Acute Lymphoblastic Leukaemia/Refractory Childhood Acute Lymphoblastic Leukaemia/B-cell Acute Lymphoblastic Leukaemia | Phase I/II | Preliminary results indicate promising safety and efficacy profiles. |
| NCT01747486 | Autologous CART-19 T cells engineered to express anti-CD19 chimeric antigen receptors | Relapsed or Refractory CLL (3rd Line) or SLL | Phase II | Demonstrated safety and potential efficacy in targeting CD19-positive cancers. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandara, S.; Raveendran, S. Current Landscape and Future Directions in Cancer Immunotherapy: Therapies, Trials, and Challenges. Cancers 2025, 17, 821. https://doi.org/10.3390/cancers17050821
Bandara S, Raveendran S. Current Landscape and Future Directions in Cancer Immunotherapy: Therapies, Trials, and Challenges. Cancers. 2025; 17(5):821. https://doi.org/10.3390/cancers17050821
Chicago/Turabian StyleBandara, Shehani, and Sreejith Raveendran. 2025. "Current Landscape and Future Directions in Cancer Immunotherapy: Therapies, Trials, and Challenges" Cancers 17, no. 5: 821. https://doi.org/10.3390/cancers17050821
APA StyleBandara, S., & Raveendran, S. (2025). Current Landscape and Future Directions in Cancer Immunotherapy: Therapies, Trials, and Challenges. Cancers, 17(5), 821. https://doi.org/10.3390/cancers17050821






