Spectroscopic Nuclear Magnetic Resonance and Fourier Transform–Infrared Approach Used for the Evaluation of Healing After Surgical Interventions for Patients with Colorectal Cancer: A Pilot Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Volunteers and Patients
2.2. Clinical Care After Surgery
2.3. Blood Plasma
2.4. 1H NMR Relaxometry
2.5. FT-IR Spectroscopy
3. Results
3.1. 1H NMR T2 Distributions
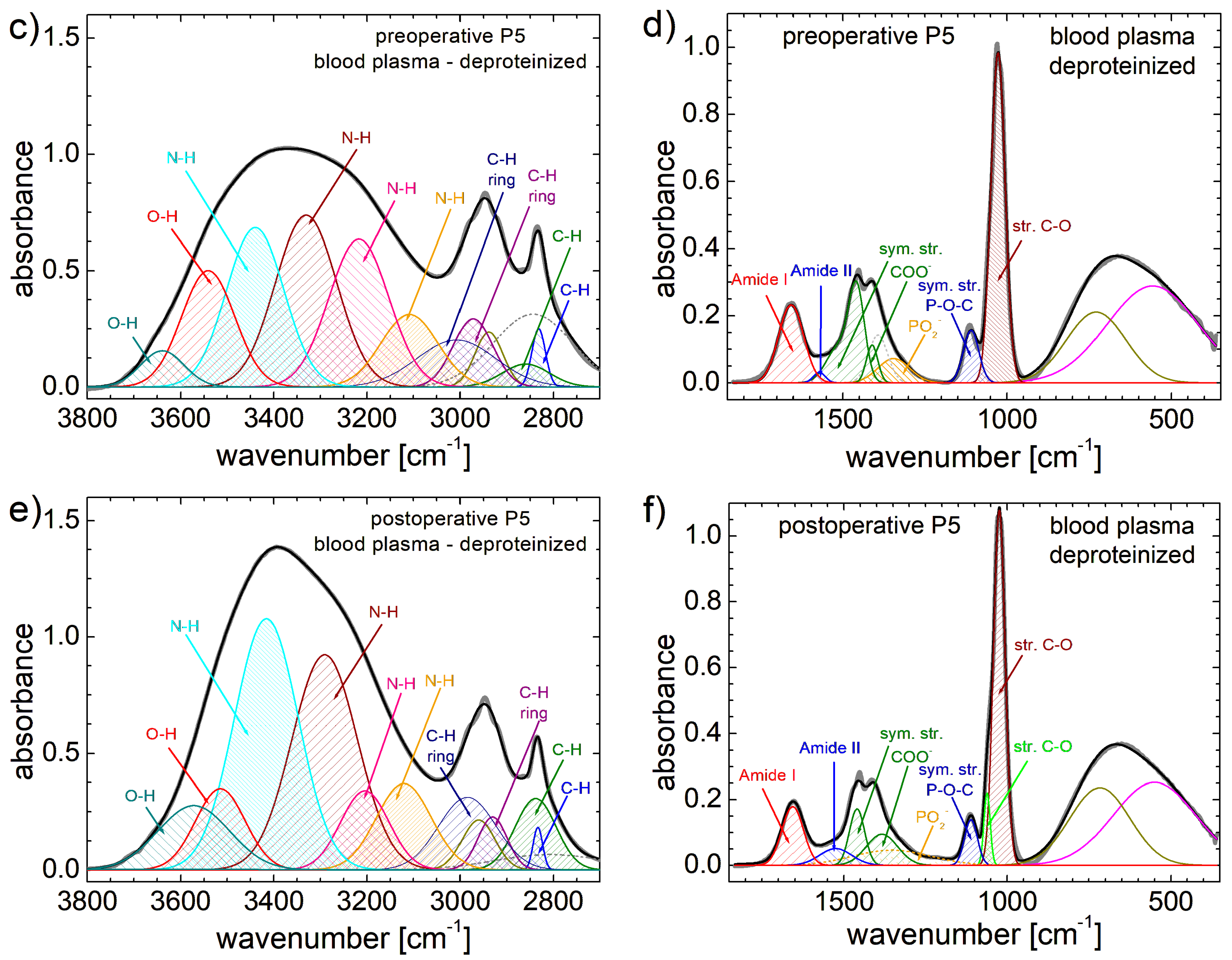
3.2. FT-IR Spectra
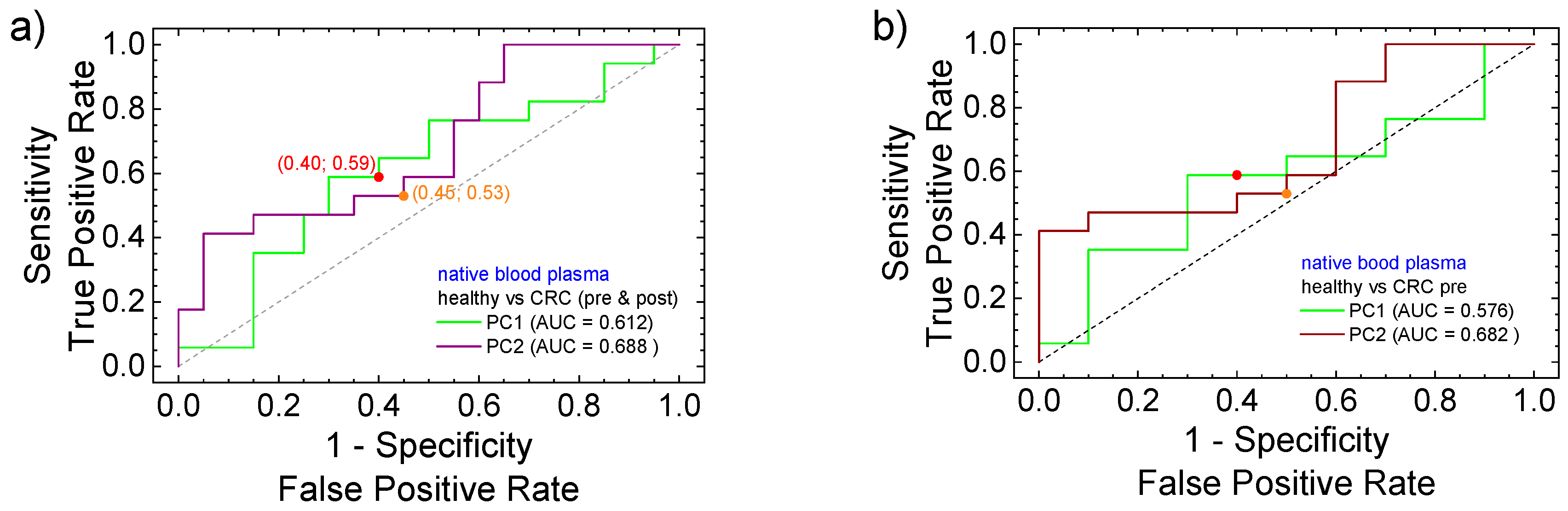
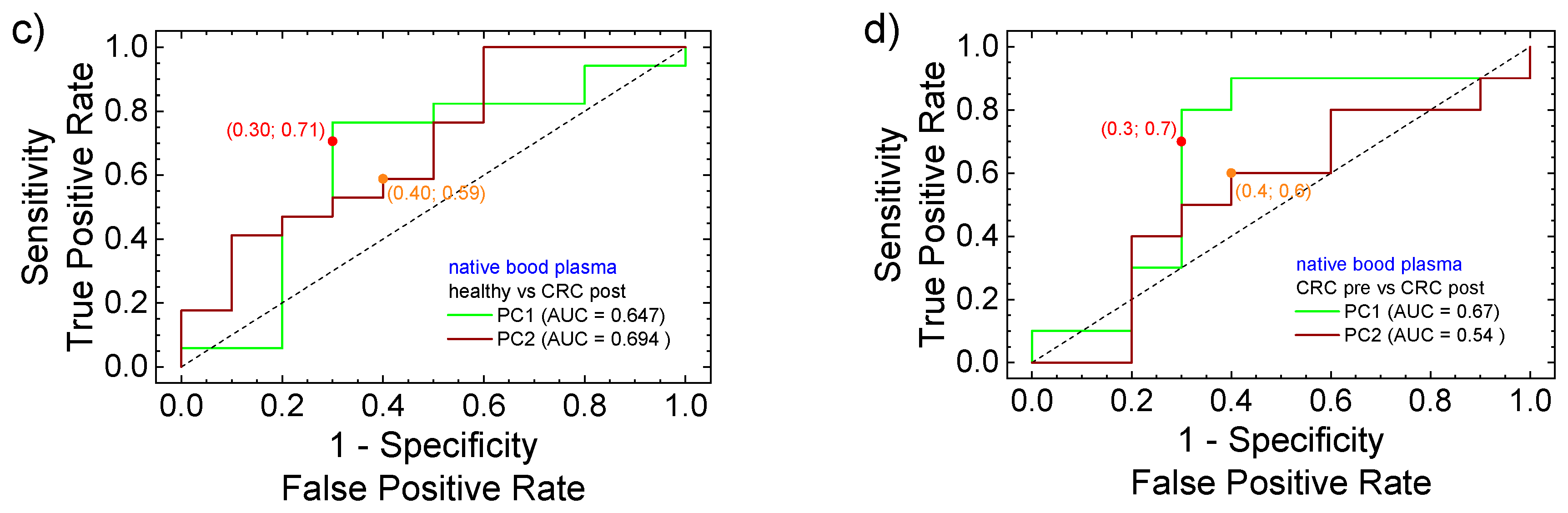
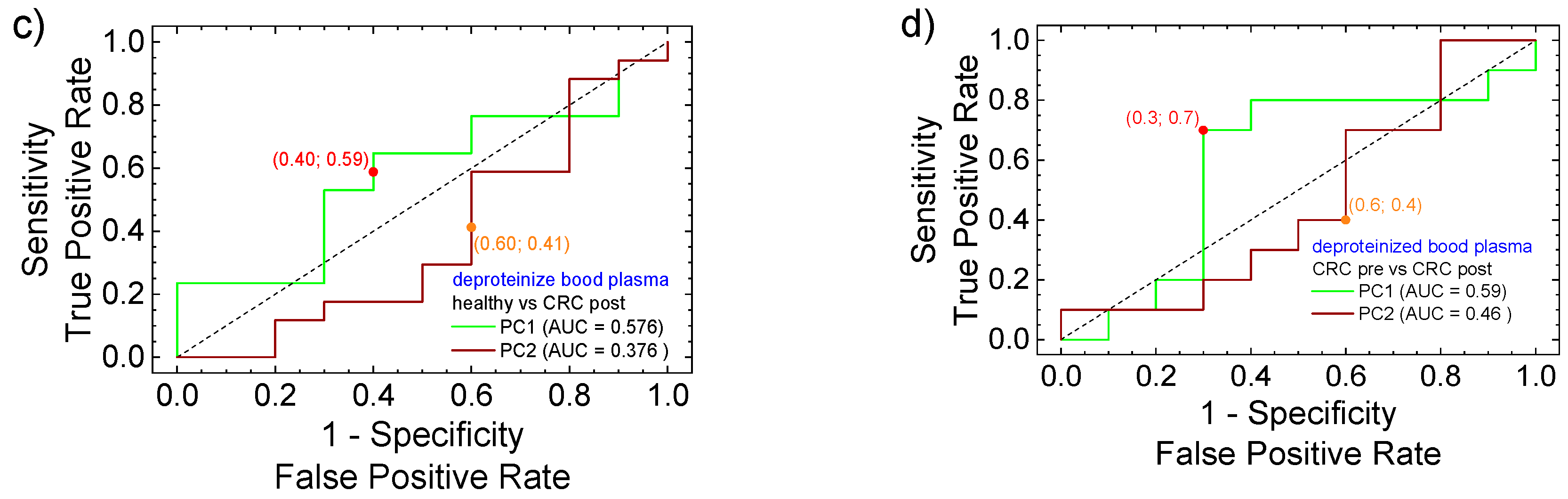
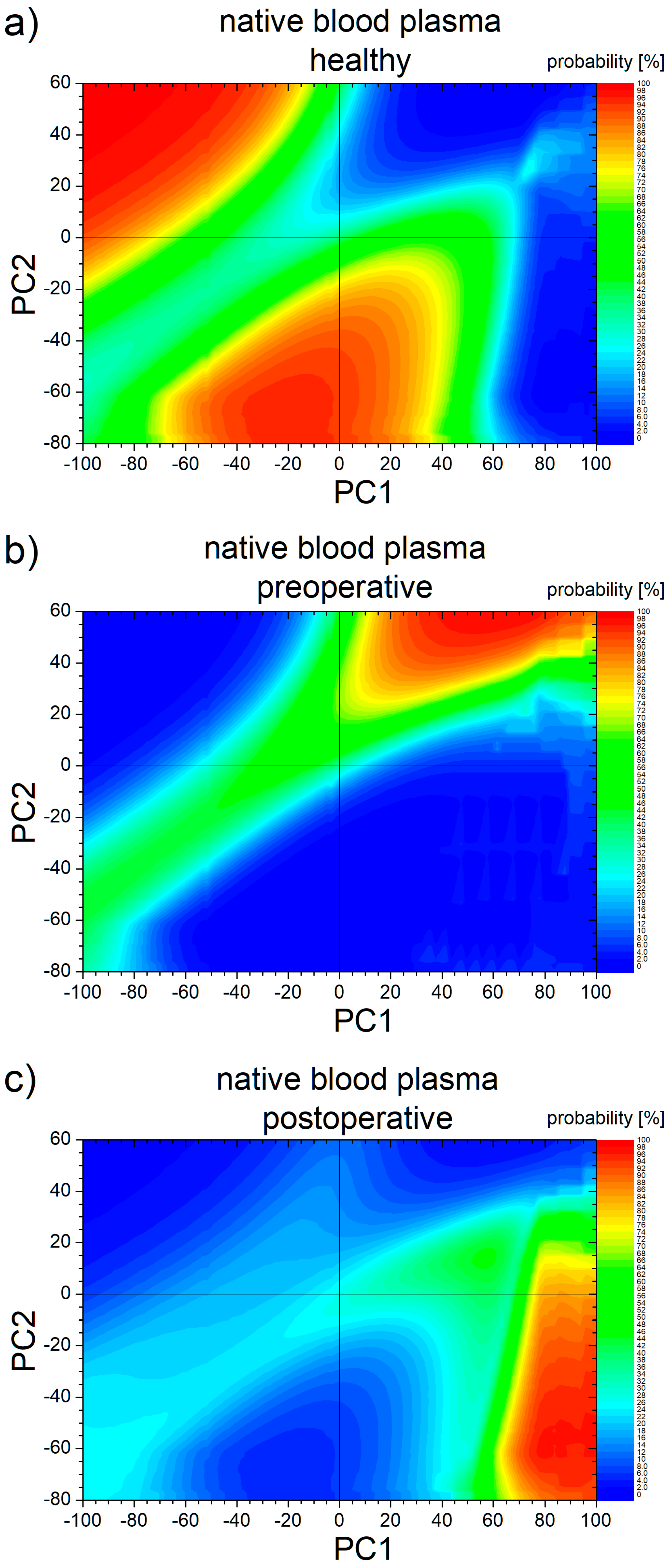
3.3. PCA Statistical Analysis and ROC Curves
3.4. Machine Learning Prediction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Bretthauer, M.; Løberg, M.; Wieszczy, P.; Kalager, M.; Emilsson, L.; Garborg, K.; Rupinski, M.; Dekker, E.; Spaander, M.; Bugajski, M.; et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N. Engl. J. Med. 2022, 387, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Lee, M.Y.; Park, J.H.; Park, D.I.; Sohn, C.I.; Choi, K.; Jung, Y.S. Serum CEA and CA 19-9 levels are associated with the presence and severity of colorectal neoplasia. Yonsei Med. J. 2017, 58, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Levin, T.R. Current and future colorectal cancer screening strategies. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 521–531. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 132. [Google Scholar]
- Ji, H.; Hu, C.; Yang, X.; Liu, Y.; Ji, G.; Ge, S.; Wang, X.; Wang, M. Lymph node metastasis in cancer progression: Molecular mechanisms, clinical significance and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 367. [Google Scholar] [CrossRef]
- Chini, A.; Manigrasso, M.; Cantore, G.; Maione, R.; Milone, M.; Maione, F.; De Palma, G.D. Can computed tomography colonography replace optical colonoscopy in detecting colorectal lesions?: State of the art. Clin. Endosc. 2022, 55, 183–190. [Google Scholar] [CrossRef]
- Kranenburg, O.; Speeten, K.V.D.; Hingh, I.D. Peritoneal metastases from colorectal cancer: Defining and addressing the challenges. Front. Oncol. 2021, 11, 650098. [Google Scholar] [CrossRef]
- Yin, B.; Mi, J.Y.; Zhai, H.L.; Zhao, B.Q.; Bi, K.X. An effective approach to the early diagnosis of colorectal cancer based on three-dimensional fluorescence spectra of human blood plasma. J. Pharm. Biomed. Anal. 2021, 193, 113757. [Google Scholar] [CrossRef]
- Hughes, C.; Brown, M.; Clemens, G.; Henderson, A.; Monjardez, G.; Clarke, N.W.; Gardner, P. Assessing the challenges of Fourier transform infrared spectroscopic analysis of blood serum. J. Biophotonics 2014, 7, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Barlev, E.; Zelig, U.; Bar, O.; Segev, C.; Mordechai, S.; Kapelushnik, J.; Nathan, I.; Flomen, F.; Kashtan, H.; Dickman, R.; et al. A novel method for screening colorectal cancer by infrared spectroscopy of peripheral blood mononuclear cells and plasma. J. Gastroenterol. 2016, 51, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Tugrul, F.; Geyik, G.A.; Kaya, B.Y.; Cengiz, B.P.; Elmas, S.N.K.; Yilmaz, I.; Arslan, F.N. A biospectroscopic approach toward colorectal cancer diagnosis from bodily fluid samples via ATR–MIR spectroscopy combined with multivariate data analysis. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2024, 304, 123342. [Google Scholar] [CrossRef]
- Drăgan, L.R.; Andras, D.; Fechete, R. Fourier transform infrared (FT-IR) spectroscopy and proton nuclear magnetic resonance (1H NMR) relaxometry and diffusometry for the identification of colorectal cancer in blood plasma. Anal. Lett. 2023, 56, 286–302. [Google Scholar] [CrossRef]
- Li, Q.Q.; Hao, C.; Kang, X.; Zhang, J.; Sun, X.; Wang, W.; Zeng, H. Colorectal cancer and colitis diagnosis using Fourier transform infrared spectroscopy and an improved K-nearest-neighbour classifier. Sensors 2017, 17, 2739. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Shi, X.; Zhang, Y. The use of FTIR-ATR spectrometry for evaluation of surgical resection margin in colorectal cancer: A pilot study of 56 samples. J. Spectrosc. 2014, 2014, 213890. [Google Scholar] [CrossRef]
- Salmana, A.; Shufan, E.; Sahu, R.K.; Mordechai, S.; Sebbag, G. Insights on colorectal cancer relapse by infrared microscopy from anastomosis tissues: Further analysis. Vib. Spectrosc. 2016, 83, 17–25. [Google Scholar] [CrossRef]
- Depciuch, J.; Kaznowska, E.; Koziorowska, A.; Cebulski, J. Verification of the effectiveness of the Fourier transform infrared spectroscopy computational model for colorectal cancer. J. Pharm. Biomed. Anal. 2017, 145, 611–615. [Google Scholar] [CrossRef]
- Nallala, J.; Jeynes, C.; Saunders, S.; Smart, N.; Lloyd, G.; Riley, L.; Salmon, D.; Stone, N. Characterization of colorectal mucus using infrared spectroscopy: A potential target for bowel cancer screening and diagnosis. Lab. Investig. 2020, 100, 1102–1110. [Google Scholar] [CrossRef]
- Kondepati, V.R.; Heise, H.M.; Oszinda, T.; Mueller, R.; Keese, M.; Backhaus, J. Detection of structural disorders in colorectal cancer DNA with Fourier-transform infrared spectroscopy. Vib. Spectrosc. 2008, 46, 150–157. [Google Scholar] [CrossRef]
- Piva, J.A.A.C.; Silva, J.L.R.; Raniero, L.; Martin, A.A.; Bohr, H.G.; Jalkanen, K.J. Overview of the use of theory to understand infrared and Raman spectra and images of biomolecules: Colorectal cancer as an example. Theor. Chem. Acc. 2011, 130, 1261–1273. [Google Scholar] [CrossRef]
- Moisoiu, V.; Iancu, S.D.; Stefancu, A.; Moisoiu, T.; Pardini, B.; Dragomir, M.P.; Crisan, N.; Avram, L.; Crisan, D.; Andras, I.; et al. SERS liquid biopsy: An emerging tool for medical diagnosis. Colloids Surf. B Biointerfaces 2021, 208, 112064. [Google Scholar] [CrossRef] [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Software; Surveillance Research Program, National Cancer Institute: Bethesda, MD, USA, 2019. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: National Program of Cancer Registries (NPCR) and SEER Incidence US Cancer Statistics (USCS) File for Delay Adjustment-1999-2016-jbk 072919; Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute: Bethesda, MD, USA, 2019. [Google Scholar]
- Clegg, L.X.; Feuer, E.J.; Midthune, D.N.; Fay, M.P.; Hankey, B.F. Impact of reporting delay and reporting error on cancer incidence rates and trends. J. Natl. Cancer Inst. 2002, 94, 1537–1545. [Google Scholar] [CrossRef]
- Miller, B.A.; Scoppa, S.M.; Feuer, E.J. Racial/ethnic patterns in lifetime and age-conditional risk estimates for selected cancers. Cancer 2006, 106, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Statistical Research and Applications Branch, National Cancer Institute. DevCan—Probability of Developing or Dying of Cancer Software; Version 6.7.7; Surveillance Research Program, Statistical Methodology and Applications, National Cancer Institute: Bethesda, MD, USA, 2019. [Google Scholar]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.D.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef] [PubMed]
- Salmerón, A.M.; Tristán, A.I.; Abreu, A.C.; Fernández, I. Serum colorectal cancer biomarkers unraveled by NMR metabolomics: Past, present, and future. Anal. Chem. 2022, 94, 417–430. [Google Scholar] [CrossRef]
- Saorin, A.; Di Gregorio, E.; Buonadonna, A.; Miolo, G.; Corona, G. The riddle of cetuximab-related skin toxicity: 1H-NMR sebum analysis revealed dynamic lipid alterations associated with skin toxicity development in metastatic colorectal cancer patients. Cancers 2022, 14, 5308. [Google Scholar] [CrossRef]
- Gu, J.; Xiao, Y.; Shu, D.; Liang, X.; Hu, X.; Xie, Y.; Lin, D.; Li, H. Metabolomics analysis in serum from patients with colorectal polyp and colorectal cancer by 1H-NMR spectrometry. Dis. Markers 2019, 2019, 3491852. [Google Scholar] [CrossRef]
- Pacholczyk-Sienicka, B.; Fabiańska, A.; Pasz-Walczak, G.; Kordek, R.; Jankowski, S. Prediction of survival for patients with advanced colorectal cancer using 1H high-resolution magic angle spinning nuclear MR spectroscopy. J. Magn. Reson. Imaging 2015, 41, 1669–1674. [Google Scholar] [CrossRef]
- Kim, E.R.; Kwon, H.N.; Nam, H.; Kim, J.J.; Park, S.; Kim, Y.H. Urine-NMR metabolomics for screening of advanced colorectal adenoma and early stage colorectal cancer. Sci. Rep. 2019, 9, 4786. [Google Scholar] [CrossRef]
- Dávid, E.R.; Fechete, R.; Sfrângeu, S.; Moldovan, D.; Chelcea, R.I.; Morar, I.A.; Stamatian, F.; Kovács, T.; Popoi, P. In vivo 1H nuclear magnetic resonance spectroscopy and relaxometry maps of the human female pelvis. Anal. Lett. 2018, 52, 54–77. [Google Scholar] [CrossRef]
- Dávid, E.R.; Nagy, M.V.; Stamatian, F.V.; Kovács, T.; Bura, V.; Morar, I.A.; Pintican, R.M.; Fechete, R. In vivo 1H magnetic resonance spectroscopy of woman’s normal and cancerous uterus. Obstet. Şi Ginecol. 2018, 66, 105–112. [Google Scholar]
- Pintican, R.; Fechete, R.; Boca, B.; Cambrea, M.; Leonte, T.; Camuescu, O.; Gherman, D.; Bene, I.; Ciule, L.D.; Ciortea, C.A.; et al. Predicting the early response to neoadjuvant therapy with breast MR morphological, functional and relaxometry features—A pilot study. Cancers 2022, 14, 5866. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-P.; Ruan, C.-P.; Wang, H.; Hu, Z.-Q.; Fang, M.; Gu, X.; Ji, J.; Zhao, J.-Y.; Gao, C.-F. Identification and assessment of new biomarkers for colorectal cancer with serum N-glycan profiling. Cancer 2012, 118, 639–650. [Google Scholar] [CrossRef]
- Tham, C.; Chew, M.; Soong, R.; Lim, J.; Ang, M.; Tang, C.; Zhao, Y.; Ong, S.Y.K.; Liu, Y. Postoperative serum methylation levels of TAC1 and SEPT9 are independent predictors of recurrence and survival of patients with colorectal cancer. Cancer 2014, 120, 3131–3141. [Google Scholar] [CrossRef]
- Loughrey, M.B.; Webster, F.; Arends, M.J.; Brown, I.; Burgart, L.J.; Cunningham, C.; Flejou, J.F.; Kakar, S.; Kirsch, R.; Kojima, M.; et al. Dataset for Pathology Reporting of Colorectal Cancer: Recommendations From the International Collaboration on Cancer Reporting (ICCR). Ann. Surg. 2022, 275, e549–e561. [Google Scholar] [CrossRef]
- Turaga, A.H. Enhanced Recovery After Surgery (ERAS) Protocols for Improving Outcomes for Patients Undergoing Major Colorectal Surgery. Cureus 2023, 15, e41755. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, E.; Villalobos-Hernandez, E.C.; Fiorda-Diaz, J.; Harzman, A.; Christofi, F.L. Postoperative Ileus and Postoperative Gastrointestinal Tract Dysfunction: Pathogenic Mechanisms and Novel Treatment Strategies Beyond Colorectal Enhanced Recovery After Surgery Protocols. Front. Pharmacol. 2020, 11, 583422. [Google Scholar] [CrossRef]
- Deshpande, S.; Robertson, B. Perioperative Pain Management in Colorectal Surgery. Surgery 2020, 38, 303–309. [Google Scholar] [CrossRef]
- Fechete, R.; Demco, D.E.; Zhu, X.; Tillmann, W.; Vinokur, R.; Möller, M. Water States and Dynamics in Perfluorinated Ionomer Proton Exchange Membranes by 1H One- and Two-Dimensional NMR Spectroscopy, Relaxometry, and Diffusometry. Chem. Phys. Lett. 2014, 597, 6–15. [Google Scholar] [CrossRef]
- Sipos, R.S.; Fechete, R.; Moldovan, D.; Sus, I.; Pávai, Z.; Demco, D.E. Ovariectomy-Induced Osteoporosis Evaluated by 1H One- and Two-Dimensional NMR Transverse Relaxometry. Appl. Magn. Reson. 2016, 47, 1419–1437. [Google Scholar] [CrossRef]
- Fechete, R.; Morar, I.A.; Moldovan, D.; Chelcea, R.I.; Crainic, R.; Nicoara, S.C. Fourier and Laplace-like Low-Field NMR Spectroscopy: The Perspectives of Multivariate and Artificial Neural Networks Analyses. J. Magn. Reson. 2021, 324, 106915. [Google Scholar] [CrossRef]
- Venkataramanan, L.; Song, Y.Q.; Hürlimann, M.D. Solving Fredholm Integrals of the First Kind with Tensor Product Structure in 2 and 2.5 Dimensions. IEEE Trans. Signal Process. 2002, 50, 1017–1026. [Google Scholar] [CrossRef]
- Hürlimann, M.D.; Flaum, M.; Venkataramanan, L.; Flaum, C.; Freedman, R.; Hirasaki, G.J. Diffusion-Relaxation Distribution Functions of Sedimentary Rocks in Different Saturation States. Magn. Reson. Imaging 2003, 21, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Q.; Venkataramanan, L.; Hürlimann, M.D.; Flaum, M.; Frulla, P.; Straley, C. T(1)-T(2) Correlation Spectra Obtained Using a Fast Two-Dimensional Laplace Inversion. J. Magn. Reson. 2002, 154, 261–268. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, D.I. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
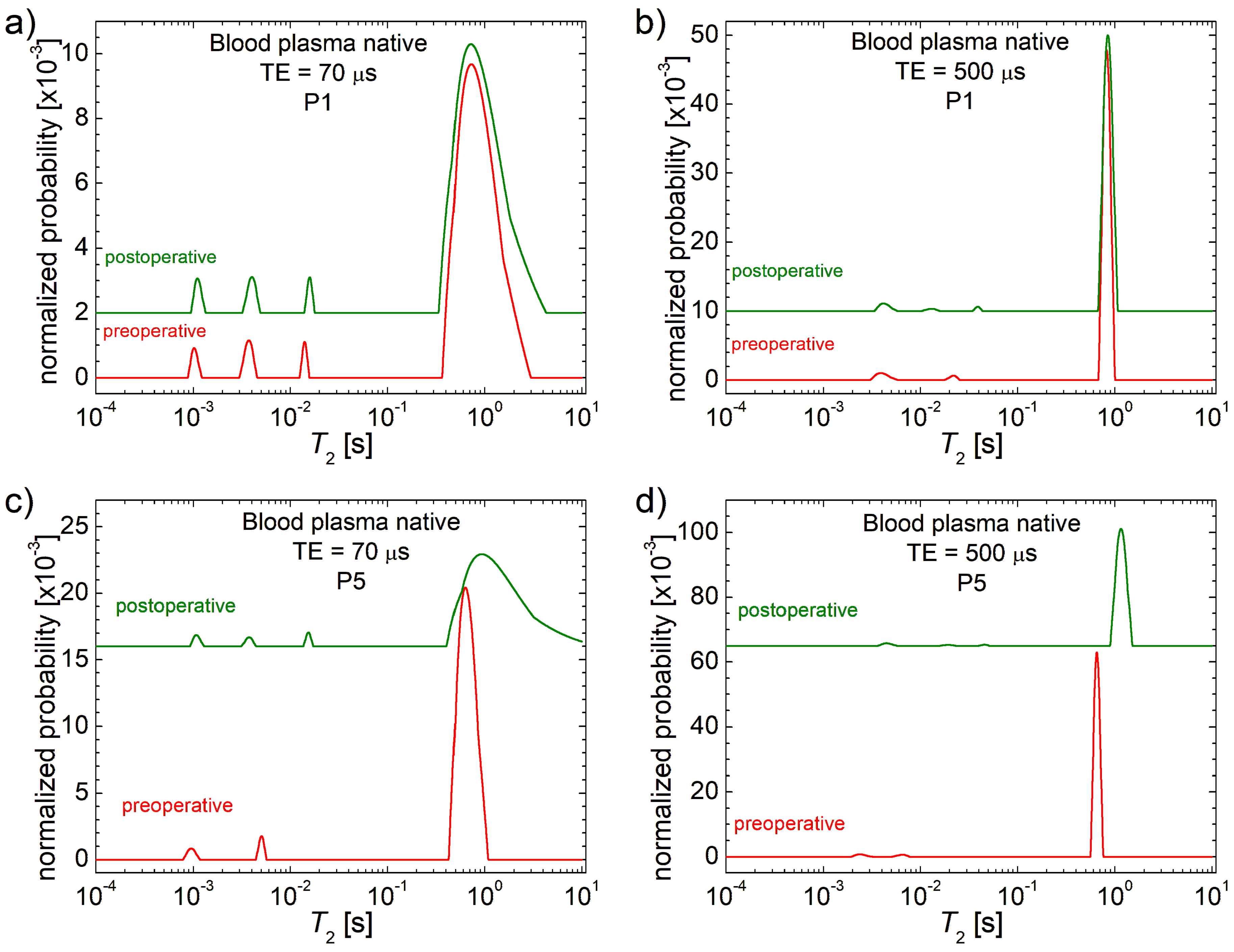
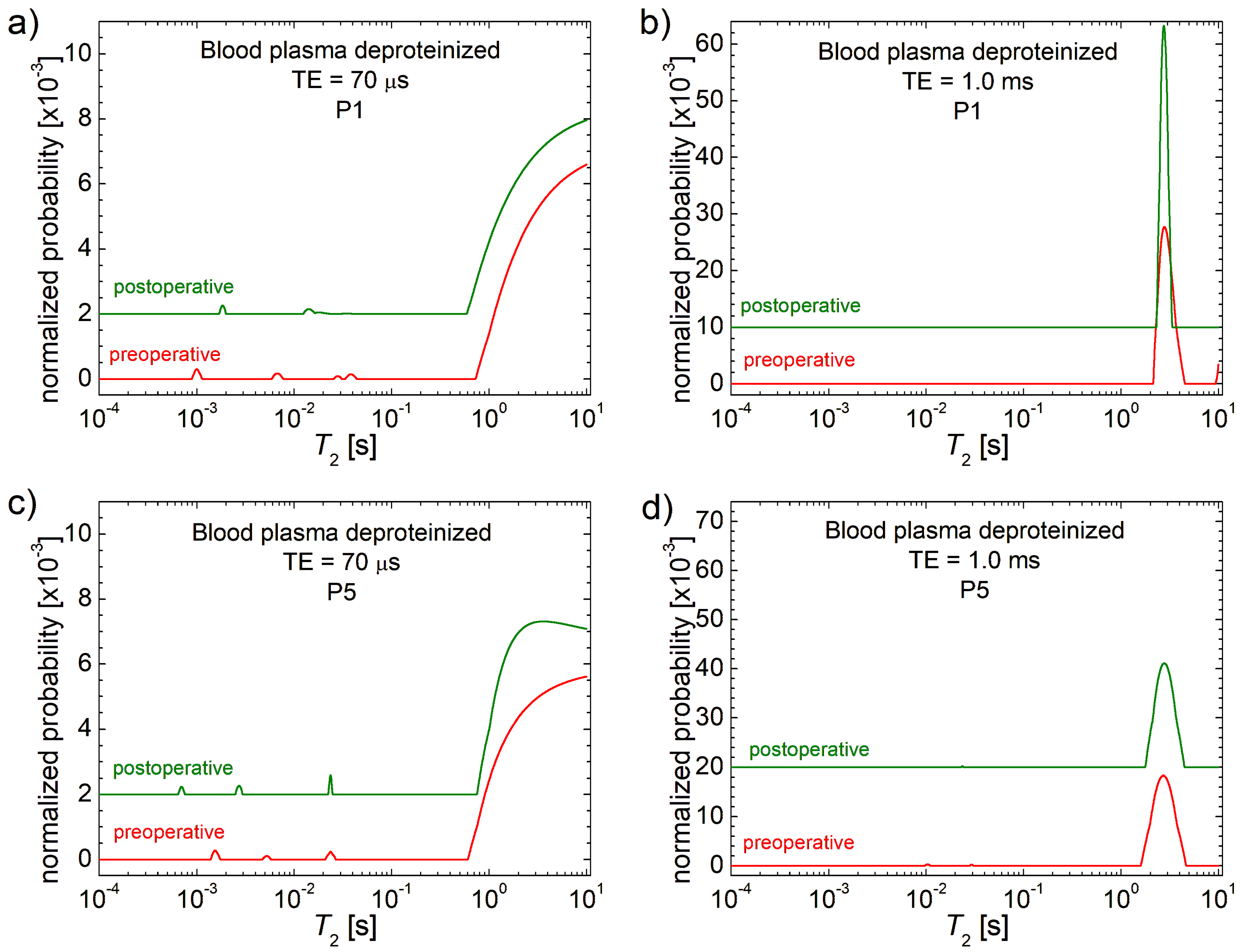
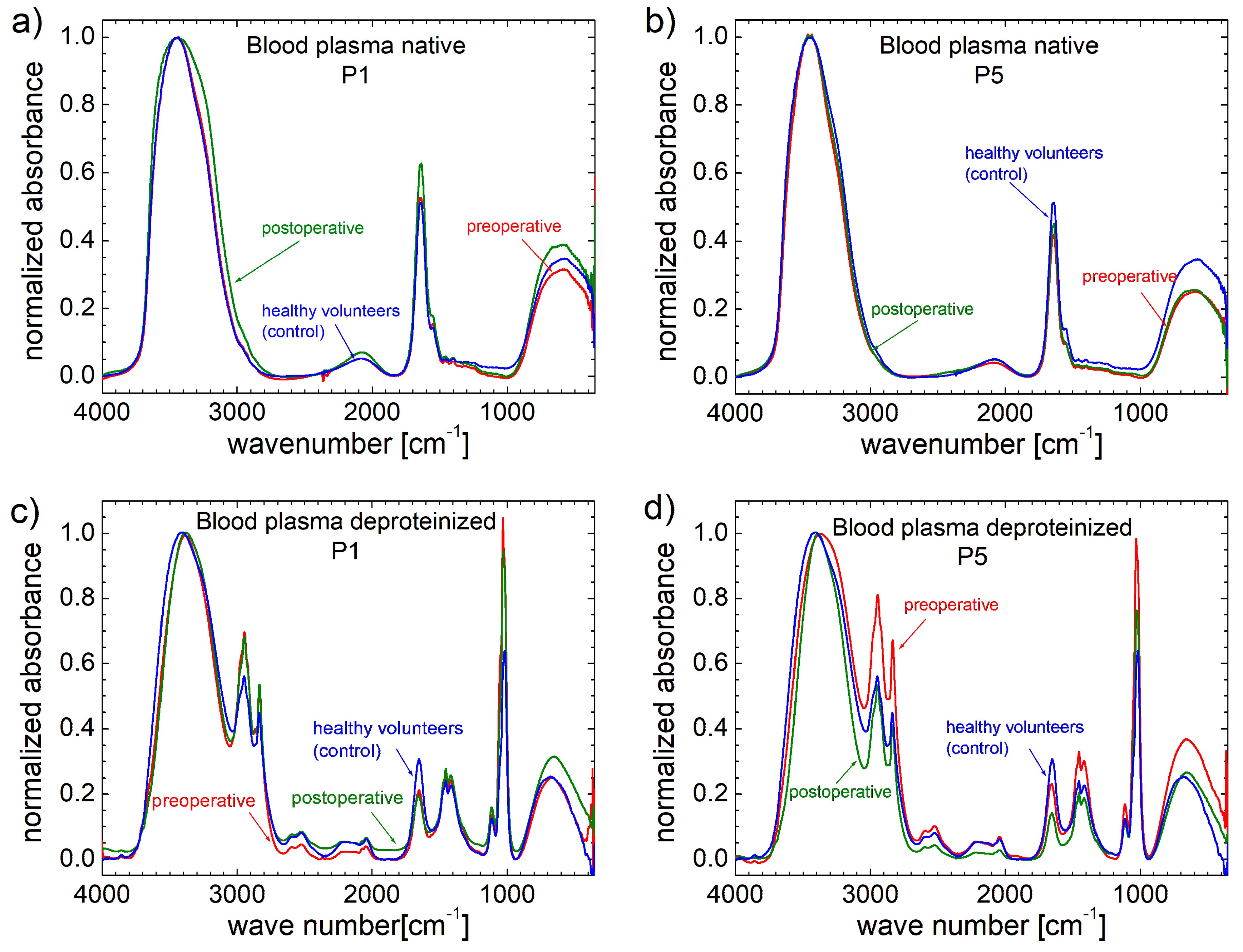
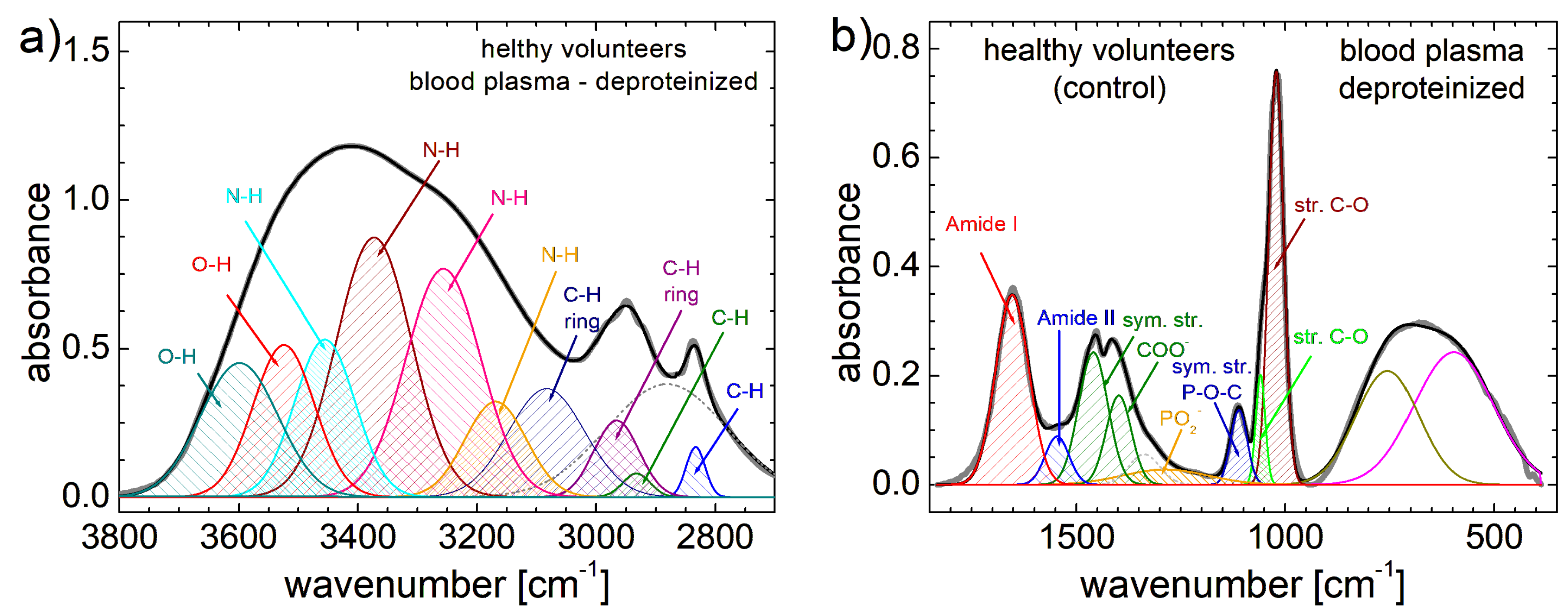


| Patient | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | F | M | M | M | M | M | F | M | F | M |
| Age (years) | 45 | 67 | 66 | 67 | 81 | 67 | 72 | 81 | 55 | 75 |
| Environment of Origin | urban | urban | rural | urban | urban | urban | urban | urban | urban | urban |
| ADK diagnoses | ML-R | L-R | S-C | LGM-C | L-R | MD-CRC | MD-R | CR | MD-C | WD-C |
| PET-CT/CT | PET-CT | CT | CT | CT | CT | CT | CT | CT | CT | CT |
| Neoadjuvant Treatment | RCT | RCT | RCT | NO | RCT | NO | RCT | NO | NO | NO |
| Smoking | no | yes | yes | no | no | yes | no | no | yes | yes |
| BMI categories | nw | uw | uw | uw | nw | nw | uw | nw | uw | uw |
| Histology | G2 | G1 | G2 | G2 | G2 | G2 | G2 | G1 | G2 | G1 |
| Stage of diagnosis after surgery | I | II | IV | III | 0 | III | I | I | III | III |
| Appetite Loss | yes | yes | yes | yes | no | yes | yes | no | yes | yes |
| Weight Loss | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes |
| Fever | no | no | no | yes | no | no | no | no | no | no |
| Rectal Bleeding | yes | yes | no | no | yes | yes | yes | yes | yes | yes |
| Intestinal Transit Disorders | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes |
| Drinking Alcohol | no | rarely | yes | no | rarely | rarely | no | rarely | rarely | rarely |
| Diabetes | dz type II | no | no | dz type II | no | dz type II | no | no | no | no |
| Anemia | no | no | yes | no | yes | no | no | no | yes | yes |
| Hemorrhoids | internal | no | no | no | internal | internal | Int. & ext. | internal | no | internal |
| Iron serum (µg/dL) | 82 | 113 | 25 | 30 | 40 | 60 | 67 | 33 | 10 | 8 |
| Hg preoperative (g/dL) | 13.5 | 15.9 | 10.5 | 13.3 | 10.2 | 13.0 | 12.5 | 13.4 | 8.9 | 7.9 |
| Hg 7 days (g/dL) postoperative | 12.5 | 13.5 | 10.2 | 12.2 | 9.9 | 12.9 | 10.5 | 11.3 | 11.6 | 10.7 |
| RDW-SD preoperative (fL) | 49.9 | 45.8 | 48.4 | 53.2 | 46.8 | 45.4 | 48.3 | 54 | 79.3 | 44.1 |
| RDW-SD at 7 days postoperative (fL) | 47 | 43.1 | 53.8 | 51.1 | 45.6 | 45.5 | 47.3 | 53.2 | 89.9 | 66.4 |
| Rayan score | 1 | 3 | - | - | 0 | - | 3 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Șaitiș, L.R.; Andras, D.; Pop, I.-A.; Șaitiș, C.; Crainic, R.; Fechete, R. Spectroscopic Nuclear Magnetic Resonance and Fourier Transform–Infrared Approach Used for the Evaluation of Healing After Surgical Interventions for Patients with Colorectal Cancer: A Pilot Study. Cancers 2025, 17, 887. https://doi.org/10.3390/cancers17050887
Șaitiș LR, Andras D, Pop I-A, Șaitiș C, Crainic R, Fechete R. Spectroscopic Nuclear Magnetic Resonance and Fourier Transform–Infrared Approach Used for the Evaluation of Healing After Surgical Interventions for Patients with Colorectal Cancer: A Pilot Study. Cancers. 2025; 17(5):887. https://doi.org/10.3390/cancers17050887
Chicago/Turabian StyleȘaitiș, Lavinia Raluca, David Andras, Ioana-Alina Pop, Cătălin Șaitiș, Ramona Crainic, and Radu Fechete. 2025. "Spectroscopic Nuclear Magnetic Resonance and Fourier Transform–Infrared Approach Used for the Evaluation of Healing After Surgical Interventions for Patients with Colorectal Cancer: A Pilot Study" Cancers 17, no. 5: 887. https://doi.org/10.3390/cancers17050887
APA StyleȘaitiș, L. R., Andras, D., Pop, I.-A., Șaitiș, C., Crainic, R., & Fechete, R. (2025). Spectroscopic Nuclear Magnetic Resonance and Fourier Transform–Infrared Approach Used for the Evaluation of Healing After Surgical Interventions for Patients with Colorectal Cancer: A Pilot Study. Cancers, 17(5), 887. https://doi.org/10.3390/cancers17050887





