Mutant KRAS and GATA6 Stratify Survival in Patients Treated with Chemotherapy for Pancreatic Adenocarcinoma: A Prospective Cohort Study
Simple Summary
Abstract
1. Introduction
2. Methods
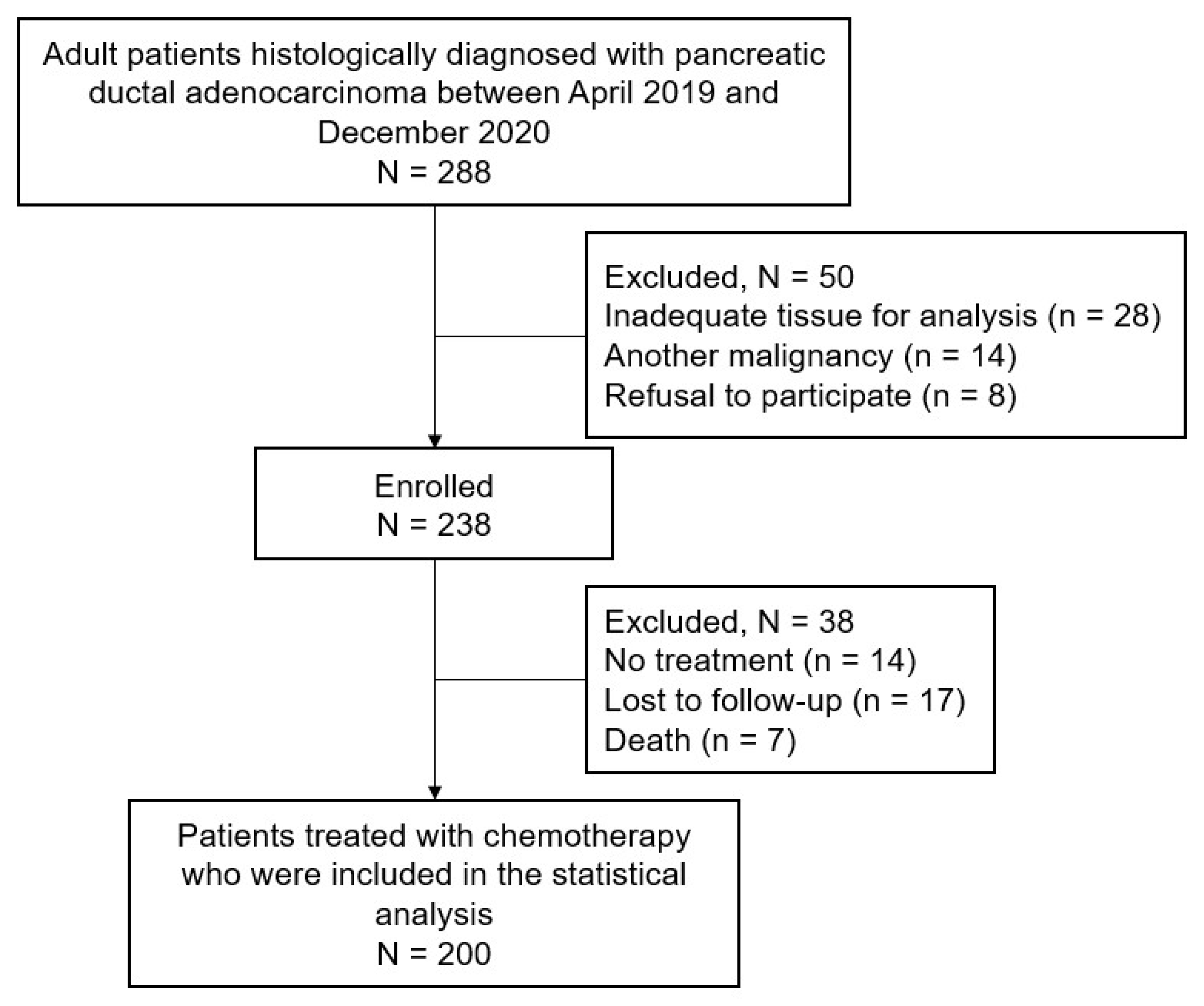
2.1. Patient Population
2.2. Treatment Strategy
2.3. Blood Biomarkers
2.4. Tissue Biomarkers
2.5. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Baseline Distribution of Candidate Biomarkers
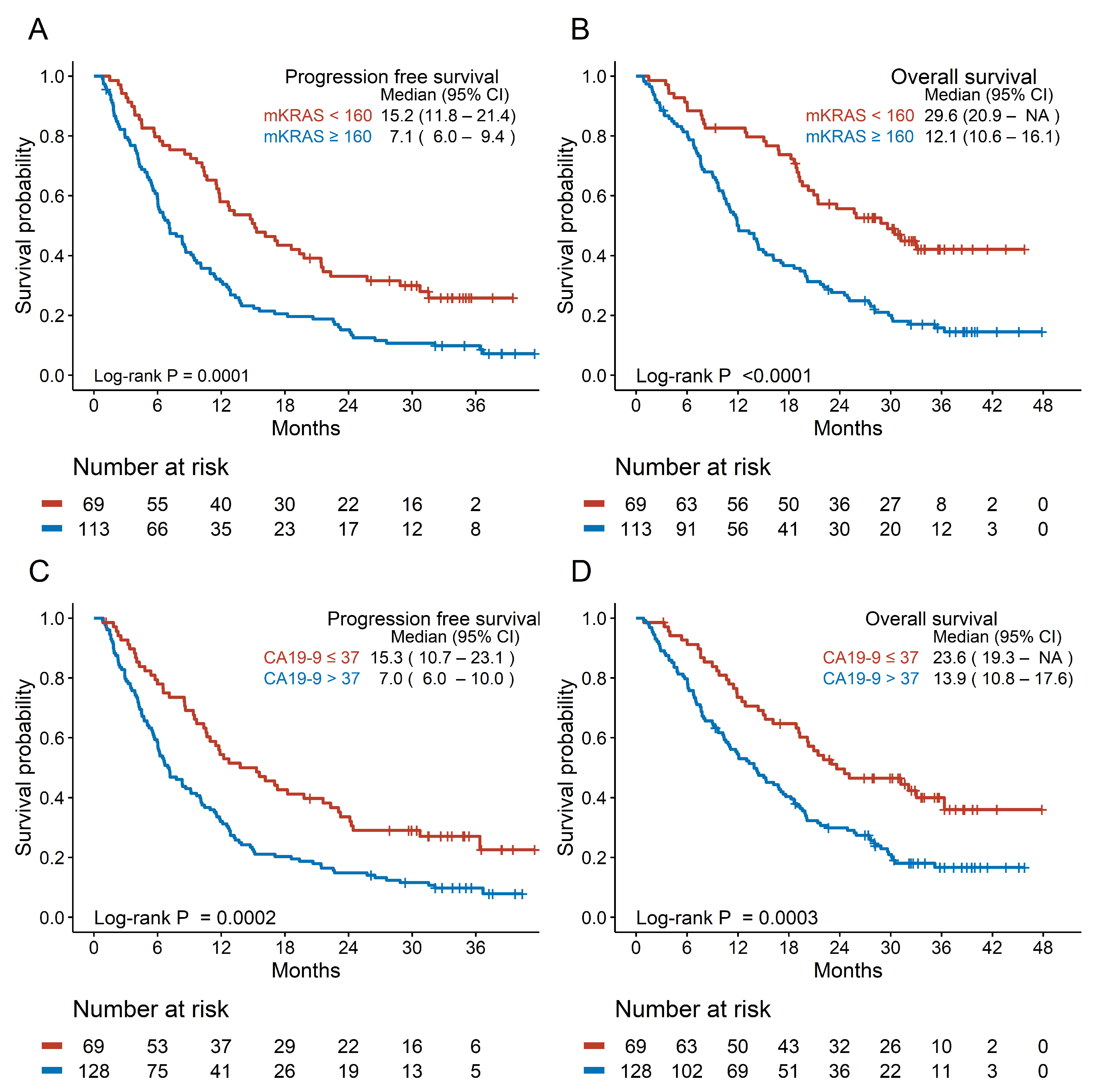
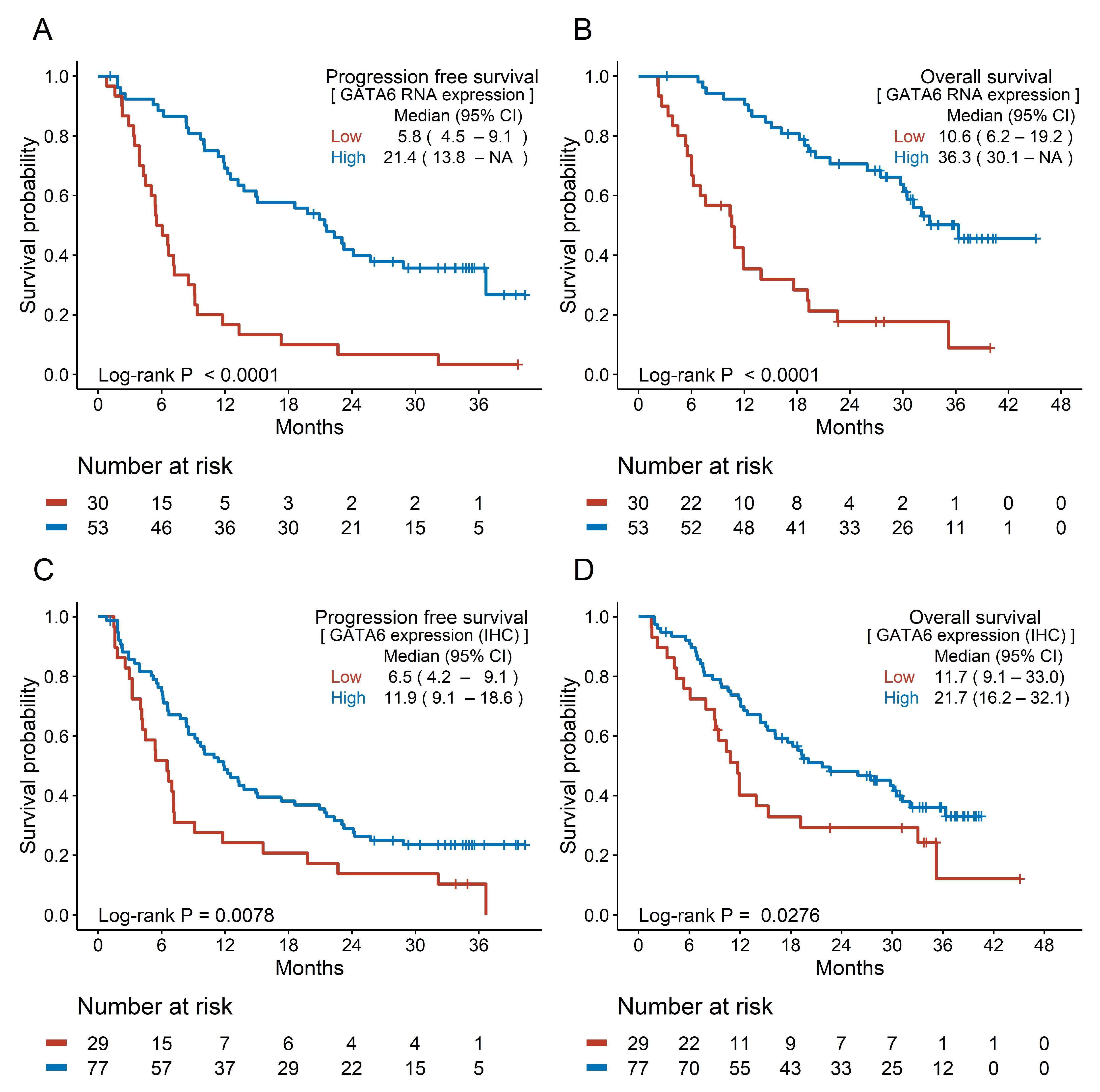
3.3. Prognostic Efficacy of Candidate Biomarkers
3.4. Cox Regression Analysis of Survival
3.5. Subgroup Analysis Based on the Treatment Setting
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pourshams, A.; Sepanlou, S.G.; Ikuta, K.S.; Bisignano, C.; Safiri, S.; Roshandel, G.; Sharif, M.; Khatibian, M.; Fitzmaurice, C.; Nixon, M.R.; et al. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019, 4, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lok, V.; Ngai, C.H.; Zhang, L.; Yuan, J.; Lao, X.Q.; Ng, K.; Chong, C.; Zheng, Z.J.; Wong, M.C.S. Worldwide Burden of, Risk Factors for, and Trends in Pancreatic Cancer. Gastroenterology 2021, 160, 744–754. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.L.; Chone, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- Miyasaka, Y.; Ohtsuka, T.; Kimura, R.; Matsuda, R.; Mori, Y.; Nakata, K.; Kakihara, D.; Fujimori, N.; Ohno, T.; Oda, Y.; et al. Neoadjuvant Chemotherapy with Gemcitabine Plus Nab-Paclitaxel for Borderline Resectable Pancreatic Cancer Potentially Improves Survival and Facilitates Surgery. Ann. Surg. Oncol. 2019, 26, 1528–1534. [Google Scholar] [CrossRef]
- Murphy, J.E.; Wo, J.Y.; Ryan, D.P.; Jiang, W.; Yeap, B.Y.; Drapek, L.C.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; et al. Total Neoadjuvant Therapy With FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2018, 4, 963–969. [Google Scholar] [CrossRef]
- Tempero, M.; O'Reilly, E.; Van Cutsem, E.; Berlin, J.; Philip, P.; Goldstein, D.; Tabernero, J.; Borad, M.; Bachet, J.; Parner, V.; et al. LBA-1 Phase 3 APACT trial of adjuvant nab-paclitaxel plus gemcitabine (nab-P + Gem) vs gemcitabine (Gem) alone in patients with resected pancreatic cancer (PC): Updated 5-year overall survival. Ann. Oncol. 2021, 32, S226. [Google Scholar] [CrossRef]
- Berger, A.C.; Garcia, M., Jr.; Hoffman, J.P.; Regine, W.F.; Abrams, R.A.; Safran, H.; Konski, A.; Benson, A.B., 3rd; MacDonald, J.; Willett, C.G. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: A prospective validation by RTOG 9704. J. Clin. Oncol. 2008, 26, 5918–5922. [Google Scholar] [CrossRef] [PubMed]
- Bernard, V.; Kim, D.U.; San Lucas, F.A.; Castillo, J.; Allenson, K.; Mulu, F.C.; Stephens, B.M.; Huang, J.; Semaan, A.; Guerrero, P.A.; et al. Circulating Nucleic Acids Are Associated with Outcomes of Patients with Pancreatic Cancer. Gastroenterology 2019, 156, 108–118.e104. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, B.K.; Seo, J.H.; Choi, J.; Choi, J.W.; Lee, C.K.; Chung, J.B.; Park, Y.; Kim, D.W. Carbohydrate antigen 19-9 elevation without evidence of malignant or pancreatobiliary diseases. Sci. Rep. 2020, 10, 8820. [Google Scholar] [CrossRef]
- Haas, M.; Ormanns, S.; Baechmann, S.; Remold, A.; Kruger, S.; Westphalen, C.B.; Siveke, J.T.; Wenzel, P.; Schlitter, A.M.; Esposito, I.; et al. Extended RAS analysis and correlation with overall survival in advanced pancreatic cancer. Br. J. Cancer 2017, 116, 1462–1469. [Google Scholar] [CrossRef]
- Kim, M.K.; Woo, S.M.; Park, B.; Yoon, K.A.; Kim, Y.H.; Joo, J.; Lee, W.J.; Han, S.S.; Park, S.J.; Kong, S.Y. Prognostic Implications of Multiplex Detection of KRAS Mutations in Cell-Free DNA from Patients with Pancreatic Ductal Adenocarcinoma. Clin. Chem. 2018, 64, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Perkhofer, L.; Gout, J.; Roger, E.; Kude de Almeida, F.; Baptista Simões, C.; Wiesmüller, L.; Seufferlein, T.; Kleger, A. DNA damage repair as a target in pancreatic cancer: State-of-the-art and future perspectives. Gut 2021, 70, 606. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.M.; Kim, M.K.; Park, B.; Cho, E.H.; Lee, T.R.; Ki, C.S.; Yoon, K.A.; Kim, Y.H.; Choi, W.; Kim, D.Y.; et al. Genomic Instability of Circulating Tumor DNA as a Prognostic Marker for Pancreatic Cancer Survival: A Prospective Cohort Study. Cancers 2021, 13, 5466. [Google Scholar] [CrossRef]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.M.; Gingras, M.C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.; Quinn, M.C.; et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Collisson, E.A.; Sadanandam, A.; Olson, P.; Gibb, W.J.; Truitt, M.; Gu, S.; Cooc, J.; Weinkle, J.; Kim, G.E.; Jakkula, L.; et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med. 2011, 17, 500–503. [Google Scholar] [CrossRef]
- Farrell, J.J.; Elsaleh, H.; Garcia, M.; Lai, R.; Ammar, A.; Regine, W.F.; Abrams, R.; Benson, A.B.; Macdonald, J.; Cass, C.E.; et al. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology 2009, 136, 187–195. [Google Scholar] [CrossRef]
- Wei, C.H.; Gorgan, T.R.; Elashoff, D.A.; Hines, O.J.; Farrell, J.J.; Donahue, T.R. A meta-analysis of gemcitabine biomarkers in patients with pancreaticobiliary cancers. Pancreas 2013, 42, 1303–1310. [Google Scholar] [CrossRef]
- Capello, M.; Fahrmann, J.F.; Perez, M.V.R.; Vykoukal, J.V.; Irajizad, E.; Tripathi, S.C.; Roife, D.; Bantis, L.E.; Kang, Y.a.; Kundnani, D.L.; et al. CES2 Expression in Pancreatic Adenocarcinoma Is Predictive of Response to Irinotecan and Is Associated With Type 2 Diabetes. JCO Precis. Oncol. 2020, 4, 426–436. [Google Scholar] [CrossRef]
- Wang, F.; Xia, X.; Yang, C.; Shen, J.; Mai, J.; Kim, H.C.; Kirui, D.; Kang, Y.; Fleming, J.B.; Koay, E.J.; et al. SMAD4 Gene Mutation Renders Pancreatic Cancer Resistance to Radiotherapy through Promotion of Autophagy. Clin. Cancer Res. 2018, 24, 3176–3185. [Google Scholar] [CrossRef]
- Ryu, K.H.; Park, S.; Chun, J.W.; Cho, E.; Choi, J.; Lee, D.E.; Shim, H.; Kim, Y.H.; Han, S.S.; Park, S.J.; et al. Prevalence and Risk Factors of Germline Pathogenic Variants in Pancreatic Ductal Adenocarcinoma. Cancer Res. Treat. 2023, 55, 1303–1312. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Chan-Seng-Yue, M.; Kim, J.C.; Wilson, G.W.; Ng, K.; Figueroa, E.F.; O’Kane, G.M.; Connor, A.A.; Denroche, R.E.; Grant, R.C.; McLeod, J.; et al. Transcription phenotypes of pancreatic cancer are driven by genomic events during tumor evolution. Nat. Genet. 2020, 52, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Guven, D.C.; Sahin, T.K.; Yildirim, H.C.; Aktepe, O.H.; Dizdar, O.; Yalcin, S. A systematic review and meta-analysis of the association between circulating tumor DNA (ctDNA) and prognosis in pancreatic cancer. Crit. Rev. Oncol./Hematol. 2021, 168, 103528. [Google Scholar] [CrossRef]
- Sivapalan, L.; Kocher, H.M.; Ross-Adams, H.; Chelala, C. Molecular profiling of ctDNA in pancreatic cancer: Opportunities and challenges for clinical application. Pancreatology 2021, 21, 363–378. [Google Scholar] [CrossRef]
- Pietrasz, D.; Pécuchet, N.; Garlan, F.; Didelot, A.; Dubreuil, O.; Doat, S.; Imbert-Bismut, F.; Karoui, M.; Vaillant, J.-C.; Taly, V.; et al. Plasma Circulating Tumor DNA in Pancreatic Cancer Patients Is a Prognostic Marker. Clin. Cancer Res. 2017, 23, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhang, Q.; Li, X.; Su, W.; Li, G.; Ma, T.; Gao, S.; Lou, J.; Que, R.; Zheng, L.; et al. Monitoring Tumor Burden in Response to FOLFIRINOX Chemotherapy Via Profiling Circulating Cell-Free DNA in Pancreatic Cancer. Mol. Cancer Ther. 2019, 18, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Norton, C.; Shaw, M.S.; Rubnitz, Z.; Smith, J.; Soares, H.P.; Nevala-Plagemann, C.D.; Garrido-Laguna, I.; Florou, V. KRAS Mutation Status and Treatment Outcomes in Patients With Metastatic Pancreatic Adenocarcinoma. JAMA Netw. Open 2025, 8, e2453588. [Google Scholar] [CrossRef]
- Duan, K.; Jang, G.H.; Grant, R.C.; Wilson, J.M.; Notta, F.; O’Kane, G.M.; Knox, J.J.; Gallinger, S.; Fischer, S. The value of GATA6 immunohistochemistry and computer-assisted diagnosis to predict clinical outcome in advanced pancreatic cancer. Sci. Rep. 2021, 11, 14951. [Google Scholar] [CrossRef]
- O’Kane, G.M.; Grunwald, B.T.; Jang, G.H.; Masoomian, M.; Picardo, S.; Grant, R.C.; Denroche, R.E.; Zhang, A.; Wang, Y.; Lam, B.; et al. GATA6 Expression Distinguishes Classical and Basal-like Subtypes in Advanced Pancreatic Cancer. Clin. Cancer Res. 2020, 26, 4901–4910. [Google Scholar] [CrossRef]
- Kasi, P.M.; Couch, F.; Bamlet, W.R.; Hu, C.; Hart, S.; Polley, E.; Petersen, G.M.; McWilliams, R.R. Germline BRCA1/2, PALB2, and ATM mutations in 3030 patients with pancreatic adenocarcinoma: Survival analysis of carriers and noncarriers. J. Clin. Oncol. 2018, 36, 280. [Google Scholar] [CrossRef]
- Yadav, S.; Kasi, P.M.; Bamlet, W.R.; Ho, T.P.; Polley, E.C.; Hu, C.; Hart, S.N.; Rabe, K.G.; Boddicker, N.J.; Gnanaolivu, R.D.; et al. Effect of Germline Mutations in Homologous Recombination Repair Genes on Overall Survival of Patients with Pancreatic Adenocarcinoma. Clin. Cancer Res. 2020, 26, 6505–6512. [Google Scholar] [CrossRef] [PubMed]
| Variable | N = 200 | |
|---|---|---|
| Age (years) | Median (range) | 65 (45–90) |
| Sex | Female | 90 (45.0%) |
| Male | 110 (55.0%) | |
| ECOG-PS | 0 | 128 (64.0%) |
| 1 or more | 72 (36.0%) | |
| Tumor location | Head or neck or uncinate process | 92 (46.0%) |
| body or tail | 108 (54.0%) | |
| Stage | Resectable | 46 (23.0%) |
| Borderline resectable/locally advanced | 58 (29.0%) | |
| Metastatic/recurrent | 96 (48.0%) | |
| Surgery | No | 125 (62.5%) |
| Yes | 75 (37.5%) | |
| Chemotherapy | Neoadjuvant | 21 (10.5%) |
| Adjuvant | 45 (22.5%) | |
| Palliative | 134 (67.0%) | |
| Regimen | FOLFIRINOX | 131 (65.5%) |
| Gemcitabine + nab-paclitaxel | 40 (20.0%) | |
| Other 5-fluorouracil-based | 12 (6.0%) | |
| Other gemcitabine-based | 17 (8.5%) | |
| Variable | N | Progression-Free Survival | Overall Survival | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event | Univariable | Adjusted for Stage | Event | Univariable | Adjusted for Stage | Adjusted for Sex and Stage | ||||||||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |||||
| CA19-9 level (U/mL) | ≤37 | 69 | 50 | 1 (ref) | 1 (ref) | 40 | 1 (ref) | 1 (ref) | 1 (ref) | |||||
| >37 | 128 | 116 | 1.866 (1.336–2.605) | <0.001 | 1.647 (1.177–2.306) | 0.0036 | 103 | 1.942 (1.345–2.802) | <0.001 | 1.803 (1.248–2.605) | 0.0017 | 1.764 (1.22–2.551) | 0.0026 | |
| mKRAS ctDNA concentration | <160 | 69 | 50 | 1 (ref) | 1 (ref) | 37 | 1 (ref) | 1 (ref) | 1 (ref) | |||||
| (copies/mL) | ≥160 | 113 | 103 | 1.965 (1.398–2.763) | <0.001 | 1.508 (1.052–2.161) | 0.0253 | 94 | 2.344 (1.598–3.437) | <0.001 | 1.796 (1.203–2.681) | 0.0042 | 1.813 (1.215–2.704) | 0.0036 |
| BRCA mutation | Not detected | 175 | 151 | 1 (ref) | 1 (ref) | 131 | 1 (ref) | 1 (ref) | 1 (ref) | |||||
| VUS/PV | 22 | 15 | 0.717 (0.421–1.219) | 0.219 | 0.724 (0.424–1.235) | 0.235 | 12 | 0.650 (0.360–1.175) | 0.153 | 0.640 (0.354–1.158) | 0.140 | 0.694 (0.382–1.261) | 0.230 | |
| ATM mutation | ND | 182 | 155 | 1 (ref) | 1 (ref) | 133 | 1 (ref) | 1 (ref) | 1 (ref) | |||||
| VUS/PV | 15 | 11 | 0.707 (0.384–1.305) | 0.267 | 0.739 (0.401–1.365) | 0.334 | 10 | 0.778 (0.409–1.479) | 0.443 | 0.796 (0.418–1.516) | 0.487 | 0.823 (0.432–1.568) | 0.553 | |
| hENT1 | Negative | 40 | 33 | 1 (ref) | 1 (ref) | 31 | 1 (ref) | 1 (ref) | 1 (ref) | |||||
| Positive | 88 | 72 | 0.905 (0.598–1.368) | 0.6354 | 0.904 (0.598–1.367) | 0.6321 | 60 | 0.748 (0.484–1.156) | 0.1909 | 0.682 (0.440–1.055) | 0.0853 | 0.706 (0.455–1.093) | 0.1184 | |
| DCK | Negative | 48 | 43 | 1 (ref) | 1 (ref) | 41 | 1 (ref) | 1 (ref) | 1 (ref) | |||||
| Positive | 80 | 62 | 0.724 (0.490–1.070) | 0.1056 | 1.045 (0.692–1.579) | 0.8327 | 50 | 0.575 (0.380–0.870) | 0.0088 | 0.788 (0.513–1.210) | 0.2767 | 0.788 (0.513–1.21) | 0.2767 | |
| CES2 | Low | 93 | 76 | 1 (ref) | 1 (ref) | 64 | 1 (ref) | 1 (ref) | 1 (ref) | |||||
| High | 32 | 23 | 0.772 (0.484–1.231) | 0.2774 | 0.850 (0.532–1.358) | 0.4966 | 19 | 0.769 (0.461–1.284) | 0.3154 | 0.838 (0.501–1.400) | 0.4990 | 0.978 (0.579–1.652) | 0.9328 | |
| GATA6 (RNA) | Low | 30 | 29 | 1 (ref) | 1 (ref) | 25 | 1 (ref) | 1 (ref) | 1 (ref) | |||||
| High | 53 | 34 | 0.266 (0.159–0.447) | <0.0001 | 0.336 (0.195–0.582) | <0.0001 | 24 | 0.225 (0.126–0.402) | <0.0001 | 0.304 (0.165–0.560) | 0.0001 | 0.269 (0.144–0.502) | <0.0001 | |
| GATA6 (Tissue) | Low | 29 | 27 | 1 (ref) | 1 (ref) | 22 | 1 (ref) | 1 (ref) | 1 (ref) | |||||
| High | 77 | 58 | 0.541 (0.341–0.857) | 0.0088 | 0.968 (0.587–1.596) | 0.8970 | 47 | 0.568 (0.341–0.945) | 0.0294 | 1.301 (0.749–2.260) | 0.3507 | 1.302 (0.755–2.243) | 0.3423 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, J.W.; Lee, D.-e.; Han, N.; Heo, S.; Kim, H.; Lee, M.R.; Park, H.M.; Han, S.-S.; Park, S.-J.; Kim, T.H.; et al. Mutant KRAS and GATA6 Stratify Survival in Patients Treated with Chemotherapy for Pancreatic Adenocarcinoma: A Prospective Cohort Study. Cancers 2025, 17, 896. https://doi.org/10.3390/cancers17050896
Chun JW, Lee D-e, Han N, Heo S, Kim H, Lee MR, Park HM, Han S-S, Park S-J, Kim TH, et al. Mutant KRAS and GATA6 Stratify Survival in Patients Treated with Chemotherapy for Pancreatic Adenocarcinoma: A Prospective Cohort Study. Cancers. 2025; 17(5):896. https://doi.org/10.3390/cancers17050896
Chicago/Turabian StyleChun, Jung Won, Dong-eun Lee, Nayoung Han, SooBeen Heo, Hyeji Kim, Mi Rim Lee, Hyeong Min Park, Sung-Sik Han, Sang-Jae Park, Tae Hyun Kim, and et al. 2025. "Mutant KRAS and GATA6 Stratify Survival in Patients Treated with Chemotherapy for Pancreatic Adenocarcinoma: A Prospective Cohort Study" Cancers 17, no. 5: 896. https://doi.org/10.3390/cancers17050896
APA StyleChun, J. W., Lee, D.-e., Han, N., Heo, S., Kim, H., Lee, M. R., Park, H. M., Han, S.-S., Park, S.-J., Kim, T. H., Lee, W. J., Kim, Y.-H., Kong, S.-Y., & Woo, S. M. (2025). Mutant KRAS and GATA6 Stratify Survival in Patients Treated with Chemotherapy for Pancreatic Adenocarcinoma: A Prospective Cohort Study. Cancers, 17(5), 896. https://doi.org/10.3390/cancers17050896







