Bioactive Compounds Targeting Dihydroceramide and Their Therapeutic Potential in Cancer Treatment
Simple Summary
Abstract
1. Introduction
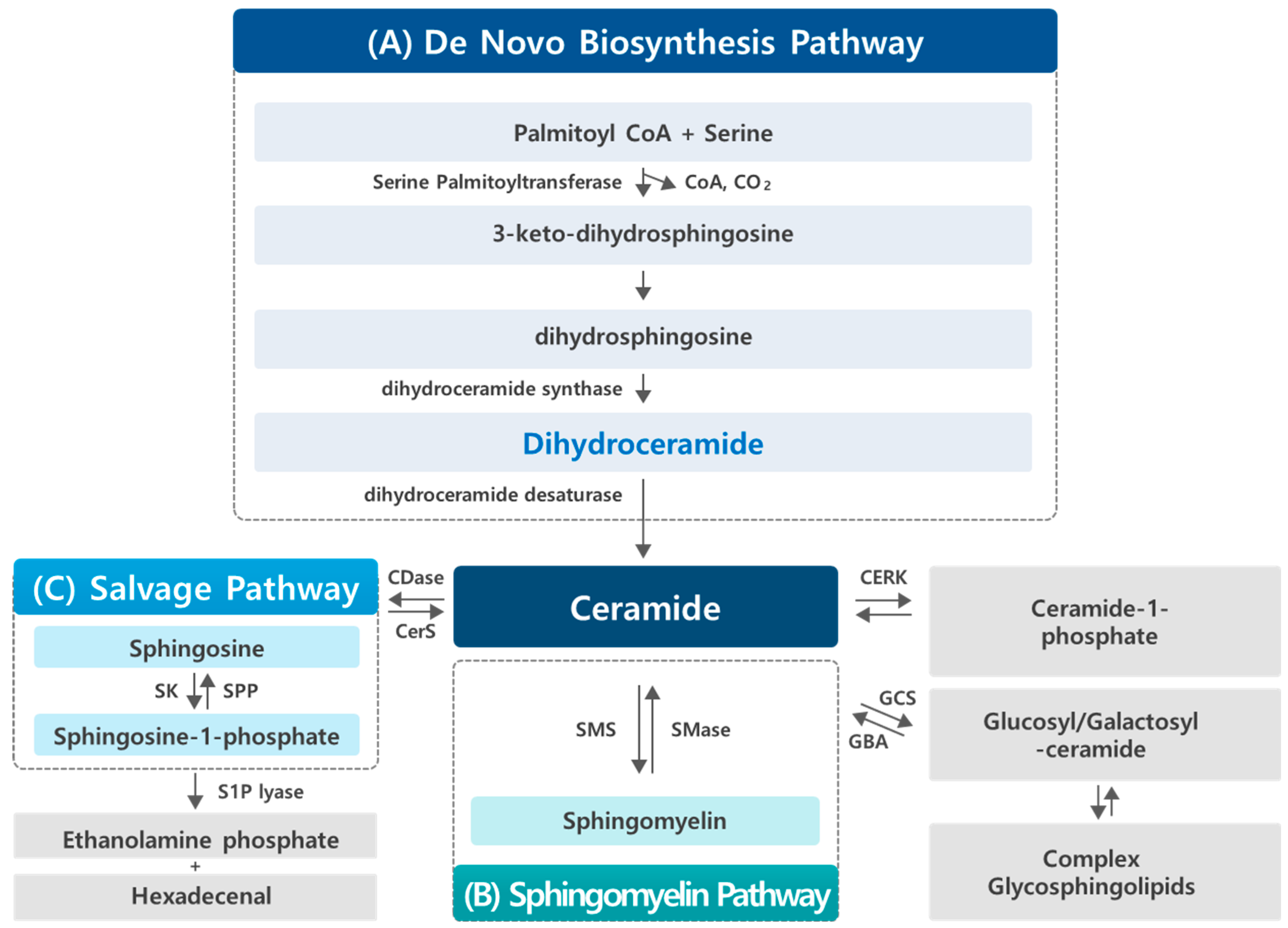
2. Sphingolipid Metabolism
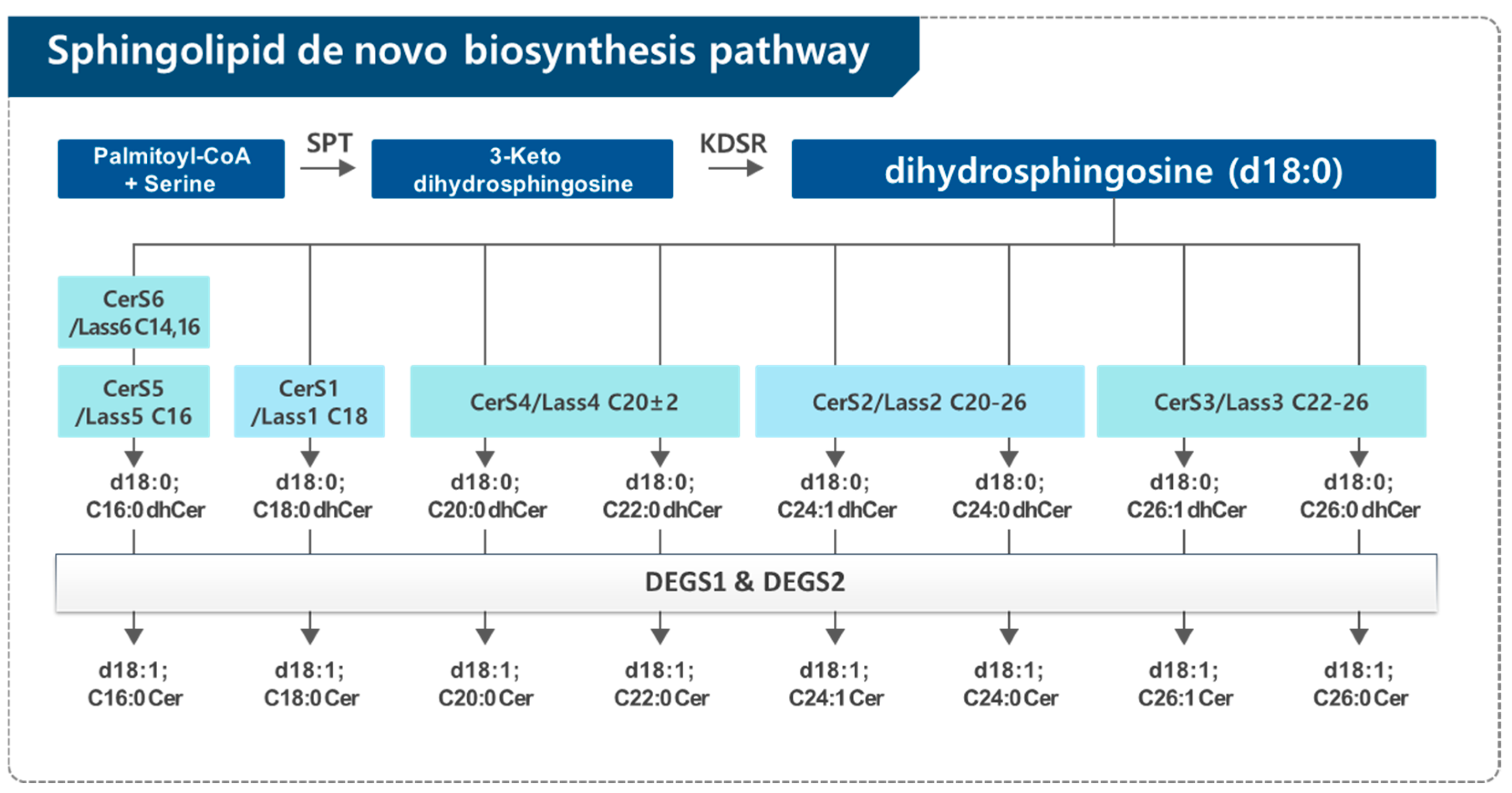
2.1. Dihydroceramide Generation in the De Novo Synthesis Pathway of Sphingolipid
2.2. Dihydroceramide Desaturase (DEGS)
3. Findings and Biological Activities of Dihydroceramide
4. Impact of Bioactive Compounds on Dihydroceramide: Focus on Cancer
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar]
- Li, R.Z.; Wang, X.R.; Wang, J.; Xie, C.; Wang, X.X.; Pan, H.D.; Meng, W.Y.; Liang, T.L.; Li, J.X.; Yan, P.Y.; et al. The key role of sphingolipid metabolism in cancer: New therapeutic targets, diagnostic and prognostic values, and anti-tumor immunotherapy resistance. Front. Oncol. 2022, 12, 941643. [Google Scholar] [CrossRef]
- Janneh, A.H.; Ogretmen, B. Targeting Sphingolipid Metabolism as a Therapeutic Strategy in Cancer Treatment. Cancers 2022, 14, 2183. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.M.; Li, Y.; Chaurasia, B.; Kaddai, V.A.; Summers, S.A. Dihydroceramides: From Bit Players to Lead Actors. J. Biol. Chem. 2015, 290, 15371–15379. [Google Scholar] [CrossRef]
- Lachkar, F.; Ferré, P.; Foufelle, F.; Papaioannou, A. Dihydroceramides: Their emerging physiological roles and functions in cancer and metabolic diseases. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E122–E130. [Google Scholar] [CrossRef] [PubMed]
- Fabrias, G.; Muñoz-Olaya, J.; Cingolani, F.; Signorelli, P.; Casas, J.; Gagliostro, V.; Ghidoni, R. Dihydroceramide desaturase and dihydrosphingolipids: Debutant players in the sphingolipid arena. Prog. Lipid Res. 2012, 51, 82–94. [Google Scholar] [CrossRef]
- Zheng, W.; Kollmeyer, J.; Symolon, H.; Momin, A.; Munter, E.; Wang, E.; Kelly, S.; Allegood, J.C.; Liu, Y.; Peng, Q.; et al. Ceramides and other bioactive sphingolipid backbones in health and disease: Lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim. Biophys. Acta 2006, 1758, 1864–1884. [Google Scholar] [CrossRef]
- Wang, H.; Maurer, B.J.; Liu, Y.Y.; Wang, E.; Allegood, J.C.; Kelly, S.; Symolon, H.; Liu, Y.; Merrill, A.H., Jr.; Gouazé-Andersson, V.; et al. N-(4-Hydroxyphenyl)retinamide increases dihydroceramide and synergizes with dimethylsphingosine to enhance cancer cell killing. Mol. Cancer Ther. 2008, 7, 2967–2976. [Google Scholar] [CrossRef]
- Merrill, A.H., Jr. De novo sphingolipid biosynthesis: A necessary, but dangerous, pathway. J. Biol. Chem. 2002, 277, 25843–25846. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, C.Y. The Role of Vitamin E Isoforms and Metabolites in Cancer Prevention: Mechanistic Insights into Sphingolipid Metabolism Modulation. Nutrients 2024, 16, 4115. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Many ceramides. J. Biol. Chem. 2011, 286, 27855–27862. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.; van Echten-Deckert, G.; Rother, J.; Sandhoff, K.; Wang, E.; Merrill, A.H., Jr. Characterization of ceramide synthesis. A dihydroceramide desaturase introduces the 4,5-trans-double bond of sphingosine at the level of dihydroceramide. J. Biol. Chem. 1997, 272, 22432–22437. [Google Scholar] [CrossRef] [PubMed]
- Ternes, P.; Franke, S.; Zahringer, U.; Sperling, P.; Heinz, E. Identification and characterization of a sphingolipid delta 4-desaturase family. J. Biol. Chem. 2002, 277, 25512–25518. [Google Scholar] [CrossRef]
- Triola, G.; Fabrias, G.; Casas, J.; Llebaria, A. Synthesis of cyclopropene analogues of ceramide and their effect on dihydroceramide desaturase. J. Org. Chem. 2003, 68, 9924–9932. [Google Scholar] [CrossRef]
- Triola, G.; Fabrias, G.; Dragusin, M.; Niederhausen, L.; Broere, R.; Llebaria, A.; van Echten-Deckert, G. Specificity of the dihydroceramide desaturase inhibitor N-[(1R,2S)-2-hydroxy-1-hydroxymethyl-2-(2-tridecyl-1-cyclopropenyl)ethyl]octanami de (GT11) in primary cultured cerebellar neurons. Mol. Pharmacol. 2004, 66, 1671–1678. [Google Scholar] [CrossRef]
- Bielawska, A.; Crane, H.M.; Liotta, D.; Obeid, L.M.; Hannun, Y.A. Selectivity of ceramide-mediated biology. Lack of activity of erythro-dihydroceramide. J. Biol. Chem. 1993, 268, 26226–26232. [Google Scholar] [CrossRef]
- Ahn, E.H.; Schroeder, J.J. Sphingoid bases and ceramide induce apoptosis in HT-29 and HCT-116 human colon cancer cells. Exp. Biol. Med. 2002, 227, 345–353. [Google Scholar] [CrossRef]
- Gagliostro, V.; Casas, J.; Caretti, A.; Abad, J.L.; Tagliavacca, L.; Ghidoni, R.; Fabrias, G.; Signorelli, P. Dihydroceramide delays cell cycle G1/S transition via activation of ER stress and induction of autophagy. Int. J. Biochem. Cell Biol. 2012, 44, 2135–2143. [Google Scholar] [CrossRef]
- Rahmaniyan, M.; Curley, R.W., Jr.; Obeid, L.M.; Hannun, Y.A.; Kraveka, J.M. Identification of dihydroceramide desaturase as a direct in vitro target for fenretinide. J. Biol. Chem. 2011, 286, 24754–24764. [Google Scholar] [CrossRef]
- Siddique, M.M.; Li, Y.; Wang, L.; Ching, J.; Mal, M.; Ilkayeva, O.; Wu, Y.J.; Bay, B.H.; Summers, S.A. Ablation of dihydroceramide desaturase 1, a therapeutic target for the treatment of metabolic diseases, simultaneously stimulates anabolic and catabolic signaling. Mol. Cell. Biol. 2013, 33, 2353–2369. [Google Scholar] [CrossRef] [PubMed]
- Merrill, A.H., Jr.; Sullards, M.C.; Allegood, J.C.; Kelly, S.; Wang, E. Sphingolipidomics: High-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods 2005, 36, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Kraveka, J.M.; Li, L.; Szulc, Z.M.; Bielawski, J.; Ogretmen, B.; Hannun, Y.A.; Obeid, L.M.; Bielawska, A. Involvement of dihydroceramide desaturase in cell cycle progression in human neuroblastoma cells. J. Biol. Chem. 2007, 282, 16718–16728. [Google Scholar] [CrossRef]
- Hernández-Tiedra, S.; Fabriàs, G.; Dávila, D.; Salanueva, Í.J.; Casas, J.; Montes, L.R.; Antón, Z.; García-Taboada, E.; Salazar-Roa, M.; Lorente, M.; et al. Dihydroceramide accumulation mediates cytotoxic autophagy of cancer cells via autolysosome destabilization. Autophagy 2016, 12, 2213–2229. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Guardiola, P.; Casas, J.; Megías-Roda, E.; Solé, S.; Perez-Montoyo, H.; Yeste-Velasco, M.; Erazo, T.; Diéguez-Martínez, N.; Espinosa-Gil, S.; Muñoz-Pinedo, C.; et al. The anti-cancer drug ABTL0812 induces ER stress-mediated cytotoxic autophagy by increasing dihydroceramide levels in cancer cells. Autophagy 2021, 17, 1349–1366. [Google Scholar] [CrossRef]
- Signorelli, P.; Munoz-Olaya, J.M.; Gagliostro, V.; Casas, J.; Ghidoni, R.; Fabrias, G. Dihydroceramide intracellular increase in response to resveratrol treatment mediates autophagy in gastric cancer cells. Cancer Lett. 2009, 282, 238–243. [Google Scholar] [CrossRef]
- Tzou, F.Y.; Su, T.Y.; Lin, W.S.; Kuo, H.C.; Yu, Y.L.; Yeh, Y.H.; Liu, C.C.; Kuo, C.H.; Huang, S.Y.; Chan, C.C. Dihydroceramide desaturase regulates the compartmentalization of Rac1 for neuronal oxidative stress. Cell Rep. 2021, 35, 108972. [Google Scholar] [CrossRef]
- Idkowiak-Baldys, J.; Apraiz, A.; Li, L.; Rahmaniyan, M.; Clarke, C.J.; Kraveka, J.M.; Asumendi, A.; Hannun, Y.A. Dihydroceramide desaturase activity is modulated by oxidative stress. Biochem. J. 2010, 427, 265–274. [Google Scholar] [CrossRef]
- Devlin, C.M.; Lahm, T.; Hubbard, W.C.; Van Demark, M.; Wang, K.C.; Wu, X.; Bielawska, A.; Obeid, L.M.; Ivan, M.; Petrache, I. Dihydroceramide-based response to hypoxia. J. Biol. Chem. 2011, 286, 38069–38078. [Google Scholar] [CrossRef]
- Wang, Y.; Park, N.Y.; Jang, Y.; Ma, A.; Jiang, Q. Vitamin E gamma-Tocotrienol Inhibits Cytokine-Stimulated NF-kappaB Activation by Induction of Anti-Inflammatory A20 via Stress Adaptive Response Due to Modulation of Sphingolipids. J. Immunol. 2015, 195, 126–133. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, Q. Vitamin E δ-tocotrienol inhibits TNF-α-stimulated NF-κB activation by up-regulation of anti-inflammatory A20 via modulation of sphingolipid including elevation of intracellular dihydroceramides. J. Nutr. Biochem. 2019, 64, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wong, J.; Fyrst, H.; Saba, J.D.; Ames, B.N. gamma-Tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc. Natl. Acad. Sci. USA 2004, 101, 17825–17830. [Google Scholar] [CrossRef]
- Gopalan, A.; Yu, W.; Jiang, Q.; Jang, Y.; Sanders, B.G.; Kline, K. Involvement of de novo ceramide synthesis in gamma-tocopherol and gamma-tocotrienol-induced apoptosis in human breast cancer cells. Mol. Nutr. Food Res. 2012, 56, 1803–1811. [Google Scholar] [CrossRef]
- Jang, Y.; Rao, X.; Jiang, Q. Gamma-tocotrienol profoundly alters sphingolipids in cancer cells by inhibition of dihydroceramide desaturase and possibly activation of sphingolipid hydrolysis during prolonged treatment. J. Nutr. Biochem. 2017, 46, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Stiban, J.; Fistere, D.; Colombini, M. Dihydroceramide hinders ceramide channel formation: Implications on apoptosis. Apoptosis 2006, 11, 773–780. [Google Scholar] [CrossRef]
- Breen, P.; Joseph, N.; Thompson, K.; Kraveka, J.M.; Gudz, T.I.; Li, L.; Rahmaniyan, M.; Bielawski, J.; Pierce, J.S.; Buren, E.V.A.N.; et al. Dihydroceramide desaturase knockdown impacts sphingolipids and apoptosis after photodamage in human head and neck squamous carcinoma cells. Anticancer Res. 2013, 33, 77–84. [Google Scholar]
- Boppana, N.B.; DeLor, J.S.; Van Buren, E.; Bielawska, A.; Bielawski, J.; Pierce, J.S.; Korbelik, M.; Separovic, D. Enhanced apoptotic cancer cell killing after Foscan photodynamic therapy combined with fenretinide via de novo sphingolipid biosynthesis pathway. J. Photochem. Photobiol. B 2016, 159, 191–195. [Google Scholar] [CrossRef]
- Schiffmann, S.; Sandner, J.; Schmidt, R.; Birod, K.; Wobst, I.; Schmidt, H.; Angioni, C.; Geisslinger, G.; Grosch, S. The selective COX-2 inhibitor celecoxib modulates sphingolipid synthesis. J. Lipid Res. 2009, 50, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Venant, H.; Rahmaniyan, M.; Jones, E.E.; Lu, P.; Lilly, M.B.; Garrett-Mayer, E.; Drake, R.R.; Kraveka, J.M.; Smith, C.D.; Voelkel-Johnson, C. The Sphingosine Kinase 2 Inhibitor ABC294640 Reduces the Growth of Prostate Cancer Cells and Results in Accumulation of Dihydroceramides In Vitro and In Vivo. Mol. Cancer Ther. 2015, 14, 2744–2752. [Google Scholar] [CrossRef]
- Jang, Y.; Park, N.Y.; Rostgaard-Hansen, A.L.; Huang, J.; Jiang, Q. Vitamin E metabolite 13′-carboxychromanols inhibit pro-inflammatory enzymes, induce apoptosis and autophagy in human cancer cells by modulating sphingolipids and suppress colon tumor development in mice. Free Radic. Biol. Med. 2016, 95, 190–199. [Google Scholar] [CrossRef]
| Year and Author | Type of Cancer | Treatment | Sphingolipid Levels | Mechanisms/ Results |
|---|---|---|---|---|
| 2004; Jiang et al. [32] | Human prostate (LNCaP, PC-3) and lung (A549) cancer cells | Vitamin E; γ-tocopherol: 50 μM, 1~3 days | dhCer ↑ dhSph ↑ | Apoptosis induction/cell death |
| 2006; Zheng et al. [8] | Human prostate cancer cells (DU145) | Fenretinide (4-HPR): 10 μM, 24 h | dhCer ↑ | Autophagy induction |
| 2008; Wang et al. [9] | Human cancer cells (MCF-7/AdrR, HL-60, HT-29) | Fenretinide (4-HPR): 10 μM, 24 h | dhCer ↑, dhSph ↑ | De novo sphingolipid synthesis ↑ DEGS inhibition |
| 2009; Signorelli et al. [26] | Human gastric epithelial cancer cells (HGC-27) | Resveratrol: 50 μM, 16 h (DEGS inhibitor; XM462) | dhCer (C14:0, 16:0, 18:0, 20:0, 22:0, 24:0, 24:1-dhCer) ↑ (Cer levels increased only slightly) | Inhibition of DEGS/ autophagy induction (no sign of cell death) |
| 2009; Schiffmann et al. [38] | Human colon (HCT116, HCA-7, HT-29), cervix (HeLa), lung (A549), breast (MDA-MB231, MCF-7), embryonic kidney (HEK293) cancer cells | Celecoxib: 20, 40, 60, 80 μM, 2 h (Methyl celecoxib 80 μM) | dhCer (C16:0, 24:0, 24:1-dhCer) ↑ dhSph ↑ Cer (C24:0, 24:1-Cer) ↓ | De novo sphingolipid synthesis ↑, DEGS inhibition/anti-proliferation |
| 2012; Gopalan et al. [33] | Human breast cancer cells (MCF-7) | Vitamin E; γ-tocopherol (40 μM) and γ-tocotrienol (10 μM): 2~3 days | dhCer ↑ Cer ↑ | JNK/CHOP/DR5-mediated apoptosis induction |
| 2012; Gagliostro et al. [19] | Human gastric cancer cells (HCG27) | DEGS inhibitor; XM462): 8 μM, 8~24 h | dhCer ↑ | ER stress, autophagy ↑/delayed cell cycle G1/S transition |
| 2013; Venant et al. [39] | Murine castration-resistant prostate cancer cells (TRAMP-C2) | ABC294640; sphingosine kinase 2 inhibitor: 10~30 μmol/L, 72 h | dhCer ↑ | Inhibition of DEGS activity/reduction in cancer cell growth |
| 2016; Jang et al. [40] | Human colon cancer cells (HCT116) | Vitamin E metabolite; 13′-carboxychromanols: 10, 20 μM, 1~16 h | dhCer (C16:0, 24:0, 24:1-dhCer) ↑ dhSph ↑ Cer (C24:0, 24:1-Cer) ↓ | Inhibition of DEGS activity/apoptosis and autophagy induction |
| 2016; Hernàndez-Tiedra et al. [24] | U87MG MEFs cancer cells | THC (∆9-Tetrahydrocannabinol; a component of marijuana): 6 μM, 6 h | dhCer (C16:0, 24:0, 24:1-dhCer) ↑ Cer ↓ | Autophagy-mediated cancer cell death |
| 2017; Jang et al. [34] | Human colon (HCT116), breast (MCF-7) cancer cells | Vitamin E; γ-tocotrienol: 20 μM, 8, 16, 24 h | dhCer, dhSph ↑ Cer 8, 16 h ↓ SM ↓, | Inhibition of DEGS activity/early apoptosis and autophagy induction |
| 2021; Munoz-Guordiola et al. [25] | Human pancreatic (MiaPaca-2) and lung (A549) cancer cells | ABTL0812; anticancer drug: 40~100 μM, 1~24 h | dhCer ↑ | Impaired DEGS1 activity/ ER stress-mediated cytotoxic autophagy induction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, Y. Bioactive Compounds Targeting Dihydroceramide and Their Therapeutic Potential in Cancer Treatment. Cancers 2025, 17, 909. https://doi.org/10.3390/cancers17050909
Jang Y. Bioactive Compounds Targeting Dihydroceramide and Their Therapeutic Potential in Cancer Treatment. Cancers. 2025; 17(5):909. https://doi.org/10.3390/cancers17050909
Chicago/Turabian StyleJang, Yumi. 2025. "Bioactive Compounds Targeting Dihydroceramide and Their Therapeutic Potential in Cancer Treatment" Cancers 17, no. 5: 909. https://doi.org/10.3390/cancers17050909
APA StyleJang, Y. (2025). Bioactive Compounds Targeting Dihydroceramide and Their Therapeutic Potential in Cancer Treatment. Cancers, 17(5), 909. https://doi.org/10.3390/cancers17050909






