The Chimeric Antigen Receptor T Cell Target Claudin 6 Is a Marker for Early Organ-Specific Epithelial Progenitors and Is Expressed in Some Pediatric Solid Tumor Entities
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
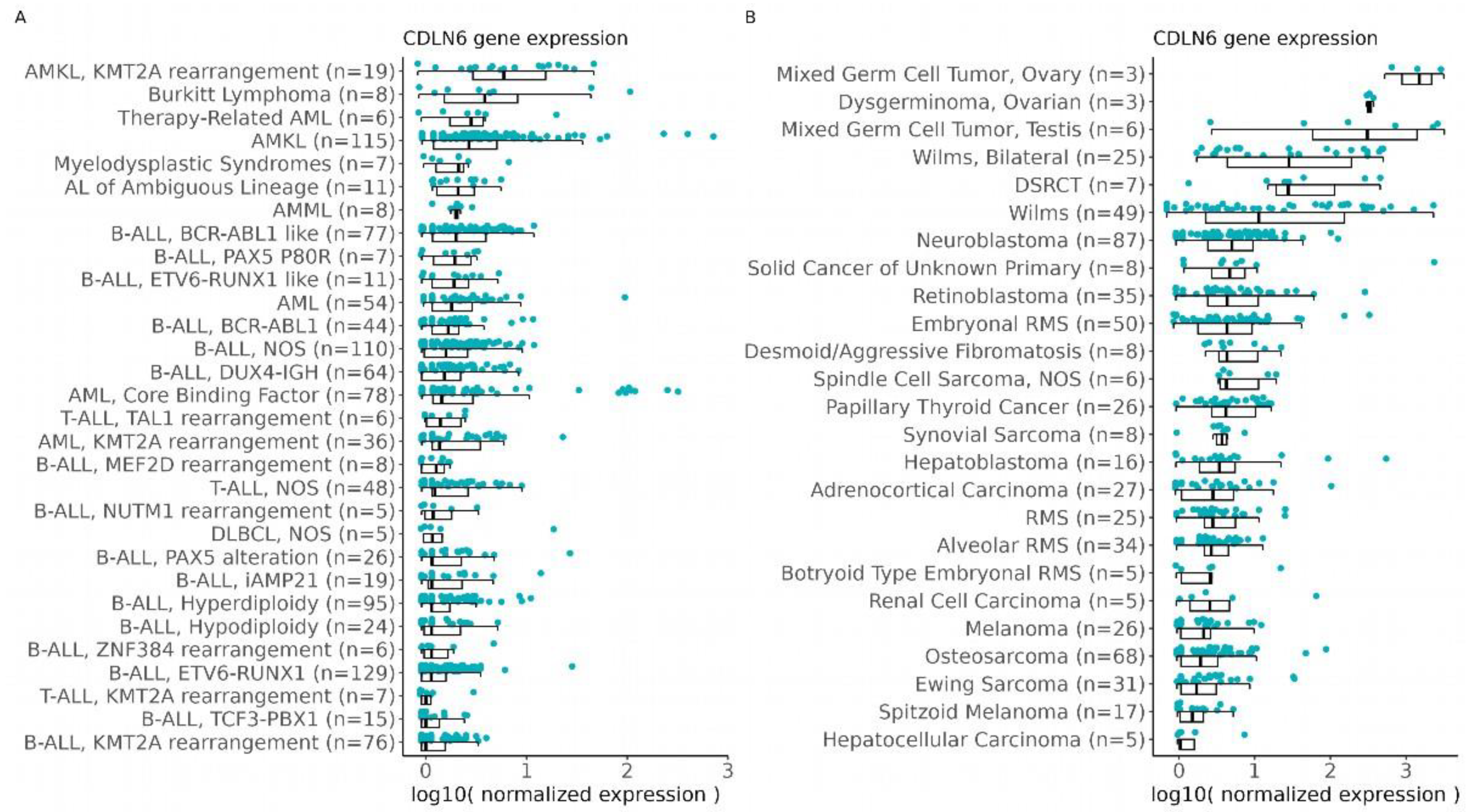
2.2. CLDN6 Gene Expression Across Cancer Types
2.3. CLDN6 Staining
2.4. qRT-PCR Expression Analysis
3. Results
3.1. CLDN6 Expression Is Predicted in Solid and Blood Cancer in the Pediatric Population
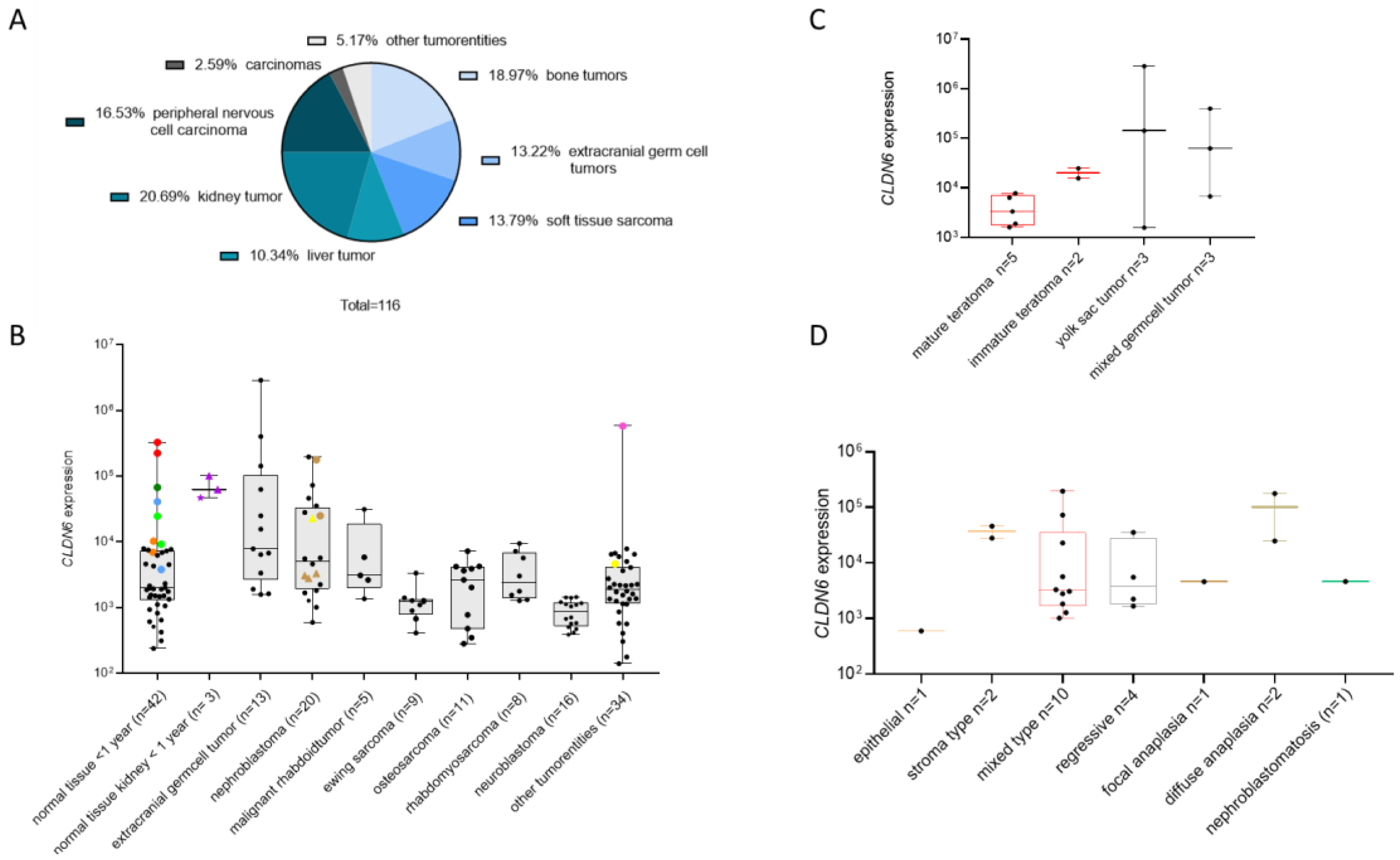
3.2. CLDN6 mRNA Is Present in Normal Tissues of Infants and in Pediatric Tumors
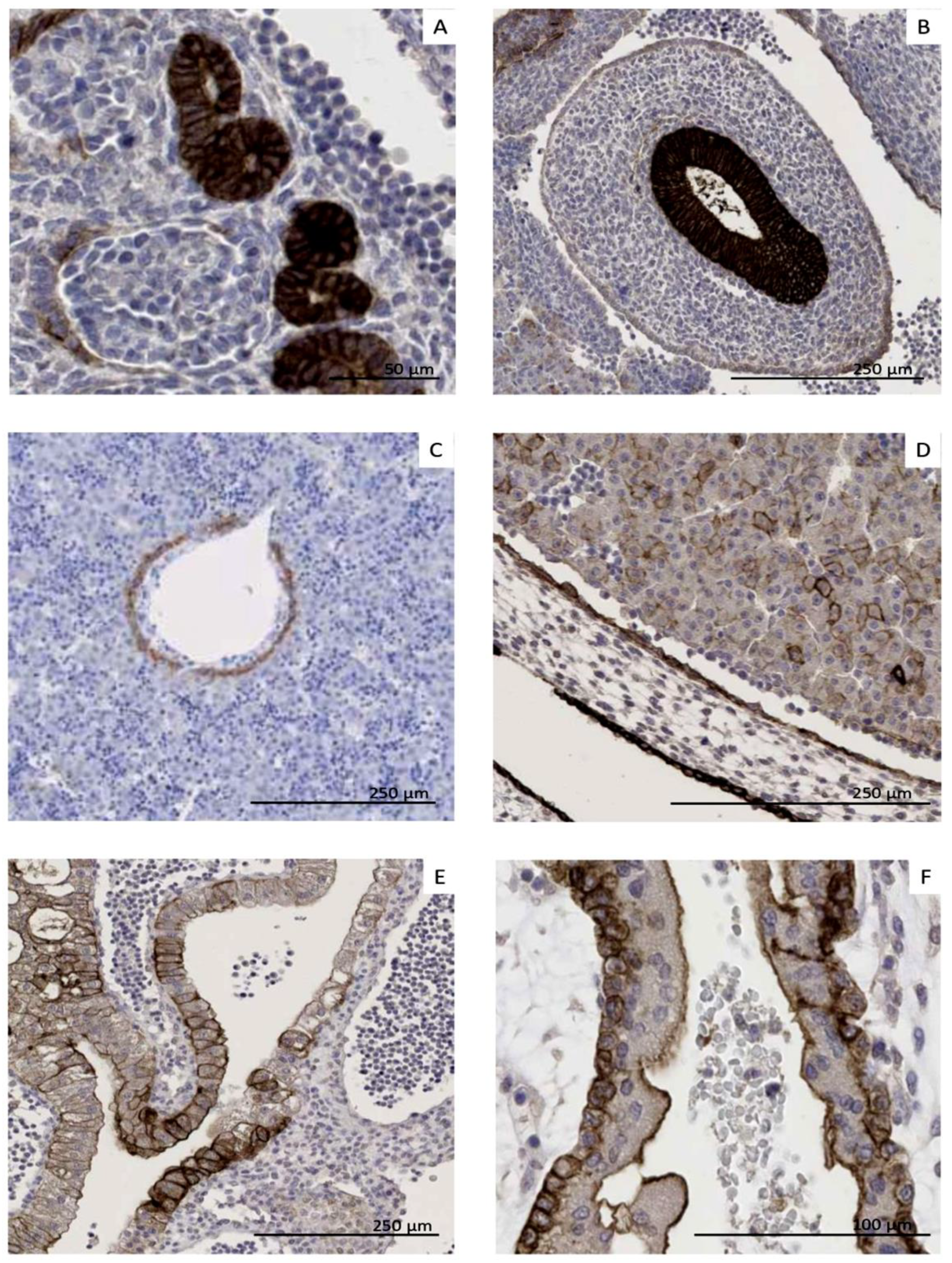
3.3. Organ–Specific Presence of CLDN6-Positive Epithelial Precursors
3.4. CLDN6 Expression Is Lost After Birth
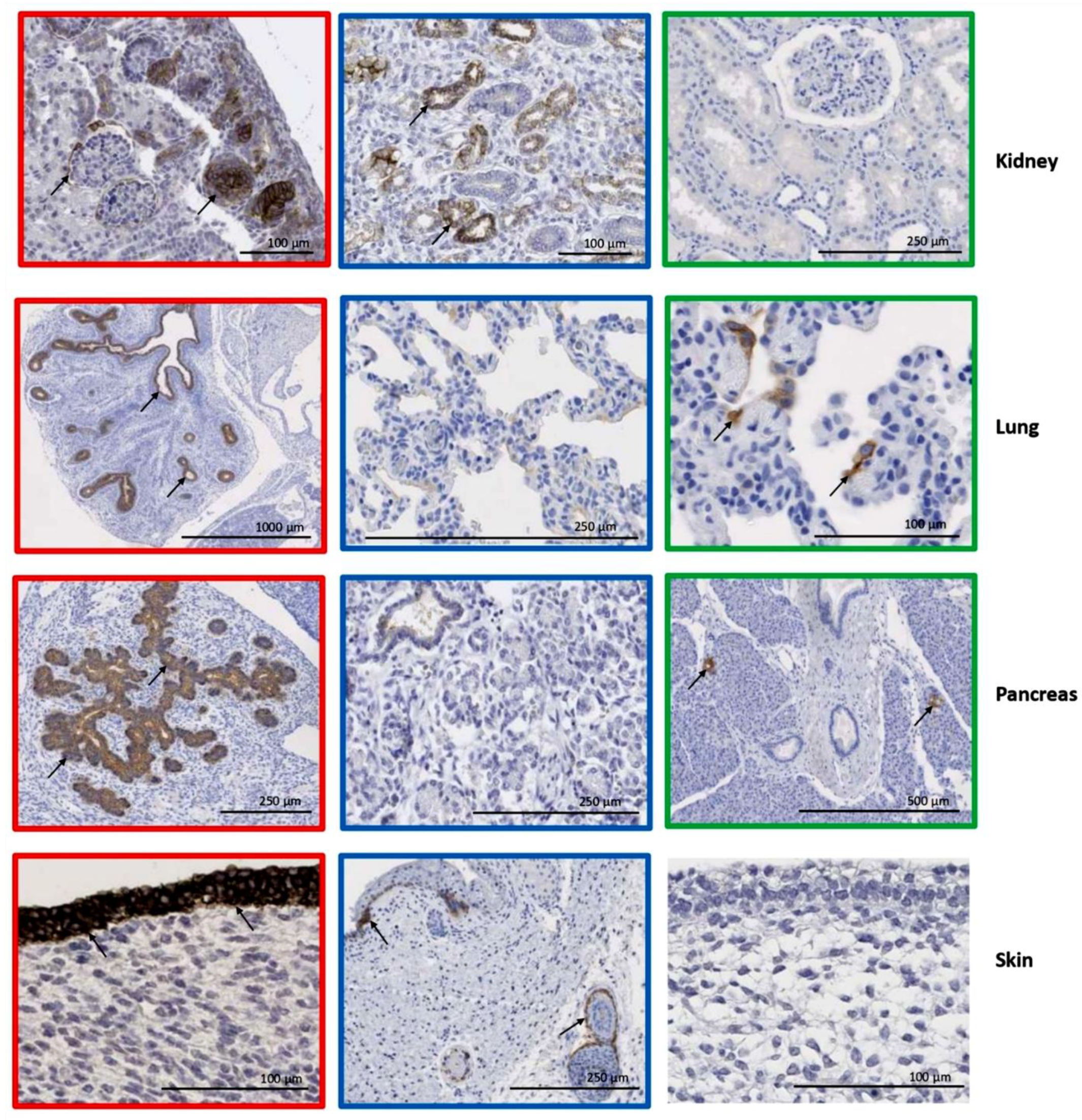
| Localization/Differentiation | Prenatal Development (n = 14) | Postnatal Development (n = 29) | ||||||
|---|---|---|---|---|---|---|---|---|
| I Trimester | II Trimester | III Trimester | 0–1 Mo | 1–12 Mo | 1–5 y | 6–12 y | 13–18 y | |
| Cortex | ||||||||
| Undifferentiated cells (blastema) | 1.1 ± 1.0 | 0 | 0 | 0 | - | - | - | - |
| Epithelial cells | ||||||||
| Tubuli | 81.3 ± 7.0 | 34.3 ± 21.5 | 10.7 ± 8.2 | 5.0 ± 0.5 | 7.3 ± 4.5 | 5.4 ± 2.7 | 0.2 ± 0.4 | 0 |
| Bowman’s capsule | 14.0 ± 8.5 | 9.3 ± 13.9 | 0 | 1.0 ± 0.3 | 0.3 ± 0.8 | 0 | 0 | 0 |
| Stromal cells | ||||||||
| Glomerular capillaries | 3.0 ± 0.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Connective tissue cells | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Medulla | ||||||||
| Epithelial cells | ||||||||
| Collecting Tubuli | 92.5 ± 3.5 | 34.3 ± 27.8 | 8.3 ± 2.9 | 5.0 ± 0.5 | 2.7 ± 3.3 | 0.7 ± 1.7 | 0 | 0 |
| Stromal cells | ||||||||
| Connective tissue cells | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
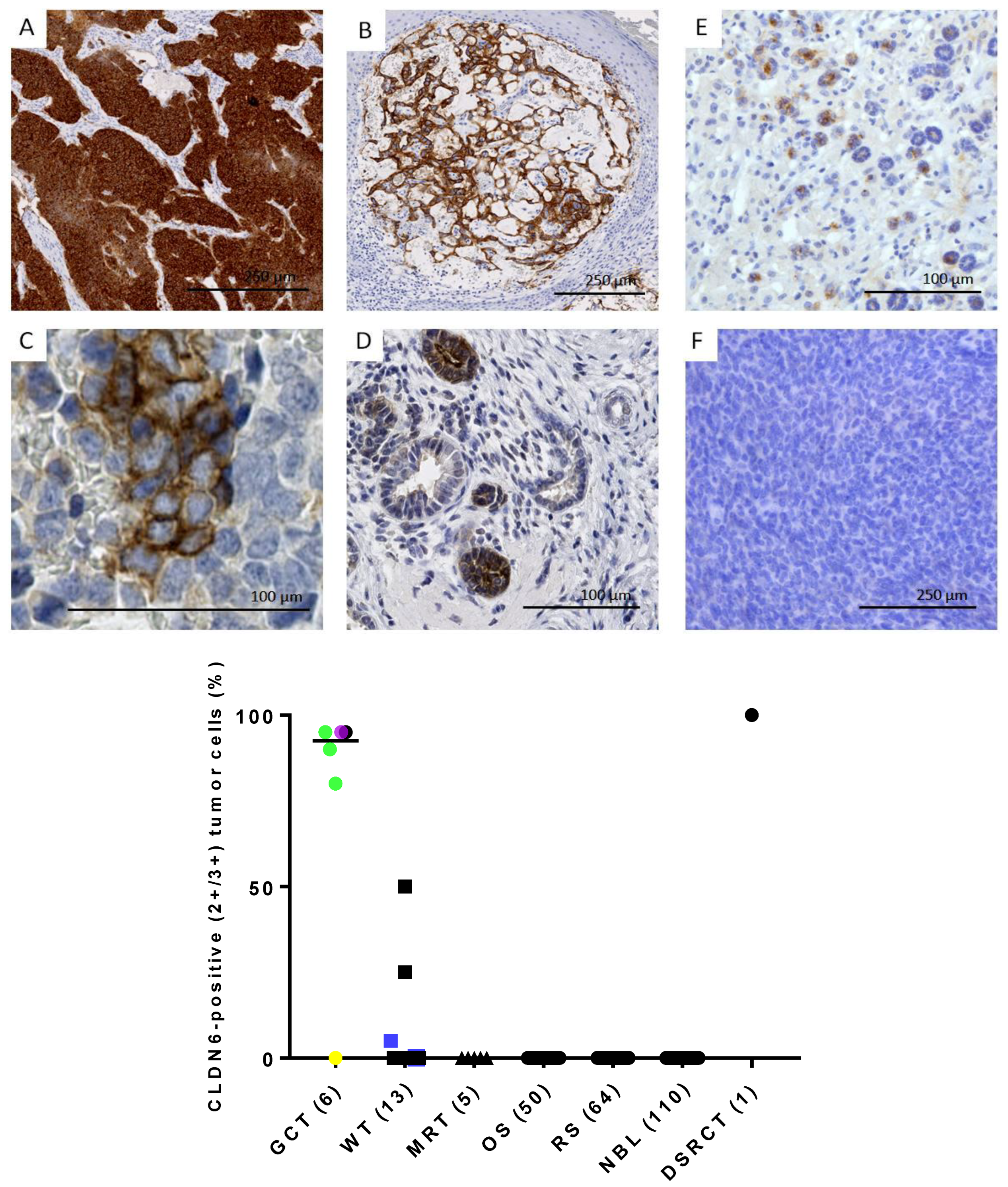
3.5. CLDN6 Is Expressed at the Protein Level in GCTs, DSRCTs and WTs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CLDN6 | Claudin 6 |
| DSRCT | desmoplastic small round cell tumor |
| GCT | Germ cell tumors |
| MRT | malignant rhabdoid tumors |
| WT | Wilms tumors |
| RMS | Rhabdomyosarcoma |
References
- Erdmann, F.; Kaatsch, P.; Grabow, D.; Spix, C. German Childhood Cancer Registry—Annual Report 2019 (1980–2018); Institute of Medical Biostatistics, Epidemiology and Informatics (IMBEI) at the University Medical Center of the Johannes Gutenberg University: Mainz, Germany, 2020. [Google Scholar]
- Ladenstein, R.; Potschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Yaniv, I.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): A multicentre, randomised, phase 3 trial. Lancet. Oncol. 2018, 19, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Minard-Colin, V.; Auperin, A.; Pillon, M.; Burke, G.A.A.; Barkauskas, D.A.; Wheatley, K.; Delgado, R.F.; Alexander, S.; Uyttebroeck, A.; Bollard, C.M.; et al. Rituximab for High-Risk, Mature B-Cell Non-Hodgkin's Lymphoma in Children. N. Engl. J. Med. 2020, 382, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- McLeod, C.; Gout, A.M.; Zhou, X.; Thrasher, A.; Rahbarinia, D.; Brady, S.W.; Macias, M.; Birch, K.; Finkelstein, D.; Sunny, J.; et al. St. Jude Cloud: A Pediatric Cancer Genomic Data-Sharing Ecosystem. Cancer Discov. 2021, 11, 1082–1099. [Google Scholar] [CrossRef]
- GTEx Consortium. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 2020, 369, 1318–1330. [Google Scholar] [CrossRef]
- Del Bufalo, F.; De Angelis, B.; Caruana, I.; Del Baldo, G.; De Ioris, M.A.; Serra, A.; Mastronuzzi, A.; Cefalo, M.G.; Pagliara, D.; Amicucci, M.; et al. GD2-CART01 for Relapsed or Refractory High-Risk Neuroblastoma. N. Engl. J. Med. 2023, 388, 1284–1295. [Google Scholar] [CrossRef]
- Majzner, R.G.; Ramakrishna, S.; Yeom, K.W.; Patel, S.; Chinnasamy, H.; Schultz, L.M.; Richards, R.M.; Jiang, L.; Barsan, V.; Mancusi, R.; et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 2022, 603, 934–941. [Google Scholar] [CrossRef]
- Flugel, C.L.; Majzner, R.G.; Krenciute, G.; Dotti, G.; Riddell, S.R.; Wagner, D.L.; Abou-El-Enein, M. Overcoming on-target, off-tumour toxicity of CAR T cell therapy for solid tumours. Nat. Rev. Clin. Oncol. 2023, 20, 49–62. [Google Scholar] [CrossRef]
- Turksen, K.; Troy, T.C. Claudin-6: A novel tight junction molecule is developmentally regulated in mouse embryonic epithelium. Dev. Dyn. 2001, 222, 292–300. [Google Scholar] [CrossRef]
- Abuazza, G.; Becker, A.; Williams, S.S.; Chakravarty, S.; Truong, H.T.; Lin, F.; Baum, M. Claudins 6, 9, and 13 are developmentally expressed renal tight junction proteins. Am. J. Physiol. Renal. Physiol. 2006, 291, F1132–F1141. [Google Scholar] [CrossRef]
- Reinhard, K.; Rengstl, B.; Oehm, P.; Michel, K.; Billmeier, A.; Hayduk, N.; Klein, O.; Kuna, K.; Ouchan, Y.; Woll, S.; et al. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science 2020, 367, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Mackensen, A.; Haanen, J.; Koenecke, C.; Alsdorf, W.; Wagner-Drouet, E.; Borchmann, P.; Heudobler, D.; Ferstl, B.; Klobuch, S.; Bokemeyer, C.; et al. CLDN6-specific CAR-T cells plus amplifying RNA vaccine in relapsed or refractory solid tumors: The phase 1 BNT211-01 trial. Nat. Med. 2023, 29, 2844–2853. [Google Scholar] [CrossRef] [PubMed]
- Stadler, C.R.; Ellinghaus, U.; Fischer, L.; Bahr-Mahmud, H.; Rao, M.; Lindemann, C.; Chaturvedi, A.; Scharf, C.; Biermann, I.; Hebich, B.; et al. Preclinical efficacy and pharmacokinetics of an RNA-encoded T cell-engaging bispecific antibody targeting human claudin 6. Sci. Transl. Med. 2024, 16, eadl2720. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.M.; Yankovich, T.; Le, P.; Martinez, D.; Santi, M.; Biegel, J.A.; Pawel, B.R.; Judkins, A.R. Claudin-6 is a nonspecific marker for malignant rhabdoid and other pediatric tumors. Am. J. Surg. Pathol. 2012, 36, 73–80. [Google Scholar] [CrossRef]
- Birks, D.K.; Kleinschmidt-DeMasters, B.K.; Donson, A.M.; Barton, V.N.; McNatt, S.A.; Foreman, N.K.; Handler, M.H. Claudin 6 is a positive marker for atypical teratoid/rhabdoid tumors. Brain Pathol. 2010, 20, 140–150. [Google Scholar] [CrossRef]
- Antonelli, M.; Hasselblatt, M.; Haberler, C.; Di Giannatale, A.; Garre, M.L.; Donofrio, V.; Lauriola, L.; Ridola, V.; Arcella, A.; Fruhwald, M.; et al. Claudin-6 is of limited sensitivity and specificity for the diagnosis of atypical teratoid/rhabdoid tumors. Brain Pathol. 2011, 21, 558–563. [Google Scholar] [CrossRef]
- Ushiku, T.; Shinozaki-Ushiku, A.; Maeda, D.; Morita, S.; Fukayama, M. Distinct expression pattern of claudin-6, a primitive phenotypic tight junction molecule, in germ cell tumours and visceral carcinomas. Histopathology 2012, 61, 1043–1056. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jager, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Qi, C.; Gong, J.; Li, J.; Liu, D.; Qin, Y.; Ge, S.; Zhang, M.; Peng, Z.; Zhou, J.; Cao, Y.; et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: Phase 1 trial interim results. Nat. Med. 2022, 28, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Liu, C.; Gong, J.; Liu, D.; Wang, X.; Zhang, P.; Qin, Y.; Ge, S.; Zhang, M.; Peng, Z.; et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: Phase 1 trial final results. Nat. Med. 2024, 30, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, X.; Li, Y.; Ruan, Y.; Lu, Y.; Yang, M.; Lin, D.; Song, P.; Guo, Y.; Zhao, S.; et al. DNA methylation of claudin-6 promotes breast cancer cell migration and invasion by recruiting MeCP2 and deacetylating H3Ac and H4Ac. J. Exp. Clin. Cancer Res. 2016, 35, 120. [Google Scholar] [CrossRef]
- Wang, L.; Xue, Y.; Shen, Y.; Li, W.; Cheng, Y.; Yan, X.; Shi, W.; Wang, J.; Gong, Z.; Yang, G.; et al. Claudin 6: A novel surface marker for characterizing mouse pluripotent stem cells. Cell Res. 2012, 22, 1082–1085. [Google Scholar] [CrossRef]
- Ben-David, U.; Nudel, N.; Benvenisty, N. Immunologic and chemical targeting of the tight-junction protein Claudin-6 eliminates tumorigenic human pluripotent stem cells. Nat. Commun. 2013, 4, 1992. [Google Scholar] [CrossRef]
- Kohmoto, T.; Masuda, K.; Shoda, K.; Takahashi, R.; Ujiro, S.; Tange, S.; Ichikawa, D.; Otsuji, E.; Imoto, I. Claudin-6 is a single prognostic marker and functions as a tumor-promoting gene in a subgroup of intestinal type gastric cancer. Gastric Cancer 2020, 23, 403–417. [Google Scholar] [CrossRef]
- Yang, M.; Li, Y.; Ruan, Y.; Lu, Y.; Lin, D.; Xie, Y.; Dong, B.; Dang, Q.; Quan, C. CLDN6 enhances chemoresistance to ADM via AF-6/ERKs pathway in TNBC cell line MDAMB231. Mol. Cell Biochem. 2018, 443, 169–180. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, C.; Li, Y.; Liu, K.; Zhao, Q.; Ouyang, L. Identification of Claudin-6 as a Molecular Biomarker in Pan-Cancer Through Multiple Omics Integrative Analysis. Front. Cell. Dev. Biol. 2021, 9, 726656. [Google Scholar] [CrossRef]
- Waqar, S.H.B.; Ali, H. Changing incidence and survival of desmoplastic small round cell tumor in the USA. Bayl. Univ. Med. Cent. Proc. 2022, 35, 415–419. [Google Scholar] [CrossRef]
- Scheer, M.; Vokuhl, C.; Blank, B.; Hallmen, E.; von Kalle, T.; Munter, M.; Wessalowski, R.; Hartwig, M.; Sparber-Sauer, M.; Schlegel, P.G.; et al. Desmoplastic small round cell tumors: Multimodality treatment and new risk factors. Cancer Med. 2019, 8, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, N.G. Desmoplastic small round cell tumor: II: An ultrastructural and immunohistochemical study with emphasis on new immunohistochemical markers. Am. J. Surg. Pathol. 1998, 22, 1314–1327. [Google Scholar] [CrossRef] [PubMed]
- Gedminas, J.M.; Chasse, M.H.; McBrairty, M.; Beddows, I.; Kitchen-Goosen, S.M.; Grohar, P.J. Desmoplastic small round cell tumor is dependent on the EWS-WT1 transcription factor. Oncogenesis 2020, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.T.; Calaminus, G.; Reinhard, H.; Gutjahr, P.; Kremens, B.; Harms, D.; Gobel, U. Primary mediastinal germ cell tumors in children and adolescents: Results of the German cooperative protocols MAKEI 83/86, 89, and 96. J. Clin. Oncol. 2000, 18, 832–839. [Google Scholar] [CrossRef]
- Frazier, A.L.; Hale, J.P.; Rodriguez-Galindo, C.; Dang, H.; Olson, T.; Murray, M.J.; Amatruda, J.F.; Thornton, C.; Arul, G.S.; Billmire, D.; et al. Revised risk classification for pediatric extracranial germ cell tumors based on 25 years of clinical trial data from the United Kingdom and United States. J. Clin. Oncol. 2015, 33, 195–201. [Google Scholar] [CrossRef]
- Arnold, M.A.; Schoenfield, L.; Limketkai, B.N.; Arnold, C.A. Diagnostic pitfalls of differentiating desmoplastic small round cell tumor (DSRCT) from Wilms tumor (WT): Overlapping morphologic and immunohistochemical features. Am. J. Surg. Pathol. 2014, 38, 1220–1226. [Google Scholar] [CrossRef]
- Armstrong, J.F.; Pritchard-Jones, K.; Bickmore, W.A.; Hastie, N.D.; Bard, J.B. The expression of the Wilms' tumour gene, WT1, in the developing mammalian embryo. Mech. Dev. 1993, 40, 85–97. [Google Scholar] [CrossRef]
- Miyagawa, K.; Kent, J.; Moore, A.; Charlieu, J.P.; Little, M.H.; Williamson, K.A.; Kelsey, A.; Brown, K.W.; Hassam, S.; Briner, J.; et al. Loss of WT1 function leads to ectopic myogenesis in Wilms’ tumour. Nat. Genet. 1998, 18, 15–17. [Google Scholar] [CrossRef]
- Jahn, N.; Terzer, T.; Strang, E.; Dolnik, A.; Cocciardi, S.; Panina, E.; Corbacioglu, A.; Herzig, J.; Weber, D.; Schrade, A.; et al. Genomic heterogeneity in core-binding factor acute myeloid leukemia and its clinical implication. Blood Adv. 2020, 4, 6342–6352. [Google Scholar] [CrossRef]
- McNulty, M.; Crispino, J.D. Acute Megakaryocytic Leukemia. Cold Spring Harb Perspect. Med. 2020, 10, a034884. [Google Scholar] [CrossRef]
- Hagen, S.J. Non-canonical functions of claudin proteins: Beyond the regulation of cell-cell adhesions. Tissue Barriers 2017, 5, e1327839. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Chin, V.; Fairfax, K.; Moutinho, C.; Suan, D.; Ji, H.; Powell, J.E. Transitioning single-cell genomics into the clinic. Nat. Rev. Genet. 2023, 24, 573–584. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.S.J.; O'Brien, N.A.; Hoffstrom, B.; Gong, K.; Lu, M.; Zhang, J.; Luo, T.; Liang, M.; Jia, W.; Hong, J.J.; et al. Preclinical Efficacy of the Antibody-Drug Conjugate CLDN6-23-ADC for the Treatment of CLDN6-Positive Solid Tumors. Clin. Cancer Res. 2023, 29, 2131–2143. [Google Scholar] [CrossRef] [PubMed]
- Stadler, C.R.; Bahr-Mahmud, H.; Plum, L.M.; Schmoldt, K.; Kolsch, A.C.; Tureci, O.; Sahin, U. Characterization of the first-in-class T-cell-engaging bispecific single-chain antibody for targeted immunotherapy of solid tumors expressing the oncofetal protein claudin 6. Oncoimmunology 2016, 5, e1091555. [Google Scholar] [CrossRef]
- Screnci, B.; Stafford, L.J.; Barnes, T.; Shema, K.; Gilman, S.; Wright, R.; Al Absi, S.; Phillips, T.; Azuelos, C.; Slovik, K.; et al. Antibody specificity against highly conserved membrane protein Claudin 6 driven by single atomic contact point. iScience 2022, 25, 105665. [Google Scholar] [CrossRef]
- Higashi, A.Y.; Higashi, T.; Furuse, K.; Ozeki, K.; Furuse, M.; Chiba, H. Claudin-9 constitutes tight junctions of folliculo-stellate cells in the anterior pituitary gland. Sci. Rep. 2021, 11, 21642. [Google Scholar] [CrossRef]
- Downing, J.R.; Wilson, R.K.; Zhang, J.; Mardis, E.R.; Pui, C.H.; Ding, L.; Ley, T.J.; Evans, W.E. The Pediatric Cancer Genome Project. Nat. Genet. 2012, 44, 619–622. [Google Scholar] [CrossRef]
- Newman, S.; Nakitandwe, J.; Kesserwan, C.A.; Azzato, E.M.; Wheeler, D.A.; Rusch, M.; Shurtleff, S.; Hedges, D.J.; Hamilton, K.V.; Foy, S.G.; et al. Genomes for Kids: The Scope of Pathogenic Mutations in Pediatric Cancer Revealed by Comprehensive DNA and RNA Sequencing. Cancer Discov. 2021, 11, 3008–3027. [Google Scholar] [CrossRef]
- Rusch, M.; Nakitandwe, J.; Shurtleff, S.; Newman, S.; Zhang, Z.; Edmonson, M.N.; Parker, M.; Jiao, Y.; Ma, X.; Liu, Y.; et al. Clinical cancer genomic profiling by three-platform sequencing of whole genome, whole exome and transcriptome. Nat. Commun. 2018, 9, 3962. [Google Scholar] [CrossRef]
- Schwartz, J.R.; Ma, J.; Kamens, J.; Westover, T.; Walsh, M.P.; Brady, S.W.; Robert Michael, J.; Chen, X.; Montefiori, L.; Song, G.; et al. The acquisition of molecular drivers in pediatric therapy-related myeloid neoplasms. Nat. Commun. 2021, 12, 985. [Google Scholar] [CrossRef]
| Nr | Tissues | qRT-PCR | Immunohistochemistry | |||
|---|---|---|---|---|---|---|
| <1 y | <1 y | 1–5 y | 6–12 y | 13–18 y | ||
| 1 | Adipose tissue skin | 4 | 2 | 2 | 2 | |
| 2 | Adipose tissue visceral | 1 | 1 | 2 | 2 | 2 |
| 3 | Appendix | 1 | 1 | 1 | 1 | 1 |
| 4 | Connective tissue visceral | 2 | 2 | 2 | ||
| 5 | Blood vessels | 1 | 1 | 2 (n = 2) | 2 | 2 |
| 6 | Adrenal gland | 1 | 2 (n = 2) | 2 | 2 | |
| 7 | Bladder | |||||
| 8 | Bone Marrow | 2 (n = 2) | 2 | 2 | 2 | 2 |
| 9 | Bone growth plate | 2 (n = 2) | 2 | 2 | 2 | |
| 10 | Colon | 2 | 2 | 2 | 2 | 2 |
| 11 | Duodenum | 2 | 1 | 1 | 1 | |
| 12 | Esophagus | 2 (n = 2) | 2 (n = 2) | 1 | 1 | 1 |
| 13 | Epididymis | 1 | 2 | 2 | 2 | 2 |
| 14 | Fallopian tube | 2 (n = 2) | 1 | 1 | 1 | |
| 15 | Gall Bladder | 1 | 2 | 1 | 1 | 1 |
| 16 | Gastrointestinal tract | |||||
| 17 | Heart | 3 (n = 2) | 2 | 4 | 4 | 4 |
| 18 | Ileum | 1 | 1 | 1 | 1 | 1 |
| 19 | Kidney Cortex | 1 | 2 | 2 | 2 | 2 |
| 20 | Kidney Medulla | 2 (n = 2) | 2 | 2 | 2 | 2 |
| 21 | Kidney | |||||
| 22 | Liver | 2 | 2 | 2 | 2 | |
| 23 | Lung | 2 (n = 2) | 2 | 2 | 2 | 2 |
| 24 | Lymph node | 2 | 2 | 2 | 2 | |
| 25 | Nerve | 1 | 2 (n = 2) | 2 (n = 2) | 2 | 2 |
| 26 | Ovary | 2 (n = 2) | 2 | 2 | ||
| 27 | Pancreas | 2 (n = 2) | 2 | 2 | 2 | |
| 28 | Placenta 8w | 2 (n = 2) | ||||
| 29 | Placenta 40W | 2 | ||||
| 30 | Prostate | 2 | 1 | |||
| 31 | Rectum | 2 (n = 2) | ||||
| 32 | Renal pelvis | 1 | ||||
| 33 | Retina | 1 | ||||
| 34 | Salivary Gland | 2 | 1 | 1 | 1 | |
| 35 | Skin | 2 (n = 2) | 2 (n = 2) | 2 | 2 | 2 |
| 36 | Small intestine | 2 | 2 | 2 | ||
| 37 | Spleen | 2 (n = 2) | 2 (n = 2) | 1 | 1 | 1 |
| 38 | Stomach | 1 | 2 (n = 2) | 1 | 1 | 1 |
| 39 | Smooth muscle | 4 (n = 3) | 1 | 1 | 1 | |
| 40 | Skeleat muscle | 5 (n = 5) | 2 (n = 2) | 2 (n = 2) | 2 (n = 2) | 2 |
| 41 | Striated muscle | |||||
| 42 | Testis | 3 (n = 3) | 2 | 2 | 2 | 2 |
| 43 | Thymus | 2 (n = 2) | 2 | 2 | 2 | 2 |
| 44 | Thyroid | 2 (n = 2) | 2 | 2 | 2 | |
| 45 | Tibia | |||||
| 46 | Tonsil | 2 (n = 2) | 2 | 2 | 2 | |
| 47 | Trachea | 1 | 1 | 1 | 1 | |
| 48 | Ureter | 2 (n = 2) | 2 | 2 | 2 (n = 2) | 2 |
| 49 | Uterus | 2 (n = 2) | 2 (n = 2) | 2 | 2 | |
| 50 | Vagina | 2 | 2 (n = 2) | 2 | 2 | |
| 51 | Bronchus | 1 | 1 | 2 | 1 | |
| 52 | Sigmoid colon | 2 (n = 2) | ||||
| qRT–PCR | IHC | ||||
|---|---|---|---|---|---|
| Entity | Tissues | Age | Tissues | Age | Comment |
| extracranial germ cells tumors | 13 (n = 11) | 8 (0–9) | 7 (n = 5) | 4 (1–14) | One sample from relapse |
| nephroblastoma | 20 (n = 15) | 3 (0–12) | 13(n = 10) | 6 (1–10) | Three samples from metastasis, two samples from relapse |
| malignant rhabdoid tumor | 5 (n = 2) | 2 | 5 (n = 4) | 2 (1–16) | |
| Ewing sarcoma | 9 (n = 8) | 14 (4–16) | 0 | ||
| osteosarcoma | 11 (n = 8) | 16 (10–20) | 50 (n = 12) | 16 (11–21) | Eleven samples from lung metastasis, 12 samples from relapse (six soft tissue periprosthetic, four lung, two thoracic wall and lung) |
| rhabdomyosarcoma | 8 (n = 6) | 4.5 (1–13) | 64 (n = 21) | 5 (1–15) | one sample from lymphnode metastasis |
| neuroblastoma | 16 (n = 13) | 2.5 (0–10) | 110 (n = 24) | 1 (0–12) | Twenty-nine samples from metastasis, 18 samples from relapse |
| Other tumorentities: | 34 (n = 25) | ||||
| paraganglioma | 2 (n = 2) | 11.5 (9–14) | 0 | ||
| liver sarcoma | 2 (n = 1) | 15 | 0 | ||
| adamantinoma | 2 (n = 1) | 4 | 0 | ||
| hepatoblastoma | 6 (n = 4) | 5.5 | 0 | ||
| adrenocortical tumor | 2 (n = 2) | 7 (3–11) | 0 | ||
| pleuroplumonary blastoma | 6 (n = 1) | 5 (5–6) | 0 | ||
| neurinoma | 1 | 15 | 0 | ||
| fibromyxoid sarcoma | 1 | 14 | 0 | ||
| undifferentiated sarcoma | 2 (n = 2) | 15 (1–16) | 0 | ||
| fibrosarcoma | 1 | 1 (4) | 0 | ||
| HCC | 1 | 17 | 0 | ||
| kidney cell carcinoma | 1 | 11 | 0 | ||
| mesoblastic nephroma | 3 (n = 3) | 1 (1–10) | 2 (n = 2) | 1 | |
| GIST | 1 | 16 | 0 | ||
| neuroendocrine tumor | 1 | 11 | 0 | ||
| DSRCT | 1 | 16 | 1 | 16 | |
| nephroblastomatosis | 1 | 3 | 1 | 3 | |
| Localization (n) | <10 w | 0<1 y | 1–5 y | 6–12 y | 13–18 y | Localization | <10 w | 0<1 y | 1–5 y | 6–12 y | 13–18 y |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Epithelium | Lymphoid tissue | ||||||||||
| Esophagus (5) | Spleen (5) | ||||||||||
| Intestinum (20) | 100% | Tonsils (8) | |||||||||
| Liver ducts (9) | 90% | 1–4% | 0–1% | Thymus (8) | |||||||
| Liver hepatocytes (9) | 10% | Lymph nodes (8) | |||||||||
| Gall bladder (5) | |||||||||||
| Pancreas ducts (7) | 100% | 2% | 0–2% | Other tissue | |||||||
| Pancreas acini (6) | 2–5% | 0–1% | Heart (8) | ||||||||
| Pancreas islets (6) | Adipose tissue (10) | ||||||||||
| Lung (9) | 100% | 0–2% | 0–4% | Connective tissue (8) | |||||||
| Bronchus (5) | Smooth muscle (9) | ||||||||||
| Kidney (41) | 88% | 0–5% | Skeletal muscle (9) | ||||||||
| Prostate (3) | Peripheral nerves (7) | ||||||||||
| Ureter (8) | Endothelium | 1–11% | |||||||||
| Salivary Gland (5) | Blood cells | ||||||||||
| Skin (9) | 100% | 5–10% | 0–2% | ||||||||
| Mesothelial cells (1) | 50% | ||||||||||
| Thyroid gland (8) | 0–1% | ||||||||||
| Adrenal gland (8) | |||||||||||
| Ovary (6) | |||||||||||
| Testis (8) | 1% | 1–3% | |||||||||
| Epididymis (8) | |||||||||||
| Uterus (8) | |||||||||||
| Fallopian tube (5) | |||||||||||
| Vagina (8) | |||||||||||
| Placenta (1) | 100% | ||||||||||
| Yolk sac (1) | 50% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seidmann, L.; Wingerter, A.; Oliver Metzig, M.; Bornas, A.; El Malki, K.; Ustjanzew, A.; Ortmüller, F.; Kamyshanskiy, Y.; Kindler, T.; Laible, M.; et al. The Chimeric Antigen Receptor T Cell Target Claudin 6 Is a Marker for Early Organ-Specific Epithelial Progenitors and Is Expressed in Some Pediatric Solid Tumor Entities. Cancers 2025, 17, 920. https://doi.org/10.3390/cancers17060920
Seidmann L, Wingerter A, Oliver Metzig M, Bornas A, El Malki K, Ustjanzew A, Ortmüller F, Kamyshanskiy Y, Kindler T, Laible M, et al. The Chimeric Antigen Receptor T Cell Target Claudin 6 Is a Marker for Early Organ-Specific Epithelial Progenitors and Is Expressed in Some Pediatric Solid Tumor Entities. Cancers. 2025; 17(6):920. https://doi.org/10.3390/cancers17060920
Chicago/Turabian StyleSeidmann, Larissa, Arthur Wingerter, Marie Oliver Metzig, Angelina Bornas, Khalifa El Malki, Arsenij Ustjanzew, Franziska Ortmüller, Yevgeniy Kamyshanskiy, Thomas Kindler, Mark Laible, and et al. 2025. "The Chimeric Antigen Receptor T Cell Target Claudin 6 Is a Marker for Early Organ-Specific Epithelial Progenitors and Is Expressed in Some Pediatric Solid Tumor Entities" Cancers 17, no. 6: 920. https://doi.org/10.3390/cancers17060920
APA StyleSeidmann, L., Wingerter, A., Oliver Metzig, M., Bornas, A., El Malki, K., Ustjanzew, A., Ortmüller, F., Kamyshanskiy, Y., Kindler, T., Laible, M., Mohr, X., Henninger, N., Russo, A., Beck, O., Alt, F., Wehling, P., Roth, W., Paret, C., & Faber, J. (2025). The Chimeric Antigen Receptor T Cell Target Claudin 6 Is a Marker for Early Organ-Specific Epithelial Progenitors and Is Expressed in Some Pediatric Solid Tumor Entities. Cancers, 17(6), 920. https://doi.org/10.3390/cancers17060920







