Optimizing Surgical Management of Anterior Skull Base Meningiomas: Imaging Modalities, Key Surgical Considerations, and Risk Mitigation Strategies
Simple Summary
Abstract
1. Introduction
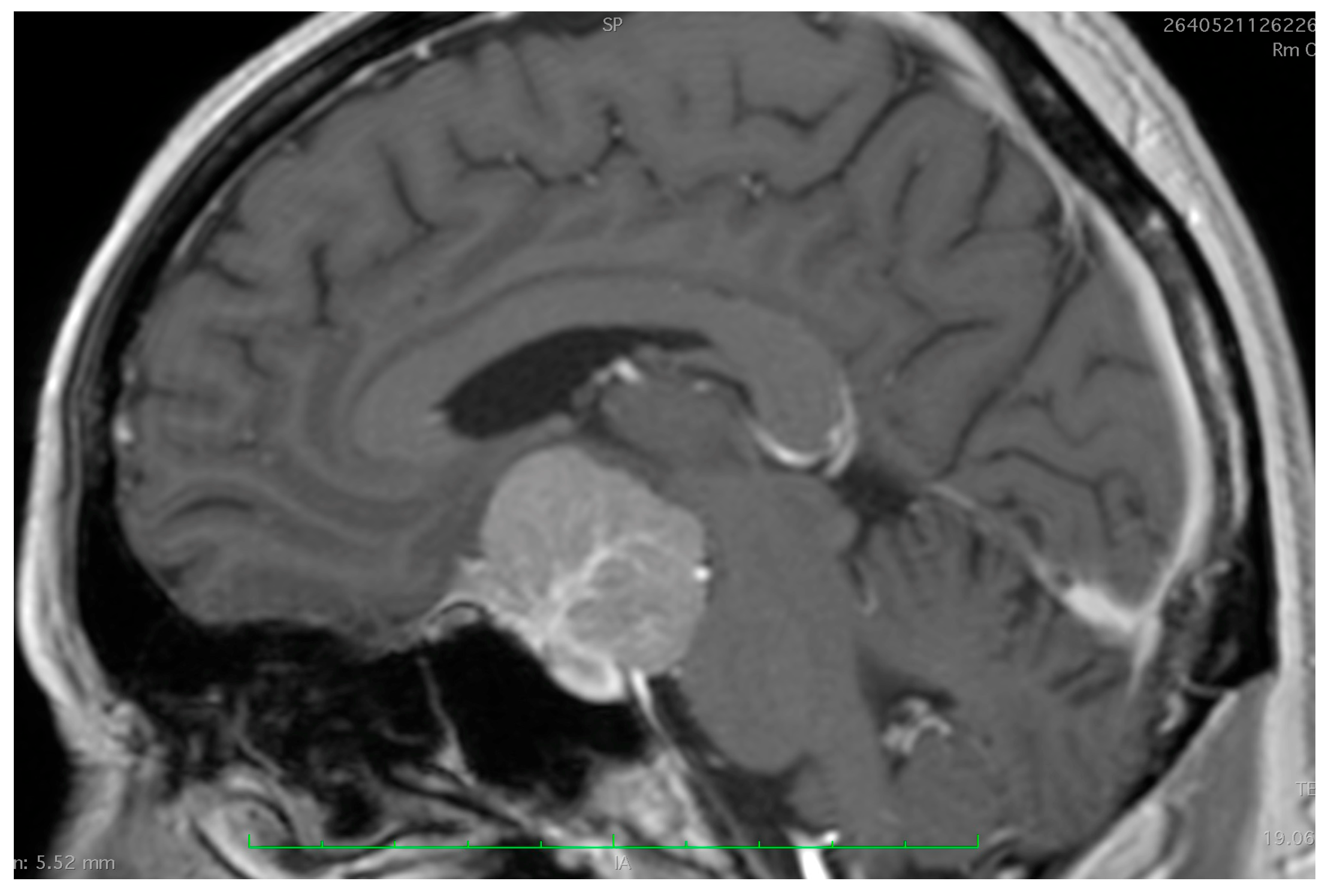
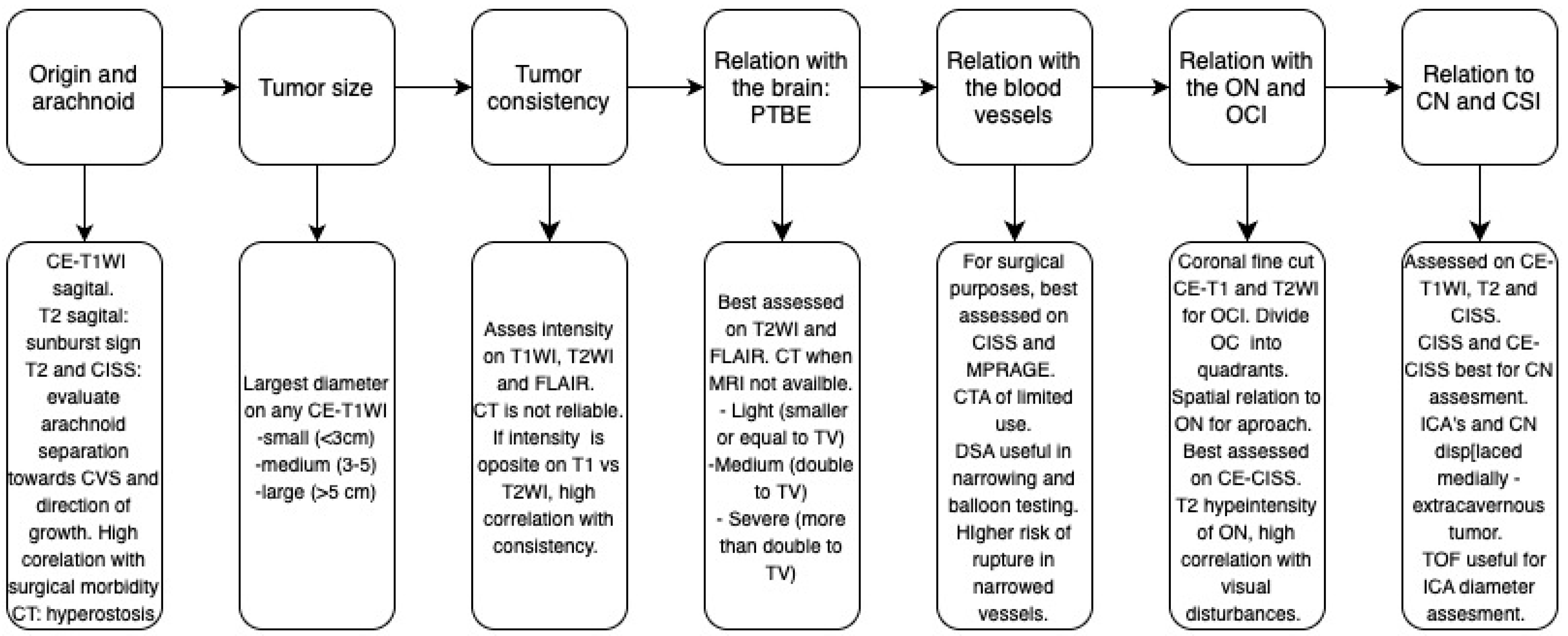
2. Assessment of Tumor Origin and the Presence of an Arachnoid Cleavage Plane
2.1. Surgical Significance
2.2. Imaging Evaluation
2.3. Key Surgical Considerations
- ○
- Basal devascularization: Initiating resection with basal devascularization at the tumor’s origin is a critical first step to achieving complete resection.
- ○
- Extradural devascularization: For clinoid and alar meningiomas approached through a frontolateral or pterional approach, this technique reduces blood loss and softens the tumor, facilitating removal.
- ○
- Intradural devascularization: In tuberculum and diaphragma sellae meningiomas, this should be performed only after identifying major regional anatomical structures.
- ○
- Follow the arachnoid: The arachnoid plane, its fibrous structures, and compartmentations direct the tumor growth and provide a pathway for tumor resection while protecting vascular and nerve structures covered by their own arachnoid.
- ○
- The choice between a “skull base” or “vascular” approach for these tumors remains a topic of ongoing debate [24]. In cases without significant extradural extension, the frontolateral approach is a highly suitable option for most tumors (Figure 3). It provides an optimal balance of exposure, versatility, and simplicity while minimizing the risks associated with more complex skull base techniques [32].
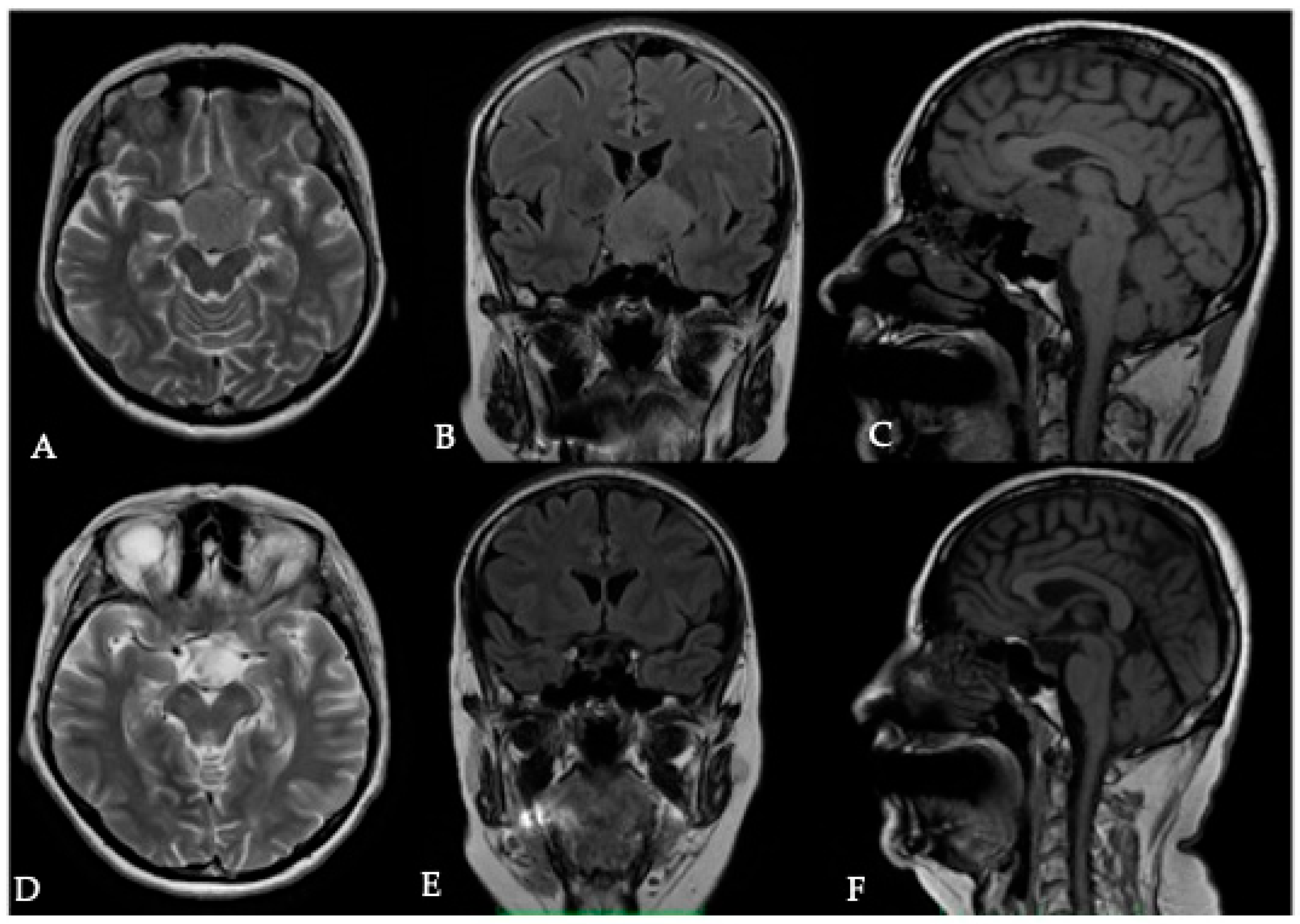
3. Tumor Size
3.1. Surgical Significance
3.2. Imaging Evaluation
3.3. Key Surgical Considerations
- ○
- Tumor size affects both intraoperative and postoperative management.
- ○
- Large meningiomas often show variable consistency: denser at the core/base and softer at the periphery.
- ○
- Larger tumors provide more workspace as volume decreases, allowing multi-directional approaches.
- ○
- Postoperative issues in large meningiomas include regional circulatory changes causing venous hemorrhages, ischemia, or cerebral edema.
- ○
- CSF compensates for the tumor space, potentially causing pseudo meningocele or CSF fistula. Issues are typically managed with lumbar drainage, compressive dressing, and diuretics; persistent hypersecretion may require subgaleo-peritoneal drainage.
4. Assessment of Tumor Consistency
4.1. Surgical Significance
4.2. Imaging Evaluation
4.3. Key Surgical Considerations
- ○
- Soft tumors: Associated with a higher chance of GTR. Risks include arterial vessel encasement—avoid arterial trunk coagulation until intratumoral trajectory is clear; strictly coagulate tumor feeders.
- ○
- Firm/calcified tumors: Pose challenges for volume reduction and dissection of adjacent structures; initial basal devascularization is critical, with calcified tumors requiring progressive drilling and immediate sealing of vascular canals with bone wax.
- ○
- In cases where injury to critical structures could lead to significant neurological deficits, leaving a small portion of the tumor on the affected tissue may be a safer approach.
5. Assessment of Peritumoral Brain Edema (PTBE)
5.1. Surgical Significance
5.2. Imaging Evaluation
5.3. Key Surgical Considerations
- ○
- The presence of PTBE should serve as an indicator of potential arachnoid disruption; exercise greater caution during tumor dissection from surrounding cerebral tissue.
- ○
- Infiltration of the pia mater, with a glial pseudocapsule and tortuous arterialized vessels, suggests a complex PTBE mechanism.
- ○
- Edema is a better indicator of a higher risk of postoperative neurological worsening rather than tumor resectability.
- ○
- A two-staged approach, where an initial surgery leaves a shell of the tumor in place and a second surgery for complete removal is performed after PTBE subsides, has been described for OGMs [55]. Its advantages are largely theoretical, as PTBE may persist for years even after the complete removal of a meningioma.
6. Vascular Encasement
6.1. Surgical Significance
6.2. Imaging Evaluation
6.3. Key Surgical Considerations
- ○
- Imaging suggests the tumor’s relationship with adjacent vessels, but intraoperative findings determine resectability without vascular injury.
- ○
- Tumors grow within their own space, displacing or partially encompassing arterial trunks, protected by the arachnoid layer. Progressive debulking allows the delicate mobilization of smaller fragments through windows created by vascular structures.
- ○
- Leave a thin tumor layer if detachment risks vascular damage.
- ○
- Perforating arteries, usually on the tumor surface, can be delicately detached within the arachnoid plane; avoid coagulation near perforator origins to prevent shrinkage.
- ○
- ACI injury causes severe bleeding and obstructs visibility. Temporary vessel clipping enables assessment and action: Direct vascular wall suturing is sometimes possible.
- ○
- Microclip placement for small lateral lacerations to stop bleeding is often more effective than emergency anastomosis. In cases in which this is necessary, the options include termino-terminal anastomosis for the ACA trunk and termino-lateral anastomosis for major arteries arising from the ICA.
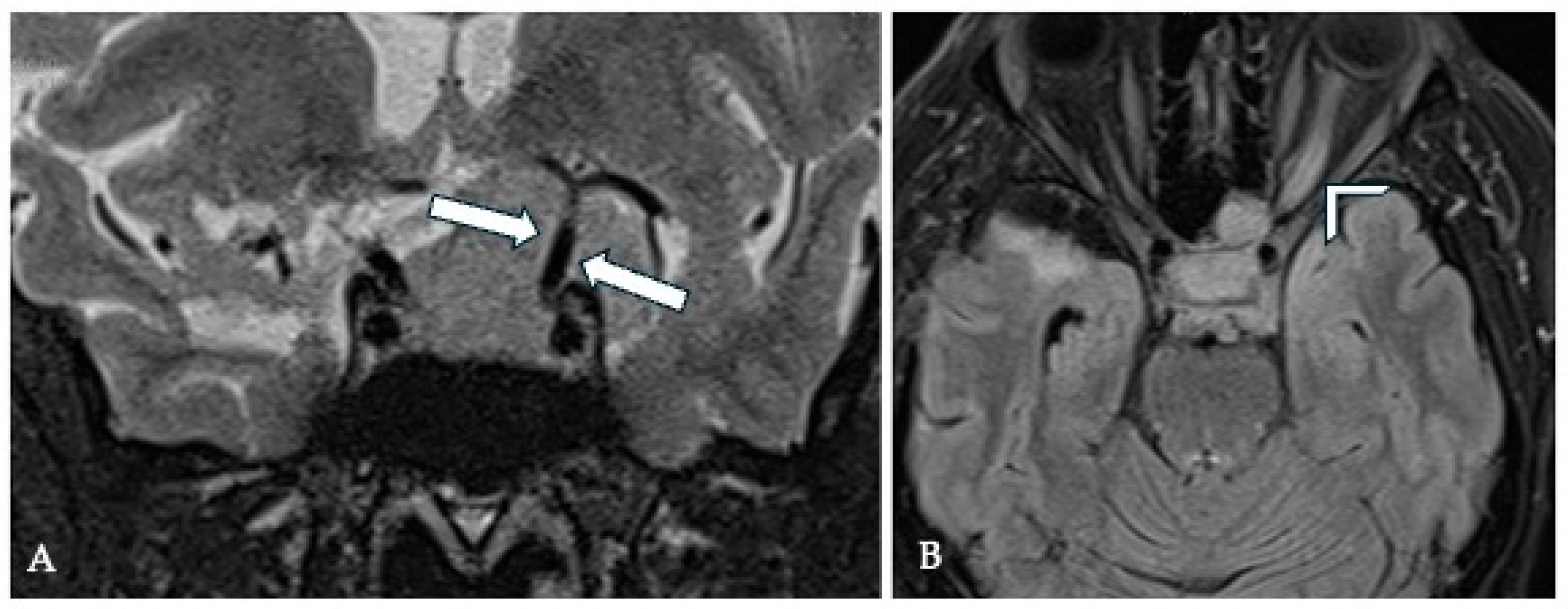
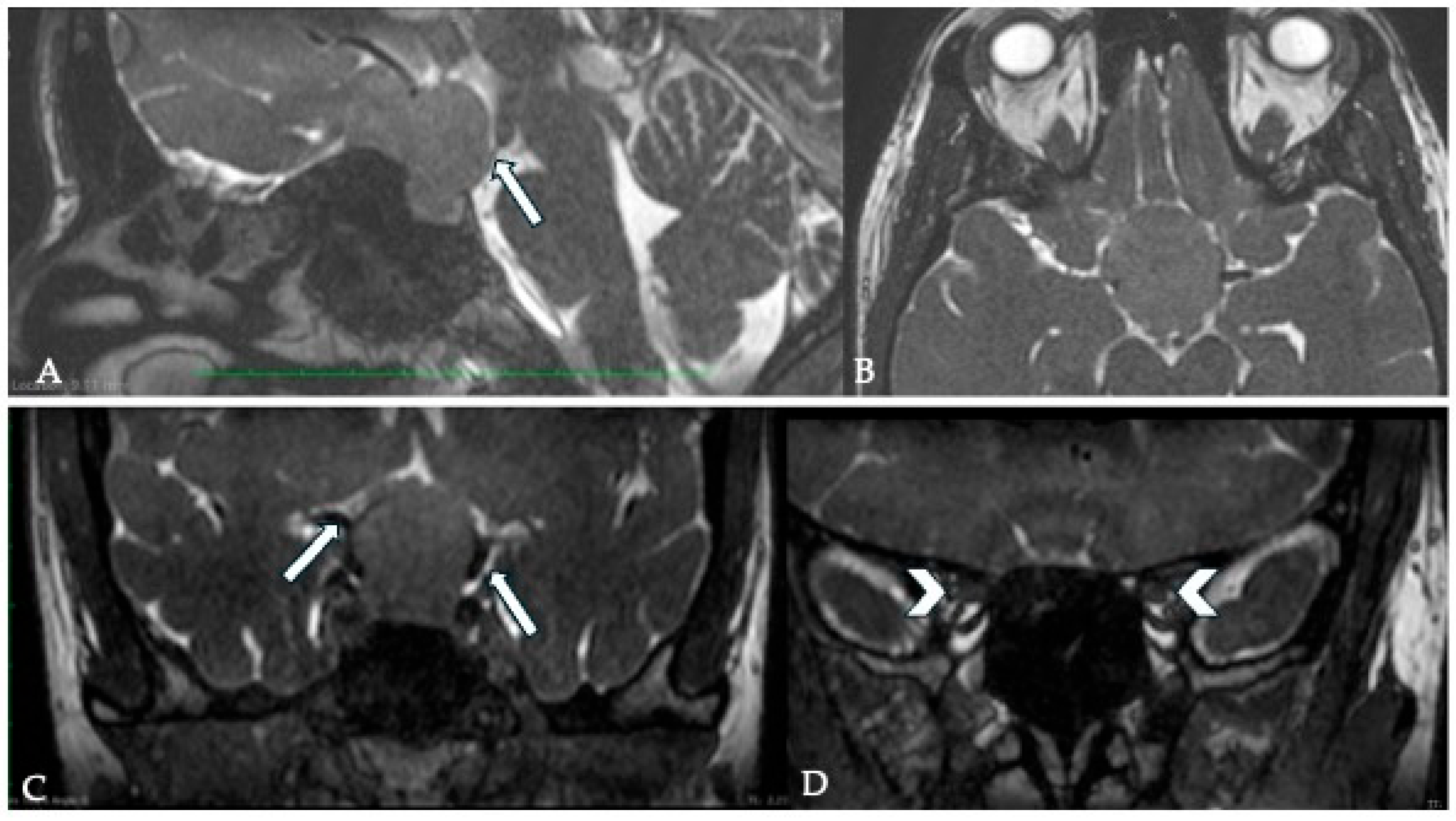
7. Optic Canal Involvement (OCI) and Optic Nerve (ON) Compression
7.1. Surgical Significance
7.2. Imaging Evaluation
7.3. Key Surgical Considerations
- ○
- The arachnoid at the interface with the ON should be preserved during surgery, as this has been linked to an improved visual recovery after surgery [20].
- ○
- Opening of the OC is essential for tumor extension into it but should be avoided routinely to prevent unnecessary complications.
- ○
- A bulging optic nerve suggests a fragment beneath it and warrants further inspection.
- ○
- Gentle canal opening enables detachment of intracanal tumor fragments, guided by the preserved arachnoid plane.
- ○
- Prolonged compression may erode the arachnoid layers, requiring canal opening and tumor mass reduction to protect the ON.
8. Cavernous Sinus Involvement (CSI)
8.1. Surgical Significance
8.2. Imaging Evaluation
8.3. Key Surgical Considerations
- ○
- Complete resection of intracavernous meningiomas is risky due to cranial nerve injury, ICA laceration, and cavernous sinus bleeding.
- ○
- Extracavernous portion reduction suffices for optimal neurological outcomes.
- ○
- Decompression via tumor tracking into the cavernous sinus relieves pain.
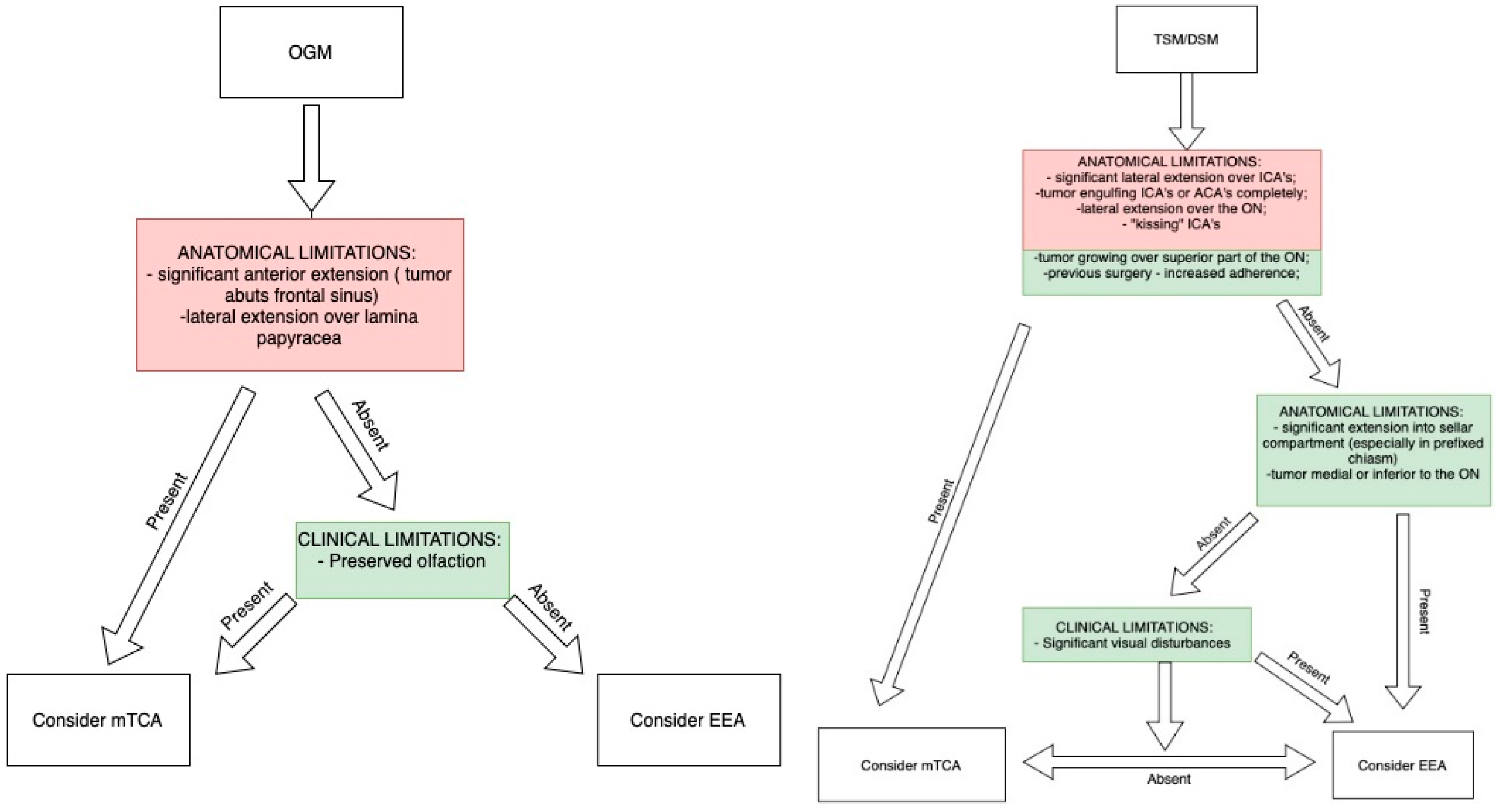
9. Importance of Preoperative Imaging and Clinical Characteristics for Approach Selection
10. Conclusions
- Basal and Extradural Devascularization: Initiate resection with basal devascularization. For clinoid and alar meningiomas, extradural devascularization minimizes blood loss and softens the tumor.
- Intradural Devascularization: Crucial for tuberculum and diaphragma sellae meningiomas after identifying key anatomical structures.
- Arachnoid-Guided Resection: Following the arachnoid plane protects critical vascular and nerve structures.
- Approach Selection: The frontolateral approach balances exposure and safety for tumors without significant extradural extension. The EEA is best for visual preservation in tumors without extensive lateral extension and without vascular encasement.
- Tumor Size: Larger tumors allow multi-directional resection but pose postoperative risks like edema and CSF leaks.
- Soft vs. Firm Tumors: Soft tumors have higher GTR rates but a higher risk of vessel encasement; firm/calcified tumors require patience, drilling, patience and cautious dissection.
- Peritumoral Brain Edema (PTBE): Indicates potential arachnoid disruption and increased risk of neurological deficits. Staged resection may be considered.
- Vascular Considerations: Tumors displace rather than invade vessels. Preserve the arachnoid layer, debulk progressively, and avoid excessive coagulation near perforators.
- Optic Nerve Protection: Preserve the arachnoid interface for better visual outcomes; open the optic canal only when necessary.
- Cavernous Sinus Involvement: Total resection is high-risk; extracavernous reduction suffices for symptom relief.
Author Contributions
Funding
Conflicts of Interest
References
- Rachinger, W.; Grau, S.; Tonn, J.-C. Different Microsurgical Approaches to Meningiomas of the Anterior Cranial Base. Acta Neurochir. 2010, 152, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Starnoni, D.; Tuleasca, C.; Giammattei, L.; Cossu, G.; Bruneau, M.; Berhouma, M.; Cornelius, J.F.; Cavallo, L.; Froelich, S.; Jouanneau, E.; et al. Surgical Management of Anterior Clinoidal Meningiomas: Consensus Statement on Behalf of the EANS Skull Base Section. Acta Neurochir. 2021, 163, 3387–3400. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.M.; Effendi, S.T.; DeMonte, F. Tuberculum Sellae Meningiomas: Evolving Surgical Strategies. Curr. Surg. Rep. 2014, 2, 73. [Google Scholar] [CrossRef]
- Bowers, C.A.; Altay, T.; Couldwell, W.T. Surgical Decision-Making Strategies in Tuberculum Sellae Meningioma Resection. Neurosurg. Focus 2011, 30, E1. [Google Scholar] [CrossRef]
- Watts, J.; Box, G.; Galvin, A.; Brotchie, P.; Trost, N.; Sutherland, T. Magnetic Resonance Imaging of Meningiomas: A Pictorial Review. Insights Imaging 2014, 5, 113–122. [Google Scholar] [CrossRef]
- Huang, R.Y.; Bi, W.L.; Griffith, B.; Kaufmann, T.J.; la Fougère, C.; Schmidt, N.O.; Tonn, J.C.; Vogelbaum, M.A.; Wen, P.Y.; Aldape, K.; et al. Imaging and Diagnostic Advances for Intracranial Meningiomas. Neuro Oncol. 2019, 21, i44–i61. [Google Scholar] [CrossRef]
- Sughrue, M.E.; Sayegh, E.T.; Parsa, A.T. Towards a Hypermodern Theory of Meningioma Surgery. Clin. Neurol. Neurosurg. 2014, 126, 69–75. [Google Scholar] [CrossRef]
- Al-Mefty, O. Clinoidal meningiomas. J. Neurosurg. 1990, 73, 840–849. [Google Scholar] [CrossRef]
- Xu, T.; Yan, Y.; Evins, A.I.; Gong, Z.; Jiang, L.; Sun, H.; Cai, L.; Wang, H.; Li, W.; Lu, Y.; et al. Anterior Clinoidal Meningiomas: Meningeal Anatomical Considerations and Surgical Implications. Front. Oncol. 2020, 10, 634. [Google Scholar] [CrossRef]
- Nanda, A.; Konar, S.K.; Maiti, T.K.; Bir, S.C.; Guthikonda, B. Stratification of Predictive Factors to Assess Resectability and Surgical Outcome in Clinoidal Meningioma. Clin. Neurol. Neurosurg. 2016, 142, 31–37. [Google Scholar] [CrossRef]
- Bassiouni, H.; Asgari, S.; Erol Sandalcioglu, I.; Seifert, V.; Stolke, D.; Marquardt, G. Anterior Clinoidal Meningiomas: Functional Outcome after Microsurgical Resection in a Consecutive Series of 106 Patients—Clinical Article. J. Neurosurg. 2009, 111, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.H.; McDermott, M.W. Tuberculum Sellae Meningiomas. Neurosurg. Focus 2003, 14, 1–6. [Google Scholar] [CrossRef]
- Ajlan, A.M.; Choudhri, O.; Hwang, P.; Harsh, G. Meningiomas of the Tuberculum and Diaphragma Sellae. J. Neurol. Surg. B Skull Base 2015, 76, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Chotai, S.; Yi, L.; Jun, P.; Qi, S. Tuberculum Sellae Meningiomas—Radiological Classification and Its Clinical Significance. J. Neurol. Surg. B Skull Base 2014, 75, A089. [Google Scholar] [CrossRef]
- Goel, A.; Ch, M.; Muzumdar, D.; Ketan, I.; Desai, I. Tuberculum sellae meningioma: A report on management on the basis of a surgical experience with 70 patients. Neurosurgery 2002, 51, 1358–1364. [Google Scholar] [CrossRef]
- Mortazavi, M.M.; Da Silva, H.B.; Ferreira, M.; Barber, J.K.; Pridgeon, J.S.; Sekhar, L.N. Planum Sphenoidale and Tuberculum Sellae Meningiomas: Operative Nuances of a Modern Surgical Technique with Outcome and Proposal of a New Classification System. World Neurosurg. 2016, 86, 270–286. [Google Scholar] [CrossRef]
- Nanda, A.; Javalkar, V.; Banerjee, A.D. Petroclival Meningiomas: Study on Outcomes, Complications and Recurrence Rates. J. Neurosurg. 2011, 114, 1268–1277. [Google Scholar] [CrossRef]
- Sekhar, L.N.; Wright, D.C.; Richardson, R.; Monacci, W. Petroclival and Foramen Magnum Meningiomas: Surgical Approaches and Pitfalls. J. Neurooncol. 1996, 29, 249–259. [Google Scholar] [CrossRef]
- Carlson, A.P.; Stippler, M.; Myers, O. Predictive Factors for Vision Recovery after Optic Nerve Decompression for Chronic Compressive Neuropathy: Systematic Review and Meta-Analysis. J. Neurol. Surg. B Skull Base 2013, 74, 20–38. [Google Scholar] [CrossRef]
- Kimura, Y.; Wanibuchi, M.; Akiyama, Y.; Mikami, T.; Mikuni, N. Preserved Arachnoid Membrane Acts as a Predictor of Postoperative Visual Improvement in Clinoidal Meningioma. Clin. Neurol. Neurosurg. 2021, 208, 106874. [Google Scholar] [CrossRef]
- Jallo, G.I.; Benjamin, V. Tuberculum sellae meningiomas: Microsurgical anatomy and surgical technique. Neurosurgery 2002, 51, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Florian, I.S.; Ungureanu, G.; Florian, A. The Role of the Basal Cisterns in the Development of Posterior Fossa Skull Base Meningiomas. Rom. Neurosurg. 2016, 30, 321–328. [Google Scholar] [CrossRef]
- Pamir, M.N.; Belirgen, M.; Özduman, K.; Kiliç, T.; Özek, M. Anterior Clinoidal Meningiomas: Analysis of 43 Consecutive Surgically Treated Cases. Acta Neurochir. 2008, 150, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Giammattei, L.; Starnoni, D.; Cossu, G.; Bruneau, M.; Cavallo, L.M.; Cappabianca, P.; Meling, T.R.; Jouanneau, E.; Schaller, K.; Benes, V.; et al. Surgical Management of Tuberculum Sellae Meningiomas: Myths, Facts, and Controversies. Acta Neurochir. 2020, 162, 631–640. [Google Scholar] [CrossRef]
- Goyal, N.; Kakkar, A.; Sarkar, C.; Agrawal, D. Does Bony Hyperostosis in Intracranial Meningioma Signify Tumor Invasion? A Radio-Pathologic Study. Neurol. India 2012, 60, 50. [Google Scholar] [CrossRef]
- Pieper, D.R.; Al-Mefty, O.; Hanada, Y.; Buechner, D. Hyperostosis Associated with Meningioma of the Cranial Base: Secondary Changes or Tumor Invasion. Neurosurgery 1999, 44, 742–746. [Google Scholar] [CrossRef]
- May, M.; Sedlak, V.; Pecen, L.; Priban, V.; Buchvald, P.; Fiedler, J.; Vaverka, M.; Lipina, R.; Reguli, S.; Malik, J.; et al. Role of Risk Factors, Scoring Systems, and Prognostic Models in Predicting the Functional Outcome in Meningioma Surgery: Multicentric Study of 552 Skull Base Meningiomas. Neurosurg. Rev. 2023, 46, 124. [Google Scholar] [CrossRef]
- Hingwala, D.; Chatterjee, S.; Kesavadas, C.; Thomas, B.; Kapilamoorthy, T.R. Applications of 3D CISS Sequence for Problem Solving in Neuroimaging. Indian J. Radiol. Imaging 2011, 21, 90–97. [Google Scholar] [CrossRef]
- Watanabe, K.; Kakeda, S.; Yamamoto, J.; Watanabe, R.; Nishimura, J.; Ohnari, N.; Nishizawa, S.; Korogi, Y. Delineation of Optic Nerves and Chiasm in Close Proximity to Large Suprasellar Tumors with Contrast-Enhanced FIESTA MR Imaging. Radiology 2012, 264, 852–858. [Google Scholar] [CrossRef]
- Xie, T.; Zhang, X.-B.; Yun, H.; Hu, F.; Yu, Y.; Gu, Y. 3D-FIESTA MR Images Are Useful in the Evaluation of the Endoscopic Expanded Endonasal Approach for Midline Skull-Base Lesions. Acta Neurochir. 2011, 153, 12–18. [Google Scholar] [CrossRef]
- Yamamoto, J.; Kakeda, S.; Takahashi, M.; Aoyama, Y.; Soejima, Y.; Saito, T.; Akiba, D.; Korogi, Y.; Nishizawa, S. Dural Attachment of Intracranial Meningiomas: Evaluation with Contrast-Enhanced Three-Dimensional Fast Imaging with Steady-State Acquisition (FIESTA) at 3 T. Neuroradiology 2011, 53, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Samii, M.; Gerganov, V.M. Surgery of Extra-Axial Tumors of the Cerebral Base. Neurosurgery 2008, 62, SHC1153–SHC1168. [Google Scholar] [CrossRef]
- GOEL, A.; GUPTA, S.; DESAI, K. New Grading System to Predict Resectability of Anterior Clinoid Meningiomas. Neurol. Med. Chir. 2000, 40, 610–617. [Google Scholar] [CrossRef]
- Rosenstein, J.; Symon, L. Surgical Management of Suprasellar Meningioma. J. Neurosurg. 1984, 61, 642–648. [Google Scholar] [CrossRef]
- Gregorius, F.K.; Hepler, R.S.; Stern, W.E. Loss and Recovery of Vision with Suprasellar Meningiomas. J. Neurosurg. 1975, 42, 69–75. [Google Scholar] [CrossRef]
- Nakamura, M.; Roser, F.; Struck, M.; Vorkapic, P.; Samii, M. Tuberculum Sellae Meningiomas: Clinical Outcome Considering Different Surgical Approaches. Neurosurgery 2006, 59, 1019–1028. [Google Scholar] [CrossRef]
- Nanda, A.; Ambekar, S.; Javalkar, V.; Sharma, M. Technical Nuances in the Management of Tuberculum Sellae and Diaphragma Sellae Meningiomas. Neurosurg. Focus 2013, 35, E7. [Google Scholar] [CrossRef]
- Liu, D.; Yuan, X.; Liu, Q.; Jiang, X.; Jiang, W.; Peng, Z.; Ding, X.; Luo, D. Large Medial Sphenoid Wing Meningiomas: Long-Term Outcome and Correlation with Tumor Size after Microsurgical Treatment in 127 Consecutive Cases. Turk. Neurosurg. 2012, 22, 547–557. [Google Scholar] [CrossRef]
- Mathiesen, T.; Kihlström, L. Visual Outcome of Tuberculum Sellae Meningiomas after Extradural Optic Nerve Decompression. Neurosurgery 2006, 59, 570–576. [Google Scholar] [CrossRef]
- Chokyu, I.; Goto, T.; Ishibashi, K.; Nagata, T.; Ohata, K. Bilateral Subfrontal Approach for Tuberculum Sellae Meningiomas in Long-Term Postoperative Visual Outcome. J. Neurosurg. 2011, 115, 802–810. [Google Scholar] [CrossRef]
- Roberti, F.; Sekhar, L.N.; Kalavakonda, C.; Wright, D.C. Posterior Fossa Meningiomas: Surgical Experience in 161 Cases. Surg. Neurol. 2001, 56, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Zada, G.; Yashar, P.; Robison, A.; Winer, J.; Khalessi, A.; Mack, W.J.; Giannotta, S.L. A Proposed Grading System for Standardizing Tumor Consistency of Intracranial Meningiomas. Neurosurg. Focus 2013, 35, E1. [Google Scholar] [CrossRef] [PubMed]
- Kendall, B.; Pullicino, P. Comparison of Consistency of Meningiomas and CT Appearances. Neuroradiology 1979, 18, 173–176. [Google Scholar] [CrossRef]
- Sitthinamsuwan, B.; Khampalikit, I.; Nunta-aree, S.; Srirabheebhat, P.; Witthiwej, T.; Nitising, A. Predictors of Meningioma Consistency: A Study in 243 Consecutive Cases. Acta Neurochir. 2012, 154, 1383–1389. [Google Scholar] [CrossRef]
- Meyer, F.; Hoover, J.; Morris, J. Use of Preoperative Magnetic Resonance Imaging T1 and T2 Sequences to Determine Intraoperative Meningioma Consistency. Surg. Neurol. Int. 2011, 2, 142. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Leever, J.; Chamoun, R. Predicting Consistency of Meningioma by Magnetic Resonance Imaging. J. Neurol. Surg. B Skull Base 2015, 76, 225–229. [Google Scholar] [CrossRef]
- Shiroishi, M.S.; Cen, S.Y.; Tamrazi, B.; D’Amore, F.; Lerner, A.; King, K.S.; Kim, P.E.; Law, M.; Hwang, D.H.; Boyko, O.B.; et al. Predicting Meningioma Consistency on Preoperative Neuroimaging Studies. Neurosurg. Clin. N. Am. 2016, 27, 145–154. [Google Scholar] [CrossRef]
- Hughes, J.D.; Fattahi, N.; Van Gompel, J.; Arani, A.; Meyer, F.; Lanzino, G.; Link, M.J.; Ehman, R.; Huston, J. Higher-Resolution Magnetic Resonance Elastography in Meningiomas to Determine Intratumoral Consistency. Neurosurgery 2015, 77, 653–659. [Google Scholar] [CrossRef]
- Almefty, R.; Dunn, I.F.; Pravdenkova, S.; Abolfotoh, M.; Al-Mefty, O. True Petroclival Meningiomas: Results of Surgical Management. Clinical Article. J. Neurosurg. 2014, 120, 40–51. [Google Scholar] [CrossRef]
- Carvalho, G.A.; Matthies, C.; Tatagiba, M.; Eghbal, R.; Samii, M. Impact of Computed Tomographic and Magnetic Resonance Imaging Findings on Surgical Outcome in Petroclival Meningiomas. Neurosurgery 2000, 47, 1287–1294; discussion 1294–1295. [Google Scholar] [CrossRef]
- Seifert, V. Clinical Management of Petroclival Meningiomas and the Eternal Quest for Preservation of Quality of Life: Personal Experiences over a Period of 20 Years. Acta Neurochir. 2010, 152, 1099–1116. [Google Scholar] [CrossRef] [PubMed]
- Berhouma, M.; Jacquesson, T.; Jouanneau, E.; Cotton, F. Pathogenesis of Peri-Tumoral Edema in Intracranial Meningiomas. Neurosurg. Rev. 2019, 42, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Kshettry, V.R.; Selman, W.R.; Bambakidis, N.C. Peritumoral Brain Edema in Intracranial Meningiomas: The Emergence of Vascular Endothelial Growth Factor-Directed Therapy. Neurosurg. Focus 2013, 35, E2. [Google Scholar] [CrossRef] [PubMed]
- Ahmeti, H.; Caliebe, A.; Röcken, C.; Jansen, O.; Mehdorn, M.H.; Synowitz, M. Impact of Peritumoral Brain Edema on Pre- and Postoperative Clinical Conditions and on Long-Term Outcomes in Patients with Intracranial Meningiomas. Eur. J. Med. Res. 2023, 28, 40. [Google Scholar] [CrossRef]
- Marquardt, G.; Quick-Weller, J.; Tritt, S.; Baumgarten, P.; Senft, C.; Seifert, V. Two-Step Staged Resection of Giant Olfactory Groove Meningiomas. Acta Neurochir. 2021, 163, 3425–3431. [Google Scholar] [CrossRef]
- Sapkota, M.R.; Yang, Z.; Zhu, D.; Zhang, Y.; Yuan, T.; Gao, J.; Si, T.; Wang, J. Evaluation of Epidemiologic Factors, Radiographic Features, and Pathologic Findings for Predicting Peritumoral Brain Edema in Meningiomas. J. Magn. Reson. Imaging 2020, 52, 174–182. [Google Scholar] [CrossRef]
- Nakano, T.; Asano, K.; Miura, H.; Itoh, S.; Suzuki, S. Meningiomas with Brain Edema. Clin. Imaging 2002, 26, 243–249. [Google Scholar] [CrossRef]
- Tamiya, T.; Ono, Y.; Matsumoto, K.; Ohmoto, T. Peritumoral Brain Edema in Intracranial Meningiomas: Effects of Radiological and Histological Factors. Neurosurgery 2001, 49, 1046–1052. [Google Scholar] [CrossRef]
- Domingues, P.H.; Sousa, P.; Otero, Á.; Gonçalves, J.M.; Ruiz, L.; De Oliveira, C.; Lopes, M.C.; Orfao, A.; Tabernero, M.D. Proposal for a New Risk Stratification Classification for Meningioma Based on Patient Age, WHO Tumor Grade, Size, Localization, and Karyotype. Neuro Oncol. 2014, 16, 735–747. [Google Scholar] [CrossRef]
- Ungureanu, G.; Serban, L.-N.; Beni, L.; Florian, S.-I. Enhancing Patient Comprehension in Skull-Base Meningioma Surgery through 3D Volumetric Reconstructions: A Cost-Effective Approach. J. Pers. Med. 2024, 14, 982. [Google Scholar] [CrossRef]
- Khan, D.Z.; Muskens, I.S.; Mekary, R.A.; Zamanipoor Najafabadi, A.H.; Helmy, A.E.; Reisch, R.; Broekman, M.L.D.; Marcus, H.J. The Endoscope-Assisted Supraorbital “Keyhole” Approach for Anterior Skull Base Meningiomas: An Updated Meta-Analysis. Acta Neurochir. 2021, 163, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Shiferaw, M.Y.; Baleh, A.S.; Gizaw, A.; Teklemariam, T.L.; Aklilu, A.T.; Awedew, A.F.; Anley, D.T.; Mekuria, B.H.; Yesuf, E.F.; Yigzaw, M.A.; et al. Predictors of Operative Ischemic Cerebrovascular Complications in Skull Base Tumor Resections: Experience in Low-Resource Setting. Neurooncol. Pract. 2024, 11, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Van Der Veken, J.; Simons, M.; Mulcahy, M.J.; Wurster, C.; Harding, M.; Van Velthoven, V. The Surgical Management of Intraoperative Intracranial Internal Carotid Artery Injury in Open Skull Base Surgery—A Systematic Review. Neurosurg. Rev. 2022, 45, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- AlQahtani, A.; London, N.R.; Castelnuovo, P.; Locatelli, D.; Stamm, A.; Cohen-Gadol, A.A.; Elbosraty, H.; Casiano, R.; Morcos, J.; Pasquini, E.; et al. Assessment of Factors Associated With Internal Carotid Injury in Expanded Endoscopic Endonasal Skull Base Surgery. JAMA Otolaryngol.—Head Neck Surg. 2020, 146, 364. [Google Scholar] [CrossRef]
- Ishikawa, M.; Nishi, S.; Aoki, T.; Takase, T.; Wada, E.; Oowaki, H.; Katsuki, T.; Fukuda, H. Predictability of Internal Carotid Artery (ICA) Dissectability in Cases Showing ICA Involvement in Parasellar Meningioma. J. Clin. Neurosci. 2001, 8 (Suppl. S1), 22–25. [Google Scholar] [CrossRef]
- Ungureanu, G.; Florian, A.; Florian, S.I. The Impact of Arachnoid Structures on Skull-Base Meningioma Surgical Management: A Radiological Analysis and Narrative Review. J. Med. Life 2024, 17, 682–689. [Google Scholar] [CrossRef]
- Heth, J.A.; Al-Mefty, O. Cavernous Sinus Meningiomas. Neurosurg. Focus 2003, 14, 1–9. [Google Scholar] [CrossRef]
- Guggenberger, K.; Krafft, A.J.; Ludwig, U.; Vogel, P.; Elsheik, S.; Raithel, E.; Forman, C.; Dovi-Akué, P.; Urbach, H.; Bley, T.; et al. High-Resolution Compressed-Sensing T1 Black-Blood MRI. Clin. Neuroradiol. 2021, 31, 207–216. [Google Scholar] [CrossRef]
- Seol, H.J.; Park, H.Y.; Nam, D.H.; Kong, D.S.; Lee, J.I.; Kim, J.H.; Park, K. Clinical Outcomes of Tuberculum Sellae Meningiomas Focusing on Reversibility of Postoperative Visual Function. Acta Neurochir. 2013, 155, 25–31. [Google Scholar] [CrossRef]
- Margalit, N.; Kesler, A.; Ezer, H.; Freedman, S.; Ram, Z. Tuberculum and Diaphragma Sella Meningioma—Surgical Technique and Visual Outcome in a Series of 20 Cases Operated over a 2.5-Year Period. Acta Neurochir. 2007, 149, 1199–1204. [Google Scholar] [CrossRef]
- Zevgaridis, D.; Medele, R.J.; Müller, A.; Hischa, A.C.; Steiger, H.-J. Meningiomas of the Sellar Region Presenting with Visual Impairment: Impact of Various Prognostic Factors on Surgical Outcome in 62 Patients. Acta Neurochir. 2001, 143, 471–476. [Google Scholar] [CrossRef]
- Song, S.W.; Kim, Y.H.; Kim, J.W.; Park, C.K.; Kim, J.E.; Kim, D.G.; Koh, Y.C.; Jung, H. won Outcomes After Transcranial and Endoscopic Endonasal Approach for Tuberculum Meningiomas—A Retrospective Comparison. World Neurosurg. 2018, 109, e434–e445. [Google Scholar] [CrossRef] [PubMed]
- Sade, B.; Lee, J.H. High Incidence of Optic Canal Involvement in Tuberculum Sellae Meningiomas: Rationale for Aggressive Skull Base Approach. Surg. Neurol. 2009, 72, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.W.; You, W.; Guo, A.S.; Lin, Z.R.; Wang, Y.Z. Efficiency and Safety of Optic Canal Unroofing in Tuberculum Sellae Meningiomas: A Meta-Analysis and Systematic Review. Neurosurg. Rev. 2023, 46, 240. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.; Nader, R.; Al-Mefty, O. Optic Canal Involvement in Tuberculum Sellae Meningiomas. Oper. Neurosurg. 2010, 67, ons108–ons119. [Google Scholar] [CrossRef]
- Taha, A.N.M.; Erkmen, K.; Dunn, I.F.; Pravdenkova, S.; Al-Mefty, O. Meningiomas Involving the Optic Canal: Pattern of Involvement and Implications for Surgical Technique. Neurosurg. Focus 2011, 30, E12. [Google Scholar] [CrossRef]
- Nimmannitya, P.; Goto, T.; Terakawa, Y.; Sato, H.; Kawashima, T.; Morisako, H.; Ohata, K. Characteristic of Optic Canal Invasion in 31 Consecutive Cases with Tuberculum Sellae Meningioma. Neurosurg. Rev. 2016, 39, 691–697. [Google Scholar] [CrossRef]
- Spektor, S.; Dotan, S.; Mizrahi, C.J. Safety of Drilling for Clinoidectomy and Optic Canal Unroofing in Anterior Skull Base Surgery. Acta Neurochir. 2013, 155, 1017–1024. [Google Scholar] [CrossRef]
- Mariniello, G.; De Divitiis, O.; Bonavolontà, G.; Maiuri, F. Surgical Unroofing of the Optic Canal and Visual Outcome in Basal Meningiomas. Acta Neurochir. 2013, 155, 77–84. [Google Scholar] [CrossRef]
- Bassiouni, H.; Asgari, S.; Stolke, D. Tuberculum Sellae Meningiomas: Functional Outcome in a Consecutive Series Treated Microsurgically. Surg. Neurol. 2006, 66, 37–44. [Google Scholar] [CrossRef]
- Inoue, K.; Seker, A.; Osawa, S.; Alencastro, L.F.; Matsushima, T.; Rhoton, A.L. Microsurgical and endoscopic anatomy of the supratentorial arachnoidal membranes and cisterns. Neurosurgery 2009, 65, 644–665. [Google Scholar] [CrossRef] [PubMed]
- Lü, J. Arachnoid Membrane: The First and Probably the Last Piece of the Roadmap. Surg. Radiol. Anat. 2015, 37, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hong, S.H.; Cho, Y.H.; Kim, J.H.; Kim, C.J. Anatomical Origin of Tuberculum Sellae Meningioma: Off-Midline Location and Its Clinical Implications. World Neurosurg. 2016, 89, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Makarenko, S.; Carreras, E.M.; Akagami, R. Craniotomy for Perisellar Meningiomas: Comparison of Simple (Appropriate for Endoscopic Approach) versus Complex Anatomy and Surgical Outcomes. J. Neurosurg. 2017, 126, 1191–1200. [Google Scholar] [CrossRef]
- Borghei-Razavi, H.; Lee, J.; Ibrahim, B.; Muhsen, B.A.; Raghavan, A.; Wu, I.; Poturalski, M.; Stock, S.; Karakasis, C.; Adada, B.; et al. Accuracy and Interrater Reliability of CISS Versus Contrast-Enhanced T1-Weighted VIBE for the Presence of Optic Canal Invasion in Tuberculum Sellae Meningiomas. World Neurosurg. 2021, 148, e502–e507. [Google Scholar] [CrossRef]
- Hayashi, Y.; Kita, D.; Fukui, I.; Sasagawa, Y.; Oishi, M.; Tachibana, O.; Ueda, F.; Nakada, M. Preoperative Evaluation of the Interface Between Tuberculum Sellae Meningioma and the Optic Nerves on Fast Imaging with Steady-State Acquisition for Extended Endoscopic Endonasal Transsphenoidal Surgery. World Neurosurg. 2017, 103, 153–160. [Google Scholar] [CrossRef]
- Sindou, M.; Wydh, E.; Jouanneau, E.; Nebbal, M.; Lieutaud, T. Long-Term Follow-up of Meningiomas of the Cavernous Sinus after Surgical Treatment Alone. J. Neurosurg. 2007, 107, 937–944. [Google Scholar] [CrossRef]
- Abdel Aziz, K.M.; Froelich, S.C.; Dagnew, E.; Jean, W.; Breneman, J.C.; Zuccarello, M.; van Loveren, H.R.; Tew, J.M. Large sphenoid wing meningiomas involving the cavernous sinus: Conservative surgical strategies for better functional outcomes. Neurosurgery 2004, 54, 1375–1384. [Google Scholar] [CrossRef]
- DeMonte, F.; Smith, H.K.; Al-Mefty, O. Outcome of Aggressive Removal of Cavernous Sinus Meningiomas. J. Neurosurg. 1994, 81, 245–251. [Google Scholar] [CrossRef]
- Spiegelmann, R.; Cohen, Z.R.; Nissim, O.; Alezra, D.; Pfeffer, R. Cavernous Sinus Meningiomas: A Large LINAC Radiosurgery Series. J. Neurooncol. 2010, 98, 195–202. [Google Scholar] [CrossRef]
- Pamir, M.N.; Kılıç, T.; Bayraklı, F.; Peker, S. Changing Treatment Strategy of Cavernous Sinus Meningiomas: Experience of a Single Institution. Surg. Neurol. 2005, 64, S58–S66. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, A.; Tuleasca, C.; Liščák, R.; Motti, E.; Lindquist, C.; Radatz, M.; Gatterbauer, B.; Lippitz, B.E.; Martínez Álvarez, R.; Martínez Moreno, N.; et al. Stereotactic Radiosurgery for Benign Cavernous Sinus Meningiomas: A Multicentre Study and Review of the Literature. Cancers 2022, 14, 4047. [Google Scholar] [CrossRef] [PubMed]
- Corniola, M.V.; Roche, P.H.; Bruneau, M.; Cavallo, L.M.; Daniel, R.T.; Messerer, M.; Froelich, S.; Gardner, P.A.; Gentili, F.; Kawase, T.; et al. Management of Cavernous Sinus Meningiomas: Consensus Statement on Behalf of the EANS Skull Base Section. Brain Spine 2022, 2, 100864. [Google Scholar] [CrossRef]
- Masalha, W.; Heiland, D.H.; Steiert, C.; Krüger, M.T.; Schnell, D.; Heiland, P.; Bissolo, M.; Grosu, A.-L.; Schnell, O.; Beck, J.; et al. Management of Medial Sphenoid Wing Meningioma Involving the Cavernous Sinus: A Single-Center Series of 105 Cases. Cancers 2022, 14, 2201. [Google Scholar] [CrossRef]
- Kano, H.; Park, K.J.; Kondziolka, D.; Iyer, A.; Liu, X.; Tonetti, D.; Flickinger, J.C.; Lunsford, L.D. Does Prior Microsurgery Improve or Worsen the Outcomes of Stereotactic Radiosurgery for Cavernous Sinus Meningiomas? Neurosurgery 2013, 73, 401–410. [Google Scholar] [CrossRef]
- Hayashi, M.; Chernov, M.F.; Tamura, N.; Yomo, S.; Tamura, M.; Horiba, A.; Izawa, M.; Muragaki, Y.; Iseki, H.; Okada, Y.; et al. Usefulness of the Advanced Neuroimaging Protocol Based on Plain and Gadolinium-Enhanced Constructive Interference in Steady State Images for Gamma Knife Radiosurgery and Planning Microsurgical Procedures for Skull Base Tumors; Springer: Vienna, Austria, 2013; pp. 167–178. [Google Scholar]
- Cavallaro, M.; Coglitore, A.; Tessitore, A.; Galletta, K.; Frosina, L.; Cuffari, A.; Ingrassia, R.; Scarcella, S.C.; Caponnetto, M.; Longo, M.; et al. Three-Dimensional Constructive Interference in Steady State (3D CISS) Imaging and Clinical Applications in Brain Pathology. Biomedicines 2022, 10, 2997. [Google Scholar] [CrossRef]
- Blitz, A.M.; Macedo, L.L.; Chonka, Z.D.; Ilica, A.T.; Choudhri, A.F.; Gallia, G.L.; Aygun, N. High-Resolution Ciss Mr Imaging with and without Contrast for Evaluation of the Upper Cranial Nerves. Segmental Anatomy and Selected Pathologic Conditions of the Cisternal through Extraforaminal Segments. Neuroimaging Clin. N. Am. 2014, 24, 17–34. [Google Scholar] [CrossRef]
- Bladowska, J.; Biel, A.; Zimny, A.; Lubkowska, K.; Bednarek-Tupikowska, G.; Sozanski, T.; Zaleska-Dorobisz, U.; Sasiadek, M. Are T2-Weighted Images More Useful than T1-Weighted Contrast-Enhanced Images in Assessment of Postoperative Sella and Parasellar Region? Med. Sci. Monit. 2011, 17, MT83–MT90. [Google Scholar] [CrossRef]
- Micko, A.S.G.; Wöhrer, A.; Wolfsberger, S.; Knosp, E. Invasion of the Cavernous Sinus Space in Pituitary Adenomas: Endoscopic Verification and Its Correlation with an MRI-Based Classification. J. Neurosurg. 2015, 122, 803–811. [Google Scholar] [CrossRef]
- Youngerman, B.E.; Banu, M.A.; Gerges, M.M.; Odigie, E.; Tabaee, A.; Kacker, A.; Anand, V.K.; Schwartz, T.H. Endoscopic Endonasal Approach for Suprasellar Meningiomas: Introduction of a New Scoring System to Predict Extent of Resection and Assist in Case Selection with Long-Term Outcome Data. J. Neurosurg. 2021, 135, 113–125. [Google Scholar] [CrossRef]
- Mascarella, M.A.; Tewfik, M.A.; Aldosari, M.; Sirhan, D.; Zeitouni, A.; Di Maio, S. A Simple Scoring System to Predict the Resectability of Skull Base Meningiomas via an Endoscopic Endonasal Approach. World Neurosurg. 2016, 91, 582–591.e1. [Google Scholar] [CrossRef] [PubMed]
- Muskens, I.S.; Zamanipoor Najafabadi, A.H.; Broekman, M.L.D. The Endoscopic Endonasal Approach or Microscopic Transcranial Approach for Anterior Skull Base Meningiomas—It Is All about Right Indication Rather than Superiority. Acta Neurochir. 2020, 162, 75–76. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.J.; Jahangiri, A.; Garcia, R.M.; George, J.R.; Sughrue, M.E.; McDermott, M.W.; El-Sayed, I.H.; Aghi, M.K. Endoscopic Surgery for Tuberculum Sellae Meningiomas: A Systematic Review and Meta-Analysis. Neurosurg. Rev. 2013, 36, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, A.G.; Cappelletti, M.; Fazzolari, B.; Marotta, N.; Delfini, R. Frontobasal Midline Meningiomas: Is It Right To Shed Doubt on the Transcranial Approaches? Updates and Review of the Literature. World Neurosurg. 2016, 88, 374–382. [Google Scholar] [CrossRef]
- Roa Montes de Oca, J.C.; Gonçalves Estella, J.M.; Nieto-Librero, A.B.; Galindo-Villardón, P.; Roa Ramírez, C.J.; Gonçalves Sánchez, J.; Berhouma, M.; Cornelius, J.F.; Daniel, R.T.; Zazpe, I.; et al. Olfactory Groove Meningiomas: Comprehensive Assessment between the Different Microsurgical Transcranial Approaches and the Endoscopic Endonasal Approaches, Systematic Review and Metanalysis on Behalf of the EANS Skull Base Section. Brain Spine 2022, 2, 101661. [Google Scholar] [CrossRef]
- Muskens, I.S.; Briceno, V.; Ouwehand, T.L.; Castlen, J.P.; Gormley, W.B.; Aglio, L.S.; Zamanipoor Najafabadi, A.H.; van Furth, W.R.; Smith, T.R.; Mekary, R.A.; et al. The Endoscopic Endonasal Approach Is Not Superior to the Microscopic Transcranial Approach for Anterior Skull Base Meningiomas—A Meta-Analysis. Acta Neurochir. 2018, 160, 59–75. [Google Scholar] [CrossRef]
- Zamanipoor Najafabadi, A.H.; Khan, D.Z.; Muskens, I.S.; Broekman, M.L.D.; Dorward, N.L.; Van Furth, W.R.; Marcus, H.J. Trends in Cerebrospinal Fluid Leak Rates Following the Extended Endoscopic Endonasal Approach for Anterior Skull Base Meningioma: A Meta-Analysis over the Last 20 Years. Acta Neurochir. 2021, 163, 711–719. [Google Scholar] [CrossRef]
- Wang, E.W.; Gardner, P.A.; Zanation, A.M. International Consensus Statement on Endoscopic Skull-Base Surgery: Executive Summary. Int. Forum Allergy Rhinol. 2019, 9, S127–S144. [Google Scholar] [CrossRef]
- Kshettry, V.R.; Elshazly, K.; Evans, J.J. Endoscopic Transnasal Surgery for Planum and Tuberculum Sella Meningiomas: Decision-Making, Technique and Outcomes. CNS Oncol. 2016, 5, 211–222. [Google Scholar] [CrossRef]
- Nangarwal, B.; Gosal, J.S.; Das, K.K.; Khatri, D.; Bhaisora, K.; Verma, P.K.; Sardhara, J.; Mehrotra, A.; Srivastava, A.K.; Jaiswal, A.K.; et al. Anterior Skull Base Meningioma: Surgical Approach and Complication Avoidance. J. Neurol. Surg. B Skull Base 2023, 84, 038–050. [Google Scholar] [CrossRef]
- Mastantuoni, C.; Cavallo, L.M.; Esposito, F.; D’avella, E.; de Divitiis, O.; Somma, T.; Bocchino, A.; Fabozzi, G.L.; Cappabianca, P.; Solari, D. Midline Skull Base Meningiomas: Transcranial and Endonasal Perspectives. Cancers 2022, 14, 2878. [Google Scholar] [CrossRef] [PubMed]
- Ottenhausen, M.; Rumalla, K.; Alalade, A.F.; Nair, P.; La Corte, E.; Younus, I.; Forbes, J.A.; Nsir, A.B.; Banu, M.A.; Tsiouris, A.J.; et al. Decision-Making Algorithm for Minimally Invasive Approaches to Anterior Skull Base Meningiomas. Neurosurg. Focus 2018, 44, E7. [Google Scholar] [CrossRef] [PubMed]
- Sankhla, S.K.; Jayashankar, N.; Khan, M.A.; Khan, G.M. Surgical Management of Tuberculum Sellae Meningioma. Neurol. India 2021, 69, 1592–1600. [Google Scholar] [CrossRef]
- Schroeder, L.A.; Starreveld, Y.P. Outcomes of Endoscopic Endonasal Surgery for Tuberculum Sellae and Planum Sphenoidale Meningiomas: A Retrospective Study. Can. J. Neurol. Sci. J. Can. Des Sci. Neurol. 2024, 1–6. [Google Scholar] [CrossRef]
- Cohen, D.M.; Borghei-Razavi, H.; Kshettry, V.R.; Recinos, P.F. The Endoscopic Endonasal Approach or Microscopic Transcranial Approach for Anterior Skull Base Meningiomas—It Is All about Right Indication Rather than Superiority. Acta Neurochir. 2020, 162, 77–78. [Google Scholar] [CrossRef]
- Nuru, M.; Chandra, A.; Aghi, M.K. Combined Endoscopic Endonasal and Transcranial Approach to Complex Intracranial Lesions. In Integrated Management of Complex Intracranial Lesions; Cambridge University Press: Cambridge, UK, 2021; pp. 35–50. [Google Scholar]
- De Simone, M.; Zoia, C.; Choucha, A.; Kong, D.-S.; De Maria, L. The Transorbital Approach: A Comprehensive Review of Targets, Surgical Techniques, and Multiportal Variants. J. Clin. Med. 2024, 13, 2712. [Google Scholar] [CrossRef]
- Karımzada, G.; Evleksiz Karımzada, D.; Erol, G.; Gülsuna, B.; Kuzucu, P.; Güngör, A.; Kutlay, A.M.; Şahin, M.M.; Çeltikçi, E. Transorbital Neuroendoscopic Surgery for Treatment of Sphenoid Wing Meningiomas Extending to the Cavernous Sinus: Clinical Implications and a Technical Illustration. Neurosurg. Focus 2024, 56, E8. [Google Scholar] [CrossRef]


| OGM | TSM | |||
|---|---|---|---|---|
| EEA | mTCA | EEA | mTCA | |
| GTR | 70–80.7% | 84.7–98.1% | 79–88% | 87–92% |
| 30-day mortality | 0–4.2% | 0.3–3.9% | 0–1.7% | 0–1.7% |
| Visual Improvement | 64–87% | 12–82.2% | 71.9–92% | 57.9–71.9% |
| Olfaction Preservation | 0% | 17.8–29% | NA | NA |
| CSF Leak | 14.4–25.7% | 1.6–10.5% | 5.3–13.1 | 0–5.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungureanu, G.; Serban, L.-N.; Florian, S.-I. Optimizing Surgical Management of Anterior Skull Base Meningiomas: Imaging Modalities, Key Surgical Considerations, and Risk Mitigation Strategies. Cancers 2025, 17, 987. https://doi.org/10.3390/cancers17060987
Ungureanu G, Serban L-N, Florian S-I. Optimizing Surgical Management of Anterior Skull Base Meningiomas: Imaging Modalities, Key Surgical Considerations, and Risk Mitigation Strategies. Cancers. 2025; 17(6):987. https://doi.org/10.3390/cancers17060987
Chicago/Turabian StyleUngureanu, Gheorghe, Larisa-Nicoleta Serban, and Stefan-Ioan Florian. 2025. "Optimizing Surgical Management of Anterior Skull Base Meningiomas: Imaging Modalities, Key Surgical Considerations, and Risk Mitigation Strategies" Cancers 17, no. 6: 987. https://doi.org/10.3390/cancers17060987
APA StyleUngureanu, G., Serban, L.-N., & Florian, S.-I. (2025). Optimizing Surgical Management of Anterior Skull Base Meningiomas: Imaging Modalities, Key Surgical Considerations, and Risk Mitigation Strategies. Cancers, 17(6), 987. https://doi.org/10.3390/cancers17060987







