Evolution of Neo-RAS-WT in Circulating Tumor DNA from First-Line to Subsequent Therapies in Metastatic Colorectal Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Longitudinal Monitoring of RAS Status in Plasma ctDNA During First-Line Treatment
3.3. Longitudinal Monitoring of RAS Status in Plasma ctDNA During Second-Line Treatment
3.4. Longitudinal Monitoring of RAS Status in Plasma ctDNA During Third-Line Treatment
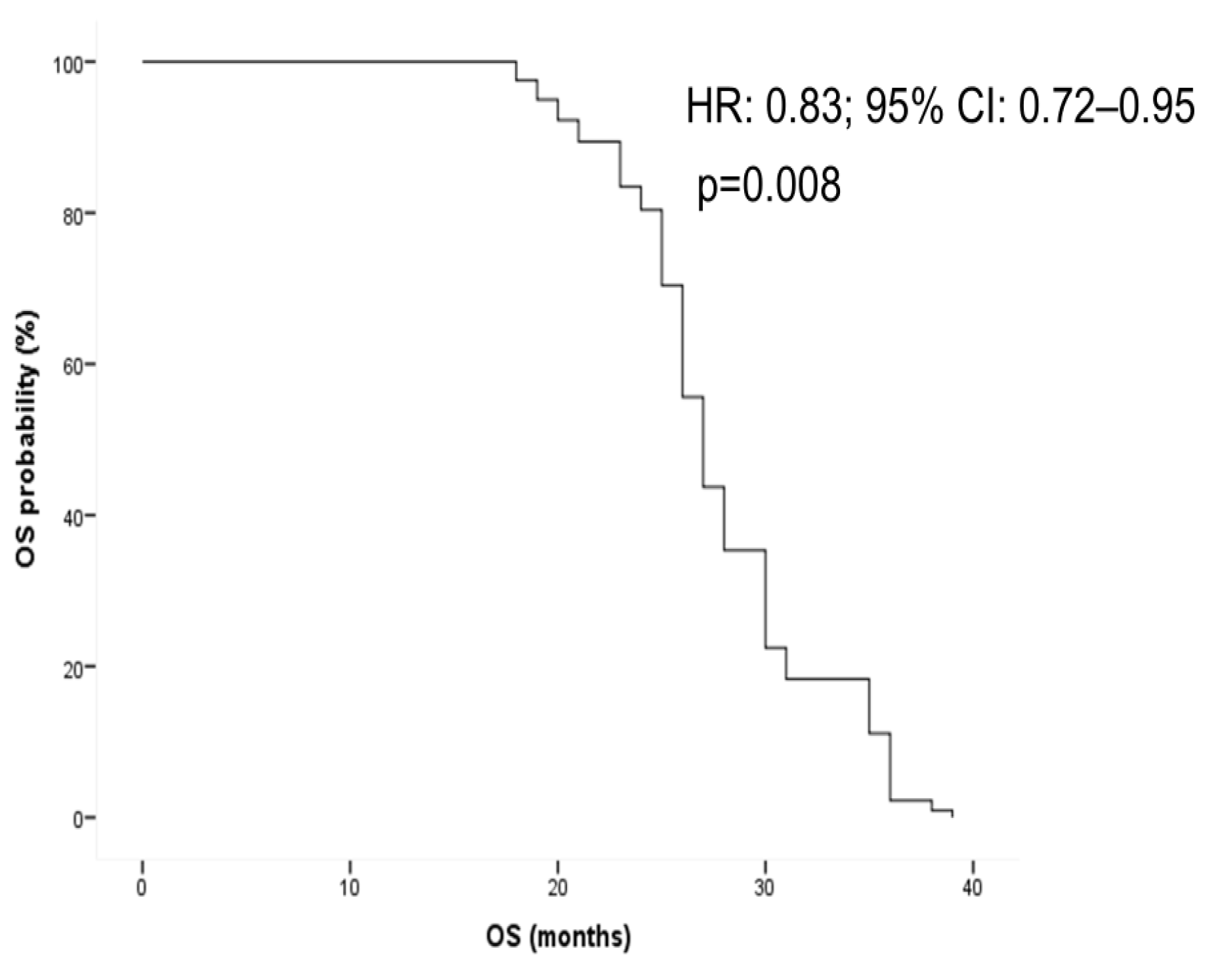
3.5. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Metastatic colorectal cancer | mCRC |
| Circulating tumor DNA | ctDNA |
| Progression-free survival | PFS |
| Overall survival | OS |
| Epidermal growth factor receptor | EGFR |
| Wild-type | WT |
References
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Jeantet, M.; Tougeron, D.; Tachon, G.; Cortes, U.; Archambaut, C.; Fromont, G.; Karayan-Tapon, L. High Intra- and Inter-Tumoral Heterogeneity of RAS Mutations in Colorectal Cancer. Int. J. Mol. Sci. 2016, 17, 2015. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, D.S.; Barriuso, J.; Mullamitha, S.; Saunders, M.P.; O’Dwyer, S.T.; Aziz, O. Biomarker concordance between primary colorectal cancer and its metastases. EBioMedicine 2019, 40, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Malla, M.; Loree, J.M.; Kasi, P.M.; Parikh, A.R. Using Circulating Tumor DNA in Colorectal Cancer: Current and Evolving Practices. J. Clin. Oncol. 2022, 40, 2846–2857. [Google Scholar] [CrossRef]
- Vidal, J.; Muinelo, L.; Dalmases, A.; Jones, F.; Edelstein, D.; Iglesias, M.; Orrillo, M.; Abalo, A.; Rodríguez, C.; Brozos, E.; et al. Plasma ctDNA RAS mutation analysis for the diagnosis and treatment monitoring of metastatic colorectal cancer patients. Ann. Oncol. 2017, 28, 1325–1332. [Google Scholar] [CrossRef]
- Pesola, G.; Epistolio, S.; Cefalì, M.; Trevisi, E.; De Dosso, S.; Frattini, M. Neo-RAS Wild Type or RAS Conversion in Metastatic Colorectal Cancer: A Comprehensive Narrative Review. Cancers 2024, 16, 3923. [Google Scholar] [CrossRef]
- Osumi, H.; Shinozaki, E.; Nakamura, Y.; Esaki, T.; Yasui, H.; Taniguchi, H.; Satake, H.; Sunakawa, Y.; Komatsu, Y.; Kagawa, Y.; et al. Clinical features associated with NeoRAS wild-type metastatic colorectal cancer A SCRUM-Japan GOZILA substudy. Nat. Commun. 2024, 15, 5885. [Google Scholar] [CrossRef]
- Osumi, H.; Ishizuka, N.; Takashima, A.; Kumekawa, Y.; Nakano, D.; Shiozawa, M.; Denda, T.; Sawada, R.; Ouchi, K.; Wakatsuki, T.; et al. Multicentre single-arm phase II trial evaluating the safety and effiCacy of Panitumumab and iRinOtecan in NeoRAS Wild-type mEtaStatic colorectal cancer patientS (C-PROWESS trial): Study protocol. BMJ Open 2022, 12, e063071. [Google Scholar] [CrossRef]
- Fernández Montes, A.; Martínez Lago, N.; de la Cámara Gómez, J.; Covela Rúa, M.; Cousillas Castiñeiras, A.; Gonzalez Villarroe, P.; Méndez Méndez, J. FOLFIRI plus panitumumab as second-line treatment in mutated RAS metastatic colorectal cancer patients who converted to wild type RAS after receiving first-line FOLFOX/CAPOX plus bevacizumab-based treatment: Phase II CONVERTIX trial. Ann. Oncol. 2019, 30 (Suppl. S4), iv23–iv24. [Google Scholar]
- Bachet, J.B.; Bouché, O.; Taieb, J.; Dubreuil, O.; Garcia, M.L.; Meurisse, A.; Normand, C.; Gornet, J.M.; Artru, P.; Louafi, S.; et al. RAS mutation analysis in circulating tumor DNA from patients with metastatic colorectal cancer: The AGEO RASANC prospective multicenter study. Ann. Oncol. 2018, 29, 1211–1219. [Google Scholar] [CrossRef]
- Sunakawa, Y.; Satake, H.; Usher, J.; Jaimes, Y.; Miyamoto, Y.; Nakamura, M.; Kataoka, M.; Shiozawa, M.; Takagane, A.; Terazawa, T.; et al. Dynamic changes in RAS gene status in circulating tumour DNA: A phase II trial of first-line FOLFOXIRI plus bevacizumab for RAS-mutant metastatic colorectal cancer (JACCRO CC-11). ESMO Open 2022, 7, 100512. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Huang, Y.S.; Wu, H.X.; Wang, Z.X.; Jin, Y.; Yao, Y.C.; Chen, Y.X.; Zhao, Q.; Chen, S.; He, M.M.; et al. Genomic temporal heterogeneity of circulating tumour DNA in unresectable metastatic colorectal cancer under first-line treatment. Gut 2022, 71, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Nicolazzo, C.; Magri, V.; Marino, L.; Belardinilli, F.; Di Nicolantonio, F.; De Renzi, G.; Caponnetto, S.; De Meo, M.; Giannini, G.; Santini, D.; et al. Genomic landscape and survival analysis of ctDNA “neo-RAS wild-type” patients with originally RAS mutant metastatic colorectal cancer. Front. Oncol. 2023, 13, 1160673. [Google Scholar] [CrossRef]
- Moati, E.; Blons, H.; Taly, V.; Garlan, F.; Wang-Renault, S.F.; Pietrasz, D.; Didelot, A.; Garrigou, S.; Saint, A.; Pernot, S.; et al. Plasma clearance of RAS mutation under therapeutic pressure is a rare event in metastatic colorectal cancer. Int. J. Cancer 2020, 147, 1185–1189. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, J.W.; Kim, H.G.; Hwang, S.H.; Kim, K.J.; Lee, J.H.; Seo, J.; Kang, M.; Jung, E.H.; Suh, K.J.; et al. Longitudinal Comparative Analysis of Circulating Tumor DNA and Matched Tumor Tissue DNA in Patients with Metastatic Colorectal Cancer Receiving Palliative First-Line Systemic Anti-Cancer Therapy. Cancer Res. Treat. 2024, 56, 1171–1182. [Google Scholar] [CrossRef]
- Nicolazzo, C.; Barault, L.; Caponnetto, S.; De Renzi, G.; Belardinilli, F.; Bottillo, I.; Bargiacchi, S.; Macagno, M.; Grammatico, P.; Giannini, G.; et al. True conversions from RAS mutant to RAS wild-type in circulating tumor DNA from metastatic colorectal cancer patients as assessed by methylation and mutational signature. Cancer Lett. 2021, 507, 89–96. [Google Scholar] [CrossRef]
- Nicolazzo, C.; Belardinilli, F.; Vestri, A.; Magri, V.; De Renzi, G.; De Meo, M.; Caponnetto, S.; Di Nicolantonio, F.; Cortesi, E.; Giannini, G.; et al. RAS Mutation Conversion in Bevacizumab-Treated Metastatic Colorectal Cancer Patients: A Liquid Biopsy Based Study. Cancers 2022, 14, 802. [Google Scholar] [CrossRef]
- Epistolio, S.; Cefalì, M.; Spina, P.; Molinari, F.; Movilia, A.; Cergnul, M.; Mazzucchelli, L.; De Dosso, S.; Frattini, M.; Saletti, P. Occurence of RAS reversion in metastatic colorectal cancer patients treated with bevacizumab. Oncotarget 2021, 12, 1046–1056. [Google Scholar] [CrossRef]
- Klein-Scory, S.; Wahner, I.; Maslova, M.; Al-Sewaidi, Y.; Pohl, M.; Mika, T.; Ladigan, S.; Schroers, R.; Baraniskin, A. Evolution of RAS Mutational Status in Liquid Biopsies During First-Line Chemotherapy for Metastatic Colorectal. Cancer Front. Oncol. 2020, 10, 1115. [Google Scholar] [CrossRef]
- Raimondi, C.; Nicolazzo, C.; Belardinilli, F.; Loreni, F.; Gradilone, A.; Mahdavian, Y.; Gelibter, A.; Giannini, G.; Cortesi, E.; Gazzaniga, P. Transient Disappearance of RAS Mutant Clones in Plasma: A Counterintuitive Clinical Use of EGFR Inhibitors in RAS Mutant Metastatic Colorectal Cancer. Cancers 2019, 11, 42. [Google Scholar] [CrossRef]
- Osumi, H.; Takashima, A.; Ooki, A.; Yoshinari, Y.; Wakatsuki, T.; Hirano, H.; Nakayama, I.; Okita, N.; Sawada, R.; Ouchi, K.; et al. A multi-institutional observational study evaluating the incidence and the clinicopathological characteristics of NeoRAS wild-type metastatic colorectal cancer. Transl. Oncol. 2023, 35, 101718. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.; Willis, J.; Parseghian, C.M.; Raghav, K.P.S.; Johnson, B.; Dasari, A.; Stone, D.; Jeyakumar, N.; Coker, O.; Raymond, V.M.; et al. NeoRAS: Incidence of RAS reversion from RAS mutated to RAS wild type. J. Clin. Oncol. 2020, 38 (Suppl. S4), 180. [Google Scholar]
- Gazzaniga, P.; Raimondi, C.; Urbano, F.; Cortesi, E. EGFR Inhibitor as Second-Line Therapy in a Patient With Mutant RAS Metastatic Colorectal Cancer: Circulating Tumor DNA to Personalize Treatment. JCO Precis. Oncol. 2018, 2, 1–6. [Google Scholar] [CrossRef]
- Bouchahda, M.; Saffroy, R.; Karaboué, A.; Hamelin, J.; Innominato, P.; Saliba, F.; Levi, F.; Bosselut, N.; Lemoine, A. Efficacy of an anti-EGFR after ctDNA conversion from mutated RAS status of metastatic colorectal cancer: Results of a pilot study. J. Clin. Oncol. 2021, 39 (Suppl. S15), e15574. [Google Scholar]
- Sato, S.; Mikayama, Y.O.; Shiozawa, M.; Nukada, S.; Iguchi, K.; Okamoto, H.; Kohmura, T.; Kazama, K.; Tanaka, K.; Oshima, T.; et al. Chemotherapy-induced Reversion of Mutant RAS to Wild-type RAS in Metastatic Colorectal Cancer. Anticancer Res. 2022, 42, 2625–2635. [Google Scholar] [CrossRef]
- Osumi, H.; Vecchione, L.; Keilholz, U.; Vollbrecht, C.; Alig, A.H.S.; von Einem, J.C.; Stahler, A.; Striefler, J.K.; Kurreck, A.; Kind, A.; et al. NeoRAS wild-type in metastatic colorectal cancer: Myth or truth?-Case series and review of the literature. Eur. J. Cancer 2021, 153, 86–95. [Google Scholar] [CrossRef]
- Gramaça, J.; Fernandes, I.G.; Trabulo, C.; Gonçalves, J.; Dos Santos, R.G.; Baptista, A.; Pina, I. Emerging role of liquid biopsy in rat sarcoma virus mutated metastatic colorectal cancer: A case report. World J. Gastrointest. Oncol. 2024, 16, 234–243. [Google Scholar] [CrossRef]
- Harada, K.; Yuki, S.; Kawamoto, Y.; Nakamura, T.; Kaneko, S.; Ishida, K.; Sakamoto, N.; Komatsu, Y. Anti-epidermal growth factor receptor treatment for patients with NeoRAS wild-type metastatic colorectal cancer: A case report of two cases. Ther. Adv. Med. Oncol. 2023, 15, 17588359231216090. [Google Scholar] [CrossRef]
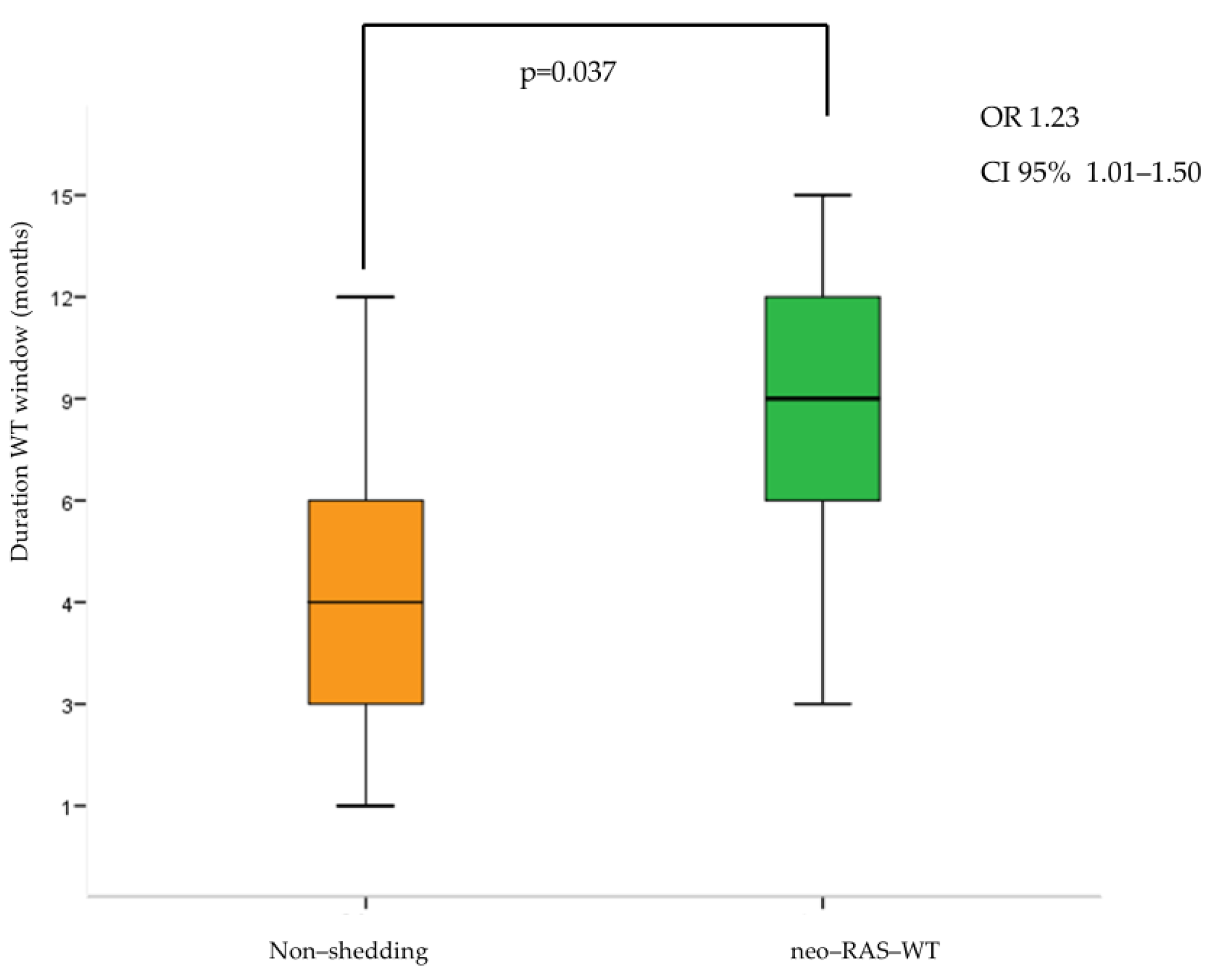
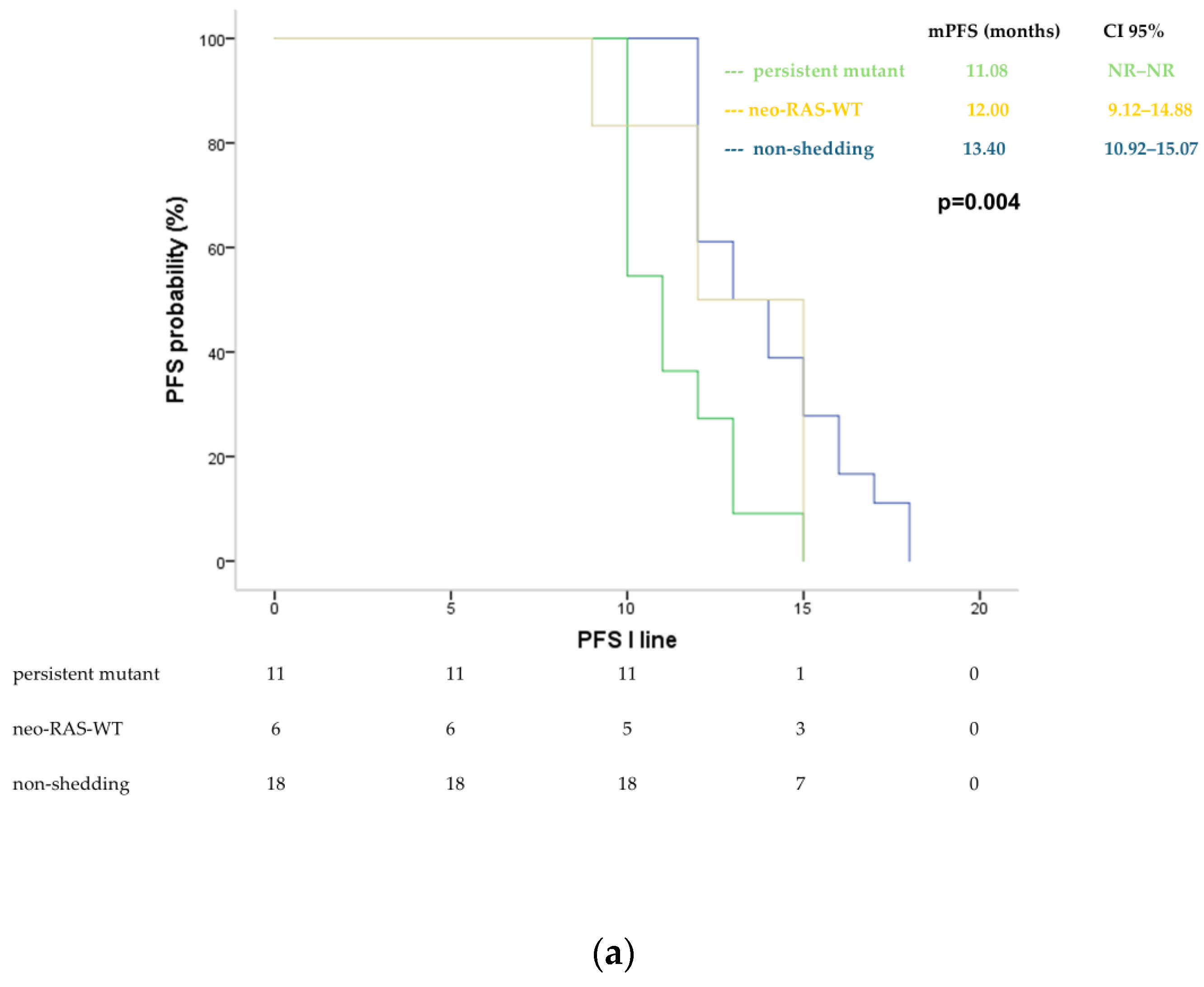

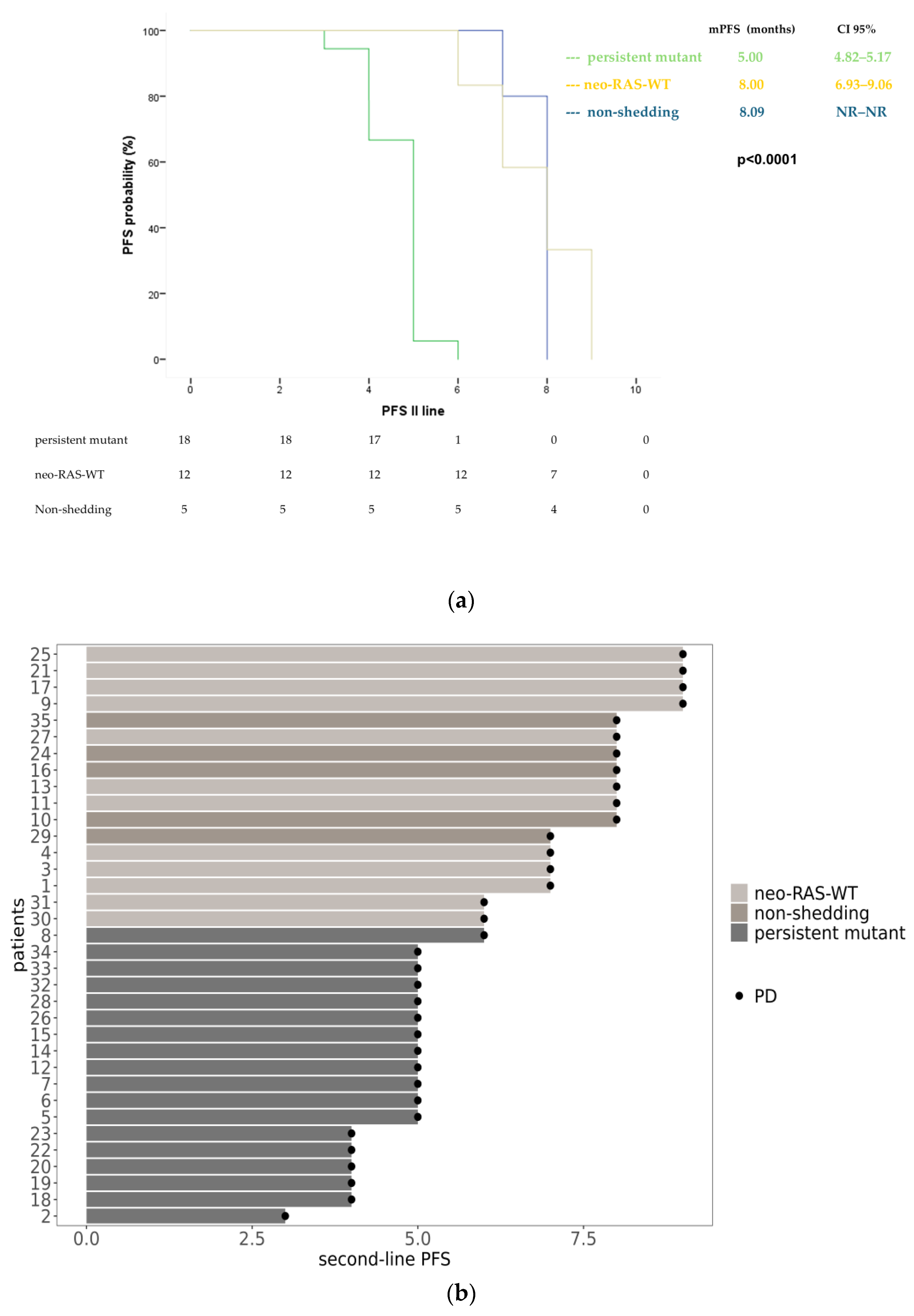

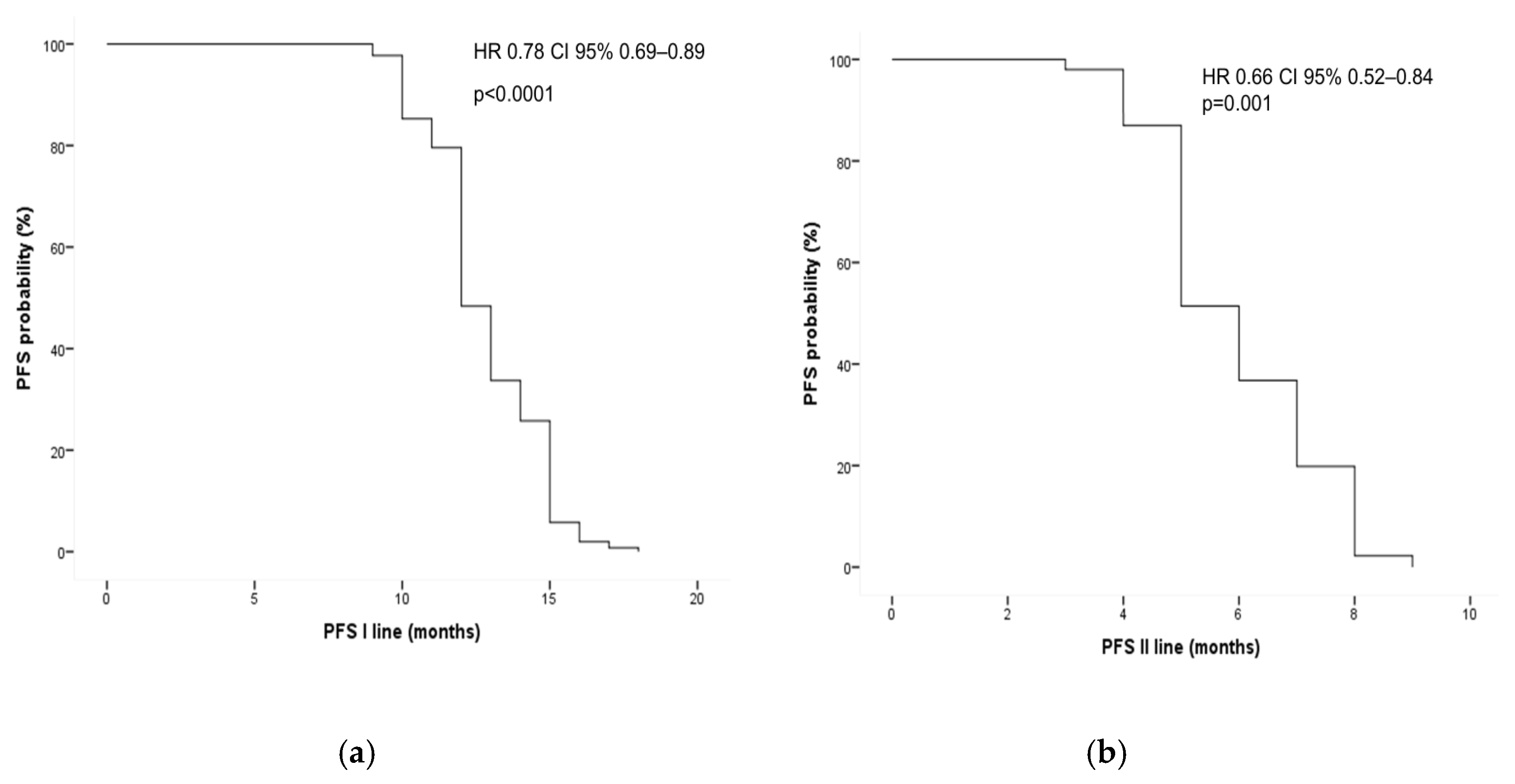
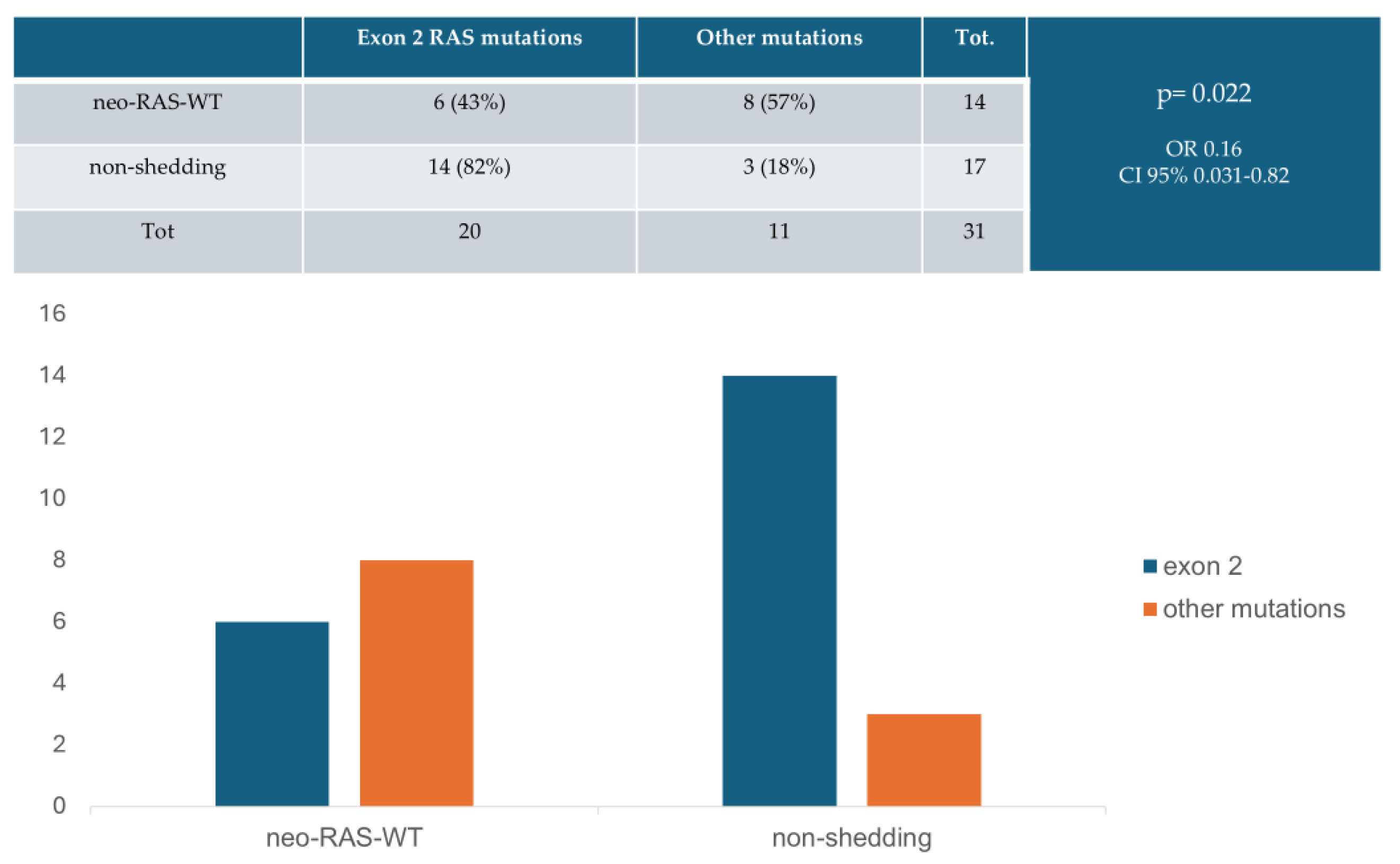
| Neo-RAS-WT | Non-Shedding | Persistent Mutant | |
|---|---|---|---|
| First-line | 6 (17%) | 18 (51%) | 11 (32%) |
| PD (first-line) | 6 (17%) | 0 (0%) | 29 (83%) |
| Second-line | 12 (34%) | 5 (14%) | 18 (52%) |
| PD (second-line) | 11 (31%) | 0 (0%) | 24 (69%) |
| Third-line | 3 (8.5%) | 3 (8.5%) | 29 (83%) |
| PD (third-line) | 2 (6%) | 3 (8%) | 30 (86%) |
| Neo-RAS-WT | Non-Shedding | |
|---|---|---|
| First line | 5.5 months (3–9) | 4.7 months (1–9) |
| Second line | 5.6 months (3–7) | 2.8 months (2–3) |
| Third line | 4 months (3–6) | 4 months (3–6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siringo, M.; De Meo, M.; Bottillo, I.; Grammatico, P.; Cortesi, E.; Nicolazzo, C.; Gazzaniga, P. Evolution of Neo-RAS-WT in Circulating Tumor DNA from First-Line to Subsequent Therapies in Metastatic Colorectal Cancer. Cancers 2025, 17, 1070. https://doi.org/10.3390/cancers17071070
Siringo M, De Meo M, Bottillo I, Grammatico P, Cortesi E, Nicolazzo C, Gazzaniga P. Evolution of Neo-RAS-WT in Circulating Tumor DNA from First-Line to Subsequent Therapies in Metastatic Colorectal Cancer. Cancers. 2025; 17(7):1070. https://doi.org/10.3390/cancers17071070
Chicago/Turabian StyleSiringo, Marco, Michela De Meo, Irene Bottillo, Paola Grammatico, Enrico Cortesi, Chiara Nicolazzo, and Paola Gazzaniga. 2025. "Evolution of Neo-RAS-WT in Circulating Tumor DNA from First-Line to Subsequent Therapies in Metastatic Colorectal Cancer" Cancers 17, no. 7: 1070. https://doi.org/10.3390/cancers17071070
APA StyleSiringo, M., De Meo, M., Bottillo, I., Grammatico, P., Cortesi, E., Nicolazzo, C., & Gazzaniga, P. (2025). Evolution of Neo-RAS-WT in Circulating Tumor DNA from First-Line to Subsequent Therapies in Metastatic Colorectal Cancer. Cancers, 17(7), 1070. https://doi.org/10.3390/cancers17071070









