Radiation-Induced Fibrosis in Head and Neck Cancer: Challenges and Future Therapeutic Strategies for Vocal Fold Treatments
Simple Summary
Abstract
1. Introduction

1.1. Complexity and Treatment Modalities
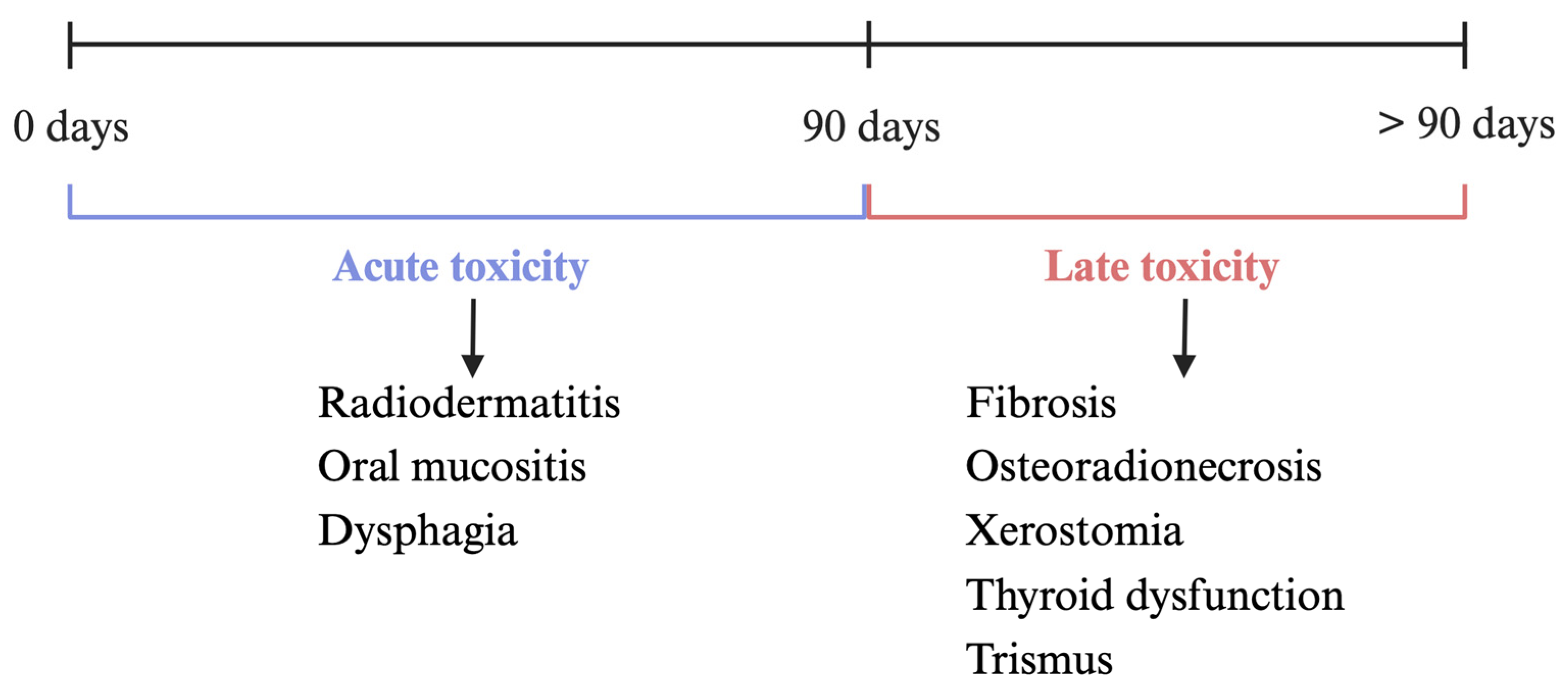
1.2. Mechanisms and Complications of Radiotherapy
2. Radiation-Induced Fibrosis
2.1. Current and Experimental Treatments for Radiation-Induced Fibrosis
2.1.1. Systemic Therapies
2.1.2. Topical Treatments
2.1.3. Mechanical Treatments
2.1.4. Experimental Treatments
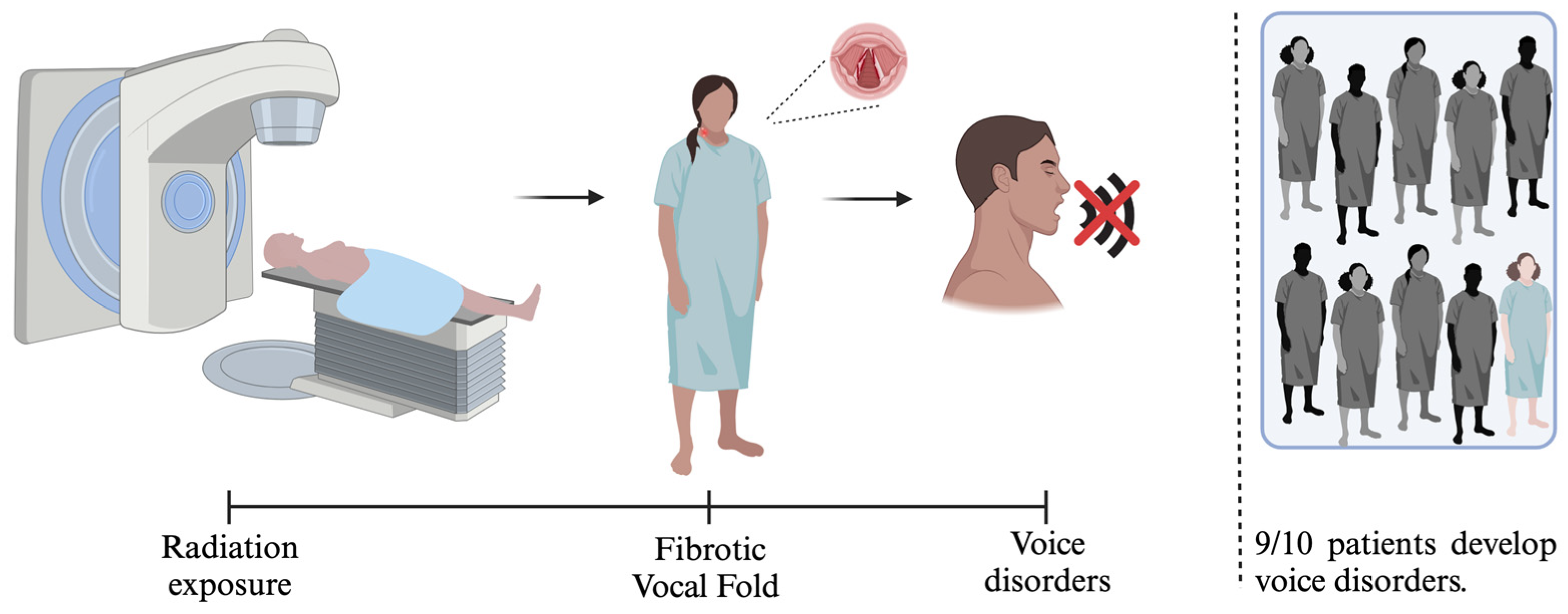
3. VF Fibrosis and Treatment
3.1. Traditional VF Treatments
3.2. Novel VF Treatments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Starzyńska, A.; Sobocki, B.K.; Alterio, D. Current Challenges in Head and Neck Cancer Management. Cancers 2022, 14, 358. [Google Scholar] [CrossRef] [PubMed]
- Gilyoma, J.M.; Rambau, P.F.; Masalu, N.; Kayange, N.M.; Chalya, P.L. Head and neck cancers: A clinico-pathological profile and management challenges in a resource-limited setting. BMC Res. Notes 2015, 8, 772. [Google Scholar]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Méd Sci. 2023, 11, 42. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, X.; Chen, Z.; Du, J.; Wu, Y. Head and Neck Squamous Cell Carcinoma: Risk Factors, Molecular Alterations, Immunology and Peptide Vaccines. Int. J. Pept. Res. Ther. 2022, 28, 19. [Google Scholar] [PubMed]
- Fakhry, C.; D’Souza, G. Discussing the diagnosis of HPV-OSCC: Common questions and answers. Oral Oncol. 2013, 49, 863–871. [Google Scholar]
- Pisani, P.; Airoldi, M.; Allais, A.; Valletti, P.A.; Battista, M.; Benazzo, M.; Briatore, R.; Cacciola, S.; Cocuzza, S.; Colombo, A.; et al. Metastatic disease in head & neck oncology. Acta Otorhinolaryngol. Ital. 2020, 40 (Suppl. S1), S1–S86. [Google Scholar]
- Lazure, K.E.; Lydiatt, W.M.; Denman, D.; Burke, W.J. Association between depression and survival or disease recurrence in patients with head and neck cancer enrolled in a depression prevention trial. Head Neck 2009, 31, 888–892. [Google Scholar] [CrossRef]
- Rieke, K.; Schmid, K.K.; Lydiatt, W.; Houfek, J.; Boilesen, E.; Watanabe-Galloway, S. Depression and survival in head and neck cancer patients. Oral Oncol. 2017, 65, 76–82. [Google Scholar] [CrossRef]
- Kam, D.; Salib, A.; Gorgy, G.; Patel, T.D.; Carniol, E.T.; Eloy, J.A.; Baredes, S.; Park, R.C.W. Incidence of Suicide in Patients With Head and Neck Cancer. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 1075–1081. [Google Scholar] [CrossRef]
- Taylor, K.J.; Amdal, C.D.; Bjordal, K.; Astrup, G.L.; Herlofson, B.B.; Duprez, F.; Gama, R.R.; Jacinto, A.; Hammerlid, E.; Scricciolo, M.; et al. Serious Long-Term Effects of Head and Neck Cancer from the Survivors’ Point of View. Healthcare 2023, 11, 906. [Google Scholar] [CrossRef]
- Zebralla, V.; Wichmann, G.; Pirlich, M.; Hammermüller, C.; Berger, T.; Zimmermann, K.; Neumuth, T.; Mehnert-Theuerkauf, A.; Dietz, A.; Hinz, A.; et al. Dysphagia, voice problems, and pain in head and neck cancer patients. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 3985–3994. [Google Scholar]
- WHO Estimated Number of New Cases from 2022 to 2030. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/tables (accessed on 3 August 2024).
- Payten, C.L.; Chiapello, G.; Weir, K.A.; Madill, C.J. Frameworks, Terminology and Definitions Used for the Classification of Voice Disorders: A Scoping Review. J. Voice 2024, 38, 1070–1087. [Google Scholar] [PubMed]
- Kimball, E.E.; Rousseau, B. Mechanotransduction in the Vocal Fold Microenvironment: A Narrative Review. J. Speech Lang. Hear. Res. 2024, 67, 2128–2138. [Google Scholar]
- Kumai, Y. Pathophysiology of Fibrosis in the Vocal Fold: Current Research, Future Treatment Strategies, and Obstacles to Restoring Vocal Fold Pliability. Int. J. Mol. Sci. 2019, 20, 2551. [Google Scholar] [CrossRef]
- Morrison, M.; Rammage, L.; Nichol, H.; Pullan, B.; May, P.; Salkeld, L. The Management of Voice Disorders; Springer: Berlin/Heidelberg, Germany, 2013; pp. 161–200. [Google Scholar]
- Zhang, S.; Zeng, N.; Yang, J.; He, J.; Zhu, F.; Liao, W.; Xiong, M.; Li, Y. Advancements of radiotherapy for recurrent head and neck cancer in modern era. Radiat. Oncol. 2023, 18, 166. [Google Scholar] [PubMed]
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and neck cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef]
- Alfouzan, A.F. Radiation therapy in head and neck cancer. Saudi Méd. J. 2021, 42, 247–254. [Google Scholar]
- Creak, A.L.; Harrington, K.; Nutting, C. Treatment of recurrent head and neck cancer: Re-irradiation or chemotherapy? Clin. Oncol. 2005, 17, 138–147. [Google Scholar]
- Ionna, F.; Bossi, P.; Guida, A.; Alberti, A.; Muto, P.; Salzano, G.; Ottaiano, A.; Maglitto, F.; Leopardo, D.; Felice, M.D.; et al. Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck: A Big and Intriguing Challenge Which May Be Resolved by Integrated Treatments Combining Locoregional and Systemic Therapies. Cancers 2021, 13, 2371. [Google Scholar] [CrossRef]
- Galbiatti, A.L.S.; Padovani-Junior, J.A.; Maníglia, J.V.; Rodrigues, C.D.S.; Pavarino, É.C.; Goloni-Bertollo, E.M. Head and neck cancer: Causes, prevention and treatment. Braz. J. Otorhinolaryngol. 2013, 79, 239–247. [Google Scholar]
- Dormand, E.; Banwell, P.E.; Goodacre, T.E. Radiotherapy and wound healing. Int. Wound J. 2005, 2, 112–127. [Google Scholar]
- Ordoñez, R.; Otero, A.; Jerez, I.; Medina, J.A.; Lupiañez-Pérez, Y.; Gomez-Millan, J. Role of radiotherapy in the treatment of metastatic head and neck cancer. OncoTargets Ther. 2019, 12, 677–683. [Google Scholar]
- Yeh, S.A. Radiotherapy for Head and Neck Cancer. Semin. Plast. Surg. 2010, 24, 127–136. [Google Scholar] [PubMed]
- Rocha, P.H.P.; Reali, R.M.; Decnop, M.; Souza, S.A.; Teixeira, L.A.B.; Júnior, A.L.; Sarpi, M.O.; Cintra, M.B.; Pinho, M.C.; Garcia, M.R.T. Adverse Radiation Therapy Effects in the Treatment of Head and Neck Tumors. RadioGraphics 2022, 42, 806–821. [Google Scholar]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 2020, 6, 92. [Google Scholar]
- Taylor, A.; Powell, M.E.B. Intensity-modulated radiotherapy—What is it? Cancer Imaging 2004, 4, 68–73. [Google Scholar]
- Rehman, J.U.; Zahra; Ahmad, N.; Khalid, M.; Asghar, H.N.U.H.K.; Gilani, Z.A.; Ullah, I.; Nasar, G.; Akhtar, M.M.; Usmani, M.N. Intensity modulated radiation therapy: A review of current practice and future outlooks. J. Radiat. Res. Appl. Sci. 2018, 11, 361–367. [Google Scholar]
- Bała, K.; Samovich, Y.; Dorobisz, K. Proton Therapy in The Treatment of Head And Neck Cancers—Review. Curr. Oncol. Rep. 2024, 26, 1380–1387. [Google Scholar] [PubMed]
- Chang, C.L.; Lin, K.C.; Chen, W.M.; Shia, B.C.; Wu, S.Y. Comparing the oncologic outcomes of proton therapy and intensity-modulated radiation therapy for head and neck squamous cell carcinoma. Radiother. Oncol. 2024, 190, 109971. [Google Scholar] [CrossRef]
- Glastonbury, C.M.; Parker, E.E.; Hoang, J.K. The Postradiation Neck: Evaluating Response to Treatment and Recognizing Complications. Am. J. Roentgenol. 2010, 195, W164–W171. [Google Scholar]
- Johns, M.M.; Kolachala, V.; Berg, E.; Muller, S.; Creighton, F.X.; Branski, R.C. Radiation fibrosis of the vocal fold: From man to mouse. Laryngoscope 2012, 122, SS107–SS125. [Google Scholar] [CrossRef] [PubMed]
- Radvansky, L.J.; Pace, M.B.; Siddiqui, A. Prevention and management of radiation-induced dermatitis, mucositis, and xerostomia. Am. J. Health-Syst. Pharm. 2013, 70, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Early side effects of radiation treatment for head and neck cancer. Cancer Radiothér. 2021, 25, 507–513. [Google Scholar] [CrossRef]
- Bhandari, S.; Soni, B.W.; Bahl, A.; Ghoshal, S. Radiotherapy-induced oral morbidities in head and neck cancer patients. Spéc. Care Dent. 2020, 40, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Moslemi, D.; Nokhandani, A.M.; Otaghsaraei, M.T.; Moghadamnia, Y.; Kazemi, S.; Moghadamnia, A.A. Management of chemo/radiation-induced oral mucositis in patients with head and neck cancer: A review of the current literature. Radiother. Oncol. 2016, 120, 13–20. [Google Scholar] [CrossRef]
- Petersson, K.; Finizia, C.; Tuomi, L. Predictors of severe dysphagia following radiotherapy for head and neck cancer. Laryngoscope Investig. Otolaryngol. 2021, 6, 1395–1405. [Google Scholar] [CrossRef]
- Cooper, J.S.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Campbell, B.H.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Postoperative Concurrent Radiotherapy and Chemotherapy for High-Risk Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2004, 350, 1937–1944. [Google Scholar] [CrossRef]
- Madrid, C.; Abarca, M.; Bouferrache, K. Osteoradionecrosis: An update. Oral Oncol. 2010, 46, 471–474. [Google Scholar] [CrossRef]
- Sroussi, H.Y.; Epstein, J.B.; Bensadoun, R.; Saunders, D.P.; Lalla, R.V.; Migliorati, C.A.; Heaivilin, N.; Zumsteg, Z.S. Common oral complications of head and neck cancer radiation therapy: Mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017, 6, 2918–2931. [Google Scholar] [CrossRef]
- Brook, I. Late side effects of radiation treatment for head and neck cancer. Radiat. Oncol. J. 2020, 38, 84–92. [Google Scholar] [CrossRef]
- Memtsa, P.T.; Tolia, M.; Tzitzikas, I.; Bizakis, J.; Pistevou-Gombaki, K.; Charalambidou, M.; Iliopoulou, C.; Kyrgias, G. Assessment of xerostomia and its impact on quality of life in head and neck cancer patients undergoing radiation therapy. Mol. Clin. Oncol. 2017, 6, 789–793. [Google Scholar] [CrossRef]
- Ramia, P.; Bodgi, L.; Mahmoud, D.; Mohammad, M.A.; Youssef, B.; Kopek, N.; Al-Shamsi, H.; Dagher, M.; Abu-Gheida, I. Radiation-Induced Fibrosis in Patients with Head and Neck Cancer: A Review of Pathogenesis and Clinical Outcomes. Clin. Med. Insights Oncol. 2022, 16, 11795549211036898. [Google Scholar] [PubMed]
- Bach, C.A.; Wagner, I.; Lachiver, X.; Baujat, B.; Chabolle, F. Botulinum toxin in the treatment of post-radiosurgical neck contracture in head and neck cancer: A novel approach. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2012, 129, 6–10. [Google Scholar] [PubMed]
- Bourgier, C.; Auperin, A.; Rivera, S.; Boisselier, P.; Petit, B.; Lang, P.; Lassau, N.; Taourel, P.; Tetreau, R.; Azria, D.; et al. Pravastatin Reverses Established Radiation-Induced Cutaneous and Subcutaneous Fibrosis in Patients With Head and Neck Cancer: Results of the Biology-Driven Phase 2 Clinical Trial Pravacur. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 365–373. [Google Scholar]
- Shaw, S.M.; Skoretz, S.A.; O’Sullivan, B.; Hope, A.; Liu, L.W.C.; Martino, R. Valid and reliable techniques for measuring fibrosis in patients with head and neck cancer postradiotherapy: A systematic review. Head Neck 2016, 38, E2322–E2334. [Google Scholar]
- Deng, J.; Wulff-Burchfield, E.M.; Murphy, B.A. Late Soft Tissue Complications of Head and Neck Cancer Therapy: Lymphedema and Fibrosis. JNCI Monogr. 2019, 2019, lgz005. [Google Scholar]
- Borrego-Soto, G.; Ortiz-López, R.; Rojas-Martínez, A. Ionizing radiation-induced DNA injury and damage detection in patients with breast cancer. Genet. Mol. Biol. 2015, 38, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, A.; Greenberger, J.S.; Rubin, P.J. Understanding the mechanism of radiation induced fibrosis and therapy options. Pharmacol. Ther. 2019, 204, 107399. [Google Scholar]
- Guipaud, O.; Jaillet, C.; Clément-Colmou, K.; François, A.; Supiot, S.; Milliat, F. The importance of the vascular endothelial barrier in the immune-inflammatory response induced by radiotherapy. Br. J. Radiol. 2018, 91, 20170762. [Google Scholar] [CrossRef]
- Wang, B.; Wei, J.; Meng, L.; Wang, H.; Qu, C.; Chen, X.; Xin, Y.; Jiang, X. Advances in pathogenic mechanisms and management of radiation-induced fibrosis. Biomed. Pharmacother. 2020, 121, 109560. [Google Scholar]
- François, A.; Milliat, F.; Guipaud, O.; Benderitter, M. Inflammation and Immunity in Radiation Damage to the Gut Mucosa. BioMed Res. Int. 2013, 2013, 123241. [Google Scholar] [CrossRef]
- Straub, J.M.; New, J.; Hamilton, C.D.; Lominska, C.; Shnayder, Y.; Thomas, S.M. Radiation-induced fibrosis: Mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 2015, 141, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, S.; Bao, S.; Yao, L.; Wen, Z.; Xu, L.; Chen, X.; Guo, S.; Pang, H.; Zhou, Y.; et al. Deciphering the fibrotic process: Mechanism of chronic radiation skin injury fibrosis. Front. Immunol. 2024, 15, 1338922. [Google Scholar] [CrossRef]
- Azzam, P.; Mroueh, M.; Francis, M.; Daher, A.A.; Zeidan, Y.H. Radiation-induced neuropathies in head and neck cancer: Prevention and treatment modalities. Ecancermedicalscience 2020, 14, 1133. [Google Scholar] [CrossRef]
- Sanguineti, G.; Ricchetti, F.; McNutt, T.; Wu, B.; Fiorino, C. Dosimetric Predictors of Dysphonia after Intensity-modulated Radiotherapy for Oropharyngeal Carcinoma. Clin. Oncol. 2014, 26, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Hutcheson, K.A.; Garden, A.S.; Mott, F.E.; Goepfert, R.P.; Duvall, A.; Fuller, C.D.; Lai, S.Y.; Gunn, G.B.; Sturgis, E.M.; et al. Association of Risk Factors With Patient-Reported Voice and Speech Symptoms Among Long-term Survivors of Oropharyngeal Cancer. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Ohno, K.; Yamashita, Y.; Takahashi, K. Factors influencing postoperative speech function of tongue cancer patients following reconstruction with fasciocutaneous/myocutaneous flaps—A multicenter study. Int. J. Oral Maxillofac. Surg. 2007, 36, 601–609. [Google Scholar] [CrossRef]
- Dwivedi, R.C.; Kazi, R.A.; Agrawal, N.; Nutting, C.M.; Clarke, P.M.; Kerawala, C.J.; Rhys-Evans, P.H.; Harrington, K.J. Evaluation of speech outcomes following treatment of oral and oropharyngeal cancers. Cancer Treat. Rev. 2009, 35, 417–424. [Google Scholar] [CrossRef]
- Machtay, M.; Moughan, J.; Trotti, A.; Garden, A.S.; Weber, R.S.; Cooper, J.S.; Forastiere, A.; Ang, K.K. Factors Associated With Severe Late Toxicity After Concurrent Chemoradiation for Locally Advanced Head and Neck Cancer: An RTOG Analysis. J. Clin. Oncol. 2008, 26, 3582–3589. [Google Scholar] [CrossRef]
- Gomez-Millan, J. Radiation therapy in the elderly: More side effects and complications? Crit. Rev. Oncol. Hematol. 2009, 71, 70–78. [Google Scholar] [CrossRef]
- Dornfeld, K.; Simmons, J.R.; Karnell, L.; Karnell, M.; Funk, G.; Yao, M.; Wacha, J.; Zimmerman, B.; Buatti, J.M. Radiation Doses to Structures Within and Adjacent to the Larynx are Correlated With Long-Term Diet- and Speech-Related Quality of Life. Int. J. Radiat. Oncol. BiolPhys. 2007, 68, 750–757. [Google Scholar]
- Yu, Z.; Xu, C.; Song, B.; Zhang, S.; Chen, C.; Li, C.; Zhang, S. Tissue fibrosis induced by radiotherapy: Current understanding of the molecular mechanisms, diagnosis and therapeutic advances. J. Transl. Med. 2023, 21, 708. [Google Scholar] [PubMed]
- Andreassen, C.N.; Overgaard, J.; Alsner, J.; Overgaard, M.; Herskind, C.; Cesaretti, J.A.; Atencio, D.P.; Green, S.; Formenti, S.C.; Stock, R.G.; et al. ATM sequence variants and risk of radiation-induced subcutaneous fibrosis after postmastectomy radiotherapy. Int. J. Radiat. Oncol. BiolPhys. 2006, 64, 776–783. [Google Scholar]
- Price, M.L.; Lai, Y.; Marcus, K.L.; Robertson, J.B.; Lascelles, B.D.X.; Nolan, M.W. Early radiation-induced oral pain signaling responses are reduced with pentoxifylline treatment. Vet. Radiol. Ultrasound 2021, 62, 255–263. [Google Scholar] [PubMed]
- Patel, V.; McGurk, M. Use of pentoxifylline and tocopherol in radiation-induced fibrosis and fibroatrophy. Br. J. Oral Maxillofac. Surg. 2017, 55, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Karemore, T.; Motwani, M. Evaluation of the effect of newer antioxidant lycopene in the treatment of oral submucous fibrosis. Indian J. Dent. Res. 2012, 23, 524. [Google Scholar] [CrossRef]
- Tyagi, H.; Lakhanpal, M.; Dhillon, M.; Baduni, A.; Goel, A.; Banga, A. Efficacy of therapeutic ultrasound with soft tissue mobilization in patients of oral submucous fibrosis. J. Indian Acad. Oral Med. Radiol. 2018, 30, 349. [Google Scholar]
- Horton, J.A.; Chung, E.J.; Hudak, K.E.; Sowers, A.; Thetford, A.; White, A.O.; Mitchell, J.B.; Citrin, D.E. Inhibition of radiation-induced skin fibrosis with imatinib. Int. J. Radiat. Biol. 2013, 89, 162–170. [Google Scholar] [CrossRef]
- Duffield, J.S.; Lupher, M.; Thannickal, V.J.; Wynn, T.A. Host Responses in Tissue Repair and Fibrosis. Pathol. Mech. Dis. 2013, 8, 241–276. [Google Scholar]
- Tu, J.; Chen, X.; Li, C.; Liu, C.; Huang, Y.; Wang, X.; Liang, H.; Yuan, X. Nintedanib Mitigates Radiation-Induced Pulmonary Fibrosis by Suppressing Epithelial Cell Inflammatory Response and Inhibiting Fibroblast-to-Myofibroblast Transition. Int. J. Biol. Sci. 2024, 20, 3353–3371. [Google Scholar]
- Tangella, A.V. The Evolving Role of Intra-arterial Chemotherapy in Adult and Pediatric Cancers: A Comprehensive Review. Cureus 2023, 15, e46631. [Google Scholar] [PubMed]
- Rao, K.; Kalapurakal, S.; Chalasani, P.; Robinson, K.; Malone, J.; Clausen, C.; Ronen, O.; Dhiwakar, M.; Shevlin, B.; Robbins, K.T. A phase II study of intra-arterial cisplatin with concurrent radiation and erlotinib for locally advanced head and neck cancer. Cancer Chemother. Pharmacol. 2013, 72, 545–552. [Google Scholar] [PubMed]
- Yu, K.H.; Yu, S.C.H.; Hui, E.P.; Kam, M.K.M.; Vlantis, A.C.; Yuen, E.; Chan, A.T.C. Accelerated fractionation radiotherapy and late intensification with 2 intra-arterial cisplatin infusions for locally advanced head and neck squamous cell carcinoma. Head Neck 2010, 32, 913–920. [Google Scholar]
- Homma, A.; Onimaru, R.; Matsuura, K.; Robbins, K.T.; Fujii, M. Intra-arterial chemoradiotherapy for head and neck cancer. Jpn. J. Clin. Oncol. 2016, 46, 4–12. [Google Scholar] [CrossRef]
- Landeen, K.C.; Spanos, W.C.; Gromer, L. Topical superoxide dismutase in posttreatment fibrosis in patients with head and neck cancer. Head Neck 2018, 40, 1400–1405. [Google Scholar] [CrossRef]
- Pérez, I.M.M.; Pérez, S.E.M.; García, R.P.; Lupgens D de, Z.; Martínez, G.B.; González, C.R.; Yáñez, N.K.; Hernández, F.R. Exercise-based rehabilitation on functionality and quality of life in head and neck cancer survivors. A systematic review and meta-analysis. Sci. Rep. 2023, 13, 8523. [Google Scholar]
- Peng, D.; Fu, M.; Wang, M.; Wei, Y.; Wei, X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol. Cancer 2022, 21, 104. [Google Scholar] [CrossRef]
- Shi, N.; Wang, Z.; Zhu, H.; Liu, W.; Zhao, M.; Jiang, X.; Zhao, J.; Ren, C.; Zhang, Y.; Luo, L. Research progress on drugs targeting the TGF-β signaling pathway in fibrotic diseases. Immunol. Res. 2022, 70, 276–288. [Google Scholar]
- Dadrich, M.; Nicolay, N.H.; Flechsig, P.; Bickelhaupt, S.; Hoeltgen, L.; Roeder, F.; Hauser, K.; Tietz, A.; Jenne, J.; Lopez, R.; et al. Combined inhibition of TGFβ and PDGF signaling attenuates radiation-induced pulmonary fibrosis. OncoImmunology 2016, 5, e1123366. [Google Scholar]
- Chen, Z.; Wu, Z.; Ning, W. Advances in Molecular Mechanisms and Treatment of Radiation-Induced Pulmonary Fibrosis. Transl. Oncol. 2019, 12, 162–169. [Google Scholar]
- Li, Z.J.; Wang, L.Q.; Li, Y.Z.; Wang, C.Y.; Huang, J.Z.; Yu, N.Z.; Long, X. Application of adipose-derived stem cells in treating fibrosis. World J. Stem Cells 2021, 13, 1747–1761. [Google Scholar] [CrossRef] [PubMed]
- Almadori, A.; Butler, P.E. Scarring and Skin Fibrosis Reversal with Regenerative Surgery and Stem Cell Therapy. Cells 2024, 13, 443. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, L.S.Y.; Duddempudi, P.K.; Yang, W.L.; Tamarat, R.; Guha, C. Extracellular Vesicles for the Treatment of Radiation Injuries. Front. Pharmacol. 2021, 12, 662437. [Google Scholar] [CrossRef]
- Musielak, M.; Suchorska, W.M.; Fundowicz, M.; Milecki, P.; Malicki, J. Future Perspectives of Proton Therapy in Minimizing the Toxicity of Breast Cancer Radiotherapy. J. Pers. Med. 2021, 11, 410. [Google Scholar] [CrossRef]
- Fijardo, M.; Kwan, J.Y.Y.; Bissey, P.A.; Citrin, D.E.; Yip, K.W.; Liu, F.F. The clinical manifestations and molecular pathogenesis of radiation fibrosis. eBioMedicine 2024, 103, 105089. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chang, J.Y. Proton therapy in clinical practice. Chin. J. Cancer. 2011, 30, 315–326. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022, 10, 15–31. [Google Scholar] [CrossRef]
- Ahmad, K.; Shaikh, S.; Chun, H.J.; Ali, S.; Lim, J.H.; Ahmad, S.S.; Lee, E.J.; Choi, I. Extracellular matrix: The critical contributor to skeletal muscle regeneration—A comprehensive review. Inflamm. Regen. 2023, 43, 58. [Google Scholar] [CrossRef]
- Pal, S.; Chaudhari, R.; Baurceanu, I.; Hill, B.J.; Nagy, B.A.; Wolf, M.T. Extracellular matrix scaffold-assisted tumor vaccines induce tumor regression and long-term immune memory. bioRxiv 2023. bioRxiv: 2023.09.12.557449. [Google Scholar] [CrossRef]
- Badylak, S.F.; Hoppo, T.; Nieponice, A.; Gilbert, T.W.; Davison, J.M.; Jobe, B.A. Esophageal Preservation in Five Male Patients After Endoscopic Inner-Layer Circumferential Resection in the Setting of Superficial Cancer: A Regenerative Medicine Approach with a Biologic Scaffold. Tissue Eng. Part A 2011, 17, 1643–1650. [Google Scholar] [CrossRef]
- Membreno, P.V.; Eid, A.A.; Vanison, C.C.; Gillespie, M.B.; Gleysteen, J.P. Porcine small intestine graft for reconstruction of oral defects. Laryngoscope Investig. Otolaryngol. 2021, 6, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical applications of stem cell-derived exosomes. Signal Transduct. Target. Ther. 2024, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Ilg, M.M.; Bustin, S.A.; Ralph, D.J.; Cellek, S. TGF-β1 induces formation of TSG-6-enriched extracellular vesicles in fibroblasts which can prevent myofibroblast transformation by modulating Erk1/2 phosphorylation. Sci. Rep. 2024, 14, 12389. [Google Scholar]
- Mora-Navarro, C.; Badileanu, A.; Martins, A.M.G.; Ozpinar, E.W.; Gaffney, L.; Huntress, I.; Harrell, E.; Enders, J.R.; Peng, X.; Branski, R.C.; et al. Porcine Vocal Fold Lamina Propria-Derived Biomaterials Modulate TGF-β1-Mediated Fibroblast Activation in Vitro. Acs Biomater. Sci. Eng. 2020, 6, 1690–1703. [Google Scholar] [PubMed]
- Varghese, J.J.; Schmale, I.L.; Mickelsen, D.; Hansen, M.E.; Newlands, S.D.; Benoit, D.S.W.; Korshunov, V.A.; Ovitt, C.E. Localized Delivery of Amifostine Enhances Salivary Gland Radioprotection. J. Dent. Res. 2018, 97, 1252–1259. [Google Scholar]
- FDA. Ethyol® (Amifostine) for Injection. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020221s033lbl.pdf (accessed on 26 August 2024).
- Singh, V.K.; Seed, T.M. The efficacy and safety of amifostine for the acute radiation syndrome. Expert Opin. Drug Saf. 2019, 18, 1077–1090. [Google Scholar]
- Tanigami, Y.; Kawai, Y.; Kaba, S.; Uozumi, R.; Ohnishi, H.; Kita, T.; Omori, K.; Kishimoto, Y. Establishment of a radiation-induced vocal fold fibrosis mouse model. Biochem. Biophys. Res. Commun. 2022, 601, 31–37. [Google Scholar]
- Lazarus, C.L. Effects of chemoradiotherapy on voice and swallowing. Curr. Opin. Otolaryngol. Head Neck Surg. 2009, 17, 172–178. [Google Scholar]
- Wu, P.; Wang, C. Risk factors for postoperative vocal fold fibrosis following microlaryngeal surgery. Laryngoscope Investig. Otolaryngol. 2023, 8, 1324–1327. [Google Scholar]
- Park, S.J.; Park, Y.H.; Jeong, W.J.; Cha, W. Prolonged persistence of hyaluronic acid after suboptimal vocal fold injection. Ear Nose Throat J. 2022, 103, 014556132210826. [Google Scholar]
- Chhetri, D.K.; Mendelsohn, A.H. Hyaluronic acid for the treatment of vocal fold scars. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 498–502. [Google Scholar] [PubMed]
- Kwon, S.; Choi, H.; Park, C.; Choi, S.; Kim, E.; Kim, S.W.; Kim, C.S.; Koo, H. In vivo vocal fold augmentation using an injectable polyethylene glycol hydrogel based on click chemistry. Biomater. Sci. 2020, 9, 108–115. [Google Scholar]
- Jimenez-Vergara, A.C.; Munoz-Pinto, D.J.; Becerra-Bayona, S.; Wang, B.; Iacob, A.; Hahn, M.S. Influence of glycosaminoglycan identity on vocal fold fibroblast behavior. Acta Biomater. 2011, 7, 3964–3972. [Google Scholar] [PubMed]
- Kazemirad, S.; Heris, H.K.; Mongeau, L. Viscoelasticity of hyaluronic acid-gelatin hydrogels for vocal fold tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 283–290. [Google Scholar]
- Wang, J.; Mao, W.; Fang, R.; Wei, C.; He, P. Use of 532 nm Potassium Titanyl Phosphate Laser on Vocal Fold Scars Under Topical Anesthesia: A Pilot Study. Ann. Otol. Rhinol. Laryngol. 2022, 131, 715–723. [Google Scholar] [PubMed]
- Zhang, J.; Zhen, R.; Wei, C. Potassium titanyl phosphate laser-induced inflammatory response and extracellular matrix turnover in rabbit vocal fold scar. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 1525–1532. [Google Scholar]
- Antar, S.A.; Saleh, M.A.; Al-Karmalawy, A.A. Investigating the possible mechanisms of pirfenidone to be targeted as a promising anti-inflammatory, anti-fibrotic, anti-oxidant, anti-apoptotic, anti-tumor, and/or anti-SARS-CoV-2. Life Sci. 2022, 309, 121048. [Google Scholar]
- Yamada, T.; Kumai, Y.; Kodama, H.; Nishimoto, K.; Miyamaru, S.; Onoue, S.; Orita, Y. Effect of pirfenidone injection on ferret vocal fold scars: A preliminary in vivo study. Laryngoscope 2020, 130, 726–731. [Google Scholar] [CrossRef]
- Weissberg, J.B.; Son, Y.H.; Papac, R.J.; Sasaki, C.; Fischer, D.B.; Lawrence, R.; Rockwell, S.; Sartorelli, A.C.; Fischer, J.J. Randomized clinical trial of mitomycin c as an adjunct to radiotherapy in head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 3–9. [Google Scholar]
- Haffty, B.G.; Son, Y.H.; Sasaki, C.T.; Papac, R.; Fischer, D.; Rockwell, S.; Sartorelli, A.; Fischer, J.J. Mitomycin C as an adjunct to postoperative radiation therapy in squamous cell carcinoma of the head and neck: Results from two randomized clinical trials. Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 241–250. [Google Scholar]
- Szabó, D.; Kovács, D.; Endrész, V.; Igaz, N.; Jenovai, K.; Spengler, G.; Tiszlavicz, L.; Molnár, J.; Burián, K.; Kiricsi, M.; et al. Antifibrotic effect of mitomycin-C on human vocal cord fibroblasts. Laryngoscope 2019, 129, E255–E262. [Google Scholar] [PubMed]
- Roh, J.L.; Yoon, Y.H. Prevention of Anterior Glottic Stenosis After Bilateral Vocal Fold Stripping With Mitomycin C. Arch. Otolaryngol. Head Neck Surg. 2005, 131, 690–695. [Google Scholar] [PubMed]
- Svistushkin, M.V.; Kotova, S.; Shpichka, A.; Starostina, S.; Shekhter, A.; Bikmulina, P.; Nikiforova, A.; Zolotova, A.; Royuk, V.; Kochetkov, P.A.; et al. Stem cell therapy for vocal fold regeneration after scarring: A review of experimental approaches. Stem Cell Res. Ther. 2022, 13, 176. [Google Scholar] [PubMed]
- Wingstrand, V.L.; Larsen, C.G.; Jensen, D.H.; Bork, K.; Sebbesen, L.; Balle, J.; Fischer-Nielsen, A.; Buchwald, C.V. Mesenchymal Stem Cell Therapy for the Treatment of Vocal Fold Scarring: A Systematic Review of Preclinical Studies. PLoS ONE 2016, 11, e0162349. [Google Scholar]
- Svistushkin, M.; Shpichka, A.; Bikmulina, P.; Fayzullin, A.; Zolotova, A.; Kosheleva, N.; Selezneva, L.; Shavkuta, B.; Lobacheva, V.; Nikiforova, A.; et al. Vocal fold restoration after scarring: Biocompatibility and efficacy of an MSC-based bioequivalent. Stem Cell Res. Ther. 2023, 14, 303. [Google Scholar]

| Systemic Therapies | Mechanisms | References |
|---|---|---|
| Pentoxifylline with vitamin E | Inhibition of intracellular signaling in response to TGF-β (family of protein) | [44,67] |
| Lycopene | Radical scavenger | [68] |
| Imatinib | Inhibit PDGF receptor | [70] |
| Pravastatin | Antifibrotic potential | [46] |
| Nintedanib | Inhibit pathways suck as TGF-β/Smad, PI3K/AKT/mTOR, and MAPK | [72] |
| Experimental Treatments | Examples | Mechanisms | References |
|---|---|---|---|
| TGF-β1 receptor kinase inhibitors | LY2157299, EW-7197, LY2109761, SKI2162, and GC1008 | Reduce inflammation and fibrosis by blocking TGF-β1 signaling | [54,64,79] |
| PDGF inhibitors | SU9518 and SU14816 | Reduce excess scar tissue | [81,82] |
| Stem cell therapy | Adipose tissue-derived stem cells | Suppress TGF-β1 expression | [84] |
| Advances in radiation techniques | Intensity-modulated radiation therapy | High doses are delivered to the tumor site | [86,87] |
| Extracellular vesicles (EVs) | EVs derived from bone marrow mesenchymal stem cells (MSCs) | Promote cell proliferation and reduce apoptosis | [85] |
| EVs derived from primed cells, such as interferon-gamma MSCs or fibroblasts | EVs isolated from primed cells have been shown to modulate the TGF-β1 pathway through their molecular cargos | [94,95] | |
| Matrix-bound vesicles (BVs) | MBVs and macromolecules isolated from the VF lamina propria ECM have been reported to inhibit alpha-smooth muscle actin in fibroblasts stimulated through TGF-β1 | [96] | |
| Decellularized ECM scaffolds | Use in intramucosal adenocarcinoma, oral and oropharyngeal defects | Promotes tissue healing | [92,93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimenez-Socha, M.; Dion, G.R.; Mora-Navarro, C.; Wang, Z.; Nolan, M.W.; Freytes, D.O. Radiation-Induced Fibrosis in Head and Neck Cancer: Challenges and Future Therapeutic Strategies for Vocal Fold Treatments. Cancers 2025, 17, 1108. https://doi.org/10.3390/cancers17071108
Jimenez-Socha M, Dion GR, Mora-Navarro C, Wang Z, Nolan MW, Freytes DO. Radiation-Induced Fibrosis in Head and Neck Cancer: Challenges and Future Therapeutic Strategies for Vocal Fold Treatments. Cancers. 2025; 17(7):1108. https://doi.org/10.3390/cancers17071108
Chicago/Turabian StyleJimenez-Socha, Maria, Gregory R. Dion, Camilo Mora-Navarro, Ziyu Wang, Michael W. Nolan, and Donald O. Freytes. 2025. "Radiation-Induced Fibrosis in Head and Neck Cancer: Challenges and Future Therapeutic Strategies for Vocal Fold Treatments" Cancers 17, no. 7: 1108. https://doi.org/10.3390/cancers17071108
APA StyleJimenez-Socha, M., Dion, G. R., Mora-Navarro, C., Wang, Z., Nolan, M. W., & Freytes, D. O. (2025). Radiation-Induced Fibrosis in Head and Neck Cancer: Challenges and Future Therapeutic Strategies for Vocal Fold Treatments. Cancers, 17(7), 1108. https://doi.org/10.3390/cancers17071108







