Treatment Selection for Patients with HER2-Negative Metastatic Gastric Cancer Expressing Claudin 18.2 and PD-L1
Simple Summary
Abstract
1. Introduction
2. Biomarkers in HER2-Negative Gastric Cancer
2.1. PD-L1 CPS
2.2. MSI-H/dMMR
2.3. Claudin 18.2
3. ICI Plus Chemotherapy for HER2-Negative Gastric Cancer
4. Zolbetuximab Plus Chemotherapy for CLDN-Positive Gastric Cancer
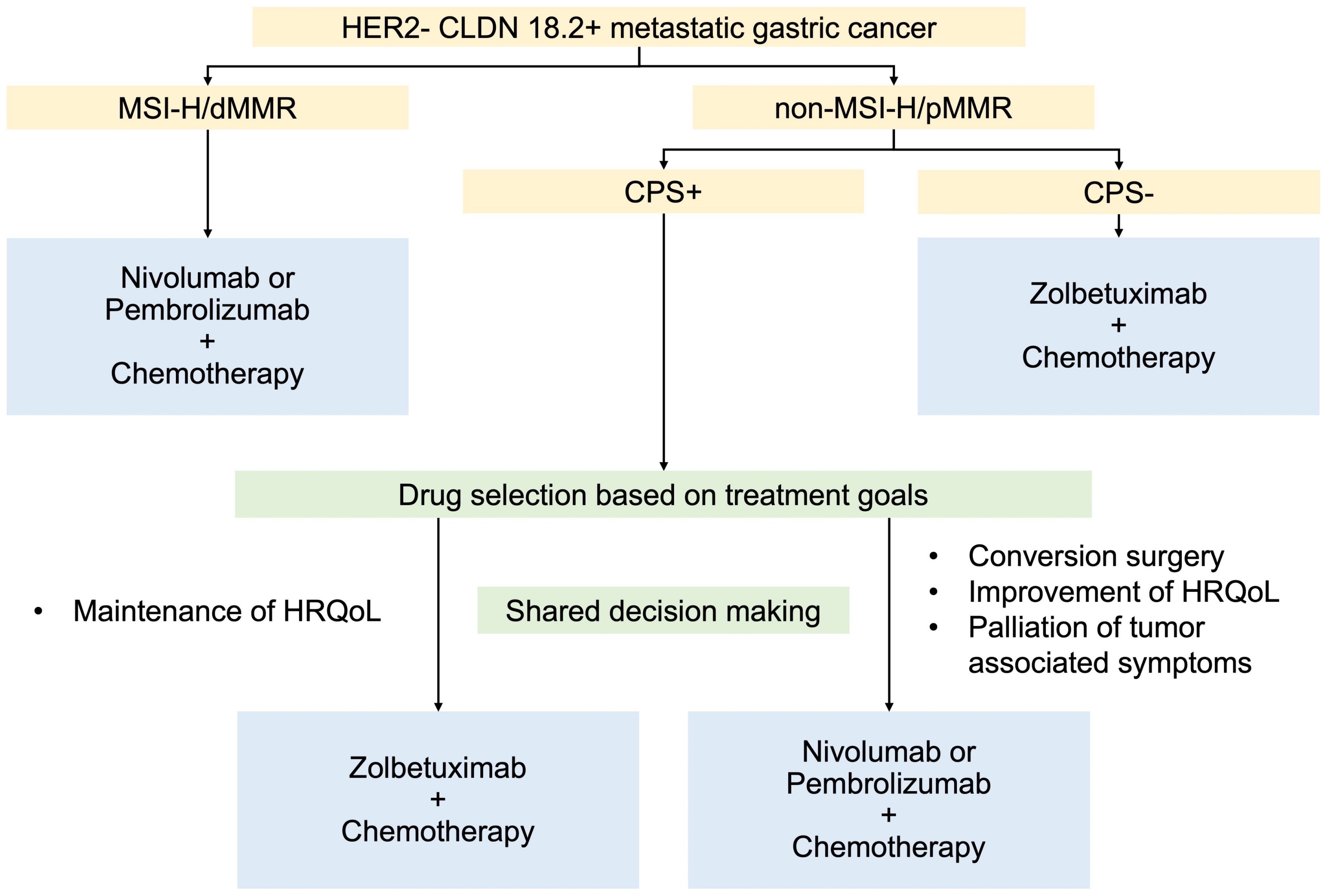
5. Proposed Treatment Selection for HER2-Negative mGCs Expressing CLDN 18.2 and PD-L1
5.1. For MSI-H/dMMR
5.2. For Non-MSI-H/Proficient Mismatch Repair (pMMR)
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, M.A.; Kennedy, E.B.; Alarcon-Rozas, A.E.; Alcindor, T.; Bartley, A.N.; Malowany, A.B.; Bhadkamkar, N.A.; Deighton, D.C.; Janjigian, Y.; Karippot, A.; et al. Immunotherapy and Targeted Therapy for Advanced Gastroesophageal Cancer: ASCO Guideline. J. Clin. Oncol. 2023, 41, 1470–1491. [Google Scholar] [CrossRef]
- European Society for Medical Oncology. Upper Gastrointestinal Cancers Pocket Guideline; European Society for Medical Oncology: Lugano, Switzerland, 2024. [Google Scholar]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021. Gastric Cancer 2023, 26, 1–25. [Google Scholar] [CrossRef]
- Shitara, K.; Fleitas, T.; Kawakami, H.; Curigliano, G.; Narita, Y.; Wang, F.; Wardhani, S.O.; Basade, M.; Rha, S.Y.; Wan Zamaniah, W.I.; et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with gastric cancer. ESMO Open 2024, 9, 102226. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Kawazoe, A.; Bai, Y.; Xu, J.; Lonardi, S.; Metges, J.P.; Yanez Weber, P.E.; Wyrwicz, L.S.; Shen, L.; Ostapenko, Y.V.; et al. 1511O Pembrolizumab plus trastuzumab and chemotherapy for HER2+ metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: Survival results from the phase III, randomized, double-blind, placebo-controlled KEYNOTE-811 study. Ann. Oncol. 2023, 34, S851–S852. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.K.; Chen, L.T.; Ryu, M.H.; Oh, D.Y.; Oh, S.C.; Chung, H.C.; Lee, K.W.; Omori, T.; Shitara, K.; Sakuramoto, S.; et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 234–247. [Google Scholar] [CrossRef]
- Rha, S.Y.; Oh, D.Y.; Yanez, P.; Bai, Y.; Ryu, M.H.; Lee, J.; Rivera, F.; Alves, G.V.; Garrido, M.; Shiu, K.K.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1181–1195. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology; Gastric Cancer Version 5; NCCN: Plymouth Meeting, PA, USA, 2024. [Google Scholar]
- Niimi, T.; Nagashima, K.; Ward, J.M.; Minoo, P.; Zimonjic, D.B.; Popescu, N.C.; Kimura, S. claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol. Cell Biol. 2001, 21, 7380–7390. [Google Scholar] [CrossRef]
- Sahin, U.; Koslowski, M.; Dhaene, K.; Usener, D.; Brandenburg, G.; Seitz, G.; Huber, C.; Türeci, O. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin. Cancer. Res. 2008, 14, 7624–7634. [Google Scholar] [CrossRef]
- Shitara, K.; Lordick, F.; Bang, Y.J.; Enzinger, P.; Ilson, D.; Shah, M.A.; Van Cutsem, E.; Xu, R.H.; Aprile, G.; Xu, J.; et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2023, 401, 1655–1668. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Shitara, K.; Ajani, J.A.; Bang, Y.J.; Enzinger, P.; Ilson, D.; Lordick, F.; Van Cutsem, E.; Gallego Plazas, J.; Huang, J.; et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: The randomized, phase 3 GLOW trial. Nat. Med. 2023, 29, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Kawazoe, A.; Mishima, S.; Nakamura, Y.; Kotani, D.; Kuboki, Y.; Bando, H.; Kojima, T.; Doi, T.; Yoshino, T.; et al. Comprehensive clinical and molecular characterization of claudin 18.2 expression in advanced gastric or gastroesophageal junction cancer. ESMO Open 2023, 8, 100762. [Google Scholar] [CrossRef]
- Shitara, K.; Xu, R.H.; Ajani, J.A.; Moran, D.; Guerrero, A.; Li, R.; Pavese, J.; Matsangou, M.; Bhattacharya, P.; Ueno, Y.; et al. Global prevalence of claudin 18 isoform 2 in tumors of patients with locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma. Gastric Cancer 2024, 27, 1058–1068. [Google Scholar] [CrossRef]
- Pellino, A.; Brignola, S.; Riello, E.; Niero, M.; Murgioni, S.; Guido, M.; Nappo, F.; Businello, G.; Sbaraglia, M.; Bergamo, F.; et al. Association of CLDN18 Protein Expression with Clinicopathological Features and Prognosis in Advanced Gastric and Gastroesophageal Junction Adenocarcinomas. J. Pers. Med. 2021, 11, 95. [Google Scholar] [CrossRef]
- Jia, K.; Chen, Y.; Sun, Y.; Hu, Y.; Jiao, L.; Ma, J.; Yuan, J.; Qi, C.; Li, Y.; Gong, J.; et al. Multiplex immunohistochemistry defines the tumor immune microenvironment and immunotherapeutic outcome in CLDN18.2-positive gastric cancer. BMC Med. 2022, 20, 223. [Google Scholar] [CrossRef]
- Kim, H.D.; Shin, J.; Hyung, J.; Lee, H.; Moon, M.; Ma, J.; Park, Y.S.; Ryu, M.H. Survival outcomes of patients with gastric cancer treated with first-line nivolumab plus chemotherapy based on claudin 18.2 expression. Gastric Cancer 2025, 28, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Ajani, J.A.; Moehler, M.; Shen, L.; Garrido, M.; Gallardo, C.; Wyrwicz, L.; Yamaguchi, K.; Cleary, J.M.; Elimova, E.; et al. First-Line Nivolumab Plus Chemotherapy for Advanced Gastric, Gastroesophageal Junction, and Esophageal Adenocarcinoma: 3-Year Follow-Up of the Phase III CheckMate 649 Trial. J. Clin. Oncol. 2024, 42, 2012–2020. [Google Scholar] [CrossRef]
- Shitara, K.; Shah Manish, A.; Lordick, F.; Van Cutsem, E.; Ilson David, H.; Klempner Samuel, J.; Kang, Y.-K.; Lonardi, S.; Hung, Y.-P.; Yamaguchi, K.; et al. Zolbetuximab in Gastric or Gastroesophageal Junction Adenocarcinoma. N. Engl. J. Med. 2024, 391, 1159–1162. [Google Scholar] [CrossRef]
- Yoon, H.H.; Jin, Z.; Kour, O.; Kankeu Fonkoua, L.A.; Shitara, K.; Gibson, M.K.; Prokop, L.J.; Moehler, M.; Kang, Y.K.; Shi, Q.; et al. Association of PD-L1 Expression and Other Variables with Benefit from Immune Checkpoint Inhibition in Advanced Gastroesophageal Cancer: Systematic Review and Meta-analysis of 17 Phase 3 Randomized Clinical Trials. JAMA Oncol. 2022, 8, 1456–1465. [Google Scholar] [CrossRef]
- Formica, V.; Morelli, C.; Fornaro, L.; Riondino, S.; Rofei, M.; Fontana, E.; Smyth, E.C.; Roselli, M.; Arkenau, H.T. PD-L1 thresholds predict efficacy of immune checkpoint inhibition in first-line treatment of advanced gastroesophageal adenocarcinoma. A systematic review and meta-analysis of seven phase III randomized trials. ESMO Open 2024, 9, 103967. [Google Scholar] [CrossRef] [PubMed]
- Klempner, S.J.; Cowden, E.S.; Cytryn, S.L.; Fassan, M.; Kawakami, H.; Shimada, H.; Tang, L.H.; Wagner, D.C.; Yatabe, Y.; Savchenko, A.; et al. PD-L1 Immunohistochemistry in Gastric Cancer: Comparison of Combined Positive Score and Tumor Area Positivity Across 28-8, 22C3, and SP263 Assays. JCO Precis. Oncol. 2024, 8, e2400230. [Google Scholar] [CrossRef]
- Narita, Y.; Sasaki, E.; Masuishi, T.; Taniguchi, H.; Kadowaki, S.; Ito, S.; Yatabe, Y.; Muro, K. PD-L1 immunohistochemistry comparison of 22C3 and 28-8 assays for gastric cancer. J. Gastrointest. Oncol. 2021, 12, 2696–2705. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Kim, K.M. PD-L1 expression in gastric cancer: Interchangeability of 22C3 and 28-8 pharmDx assays for responses to immunotherapy. Mod. Pathol. 2021, 34, 1719–1727. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, J.Y.; Seo, A.N. Biomarkers for Predicting Response to Personalized Immunotherapy in Gastric Cancer. Diagnostics 2023, 13, 2782. [Google Scholar] [CrossRef]
- Kim, B.; Kang, S.Y.; Kim, K.M. DNA-protein biomarkers for immunotherapy in the era of precision oncology. J. Pathol. Transl. Med. 2021, 55, 26–32. [Google Scholar] [CrossRef]
- Zou, W.; Wolchok, J.D.; Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016, 8, 328rv4. [Google Scholar] [CrossRef] [PubMed]
- Marin-Acevedo, J.A.; Kimbrough, E.O.; Lou, Y. Next generation of immune checkpoint inhibitors and beyond. J. Hematol. Oncol. 2021, 14, 45. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef] [PubMed]
- Kulangara, K.; Guerrero, L.; Posch, A.; Boyer, S.; Hanks, D.A.; Carnahan, J.; Wang, J.; Lunceford, J.; Savage, M.; Marton, M.J.; et al. Investigation of PD-L1 expression and response to pembrolizumab (pembro) in gastric cancer (GC) and cervical cancer (CC) using combined positive score (CPS) and tumor proportion score (TPS). J. Clin. Oncol. 2018, 36, 4065. [Google Scholar] [CrossRef]
- Lei, M.; Siemers, N.; Pandya, D.; Chang, H.; Sanchez, T.; Dorange, C.; Harbison, C.; Szabo, P.M.; Janjigian, Y.; Ott, P.A.; et al. Abstract 2673: Association of PD-L1 combined positive score and immune gene signatures with efficacy of nivolumab (NIVO) ± ipilimumab (IPI) in patients with metastatic gastroesophageal cancer (mGEC). Cancer Res. 2019, 79, 2673. [Google Scholar] [CrossRef]
- Zhou, K.I.; Peterson, B.; Serritella, A.; Thomas, J.; Reizine, N.; Moya, S.; Tan, C.; Wang, Y.; Catenacci, D.V.T. Spatial and Temporal Heterogeneity of PD-L1 Expression and Tumor Mutational Burden in Gastroesophageal Adenocarcinoma at Baseline Diagnosis and after Chemotherapy. Clin. Cancer Res. 2020, 26, 6453–6463. [Google Scholar] [CrossRef]
- Park, Y.; Koh, J.; Na, H.Y.; Kwak, Y.; Lee, K.W.; Ahn, S.H.; Park, D.J.; Kim, H.H.; Lee, H.S. PD-L1 Testing in Gastric Cancer by the Combined Positive Score of the 22C3 PharmDx and SP263 Assay with Clinically Relevant Cut-offs. Cancer Res. Treat. 2020, 52, 661–670. [Google Scholar] [CrossRef]
- Kim, H.D.; Shin, J.; Song, I.H.; Hyung, J.; Lee, H.; Ryu, M.H.; Park, Y.S. Discordant PD-L1 results between 28-8 and 22C3 assays are associated with outcomes of gastric cancer patients treated with nivolumab plus chemotherapy. Gastric Cancer 2024, 27, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Sahin, I.H.; Akce, M.; Alese, O.; Shaib, W.; Lesinski, G.B.; El-Rayes, B.; Wu, C. Immune checkpoint inhibitors for the treatment of MSI-H/MMR-D colorectal cancer and a perspective on resistance mechanisms. Br. J. Cancer 2019, 121, 809–818. [Google Scholar] [CrossRef]
- Dedeurwaerdere, F.; Claes, K.B.; Van Dorpe, J.; Rottiers, I.; Van der Meulen, J.; Breyne, J.; Swaerts, K.; Martens, G. Comparison of microsatellite instability detection by immunohistochemistry and molecular techniques in colorectal and endometrial cancer. Sci. Rep. 2021, 11, 12880. [Google Scholar] [CrossRef]
- Komori, A.; Hironaka, S.; Kadowaki, S.; Mitani, S.; Furuta, M.; Kawakami, T.; Makiyama, A.; Takegawa, N.; Sugiyama, K.; Hirano, H.; et al. Prevalence and clinicopathological features of microsatellite instability-high metastatic or recurrent gastric and esophagogastric junction cancer: WJOG13320GPS. Gastric Cancer 2025, 28, 301–308. [Google Scholar] [CrossRef]
- Ooki, A.; Osumi, H.; Yoshino, K.; Yamaguchi, K. Potent therapeutic strategy in gastric cancer with microsatellite instability-high and/or deficient mismatch repair. Gastric Cancer 2024, 27, 907–931. [Google Scholar] [CrossRef]
- Balmaceda, N.B.; Kim, S.S. Evolving Strategies in the Management of Microsatellite Instability-High/Mismatch Repair Deficient Esophagogastric Adenocarcinoma. Curr. Oncol. Rep. 2025, 27, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Wotherspoon, A.; Peckitt, C.; Gonzalez, D.; Hulkki-Wilson, S.; Eltahir, Z.; Fassan, M.; Rugge, M.; Valeri, N.; Okines, A.; et al. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol. 2017, 3, 1197–1203. [Google Scholar] [CrossRef]
- Nakayama, I.; Shinozaki, E.; Kawachi, H.; Sasaki, T.; Yunokawa, M.; Tomomatsu, J.; Yuasa, T.; Kitazono, S.; Kobayashi, K.; Hayakawa, K.; et al. Implementation of microsatellite instability testing for the assessment of solid tumors in clinical practice. Cancer Med. 2023, 12, 7932–7940. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ma, W.; Jiang, C.; Li, N.; Xu, X.; Ding, Y.; Jiang, H. Heterogeneity and Adjuvant Therapeutic Approaches in MSI-H/dMMR Resectable Gastric Cancer: Emerging Trends in Immunotherapy. Ann. Surg. Oncol. 2023, 30, 8572–8587. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Shitara, K.; Moehler, M.H.; Ajani, J.A.; Shen, L.; Garrido, M.; Gallardo, C.; Wyrwicz, L.S.; Yamaguchi, K.; Cleary, J.M.; Elimova, E.; et al. Nivolumab (NIVO) + chemotherapy (chemo) vs chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma (GC/GEJC/EAC): 4 year (yr) follow-up of CheckMate 649. J. Clin. Oncol. 2024, 42, 306. [Google Scholar] [CrossRef]
- Chao, J.; Fuchs, C.S.; Shitara, K.; Tabernero, J.; Muro, K.; Van Cutsem, E.; Bang, Y.J.; De Vita, F.; Landers, G.; Yen, C.J.; et al. Assessment of Pembrolizumab Therapy for the Treatment of Microsatellite Instability-High Gastric or Gastroesophageal Junction Cancer Among Patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 Clinical Trials. JAMA Oncol. 2021, 7, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Nakayama, I.; Qi, C.; Chen, Y.; Nakamura, Y.; Shen, L.; Shitara, K. Claudin 18.2 as a novel therapeutic target. Nat. Rev. Clin. Oncol. 2024, 21, 354–369. [Google Scholar] [CrossRef]
- Furuse, M.; Fujita, K.; Hiiragi, T.; Fujimoto, K.; Tsukita, S. Claudin-1 and -2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 1998, 141, 1539–1550. [Google Scholar] [CrossRef]
- Hayashi, D.; Tamura, A.; Tanaka, H.; Yamazaki, Y.; Watanabe, S.; Suzuki, K.; Suzuki, K.; Sentani, K.; Yasui, W.; Rakugi, H.; et al. Deficiency of claudin-18 causes paracellular H+ leakage, up-regulation of interleukin-1β, and atrophic gastritis in mice. Gastroenterology 2012, 142, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Günzel, D.; Yu, A.S. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef] [PubMed]
- Jun, K.H.; Kim, J.H.; Jung, J.H.; Choi, H.J.; Chin, H.M. Expression of claudin-7 and loss of claudin-18 correlate with poor prognosis in gastric cancer. Int. J. Surg. 2014, 12, 156–162. [Google Scholar] [CrossRef]
- Sentani, K.; Oue, N.; Tashiro, T.; Sakamoto, N.; Nishisaka, T.; Fukuhara, T.; Taniyama, K.; Matsuura, H.; Arihiro, K.; Ochiai, A.; et al. Immunohistochemical staining of Reg IV and claudin-18 is useful in the diagnosis of gastrointestinal signet ring cell carcinoma. Am. J. Surg. Pathol. 2008, 32, 1182–1189. [Google Scholar] [CrossRef]
- Lordick, F.; Van Cutsem, E.; Shitara, K.; Xu, R.H.; Ajani, J.A.; Shah, M.A.; Oh, M.; Ganguli, A.; Chang, L.; Rhoten, S.; et al. Health-related quality of life in patients with CLDN18.2-positive, locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma: Results from the SPOTLIGHT and GLOW clinical trials. ESMO Open 2024, 9, 103663. [Google Scholar] [CrossRef]
- Sahin, U.; Türeci, Ö.; Manikhas, G.; Lordick, F.; Rusyn, A.; Vynnychenko, I.; Dudov, A.; Bazin, I.; Bondarenko, I.; Melichar, B.; et al. FAST: A randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann. Oncol. 2021, 32, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Sawada, N. Tight junction-related human diseases. Pathol. Int. 2013, 63, 1–12. [Google Scholar] [CrossRef]
- Moran, D.; Maurus, D.; Rohde, C.; Arozullah, A. 103P—Prevalence of CLDN18.2, HER2 and PD-L1 in gastric cancer samples. Ann. Oncol. 2018, 29, viii32. [Google Scholar] [CrossRef]
- Inamoto, R.; Takahashi, N.; Yamada, Y. Claudin18.2 in Advanced Gastric Cancer. Cancers 2023, 15, 5742. [Google Scholar] [CrossRef]
- Xu, B.; Liu, F.; Liu, Q.; Shi, T.; Wang, Z.; Wu, N.; Xu, X.; Li, L.; Fan, X.; Yu, L.; et al. Highly expressed Claudin18.2 as a potential therapeutic target in advanced gastric signet-ring cell carcinoma (SRCC). J. Gastrointest. Oncol. 2020, 11, 1431–1439. [Google Scholar] [CrossRef]
- Rohde, C.; Yamaguchi, R.; Mukhina, S.; Sahin, U.; Itoh, K.; Türeci, Ö. Comparison of Claudin 18.2 expression in primary tumors and lymph node metastases in Japanese patients with gastric adenocarcinoma. Jpn. J. Clin. Oncol. 2019, 49, 870–876. [Google Scholar] [CrossRef]
- Choi, E.; Shin, J.; Ryu, M.H.; Kim, H.D.; Park, Y.S. Heterogeneity of claudin 18.2 expression in metastatic gastric cancer. Sci. Rep. 2024, 14, 17648. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Ye, W.; Xiao, R.; Silvin, C.; Padget, M.; Hodge, J.W.; Van Waes, C.; Schmitt, N.C. Cisplatin and oxaliplatin induce similar immunogenic changes in preclinical models of head and neck cancer. Oral Oncol. 2019, 95, 127–135. [Google Scholar] [CrossRef]
- Kroemer, G.; Senovilla, L.; Galluzzi, L.; André, F.; Zitvogel, L. Natural and therapy-induced immunosurveillance in breast cancer. Nat. Med. 2015, 21, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- de Biasi, A.R.; Villena-Vargas, J.; Adusumilli, P.S. Cisplatin-induced antitumor immunomodulation: A review of preclinical and clinical evidence. Clin. Cancer Res. 2014, 20, 5384–5391. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, L.; Zhang, J.; Wu, H.; Han, E.; Guo, Q. Chemoimmunotherapy by combining oxaliplatin with immune checkpoint blockades reduced tumor burden in colorectal cancer animal model. Biochem. Biophys. Res. Commun. 2017, 487, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jiang, H.; Pan, Y.; Gu, K.; Cang, S.; Han, L.; Shu, Y.; Li, J.; Zhao, J.; Pan, H.; et al. Sintilimab Plus Chemotherapy for Unresectable Gastric or Gastroesophageal Junction Cancer: The ORIENT-16 Randomized Clinical Trial. Jama 2023, 330, 2064–2074. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, H.; Pan, Y.; Gu, K.; Cang, S.; Han, L.; Shu, Y.; Li, J.; Zhao, J.; Pan, H.; et al. Abstract CT078: First-line treatment with sintilimab (sin) vs placebo in combination with chemotherapy (chemo) in patients (pts) with unresectable gastric or gastroesophageal junction (G/GEJ) cancer: Final overall survival (OS) results from the randomized, phase III ORIENT-16 trial. Cancer Res. 2023, 83, CT078. [Google Scholar] [CrossRef]
- Leone, A.G.; Mai, A.S.; Fong, K.Y.; Yap, D.W.T.; Kato, K.; Smyth, E.; Moehler, M.; Seong, J.T.C.; Sundar, R.; Zhao, J.J.; et al. Immune checkpoint inhibitors in advanced gastroesophageal adenocarcinoma: A series of patient-level meta-analyses in different programmed death-ligand 1 subgroups. ESMO Open 2024, 9, 103962. [Google Scholar] [CrossRef]
- Shitara, K.; Van Cutsem, E.; Bang, Y.J.; Fuchs, C.; Wyrwicz, L.; Lee, K.W.; Kudaba, I.; Garrido, M.; Chung, H.C.; Lee, J.; et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients with First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1571–1580. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Q.; Tang, L.L.; Mao, Y.P.; Li, W.F.; Chen, L.; Zhang, Y.; Guo, Y.; Liu, Q.; Sun, Y.; Xu, C.; et al. The Pattern of Time to Onset and Resolution of Immune-Related Adverse Events Caused by Immune Checkpoint Inhibitors in Cancer: A Pooled Analysis of 23 Clinical Trials and 8,436 Patients. Cancer Res. Treat. 2021, 53, 339–354. [Google Scholar] [CrossRef]
- Xie, X.; Wang, L.; Li, Y.; Xu, Y.; Wu, J.; Lin, X.; Lin, W.; Mai, Q.; Chen, Z.; Zhang, J.; et al. Multi-organ Immune-Related Adverse Event Is a Risk Factor of Immune Checkpoint Inhibitor-Associated Myocarditis in Cancer Patients: A Multi-center Study. Front. Immunol. 2022, 13, 879900. [Google Scholar] [CrossRef] [PubMed]
- Couey, M.A.; Bell, R.B.; Patel, A.A.; Romba, M.C.; Crittenden, M.R.; Curti, B.D.; Urba, W.J.; Leidner, R.S. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: Diagnostic hazard of autoimmunity at a distance. J. Immunother. Cancer 2019, 7, 165. [Google Scholar] [CrossRef]
- Moehler, M.; Xiao, H.; Blum, S.I.; Elimova, E.; Cella, D.; Shitara, K.; Ajani, J.A.; Janjigian, Y.Y.; Garrido, M.; Shen, L.; et al. Health-Related Quality of Life with Nivolumab Plus Chemotherapy Versus Chemotherapy in Patients with Advanced Gastric/Gastroesophageal Junction Cancer or Esophageal Adenocarcinoma from CheckMate 649. J. Clin. Oncol. 2023, 41, 5388–5399. [Google Scholar] [CrossRef] [PubMed]
- Lowery, M.; Wyrwicz, L.; Oh, D.Y.; Shiu, K.K.; Yanez Weber, P.; Bai, Y.; Lee, J.; Rivera, F.; Alves, G.; Garrido, M.; et al. 1516P Health-related quality of life (hrqol) analysis from KEYNOTE-859: First-line (1L) pembrolizumab (pembro) + chemotherapy (chemo) for advanced HER2-negative gastric or gastroesophageal junction (G/GEJ) adenocarcinoma. Ann. Oncol. 2023, 34, S854–S855. [Google Scholar] [CrossRef]
- Sahin, U.; Schuler, M.; Richly, H.; Bauer, S.; Krilova, A.; Dechow, T.; Jerling, M.; Utsch, M.; Rohde, C.; Dhaene, K.; et al. A phase I dose-escalation study of IMAB362 (Zolbetuximab) in patients with advanced gastric and gastro-oesophageal junction cancer. Eur. J. Cancer 2018, 100, 17–26. [Google Scholar] [CrossRef]
- Mitnacht-Kraus, R.; Kreuzberg, M.; Utsch, M.; Sahin, U.; Türeci, Ö. 378P—Preclinical characterization of IMAB362 for the treatment of gastric carcinoma. Ann. Oncol. 2017, 28, v126. [Google Scholar] [CrossRef]
- Türeci, Ö.; Mitnacht-Kraus, R.; Wöll, S.; Yamada, T.; Sahin, U. Characterization of zolbetuximab in pancreatic cancer models. Oncoimmunology 2019, 8, e1523096. [Google Scholar] [CrossRef]
- Türeci, O.; Sahin, U.; Schulze-Bergkamen, H.; Zvirbule, Z.; Lordick, F.; Koeberle, D.; Thuss-Patience, P.; Ettrich, T.; Arnold, D.; Bassermann, F.; et al. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: The MONO study. Ann. Oncol. 2019, 30, 1487–1495. [Google Scholar] [CrossRef]
- Shitara, K.; Pophale, R.; Matsangou, M.; Park, J.W.; Oh, M.; Bhattacharya, P.P.; Ranganath, R. Management of nausea and vomiting (N/V) following first-line (1L) zolbetuximab + chemotherapy treatment in claudin-18.2 (CLDN18.2)+, HER2−, locally advanced (LA) unresectable or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: Analysis from the phase 3 SPOTLIGHT and GLOW studies. J. Clin. Oncol. 2024, 42, 372. [Google Scholar] [CrossRef]
- Kinugasa, F.; Kajikawa, S.; Weng, J.; Ugawa, T.; Fushiki, H.; Yamanaka, Y.; Nagata, M.; Haggerty, G.; Akuzawa, S.; Nakazawa, T.; et al. Effect of antiemetics on zolbetuximab-induced gastric injury and emesis in ferrets. J. Pharmacol. Sci. 2024, 156, 161–170. [Google Scholar] [CrossRef]
- Fei, S.; Lu, Y.; Chen, J.; Qi, J.; Wu, W.; Wang, B.; Han, Y.; Wang, K.; Han, X.; Zhou, H.; et al. Efficacy of PD-1 Inhibitors in First-Line Treatment for Advanced Gastroesophageal Junction and Gastric Cancer by Subgroups: A Systematic Review and Meta-Analysis. Chemotherapy 2023, 68, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Yasufuku, I.; Terashima, M.; Young Rha, S.; Moon Bae, J.; Li, G.; Katai, H.; Watanabe, M.; Seto, Y.; Hoon Noh, S.; et al. International Retrospective Cohort Study of Conversion Therapy for Stage IV Gastric Cancer 1 (CONVO-GC-1). Ann. Gastroenterol. Surg. 2022, 6, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Illerhaus, G.; Martens, U.M.; Stoehlmacher, J.; Schmalenberg, H.; Luley, K.B.; Prasnikar, N.; Egger, M.; et al. Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients with Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial. JAMA Oncol. 2017, 3, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Yasufuku, I.; Tsuchiya, H.; Fujibayashi, S.; Okumura, N.; Sengoku, Y.; Fukada, M.; Asai, R.; Sato, Y.; Tajima, J.Y.; Kiyama, S.; et al. Oligometastasis of Gastric Cancer: A Review. Cancers 2024, 16, 673. [Google Scholar] [CrossRef]
- Katsumata, K.; Morimoto, Y.; Aoyama, J.; Yamada, T.; Katsuki, Y.; Nishiyama, R.; Egawa, T. Conversion surgery for gastric remnant cancer with liver metastasis after nivolumab combination chemotherapy achieving pathological complete response: A case report and literature review. Surg. Case Rep. 2024, 10, 107. [Google Scholar] [CrossRef]
- Izumo, W.; Hosoda, K.; Kuramochi, H.; Nakajima, G.; Maeda, S.; Ito, S.; Nagashima, Y.; Itabashi, M. A Case of Pathologically Complete Response After Nivolumab Combined with Chemotherapy in a Gastric Cancer Patient with Virchow’s Lymph Node Metastasis. Clin. Exp. Gastroenterol. 2023, 16, 107–115. [Google Scholar] [CrossRef]
- Toyota, K.; Hashimoto, Y.; Sakashita, Y.; Yokoyama, Y.; Murakami, Y.; Takahashi, S.; Miyamoto, K. Pathological Complete Response to Nivolumab, S1, Oxaliplatin, and Radiation in a Patient with Gastric Cancer: A Case Report. J. Gastrointest. Cancer 2023, 54, 1000–1002. [Google Scholar] [CrossRef]
- van Kleef, J.J.; Ter Veer, E.; van den Boorn, H.G.; Schokker, S.; Ngai, L.L.; Prins, M.J.; Mohammad, N.H.; van de Poll-Franse, L.V.; Zwinderman, A.H.; van Oijen, M.G.H.; et al. Quality of Life During Palliative Systemic Therapy for Esophagogastric Cancer: Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2020, 112, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Eisenhauer, E.A.; Booth, C.M. The Time Toxicity of Cancer Treatment. J. Clin. Oncol. 2022, 40, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Charles, C.; Gafni, A.; Whelan, T. Shared decision-making in the medical encounter: What does it mean? (or it takes at least two to tango). Soc. Sci. Med. 1997, 44, 681–692. [Google Scholar] [CrossRef]
- Barry, M.J.; Edgman-Levitan, S. Shared decision making—Pinnacle of patient-centered care. N. Engl. J. Med. 2012, 366, 780–781. [Google Scholar] [CrossRef] [PubMed]
- Klempner, S.J.; Lee, K.W.; Shitara, K.; Metges, J.P.; Lonardi, S.; Ilson, D.H.; Fazio, N.; Kim, T.Y.; Bai, L.Y.; Moran, D.; et al. ILUSTRO: Phase II Multicohort Trial of Zolbetuximab in Patients with Advanced or Metastatic Claudin 18.2-Positive Gastric or Gastroesophageal Junction Adenocarcinoma. Clin. Cancer Res. 2023, 29, 3882–3891. [Google Scholar] [CrossRef]
- Rha, S.Y.; Zhang, Y.; Elme, A.; Pazo Cid, R.; Alacacioglu, A.; Ziogas, D.C.; Shitara, K.; Ranceva, A.; Nemecek, R.; Santoro, A.; et al. Prevalence of FGFR2b Protein Overexpression in Advanced Gastric Cancers During Prescreening for the Phase III FORTITUDE-101 Trial. JCO Precis. Oncol. 2025, 9, e2400710. [Google Scholar] [CrossRef]
- Catenacci, D.V.; Tesfaye, A.; Tejani, M.; Cheung, E.; Eisenberg, P.; Scott, A.J.; Eng, C.; Hnatyszyn, J.; Marina, N.; Powers, J.; et al. Bemarituzumab with modified FOLFOX6 for advanced FGFR2-positive gastroesophageal cancer: FIGHT Phase III study design. Future Oncol. 2019, 15, 2073–2082. [Google Scholar] [CrossRef]
- Catenacci, D.V.T.; Rasco, D.; Lee, J.; Rha, S.Y.; Lee, K.W.; Bang, Y.J.; Bendell, J.; Enzinger, P.; Marina, N.; Xiang, H.; et al. Phase I Escalation and Expansion Study of Bemarituzumab (FPA144) in Patients with Advanced Solid Tumors and FGFR2b-Selected Gastroesophageal Adenocarcinoma. J. Clin. Oncol. 2020, 38, 2418–2426. [Google Scholar] [CrossRef]
- Kang, Y.K.; Qin, S.; Lee, K.W.; Oh, S.C.; Kim, I.H.; Kim, J.G.; Li, Y.; Yan, Z.; Li, J.; Bai, L.Y.; et al. Bemarituzumab plus mFOLFOX6 as first-line treatment in East Asian patients with FGFR2b-overexpressing locally advanced or metastatic gastric/gastroesophageal junction cancer: Subgroup of FIGHT final analysis. Gastric Cancer 2024, 27, 1046–1057. [Google Scholar] [CrossRef]
- Smyth, E.C.; Chao, J.; Muro, K.; Yen, P.; Yanes, R.E.; Zahlten-Kumeli, A.; Rha, S.Y. Trial in progress: Phase 3 study of bemarituzumab + mFOLFOX6 versus placebo + mFOLFOX6 in previously untreated advanced gastric or gastroesophageal junction (GEJ) cancer with FGFR2b overexpression (FORTITUDE-101). J. Clin. Oncol. 2022, 40, TPS4164. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Van Cutsem, E.; Moehler, M.H.; Kang, Y.-K.; Yen, P.; Finger, E.; Keegan, A.; Shitara, K. Trial in progress: Phase 1b/3 study of bemarituzumab + mFOLFOX6 + nivolumab versus mFOLFOX6 + nivolumab in previously untreated advanced gastric and gastroesophageal junction (GEJ) cancer with FGFR2b overexpression (FORTITUDE-102). J. Clin. Oncol. 2022, 40, TPS4165. [Google Scholar] [CrossRef]
- Lee, K.W.; Kang, Y.K.; Chen, M.H.; Hirano, H.; Sunakawa, Y.; Kim, S.T.; Lin, C.C.; Yong, W.P.; Kawakami, T.; Oshima, T.; et al. 146P Phase (ph) Ib results of bemarituzumab (BEMA) added to capecitabine/oxaliplatin (CAPOX) or S-1/oxaliplatin (SOX) with or without nivolumab (NIVO) for previously untreated advanced gastric/gastroesophageal junction cancer (G/GEJC): FORTITUDE-103 study. Ann. Oncol. 2023, 34, S1530. [Google Scholar] [CrossRef]
- Kwak, Y.; Kim, T.Y.; Nam, S.K.; Hwang, H.J.; Han, D.; Oh, H.J.; Kong, S.H.; Park, D.J.; Oh, D.Y.; Lee, H.J.; et al. Clinicopathologic and molecular characterization of stages II-IV gastric cancer with Claudin 18.2 expression. Oncologist 2024. [Google Scholar] [CrossRef]
- Tsimafeyeu, I.; Musayeva, G.; Mahmudova, S.; Otkhozoria, N.; Abbasov, B.; Kahharov, A.; Guliyev, F. Nivolumab Combined with Chemotherapy in FGFR2 and PD-L1 Co-Expressing Metastatic Gastric Cancer: A Prospective Phase 2 NIVOFGFR2 Study. J. Gastrointest. Cancer 2025, 56, 40. [Google Scholar] [CrossRef]
| CheckMate-649 [7] | ATTRACTION-4 [8] | KEYNOTE-859 [9] | ||||
|---|---|---|---|---|---|---|
| Treatment | Nivo + CT | Nivo + CT | Pembro + CT | |||
| Chemotherapy regimen | CAPOX or FOLFOX | SOX or CAPOX | CAPOX or FP | |||
| Incidence of AEs (%) | Any Grade | Grade ≥ 3 | Any Grade | Grade ≥ 3 | Any Grade | Grade ≥ 3 |
| Any | NA | NA | NA | 18 | 27 | 8 |
| Gastrointestinal/Colitis | 34 | 5 | 35.9 | 5.8 | 3 | 2 |
| Skin related | 28 | 4 | 37.3 | 3.9 | 2 | 2 |
| Hepatic | 27 | 4 | 23.1 | 4.2 | 1 | 0.4 |
| Endocrine | 14 | 0.8 | 11.4 | 2.2 | NA | NA |
| Hypothyroidism | NA | NA | NA | NA | 15 | 0.1 |
| Hyperthyroidism | NA | NA | NA | NA | 6 | 0 |
| Thyroiditis | NA | NA | NA | NA | 1 | 0 |
| Adrenal insufficiency | NA | NA | NA | NA | 1 | 0.5 |
| Pulmonary/Pneumonitis | 5 | 2 | 3.3 | 1.1 | 3 | 1 |
| Renal/Nephritis | 4 | 0.9 | 2.5 | 0.3 | 0.5 | 0.5 |
| Jia et al. [18] | Pellino et al. [17] | Kubota et al. [15] | Combined Analysis of SPOTLIGHT and GLOW [16] | Kim et al. [19] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients, n (%) | CLDN+ | CLDN- | CLDN+ | CLDN- | CLDN+ | CLDN- | CLDN+ | CLDN- | CLDN+ | CLDN- | |
| n = 42 (52.5) | n = 38 (47.5) | n = 117 (33.4) | n = 233 (66.6) | n = 98 (24.0) | n = 310 (76.0) | n = 1730 (38.4) | n = 2777 (61.6) | n = 96 (47.1) | n = 108 (52.9) | ||
| Sex | Female | 7 (16.7) | 12 (31.6) | 37 (31.6) | 95 (40.8) | 32 (32.7) | 95 (30.6) | 637 (36.8) | 853 (30.7) | 45 (46.9) | 38 (35.2) |
| Male | 35 (83.3) | 26 (68.4) | 80 (68.4) | 138 (59.2) | 66 (67.3) | 215 (69.4) | 1093 (63.2) | 1924 (69.3) | 51 (53.1) | 70 (64.8) | |
| HER2 | positive | 9 (21.4) | 13 (34.2) | 17 (14.5) | 35 (15.0) | 15 (15.3) | 43 (13.9) | NA | NA | NA | NA |
| negative | 33 (78.6) | 25 (65.8) | 100 (85.5) | 198 (85.0) | 83 (84.7) | 267 (86.1) | NA | NA | NA | NA | |
| PD-L1 | CPS < 1 | 9 (21.4) | 8 (21.1) | 87 (74.4) | 165 (70.8) | 24 (25.8) | 68 (23.2) | NA | NA | NA | NA |
| CPS ≥ 1 | 33 (78.6) | 30 (78.9) | 30 (25.6) | 68 (29.2) | 69 (74.2) | 225 (76.8) | NA | NA | NA | NA | |
| CPS < 5 | 18 (42.9) | 16 (42.1) | 96 (82.1) | 183 (78.5) | 54 (58.1) | 142 (48.5) | 495 (82.6) | NA | 59 (62.1) | 53 (51.5) | |
| CPS ≥ 5 | 24 (57.1) | 22 (57.9) | 21 (17.9) | 50 (21.5) | 39 (41.9) | 151 (51.5) | 104 (17.4) | NA | 36 (37.9) | 50 (48.5) | |
| CPS < 10 | 23 (54.8) | 21 (55.3) | NA | NA | NA | NA | NA | NA | NA | NA | |
| CPS ≥ 10 | 19 (45.2) | 17 (44.7) | NA | NA | NA | NA | NA | NA | NA | NA | |
| MMR status | pMMR | 36 (85.7) | 33 (86.8) | 102 (87.2) | 194 (83.3) | 93 (94.9) | 291 (93.9) | NA | NA | NA | NA |
| dMMR | 6 (14.3) | 5 (13.2) | 15 (12.8) | 39 (16.7) | 5 (5.1) | 19 (6.1) | NA | NA | 9 (10.0) | 16 (16.7) | |
| Lauren classification | Diffuse type | 12 (28.6) | 22 (57.9) | 47 (40.2) | 70 (30.0) | 47 (48.0) | 137 (44.2) | 553 (33.3) | 592 (24.2) | 25 (26.0) | 25 (23.1) |
| Intestinal type | 16 (38.1) | 6 (15.8) | 54 (46.2) | 132 (56.7) | 51 (52.0) | 173 (55.8) | 308 (18.6) | 486 (19.9) | 71 (74.0) | 83 (76.9) | |
| Mixed /Other | 14 (33.3) | 10 (26.3) | 14 (12.0) | 25 (10.7) | NA | NA | 386 (23.2) | 603 (24.7) | NA | NA | |
| SPOTLIGHT [13] | GLOW [14] | CheckMate-649 [7] | ATTRACTION-4 [8] | KEYNOTE-859 [9] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | ZOL + CT | CT | ZOL + CT | CT | Nivo + CT | CT | Nivo + CT | CT | Pembro + CT | CT | |
| CT regimen | mFOLFOX6 | CAPOX | CAPOX or FOLFOX | SOX or CAPOX | CAPOX or FP | ||||||
| Patients, n | 283 | 282 | 254 | 253 | 789 | 833 | 362 | 362 | 790 | 789 | |
| median observation time, months | 22.1 | 20.9 | 17.7 | 18.4 | 47.4 | 47.3 | 26.6 | 31.0 | |||
| mOS, months | ITT polulation | 18.23 | 15.54 | 14.39 | 12.16 | 13.7 | 11.6 | 17.45 | 17.15 | 12.9 | 11.5 |
| HR for OS (95%CI) | 0.75 (0.60–0.94) | 0.771 (0.615–0.965) | 0.79 (0.71–0.88) | 0.90 (0.75–1.08) | 0.78 (0.70–0.87) | ||||||
| CPS ≥ 10 | NA | NA | NA | NA | 15.0 | 10.9 | NA | NA | 15.7 | 11.8 | |
| NA | NA | 0.66 (0.57–0.77) | NA | 0.65 (0.53–0.79) | |||||||
| CPS ≥ 5 | NA | NA | NA | NA | 14.4 | 11.1 | NA | NA | NA | NA | |
| NA | NA | 0.69 (0.60–0.79) | NA | NA | |||||||
| CPS ≥ 1 | NA | NA | NA | NA | 13.8 | 11.3 | NA | NA | 13.0 | 11.4 | |
| NA | NA | 0.75 (0.66–0.84) | NA | 0.74 (0.65–0.84) | |||||||
| mPFS, months | ITT polulation | 10.61 | 8.67 | 8.21 | 6.80 | 7.7 | 6.9 | 10.94 | 8.41 | 6.9 | 5.6 |
| HR for PFS (95%CI) | 0.75 (0.60–0.94) | 0.687 (0.544–0.866) | 0.79 (0.71–0.89) | 0.70 (0.57–0.86) | 0.76 (0.67–0.85) | ||||||
| CPS ≥ 10 | NA | NA | NA | NA | NA | NA | NA | NA | 8.1 | 5.6 | |
| NA | NA | NA | NA | 0.62 (0.51–0.76) | |||||||
| CPS ≥ 5 | NA | NA | NA | NA | 8.3 | 6.1 | NA | NA | NA | NA | |
| NA | NA | 0.70 (0.60–0.81) | NA | NA | |||||||
| CPS ≥ 1 | NA | NA | NA | NA | 7.5 | 6.9 | NA | NA | 6.9 | 5.6 | |
| NA | NA | 0.77 (0.68–0.88) | NA | 0.72 (0.63–0.82) | |||||||
| ORR, % | ITT polulation | 61 | 62 | 53.8 | 48.8 | 58 | 46 | 57 | 48 | 51 | 42 |
| CPS ≥ 10 | NA | NA | NA | NA | 59 | 45 | NA | NA | 61 | 43 | |
| CPS ≥ 5 | NA | NA | NA | NA | 60 | 45 | NA | NA | NA | NA | |
| CPS ≥ 1 | NA | NA | NA | NA | 60 | 46 | NA | NA | 52 | 43 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyajima, Y.; Kawakami, T. Treatment Selection for Patients with HER2-Negative Metastatic Gastric Cancer Expressing Claudin 18.2 and PD-L1. Cancers 2025, 17, 1120. https://doi.org/10.3390/cancers17071120
Miyajima Y, Kawakami T. Treatment Selection for Patients with HER2-Negative Metastatic Gastric Cancer Expressing Claudin 18.2 and PD-L1. Cancers. 2025; 17(7):1120. https://doi.org/10.3390/cancers17071120
Chicago/Turabian StyleMiyajima, Yusuke, and Takeshi Kawakami. 2025. "Treatment Selection for Patients with HER2-Negative Metastatic Gastric Cancer Expressing Claudin 18.2 and PD-L1" Cancers 17, no. 7: 1120. https://doi.org/10.3390/cancers17071120
APA StyleMiyajima, Y., & Kawakami, T. (2025). Treatment Selection for Patients with HER2-Negative Metastatic Gastric Cancer Expressing Claudin 18.2 and PD-L1. Cancers, 17(7), 1120. https://doi.org/10.3390/cancers17071120






