Assessing the Impact of Nutritional Status on the Quality of Life in Head and Neck Cancer Patients—The Need for Comprehensive Digital Tools
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Clinical and Demographical Variables Reported in QoL Studies
3.1.1. Sex and Age
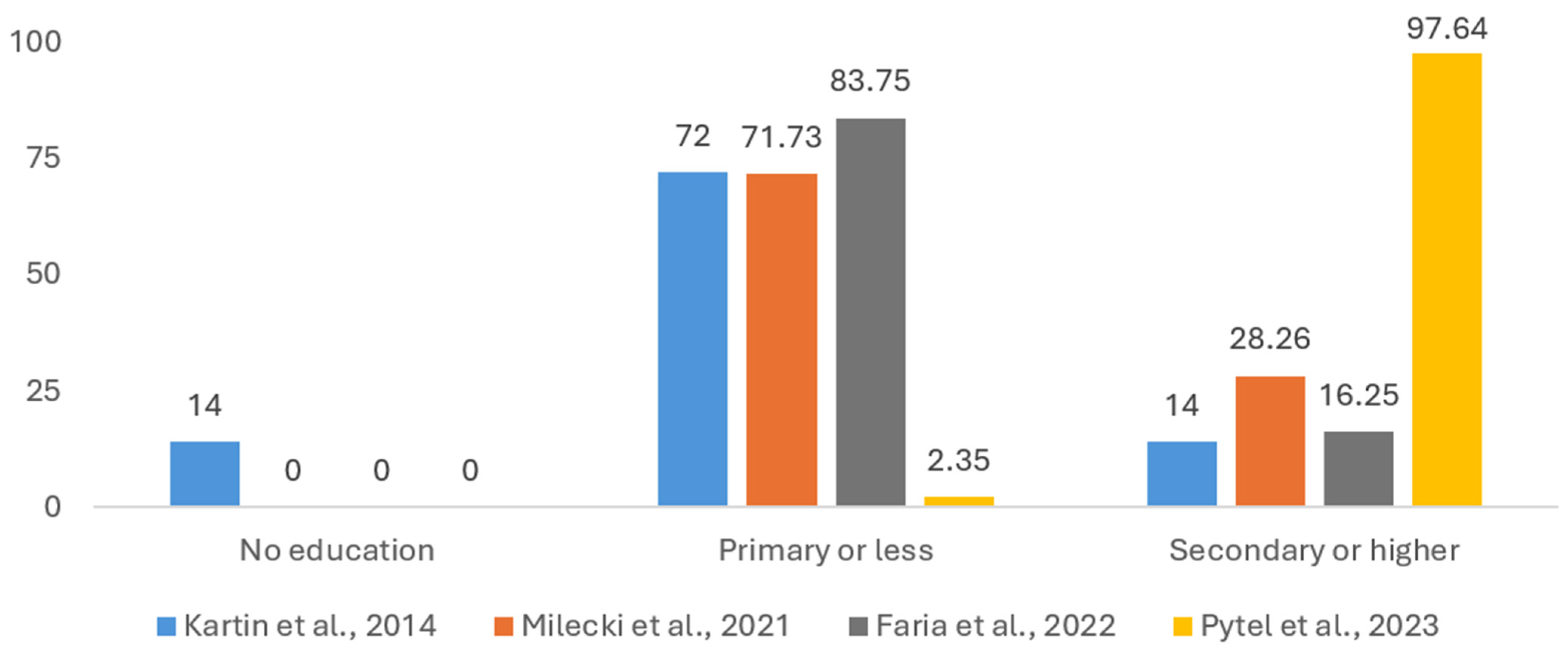
3.1.2. Patients’ Education Level
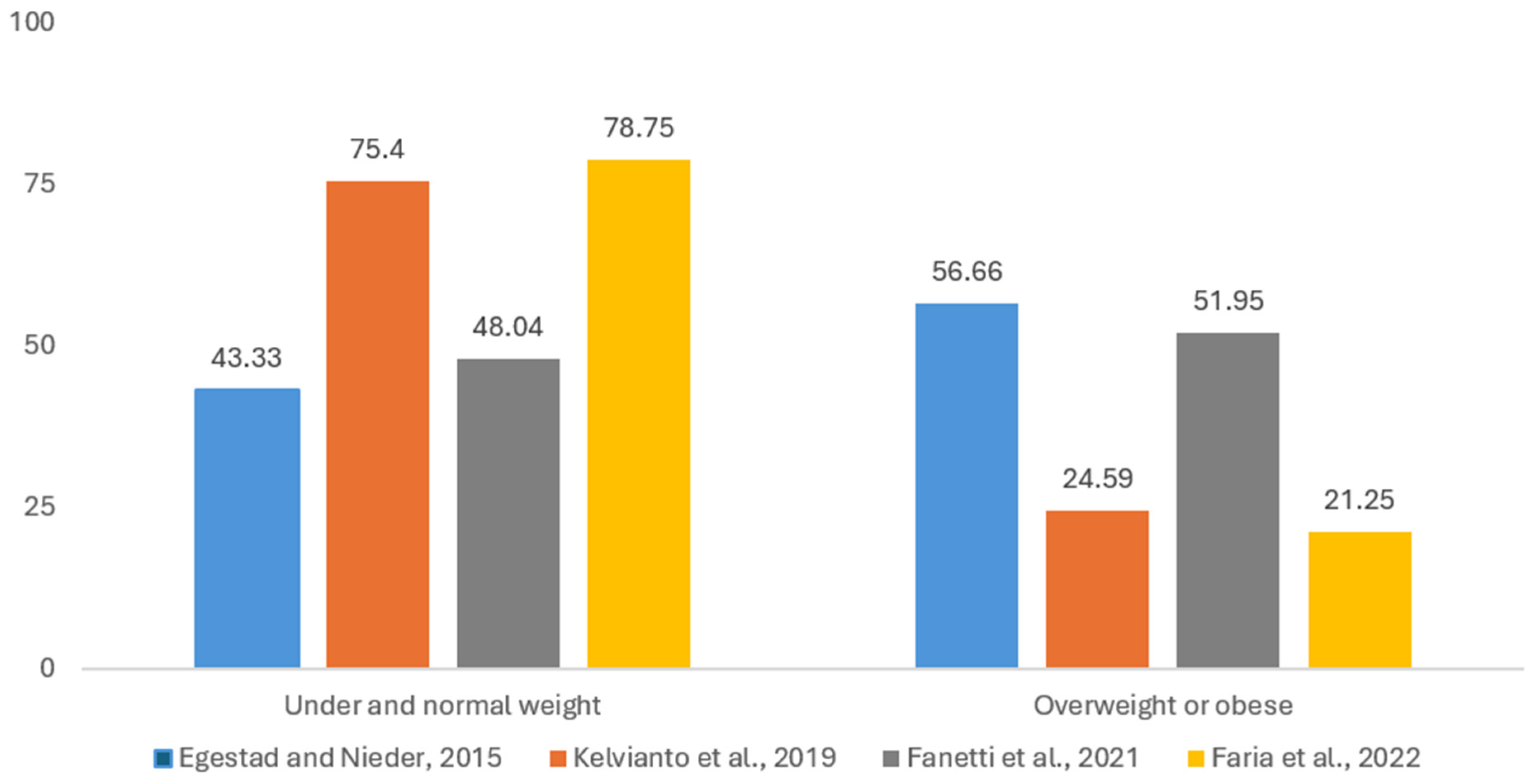
3.1.3. Weight Loss and Body Mass Index (BMI)
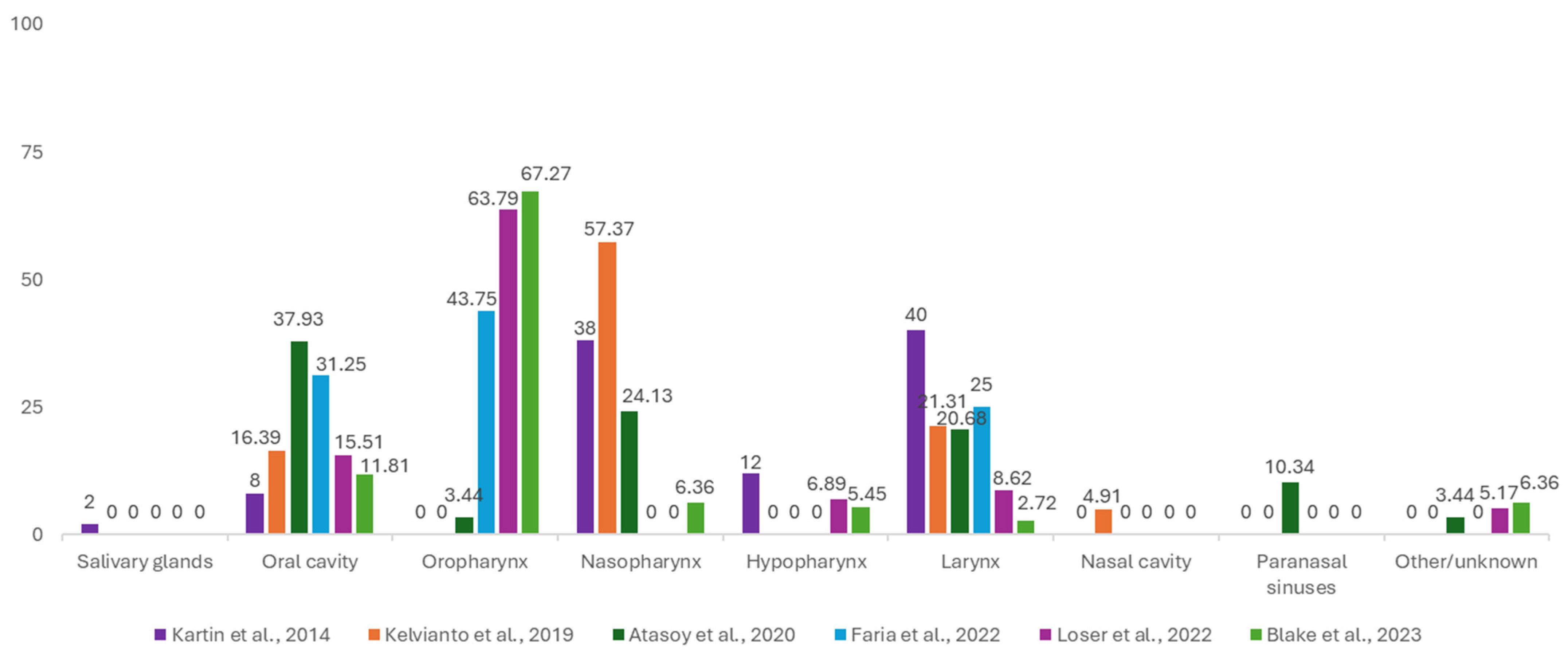
3.1.4. Tumor Sites
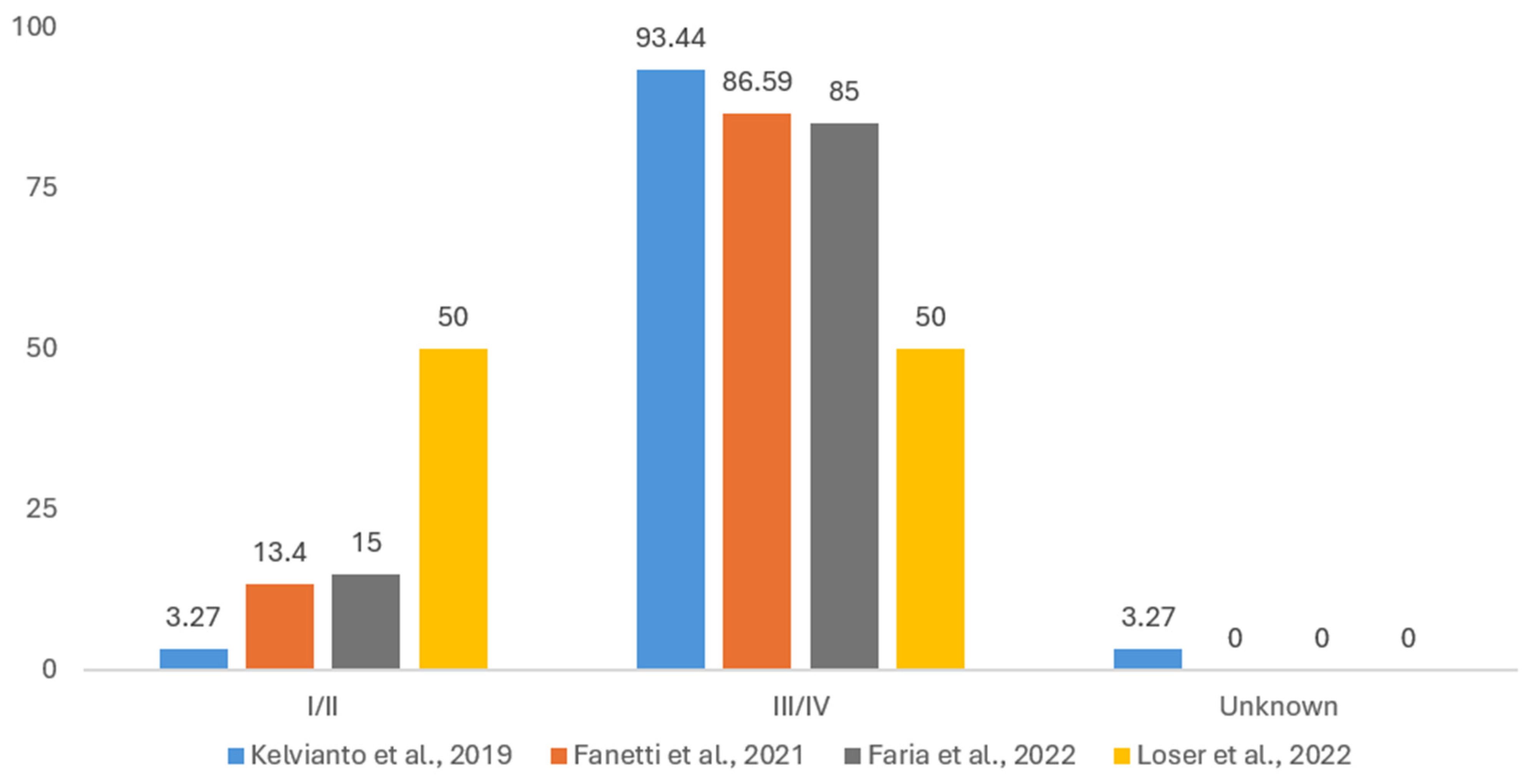
3.1.5. Disease Staging
3.1.6. Treatment Modalities
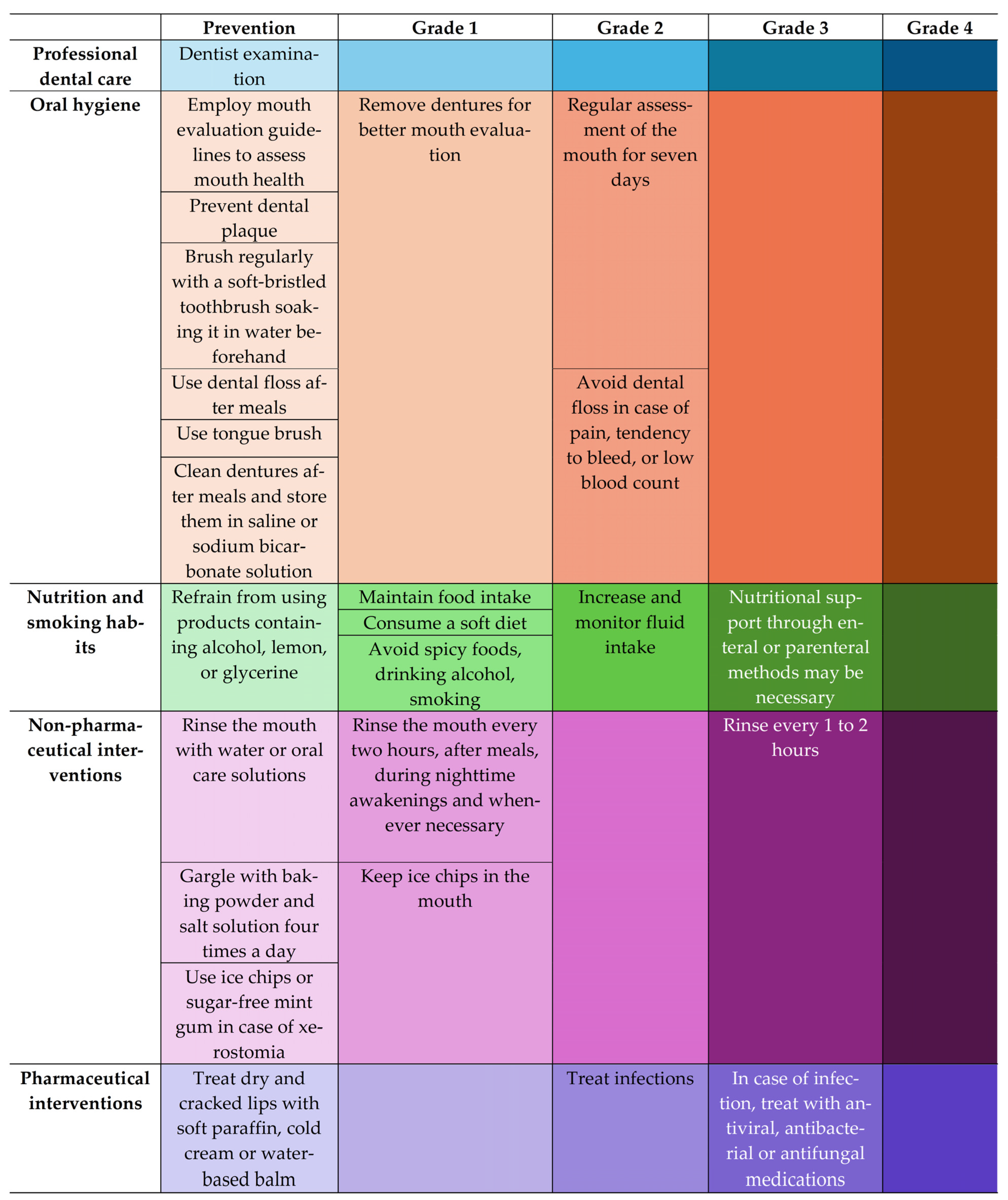
3.2. Oral Mucositis: Impact on QoL Nutritional Aspects, Prevention, Control, and Treatment
3.3. QoL Questionnaires and Associations with Nutritional Status
4. Discussion
4.1. Treatment-Related Adverse Effects Leading to Malnutrition
4.2. Diagnosis, Evaluation, and Correlates of Malnutrition
4.3. Relationships of Nutritional Status and Feeding Capacity with QoL
4.4. Prevention and Interventions—Non-Uniformity and Gaps
4.5. Bettering QoL Evalution in HNC Patients by Digital Solutions
4.5.1. Digitalization in Healthcare
4.5.2. Nutrition Status and Feeding Capacity in Current QoL Evaluations
4.5.3. Limitations of Current Data Landscape of QoL and Nutritional Status
4.5.4. Personalization of Interventions by Comprehensive Description of Disease Complexity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AE | adverse effects |
| BMI | body mass index |
| CCRT | chemoradiation therapy |
| DT | digital twin |
| EBV | Epstein–Barr virus |
| EORTC | European Organization for Research and Treatment of Cancer |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| FFM | fat-free mass |
| FFMI | fat-free mass index |
| HNCs | head and neck cancers |
| HNSCC | head and neck squamous cell carcinomas |
| HPV | Human Papillomavirus |
| IMRT | intensity-modulated radiotherapy |
| MUST | Malnutrition Universal Screening Tool |
| NRS-2002 | Nutritional Risk Screening 2002 |
| QoL | quality of life |
| RT | radiotherapy |
| UICC | Union Internationale Contre le Cancer |
References
- Huang, S.H.; O’Sullivan, B. Overview of the 8th Edition TNM Classification for Head and Neck Cancer. Curr. Treat. Options Oncol. 2017, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Li, Y.; Forastiere, A. Prognostic Factors and Long-Term Survivorship in Patients with Recurrent or Metastatic Carcinoma of the Head and Neck. Cancer 2004, 101, 2222–2229. [Google Scholar] [CrossRef] [PubMed]
- Elrefaey, S.; Massaro, M.A.; Chiocca, S.; Chiesa, F.; Ansarin, M. HPV in Oropharyngeal Cancer: The Basics to Know in Clinical Practice. Acta Otorhinolaryngol. Ital. 2014, 34, 299–309. [Google Scholar] [PubMed]
- Al-Anazi, A.E.; Alanazi, B.S.; Alshanbari, H.M.; Masuadi, E.; Hamed, M.E.; Dandachi, I.; Alkathiri, A.; Hanif, A.; Nour, I.; Fatani, H.; et al. Increased Prevalence of EBV Infection in Nasopharyngeal Carcinoma Patients: A Six-Year Cross-Sectional Study. Cancers 2023, 15, 643. [Google Scholar] [CrossRef]
- Cancer (IARC). T.I.A. for R. on Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 24 November 2024).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wissinger, E.; Griebsch, I.; Lungershausen, J.; Foster, T.; Pashos, C.L. The Economic Burden of Head and Neck Cancer: A Systematic Literature Review. Pharmacoeconomics 2014, 32, 865–882. [Google Scholar] [CrossRef]
- Vigneswaran, N.; Williams, M.D. Epidemiologic Trends in Head and Neck Cancer and Aids in Diagnosis. Oral Maxillofac. Surg. Clin. N. Am. 2014, 26, 123–141. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Joshi, P.; Dutta, S.; Chaturvedi, P.; Nair, S. Head and Neck Cancers in Developing Countries. Rambam Maimonides Med. J. 2014, 5, e0009. [Google Scholar] [CrossRef]
- Diz, P.; Meleti, M.; Diniz-Freitas, M.; Vescovi, P.; Warnakulasuriya, S.; Johnson, N.W.; Kerr, A.R. Oral and Pharyngeal Cancer in Europe: Incidence, Mortality and Trends as Presented to the Global Oral Cancer Forum. Transl. Res. Oral Oncol. 2017, 2, 2057178X17701517. [Google Scholar] [CrossRef]
- Didkowska, J. Cancer in Poland in 2019; Polish National Cancer Registry: Warszawa, Poland, 2021; pp. 76–79. [Google Scholar]
- Profil de Țară Privind Cancerul—2023. 2024. Available online: https://insp.gov.ro/2024/07/08/profil-de-tara-privind-cancerul-2023/ (accessed on 28 January 2025).
- Barajas Galindo, D.E.; Vidal-Casariego, A.; Calleja-Fernández, A.; Hernández-Moreno, A.; Pintor de la Maza, B.; Pedraza-Lorenzo, M.; Rodríguez-García, M.A.; Ávila-Turcios, D.M.; Alejo-Ramos, M.; Villar-Taibo, R.; et al. Appetite Disorders in Cancer Patients: Impact on Nutritional Status and Quality of Life. Appetite 2017, 114, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Wallengren, O.; Lundholm, K.; Bosaeus, I. Diagnostic Criteria of Cancer Cachexia: Relation to Quality of Life, Exercise Capacity and Survival in Unselected Palliative Care Patients. Support. Care Cancer 2013, 21, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Gorenc, M.; Kozjek, N.R.; Strojan, P. Malnutrition and Cachexia in Patients with Head and Neck Cancer Treated with (Chemo)Radiotherapy. Rep. Pract. Oncol. Radiother. 2015, 20, 249–258. [Google Scholar] [CrossRef]
- Pytel, A.; Zielińska, A.; Staś, J.; Chabowski, M. Quality of Life, Psychological Distress, and Nutritional Status of Polish Patients with Head and Neck Cancer Treated with Radiotherapy. J. Clin. Med. 2023, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Kelvianto, A.; Witjaksono, F.; Sekarutami, S.M. Protein Intake, Prognostic Nutritional Index and Quality of Life in Head and Neck Cancer Patients Undergoing Radiotherapy. Indones. Biomed. J. 2019, 11, 70–77. [Google Scholar] [CrossRef]
- Machiels, J.-P.; René Leemans, C.; Golusinski, W.; Grau, C.; Licitra, L.; Gregoire, V.; EHNS Executive Board; ESMO Guidelines Committee; ESTRO Executive Board. Squamous Cell Carcinoma of the Oral Cavity, Larynx, Oropharynx and Hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 1462–1475. [Google Scholar] [CrossRef]
- Pignon, J.-P.; le Maître, A.; Maillard, E.; Bourhis, J. Meta-Analysis of Chemotherapy in Head and Neck Cancer (MACH-NC): An Update on 93 Randomised Trials and 17,346 Patients. Radiother. Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef]
- Marta, G.N.; Silva, V.; de Andrade Carvalho, H.; de Arruda, F.F.; Hanna, S.A.; Gadia, R.; da Silva, J.L.F.; Correa, S.F.M.; Vita Abreu, C.E.C.; Riera, R. Intensity-Modulated Radiation Therapy for Head and Neck Cancer: Systematic Review and Meta-Analysis. Radiother. Oncol. 2014, 110, 9–15. [Google Scholar] [CrossRef]
- Van Gestel, D.; Verellen, D.; Van De Voorde, L.; de Ost, B.; De Kerf, G.; Vanderveken, O.; Van Laer, C.; Van den Weyngaert, D.; Vermorken, J.B.; Gregoire, V. The Potential of Helical Tomotherapy in the Treatment of Head and Neck Cancer. Oncologist 2013, 18, 697–706. [Google Scholar] [CrossRef]
- Peng, G.; Wang, T.; Yang, K.-Y.; Zhang, S.; Zhang, T.; Li, Q.; Han, J.; Wu, G. A Prospective, Randomized Study Comparing Outcomes and Toxicities of Intensity-Modulated Radiotherapy vs. Conventional Two-Dimensional Radiotherapy for the Treatment of Nasopharyngeal Carcinoma. Radiother. Oncol. 2012, 104, 286–293. [Google Scholar] [CrossRef]
- Kam, M.K.M.; Leung, S.-F.; Zee, B.; Chau, R.M.C.; Suen, J.J.S.; Mo, F.; Lai, M.; Ho, R.; Cheung, K.; Yu, B.K.H.; et al. Prospective Randomized Study of Intensity-Modulated Radiotherapy on Salivary Gland Function in Early-Stage Nasopharyngeal Carcinoma Patients. J. Clin. Oncol. 2007, 25, 4873–4879. [Google Scholar] [CrossRef] [PubMed]
- Nutting, C.M.; Morden, J.P.; Harrington, K.J.; Urbano, T.G.; Bhide, S.A.; Clark, C.; Miles, E.A.; Miah, A.B.; Newbold, K.; Tanay, M.; et al. Parotid-Sparing Intensity Modulated versus Conventional Radiotherapy in Head and Neck Cancer (PARSPORT): A Phase 3 Multicentre Randomised Controlled Trial. Lancet Oncol. 2011, 12, 127–136. [Google Scholar] [CrossRef]
- Gupta, T.; Agarwal, J.; Jain, S.; Phurailatpam, R.; Kannan, S.; Ghosh-Laskar, S.; Murthy, V.; Budrukkar, A.; Dinshaw, K.; Prabhash, K.; et al. Three-Dimensional Conformal Radiotherapy (3D-CRT) versus Intensity Modulated Radiation Therapy (IMRT) in Squamous Cell Carcinoma of the Head and Neck: A Randomized Controlled Trial. Radiother. Oncol. 2012, 104, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.; Banks, M.; Hughes, B.G.M.; Lin, C.; Kenny, L.M.; Bauer, J.D. New Radiotherapy Techniques Do Not Reduce the Need for Nutrition Intervention in Patients with Head and Neck Cancer. Eur. J. Clin. Nutr. 2015, 69, 1119–1124. [Google Scholar] [CrossRef]
- Moroney, L.B.; Helios, J.; Ward, E.C.; Crombie, J.; Wockner, L.F.; Burns, C.L.; Spurgin, A.-L.; Blake, C.; Kenny, L.; Hughes, B.G.M. Patterns of Dysphagia and Acute Toxicities in Patients with Head and Neck Cancer Undergoing Helical IMRT±concurrent Chemotherapy. Oral. Oncol. 2017, 64, 1–8. [Google Scholar] [CrossRef]
- Fanetti, G.; Polesel, J.; Fratta, E.; Muraro, E.; Lupato, V.; Alfieri, S.; Gobitti, C.; Minatel, E.; Matrone, F.; Caroli, A.; et al. Prognostic Nutritional Index Predicts Toxicity in Head and Neck Cancer Patients Treated with Definitive Radiotherapy in Association with Chemotherapy. Nutrients 2021, 13, 1277. [Google Scholar] [CrossRef]
- Wulff-Burchfield, E.; Dietrich, M.S.; Ridner, S.; Murphy, B.A. Late Systemic Symptoms in Head and Neck Cancer Survivors. Support. Care Cancer 2019, 27, 2893–2902. [Google Scholar] [CrossRef]
- Alterio, D.; Marvaso, G.; Ferrari, A.; Volpe, S.; Orecchia, R.; Jereczek-Fossa, B.A. Modern Radiotherapy for Head and Neck Cancer. Semin. Oncol. 2019, 46, 233–245. [Google Scholar] [CrossRef]
- Head, B.A.; Heitz, L.; Keeney, C.; Myers, J.; Appana, S.N.; Studts, J.L.; Bumpous, J.; Pfeifer, M. The Relationship between Weight Loss and Health-Related Quality of Life in Persons Treated for Head and Neck Cancer. Support. Care Cancer 2011, 19, 1511–1518. [Google Scholar] [CrossRef]
- Söderlund Schaller, A.; Dragioti, E.; Liedberg, G.M.; Larsson, B. Are Patient Education and Self-Care Advantageous for Patients with Head and Neck Cancer? A Feasibility Study. Nurs. Open 2019, 6, 1528–1541. [Google Scholar] [CrossRef]
- Liao, L.-J.; Hsu, W.-L.; Lo, W.-C.; Cheng, P.-W.; Shueng, P.-W.; Hsieh, C.-H. Health-Related Quality of Life and Utility in Head and Neck Cancer Survivors. BMC Cancer 2019, 19, 425. [Google Scholar] [CrossRef] [PubMed]
- Hammermüller, C.; Hinz, A.; Dietz, A.; Wichmann, G.; Pirlich, M.; Berger, T.; Zimmermann, K.; Neumuth, T.; Mehnert-Theuerkauf, A.; Wiegand, S.; et al. Depression, Anxiety, Fatigue, and Quality of Life in a Large Sample of Patients Suffering from Head and Neck Cancer in Comparison with the General Population. BMC Cancer 2021, 21, 94. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.J.; Soulières, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.F.; Ahn, M.-J.; Soria, A.; Machiels, J.-P.H.; Mach, N.; Mehra, R.; et al. Quality of Life With Pembrolizumab for Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: KEYNOTE-040. J. Natl. Cancer Inst. 2021, 113, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Nikoloudi, M.; Lymvaios, I.; Zygogianni, A.; Parpa, E.; Strikou, D.-A.; Tsilika, E.; Kouloulias, V.; Mystakidou, K. Quality of Life, Anxiety, and Depression in the Head-and-Neck Cancer Patients, Undergoing Intensity-Modulated Radiotherapy Treatment. Indian J. Palliat. Care 2020, 26, 54–59. [Google Scholar] [CrossRef]
- Nayak, S.G.; Pai, M.S.; George, L.S. Quality of Life of Patients with Head and Neck Cancer: A Mixed Method Study. J. Cancer Res. Ther. 2019, 15, 638–644. [Google Scholar] [CrossRef]
- Samuel, S.R.; Maiya, A.G.; Fernandes, D.J.; Guddattu, V.; Saxena, P.U.P.; Kurian, J.R.; Lin, P.-J.; Mustian, K.M. Effectiveness of Exercise-Based Rehabilitation on Functional Capacity and Quality of Life in Head and Neck Cancer Patients Receiving Chemo-Radiotherapy. Support. Care Cancer 2019, 27, 3913–3920. [Google Scholar] [CrossRef]
- Langius, J.A.E.; Doornaert, P.; Spreeuwenberg, M.D.; Langendijk, J.A.; Leemans, C.R.; van B. van der Schueren, M.A.E. Radiotherapy on the Neck Nodes Predicts Severe Weight Loss in Patients with Early Stage Laryngeal Cancer. Radiother. Oncol. 2010, 97, 80–85. [Google Scholar] [CrossRef]
- Elting, L.S.; Cooksley, C.D.; Chambers, M.S.; Garden, A.S. Risk, Outcomes, and Costs of Radiation-Induced Oral Mucositis Among Patients With Head-and-Neck Malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1110–1120. [Google Scholar] [CrossRef]
- Milecki, J.; Żmijewska-Tomczak, M.; Osmola, K.; Wierzbicka, M. The Impact of Radiotherapy on the Quality of Life in Patients with Early-Stage Clinical Head and Neck Cancer. Otolaryngol. Pol. 2021, 75, 1–8. [Google Scholar] [CrossRef]
- Wan Leung, S.; Lee, T.-F.; Chien, C.-Y.; Chao, P.-J.; Tsai, W.-L.; Fang, F.-M. Health-Related Quality of Life in 640 Head and Neck Cancer Survivors after Radiotherapy Using EORTC QLQ-C30 and QLQ-H&N35 Questionnaires. BMC Cancer 2011, 11, 128. [Google Scholar] [CrossRef]
- Mendi, A.F. A Digital Twin Case Study on Automotive Production Line. Sensors 2022, 22, 6963. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.-P.; Volpert, A.; Antonino, P.; Ahrens, T.D. Digital Patient Twins for Personalized Therapeutics and Pharmaceutical Manufacturing. Front. Digit. Health 2024, 5, 1302338. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 5 January 2025).
- Egestad, H.; Nieder, C. Differences in Quality of Life in Obese and Normal Weight Head and Neck Cancer Patients Undergoing Radiation Therapy. Support. Care Cancer 2015, 23, 1081–1090. [Google Scholar] [CrossRef]
- Kartin, P.T.; Tasci, S.; Soyuer, S.; Elmali, F. Effect of an Oral Mucositis Protocol on Quality of Life of Patients with Head and Neck Cancer Treated with Radiation Therapy. Clin. J. Oncol. Nurs. 2014, 18, E118–E125. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, B.M.; Kayhan, K.B.; Demirel, B.; Akdeniz, E. Mucositis-Induced Pain Due to Barrier Dysfunction May Have a Direct Effect on Nutritional Status and Quality of Life in Head and Neck Cancer Patients Receiving Radiotherapy. Turk. J. Oncol. 2020, 35, 40–46. [Google Scholar] [CrossRef]
- De Oliveira Faria, S.; Simões Lima, G.A.; Lopes Carvalho, A.; Nader Marta, G.; Howell, D.; Eluf-Neto, J. Clinically Significant Changes in Health-Related Quality of Life in Head and Neck Cancer Patients Following Intensive Nutritional Care during Radiotherapy. Eur. J. Oncol. Nurs. 2022, 56, 102065. [Google Scholar] [CrossRef]
- Blake, C.L.; Brown, T.E.; Pelecanos, A.; Moroney, L.B.; Helios, J.; Hughes, B.G.M.; Chua, B.; Kenny, L.M. Enteral Nutrition Support and Treatment Toxicities in Patients with Head and Neck Cancer Receiving Definitive or Adjuvant Helical Intensity-Modulated Radiotherapy with Concurrent Chemotherapy. Head Neck 2023, 45, 417–430. [Google Scholar] [CrossRef]
- Löser, A.; Avanesov, M.; Thieme, A.; Gargioni, E.; Baehr, A.; Hintelmann, K.; Tribius, S.; Krüll, A. Nutritional Status Impacts Quality of Life in Head and Neck Cancer Patients Undergoing (Chemo)Radiotherapy: Results from the Prospective HEADNUT Trial. Nutr. Cancer 2022, 74, 2887–2895. [Google Scholar]
- A Healthy Lifestyle—WHO Recommendations. Available online: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations (accessed on 28 January 2025).
- Mason, H.; DeRubeis, M.B.; Burke, N.; Shannon, M.; Karsies, D.; Wolf, G.; Eisbruch, A.; Worden, F. Symptom Management during and after Treatment with Concurrent Chemoradiotherapy for Oropharyngeal Cancer: A Review of the Literature and Areas for Future Research. World J. Clin. Oncol. 2016, 7, 220–226. [Google Scholar] [CrossRef]
- Arribas, L.; Hurtós, L.; Taberna, M.; Peiró, I.; Vilajosana, E.; Lozano, A.; Vazquez, S.; Mesia, R.; Virgili, N. Nutritional Changes in Patients with Locally Advanced Head and Neck Cancer during Treatment. Oral Oncol. 2017, 71, 67–74. [Google Scholar] [CrossRef]
- Gomes, E.P.A.A.; Aranha, A.M.F.; Borges, A.H.; Volpato, L.E.R. Head and Neck Cancer Patients’ Quality of Life: Analysis of Three Instruments. J. Dent. 2020, 21, 31–41. [Google Scholar] [CrossRef]
- Soldera, E.B.; Ortigara, G.B.; Bonzanini, L.I.L.; Schulz, R.E.; Danesi, C.C.; Antoniazzi, R.P.; Linhares Ferrazzo, K. Clinical and Sociodemographic Factors Associated with Oral Health-Related Quality of Life in Survivors of Head and Neck Cancer. Head Neck 2020, 42, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Hinz, A.; Mehnert, A.; Dégi, C.; Reissmann, D.R.; Schotte, D.; Schulte, T. The Relationship between Global and Specific Components of Quality of Life, Assessed with the EORTC QLQ-C30 in a Sample of 2019 Cancer Patients. Eur. J. Cancer Care 2017, 26, e12416. [Google Scholar] [CrossRef]
- Lewandowska, A.; Rudzki, G.; Lewandowski, T.; Próchnicki, M.; Rudzki, S.; Laskowska, B.; Brudniak, J. Quality of Life of Cancer Patients Treated with Chemotherapy. Int. J. Environ. Res. Public Health 2020, 17, 6938. [Google Scholar] [CrossRef]
- Bashir, A.; Kumar, D.; Dewan, D.; Sharma, R. Quality of Life of Head and Neck Cancer Patients before and after Cancer-Directed Treatment—A Longitudinal Study. J. Cancer Res. Ther. 2020, 16, 500–507. [Google Scholar] [CrossRef]
- Wondie, Y.; Hinz, A. Quality of Life among Ethiopian Cancer Patients. Support. Care Cancer 2020, 28, 5469–5478. [Google Scholar] [CrossRef]
- Ge, X.; Liao, Z.; Yuan, J.; Mao, D.; Li, Y.; Yu, E.; Wang, X.; Ding, Z. Radiotherapy-Related Quality of Life in Patients with Head and Neck Cancers: A Meta-Analysis. Support. Care Cancer 2020, 28, 2701–2712. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhao, X.; Zhao, Q.; Zhang, Y.; Liu, S.; Liu, Z.; Meng, L.; Xin, Y.; Jiang, X. The Effects of Early Nutritional Intervention on Oral Mucositis and Nutritional Status of Patients With Head and Neck Cancer Treated With Radiotherapy. Front. Oncol. 2020, 10, 595632. [Google Scholar] [CrossRef]
- Oba, M.K.; Innocentini, L.M.A.R.; Viani, G.; Ricz, H.M.A.; de Carvalho Reis, T.; Ferrari, T.C.; de Macedo, L.D. Evaluation of the Correlation between Side Effects to Oral Mucosa, Salivary Glands, and General Health Status with Quality of Life during Intensity-Modulated Radiotherapy for Head and Neck Cancer. Support. Care Cancer 2021, 29, 127–134. [Google Scholar] [CrossRef]
- Palma, L.F.; Gonnelli, F.A.S.; Marcucci, M.; Dias, R.S.; Giordani, A.J.; Segreto, R.A.; Segreto, H.R.C. Impact of Low-Level Laser Therapy on Hyposalivation, Salivary pH, and Quality of Life in Head and Neck Cancer Patients Post-Radiotherapy. Lasers Med. Sci. 2017, 32, 827–832. [Google Scholar] [CrossRef]
- Schaller, A.; Dragioti, E.; Liedberg, G.M.; Larsson, B. Quality of Life during Early Radiotherapy in Patients with Head and Neck Cancer and Pain. J. Pain Res. 2017, 10, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- King, M.T. The Interpretation of Scores from the EORTC Quality of Life Questionnaire QLQ-C30. Qual. Life Res. 1996, 5, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Trotti, A.; Bellm, L.A.; Epstein, J.B.; Frame, D.; Fuchs, H.J.; Gwede, C.K.; Komaroff, E.; Nalysnyk, L.; Zilberberg, M.D. Mucositis Incidence, Severity and Associated Outcomes in Patients with Head and Neck Cancer Receiving Radiotherapy with or without Chemotherapy: A Systematic Literature Review. Radiother. Oncol. 2003, 66, 253–262. [Google Scholar] [CrossRef]
- Cavusoglu, H. Evidenced based nursing in the management of oral mucositis: Review. Turk. Klin. J. Med. Sci. 2007, 27, 398–406. [Google Scholar]
- Barasch, A.; Epstein, J.B. Management of Cancer Therapy-Induced Oral Mucositis. Dermatol. Ther. 2011, 24, 424–431. [Google Scholar] [CrossRef]
- Sonis, S.T. Oral Mucositis in Cancer Therapy. J. Support. Oncol. 2004, 2, 3–8. [Google Scholar]
- Yilmaz, M.Ç. Evidence-Based Practices for Managing Mucositis. Int. J. Hematol. Oncol. 2007, 34, 241–246. [Google Scholar]
- Shih, A.; Miaskowski, C.; Dodd, M.J.; Stotts, N.A.; MacPhail, L. Mechanisms for Radiation-Induced Oral Mucositis and the Consequences. Cancer Nurs. 2003, 26, 222–229. [Google Scholar] [CrossRef]
- Silverman, S. Diagnosis and Management of Oral Mucositis. J. Support. Oncol. 2007, 5, 13–21. [Google Scholar]
- Jager-Wittenaar, H.; Dijkstra, P.U.; Vissink, A.; Langendijk, J.A.; van der Laan, B.F.A.M.; Pruim, J.; Roodenburg, J.L.N. Changes in Nutritional Status and Dietary Intake during and after Head and Neck Cancer Treatment. Head Neck 2011, 33, 863–870. [Google Scholar] [CrossRef]
- Unsal, D.; Mentes, B.; Akmansu, M.; Uner, A.; Oguz, M.; Pak, Y. Evaluation of Nutritional Status in Cancer Patients Receiving Radiotherapy: A Prospective Study. Am. J. Clin. Oncol. 2006, 29, 183. [Google Scholar] [CrossRef] [PubMed]
- Beaver, M.E.; Matheny, K.E.; Roberts, D.B.; Myers, J.N. Predictors of Weight Loss during Radiation Therapy. Otolaryngol. Head Neck Surg. 2001, 125, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Marta, G.N.; Saad, E.D. Assessment of Quality of Life in Phase III Trials of Radiotherapy in Localized or Locally Advanced Head and Neck Cancer over the Past 17 Years. Ann. Palliat. Med. 2017, 6, 730–780. [Google Scholar] [CrossRef]
- Pandit, P.; Patil, R.; Palwe, V.; Yasam, V.R.; Nagarkar, R. Predictors of Weight Loss in Patients With Head and Neck Cancer Receiving Radiation or Concurrent Chemoradiation Treated at a Tertiary Cancer Center. Nutr. Clin. Pract. 2020, 35, 1047–1052. [Google Scholar] [CrossRef]
- Sandmael, J.A.; Sand, K.; Bye, A.; Solheim, T.S.; Oldervoll, L.; Helvik, A.-S. Nutritional Experiences in Head and Neck Cancer Patients. Eur. J. Cancer Care 2019, 28, e13168. [Google Scholar] [CrossRef]
- De Sanctis, V.; Bossi, P.; Sanguineti, G.; Trippa, F.; Ferrari, D.; Bacigalupo, A.; Ripamonti, C.I.; Buglione, M.; Pergolizzi, S.; Langendjik, J.A.; et al. Mucositis in Head and Neck Cancer Patients Treated with Radiotherapy and Systemic Therapies: Literature Review and Consensus Statements. Crit. Rev. Oncol. Hematol. 2016, 100, 147–166. [Google Scholar] [CrossRef]
- Jung, Y.-S.; Park, E.-Y.; Sohn, H.-O. Oral Health Status and Oral Health-Related Quality of Life According to Presence or Absence of Mucositis in Head and Neck Cancer Patients. J. Cancer Prev. 2019, 24, 43–47. [Google Scholar] [CrossRef]
- Lee, S.-C.; Wang, T.-J.; Chu, P.-Y. Predictors of Weight Loss during and after Radiotherapy in Patients with Head and Neck Cancer: A Longitudinal Study. Eur. J. Oncol. Nurs. 2019, 39, 98–104. [Google Scholar] [CrossRef]
- Ertem, G. Nutrition of Cancer Patients and Nursing Approach. Dirim Tip. Gazetesi 2008, 83, 56–63. [Google Scholar]
- Kömürcü, S. Nutritional Problems in Head and Neck Cancer Patients. Kulak Burun Bogaz Ve Bas Boyun Cerrahisi Derg. 2004, 12, 101–108. [Google Scholar]
- Peterson, D.E. New Strategies for Management of Oral Mucositis in Cancer Patients. J. Support. Oncol. 2006, 4, 9–13. [Google Scholar] [PubMed]
- Shieh, S.H.; Wang, S.T.; Tsai, S.T.; Tseng, C.C. Mouth Care for Nasopharyngeal Cancer Patients Undergoing Radiotherapy. Oral Oncol. 1997, 33, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.; Fliedner, M.C.; Smiet, A.C.M. Management of Oral Mucositis in Patients with Cancer. Eur. J. Oncol. Nurs. 2005, 9 (Suppl. S1), S24–S32. [Google Scholar] [CrossRef]
- Çubukçu, N.Ü.; Çinar, S. Can Oral Mucositis Be Prevented in Patients Receiving Chemotherapy? Marmara Üniversitesi Saglik. Bilim. Enstitüsü Derg. 2012, 2, 155–163. [Google Scholar]
- Migliorati, C.; Hewson, I.; Lalla, R.V.; Antunes, H.S.; Estilo, C.L.; Hodgson, B.; Lopes, N.N.F.; Schubert, M.M.; Bowen, J.; Elad, S.; et al. Systematic Review of Laser and Other Light Therapy for the Management of Oral Mucositis in Cancer Patients. Support. Care Cancer 2013, 21, 333–341. [Google Scholar] [CrossRef]
- Nicolatou-Galitis, O.; Sarri, T.; Bowen, J.; Di Palma, M.; Kouloulias, V.E.; Niscola, P.; Riesenbeck, D.; Stokman, M.; Tissing, W.; Yeoh, E.; et al. Systematic Review of Amifostine for the Management of Oral Mucositis in Cancer Patients. Support. Care Cancer 2013, 21, 357–364. [Google Scholar] [CrossRef]
- Peterson, D.E.; Ohrn, K.; Bowen, J.; Fliedner, M.; Lees, J.; Loprinzi, C.; Mori, T.; Osaguona, A.; Weikel, D.S.; Elad, S.; et al. Systematic Review of Oral Cryotherapy for Management of Oral Mucositis Caused by Cancer Therapy. Support. Care Cancer 2013, 21, 327–332. [Google Scholar] [CrossRef]
- Raber-Durlacher, J.E.; von Bültzingslöwen, I.; Logan, R.M.; Bowen, J.; Al-Azri, A.R.; Everaus, H.; Gerber, E.; Gomez, J.G.; Pettersson, B.G.; Soga, Y.; et al. Systematic Review of Cytokines and Growth Factors for the Management of Oral Mucositis in Cancer Patients. Support. Care Cancer 2013, 21, 343–355. [Google Scholar] [CrossRef]
- Ranna, V.; Cheng, K.K.F.; Castillo, D.A.; Porcello, L.; Vaddi, A.; Lalla, R.V.; Bossi, P.; Elad, S.; Mucositis Study group of the Multinational Association of Supportive Care in Cancer/International Society for Oral Oncology (MASCC/ISOO). Development of the MASCC/ISOO Clinical Practice Guidelines for Mucositis: An Overview of the Methods. Support. Care Cancer 2019, 27, 3933–3948. [Google Scholar] [CrossRef]
- Lalla, R.V.; Bowen, J.; Barasch, A.; Elting, L.; Epstein, J.; Keefe, D.M.; McGuire, D.B.; Migliorati, C.; Nicolatou-Galitis, O.; Peterson, D.E.; et al. MASCC/ISOO Clinical Practice Guidelines for the Management of Mucositis Secondary to Cancer Therapy. Cancer 2014, 120, 1453–1461. [Google Scholar] [CrossRef]
- Chung, Y.-L.; Pui, N.N.M. Confounding Factors Associated with Oral Mucositis Assessment in Patients Receiving Chemoradiotherapy for Head and Neck Cancer. Support. Care Cancer 2017, 25, 2743–2751. [Google Scholar] [CrossRef] [PubMed]
- Borowski, B.; Benhamou, E.; Pico, J.L.; Laplanche, A.; Margainaud, J.P.; Hayat, M. Prevention of Oral Mucositis in Patients Treated with High-Dose Chemotherapy and Bone Marrow Transplantation: A Randomised Controlled Trial Comparing Two Protocols of Dental Care. Eur. J. Cancer Part B Oral Oncol. 1994, 30, 93–97. [Google Scholar] [CrossRef]
- McGuire, D.B.; Correa, M.E.P.; Johnson, J.; Wienandts, P. The Role of Basic Oral Care and Good Clinical Practice Principles in the Management of Oral Mucositis. Support. Care Cancer 2006, 14, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Pinna, R.; Campus, G.; Cumbo, E.; Mura, I.; Milia, E. Xerostomia Induced by Radiotherapy: An Overview of the Physiopathology, Clinical Evidence, And Management of the Oral Damage. TCRM 2015, 11, 171–188. [Google Scholar] [CrossRef]
- Gupta, N.; Pal, M.; Rawat, S.; Grewal, M.S.; Garg, H.; Chauhan, D.; Ahlawat, P.; Tandon, S.; Khurana, R.; Pahuja, A.K.; et al. Radiation-Induced Dental Caries, Prevention and Treatment—A Systematic Review. Natl. J. Maxillofac. Surg. 2015, 6, 160. [Google Scholar] [CrossRef]
- King, S.N.; Dunlap, N.E.; Tennant, P.A.; Pitts, T. Pathophysiology of Radiation-Induced Dysphagia in Head and Neck Cancer. Dysphagia 2016, 31, 339–351. [Google Scholar] [CrossRef]
- Baijens, L.W.J.; Walshe, M.; Aaltonen, L.-M.; Arens, C.; Cordier, R.; Cras, P.; Crevier-Buchman, L.; Curtis, C.; Golusinski, W.; Govender, R.; et al. European White Paper: Oropharyngeal Dysphagia in Head and Neck Cancer. Eur. Arch. Otorhinolaryngol. 2021, 278, 577–616. [Google Scholar] [CrossRef]
- Langius, J.A.E.; Zandbergen, M.C.; Eerenstein, S.E.J.; van Tulder, M.W.; Leemans, C.R.; Kramer, M.H.H.; Weijs, P.J.M. Effect of Nutritional Interventions on Nutritional Status, Quality of Life and Mortality in Patients with Head and Neck Cancer Receiving (Chemo)Radiotherapy: A Systematic Review. Clin. Nutr. 2013, 32, 671–678. [Google Scholar] [CrossRef]
- Lees, J. Incidence of Weight Loss in Head and Neck Cancer Patients on Commencing Radiotherapy Treatment at a Regional Oncology Centre. Eur. J. Cancer Care 1999, 8, 133–136. [Google Scholar] [CrossRef]
- Mekhail, T.M.; Adelstein, D.J.; Rybicki, L.A.; Larto, M.A.; Saxton, J.P.; Lavertu, P. Enteral Nutrition during the Treatment of Head and Neck Carcinoma: Is a Percutaneous Endoscopic Gastrostomy Tube Preferable to a Nasogastric Tube? Cancer 2001, 91, 1785–1790. [Google Scholar]
- Magnano, M.; Mola, P.; Machetta, G.; Maffeis, P.; Forestiero, I.; Cavagna, R.; Artino, E.; Boffano, P. The Nutritional Assessment of Head and Neck Cancer Patients. Eur. Arch. Otorhinolaryngol. 2015, 272, 3793–3799. [Google Scholar] [CrossRef] [PubMed]
- Jager-Wittenaar, H.; Dijkstra, P.U.; Vissink, A.; van der Laan, B.F.A.M.; van Oort, R.P.; Roodenburg, J.L.N. Critical Weight Loss in Head and Neck Cancer—Prevalence and Risk Factors at Diagnosis: An Explorative Study. Support. Care Cancer 2007, 15, 1045–1050. [Google Scholar] [CrossRef]
- Hofto, S.; Abbott, J.; Jackson, J.E.; Isenring, E. Investigating Adherence to Australian Nutritional Care Guidelines in Patients with Head and Neck Cancer. Cancers Head Neck 2018, 3, 6. [Google Scholar] [CrossRef]
- Hong, F.; Bosco, J.L.F.; Bush, N.; Berry, D.L. Patient Self-Appraisal of Change and Minimal Clinically Important Difference on the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30 before and during Cancer Therapy. BMC Cancer 2013, 13, 165. [Google Scholar] [CrossRef]
- Righini, C.-A.; Timi, N.; Junet, P.; Bertolo, A.; Reyt, E.; Atallah, I. Assessment of Nutritional Status at the Time of Diagnosis in Patients Treated for Head and Neck Cancer. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2013, 130, 8–14. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and Classification of Cancer Cachexia: An International Consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Pezdirec, M.; Strojan, P.; Boltezar, I.H. Swallowing Disorders after Treatment for Head and Neck Cancer. Radiol. Oncol. 2019, 53, 225–230. [Google Scholar] [CrossRef]
- Mulasi, U.; Vock, D.M.; Jager-Wittenaar, H.; Teigen, L.; Kuchnia, A.J.; Jha, G.; Fujioka, N.; Rudrapatna, V.; Patel, M.R.; Earthman, C.P. Nutrition Status and Health-Related Quality of Life Among Outpatients With Advanced Head and Neck Cancer. Nutr. Clin. Pract. 2020, 35, 1129–1137. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN Guidelines on Nutrition in Cancer Patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Bressan, V.; Stevanin, S.; Bianchi, M.; Aleo, G.; Bagnasco, A.; Sasso, L. The Effects of Swallowing Disorders, Dysgeusia, Oral Mucositis and Xerostomia on Nutritional Status, Oral Intake and Weight Loss in Head and Neck Cancer Patients: A Systematic Review. Cancer Treat. Rev. 2016, 45, 105–119. [Google Scholar] [CrossRef]
- Chasen, M.R.; Bhargava, R. A Descriptive Review of the Factors Contributing to Nutritional Compromise in Patients with Head and Neck Cancer. Support. Care Cancer 2009, 17, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Soeters, P.B.; Reijven, P.L.M.; Schueren, M.A.E. van B. van der; Schols, J.M.G.A.; Halfens, R.J.G.; Meijers, J.M.M.; Gemert, W.G. van A Rational Approach to Nutritional Assessment. Clin. Nutr. 2008, 27, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN Guidelines on Definitions and Terminology of Clinical Nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Arribas, L.; Hurtós, L.; Sendrós, M.J.; Peiró, I.; Salleras, N.; Fort, E.; Sánchez-Migallón, J.M. NUTRISCORE: A New Nutritional Screening Tool for Oncological Outpatients. Nutrition 2017, 33, 297–303. [Google Scholar] [CrossRef]
- World Health Organization. Nutrition Landscape Information System (NLiS) Country Profile Indicators: Interpretation Guide, 2nd ed.; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-151695-2.
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Gossum, A.V.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic Criteria for Malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef]
- Sobotka, L.; Allison, S.P.; Forbes, A.; Meier, R.F.; Schneider, S.M.; Soeters, P.B.; Stanga, Z.; van Gossum, A.; European Society for Clinical Nutrition and Metabolism (Eds.) Basics in Clinical Nutrition, 5th ed.; Galén: Prague, Czech Republic, 2019; ISBN 978-80-7492-427-9. [Google Scholar]
- Ravasco, P.; Monteiro-Grillo, I.; Marques Vidal, P.; Camilo, M.E. Impact of Nutrition on Outcome: A Prospective Randomized Controlled Trial in Patients with Head and Neck Cancer Undergoing Radiotherapy. Head Neck 2005, 27, 659–668. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN Practical Guideline: Clinical Nutrition in Cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Delafuente, J.C. Nutrients and Immune Responses. Rheum. Dis. Clin. N. Am. 1991, 17, 203–212. [Google Scholar] [CrossRef]
- Chandra, R. Nutrition and the Immune System: An Introduction. Am. J. Clin. Nutr. 1997, 66, 460S–463S. [Google Scholar] [CrossRef]
- Capuano, G.; Grosso, A.; Gentile, P.C.; Battista, M.; Bianciardi, F.; Di Palma, A.; Pavese, I.; Satta, F.; Tosti, M.; Palladino, A.; et al. Influence of Weight Loss on Outcomes in Patients with Head and Neck Cancer Undergoing Concomitant Chemoradiotherapy. Head Neck 2008, 30, 503–508. [Google Scholar] [CrossRef]
- Copeland, E.M.; Daly, J.M.; Dudrick, S.J. Nutrition as an Adjunct to Cancer Treatment in the Adult. Cancer Res. 1977, 37, 2451–2456. [Google Scholar]
- Jager-Wittenaar, H.; Dijkstra, P.U.; Vissink, A.; van der Laan, B.F.A.M.; van Oort, R.P.; Roodenburg, J.L.N. Malnutrition and Quality of Life in Patients Treated for Oral or Oropharyngeal Cancer. Head Neck 2011, 33, 490–496. [Google Scholar] [CrossRef] [PubMed]
- van Bokhorst-de van der Schueren, M.A.; van Leeuwen, P.A.; Kuik, D.J.; Klop, W.M.; Sauerwein, H.P.; Snow, G.B.; Quak, J.J. The Impact of Nutritional Status on the Prognoses of Patients with Advanced Head and Neck Cancer. Cancer 1999, 86, 519–527. [Google Scholar] [CrossRef]
- Langius, J.A.E.; Bakker, S.; Rietveld, D.H.F.; Kruizenga, H.M.; Langendijk, J.A.; Weijs, P.J.M.; Leemans, C.R. Critical Weight Loss Is a Major Prognostic Indicator for Disease-Specific Survival in Patients with Head and Neck Cancer Receiving Radiotherapy. Br. J. Cancer 2013, 109, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Langius, J.A.E.; van Dijk, A.M.; Doornaert, P.; Kruizenga, H.M.; Langendijk, J.A.; Leemans, C.R.; Weijs, P.J.M.; Verdonck-de Leeuw, I.M. More than 10% Weight Loss in Head and Neck Cancer Patients during Radiotherapy Is Independently Associated with Deterioration in Quality of Life. Nutr. Cancer 2013, 65, 76–83. [Google Scholar] [CrossRef]
- Gellrich, N.-C.; Handschel, J.; Holtmann, H.; Krüskemper, G. Oral Cancer Malnutrition Impacts Weight and Quality of Life. Nutrients 2015, 7, 2145–2160. [Google Scholar] [CrossRef]
- Meyer, F.; Fortin, A.; Wang, C.S.; Liu, G.; Bairati, I. Predictors of Severe Acute and Late Toxicities in Patients With Localized Head-and-Neck Cancer Treated With Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1454–1462. [Google Scholar] [CrossRef]
- Regueiro, C.A.; Aragón, G.; Millán, L.; Valcárcel, F.J.; de la Torre, A.; Magallón, R. Prognostic Factors for Local Control, Regional Control and Survival in Oropharyngeal Squamous Cell Carcinoma. Eur. J. Cancer 1994, 30, 2060–2067. [Google Scholar] [CrossRef]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN Expert Group Recommendations for Action against Cancer-Related Malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef]
- Baldwin, C.; Spiro, A.; Ahern, R.; Emery, P.W. Oral Nutritional Interventions in Malnourished Patients with Cancer: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2012, 104, 371–385. [Google Scholar] [CrossRef]
- Kristensen, M.B.; Isenring, E.; Brown, B. Nutrition and Swallowing Therapy Strategies for Patients with Head and Neck Cancer. Nutrition 2020, 69, 110548. [Google Scholar] [CrossRef] [PubMed]
- Talwar, B.; Donnelly, R.; Skelly, R.; Donaldson, M. Nutritional Management in Head and Neck Cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S32–S40. [Google Scholar] [CrossRef]
- Bossola, M. Nutritional Interventions in Head and Neck Cancer Patients Undergoing Chemoradiotherapy: A Narrative Review. Nutrients 2015, 7, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Cardellini, S.; Deantoni, C.L.; Paccagnella, M.; Casirati, A.; Pontara, A.; Marinosci, A.; Tresoldi, M.; Giordano, L.; Chiara, A.; Dell’Oca, I.; et al. The Impact of Nutritional Intervention on Quality of Life and Outcomes in Patients with Head and Neck Cancers Undergoing Chemoradiation. Front. Oncol. 2024, 14, 1475930. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, M.; Liu, C.; Ye, Y.; Huang, G. Percutaneous Endoscopic Gastrostomy versus Nasogastric Tube Feeding for Patients with Head and Neck Cancer: A Systematic Review. J. Radiat. Res. 2014, 55, 559–567. [Google Scholar] [CrossRef]
- Vangelov, B.; Smee, R.I. Clinical Predictors for Reactive Tube Feeding in Patients with Advanced Oropharynx Cancer Receiving Radiotherapy ± Chemotherapy. Eur. Arch. Otorhinolaryngol. 2017, 274, 3741–3749. [Google Scholar] [CrossRef]
- Brown, T.E.; Banks, M.D.; Hughes, B.G.M.; Lin, C.Y.; Kenny, L.M.; Bauer, J.D. Randomised Controlled Trial of Early Prophylactic Feeding vs Standard Care in Patients with Head and Neck Cancer. Br. J. Cancer 2017, 117, 15–24. [Google Scholar] [CrossRef]
- Brown, T.; Banks, M.; Hughes, B.G.M.; Lin, C.; Kenny, L.; Bauer, J. Tube Feeding during Treatment for Head and Neck Cancer—Adherence and Patient Reported Barriers. Oral Oncol. 2017, 72, 140–149. [Google Scholar] [CrossRef]
- Silander, E.; Nyman, J.; Bove, M.; Johansson, L.; Larsson, S.; Hammerlid, E. Impact of Prophylactic Percutaneous Endoscopic Gastrostomy on Malnutrition and Quality of Life in Patients with Head and Neck Cancer: A Randomized Study. Head Neck 2012, 34, 1–9. [Google Scholar] [CrossRef]
- Corry, J.; Poon, W.; McPhee, N.; Milner, A.D.; Cruickshank, D.; Porceddu, S.V.; Rischin, D.; Peters, L.J. Prospective Study of Percutaneous Endoscopic Gastrostomy Tubes versus Nasogastric Tubes for Enteral Feeding in Patients with Head and Neck Cancer Undergoing (Chemo)Radiation. Head Neck 2009, 31, 867–876. [Google Scholar] [CrossRef]
- Hiatt, J.S.; Brown, T.E.; Banks, M.; Lewis, C.-A.; Bauer, J. Patient and Carer Experience of Nutrition Care throughout Treatment for Head and Neck Cancer: A Systematic Qualitative Review and Thematic Synthesis. Support. Care Cancer 2020, 28, 5633–5647. [Google Scholar] [CrossRef]
- Brahmi, R.; Boujnah, N.; Ejbali, R. Elaboration of Innovative Digital Twin Models for Healthcare Monitoring With 6G Functionalities. IEEE Access 2024, 12, 109608–109624. [Google Scholar] [CrossRef]
- Katsoulakis, E.; Wang, Q.; Wu, H.; Shahriyari, L.; Fletcher, R.; Liu, J.; Achenie, L.; Liu, H.; Jackson, P.; Xiao, Y.; et al. Digital Twins for Health: A Scoping Review. npj Digit. Med. 2024, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A. Digital Twin for Healthcare Systems. Front. Digit. Health 2023, 5, 1253050. [Google Scholar] [CrossRef]
- Subramanian, B.; Kim, J.; Maray, M.; Paul, A. Digital Twin Model: A Real-Time Emotion Recognition System for Personalized Healthcare. IEEE Access 2022, 10, 81155–81165. [Google Scholar] [CrossRef]
- Kim, T.; Kwon, H.-S.; Cho, K.; Woo, W. Holistic Patient Assessment System Using Digital Twin for XR Medical Teleconsultation. In Proceedings of the Augmented Humans International Conference 2024, New York, NY, USA, 1 May 2024; pp. 72–78. [Google Scholar]
- The World Health Organization. Quality of Life Assessment (WHOQOL): Position Paper from the World Health Organization. Soc. Sci. Med. 1995, 41, 1403–1409. [Google Scholar] [CrossRef]
- Binenbaum, Y.; Amit, M.; Billan, S.; Cohen, J.T.; Gil, Z. Minimal Clinically Important Differences in Quality of Life Scores of Oral Cavity and Oropharynx Cancer Patients. Ann. Surg. Oncol. 2014, 21, 2773–2781. [Google Scholar] [CrossRef]
- Crosby, R.D.; Kolotkin, R.L.; Williams, G.R. Defining Clinically Meaningful Change in Health-Related Quality of Life. J. Clin. Epidemiol. 2003, 56, 395–407. [Google Scholar] [CrossRef]
- Jaeschke, R.; Singer, J.; Guyatt, G.H. Measurement of Health Status: Ascertaining the Minimal Clinically Important Difference. Control. Clin. Trials 1989, 10, 407–415. [Google Scholar] [CrossRef]
- Findlay, M.; Strange, E.; Bauer, J. Maintaining Evidence-Based Guidelines Online: Update on Nutritional Management of Patients with Head and Neck Cancer. Asia-Pac. J. Clin. Oncol. 2013, 9, 61–98. [Google Scholar]
- Cellina, M.; Cè, M.; Alì, M.; Irmici, G.; Ibba, S.; Caloro, E.; Fazzini, D.; Oliva, G.; Papa, S. Digital Twins: The New Frontier for Personalized Medicine? Appl. Sci. 2023, 13, 7940. [Google Scholar] [CrossRef]
- Borggreven, P.A.; Verdonck-de Leeuw, I.M.; Muller, M.J.; Heiligers, M.L.C.H.; de Bree, R.; Aaronson, N.K.; Leemans, C.R. Quality of Life and Functional Status in Patients with Cancer of the Oral Cavity and Oropharynx: Pretreatment Values of a Prospective Study. Eur. Arch. Otorhinolaryngol. 2007, 264, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Funk, G.F.; Karnell, L.H.; Dawson, C.J.; Means, M.E.; Colwill, M.L.; Gliklich, R.E.; Alford, E.L.; Stewart, M.G. Baseline and Post-Treatment Assessment of the General Health Status of Head and Neck Cancer Patients Compared with United States Population Norms. Head Neck 1997, 19, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski, O.; Smeets, R.; Gerhards, F.; Kolk, A.; Kloss, F.; Stein, J.M.; Kasaj, A.; Koch, F.; Grosjean, M.; Riediger, D.; et al. Gender Specific Quality of Life in Patients with Oral Squamous Cell Carcinomas. Head Face Med. 2010, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Michelson, H.; Bolund, C. Health-Related Quality of Life Measured by the EORTC QLQ-C30: Reference Values From a Large Sample of the Swedish Population. Acta Oncol. 2000, 39, 477–484. [Google Scholar] [CrossRef]
- Hammerlid, E.; Ahlner-Elmqvist, M.; Bjordal, K.; Biörklund, A.; Evensen, J.; Boysen, M.; Jannert, M.; Kaasa, S.; Sullivan, M.; Westin, T. A Prospective Multicentre Study in Sweden and Norway of Mental Distress and Psychiatric Morbidity in Head and Neck Cancer Patients. Br. J. Cancer 1999, 80, 766–774. [Google Scholar] [CrossRef]
- de Leeuw, J.R.; de Graeff, A.; Ros, W.J.; Blijham, G.H.; Hordijk, G.J.; Winnubst, J.A. Prediction of Depressive Symptomatology after Treatment of Head and Neck Cancer: The Influence of Pre-Treatment Physical and Depressive Symptoms, Coping, and Social Support. Head Neck 2000, 22, 799–807. [Google Scholar] [CrossRef]
- List, M.A.; Lee Rutherford, J.; Stracks, J.; Haraf, D.; Kies, M.S.; Vokes, E.E. An Exploration of the Pretreatment Coping Strategies of Patients with Carcinoma of the Head and Neck. Cancer 2002, 95, 98–104. [Google Scholar] [CrossRef]
- de Graeff, A.; de Leeuw, J.R.; Ros, W.J.; Hordijk, G.J.; Blijham, G.H.; Winnubst, J.A. Sociodemographic Factors and Quality of Life as Prognostic Indicators in Head and Neck Cancer. Eur. J. Cancer 2001, 37, 332–339. [Google Scholar] [CrossRef]
- Duffy, S.A.; Terrell, J.E.; Valenstein, M.; Ronis, D.L.; Copeland, L.A.; Connors, M. Effect of Smoking, Alcohol, and Depression on the Quality of Life of Head and Neck Cancer Patients. Gen. Hosp. Psychiatry 2002, 24, 140–147. [Google Scholar] [CrossRef]
- Lebel, S.; Devins, G.M. Stigma in Cancer Patients Whose Behavior May Have Contributed to Their Disease. Future Oncol. 2008, 4, 717–733. [Google Scholar] [CrossRef] [PubMed]
- Pai, P.-C.; Chuang, C.-C.; Tseng, C.-K.; Tsang, N.-M.; Chang, K.-P.; Yen, T.-C.; Liao, C.-T.; Hong, J.-H.; Chang, J.T.-C. Impact of Pretreatment Body Mass Index on Patients with Head-and-Neck Cancer Treated with Radiation. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e93–e100. [Google Scholar] [CrossRef]
- Ramsay, S.E.; Whincup, P.H.; Shaper, A.G.; Wannamethee, S.G. The Relations of Body Composition and Adiposity Measures to Ill Health and Physical Disability in Elderly Men. Am. J. Epidemiol. 2006, 164, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Q.; Feng, L.-J.; Zhang, K.-P.; Tang, M.; Song, M.-M.; Ruan, G.-T.; Zhang, X.-W.; Li, W.; Zhou, F.-X.; et al. The Application of Fat-Free Mass Index for Survival Prediction in Cancer Patients With Normal and High Body Mass Index. Front. Nutr. 2021, 8, 714051. [Google Scholar] [CrossRef]
- Cereda, E.; Pedrazzoli, P.; Lobascio, F.; Masi, S.; Crotti, S.; Klersy, C.; Turri, A.; Stobäus, N.; Tank, M.; Franz, K.; et al. The Prognostic Impact of BIA-Derived Fat-Free Mass Index in Patients with Cancer. Clin. Nutr. 2021, 40, 3901–3907. [Google Scholar] [CrossRef] [PubMed]
- Schutz, Y.; Kyle, U.U.G.; Pichard, C. Fat-Free Mass Index and Fat Mass Index Percentiles in Caucasians Aged 18–98 y. Int. J. Obes. 2002, 26, 953–960. [Google Scholar] [CrossRef]
- Arab, A.; Karimi, E.; Vingrys, K.; Shirani, F. Is Phase Angle a Valuable Prognostic Tool in Cancer Patients’ Survival? A Systematic Review and Meta-Analysis of Available Literature. Clin. Nutr. 2021, 40, 3182–3190. [Google Scholar] [CrossRef]
- Kyle, U.G.; Genton, L.; Pichard, C. Low Phase Angle Determined by Bioelectrical Impedance Analysis Is Associated with Malnutrition and Nutritional Risk at Hospital Admission. Clin. Nutr. 2013, 32, 294–299. [Google Scholar] [CrossRef]
- Axelsson, L.; Silander, E.; Bosaeus, I.; Hammerlid, E. Bioelectrical Phase Angle at Diagnosis as a Prognostic Factor for Survival in Advanced Head and Neck Cancer. Eur. Arch. Otorhinolaryngol. 2018, 275, 2379–2386. [Google Scholar] [CrossRef]
- Furlan, C.; Polesel, J.; Barzan, L.; Franchin, G.; Sulfaro, S.; Romeo, S.; Colizzi, F.; Rizzo, A.; Baggio, V.; Giacomarra, V.; et al. Prognostic Significance of LINE-1 Hypomethylation in Oropharyngeal Squamous Cell Carcinoma. Clin. Epigenetics 2017, 9, 58. [Google Scholar] [CrossRef]
- Avanzo, M.; Stancanello, J.; Naqa, I.E. Beyond Imaging: The Promise of Radiomics. Phys. Medica Eur. J. Med. Phys. 2017, 38, 122–139. [Google Scholar] [CrossRef] [PubMed]
- Gabryś, H.S.; Buettner, F.; Sterzing, F.; Hauswald, H.; Bangert, M. Design and Selection of Machine Learning Methods Using Radiomics and Dosiomics for Normal Tissue Complication Probability Modeling of Xerostomia. Front. Oncol. 2018, 8, 35. [Google Scholar] [CrossRef]
- Tan, F.H.; Bai, Y.; Saintigny, P.; Darido, C. mTOR Signalling in Head and Neck Cancer: Heads Up. Cells 2019, 8, 333. [Google Scholar] [CrossRef]
- Bentzen, S.M.; Constine, L.S.; Deasy, J.O.; Eisbruch, A.; Jackson, A.; Marks, L.B.; Haken, R.K.T.; Yorke, E.D. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): An Introduction to the Scientific Issues. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S3–S9. [Google Scholar] [CrossRef]
- den Bosch, L.V.; van der Schaaf, A.; van der Laan, H.P.; Hoebers, F.J.P.; Wijers, O.B.; van den Hoek, J.G.M.; Moons, K.G.M.; Reitsma, J.B.; Steenbakkers, R.J.H.M.; Schuit, E.; et al. Comprehensive Toxicity Risk Profiling in Radiation Therapy for Head and Neck Cancer: A New Concept for Individually Optimised Treatment. Radiother. Oncol. 2021, 157, 147–154. [Google Scholar] [CrossRef]
- Desideri, I.; Loi, M.; Francolini, G.; Becherini, C.; Livi, L.; Bonomo, P. Application of Radiomics for the Prediction of Radiation-Induced Toxicity in the IMRT Era: Current State-of-the-Art. Front. Oncol. 2020, 10, 1708. [Google Scholar] [CrossRef]
- Mierzwa, M.L.; Gharzai, L.A.; Li, P.; Wilkie, J.R.; Hawkins, P.G.; Aryal, M.P.; Lee, C.; Rosen, B.; Lyden, T.; Blakely, A.; et al. Early MRI Blood Volume Changes in Constrictor Muscles Correlate With Postradiation Dysphagia. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 566–573. [Google Scholar] [CrossRef]

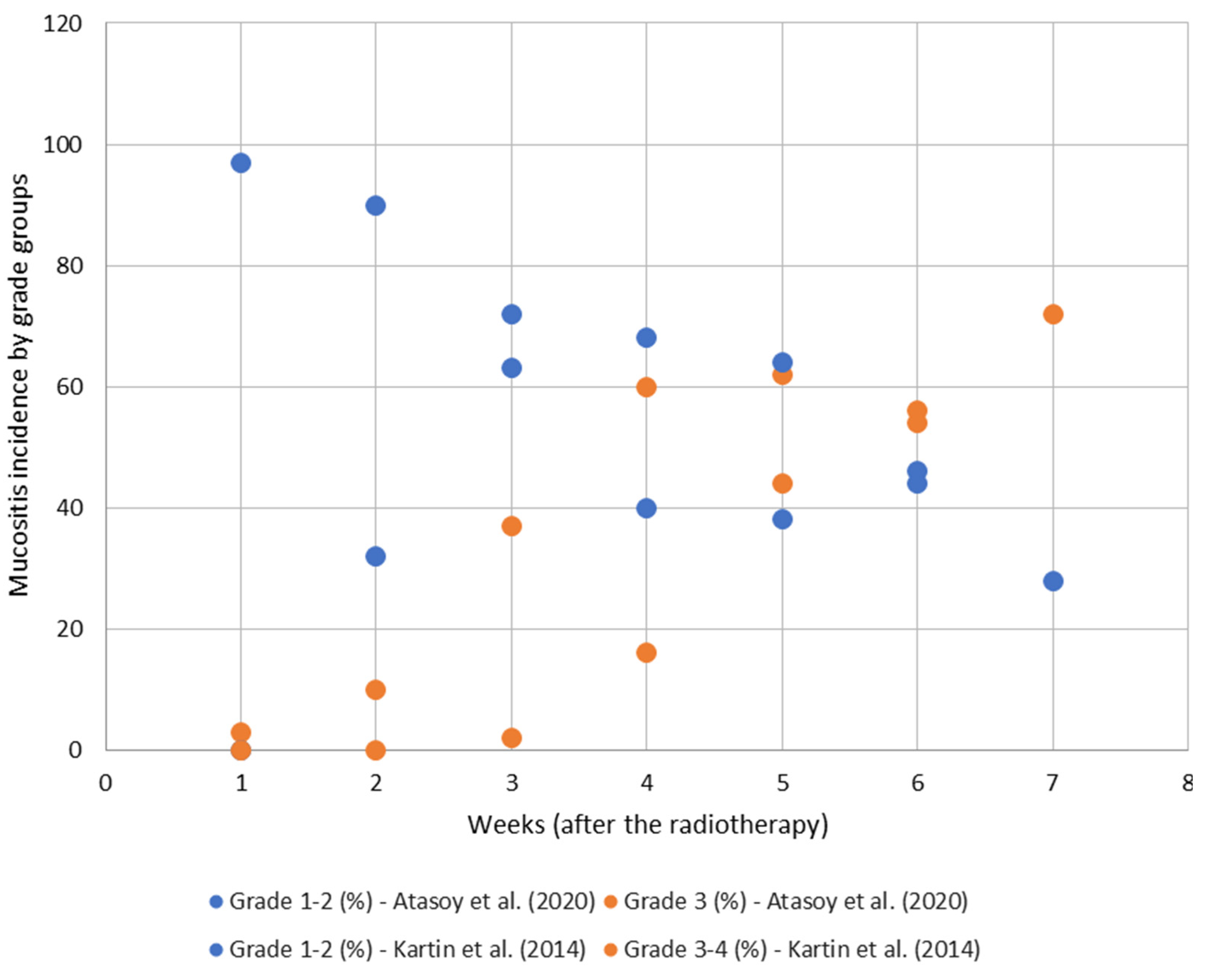
| Kartin et al., 2014 [48] | Egestad and Nieder, 2015 [47] | Kelvianto et al., 2018 [18] | Atasoy et al. 2020 [49] | Fanetti et al., 2021 [29] | ||||||||||
| N | 50 | 60 | 61 | 29 | 179 | |||||||||
| Sex | N | % | N | % | N | % | N | % | N | % | ||||
| M | 43 | 86 | 46 | 76.66 | 47 | 77.04 | 24 | 82.75 | 127 | 70.94 | ||||
| F | 7 | 14 | 14 | 23.33 | 14 | 22.95 | 5 | 17.24 | 52 | 29.05 | ||||
| Age | Age group | N | % | Mean | Age group | N | % | Mean | Age group | N | % | |||
| <39 | 5 | 10 | 60 | 18–29 | 5 | 8.2 | 56.79 | <55 | 64 | 35.8 | ||||
| 40–49 | 6 | 12 | 30–39 | 15 | 24.6 | 55–64 | 52 | 29.1 | ||||||
| 50–59 | 18 | 36 | 40–49 | 13 | 21.3 | >=65 | 63 | 35.2 | ||||||
| >60 | 21 | 42 | >=50 | 28 | 45.9 | |||||||||
| Milecki et al., 2021 [42] | De Oliveira Faria et al., 2022 [50] | Löser et al., 2022 [52] | Blake et al., 2023 [51] | Pytel et al., 2023 [17] | ||||||||||
| N | 92 | 80 | 58 | 110 | 85 | |||||||||
| Sex | N | % | N | % | N | % | N | % | N | % | ||||
| M | 74 | 80.43 | 65 | 81.25 | 41 | 70.68 | 93 | 84.54 | 55 | 64.70 | ||||
| F | 18 | 19.56 | 15 | 18.75 | 17 | 29.31 | 17 | 15.45 | 30 | 35.29 | ||||
| Age | Age group | N | % | N/A | Mean | Mean | Age group | N | % | |||||
| <60 | 57 | 62 | Malnourished—68.6 | 60.6 | 18–35 | 2 | 2.4 | |||||||
| >60 | 35 | 38 | well nourished—62.5 | 36–45 | 12 | 14.1 | ||||||||
| 46–55 | 12 | 14.1 | ||||||||||||
| 56–65 | 33 | 38.8 | ||||||||||||
| >65 | 30 | 30.6 | ||||||||||||
| Egestad and Nieder, 2015 [47] | Kelvianto et al., 2018 [18] | Atasoy et al., 2020 [49] | Fanetti et al., 2021 [29] | De Oliveira Faria et al., 2022 [50] | Löser et al., 2022 [52] | Blake et al., 2023 [51] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 60 | 61 | 29 | 179 | 80 | 58 | 110 | |||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Radiotherapy | 12 | 20.00 | 16 | 26.22 | 0 | 0 | 0 | 0 | 37 | 46.25 | 0 | 0 | 9 | 8.18 |
| Chemotherapy | 21 | 35.00 | 0 | 0 | 6 | 20.68 | 141 | 78.77 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chemoradiation | 0 | 0 | 45 | 73.77 | 25 | 86.20 | 38 | 21.22 | 43 | 53.75 | 58 | 100 | 101 | 91.81 |
| Surgery | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 18.18 |
| Egestad and Nieder, 2015 [47] | Kelvianto et al., 2018 [18] | Atasoy et al., 2020 [49] | Fanetti et al., 2021 [29] | Löser et al., 2022 [52] | Blake et al., 2023 [51] | |
|---|---|---|---|---|---|---|
| Radiotherapy | 60–70 Gy | Minimum 30–40 Gy | 60–70 Gy | (*) 70.95 Gy to PTV macro 62.70 Gy to PTV HR 56.10 Gy to PTV LR | 60–70.4 Gy | 60–70 Gy |
| Chemotherapy | Not specified | Not specified | Weekly cisplatin-based treatment for most patients; no dose specified | (**) Cisplatin at 80–100 mg/m2 q3w or (***) induction with 3 cycles of cisplatin (75 mg/m2) and DTX (75 mg/m2), 5-FU (750 mg/m2/day) and then weekly cisplatin at 40 mg/m2 during RT | Cisplatin 100 mg/m2 q3w or weekly cisplatin 40 mg/m2 or 5-FU (600 mg/m2) and MMC (10 mg/m2) | Cisplatin at 100 mg/m2 q3w or weekly cisplatin at 40 mg/m2 or cetuximab at 400 mg/m2 IV loading dose 1 week before RT and then weekly 250 mg/m2 during RT |
| Correlation Coefficient (r) | ||||
|---|---|---|---|---|
| Score Variable | Total Prot. Intake | Prot. Intake/Weight | Quality of Prot. Intake | PNI |
| General condition | 0.13 | 0.03 | −0.03 | 0.1 |
| Physical function | 0.07 | −0.08 | 0.1 | 0.4 |
| Role function | −0.2 | −0.06 | 0.03 | 0.14 |
| Emotional function | 0.2 | 0.15 | −0.05 | 0.08 |
| Cognitive function | 0.07 | 0.01 | −0.1 | 0.15 |
| Social function | 0.24 | 0.08 | −0.04 | 0.25 |
| Fatigue | −0.28 | −0.2 | 0.2 | 0.02 |
| Nausea and vomiting | −0.26 | −0.2 | 0.02 | −0.05 |
| Pain | −0.09 | −0.07 | 0.19 | −0.15 |
| Insomnia | 0.03 | 0.12 | 0.2 | −0.17 |
| Dyspnea | −0.03 | 0.1 | −0.12 | −0.26 |
| Loss of appetite | −0.3 | −0.3 | 0.2 | 0.01 |
| Constipation | −0.08 | −0.04 | 0.07 | −0.14 |
| Diarrhea | −0.09 | 0.003 | −0.03 | 0.2 |
| Financial difficulties | ||||
| Study | Kelvianto et al., 2018 [18] | Egestad and Nieder, 2015 [47] | Milecki et al., 2021 [42] | De Oliveira Faria et al., 2022 [50] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Means (time point and BMI) | Means (follow-up) | Means (age groups) | Means (by adherence to intervention) | |||||||
| Score Variable | Baseline | Treatment start, BMI ≥ 25 | Treatment start, BMI < 25 | Treatment end, BMI ≥ 25 | Treatment end, BMI < 25 | Before RT | 1 year after RT | ≤60 yr | >60 yr | Adherent | Non-adherent |
| General condition | 58.3 | 68.6 | 65.6 | 48.3 | 47.8 | 61.14 | 57.88 | 76.7 | 73.2 | ||
| Physical function | 66.7 | 83.1 | 84.5 | 69.1 | 65.3 | 79.93 | 68.72 | 72.9 | 73.1 | ||
| Role function | 66.7 | 71.1 | 76.4 | 44.8 | 43.6 | 83.15 | 75.72 | 53.1 | 50.9 | ||
| Emotional function | 75 | 81.5 | 87.2 | 75.0 | 77.2 | 67.98 | 73.40 | 75.6 | 70.7 | ||
| Cognitive function | 100 | 89.2 | 86.1 | 78.3 | 67.8 | 84.06 | 73.73 | 88.8 | 92.8 | ||
| Social function | 66.7 | 71.1 | 73.6 | 60.1 | 60.9 | 79.89 | 75.18 | 82.9 | 86.0 | ||
| Fatigue | 16.7 | 32.3 | 31.9 | 53.7 | 62.3 | 31.28 | 39.85 | 20.4 | 18.6 | ||
| Nausea and vomiting | 16.7 | 13.0 | 17.4 | 33.3 | 25.4 | 7.07 | 9.78 | 13.9 | 22.5 | ||
| Pain | 16.7 | 15.2 | 18.1 | 44.4 | 53.6 | 22.28 | 28.44 | 24.54 | 17.52 | 14.3 | 20.8 |
| Insomnia | 0 | 26.5 | 33.3 | 25.6 | 34.8 | 34.42 | 39.86 | 27.9 | 33.3 | ||
| Dyspnea | 0 | 22.5 | 16.7 | 22.2 | 30.4 | 18.6 | 10.8 | ||||
| Loss of appetite | 33.3 | 17.6 | 22.2 | 57.8 | 66.7 | 20.65 | 27.17 | 27.9 | 27.8 | ||
| Constipation | 0 | 17.2 | 20.8 | 46.7 | 47.8 | 15.58 | 21.38 | 27.9 | 19.8 | ||
| Diarrhea | 0 | 14.1 | 13.9 | 17.8 | 13.6 | 7.25 | 6.88 | 5.4 | 5.4 | ||
| Financial difficulties | 13.7 | 18.1 | 15.6 | 30.3 | 33.70 | 38.04 | 39.89 | 30.34 | 34.9 | 36.9 | |
| Correlation Coefficient (r) [18] | Regression Coefficient (β) [52] | |||||||
|---|---|---|---|---|---|---|---|---|
| Score Variable | Total Prot. Intake | Prot. Intake/Weight | Quality of Prot. Intake | PNI | MUST | NRS-2002 | HPV Status | UICC |
| Mouth pain | −0.32 | −0.17 | 0.19 | −0.7 | ||||
| Speech | −0.03 | 0.17 | 0.2 | −0.05 | ||||
| Swallowing | −0.37 | −0.15 | 0.04 | −0.2 | 16.8 | 24.9 | ||
| Senses (taste and smell) | −0.15 | −0.64 | 0.05 | −0.05 | ||||
| Teeth | 0.013 | 0.17 | 0.07 | −0.5 | ||||
| Opening mouth | −0.13 | 0.11 | 0.04 | −0.32 | ||||
| Dry mouth | −0.41 | −0.3 | 0.02 | −0.2 | ||||
| Sticky saliva | −0.32 | −0.2 | 0.04 | −0.2 | 38.1 | 36.3 | ||
| Coughing | 0.17 | 0.13 | 0.11 | −0.11 | ||||
| Feeling ill | 0.04 | 0.17 | 0.21 | 0.4 | ||||
| Weight loss | 51 | 39.1 | ||||||
| Weight gain | ||||||||
| Pain medication | ||||||||
| Feeding tube | 35.6 | |||||||
| Food supplements | ||||||||
| Appetite loss | ||||||||
| Social eating | −0.29 | −0.26 | 0.14 | 0.08 | ||||
| Social contacts | ||||||||
| Awareness of disease | ||||||||
| Sexuality | ||||||||
| Kelvianto et al., 2018 [18] | Atasoy et al., 2020 [49] | Egestad and Nieder, 2015 [47] | Milecki et al., 2021 [42] | Löser et al., 2022 [52] | De Oliveira Faria et al., 2022 [50] | Pytel et al., 2023 [17] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Median | Median | Means (time point and BMI) | Means (follow-up) | Medians (nutrition status) | Means (by adherence to intervention) | Mean (time point) | ||||||||
| Baseline | RT start | RT end | Treatment start, BMI ≥ 25 | Treatment start, BMI < 25 | Treatment end, BMI ≥ 25 | Treatment end, BMI < 25 | Before RT | 1 year after RT | Mal-nourished | Well nourished | Adherent | Non-adherent | Before RT | After RT | |
| Mouth pain | 25 | 8.33 | 33.3 | 17.2 | 18.0 | 49.4 | 48.2 | 22.16 | 28.11 | 13.9 | 16.9 | ||||
| Speech | 44.4 | 0 | 77.8 | 13.1 | 23.1 | 39.8 | 47.0 | 35.63 | 33.45 | 33.3 | 0 | 38.2 | 37.2 | 31.9 | 56.9 |
| Swallowing | 41.6 | 8.33 | 33.3 | 6.9 | 11.6 | 45.7 | 45.3 | 19.93 | 23.64 | 58.3 | 12.5 | 43.0 | 25.4 | 31.4 | 66.4 |
| Senses (taste and smell) | 50 | 100 | 0 | 14.2 | 26.7 | 50.6 | 55.8 | 19.3 | 35.69 | 24.0 | 29.3 | 26.3 | 71.2 | ||
| Teeth | 0 | 4.9 | 10.1 | 14.9 | 10.6 | 56.1 | 47.7 | 50.0 | 50.9 | ||||||
| Opening mouth | 33.3 | 17.6 | 9.3 | 40.2 | 39.1 | 22.10 | 30.07 | 27.9 | 24.3 | 50.0 | 66.5 | ||||
| Dry mouth | 66.7 | 29.4 | 41.3 | 64.4 | 62.3 | 26.81 | 51.09 | 34.1 | 27.9 | 0 | 75.9 | ||||
| Sticky saliva | 66.7 | 27.3 | 34.7 | 71.4 | 68.1 | 28.62 | 42.75 | 66.7 | 0 | 53.5 | 50.4 | 25.0 | 76.2 | ||
| Coughing | 33.3 | 20.6 | 21.3 | 35.6 | 43.5 | 41.1 | 16.2 | 33.8 | 64.1 | ||||||
| Feeling ill | 33.3 | 16.7 | 17.8 | 43.7 | 45.5 | 14.7 | 16.2 | 33.2 | 59.4 | ||||||
| Weight loss | 14.7 | 29.2 | 62.1 | 63.6 | 60.87 | 78.26 | 100 | 0 | 38.8 | 29.7 | |||||
| Weight gain | 30.3 | 43.5 | 7.4 | 4.8 | 57.61 | 60.87 | 0 | 0 | 48.5 | 48.5 | |||||
| Pain medication | 44.1 | 40.0 | 86.7 | 95.5 | 52.75 | 6.96 | 0 | 0 | 43.8 | 25.9 | |||||
| Feeding tube | 0 | 12.5 | 30.0 | 54.5 | 0 | 0 | 46.8 | 46.8 | |||||||
| Food supplements | 3.0 | 36.0 | 46.7 | 68.2 | 72.83 | 76.09 | |||||||||
| Appetite loss | 16.7 | 66.7 | |||||||||||||
| Social eating | 0 | 16.6 | 9.8 | 18.8 | 48.3 | 49.9 | 20.02 | 25.36 | 41.7 | 8.3 | 46.9 | 44.6 | 30.9 | 66.9 | |
| Social contact | 4.2 | 6.1 | 23.0 | 28.9 | 17.46 | 21.96 | 15.2 | 14.6 | 28.5 | 45.8 | |||||
| Awareness of disease | 25.00 | 28.98 | |||||||||||||
| Sexuality | 30.1 | 34.8 | 53.2 | 41.6 | 35.51 | 39.13 | 43.6 | 44.6 | 34.0 | 45.5 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anghel, R.; Bîlteanu, L.; Folea, A.-R.; Marinescu, Ș.-A.; Pisoschi, A.-M.; Alexandrescu, M.-F.; Dumachi, A.-I.; Galeș, L.-N.; Trifănescu, O.G.; Zgură, A.-F.; et al. Assessing the Impact of Nutritional Status on the Quality of Life in Head and Neck Cancer Patients—The Need for Comprehensive Digital Tools. Cancers 2025, 17, 1128. https://doi.org/10.3390/cancers17071128
Anghel R, Bîlteanu L, Folea A-R, Marinescu Ș-A, Pisoschi A-M, Alexandrescu M-F, Dumachi A-I, Galeș L-N, Trifănescu OG, Zgură A-F, et al. Assessing the Impact of Nutritional Status on the Quality of Life in Head and Neck Cancer Patients—The Need for Comprehensive Digital Tools. Cancers. 2025; 17(7):1128. https://doi.org/10.3390/cancers17071128
Chicago/Turabian StyleAnghel, Rodica, Liviu Bîlteanu, Antonia-Ruxandra Folea, Șerban-Andrei Marinescu, Aurelia-Magdalena Pisoschi, Mihai-Florin Alexandrescu, Andreea-Ionela Dumachi, Laurentia-Nicoleta Galeș, Oana Gabriela Trifănescu, Anca-Florina Zgură, and et al. 2025. "Assessing the Impact of Nutritional Status on the Quality of Life in Head and Neck Cancer Patients—The Need for Comprehensive Digital Tools" Cancers 17, no. 7: 1128. https://doi.org/10.3390/cancers17071128
APA StyleAnghel, R., Bîlteanu, L., Folea, A.-R., Marinescu, Ș.-A., Pisoschi, A.-M., Alexandrescu, M.-F., Dumachi, A.-I., Galeș, L.-N., Trifănescu, O. G., Zgură, A.-F., Șerbănescu, L.-G., Capșa, C., Charalambous, A., & Șerban, A.-I. (2025). Assessing the Impact of Nutritional Status on the Quality of Life in Head and Neck Cancer Patients—The Need for Comprehensive Digital Tools. Cancers, 17(7), 1128. https://doi.org/10.3390/cancers17071128







