Simple Summary
Venetoclax, combined with hypomethylating agents (HMAs), is a standard first-line treatment for acute myeloid leukemia (AML) patients unfit for intensive chemotherapy. However, treatment schedules used across institutions are highly heterogeneous, and an optimal venetoclax administration schedule remains unclear. Moreover, whether longer venetoclax schedules lead to improved tumor responses is still unclarified. In this study, we investigated how venetoclax plasma levels and treatment duration (≤14 days vs. >14 days) correlate with hematologic recovery and responses in a cohort of 75 AML patients treated at our institution. Our study found no correlation between the venetoclax plasma peak and trough levels or venetoclax treatment duration (≤ or >14 days) and hematologic toxicity. Relevantly, patients receiving shorter venetoclax schedules (≤14 days) had similar complete response rates as compared to patients receiving longer schedules. In line with previous reports, our results suggest that shorter (≤14 days) venetoclax schedules do not negatively impact treatment efficacy. However, prospective validation studies would be required to confirm these findings.
Abstract
(1) Background: The combination of venetoclax and hypomethylating agents (HMAs) is a standard first-line regimen for acute myeloid leukemia (AML) patients unfit for intensive chemotherapy. Since venetoclax-HMAs are usually administered until progression and delayed hematologic recovery is one of the limiting toxicities, cyclic administration including 7–14-day breaks is recommended. However, whether longer venetoclax schedules lead to higher response rates and how venetoclax pharmacokinetics correlate with toxicity and efficacy remains unclarified. In this single-center retrospective study, we analyzed how venetoclax plasma levels and treatment duration impact hematologic toxicity and treatment responses. (2) Methods: We analyzed the safety and efficacy of venetoclax-HMA combination regimens in a cohort of AML patients unfit for intensive chemotherapy treated at our institution between June 2020 and September 2023. The primary endpoint was the correlation between venetoclax plasma levels or administration schedule with hematologic recovery after the first cycle. Secondary endpoints included the following clinical outcomes: correlation with complete response (CR) status, progression-free survival, and overall survival. (3) Results: Within our cohort of 75 AML patients, we found no correlation between venetoclax plasma peak and trough levels, or venetoclax treatment duration (≤ or >14 days), and hematologic toxicity. Patients receiving shorter venetoclax schedules (≤14 days) had similar CR rates compared to patients treated with longer schedules. (4) Conclusions: Our results suggest that shorter (≤14 days) venetoclax schedules may have no negative impact on tumor responses in AML patients receiving venetoclax and HMA combinations. However, prospective validation studies would be required to confirm these findings.
1. Introduction
Acute myeloid leukemia (AML) is a hematopoietic stem cell malignancy characterized by an aggressive disease course and high lethality despite intensive treatment. For fit patients, first-line curative-intended treatment includes intensive polychemotherapy-based induction treatment, followed by consolidation with autologous or allogenic hematopoietic stem cell transplantation, depending on initial risk stratification and the initial response to induction chemotherapy [1]. However, AML frequently affects elderly patients, with a median age at diagnosis of 68 years [2]. Advanced patient age and/or coexisting comorbidities frequently preclude an intensive chemotherapy induction due to the high risk of complications and treatment-related mortality [3]. Patients unfit for intensive induction therapy are usually treated—through a non-intensive approach—with a combination of hypomethylating agents (HMAs) (azacytidine or decitabine) or low-dose cytarabine (LDAC) and the B-cell lymphoma 2 (BCL-2) inhibitor venetoclax [4,5]. Venetoclax is a selective inhibitor of the anti-apoptotic protein BCL-2, which is overexpressed in many lymphoid and myeloid malignancies, and it is crucial for the survival of AML tumor cells and involved in treatment resistance [6,7]. As compared to intensive polychemotherapy, the combination of venetoclax and HMA or LDAC is significantly less toxic, enables disease control, and delays AML progression. Patients receiving venetoclax in combination with an HMA have shown complete remission rates of 67% [8,9,10]. Relevantly, previous studies have shown that venetoclax monotherapy results in only modest anti-AML activity with a shorter duration of responses [11]. On the contrary, the synergistic combination with HMA or LDAC led to significantly higher response rates and more durable remissions [12]. Moreover, under adequate monitoring, venetoclax-based regimens can be administered in an outpatient setting, with a positive impact on patients’ quality of life. Still, myelosuppression is one of the limiting toxicities, and efforts have been made to optimize the venetoclax treatment schedule in order to minimize toxicity while maintaining efficacy. Since first-line venetoclax-based combination regimens are usually administered until progression, cyclic administration including 7–14-day breaks to enable hematologic recovery is usually recommended. Moreover, whether longer venetoclax schedules lead to higher response rates and how venetoclax pharmacokinetics correlate with toxicity and efficacy remains unclarified. Treatment schedules used across distinct institutions are highly heterogenous, and, lately, venetoclax schedules have been shortened in the clinical practice from initially 28 days to 14 and even to 7 days in 28-day cycles [13]. In this study, we analyzed how venetoclax pharmacokinetics and the administration schedule correlate with hematologic toxicity and response rates. We hypothesized that longer administration schedules negatively impact hematologic recovery, with no additional benefit on AML tumor responses.
2. Materials and Methods
2.1. Patients
This single-institution retrospective study included 75 AML patients treated with venetoclax-based combination regimens at the University Hospital of Bern, Switzerland, between 22 June 2020 and 1 September 2023. Patients who received at least one cycle of venetoclax and had at least one plasma drug level determined were included.
2.2. Study Endpoints and Data Collection
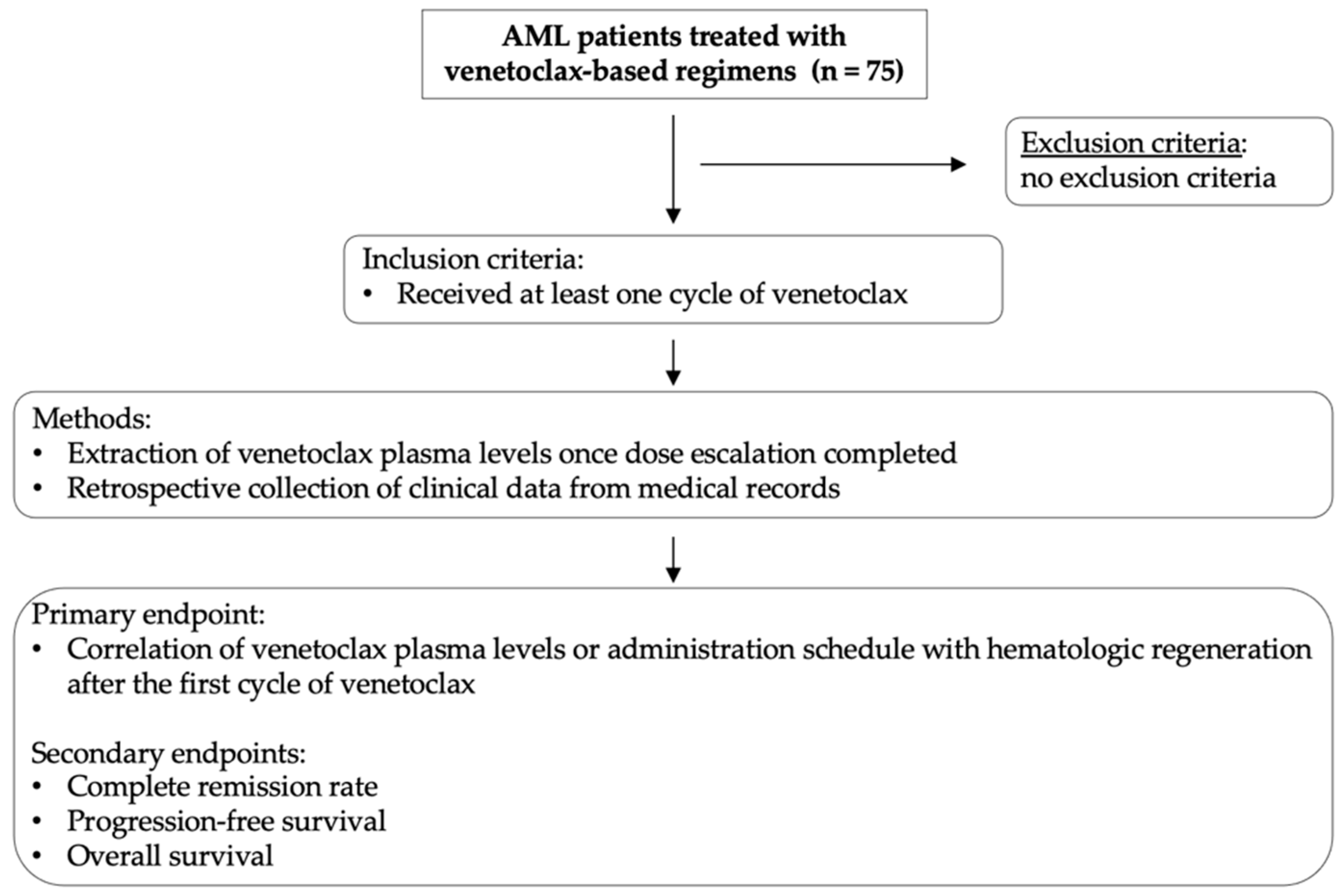
The primary endpoint of the study was the correlation between venetoclax plasma levels or administration schedule (≤14 vs. >14 days) with hematologic recovery after the first treatment cycle. The secondary endpoints included the following outcomes: complete remission (CR) rate, progression-free survival (PFS), and overall survival (OS). Risk stratification was performed according to the European Leukemia Network (ELN) 2022 guidelines [1]. For the purpose of this study, we included patients in CR with incomplete hematological regeneration within the CR group. Clinical data on patient baseline characteristics at first diagnosis, tumor responses, dates of progression and death, hematologic adverse events, as well as subsequent treatment lines were extracted from the clinical records. Study workflow and methods are summarized in Figure 1.
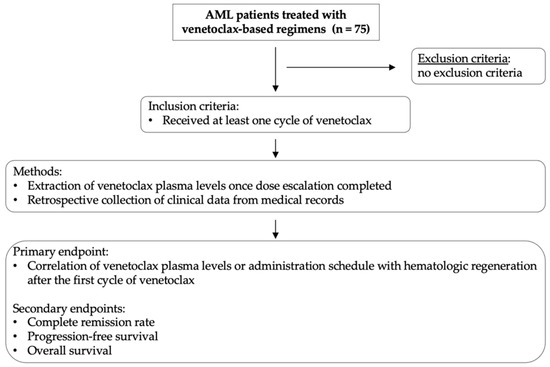
Figure 1.
Schematic diagram summarizing study workflow and research methodology. Study inclusion criteria, brief summary of methods, and study endpoints are illustrated.
2.3. Venetoclax Plasma Levels
Blood samples for measurement of venetoclax peak and trough levels were collected on day 4 or later, once venetoclax dose escalation had been completed. Peak levels were collected approximately 6 to 10 h after venetoclax administration, and trough levels immediately before. Venetoclax plasma levels were measured using ultra-high performance liquid chromatography–tandem mass spectrometry (UHPLC) with multiple reaction monitoring (MRM). Briefly, following blood extraction, samples were stored at −80 °C. Before analysis, separate stock solutions of venetoclax ((venetoclax 95% solid form) Alsachim, Illkirch Graffenstaden, France) and the isotope-labeled analogue ([2H7]-venetoclax 98% in solid form from Alsachim, Illkirch Graffenstaden, France) were prepared: venetoclax and the internal standard [2H7]-venetoclax were dissolved at a concentration of 1 mg/mL in dimethyl sulfoxide/methanol (1:1, v/v). Calibrators were prepared based on a stock solution of venetoclax at 9.50 mg/L in methanol. In the same way, an independent stock solution was prepared for the quality controls. Seven calibrator spiking solutions were prepared by diluting the stock solution with methanol to final concentrations of 0.15, 0.30, 0.59, 1.19, 2.38, 4.75, and 9.50 mg/L (undiluted) for venetoclax. The same procedure was repeated for four quality control spiking solutions with the final concentrations of 0.22, 0.89, 2.97, and 7.42 mg/L in methanol. For protein precipitation and analyte extraction from calibrators and quality controls, 25 µL of the respective spiking solutions, at the appropriate concentrations, was added to 40 µL of a DC Mass Spect Gold serum (Golden West Biologicals, Temecula, CA, USA), followed by 180 µL of acetonitrile containing the internal standard [2H7]-venetoclax. For protein precipitation and analyte extraction from patient samples, 25 µL of methanol was added to 40 µL of the serum, followed by 180 µL of acetonitrile containing the internal standard [2H7]-venetoclax. After incubation and mixing for 10 min, the samples were centrifuged at 4000 RCF and 20 °C for 15 min. Then, 80 µL of the supernatant was diluted with methanol to a final volume of 240 µL. The prepared samples were sealed and stored in the autosampler at 10 °C until analysis.
For UHPLC-MS/MS analysis, 0.5 µL of the prepared samples were injected into a reverse-phase CORTECS UPLC T3 column of 120 Å, 1.6 µm, and 2.1 mm × 100 mm (Waters Corp., Milford, MA, USA), with a gradient mobile phase comprising 0.1% ammonium acetate with 1% formic acid (A) and acetonitrile containing 0.1% ammonium acetate with 1% formic acid (B). Each sample was resolved for 3.5 min at a flow rate of 0.5 mL/min with the linear gradient 0–1.2 min from 35 to 98% B; 1.2–2.0 min 98% B; and 35% B for 1.5 min. The column temperature was 30 °C. The eluent was introduced via electrospray ionization into the mass spectrometer (LC-MS 8060NX, Shimadzu Corp., Kyoto, Japan), operating in positive ion electrospray ionization mode (ESI+). The capillary voltage was set to 1.0 kV, and the Focus Voltage to 2.0 kV. The nebulizing gas flow was 2.5 L/min, the heating gas flow was 20 L/min, and the drying gas flow was 5 L/min. The interface temperature, desolvation line temperature, and heat block temperature were set to 400 °C, 150 °C, and 500 °C, respectively. MRM conditions were optimized by adjusting the collision energy to yield the most abundant product ions for each compound, which were subsequently used for MRM analysis (see summary of optimized MS/MS parameters given in Supplementary Table S1). Data analysis was performed with Labsolution Insight LCMS (version 3.8, Shimadzu Corp., Kyoto, Japan) by analyzing the areas under the specific MRM chromatograms compared to the area of the isotope-labeled analogue. The calibration curve was constructed using venetoclax concentrations ranging from 92.8 to 5937.5 µg/L and applying weighted linear regression with a weighting factor of 1/x.
2.4. Statistical Analysis
GraphPad Prism version 10 was used for statistical analyses and the graphical representations of the data. PFS was defined as the time from the first dose of venetoclax to the occurrence of progression, loss of follow-up, or death. OS was defined as the time from the start of venetoclax therapy to the date of death. Both PFS and OS curves were generated using the Kaplan–Meier method, with statistical significance assessed using the Mantel–Cox test and the Gehan–Breslow–Wilcoxon test. Categorical data were analyzed using Fisher’s exact test, and parametric data were evaluated using the unpaired t-test. Multiple regression analysis was conducted to explore statistical correlations between several independent variables and a single dependent variable. The following variables were included in the analyses: sex, age, European LeukemiaNet (ELN) risk stratification, French–American–British (FAB) classification, AML cytogenetics, selected molecular alterations, peripheral blood counts, and parameters related to venetoclax treatment (number and duration of cycles, dose, combination with HMA or LDAC, dose reductions, and selected co-medications), tumor responses, as well as time to progression and death. p-values were rounded to two decimals, and values below 0.05 were considered statistically significant. Percentage results were rounded to whole numbers.
3. Results
3.1. Patient Baseline Characteristics
The median age at AML diagnosis was 70 years, and men slightly outnumbered women at a ratio of 1.3. Fifty-six percent of patients had a primary AML, and forty-four percent had a secondary AML. Patient classification according to the FAB system is provided in Table 1. Based on the ELN risk classification, over 75% of patients were classified within the adverse risk group; moreover, nine (12%) patients were categorized as intermediate risk and eight (11%) patients as favorable. Thirty-one (41%) patients had a normal karyotype, fourteen (24%) had a complex or both a complex and monosomal karyotype, and twenty-nine (39%) patients had other cytogenetic abnormalities. In one (1%) patient, no data on karyotype were available. Most common mutations involved ASXL1 (21%), DNMT3A (21%), IDH2 (16%), NMPM1 (20%), RUNX1 (19%), TET2 (24%), and TP53 (17%). Patient characteristics at first diagnosis, including peripheral blood counts, are summarized in Table 1. The corresponding non-aggregated clinical data are listed in Supplementary Table S2.

Table 1.
Patient baseline characteristics.
3.2. Treatment with Venetoclax
Patients received a median of four cycles of venetoclax during the study period. Fourteen (19%) patients completed only one cycle, while 15 (20%) patients received more than 10 cycles. Fifty-one percent of patients received treatment cycles lasting 28 days, 31%, 42 days, and the remaining 19% received venetoclax with variable cycle durations. Almost three-quarters of the patients underwent venetoclax therapy in combination with azacitidine, while one-quarter was treated with decitabine or other combination drugs. Only 13% of patients received the standard venetoclax dose of 400 mg daily, while most patients (60%) received an adjusted dose of 100 mg due to the simultaneous intake of posaconazole or other CYP3A4 inhibitors. During treatment, dose reductions, with a median of 40%, were primarily applied to HMA or LDAC (35% of cases), whereas venetoclax dosing was mostly reduced by shortening the cycle duration to a median of 7 days (52% of cases) (Table 2). Treatment lines before and after venetoclax-based regimens are displayed in Supplementary Table S3.

Table 2.
Details on treatment with venetoclax.
3.3. Outcome of Venetoclax Therapy
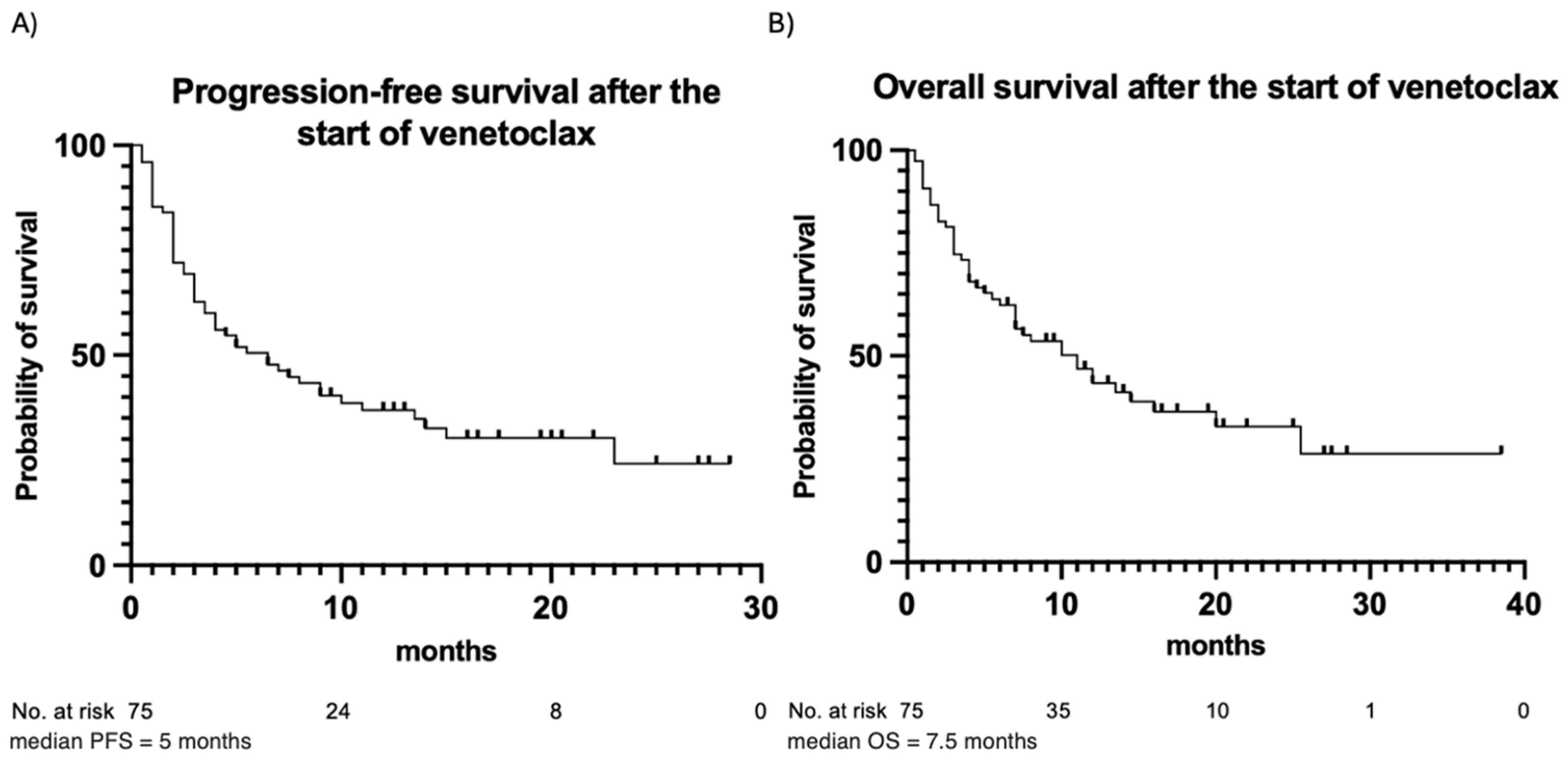
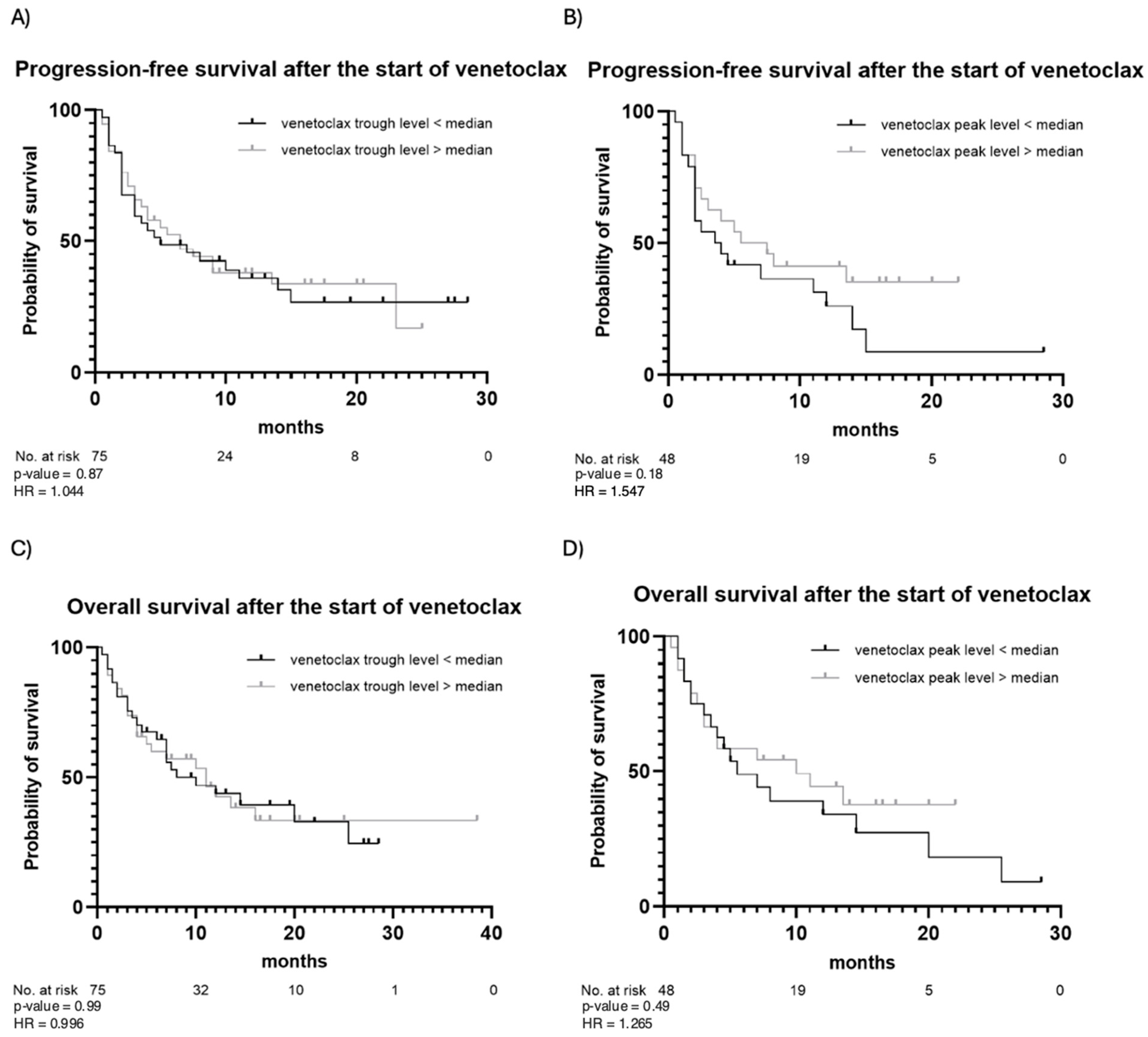
The outcomes of venetoclax therapy are summarized in Table 3. CR was achieved in 49 (65%) patients, while partial remission was observed in 10 (13%) patients. Fourteen (19%) patients had stable disease, and two (3%) patients died before response assessment. In the majority of patients (83%), the best response occurred following the first cycle of venetoclax. In the remaining cases, the best response was observed after the second (16%) or the third (1%) cycle. During the observation period, 50 (67%) patients experienced disease progression after a median of 5 months, and 45 (60%) patients died due to progression or disease-related complications. PFS and OS estimates are illustrated in Figure 2. In addition, PFS and OS curves for patients with trough and peak levels below vs. above the median are illustrated in Figure 3. No statistically significant differences in PFS or OS when stratifying by venetoclax plasma levels were observed. Patients with venetoclax trough levels below the median had a median PFS of 5 months, whereas patients with trough levels above the median showed a median PFS of 6.5 months (p-value = 0.87). The hazard ratio (HR) for this comparison was 1.044, with a 95% confidence interval (CI) from 0.599 to 1.818. The median OS was 10 months for patients with trough levels below the median and 11 months for those with trough levels above the median (p = 0.99), with an HR of 0.996 and a 95% CI from 0.555 to 1.787. A similar pattern was observed for both PFS and OS in patients with peak levels above and below the median. For PFS, the median was 3.75 months for patients with peak levels below the median and 6.5 months for those with peak levels above the median (p = 0.18). The median OS was 5.5 months for patients with peak levels below the median and 10 months for those with peak levels above the median (p = 0.49). Patients with a higher plasma peak concentration of venetoclax showed a trend toward slightly longer OS.

Table 3.
Outcomes of venetoclax therapy.
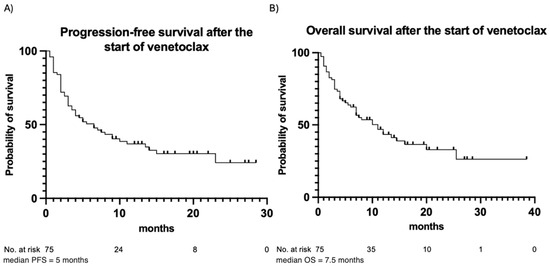
Figure 2.
Progression-free survival (A) and overall survival (B) for the entire patient cohort. Observation time (months) is plotted in the X-axis, and the probability of survival, calculated using Kaplan–Meier analysis, is plotted in the Y-axis.
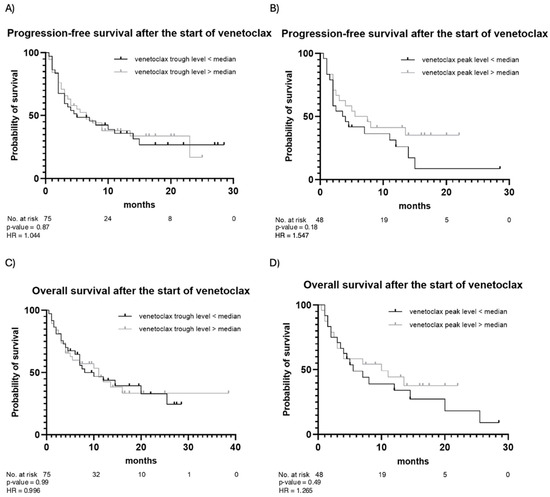
Figure 3.
Progression-free survival and overall survival after the start of venetoclax stratified by venetoclax trough (A,C) and peak (B,D) levels. Observation time (months) is plotted in the X-axis, and the probability of survival, calculated using Kaplan–Meier analysis, is plotted in the Y-axis.
3.4. Drug Levels of Venetoclax
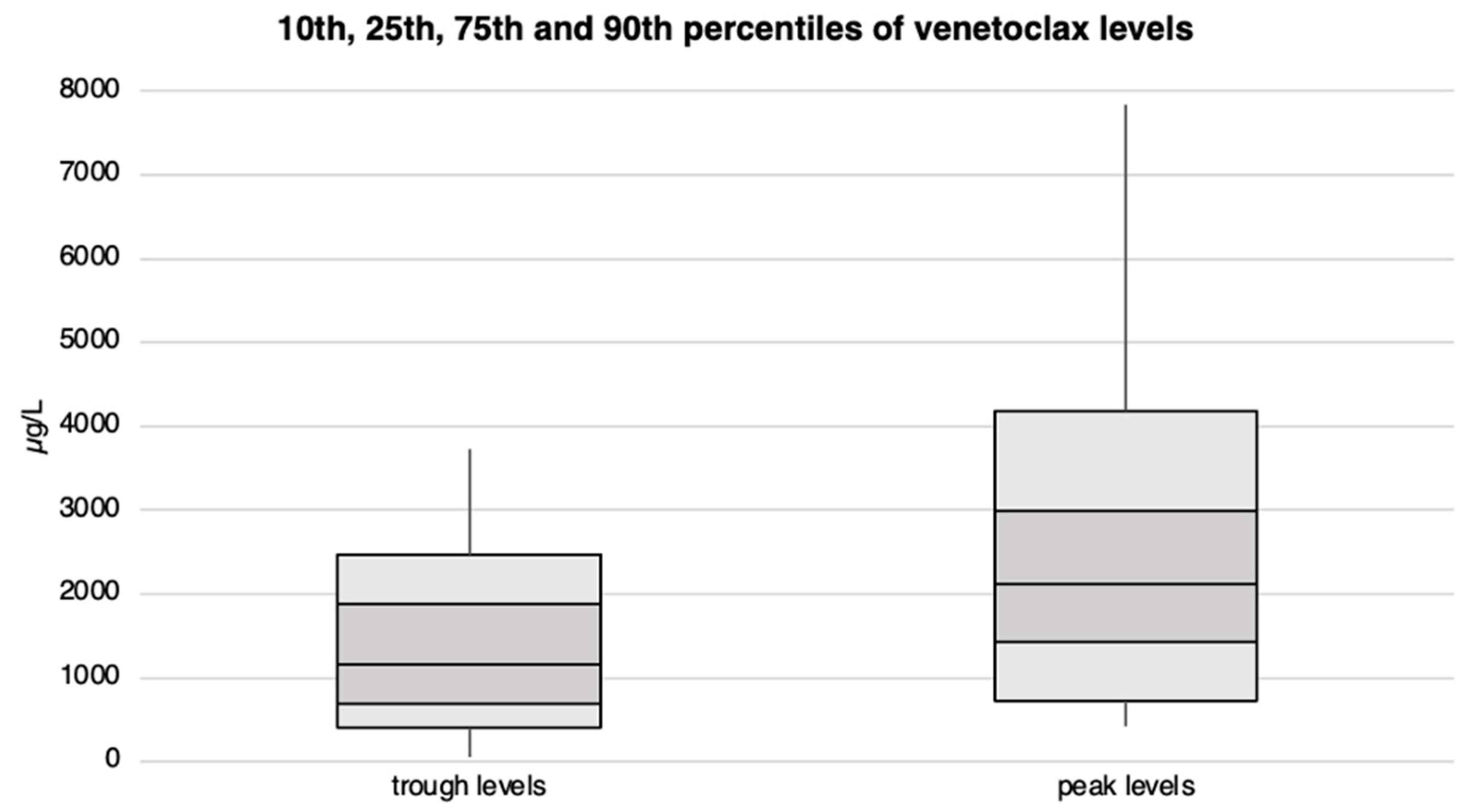
Patients exhibited venetoclax plasma trough levels ranging from approximately 60 to 3’700 µg/L, with 50% of them reaching a minimum blood concentration of at least 1’170 µg/L. Peak levels varied from 400 µg/L to over 7’800 µg/L, with the median peak level slightly exceeding 2’100 µg/L. We observed no statistical differences in venetoclax plasma levels between patients receiving 100 vs. 400 mg/day. Venetoclax trough and peak levels are illustrated in Figure 4 and Supplementary Tables S4 and S7.
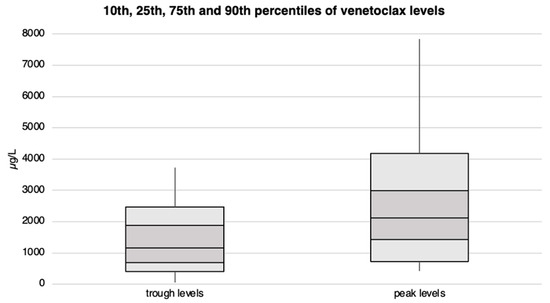
Figure 4.
Venetoclax trough and peak plasma levels with corresponding percentiles for the entire patient cohort. The concentration levels (in µg/L) are plotted on the Y-axis, while the X-axis is divided into trough and peak levels.
3.5. Impact of Remission Status and Venetoclax Plasma Levels on Hematologic Recovery
Data on the hematologic toxicities observed during the first and second cycle of venetoclax is illustrated in Supplementary Table S5. The correlation between remission status (CR versus not CR) or venetoclax plasma levels and hematologic recovery after the first cycle of venetoclax is illustrated in Table 4. Overall, 38 (51%) patients achieved CR after the first treatment cycle. Patients with CR more frequently had platelet levels over 100 G/L (p-value = 0.002) and neutrophil counts over 0.5 (p-value = 0.02), respectively, >1 G/L (p-value = 0.02) before starting the second treatment cycle, as compared to those who did not achieve a CR. Among all included patients, the median trough level was 1’220 µg/L, and the median peak level was 2’117 µg/L. No correlation was observed between the venetoclax plasma peak or trough levels and platelet levels above 100 G/L or neutrophil counts over 0.5/1 G/L before the initiation of the second treatment cycle.

Table 4.
Correlation between CR status and venetoclax plasma levels and hematologic recovery after the first cycle of venetoclax.
Table 5 displays the correlation between neutrophil and platelet dynamics before and after the first treatment cycle CR status, as well as the venetoclax trough and peak levels. Twenty-four (33%) patients had platelet levels above 100 G/L, and 48 (67%) patients had levels below 100 G/L before starting venetoclax treatment. After the first cycle, platelets increased in 36 (50%) patients over 100 G/L, while in the other 36 (50%) patients, platelet counts either remained or had decreased below 100 G/L. Again, platelet recovery over 100 G/L was positively associated with the occurrence of CR (p-value = 0.02). Further correlations between venetoclax peak and trough levels, hematologic regeneration, and CR, as well as sex, dose of venetoclax, patient age, and the combined chemotherapy agent, are presented in Supplementary Tables S6–S10.

Table 5.
Correlation between CR status or venetoclax trough and peak levels and neutrophil and platelet dynamics before and after the first treatment cycle.
The correlation between venetoclax treatment duration and remission status (CR versus no CR) or hematologic recovery is shown in Table 6. Eighteen (25%) patients received venetoclax for 14 days or less, while the remaining 54 (75%) patients were treated for more than 14 days. CR rates were similar between the two groups, with no statistically significant difference observed (p-value > 0.99). No statistically significant correlation between shorter venetoclax treatment schedules (≤14 days) and the regeneration of platelet and neutrophil levels after the first cycle of venetoclax could be shown.

Table 6.
Correlation between venetoclax treatment duration and CR status or hematologic recovery after the first cycle of venetoclax.
Table 7 displays the correlation between venetoclax treatment duration and change in neutrophil and platelet blood levels before and after the first cycle of venetoclax. No statistically significant difference between shorter (≤14 days) or longer (>14 days) venetoclax treatment schedules and change in platelet and neutrophil levels before and after the first cycle of venetoclax was found.

Table 7.
Correlation between venetoclax treatment duration and changes in neutrophil and platelet levels from before to after the first cycle of venetoclax.
3.6. Factors Influencing Response, Hematologic Regeneration, and Venetoclax Trough and Peak Levels
CR, platelet, and neutrophil counts after the first cycle of venetoclax and venetoclax peak and trough levels were each considered dependent variables. Independent variables included age, sex, ELN risk stratification, de novo vs. secondary AML, dose of venetoclax, venetoclax trough and peak levels, response after the first cycle, and neutrophil and platelet counts before starting venetoclax treatment. Achieving a CR after the first cycle of venetoclax was significantly negatively associated with having an adverse ELN risk profile. Platelet counts below 100 G/L after the first cycle positively correlated with an adverse ELN risk profile, as did platelet counts below 100 G/L before starting venetoclax and trough levels exceeding the median. A negative correlation was observed between the lack of platelet regeneration and the achievement of a CR. Other regression analyses between the dependent and independent variables showed no statistically significant association (Table 8).

Table 8.
Results of multiple linear regression analyses.
4. Discussion
The venetoclax and HMA combination is currently a standard first-line treatment for AML patients unfit for intensive chemotherapy. Given the favorable efficacy and safety profiles of these combinations, these regimens are being increasingly used in clinics. However, few data are available regarding the impact of venetoclax plasma levels on treatment response and hematologic toxicity. Moreover, while previous real-world reports have suggested that longer venetoclax schedules are associated with higher hematological toxicity, the consequences of venetoclax treatment duration on AML response rates remain unclarified. In this study, we aimed to assess the impact of venetoclax plasma levels and treatment duration on both hematologic toxicity and treatment efficacy. The pivotal phase 3 VIALE-A study assessed the efficacy and safety of the venetoclax–azacitidine combination in patients with a previously untreated AML who were unfit to receive intensive induction and consolidation therapy [8]. In this study, patients received venetoclax daily, following 28-day cycles. The results from this study highlighted a relatively high frequency of hematologic adverse events grade 3 or higher. Thrombocytopenia grade 3 or higher occurred in 45% of the patients, neutropenia in 42%, anemia in 26%, and febrile neutropenia in 42% [8]. Due to hematological toxicity, dose treatment interruptions, including the reduction in venetoclax treatment duration from 28 to 21 days, were necessary in 53% of patients [8]. Further studies showed that there is a need for venetoclax dose reduction in patients receiving concomitant treatment with strong CYP324 inhibitors, such as azole antifungals (e.g., posaconazol) [14]. Moreover, over the past years, individual reports on alternative venetoclax schedules have suggested that shorter administration schedules might overcome the handicap of delayed hematologic recovery without negatively impacting treatment efficacy [15]. Strikingly, the results of our study showed no correlation between the venetoclax plasma peak and trough levels and hematologic toxicity or treatment efficacy. Moreover, venetoclax treatment duration (≤ or >14 days) did not correlate with improved hematologic recovery. In line with previous reports, shorter venetoclax schedules did not lead to lower rates of CRs, suggesting a lack of negative impact on treatment efficacy [16,17]. The underlying biological mechanism remains unclarified [16,17]. Previous studies in AML and chronic lymphocytic leukemia showed a lack of impact of venetoclax exposure or peak levels on treatment responses [18,19]. However, the limitations of the current study include a single-center and retrospective design, a relatively small sample size, and a heterogeneous patient cohort. A relevant strength of our study is the consistency of observed CR rates with previous studies, including the phase 3 VIALE-A trial. In our study, CR was observed in 65% of patients, almost identical to the CR rate reported in the VIALE-A study (64.7%) [8]. Due to the common limitations of a retrospective study design, prospective validation studies would be required to more systematically assess the impact of the venetoclax schedule on AML tumor responses. In this sense, the ongoing observational multicentric VALOR study (NCT05215639) might provide more robust data on the safety and efficacy of venetoclax and HMA in a real-world patient cohort.
5. Conclusions
Our study could not show a significant correlation between venetoclax plasma levels or shorter vs. longer venetoclax administration schedules and hematologic toxicity in a cohort of AML patients unfit for intensive induction chemotherapy treated with venetoclax combined with HMA or LDAC. However, the results from our study suggest that shorter (≤14 days) venetoclax schedules may have no negative impact on AML tumor responses. Given the retrospective design of the current study, larger prospective studies would be required to confirm our findings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers17071138/s1, Table S1: Summary of MS/MS parameters for venetoclax and its corresponding internal standard; Table S2: Non-aggregated clinical data of all patients; Table S3: Treatment lines before and after venetoclax-based regimens; Table S4: Trough and peak levels of venetoclax; Table S5: Hematologic toxicity observed during the first and second cycle of venetoclax; Table S6: Association of venetoclax levels with hematologic regeneration and rate of CR; Table S7: Association of venetoclax levels with sex; Table S8: Association of venetoclax levels with dose of venetoclax; Table S9: Association of venetoclax levels with patient age at first diagnosis; Table S10: Association of venetoclax levels with the combination agent.
Author Contributions
Design of study: T.P.; data analysis: A.S., D.A., U.B., H.N., C.R.L., Y.A., M.H. (Micheal Hayos) and T.P.; statistics: H.N. and T.P.; writing of the manuscript: A.S., D.A. and T.P.; providing material: T.P.; review of manuscript and approval of final version: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The retrospective study was conducted according to the guidelines of the Declaration of Helsinki and approved by the relevant Ethics Committee, Switzerland (decision number 2021-01863 and date of approval 26 November 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
No data supporting the reported results are deposited elsewhere.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.D.; DiNardo, C.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Key Statistics for Acute Myeloid Leukemia (AML). Available online: https://www.cancer.org/cancer/types/acute-myeloid-leukemia/about/key-statistics.html (accessed on 14 October 2024).
- Richard-Carpentier, G.; DiNardo, C.D. Venetoclax for the treatment of newly diagnosed acute myeloid leukemia in patients who are ineligible for intensive chemotherapy. Ther. Adv. Hematol. 2019, 10, 2040620719882822. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.H.; Strickland, S.A.; Hou, J.Z.; Fiedler, W.; Lin, T.L.; Walter, R.B.; Enjeti, A.; Tiong, I.S.; Savona, M.; Lee, S.; et al. Venetoclax Combined with Low-Dose Cytarabine for Previously Untreated Patients with Acute Myeloid Leukemia: Results from a Phase Ib/II Study. J. Clin. Oncol. 2019, 37, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Garciaz, S.; Hospital, M.A.; Alary, A.S.; Saillard, C.; Hicheri, Y.; Mothy, B.; Rey, J.; D’Incan, E.; Charbonnier, A.; Villetard, F.; et al. Azacitidine Plus Venetoclax for the Treatment of Relapsed and Newly Diagnosed Acute Myeloid Leukemia Patients. Cancers 2022, 14, 2025. [Google Scholar] [CrossRef] [PubMed]
- El-Cheikh, J.; Bidaoui, G.; Saleh, M.; Moukalled, N.; Abou Dalle, I.; Bazarbachi, A. Venetoclax: A New Partner in the Novel Treatment Era for Acute Myeloid Leukemia and Myelodysplastic Syndrome. Clin. Hematol. Int. 2023, 5, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.G.; Volpe, V.O.; Wang, C.; Ball, S.; Tobon, K.; Chan, O.; Padron, E.; Kuykendall, A.; Lancet, J.E.; Komrokji, R.; et al. Outcomes by best response with hypomethylating agent plus venetoclax in adults with previously untreated acute myeloid leukemia. Ann. Hematol. 2024, 104, 307–315. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [PubMed]
- DiNardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019, 133, 7–17. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Rausch, C.R.; Benton, C.; Kadia, T.; Jain, N.; Pemmaraju, N.; Daver, N.; Covert, W.; Marx, K.R.; Mace, M.; et al. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am. J. Hematol. 2018, 93, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Konopleva, M.; Pollyea, D.A.; Potluri, J.; Chyla, B.; Hogdal, L.; Busman, T.; McKeegan, E.; Salem, A.H.; Zhu, M.; Ricker, J.L.; et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov. 2016, 6, 1106–1117. [Google Scholar] [PubMed]
- Pollyea, D.A.; Pratz, K.W.; Jonas, B.A.; Letai, A.; Pullarkat, V.A.; Wei, A.; Letai, A.; Pullarkat, V.A.; Wei, A.; Konopleva, M.Y.; et al. Venetoclax in combination with hypomethylating agents induces rapid, deep, and durable responses in patients with AML ineligible for intensive therapy. Blood 2018, 132, 285. [Google Scholar] [CrossRef]
- Jonas, B.A.; Pollyea, D.A. How we use venetoclax with hypomethylating agents for the treatment of newly diagnosed patients with acute myeloid leukemia. Leukemia 2019, 33, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.K.; DiNardo, C.D.; Potluri, J.; Dunbar, M.; Kantarjian, H.M.; Humerickhouse, R.A.; Wong, S.L.; Menon, R.M.; Konopleva, M.Y.; Salem, A.H. Management of Venetoclax-Posaconazole Interaction in Acute Myeloid Leukemia Patients: Evaluation of Dose Adjustments. Clin. Ther. 2017, 39, 359–367. [Google Scholar] [CrossRef]
- Cui, J.; Chen, X.; Li, C.; Yan, Q.; Yuan, G. Reduced duration and dosage of venetoclax is efficient in newly diagnosed patients with acute myeloid leukemia. Am. J. Hematol. 2024, 29, 2293512. [Google Scholar]
- Karrar, O.; Abdelmagid, M.; Rana, M.; Iftikhar, M.; McCullough, K.; Al-Kali, A.; Alkhateeb, H.B.; Begna, K.H.; Elliott, M.A.; Mangaonkar, A.; et al. Venetoclax duration (14 vs. 21 vs. 28 days) in combination with hypomethylating agent in newly diagnosed acute myeloid leukemia: Comparative analysis of response, toxicity, and survival. Am. J. Hematol. 2024, 99, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Gangat, N.; Karrar, O.; Iftikhar, M.; McCullough, K.; Johnson, I.M.; Abdelmagid, M.; Abdallah, M.; Al-Kali, A.; Alkhateeb, H.B.; Begna, K.H.; et al. Venetoclax and hypomethylating agent combination therapy in newly diagnosed acute myeloid leukemia: Genotype signatures for response and survival among 301 consecutive patients. Am. J. Hematol. 2024, 99, 193–202. [Google Scholar] [PubMed]
- Badawi, M.; Chen, X.; Marroum, P.; Suleiman, A.A.; Mensing, S.; Koenigsdorfer, A.; Schiele, J.T.; Palenski, T.; Samineni, D.; Hoffman, D.; et al. Bioavailability Evaluation of Venetoclax Lower-Strength Tablets and Oral Powder Formulations to Establish Interchangeability with the 100 mg Tablet. Clin. Drug Investig. 2022, 42, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Brackman, D.; Eckert, D.; Menon, R.; Salem, A.H.; Potluri, J.; Smith, B.D.; Wei, A.H.; Hayslip, J.; Miles, D.; Mensing, S.; et al. Venetoclax exposure-efficacy and exposure-safety relationships in patients with treatment-naïve acute myeloid leukemia who are ineligible for intensive chemotherapy. Hematol. Oncol. 2022, 40, 269–279. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).