Hot Spots in Urogenital Basic Cancer Research and Clinics
Simple Summary
Abstract
1. Introduction
2. 2022 WHO Update on the Pathologic Classification of Urinary and Male Genital Tumors
2.1. Prostate Cancer
2.2. Renal Cancer
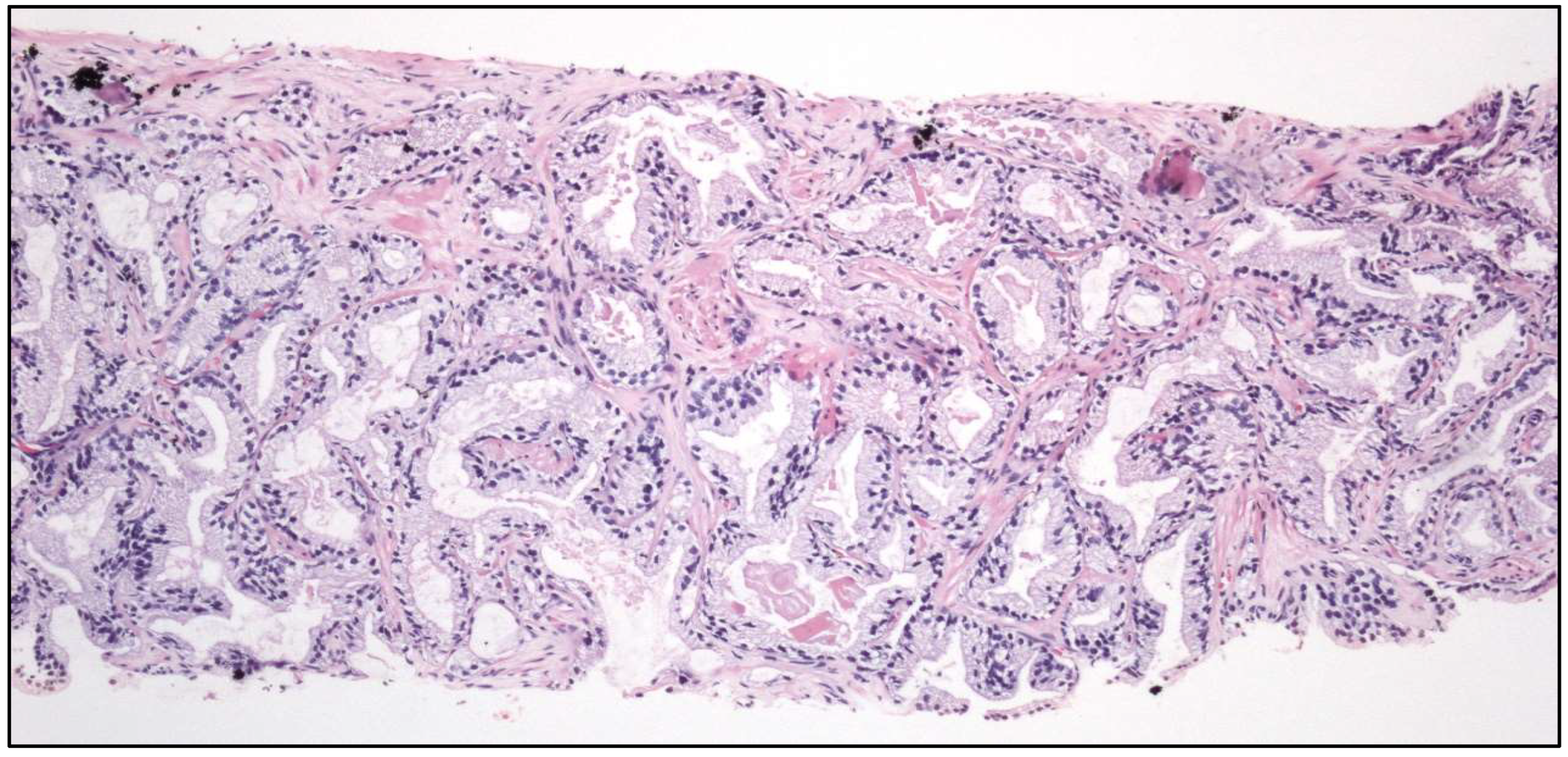
2.3. Urinary Tract Cancer
- -
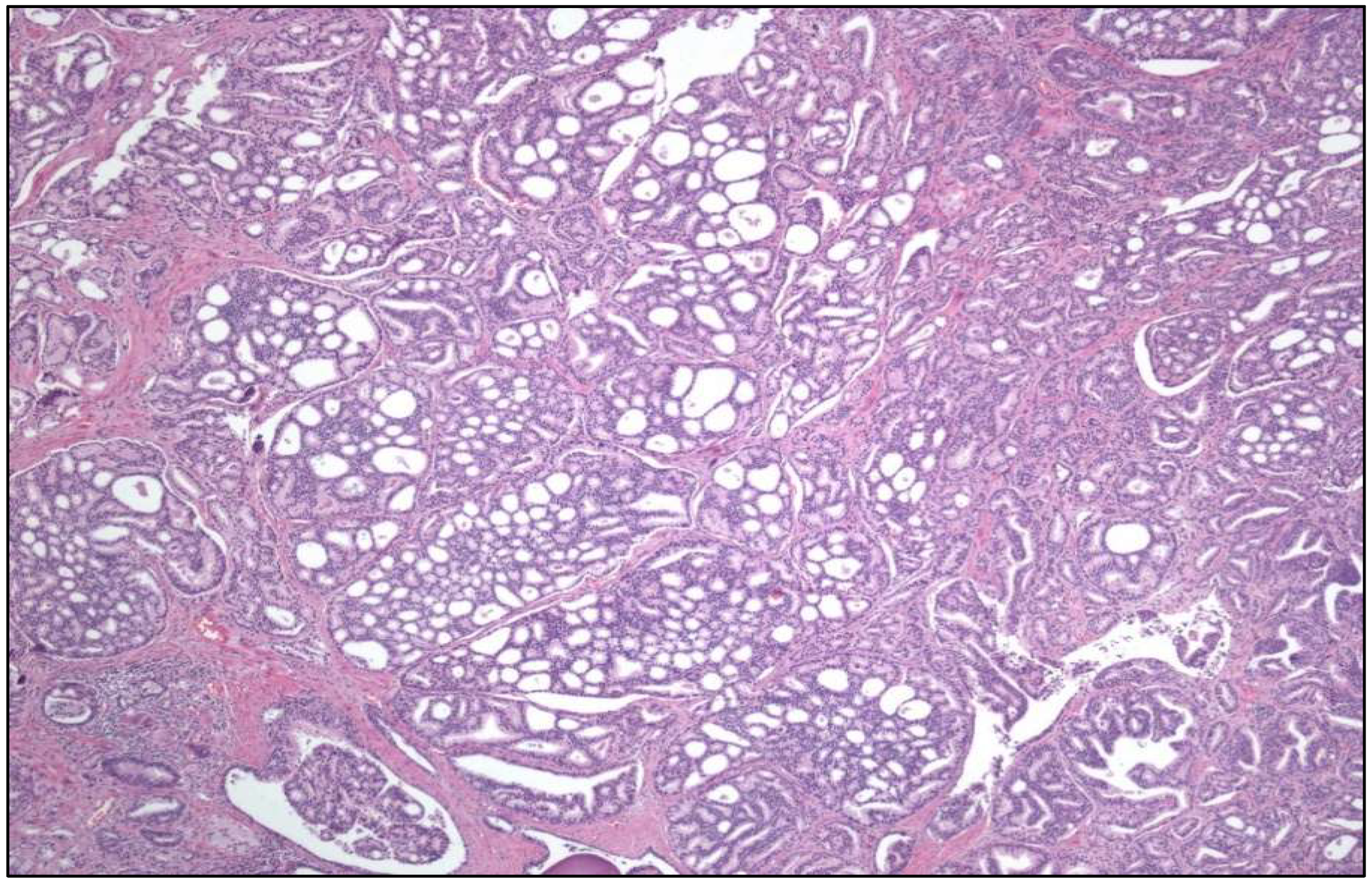
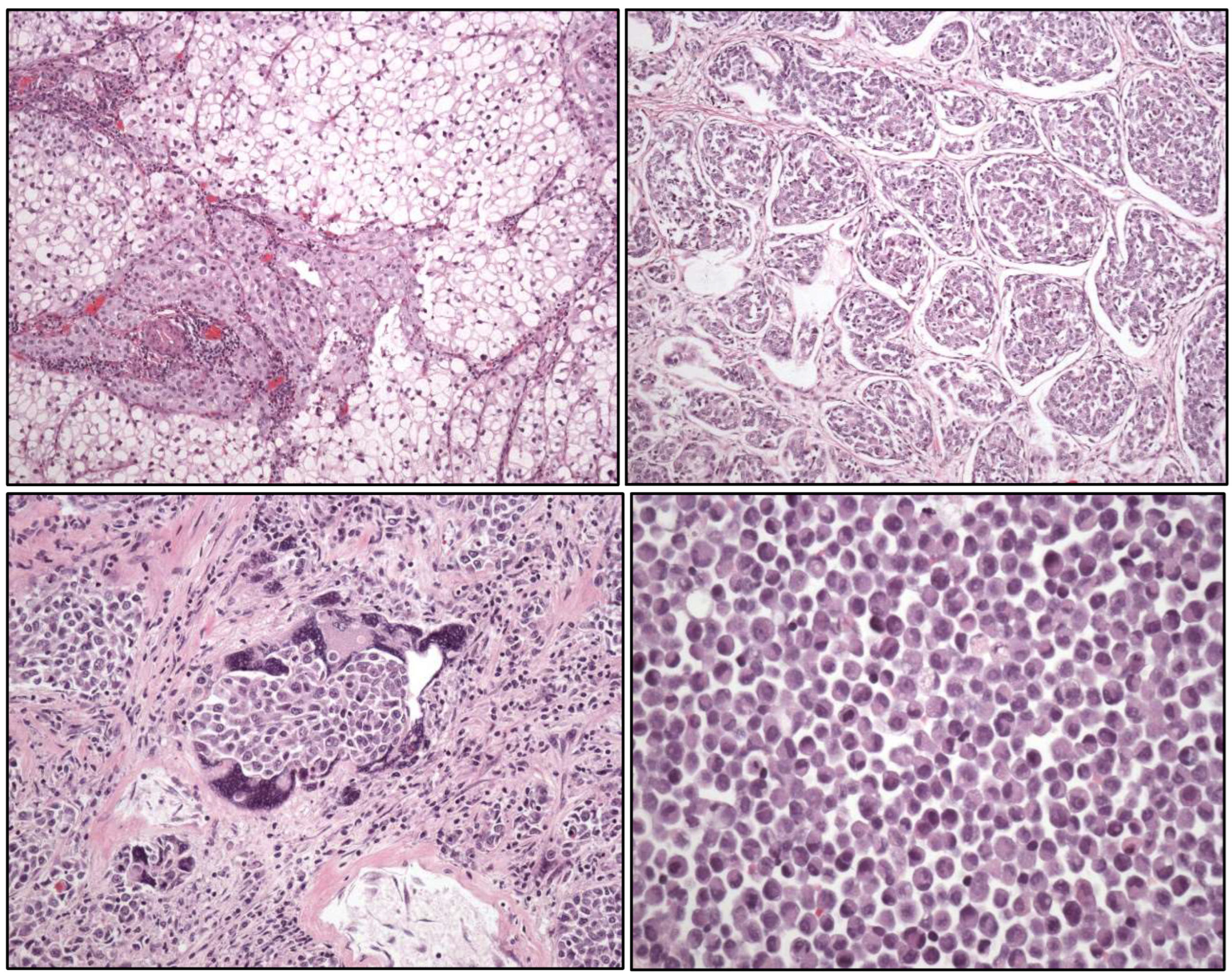
- Tubular UC
- -
- Large “nested” UC (Figure 3)
- -
- Clear cell UC is renamed as “glycogen-rich” clear cell UC (Figure 3) to distinguish it from the adenocarcinoma of Müllerian type.
- -
- Plasmacytoid UC should no longer be termed as “signet-ring cell”/“diffuse”.
- -
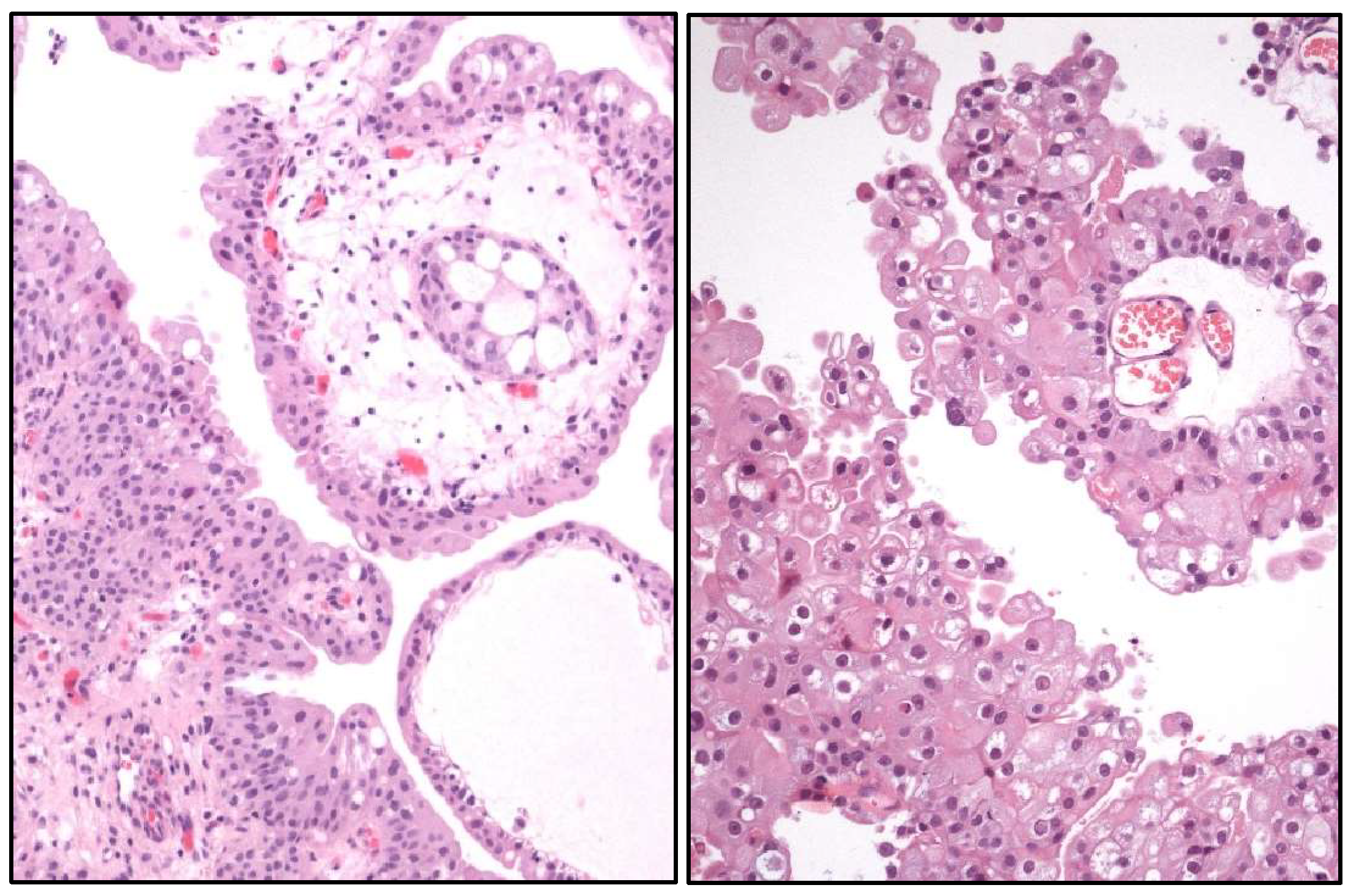
- Glandular, squamous, trophoblastic (Figure 3), Müllerian, and neuroendocrine morphologies must be specifically mentioned in the pathological report of UC, including its approximate percentage.
- -
- Micropapillary, plasmacytoid (Figure 3), and other “wolves in lamb clothes” must be recognized and specifically reported, either pure or mixed, in the pathological report of UC.
- -
- The sub-staging of pT1 carcinomas (pT1a/pT1b).
- -
- The applicability of the molecular classification (luminal, basal, basal-squamous, etc.) in muscle-invasive UC.
- -
- The clinical usefulness of FGFR3, TP53, and ERCC2 mutations.
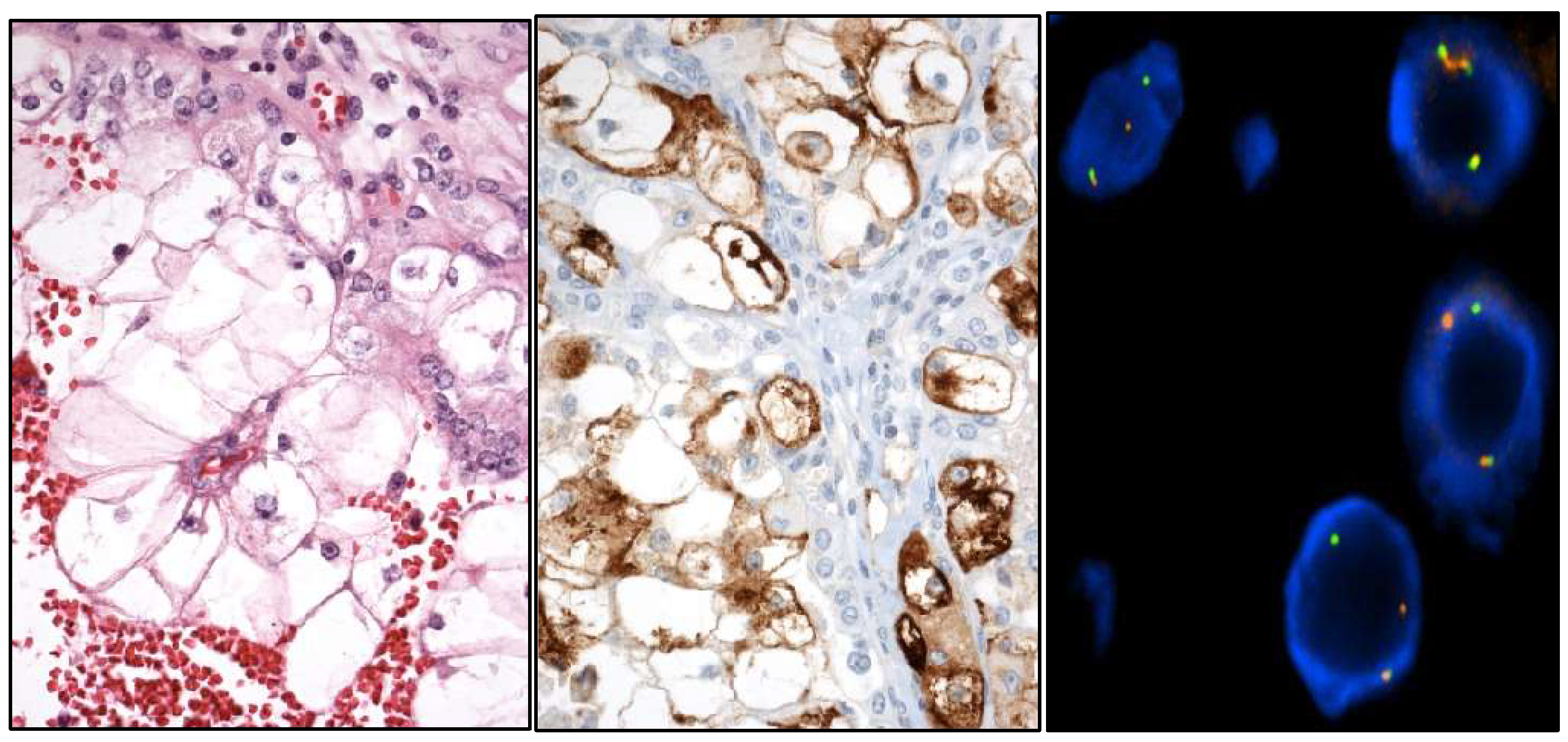
2.4. Testicular Cancer
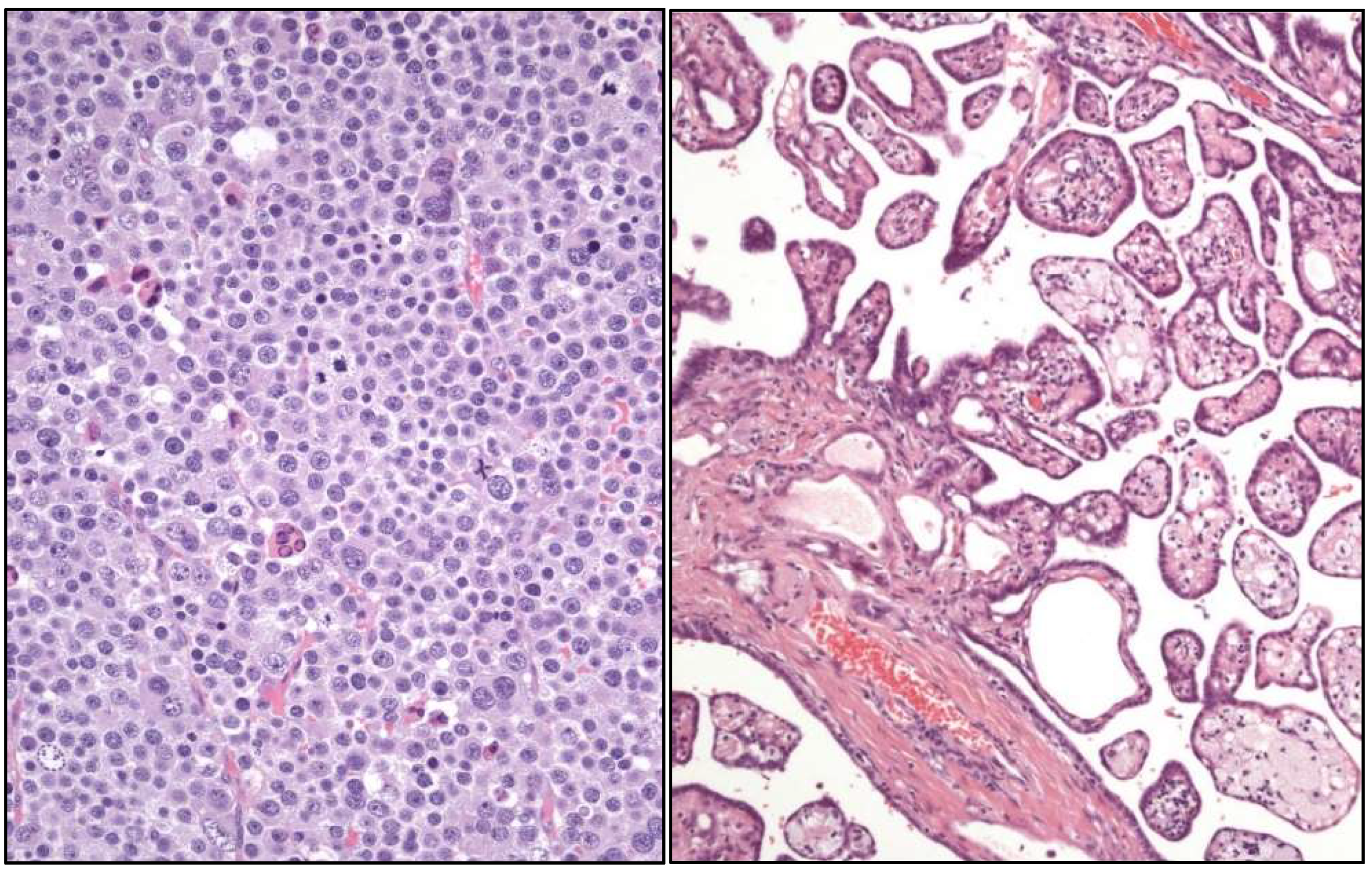
- -
- Stromal tumor with signet-ring cells.
- -
- “Myoid” gonadal stromal tumor.
- -
- Well-differentiated papillary mesothelial tumor (Figure 4).
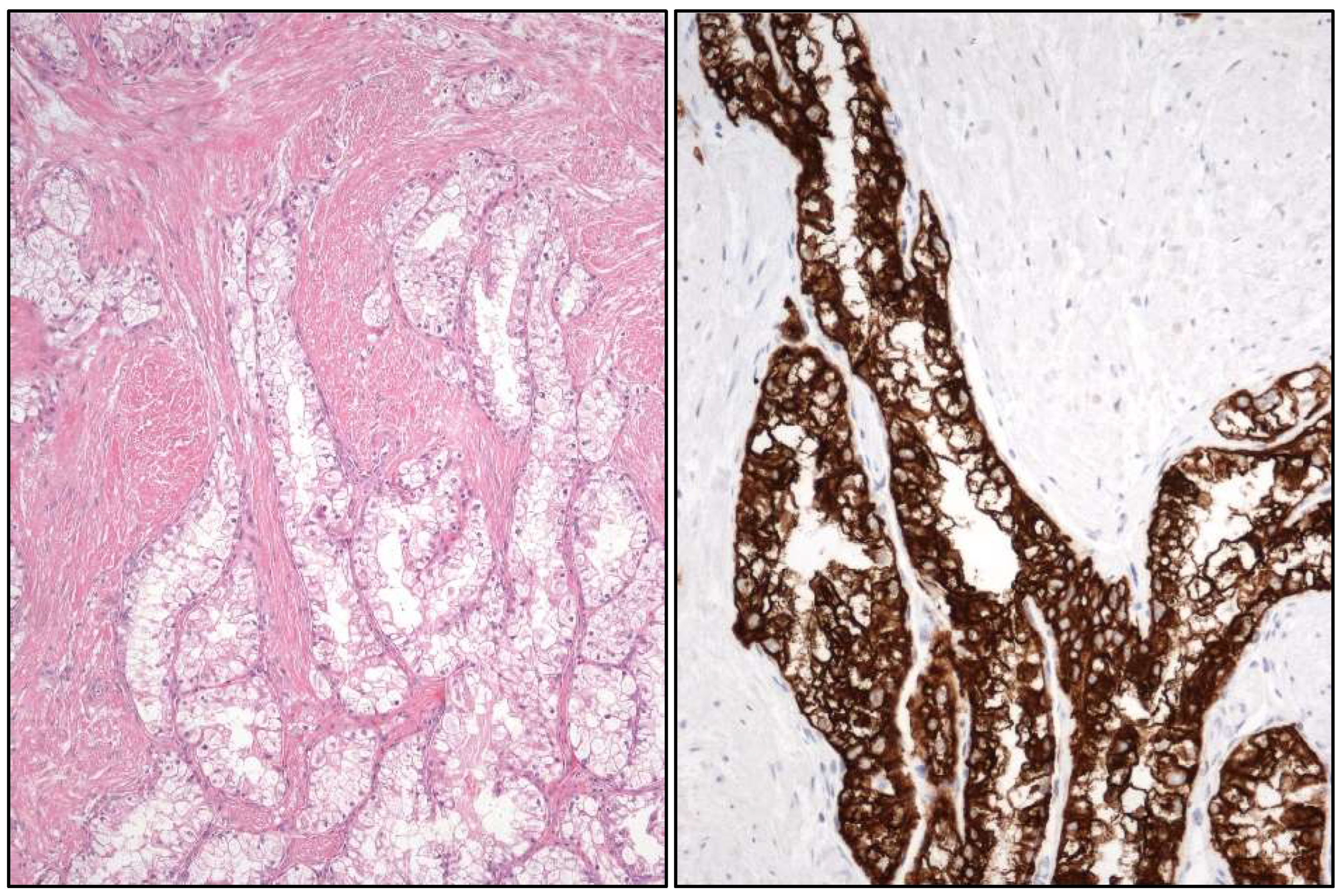
2.5. Penile Cancer
- -
- HPV-related squamous cell carcinomas are:
- ▪
- “Warty” PeCa (Figure 5).
- ▪
- Basaloid PeCa.
- ▪
- Clear cell squamous PeCa.
- ▪
- Lymphoepithelioma-like PeCa.
- -
- HPV-unrelated squamous cell PeCa are:
- -
- Squamous cell PeCa, not otherwise specified.
- -
- Mixed squamous cell PeCa.
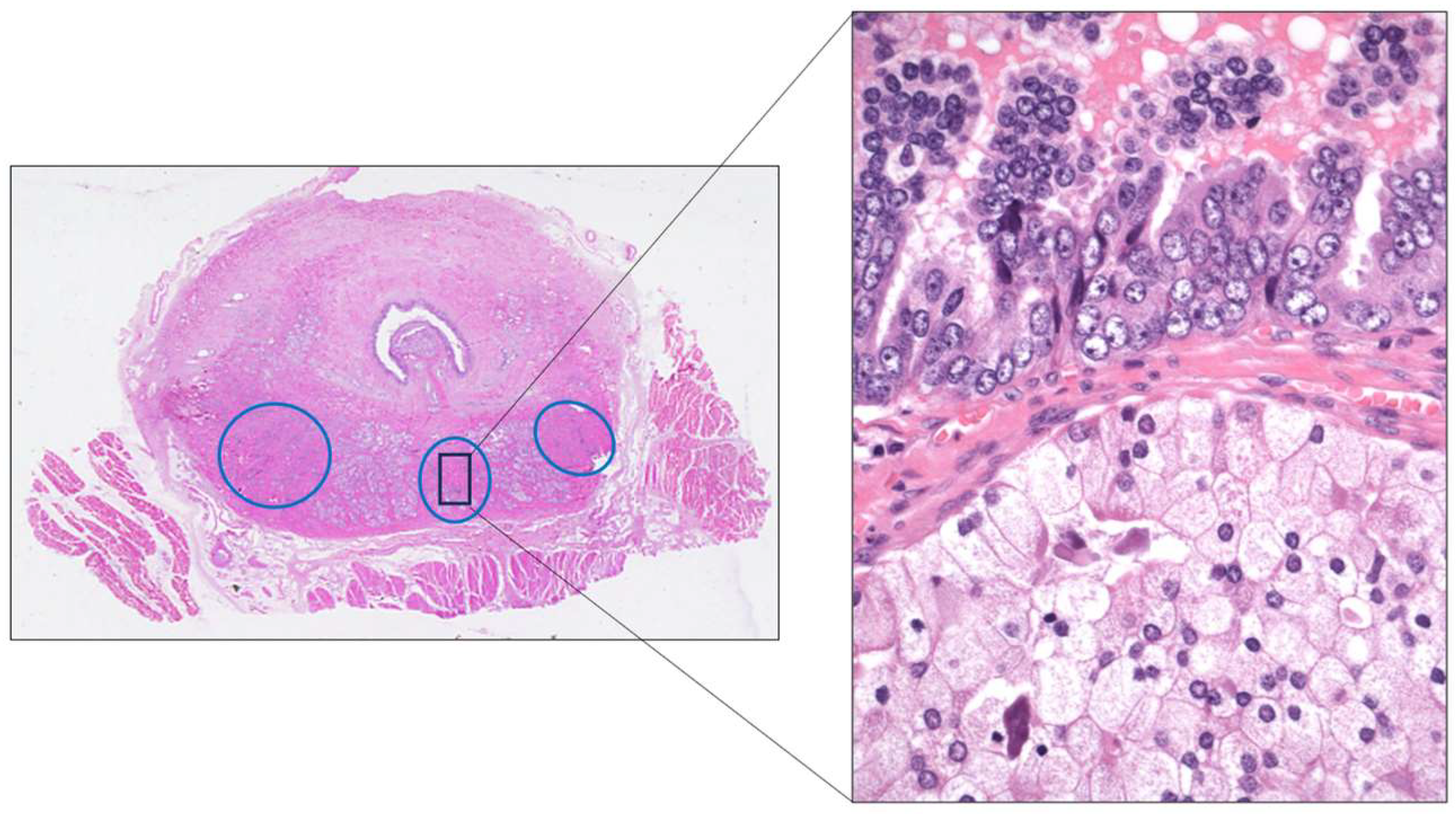
3. New Entities in Kidney Cancer
4. Urinary Cancer-Omics
4.1. Renal Cancer
4.2. Prostate Cancer
4.3. Urinary Tract Cancer
4.4. Penile Carcinoma
5. Update on Gleason Grading System
6. Targeted Therapies and Other Novel Treatments in Urologic Cancer
7. News on Non-Muscle-Invasive Urothelial Carcinoma
8. Artificial Intelligence in Urologic Cancer
9. Intratumor Heterogeneity Influences Therapeutic Failures in Urologic Neoplasms
10. Intratumor Microbiome and Its Influence in Urologic Tumor Aggressiveness
11. Ecological Principles and Mathematics Applied to Urological Cancer Study
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PCa | Prostate carcinoma |
| RCC | Renal cell carcinoma |
| CCRCC | Clear cell renal cell carcinoma |
| ITH | Intratumor heterogeneity |
| PRCC | Papillary renal cell carcinoma |
| HPV | Human papilloma virus |
| PeCa | Penile carcinoma |
| UC | Urothelial carcinoma |
| TC | Testicular carcinoma |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 26 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Netto, G.J.; Amin, M.B.; Berney, D.M.; Compérat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; et al. The 2022 World Health Organization classification of tumors of the urinary system and male genital organs-Part B: Prostate and urinary tract tumors. Eur. Urol. 2022, 82, 469–482. [Google Scholar] [CrossRef]
- Moch, H.; Amin, M.B.; Berney, D.M.; Compérat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; et al. The 2022 World Health Organization classification of tumours of the urinary system and male genital organs-Part A: Renal, penile, and testicular tumours. Eur. Urol. 2022, 82, 458–468. [Google Scholar] [CrossRef]
- Nikas, I.P.; Seide, S.; Proctor, T.; Kleinaki, Z.; Kleinaki, M.; Reynolds, J.P. The Paris System for reporting urinary cytology: A meta-analysis. J. Pers. Med. 2022, 12, 170. [Google Scholar] [CrossRef]
- Trpkov, K.; Hes, O.; Williamson, S.R.; Adeniran, A.J.; Agaimy, A.; Alaghehbandan, R.; Amin, M.B.; Argani, P.; Chen, Y.B.; Cheng, L.; et al. New developments in existing WHO entities and evolving molecular concepts: The Genitourinary Pathology Society (GUPS) update on renal neoplasia. Mod. Pathol. 2021, 34, 1392–1424. [Google Scholar] [CrossRef]
- Trpkov, K.; Williamson, S.R.; Gill, A.J.; Adeniran, A.J.; Agaimy, A.; Alaghehbandan, R.; Amin, M.B.; Argani, P.; Chen, Y.B.; Cheng, L.; et al. Novel, emerging and provisional renal entities: The Genitourinary Pathology Society (GUPS) update on renal neoplasia. Mod. Pathol. 2021, 34, 1167–1184. [Google Scholar] [CrossRef] [PubMed]
- Turajlic, S.; Xu, H.; Litchfield, K.; Rowan, A.; Horswell, S.; Chambers, T.; O’Brien, T.; López, J.I.; Watkins, T.B.; Nicol, D.; et al. Deterministic evolutionary trajectories influence primary tumor growth: TRACERx renal. Cell 2018, 173, 595–610. [Google Scholar] [CrossRef]
- Manini, C.; López-Fernández, E.; Larrinaga, G.; López, J.I. Clear cell renal cell carcinoma: A test bench for investigating tumor complexity. Cancers 2024, 16, 829. [Google Scholar] [CrossRef]
- López, J.I.; Hogan, M.F.; Sutton, B.; Church, S.E.; Angulo, J.C.; Nunes-Xavier, C.E. Distinct spatial landscapes in clear-cell renal cell carcinoma as revealed by whole transcriptome analysis. Immunooncol. Technol. 2023, 21, 100690. [Google Scholar] [CrossRef]
- De Vargas Roditi, L.; Jacobs, A.; Rueschoff, J.H.; Bankhead, P.; Chevrier, S.; Jackson, H.W.; Hermanns, T.; Fankhauser, C.D.; Poyet, C.; Chun, F.; et al. Single-cell proteomics defines the cellular heterogeneity of localized prostate cancer. Cell Rep. Med. 2022, 3, 100604. [Google Scholar] [CrossRef] [PubMed]
- Cyrta, J.; Prandi, D.; Arora, A.; Hovelson, D.H.; Sboner, A.; Rodriguez, A.; Fedrizzi, T.; Beltran, H.; Robinson, D.R.; Gopalan, A.; et al. Comparative genomics of primary prostate cancer and paired metastases: Insights from 12 molecular case studies. J. Pathol. 2022, 257, 274–284. [Google Scholar] [CrossRef]
- Yao, Z.; Xu, N.; Shang, G.; Wang, H.; Tao, H.; Wang, Y.; Qin, Z.; Tan, S.; Feng, J.; Zhu, J.; et al. Proteogenomics of different urothelial bladder cancer stages reveals distinct molecular features for papillary cancer and carcinoma in situ. Nat. Commun. 2023, 14, 5670. [Google Scholar] [CrossRef] [PubMed]
- Necchi, A.; Spiess, P.E.; Bandini, M.; Basile, G.; Grivas, P.; Bratslavsky, G.; Jacob, J.; Danzinger, N.; Lin, D.; Decker, B.; et al. Advanced squamous cell carcinomas of the pelvic and perineal region: A comprehensive genomic profiling study. Oncologist 2022, 27, 1016–1024. [Google Scholar] [CrossRef]
- Epstein, J.I.; Zelefsky, M.J.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W.; et al. A contemporary prostate cancer grading system: A validated alternative to the Gleason score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef]
- Montironi, R.; Cheng, L.; Cimadamore, A.; Mazzucchelli, R.; Scarpelli, M.; Santoni, M.; Massari, F.; Lopez-Beltran, A. Narrative review of prostate cancer grading systems: Will the Gleason scores be replaced by the Grade Groups. Transl. Androl. Urol. 2021, 10, 1530–1540. [Google Scholar] [CrossRef]
- Epstein, J.I.; Amin, M.B.; Fine, S.W.; Algaba, F.; Aron, M.; Baydar, D.E.; Lopez-Beltran, A.; Brimo, F.; Cheville, J.C.; Colecchia, M.; et al. The 2019 Genitourinary Pathology Society (GUPS) white paper on contemporary grading of prostate cancer. Arch. Pathol. Lab. Med. 2021, 145, 461–493. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I. Is Grage Group 1 (Gleason score 3+3=6) adenocarcinoma of the prostate really cancer? Curr. Opin. Urol. 2022, 32, 91–95. [Google Scholar] [CrossRef]
- Saramatunga, H.; Egevad, L.; Yaxley, J.; Perry-Keene, J.; Le Fevre, I.; Kench, J.; Matsika, A.; Bostwick, D.; Iczkowski, K.; Delahunt, B. Gleason score 3+3=6 prostatic adenocarcinoma is not benign and the current debate is unhelpful to clinicians and patients. Pathology 2024, 56, 33–38. [Google Scholar] [CrossRef]
- Lu, K.; Chiu, K.-Y.; Cheng, C.-L. Immunotherapy in genitourinary malignancy: Evolution in revolution or revolution in evolution. Cancer Treat. Res. 2022, 183, 201–223. [Google Scholar] [CrossRef]
- Schindler, N.R.; Braun, D.A. Antigenic targets in clear cell renal cell carcinoma. Kidney Cancer 2023, 7, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.A.; Bakouny, Z.; Hirsch, L.; Flippot, R.; Van Allen, E.M.; Wu, C.J.; Choueiri, T.K. Beyond conventional immune-checkpoint inhibition-novel immunotherapies for renal cell carcinoma. Nat. Rev. Clin. Oncol. 2021, 18, 199–214. [Google Scholar] [CrossRef]
- Motzer, R.J.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Shah, A.Y.; Suarez, C.; Hamzaj, A.; Porta, C.; Hocking, C.M.; et al. Nivolumab plus cabozantinib versus sunitinib in first-line treatment for advanced renal cell carcinoma (CheckMate 9ER): Long-term follow-up results from an open-label, randomized, phase 3 trial. Lancet Oncol. 2022, 23, 888–898. [Google Scholar] [CrossRef]
- Barragan-Carrillo, R.; Saad, E.; Saliby, R.M.; Sun, M.; Albiges, L.; Bex, A.; Heng, D.; Mejean, A.; Motzer, R.J.; Plimack, E.R.; et al. First and second-line treatments in metastatic renal cell carcinoma. Eur. Urol. 2025, 87, 143–154. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, M.; Raje, N. Anti-BCMA CAR T-cell therapy in multiple myeloma: Can we do better? Leukemia 2020, 34, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Zha, J.; Zhang, J.; Lu, J.; Zhang, G.; Hua, M.; Guo, W.; Yang, J.; Fan, G. A review of lactate-lactylation in malignancy: Its potential in immunotherapy. Front. Immunol. 2024, 15, 1384948. [Google Scholar] [CrossRef]
- Mjaess, G.; Karam, A.; Aoun, F.; Albisinni, S.; Roumeguère, T. Fecal microbiota transplantation for immunotherapy-resistant urological tumors: Is it time? An update of the recent literature. Cancer 2022, 128, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Quan, C.; He, Y.; Matsika, J.; Huang, J.; Liu, B.; Chen, J. Targeting gut and intratumoral microbiota: A novel strategy to improve therapy resistance in cancer with a focus on urologic tumors. Expert Opin. Biol. Ther. 2024, 24, 747–759. [Google Scholar] [CrossRef]
- Sylvester, R.J.; Rodríguez, O.; Hernández, V.; Turturica, D.; Bauerová, L.; Bruins, H.M.; Bründl, J.; van der Kwast, T.H.; Brisuda, A.; Rubio-Briones, J.; et al. European Association of Urology (EAU) Prognostic Factor Risk Groups for Non-muscle-invasive Bladder Cancer (NMIBC) Incorporating the WHO 2004/2016 and WHO 1973 Classification Systems for Grade: An Update from the EAU NMIBC Guidelines Panel. Eur. Urol. 2021, 79, 480–488. [Google Scholar] [CrossRef]
- Hannouneh, Z.A.; Hijazi, A.; Alsaleem, A.A.; Hami, S.; Kheyrbek, N.; Tanous, F.; Khaddour, K.; Abbas, A.; Alshehabi, Z. Novel immunotherapeutic options for BCG-unresponsive high-risk non-muscle-invasive bladder cancer. Cancer Med. 2023, 12, 21944–21968. [Google Scholar] [CrossRef]
- Oh, E.L.; Redfern, A.; Hayne, D. An evaluation of durvalumab across the spectrum of urothelial carcinoma. Expert Rev. Anticancer Ther. 2024, 24, 1101–1115. [Google Scholar] [CrossRef] [PubMed]
- Filon, M.; Schmidt, B. New treatment options for non-muscle-invasive bladder cancer. Am. Soc. Clin. Oncol. Educ. Book 2025, 45, e471942. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, H.W.; Ha, H.K.; Seo, H.K. Perioperative systemic therapy in muscle invasive bladder cancer: Current standard method, biomarkers and emerging strategies. Investig. Clin. Urol. 2023, 64, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Tan, A.; Shah, A.Y.; Iyer, G.; Morris, V.; Michaud, S.; Sridhar, S.S. Reevaluating the role of platinum-based chemotherapy in the evolving treatment landscape for patients with advanced urothelial carcinoma. Oncologist. 2024, 29, 1003–1013. [Google Scholar] [CrossRef]
- Lichtenegger, E.; Koll, F.; Haas, H.; Mantwill, K.; Janssen, K.-P.; Laschinger, M.; Gschwend, J.; Steiger, K.; Black, P.C.; Moskalev, I.; et al. The oncolytic adenovirus XVir-N-31 as a novel therapy in muscle-invasive bladder cancer. Hum. Gene Ther. 2019, 30, 44–56. [Google Scholar] [CrossRef]
- Mitsogiannis, I.; Tzelves, L.; Dellis, A.; Issa, H.; Papatsoris, A.; Moussa, M. Prostate cancer immunotherapy. Expert Opin. Biol. Ther. 2022, 22, 577–590. [Google Scholar] [CrossRef]
- Zhu, W.; Zeng, H.; Huang, J.; Wu, J.; Wang, Y.; Wang, Z.; Wang, H.; Luo, Y.; Lai, W. Integrated machine learning identifies epithelial cell marker genes for improving outcomes and immunotherapy in prostate cancer. J. Transl. Med. 2023, 21, 782. [Google Scholar] [CrossRef]
- Udager, A.M.; Liu, T.Y.; Skala, S.L.; Magers, M.J.; McDaniel, A.S.; Spratt, D.E.; Feng, F.Y.; Siddiqui, J.; Cao, X.; Fields, K.L.; et al. Frequent PD-L1 expression in primary and metastatic penile squamous cell carcinoma: Potential opportunities for immunotherapeutic approaches. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 1706–1712. [Google Scholar] [CrossRef]
- Gassian, N.; Frontczak, A.; Mouillet, G.; Vernerey, D.; Manseur, O.; Goujon, M.; Meurisse, A.; Berthod, D.; Robert, E.; Calcagno, F.; et al. Activity and tolerability of maintenance avelumab immunotherapy after first line polychemotherapy including platinum in patients with locally advanced or metastatic squamous cell penile carcinoma: PULSE. Bull. Cancer 2020, 107, eS16–eS21. [Google Scholar] [CrossRef]
- Garcia Del Muro, X.; Paez Lopez-Bravo, D.; Cuellar-Rivas, M.A.; Maroto, P.; Giannatempo, P.; Castellano, D.; Climent, M.A.; Valderrama, B.P.; Gomez de Liano, A.; Lopez-Montero, L.; et al. Retifanlimab in advanced penile squamous cell carcinoma: The phase 2 ORPHEUS study. Eur. Urol. Oncol. 2024, 8, 278–286. [Google Scholar] [CrossRef]
- Angulo, J.C.; Lopez, J.I.; Flores, N.; Toledo, J.D. The value of tumor spread, grading and growth pattern as morphological predictive parameters in bladder carcinoma. A critical revision of the 1987 TNM classification. J. Cancer Res. Clin. Oncol. 1993, 119, 578–593. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.I.; Angulo, J.C. Growth pattern in superficial urothelial bladder carcinomas. Histological review and clinical relevance. Int. Urol. Nephrol. 2009, 41, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.C.; Chang, Y.H.; Pan, C.C. Prognostic significance in substaging of T1 urinary bladder urothelial carcinoma on transurethral resection. Am. J. Surg. Pathol. 2012, 36, 454–461. [Google Scholar] [CrossRef]
- Angulo, J.C.; Lopez, J.I.; Grignon, D.J.; Sanchez-Chapado, M. Muscularis mucosa differentiates two populations with different prognosis in stage T1 bladder cancer. Urology 1995, 45, 47–53. [Google Scholar] [CrossRef]
- Kläger, J.; Koeller, M.C.; Oszwald, A.; Wasinger, G.; D’Andrea, D.; Compérat, E. A single-center retrospective comparison of pT1 substaging methods in bladder cancer. Virchows Arch. 2024. [Google Scholar] [CrossRef]
- Mostofi, F.K.; Sobin, L.H.; Torloni, H. Histological Typing of Urinary Bladder Tumors: International Classification of Tumors; World Health Organization: Geneva, Switzerland, 1973. [Google Scholar]
- May, M.; Brookman-Amissah, S.; Roigas, J.; Hartmann, A.; Störkel, S.; Kristiansen, G.; Gilfrich, C.; Borchardt, R.; Hoschke, B.; Kaufmann, O.; et al. Prognostic accuracy of individual uropathologists in noninvasive urinary bladder carcinoma: A multicentre study comparing the 1973 and 2004 WHO World Health Organisation classifications. Eur. Urol. 2010, 57, 850–858. [Google Scholar] [CrossRef]
- Otto, W.; Denzinger, S.; Fritsche, H.M.; Burger, M.; Wieland, W.F.; Hofstädter, F.; Hartmann, A.; Bertz, S. The WHO classification of 1973 is more suitable than the WHO classification of 2004 for predicting survival in pT1 urothelial bladder cancer. BJU Int. 2011, 107, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Anurag, M.; Strandgaard, T.; Kim, S.H.; Dou, Y.; Comperat, E.; Al-Ahmadie, H.; Inman, B.A.; Taber, A.; Nordentoft, I.; Jensen, J.B.; et al. Multiomics profiling of urothelial carcinoma in situ reveals CIS-specific gene signature and immune characteristics. iScience 2024, 27, 109179. [Google Scholar] [CrossRef]
- McNall, S.; Hooper, K.; Sullivan, T.; Rieger-Christ, K.; Clements, M. Treatment modalities for non-muscle invasive bladder cancer: An updated review. Cancers 2024, 16, 1843. [Google Scholar] [CrossRef]
- Chatrian, A.; Colling, R.T.; Browning, L.; Alham, N.K.; Sirinukunwattana, R.; Malacrino, S.; Haghighat, M.; Aberdeen, A.; Monks, A.; Moxley-Wyles, B.; et al. Artificial intelligence for advance requesting of immunohistochemistry in diagnostically uncertain prostate biopsies. Mod. Pathol. 2021, 34, 1780–1794. [Google Scholar] [CrossRef]
- Perincheri, S.; Levi, A.W.; Celli, R.; Gershkovich, P.; Rimm, D.; Morrow, J.S.; Rothrock, B.; Raciti, P.; Klimstra, D.; Sinargd, J.; et al. An independent assessment of an artificial intelligence system for prostate cancer detection shows strong diagnostic accuracy. Mod. Pathol. 2021, 34, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Bulten, W.; Kartasalo, K.; Cameron Chen, P.-H.; Ström, P.; Pinckaers, H.; Nagpal, K.; Cai, Y.; Steiner, D.F.; van Boven, H.; Vink, R.; et al. Artificial intelligence for diagnosis and Gleason grading of prostate cancer: The PANDA challenge. Nat. Med. 2022, 28, 154–163. [Google Scholar] [CrossRef]
- Wang, Y.; Xuan, Y.; Su, B.; Gao, Y.; Fan, Y.; Huang, Q.; Zhang, P.; Gu, L.; Niu, S.; Shen, D.; et al. Predicting recurrence and survival in patients with non-metastatic renal-cell carcinoma after nephrectomy: A prospective population-based study with multicenter validation. Int. J. Surg. 2024, 110, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Orton, M.R.; Hann, E.; Doran, S.J.; Shepherd, S.T.C.; Dafydd, D.A.; Spencer, C.E.; Lopez, J.I.; Albarrán-Artahona, V.; Comito, F.; Warren, H.; et al. Interpretability of radiomics models is improved when using feature group selection strategies for predicting molecular and clinical targets in clear-cell renal cell carcinoma: Insights from the TRACERx Renal study. Cancer Imaging 2023, 23, 76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, J.; Li, Z.; Yang, M.; Ye, Z. Preoperative prediction of renal fibrous capsule invasion in clear cell renal cell carcinoma using CT-based radiomics model. Br. J. Radiol. 2024, 97, 1557–1567. [Google Scholar] [CrossRef]
- Khene, Z.E.; Bigot, P.; Doumerc, N.; Ouzaid, I.; Boissier, R.; Nouhaud, F.X.; Albiges, L.; Bernhard, J.C.; Ingels, A.; Borchiellini, D.; et al. Application of machine learning models to predict recurrence after surgical resection of nonmetastatic renal cell carcinoma. Eur. Urol. Oncol. 2023, 6, 323–330. [Google Scholar] [CrossRef]
- Golkaram, M.; Kuo, F.; Gupta, S.; Carlo, M.I.; Salmans, M.L.; Vijayaraghavan, R.; Tang, C.; Makarov, V.; Rappold, P.; Blum, K.A.; et al. Spatiotemporal evolution of clear cell renal cell carcinoma microenvironment links intra-tumoral heterogeneity to immune escape. Genome Med. 2022, 14, 143. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, T.; Zhu, L.; Yao, Y.; Mei, J.; Sun, L.; Zhang, G. Using machine learning to develop preoperative model for lymph node metastasis in patients with bladder urothelial carcinoma. BMC Cancer 2024, 24, 725. [Google Scholar] [CrossRef]
- Peng, J.; Tang, Z.; Li, T.; Pan, X.; Feng, L.; Long, L. Contrast-enhanced computed tomography-based radiomics nomogram for predicting HER2 status in urothelial bladder carcinoma. Front. Oncol. 2024, 14, 1427122. [Google Scholar] [CrossRef]
- Fang, F.; Wu, L.; Luo, X.; Bu, H.; Huang, Y.; Xian, W.Y.; Lu, Z.; Li, T.; Yang, G.; Zhao, Y.; et al. Differentiation of testicular seminomas from nonseminomas based on multiphase CT radiomics combined with machine learning: A multicenter study. Eur. J. Radiol. 2024, 175, 111416. [Google Scholar] [CrossRef]
- Feng, Y.; Feng, Z.; Wang, L.; Lv, W.; Liu, Z.; Min, X.; Li, J.; Zhang, J. Comparison and analysis of multiple machine learning models for discriminating benign and malignant testicular lesions based on magnetic resonance imaging radiomics. Front. Med. 2023, 10, 1279622. [Google Scholar] [CrossRef]
- Salvi, M.; Manini, C.; López, J.I.; Fenoglio, D.; Molinari, F. Deep learning approach for accurate prostate cancer identification and stratification using combined immunostaining of cytokeratin, p63, and racemase. Comput. Med. Imaging Graph. 2023, 109, 102288. [Google Scholar] [CrossRef] [PubMed]
- Gerstung, M.; Jolly, C.; Leshchiner, I.; Dentro, S.C.; Gonzalez, S.; Rosebrock, D.; Mitchell, T.J.; Rubanova, Y.; Anur, P.; Yu, K.; et al. The evolutionary history of 2,658 cancers. Nature 2020, 578, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.; Black, J.R.M.; Reading, J.L.; Litchfield, K.; Turajlic, S.; McGranahan, N.; Jamal-Hanjani, M.; Swanton, C. Tracking cancer evolution through the disease course. Cancer Discov. 2021, 11, 916–932. [Google Scholar] [CrossRef]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Math, M.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef]
- Turajlic, S.; Xu, H.; Litchfield, K.; Rowan, A.; Chambers, T.; Lopez, J.I.; Nicol, D.; O’Brien, T.; Larkin, J.; Horswell, S.; et al. Tracking cancer evolution reveals constrained routes to metastases: TRACERx Renal. Cell 2018, 173, 581–594. [Google Scholar] [CrossRef]
- Schallenberg, S.; Dragomir, M.P.; Anders, P.; Ebner, B.; Volz, Y.; Eismann, L.; Rodler, S.; Casuscelli, J.; Buchner, A.; Klauschen, F.; et al. Intratumoral heterogeneity of molecular subtypes in muscle-invasive bladder cancer. An extensive multiregional immunohistochemical analysis. Eur. Urol. Focus 2023, 9, 788–798. [Google Scholar] [CrossRef]
- Garczyk, S.; Bischoff, F.; Schneider, U.; Golz, R.; von Rundstedt, F.C.; Knüchel, R.; Degener, S. Intratumoral heterogeneity of surrogate molecular subtypes in urothelial carcinoma in situ of the urinary bladder: Implications for prognostic stratification of high-risk non-muscle-invasive bladder cancer. Virchows Arch. 2021, 479, 325–335. [Google Scholar] [CrossRef]
- Singhal, U.; Nallandhighal, S.; Tosoian, J.J.; Hu, K.; Pham, T.M.; Stangl-Kremser, J.; Liu, C.J.; Karim, R.; Plouffe, K.R.; Morgan, T.M.; et al. Integrative multi-region molecular profiling of primary prostate cancer in men with synchronous lymph node metastasis. Nat. Commun. 2024, 15, 4341. [Google Scholar] [CrossRef]
- Singh, Y.; Barua, S.K.; Singh, V.K.; Trivedi, S.; Rajeev, T.P.; Koti, S.R.; Garg, N. Intratumoral heterogeneity, chemoresistance and lymph node landing zone prognosis in testicular tumors based on histopathological characteristics. Ann. Surg. Oncol. 2024, 31, 3544–3553. [Google Scholar] [CrossRef]
- Rhinehart, D.P.; Lai, J.; Sanin, D.E.; Vakkala, V.; Mendes, A.; Bailey, C.; Antonarakis, E.S.; Paller, C.J.; Wu, X.; Lotan, T.L.; et al. Intratumoral heterogeneity drives acquired therapy resistance in a patient with metastatic prostate cancer. NPJ Precis. Oncol. 2024, 8, 275. [Google Scholar] [CrossRef]
- van Wilpe, S.; Gorris, M.A.J.; van der Woude, L.L.; Sultan, S.; Koornstra, R.T.H.; van der Heijden, A.G.; Gerritsen, W.R.; Simons, M.; de Vries, I.J.M.; Mehra, N. Spatial and temporal heterogeneity of tumor-infiltration lymphocytes in advanced urothelial cancer. Front. Immunol. 2022, 12, 802877. [Google Scholar] [CrossRef]
- Grausenburger, R.; Herek, P.; Shariat, S.F.; Englinger, B. Recent contributions of single-cell and spatial profiling to the understanding of bladder cancer. Curr. Opin. Urol. 2024, 34, 236–243. [Google Scholar] [CrossRef]
- Song, X.; Zhu, Y.; Geng, W.; Jiao, J.; Liu, H.; Chen, R.; He, Q.; Wang, L.; Sun, X.; Qin, W.; et al. Spatial and single-cell transcriptomics reveal cellular heterogeneity and a novel cancer-promoting Treg cell subset in human clear-cell renal cell carcinoma. J. Immunother. Cancer 2025, 13, e010183. [Google Scholar] [CrossRef] [PubMed]
- Davidson, G.; Helleux, A.; Vano, Y.A.; Lindner, V.; Fattori, A.; Cerciat, M.; Elaidi, R.T.; Verkarre, V.; Sun, C.M.; Chevreau, C.; et al. Mesenchymal-like Tumor Cells and Myofibroblastic Cancer-Associated Fibroblasts Are Associated with Progression and Immunotherapy Response of Clear Cell Renal Cell Carcinoma. Cancer Res. 2023, 83, 2952–2969. [Google Scholar] [CrossRef] [PubMed]
- Calvo, I.; Fresnedo, O.; Mosteiro, L.; López, J.I.; Larrinaga, G.; Fernández, J.A. Lipid imaging mass spectrometry: Towards a new molecular histology. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2025, 1870, 159568. [Google Scholar] [CrossRef]
- Mi, H.; Sivagnanam, S.; Ho, W.J.; Zhang, S.; Bergman, D.; Deshpande, A.; Baras, A.S.; Jaffee, E.M.; Coussens, L.M.; Fertig, E.J.; et al. Computational methods and biomarker discovery strategies for spatial proteomics: A review in immuno-oncology. Brief Bioinform. 2024, 25, bbae421. [Google Scholar] [CrossRef]
- Shafighi, S.; Geras, A.; Jurzysta, B.; Sahaf Naeini, A.; Filipiuk, I.; Ra Czkowska, A.; Toosi, H.; Koperski, Ł.; Thrane, K.; Engblom, C.; et al. Integrative spatial and genomic analysis of tumor heterogeneity with Tumoroscope. Nat. Commun. 2024, 15, 9343. [Google Scholar] [CrossRef]
- Bi, K.; He, M.X.; Bakouny, Z.; Kanodia, A.; Napolitano, S.; Wu, J.; Grimaldi, G.; Braun, D.A.; Cuoco, M.S.; Mayorga, A.; et al. Tumor and immune reprogramming during immunotherapy in advanced renal cell carcinoma. Cancer Cell 2021, 39, 649–661.e5. [Google Scholar] [CrossRef]
- Galassi, C.; Chan, T.A.; Vitale, I.; Galluzzi, L. The hallmarks of cancer immune evasion. Cancer Cell 2024, 42, 1825–1863. [Google Scholar] [CrossRef]
- Xu, Y.; Li, W.; Lin, S.; Liu, B.; Wu, P.; Li, L. Fibroblast diversity and plasticity in the tumor microenvironment: Roles in immunity and relevant therapies. Cell Commun. Signal. 2023, 21, 234. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Tanaka, N.; Takamatsu, K.; Hakozaki, K.; Fukumoto, K.; Masuda, T.; Mikami, S.; Shinojima, T.; Kakimi, K.; Tsunoda, T.; et al. Multiplexed single-cell pathology reveals the association of CD8 T-cell heterogeneity with prognostic outcomes in renal cell carcinoma. Cancer Immunol. Immunother. 2021, 70, 3001–3013. [Google Scholar] [CrossRef] [PubMed]
- Larrinaga, G.; Redrado, M.; Loizaga-Iriarte, A.; Pérez-Fernández, A.; Santos-Martín, A.; Angulo, J.C.; Fernández, J.A.; Calvo, A.; López, J.I. Spatial expression of fibroblast activation protein-α in clear cell renal cell carcinomas revealed by multiplex immunoprofiling analysis of the tumor microenvironment. Cancer Immunol. Immunother. 2025, 74, 53. [Google Scholar] [CrossRef]
- Soultati, A.; Stares, M.; Swanton, C.; Larkin, J.; Turajlic, S. How should clinicians address intratumour heterogeneity in clear cell renal cell carcinoma? Curr. Opin. Urol. 2015, 25, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Cormen, T.H.; Leiserson, C.E.; Rivest, R.L.; Stein, C. Introduction to Algorithms, 3rd ed.; MIT Press: Cambridge, MA, USA, 2009; ISBN 9780262533058. [Google Scholar]
- López, J.I.; Cortés, J.M. Multisite tumor sampling: A new tumor selection method to enhance intratumor heterogeneity detection. Hum. Pathol. 2017, 64, 1–6. [Google Scholar] [CrossRef]
- Cortés, J.M.; de Petris, G.; López, J.I. Detection of intratumor heterogeneity in modern pathology: A multisite tumor sampling perspective. Front. Med. 2017, 4, 25. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–881. [Google Scholar] [CrossRef]
- Jain, T.; Sharma, P.; Are, A.C.; Vickers, S.M.; Dudeja, V. New insights into the cancer–microbiome–immune axis: Decrypting a decade of discoveries. Front. Immunol. 2021, 12, 622064. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Wu, X.; Wang, Z.; Cao, G.; Liu, K.; Yan, T. Uncovering the microbiota in renal cell carcinoma tissue using 16S rRNA gene sequencing. J. Cancer Res. Clin. Oncol. 2021, 147, 481–491. [Google Scholar] [CrossRef]
- Liss, M.A.; Chen, Y.; Rodriguez, R.; Pruthi, D.; Johnson-Pais, T.; Wang, H.; Mansour, A.; White, J.R.; Kaushik, D. Microbiome within primary tumor tissue from renal cell carcinoma may be associated with PD-L1 expression of the venous tumor thrombus. Adv. Urol. 2020, 2020, 9068068. [Google Scholar] [CrossRef]
- Russo, A.E.; Memon, A.; Ahmed, S. Bladder Cancer and the Urinary Microbiome-New Insights and Future Directions: A Review. Clin. Genitourin. Cancer 2024, 22, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhang, W.; Shen, L.; Liu, J.; Yang, F.; Maskey, N.; Wang, H.; Zhang, J.; Yan, Y.; Yao, X. Can smoking cause differences in urine microbiome in male patients with bladder cancer? A retrospective study. Front. Oncol. 2021, 11, 677605. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.; Fujita, K.; Motooka, D.; Hatano, K.; Fukae, S.; Kawamura, N.; Tomiyama, E.; Hayashi, Y.; Banno, E.; Takao, T.; et al. The gut microbiota associated with high-Gleason prostate cancer. Cancer Sci. 2021, 112, 3125–3135. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Z.; Turner, D.; Lilly, M.; Ou, T.; Jiang, W. Differential circulating fungal microbiome in prostate cancer patients compared to healthy control individuals. J. Immunol. Res. 2022, 2022, 2574964. [Google Scholar] [CrossRef]
- Sarkar, P.; Malik, S.; Banerjee, A.; Datta, C.; Pal, D.K.; Ghosh, A.; Saha, A. Differential microbial signature associated with benign prostatic hyperplasia and prostate cancer. Front. Cell. Infect. Microbiol. 2022, 12, 894777. [Google Scholar] [CrossRef] [PubMed]
- Mørup, N.; Main, A.M.; Jørgensen, N.; Daugaard, G.; Juul, A.; Almstrup, K. The seminal plasma microbiome of men with testicular ger cell tumours described by small RNA sequencing. Andrology 2023, 11, 756–769. [Google Scholar] [CrossRef]
- Reynolds, B.A.; Oli, M.W.; Oli, M.K. Eco-oncology: Applying ecological principles to understand and manage cancer. Ecol. Evol. 2020, 10, 8538–8553. [Google Scholar] [CrossRef]
- Badalamenti, G.; Fanale, D.; Incorvaia, L.; Barraco, N.; Listì, A.; Maragliano, R.; Vincenzi, B.; Calò, V.; Iovanna, J.L.; Bazan, V.; et al. Role of tumor-infiltrating lymphocytes in patients with solid tumors: Can a drop dig a stone? Cell Immunol. 2019, 343, 103753. [Google Scholar] [CrossRef]
- Hochstadt, J.; Martínez Pacheco, S.; Casanova-Acebes, M. Embracing diversity: Macrophage complexity in cancer. Trends Cancer 2025. [Google Scholar] [CrossRef]
- Errarte, P.; Larrinaga, G.; López, J.I. The role of cancer-associated fibroblasts in renal cell carcinoma. An example of tumor modulation through tumor/non-tumor cell interactions. J. Adv. Res. 2019, 21, 103–108. [Google Scholar] [CrossRef]
- Davis, A.; Gao, R.; Navin, N. Tumor evolution: Linear, branching, neutral, or punctuated? Biochim. Biophys. Acta Rev. Cancer 2017, 1867, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Vendramin, R.; Litchfield, K.; Swanton, C. Cancer evolution: Darwin and beyond. EMBO J. 2021, 40, e108389. [Google Scholar] [CrossRef] [PubMed]
- Manini, C.; López-Fernández, E.; Lawrie, C.H.; Laruelle, A.; Angulo, J.C.; López, J.I. Clear cell renal cell carcinomas with aggressive behavior display low intratumor heterogeneity at the histological level. Curr. Urol. Rep. 2022, 23, 93–97. [Google Scholar] [CrossRef]
- Zhao, Y.; Fu, X.; López, J.I.; Rowan, A.; Au, L.; Fendler, A.; Xu, H.; Horswell, S.; Shepherd, S.T.C.; Spain, L.; et al. Selection of metastasis competent subclones in the tumour interior. Nat. Ecol. Evol. 2021, 5, 1033–1045. [Google Scholar] [CrossRef]
- Dujon, A.M.; Aktipis, A.; Alix-Panabières, C.; Amend, S.R.; Boddy, A.M.; Brown, J.S.; Capp, J.P.; DeGregori, J.; Ewald, P.; Gatenby, R.; et al. Identifying key questions in the ecology and evolution of cancer. Evol. Appl. 2021, 14, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Manini, C.; López, J.I. Ecology and games in cancer: New insights into the disease. Pathologica 2022, 114, 347–351. [Google Scholar] [CrossRef]
- Wölfl, B.; te Rietmole, H.; Salvioli, M.; Kaznatcheev, A.; Thuisjman, F.; Brown, J.S.; Burgering, B.; Stanková, K. The contribution of evolutionary game theory to understanding and treating cancer. Dyn. Games Appl. 2022, 12, 313–342. [Google Scholar] [CrossRef]
- Laruelle, A.; Rocha, A.; Manini, C.; López, J.I.; Inarra, E. Effects of heterogeneity on cancer: A game theory perspective. Bull. Math. Biol. 2023, 85, 72. [Google Scholar] [CrossRef]
- Manini, C.; Laruelle, A.; Rocha, A.; López, J.I. Convergent insights into intratumor heterogeneity. Trends Cancer 2024, 10, 12–14. [Google Scholar] [CrossRef]
- Viossat, Y.; Noble, R. A theoretical analysis of tumour containment. Nat. Ecol. Evol. 2021, 5, 826–835. [Google Scholar] [CrossRef]
- Capp, J.P.; Nedelcu, A.M.; Dujon, A.M.; Roche, B.; Catania, F.; Ujvari, B.; Alix-Panabières, C.; Thomas, F. Does cancer biology rely on Parrondo’s principles? Cancers 2021, 13, 2197. [Google Scholar] [CrossRef] [PubMed]




| 2022 WHO update on the classification of urinary and male genital tumors |
| New entities in kidney cancer |
| Urinary cancer-omics |
| Gleason grading system (update) |
| Targeted therapies and other novel treatments in urologic cancer |
| News on non-muscle invasive urothelial carcinoma |
| Artificial intelligence in urologic cancer |
| Intratumor heterogeneity’s influence on therapeutic failures in urologic neoplasms |
| Intratumor microbiome and its influence on urologic tumor aggressiveness |
| Ecological principles and mathematics applied to urological cancer study |
| Luminal (positive KRT20, GATA3, and FOXA1 markers) Luminal-papillary Papillary Histology (FGFR3 mutation/fusion/amplification) Luminal-infiltrated positive EMT markers (TWIST1, ZEB1, etc.) Myofibroblast markers Wild-type p53 |
| Basal-squamous (positive KRT5, KRT6, and KRT14 and negative GATA3 and FOXA1) Carcinoma in situ Squamous differentiation Basal keratin markers (CK 5.6, etc.) Immune inflammatory infiltrates (PD-1, PD-L1, etc) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manini, C.; Larrinaga, G.; Angulo, J.C.; López, J.I. Hot Spots in Urogenital Basic Cancer Research and Clinics. Cancers 2025, 17, 1173. https://doi.org/10.3390/cancers17071173
Manini C, Larrinaga G, Angulo JC, López JI. Hot Spots in Urogenital Basic Cancer Research and Clinics. Cancers. 2025; 17(7):1173. https://doi.org/10.3390/cancers17071173
Chicago/Turabian StyleManini, Claudia, Gorka Larrinaga, Javier C. Angulo, and José I. López. 2025. "Hot Spots in Urogenital Basic Cancer Research and Clinics" Cancers 17, no. 7: 1173. https://doi.org/10.3390/cancers17071173
APA StyleManini, C., Larrinaga, G., Angulo, J. C., & López, J. I. (2025). Hot Spots in Urogenital Basic Cancer Research and Clinics. Cancers, 17(7), 1173. https://doi.org/10.3390/cancers17071173









