Can the Dose Constraints Be Trusted? Actual Dose Exposure of Bladder and Rectum During Prostate Cancer Radiotherapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Treatment
2.2. Dosimetric Evaluation Using CBCT
- CT and CBCT images were registered in the TPS and matched automatically.
- ROIs were mapped from CT to CBCT and mean Hounsfield units were measured in both images. ROIs included both femoral heads, bladder, muscle, fatty tissue and air.
- The physical density calibration curve was generated based on CT density values.
2.3. Correlation with Follow-Up Data
2.4. Statistical Analysis
3. Results
3.1. Dosimetric Evaluation
3.2. Dose-Volume Relationship
3.3. Correlation Between Toxicity and Dose
4. Discussion
4.1. How Reliable Is a Summation Plan?
4.2. Impact of OAR Volumes on Dose Distribution
4.3. Toxicities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IGRT | Image-guided radiotherapy |
| PCa | Prostate cancer |
| OAR | Organs at risk |
| CBCT | Cone-beam computed tomography |
| RT | Radiotherapy |
| EBRT | External beam radiotherapy |
| IMRT | Intensity-modulated radiotherapy |
| pCT | Planning computed tomography |
| SOP | Standard operating procedure |
| THP | Total hip replacement |
| PTV | Planning target volume |
| CTV | Clinical target volume |
| MRI | Magnetic resonance imaging |
| TPS | Treatment planning system |
| ROI | Region of interest |
| Dmax | Maximum dose |
| Dmean | Mean dose |
| DVH | Dose-volume histogram |
| GU | Genitourinary |
| GI | Gastrointestinal |
| POTD | Plan of the day |
Appendix A
| Characteristics | n (%) or Median (Range) |
|---|---|
| Number of patients | 20 |
| Median age at treatment (years) | 74 (64–86) |
| ≤65 years | 1 (5) |
| ≥65 years | 19 (95) |
| T stage | |
| T1 | 17 (85) |
| T2 | 2 (10) |
| T3 | 1 (5) |
| Gleason Score | |
| ≤ 6 | 3 (15) |
| 7 | 11 (55) |
| 8–10 | 6 (30) |
| Initial PSA (ng/mL) | |
| <10 | 10 (50) |
| 10–20 | 10 (50) |
| >20 | 0 |
| D’Amico Risk Classification | |
| Low Risk | 2 (10) |
| Intermediate Risk | 12 (60) |
| High Risk | 6 (30) |
| ECOG performance status | |
| 0 | 14 (70) |
| 1 | 5 (25) |
| 2 | 1 (5) |
| Hormone therapy | |
| ADT | 10 (50) |
| None | 10 (50) |
| Total CBCT scans | 821 |
| Correct automatic contouring | 778 (94.76) |
| Manually adjusted | 43 (5.24) |
| Parameter | Bladder | Rectum | p | |
|---|---|---|---|---|
| Absolute volumes [cm3] | Average | 186.76 | 64.82 | <0.001 |
| Standard deviation | 64.26 | 19.31 | <0.001 | |
| Range (Min–Max) | 340.31 (47.51–387.82) | 63.75 (35.44–99.19) | <0.001 | |
| Deviation from planned volumes | Average | 0.97 | 0.79 | 0.179 |
| Standard deviation | 0.37 | 0.24 | 0.079 | |
| Range (Min–Max) | 1.56 (0.19–1.75) | 1.05 (0.27–1.32) | 0.117 |
| Time | Grade | n (%) | |
|---|---|---|---|
| Genitourinary toxicity | During RT | No toxicity | 3 (15) |
| Grade 1 | 16 (80) | ||
| Grade 2 | 1 (5) | ||
| Acute toxicities | No toxicity | 4 (20) | |
| Grade 1 | 12 (60) | ||
| Grade 2 | 2 (10) | ||
| No follow-up | 2 (10) | ||
| Late toxicities | No toxicity | 7 (35) | |
| Grade 1 | 7 (35) | ||
| Grade 2 | 2 (10) | ||
| No follow-up | 4 (20) | ||
| Gastrointestinal toxicity | During RT | No toxicity | 7 (35) |
| Grade 1 | 12 (60) | ||
| Grade 2 | 1 (5) | ||
| Acute toxicities | No toxicity | 10 (10) | |
| Grade 1 | 8 (40) | ||
| No follow-up | 2 (10) | ||
| Late toxicities | No toxicity | 8 (40) | |
| Grade 1 | 5 (5) | ||
| Grade 2 | 2 (10) | ||
| Grade 3 | 1 (5) | ||
| No follow-up | 4 (20) |
| Study | Total Dose (Gy) | Fractions (n) | Acute GU Toxicity (%) | Acute GI Toxicity (%) | ||
|---|---|---|---|---|---|---|
| Grade 2 | Grade 3 | Grade 2 | Grade 3 | |||
| Guckenberger et al. [20] (n = 75) | 73.91/76.23 | 32/33 | 36 | 5 | 12 | 0 |
| Gill et al. [21] (n = 265) | 78 | 39 | 54 | 9 | 9 | 0 |
| Becker-Schiebe et al. [22] (n = 102) | 77.4 | 43 | 34 | 5 | 19 | 5 |
| Lips et al. [23] (n = 331) | 76 | 35 | 47 | 3 | 30 | 0 |
| Average | 42.8 | 5.5 | 17.5 | 1.3 | ||
| Present study (n = 20) | 79.2/80 | 44/40 | 10 | 0 | 0 | 0 |
References
- James, N.D.; Tannock, I.; N’Dow, J.; Feng, F.; Gillessen, S.; Ali, S.A.; Trujillo, B.; Al-Lazikani, B.; Attard, G.; Bray, F.; et al. The Lancet Commission on prostate cancer: Planning for the surge in cases. Lancet 2024, 403, 1683–1722. [Google Scholar] [CrossRef]
- Laughlin, B.S.; Silva, A.C.; Vora, S.A.; Keole, S.R.; Wong, W.W.; Schild, M.H.; Schild, S.E. Long-term outcomes of prostate intensity-modulated radiation therapy incorporating a simultaneous intra-prostatic MRI-directed boost. Front. Oncol. 2022, 12, 921465. [Google Scholar] [CrossRef]
- Byrne, T.E. A review of prostate motion with considerations for the treatment of prostate cancer. Med. Dosim. 2005, 30, 155–161. [Google Scholar] [CrossRef]
- Boda-Heggemann, J.; Köhler, F.; Wertz, H.; Welzel, G.; Riesenacker, N.; Schäfer, J.; Lohr, F.; Wenz, F. Fiducial-based quantification of prostate tilt using cone beam computer tomography (CBCT). Radiother. Oncol. 2007, 85, 247–250. [Google Scholar] [CrossRef]
- Ghilezan, M.J.; Jaffray, D.A.; Siewerdsen, J.H.; van Herk, M.; Shetty, A.; Sharpe, M.B.; Zafar Jafri, S.; Vicini, F.A.; Matter, R.C.; Brabbins, D.S.; et al. Prostate gland motion assessed with cine-magnetic resonance imaging (cine-MRI). Int. J. Radiat. Oncol. 2005, 62, 406–417. [Google Scholar] [CrossRef]
- Pearson, D.; Gill, S.K.; Campbell, N.; Reddy, K. Dosimetric and volumetric changes in the rectum and bladder in patients receiving CBCT-guided prostate IMRT: Analysis based on daily CBCT dose calculation. J. Appl. Clin. Med. Phys. 2016, 17, 107–117. [Google Scholar] [CrossRef]
- McParland, N.; Pearson, M.; Wong, J.; Sigur, I.; Stenger, C.; Tyldesley, S. Quantifying daily variation in volume and dose to the prostate, rectum and bladder using cone-beam computerised tomography. J. Radiother. Pract. 2014, 13, 79–86. [Google Scholar] [CrossRef]
- Richter, A.; Hu, Q.; Steglich, D.; Baier, K.; Wilbert, J.; Guckenberger, M.; Flentje, M. Investigation of the usability of conebeam CT data sets for dose calculation. Radiat. Oncol. 2008, 3, 42. [Google Scholar] [CrossRef]
- The International Commission on Radiation Units and Measurements. ICRU report Vol. 83: Prescribing, Recording, and Reporting Photon-Beam Intensity-Modulated Radiation Therapy (IMRT). J. ICRU 2010, 10, 1–106. [Google Scholar] [CrossRef]
- Michalski, J.M.; Gay, H.; Jackson, A.; Tucker, S.L.; Deasy, J.O. Radiation dose-volume effects in radiation-induced rectal injury. Int. J. Radiat. Oncol. 2010, 76, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, A.N.; Yorke, E.D.; Marks, L.B.; Eifel, P.J.; Shipley, W.U. Radiation dose-volume effects of the urinary bladder. Int. J. Radiat. Oncol. 2010, 76, 116–122. [Google Scholar] [CrossRef]
- National Institutes of Health National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0. 2017. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 12 November 2024).
- van Herk, M.; Remeijer, P.; Rasch, C.; Lebesque, J.V. The probability of correct target dosage: Dose-population histograms for deriving treatment margins in radiotherapy. Int. J. Radiat. Oncol. 2000, 47, 1121–1135. [Google Scholar] [CrossRef]
- Dang, A.; Kupelian, P.A.; Cao, M.; Agazaryan, N.; Kishan, A.U. Image-guided radiotherapy for prostate cancer. Transl. Androl. Urol. 2018, 7, 308–320. [Google Scholar] [CrossRef]
- Roch, M.; Zapatero, A.; Castro, P.; Hernández, D.; Chevalier, M.; García-Vicente, F. Dosimetric impact of rectum and bladder anatomy and intrafractional prostate motion on hypofractionated prostate radiation therapy. Clin. Transl. Oncol. 2021, 23, 2293–2301. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, Z.; Wang, J.; Hu, W. Dosimetric impact of different bladder and rectum filling during prostate cancer radiotherapy. Radiat. Oncol. 2016, 11, 103. [Google Scholar] [CrossRef]
- Fuchs, F.; Habl, G.; Devečka, M.; Kampfer, S.; Combs, S.E.; Kessel, K.A. Interfraction variation and dosimetric changes during image-guided radiation therapy in prostate cancer patients. Radiat. Oncol. J. 2019, 37, 127–133. [Google Scholar] [CrossRef]
- Ghadjar, P.; Fiorino, C.; Munck, A.; Rosenschöld, P.; Pinkawa, M.; Zilli, T.; van der Heide, U.A. ESTRO ACROP consensus guideline on the use of image guided radiation therapy for localized prostate cancer. Radiother. Oncol. 2019, 141, 5–13. [Google Scholar] [CrossRef]
- Polizzi, M.; Weiss, E.; Jan, N.; Ricco, A.; Kim, S.; Urdaneta, A.; Rosu-Bubulac, M. Rectal deformation management with IGRT in prostate radiotherapy: Can it be managed with rigid alignment alone? J. Appl. Clin. Med. Phys. 2024, 25, e14241. [Google Scholar] [CrossRef]
- Guckenberger, M.; Ok, S.; Polat, B.; Sweeney, R.A.; Flentje, M. Toxicity after intensity-modulated, image-guided radiotherapy for prostate cancer. Strahlenther. Onkol. 2010, 186, 535–543. [Google Scholar] [CrossRef]
- Gill, S.; Thomas, J.; Fox, C.; Kron, T.; Rolfo, A.; Leahy, M.; Chander, S.; Williams, S.; Tai, K.H.; Duchesne, G.M.; et al. Acute toxicity in prostate cancer patients treated with and without image-guided radiotherapy. Radiat. Oncol. 2011, 6, 145. [Google Scholar] [CrossRef] [PubMed]
- Becker-Schiebe, M.; Abaci, A.; Ahmad, T.; Hoffmann, W. Reducing radiation-associated toxicity using online image guidance (IGRT) in prostate cancer patients undergoing dose-escalated radiation therapy. Rep. Pract. Oncol. Radiother. 2016, 21, 188–194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lips, I.M.; Dehnad, H.; van Gils, C.H.; Boeken Kruger, A.E.; van der Heide, U.A.; van Vulpen, M. High-dose intensity-modulated radiotherapy for prostate cancer using daily fiducial marker-based position verification: Acute and late toxicity in 331 patients. Radiat. Oncol. 2018, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Viani, G.A.; Stefano, E.J.; Afonso, S.L. Higher-than-conventional radiation doses in localized prostate cancer treatment: A meta-analysis of randomized, controlled trials. Int. J. Radiat. Oncol. 2009, 74, 1405–1418. [Google Scholar] [CrossRef]
- Schaake, W.; van der Schaaf, A.; van Dijk, L.V.; van den Bergh, A.C.M.; Langendijk, J.A. Development of a prediction model for late urinary incontinence, hematuria, pain and voiding frequency among irradiated prostate cancer patients. PLoS ONE 2018, 13, e0197757. [Google Scholar] [CrossRef]
- Christiansen, R.L.; Dysager, L.; Hansen, C.R.; Jensen, H.R.; Schytte, T.; Nyborg, C.J.; Bertelsen, A.S.; Agergaard, S.N.; Mahmood, F.; Hansen, S.; et al. Online adaptive radiotherapy potentially reduces toxicity for high-risk prostate cancer treatment. Radiother. Oncol. 2022, 167, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Kan, M.W.; Leung, L.H.; Wong, W.; Lam, N. Radiation dose from cone beam computed tomography for image-guided radiation therapy. Int. J. Radiat. Oncol. 2008, 70, 272–279. [Google Scholar] [CrossRef]
- Ariyaratne, H.; Chesham, H.; Pettingell, J.; Alonzi, R. Image-guided radiotherapy for prostate cancer with cone beam CT: Dosimetric effects of imaging frequency and PTV margin. Radiother. Oncol. 2016, 121, 103–108. [Google Scholar] [CrossRef]
- Maund, I.F.; Benson, R.J.; Fairfoul, J.; Cook, J.; Huddart, R.; Poynter, A. Image-guided radiotherapy of the prostate using daily CBCT: The feasibility and likely benefit of implementing a margin reduction. Br. J. Radiol. 2014, 87, 20140459. [Google Scholar] [CrossRef]
- Valeriani, M.; Bracci, S.; Osti, M.F.; Falco, T.; Agolli, L.; De Sanctis, V.; Enrici, R.M. Intermediate-risk prostate cancer patients treated with androgen deprivation therapy and a hypofractionated radiation regimen with or without image guided radiotherapy. Radiat. Oncol. 2013, 8, 137. [Google Scholar] [CrossRef]
- Akin, M.; Öksüz, D.C.; Iktueren, B.; Ambarcioglu, P.; Karacam, S.; Koca, S.; Dincbas, F.Ö. Does rectum and bladder dose vary during the course of image-guided radiotherapy in the postprostatectomy setting? Tumori 2014, 100, 529–535. [Google Scholar] [CrossRef]
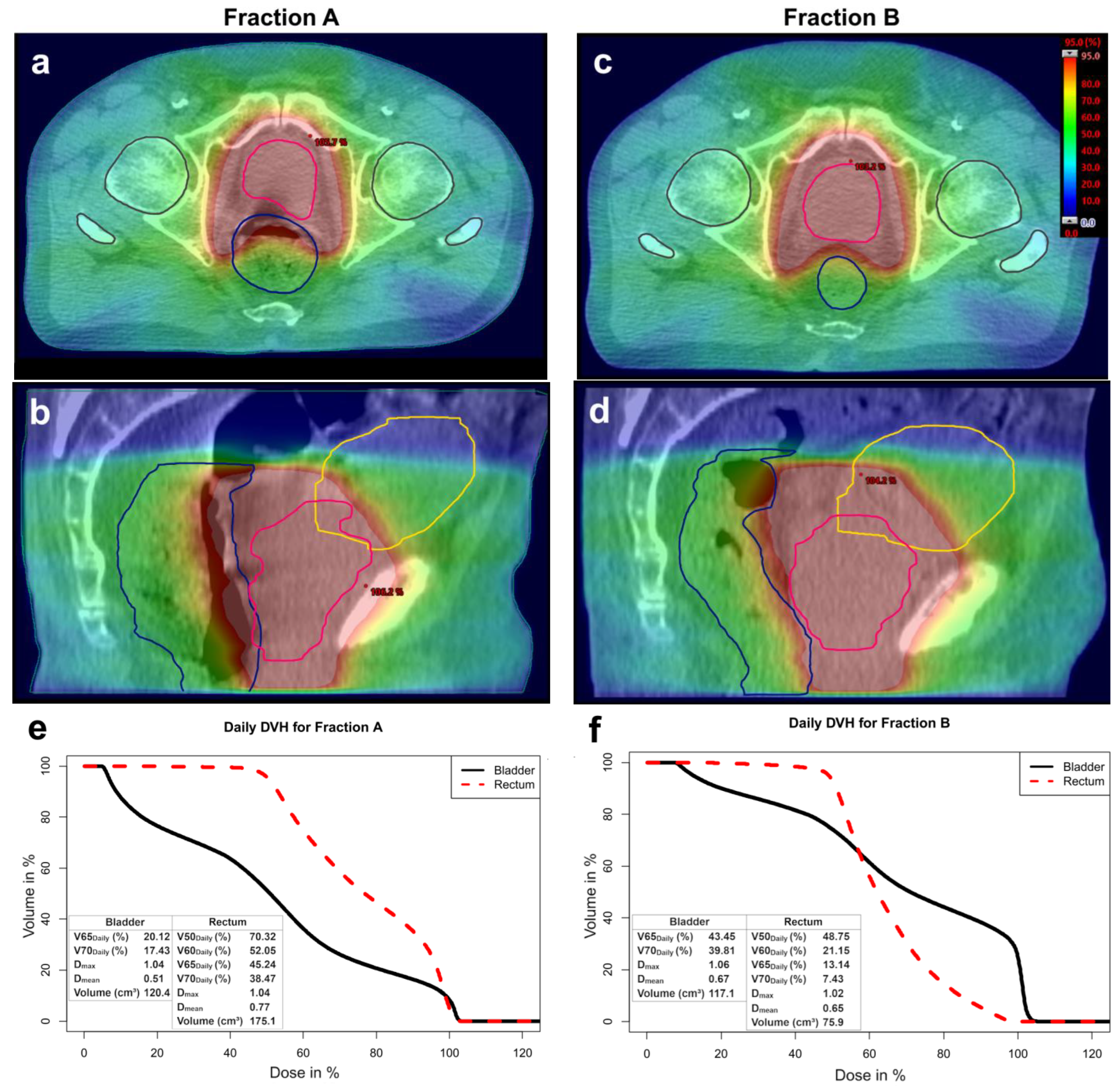
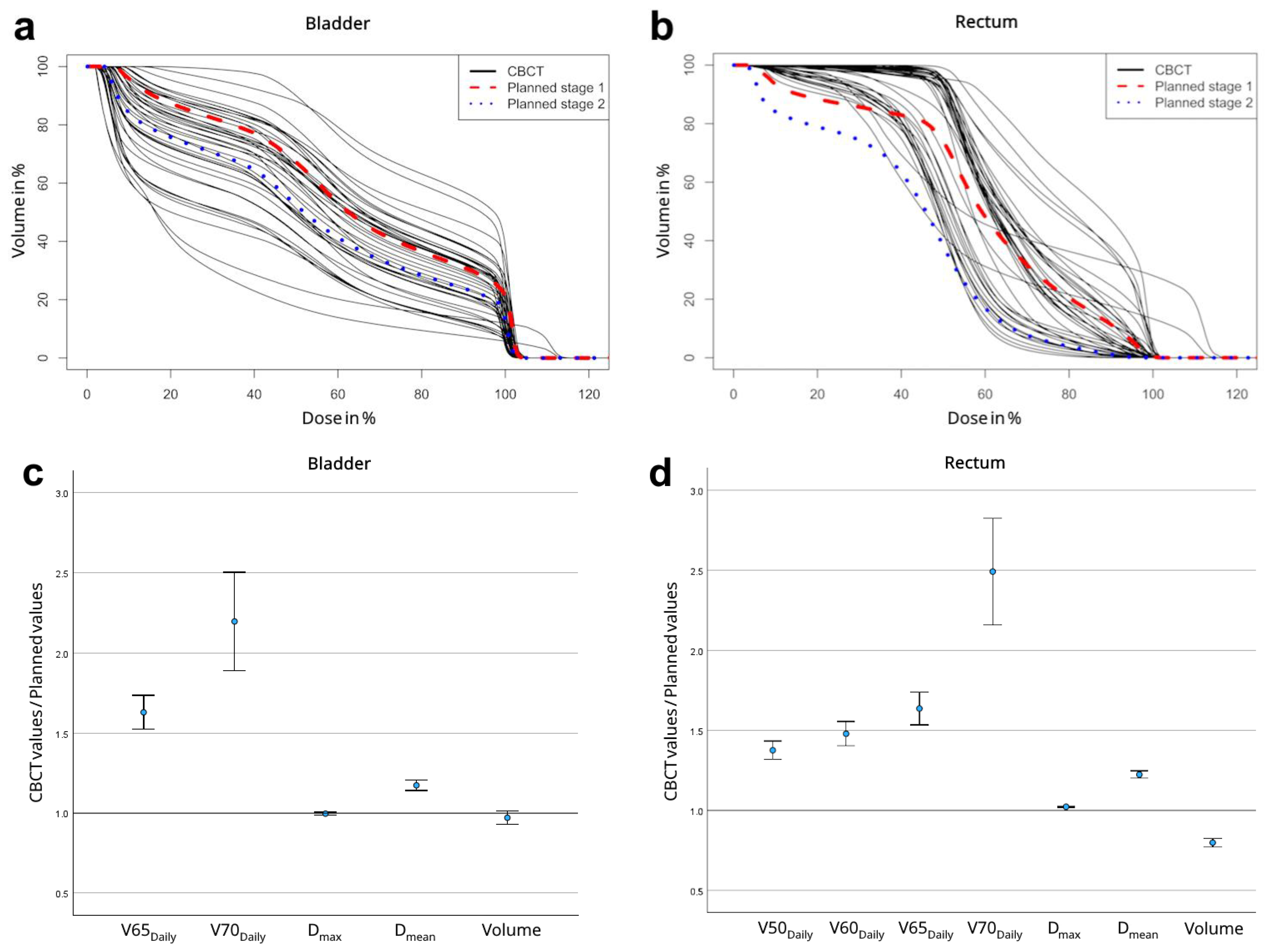
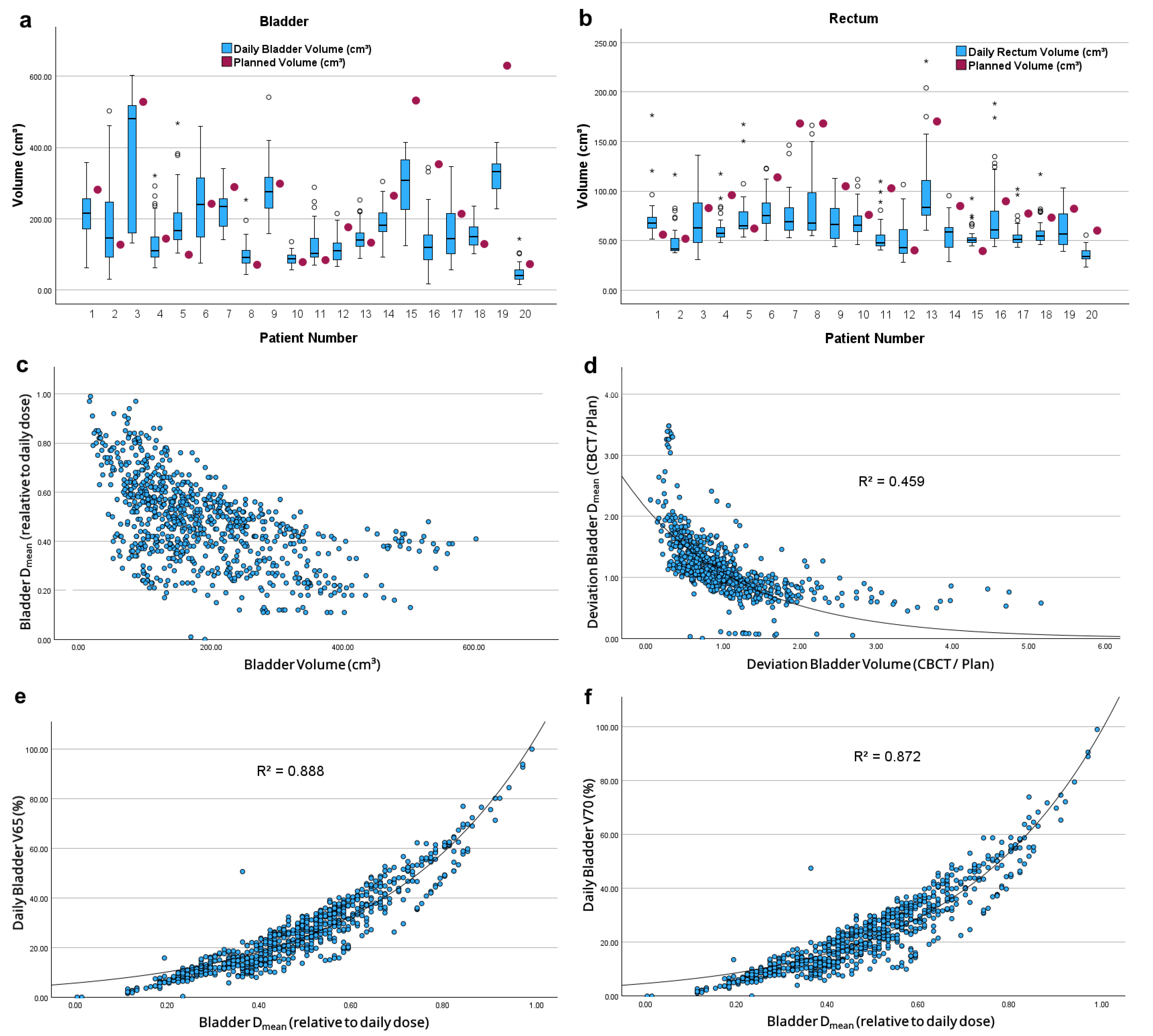
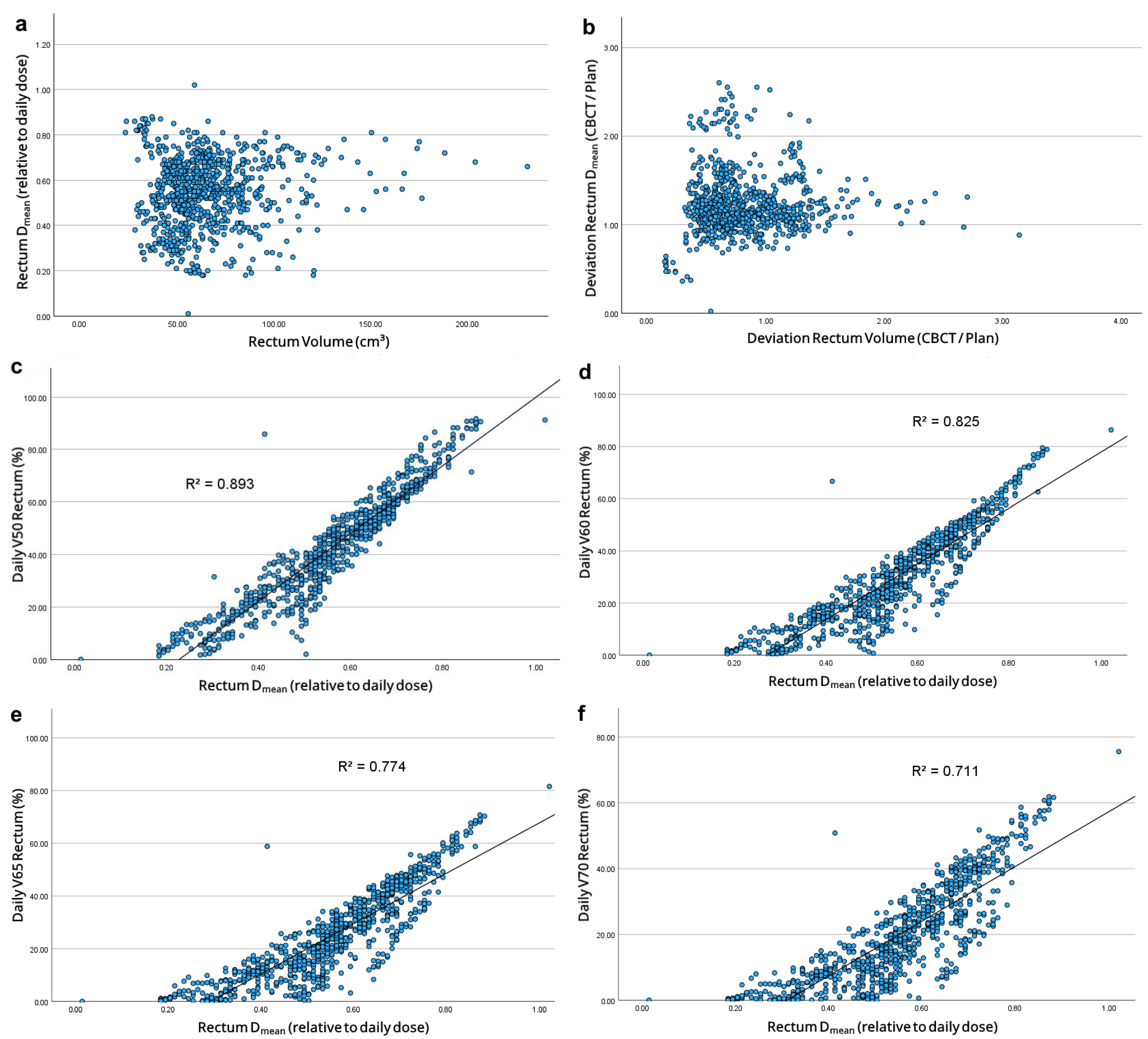
| Parameter | Initial Summation Plan | CBCT Summation Plan (n = 20) | p | |
|---|---|---|---|---|
| Bladder | (%) | 19.01 ± 8.91 | 18.17 ± 8.89 | 0.113 |
| (%) | 14.91 ± 7.81 | 13.74 ± 8.13 | 0.138 | |
| max (Gy) | 81.99 ± 1.09 | 81.02 ± 2.95 | 0.184 | |
| mean (Gy) | 35.36 ± 10.98 | 34.83 ± 10.53 | 0.150 | |
| Rectum | (%) | 32.17 ± 12.34 | 30.71 ± 12.04 | 0.021 |
| (%) | 19.80 ± 9.98 | 18.75 ± 10.15 | 0.114 | |
| (%) | 14.44 ± 8.51 | 13.40 ± 8.74 | 0.139 | |
| (%) | 9.17 ± 6.80 | 8.20 ± 7.02 | 0.170 | |
| max (Gy) | 79.36 ± 1.09 | 78.19 ± 2.82 | 0.040 | |
| mean (Gy) | 36.95 ± 6.56 | 36.45 ± 6.29 | 0.107 | |
| Parameter | Initial Summation Plan | Daily CBCT (n = 821) | p | |
| Bladder | Daily (%) | 19.01 ± 8.91 | 25.96 ± 11.43 | 0.003 |
| Daily (%) | 14.91 ± 7.81 | 23.07 ± 10.74 | <0.001 | |
| max * | 1.04 ± 0.04 | 1.05 ± 0.02 | 0.041 | |
| mean * | 0.45 ± 0.14 | 0.48 ± 0.13 | 0.246 | |
| Volume (cm3) | 237.3 ± 164.9 | 186.8 ± 85.5 | 0.042 | |
| Rectum | Daily (%) | 32.17 ± 12.34 | 42.06 ± 14.61 | <0.001 |
| Daily (%) | 19.80 ± 9.98 | 30.07 ± 11.65 | <0.001 | |
| Daily (%) | 14.44 ± 8.51 | 25.04 ± 10.36 | <0.001 | |
| Daily (%) | 9.17 ± 6.80 | 20.12 ± 9.20 | <0.001 | |
| max * | 1.01 ± 0.04 | 1.03 ± 0.02 | 0.016 | |
| mean * | 0.47 ± 0.09 | 0.55 ± 0.11 | <0.001 | |
| Volume (cm3) | 86.6 ± 35.3 | 64.8 ± 14.3 | 0.002 |
| Parameter | Genitourinary Toxicity Grade ≤ 1 (n = 16) | Genitourinary Toxicity Grade ≥ 2 (n = 4) | p | |
|---|---|---|---|---|
| Bladder | Daily (%) | 23.70 ± 9.44 | 35.01 ± 15.66 | 0.219 |
| Daily (%) | 21.14 ± 8.93 | 30.78 ± 15.24 | 0.299 | |
| max * | 1.06 ± 0.02 | 1.04 ± 0.01 | 0.059 | |
| mean * | 0.44 ± 0.10 | 0.63 ± 0.13 | 0.014 | |
| Volume (cm3) | 209.3 ± 79.1 | 96.5 ± 37.9 | 0.080 | |
| Parameter | Gastrointestinal Toxicity Grade ≤ 1 (n = 16) | Gastrointestinal Toxicity Grade ≥ 2 (n = 4) | p | |
| Rectum | Daily (%) | 42.05 ± 15.65 | 42.09 ± 11.24 | 0.777 |
| Daily (%) | 29.90 ± 12.62 | 30.78 ± 7.82 | 0.508 | |
| Daily (%) | 24.76 ± 11.19 | 26.16 ± 7.13 | 0.571 | |
| Daily (%) | 19.77 ± 9.86 | 21.55 ± 6.88 | 0.571 | |
| max * | 1.03 ± 0.02 | 1.03 ± 0.03 | 0.701 | |
| mean * | 0.55 ± 0.11 | 0.55 ± 0.04 | 0.962 | |
| Volume (cm3) | 63.9 ± 15.3 | 68.6 ± 10.5 | 0.508 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrikowski, M.; Kröger, K.; Roers, J.; Hering, D.; Lohmann, S.; Scobioala, S.; Haverkamp, U.; Eich, H.T. Can the Dose Constraints Be Trusted? Actual Dose Exposure of Bladder and Rectum During Prostate Cancer Radiotherapy. Cancers 2025, 17, 1194. https://doi.org/10.3390/cancers17071194
Petrikowski M, Kröger K, Roers J, Hering D, Lohmann S, Scobioala S, Haverkamp U, Eich HT. Can the Dose Constraints Be Trusted? Actual Dose Exposure of Bladder and Rectum During Prostate Cancer Radiotherapy. Cancers. 2025; 17(7):1194. https://doi.org/10.3390/cancers17071194
Chicago/Turabian StylePetrikowski, Marc, Kai Kröger, Julian Roers, Dominik Hering, Sebastian Lohmann, Sergiu Scobioala, Uwe Haverkamp, and Hans Theodor Eich. 2025. "Can the Dose Constraints Be Trusted? Actual Dose Exposure of Bladder and Rectum During Prostate Cancer Radiotherapy" Cancers 17, no. 7: 1194. https://doi.org/10.3390/cancers17071194
APA StylePetrikowski, M., Kröger, K., Roers, J., Hering, D., Lohmann, S., Scobioala, S., Haverkamp, U., & Eich, H. T. (2025). Can the Dose Constraints Be Trusted? Actual Dose Exposure of Bladder and Rectum During Prostate Cancer Radiotherapy. Cancers, 17(7), 1194. https://doi.org/10.3390/cancers17071194






