Distinguishing Hepatocellular Carcinoma from Cirrhotic Regenerative Nodules Using MR Cytometry
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Theory of MRI Cytometry
2.2. Validation Using Histology-Based Simulations
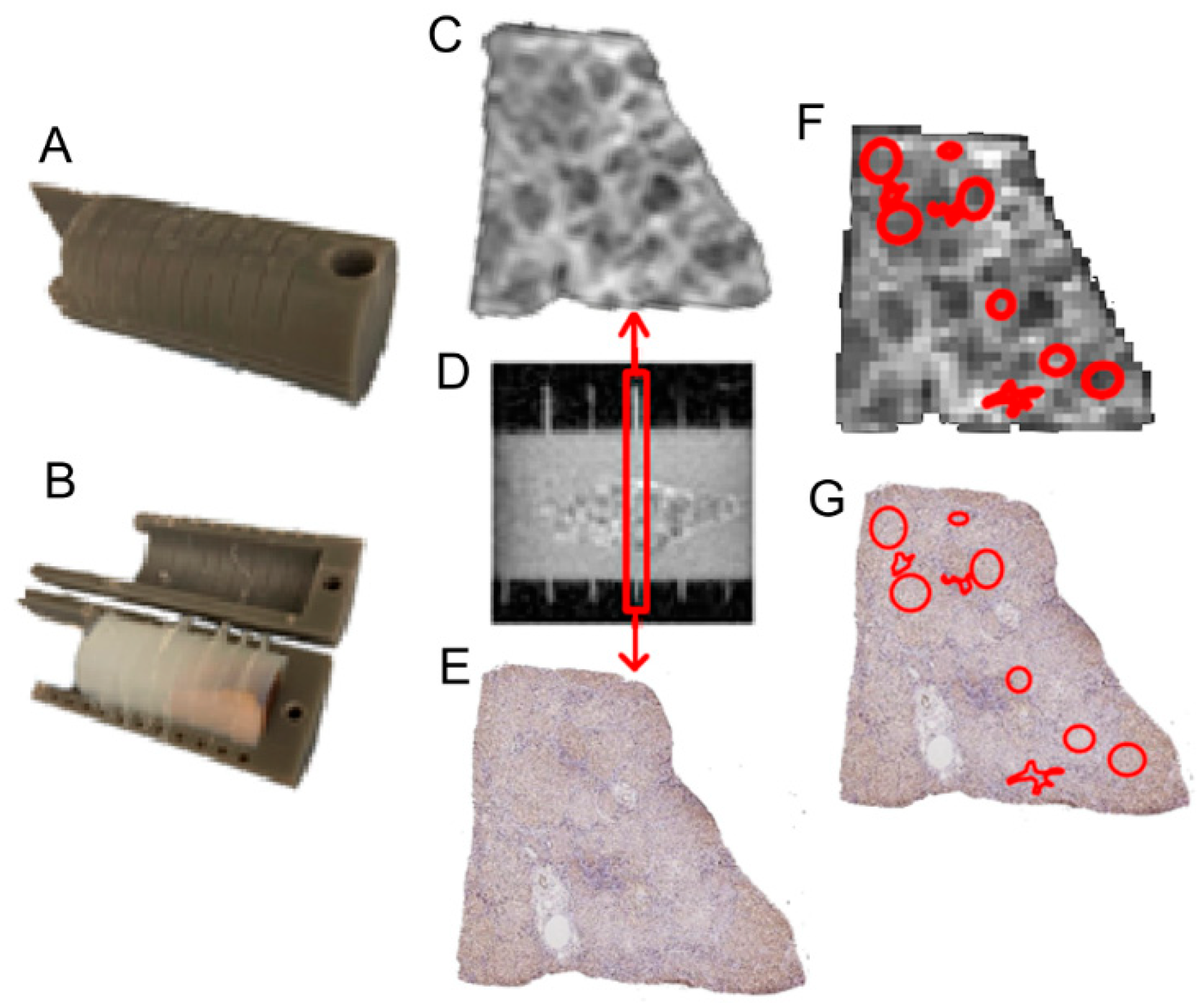
2.3. Validation Using Ex Vivo MRI Cytometry and Histology Analysis
2.3.1. Ex Vivo Imaging Protocol
2.3.2. Co-Registration Between Histology and Ex Vivo MRI
2.4. MRI Cytometry Analysis
2.5. Statistical Analysis
3. Results
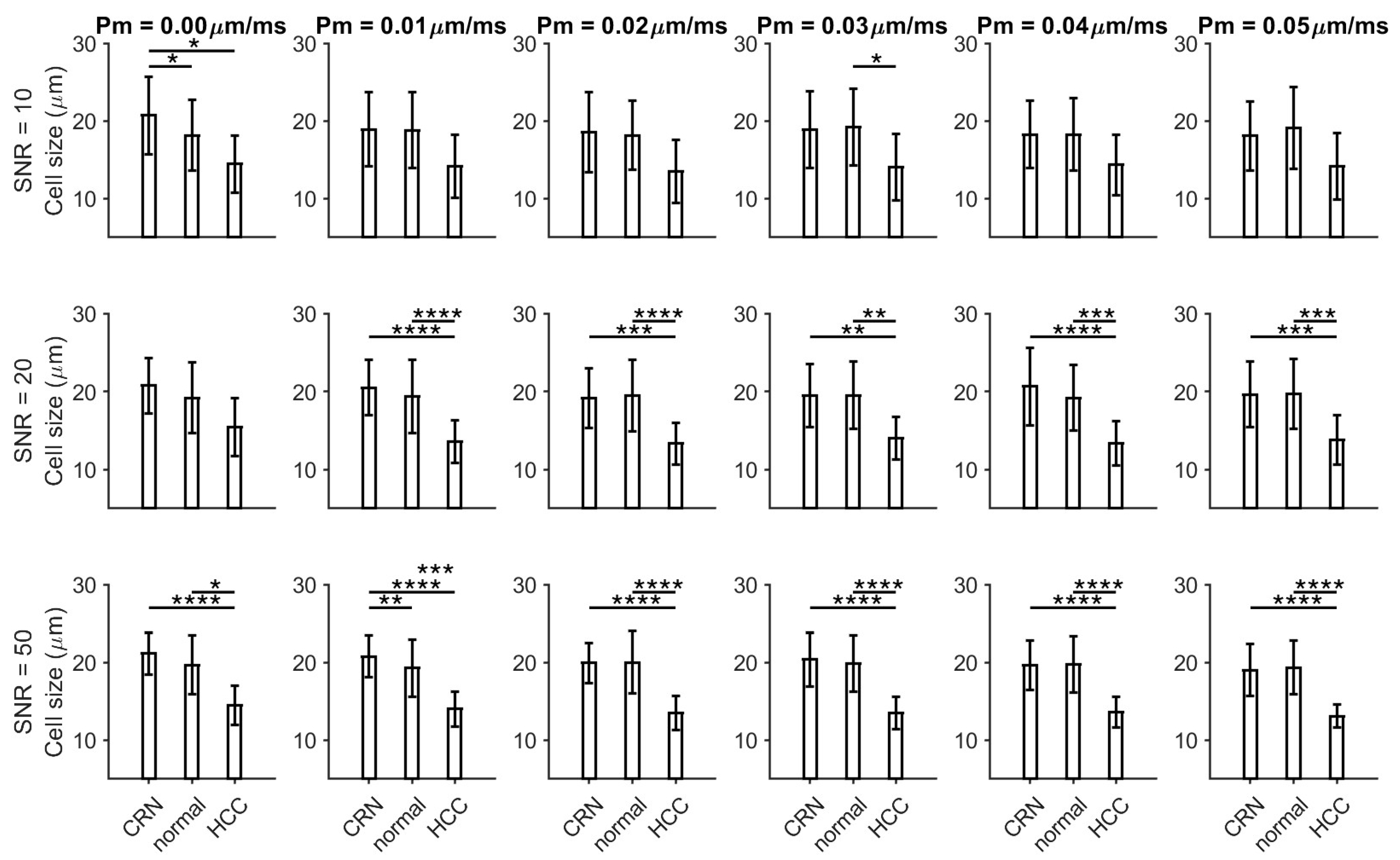
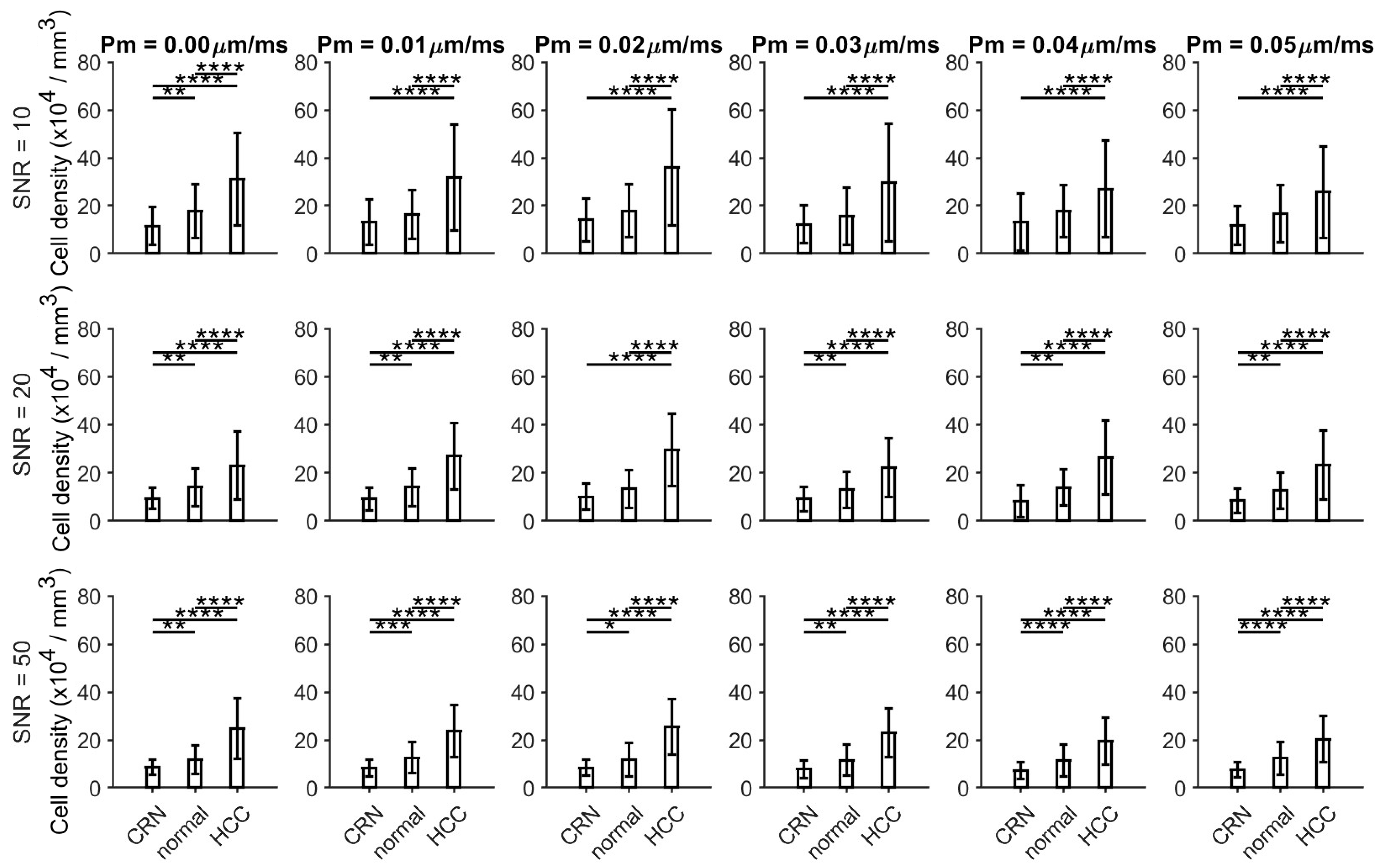
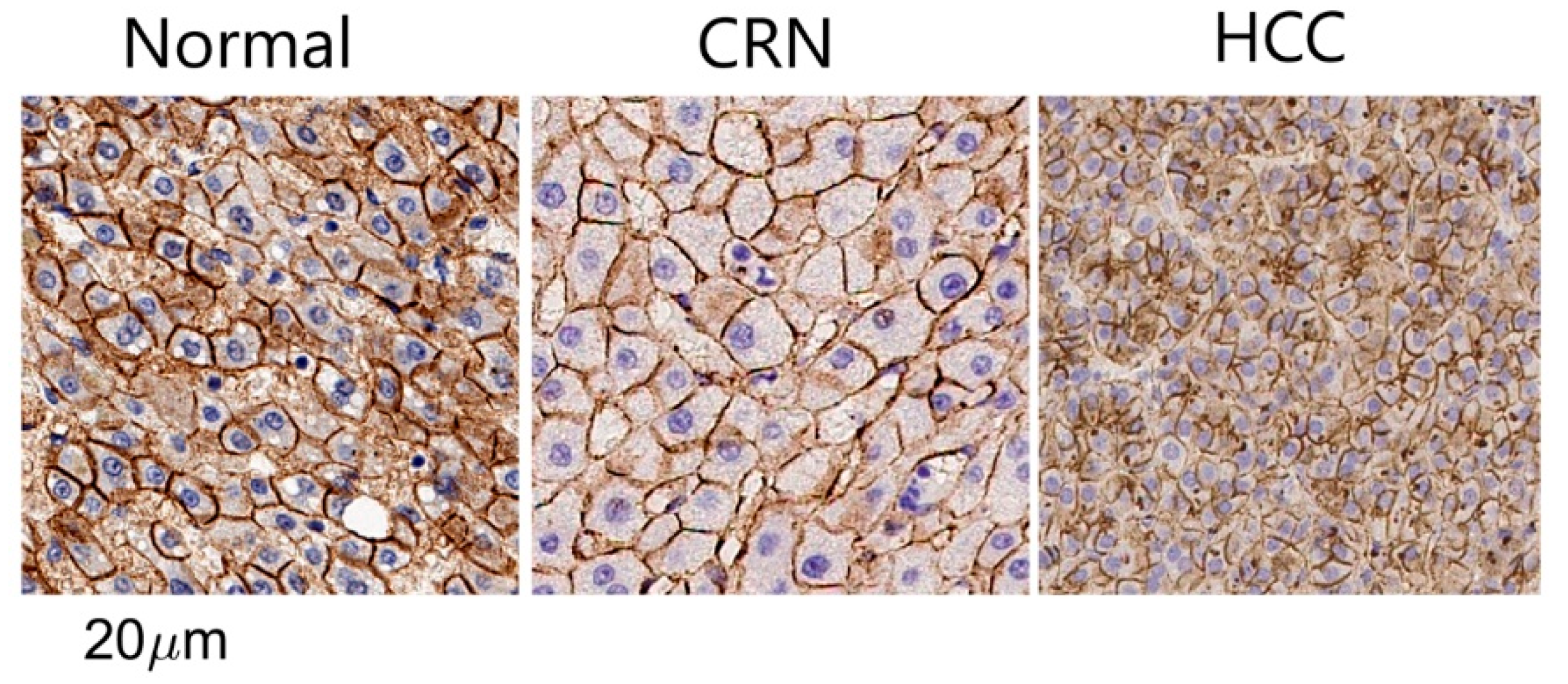
3.1. Histology-Based Simulations Confirmed Pathological Variations in Cell Size and Cellularity
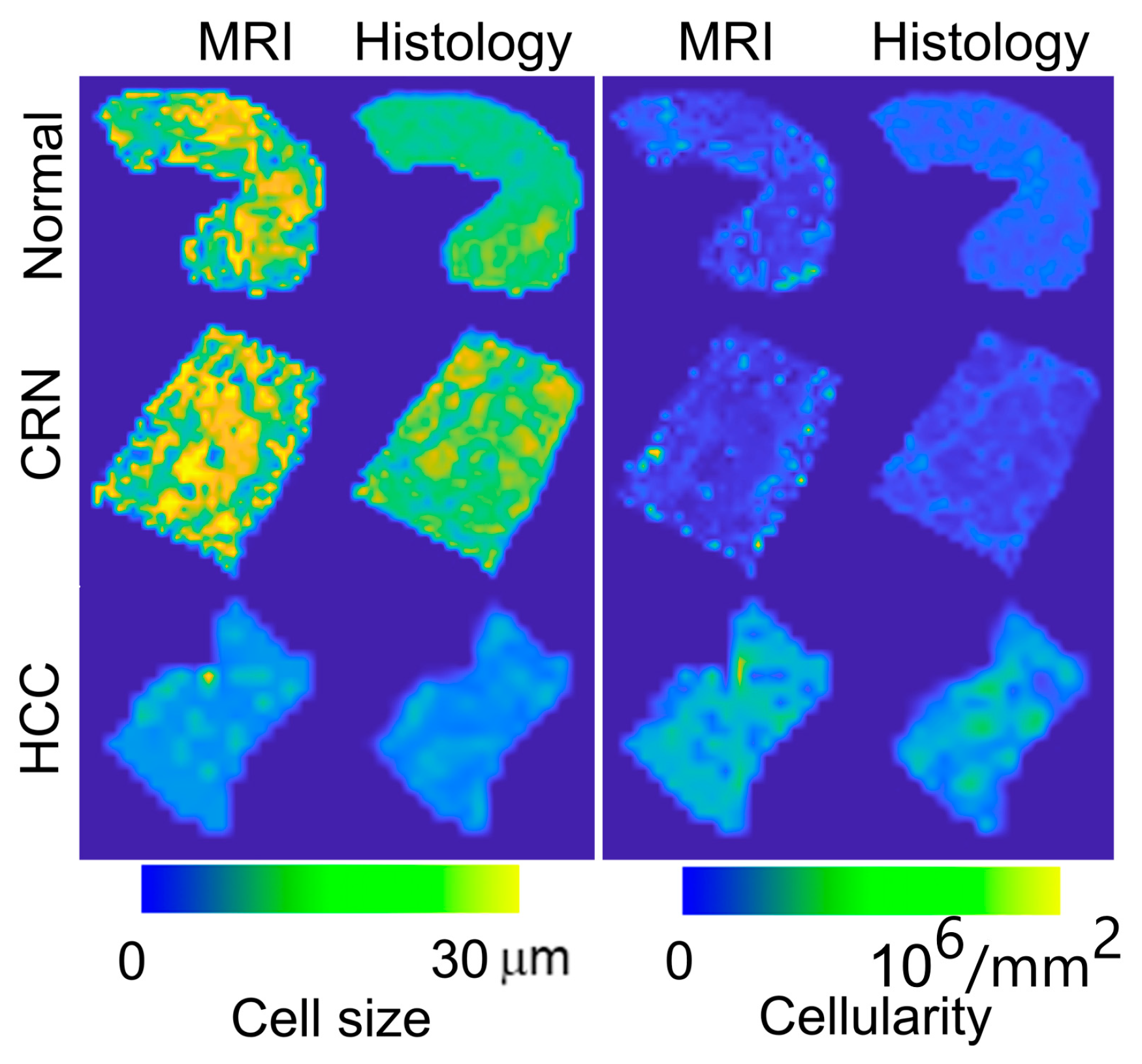
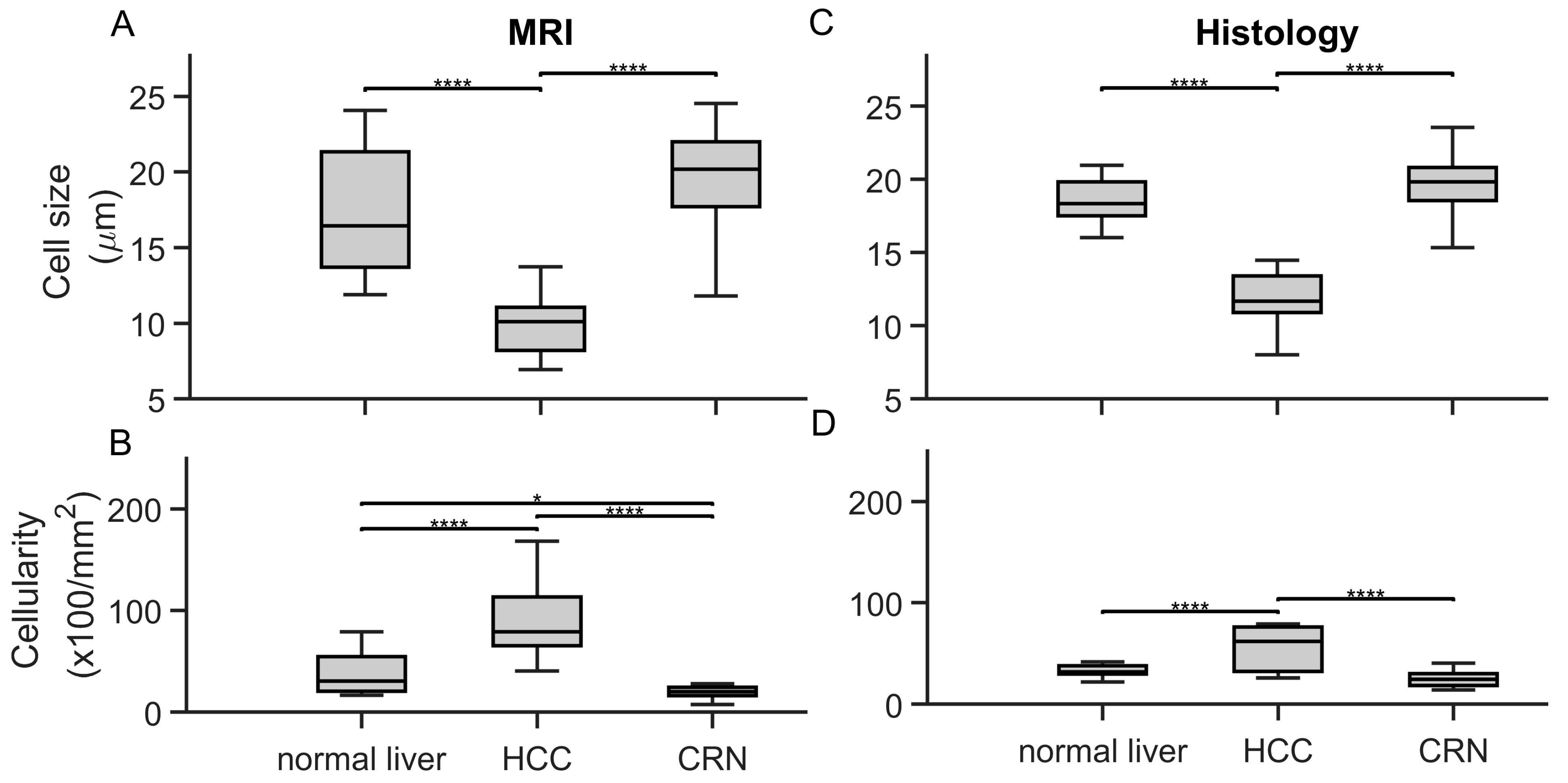
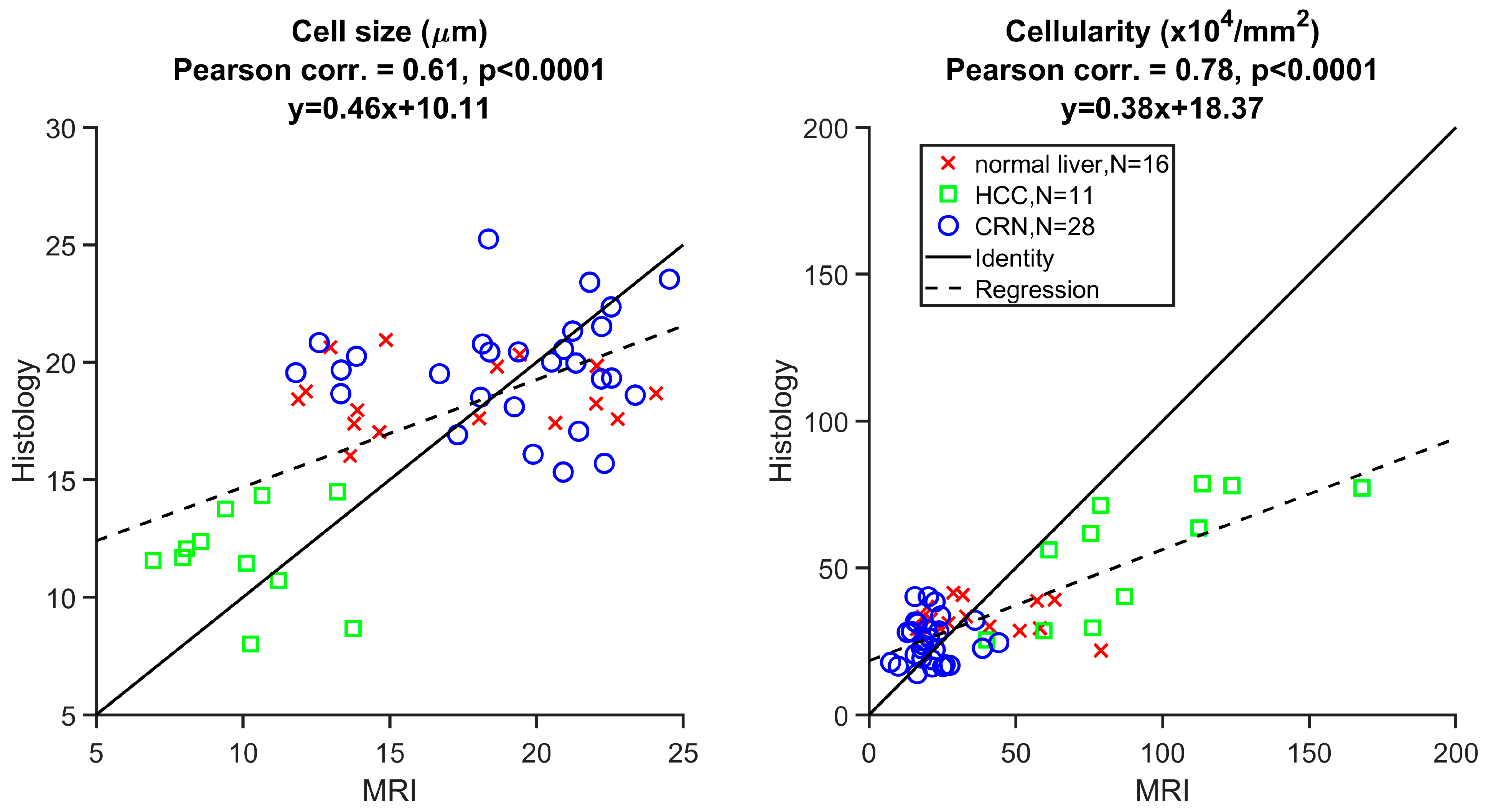
3.2. Ex Vivo MR Cytometry Characterized Different Pathological Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
- HCC: hepatocellular carcinoma
- IMPULSED: imaging microstructural parameters using limited spectrally edited diffusion
- CRN: cirrhotic regenerative nodule
- ADC: apparent diffusion coefficient
- TDS: temporal diffusion spectroscopy
- OGSE: oscillating gradient spin echo
- PGSE: pulsed gradient spin echo
References
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv. Cancer Res. 2021, 149, 1–61. [Google Scholar]
- Tsuchiya, N. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 10573–10583. [Google Scholar] [CrossRef]
- Baffy, G.; Brunt, E.M.; Caldwell, S.H. Hepatocellular carcinoma in non-alcoholic fatty liver disease: An emerging menace. J. Hepatol. 2012, 56, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Frenette, C.T.; Isaacson, A.J.; Bargellini, I.; Saab, S.; Singal, A.G. A Practical Guideline for Hepatocellular Carcinoma Screening in Patients at Risk. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 302–310. [Google Scholar] [CrossRef]
- Chou, C.-T. Differentiation between dysplastic nodule and early-stage hepatocellular carcinoma: The utility of conventional MR imaging. World J. Gastroenterol. 2013, 19, 7433–7439. [Google Scholar] [CrossRef] [PubMed]
- Chernyak, V.; Fowler, K.J.; Kamaya, A.; Kielar, A.Z.; Elsayes, K.M.; Bashir, M.R.; Kono, Y.; Do, R.K.; Mitchell, D.G.; Singal, A.G.; et al. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology 2018, 289, 816–830. [Google Scholar] [CrossRef]
- Asafo-Agyei, K.O.; Samant, H. Hepatocellular Carcinoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Schlageter, M.; Terracciano, L.M.; D’Angelo, S.; Sorrentino, P. Histopathology of hepatocellular carcinoma. World J. Gastroenterol. WJG 2014, 20, 15955–15964. [Google Scholar]
- Geramizadeh, B.; Asadi, N.; Tabei, S.Z. Cytologic Comparison Between Malignant and Regenerative Nodules in the Background of Cirrhosis. Hepat. Mon. 2012, 12, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, J.D.; Yoo, C.C.; Lai, K.K.Y.; Braun, J.; McGovern, D.P.B.; Xie, Y.; Pandol, S.J.; Lu, S.C.; Li, D. Magnetic resonance imaging for characterization of hepatocellular carcinoma metabolism. Front. Physiol. 2022, 13, 1056511. [Google Scholar] [CrossRef]
- Shenoy-Bhangle, A.; Baliyan, V.; Kordbacheh, H.; Guimaraes, A.R.; Kambadakone, A. Diffusion weighted magnetic resonance imaging of liver: Principles, clinical applications and recent updates. World J. Hepatol. 2017, 9, 1081–1091. [Google Scholar] [CrossRef]
- Yamada, S.; Morine, Y.; Ikemoto, T.; Saito, Y.; Teraoku, H.; Waki, Y.; Nakasu, C.; Shimada, M. Impact of apparent diffusion coefficient on prognosis of early hepatocellular carcinoma: A case control study. BMC Surg. 2023, 23, 6. [Google Scholar] [CrossRef]
- Gluskin, J.S.; Chegai, F.; Monti, S.; Squillaci, E.; Mannelli, L. Hepatocellular Carcinoma and Diffusion-Weighted MRI: Detection and Evaluation of Treatment Response. J. Cancer 2016, 7, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Panagiotaki, E.; Walker-Samuel, S.; Siow, B.; Johnson, S.P.; Rajkumar, V.; Pedley, R.B.; Lythgoe, M.F.; Alexander, D.C. Noninvasive Quantification of Solid Tumor Microstructure Using VERDICT MRI. Cancer Res. 2014, 74, 1902–1912. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, J.; Gore, J.C. Mapping hepatocyte size in vivo using temporal diffusion spectroscopy MRI. Magn. Reson. Med. 2020, 84, 2671–2683. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jiang, X.; Li, H.; Arlinghaus, L.R.; McKinley, E.T.; Devan, S.P.; Hardy, B.M.; Xie, J.; Kang, H.; Chakravarthy, A.B.; et al. Magnetic resonance imaging of mean cell size in human breast tumors. Magn. Reson. Med. 2019, 83, 2002–2014. [Google Scholar] [CrossRef]
- Jiang, X.; Li, H.; Xie, J.; McKinley, E.T.; Zhao, P.; Gore, J.C.; Xu, J. In vivo imaging of cancer cell size and cellularity using temporal diffusion spectroscopy. Magn. Reson. Med. 2016, 78, 156–164. [Google Scholar] [CrossRef]
- Jiang, X.; Devan, S.P.; Xie, J.; Gore, J.C.; Xu, J. Improving MR cell size imaging by inclusion of transcytolemmal water exchange. NMR Biomed. 2022, 35, e4799. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, O.; Winters, K.V.; Hoang, D.M.; Wadghiri, Y.Z.; Novikov, D.S.; Kim, S.G. Pulsed and oscillating gradient MRI for assessment of cell size and extracellular space (POMACE) in mouse gliomas. NMR Biomed. 2016, 29, 1350–1363. [Google Scholar] [CrossRef]
- Jiang, X.; Li, H.; Devan, S.P.; Gore, J.C.; Xu, J. MR cell size imaging with temporal diffusion spectroscopy. Magn. Reson. Imaging 2021, 77, 109–123. [Google Scholar] [CrossRef]
- Wu, D.; Jiang, K.; Li, H.; Zhang, Z.; Ba, R.; Zhang, Y.; Hsu, Y.-C.; Sun, Y.; Zhang, Y.-D. Time-Dependent Diffusion MRI for Quantitative Microstructural Mapping of Prostate Cancer. Radiology 2022, 303, 578–587. [Google Scholar] [CrossRef]
- Liu, F.; Wu, L.; Luo, X.; Li, S.; Wang, Y.; Zhong, W.; Feiweier, T.; Xu, J.; Shi, D.; Bao, H.; et al. Evaluating the Diagnostic Performance of MR Cytometry Imaging in Differentiating Benign and Malignant Breast Tumors. J. Magn. Reson. Imaging 2025. [Google Scholar] [CrossRef]
- Ba, R.; Wang, X.; Zhang, Z.; Li, Q.; Sun, Y.; Zhang, J.; Wu, D. Diffusion-time dependent diffusion MRI: Effect of diffusion-time on microstructural mapping and prediction of prognostic features in breast cancer. Eur. Radiol. 2023, 33, 6226–6237. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lu, Y.; Shao, W.; Zhai, W.; Song, J.; Zhang, A.; Huang, S.; Zhao, X.; Cheng, W.; Wu, F.; et al. Time-dependent diffusion MRI-based microstructural mapping for differentiating high-grade serous ovarian cancer from serous borderline ovarian tumor. Eur. J. Radiol. 2024, 178, 111622. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Cheng, J.; Lin, L.; Hu, Y.; Wang, A.; Zhang, Y.; Wang, R.; Li, Y.; Zhang, K.; et al. Microstructural diffusion MRI for differentiation of breast tumors and prediction of prognostic factors in breast cancer. Front. Oncol. 2025, 15, 1498691. [Google Scholar] [CrossRef]
- Jiang, X.; Li, H.; Xie, J.; Zhao, P.; Gore, J.C.; Xu, J. Quantification of cell size using temporal diffusion spectroscopy. Magn. Reson. Med. 2015, 75, 1076–1085. [Google Scholar] [CrossRef]
- Muriel, P. The Liver. In Liver Pathophysiology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–22. [Google Scholar] [CrossRef]
- Mendelaar, P.A.J.; Kraan, J.; Van, M.; Zeune, L.L.; Terstappen, L.W.M.M.; Hoop, E.O.; Martens, J.W.M.; Sleijfer, S. Defining the dimensions of circulating tumor cells in a large series of breast, prostate, colon, and bladder cancer patients. Mol. Oncol. 2021, 15, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Shojaie, L.; Iorga, A.; Dara, L. Cell Death in Liver Diseases: A Review. Int. J. Mol. Sci. 2020, 21, 9682. [Google Scholar] [CrossRef]
- Kärger, J.; Pfeifer, H.; Heink, W. Principles and Application of Self-Diffusion Measurements by Nuclear Magnetic Resonance. In Advances in Magnetic and Optical Resonance; Waugh, J.S., Ed.; Academic Press: Cambridge, MA, USA, 1988; Volume 12, pp. 1–89. [Google Scholar]
- Xu, J.; Does, M.D.; Gore, J.C. Numerical study of water diffusion in biological tissues using an improved finite difference method. Phys. Med. Biol. 2007, 52, N111–N126. [Google Scholar] [CrossRef]
- Li, H.; Jiang, X.; Xie, J.; Gore, J.C.; Xu, J. Impact of transcytolemmal water exchange on estimates of tissue microstructural properties derived from diffusion MRI. Magn. Reson. Med. 2016, 77, 2239–2249. [Google Scholar] [CrossRef]
- Koh, D.-M.; Collins, D.J. Diffusion-Weighted MRI in the Body: Applications and Challenges in Oncology. Am. J. Roentgenol. 2007, 188, 1622–1635. [Google Scholar] [CrossRef]
- Vachha, B.; Huang, S.Y. MRI with ultrahigh field strength and high-performance gradients: Challenges and opportunities for clinical neuroimaging at 7 T and beyond. Eur. Radiol. Exp. 2021, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Witzel, T.; Keil, B.; Scholz, A.; Davids, M.; Dietz, P.; Rummert, E.; Ramb, R.; Kirsch, J.E.; Yendiki, A.; et al. Connectome 2.0: Developing the next-generation ultra-high gradient strength human MRI scanner for bridging studies of the micro-, meso- and macro-connectome. NeuroImage 2021, 243, 118530. [Google Scholar] [CrossRef]
- Bischoff, L.M.; Endler, C.; Krausewitz, P.; Ellinger, J.; Klümper, N.; Isaak, A.; Mesropyan, N.; Kravchenko, D.; Nowak, S.; Kuetting, D.; et al. Ultra-high gradient performance 3-Tesla MRI for super-fast and high-quality prostate imaging: Initial experience. Insights Into Imaging 2024, 15, 286. [Google Scholar] [CrossRef]
- Jiang, X.; Izzy, M.; Washington, K.; Gore, J.; Xu, J. In vivo MRI characterization of pathological changes in liver microstructures. J. Hepatol. 2023, 78, S555–S556. [Google Scholar] [CrossRef]
- Li, Y.T.; Cercueil, J.-P.; Yuan, J.; Chen, W.; Loffroy, R.; Wáng, Y.X.J. Liver intravoxel incoherent motion (IVIM) magnetic resonance imaging: A comprehensive review of published data on normal values and applications for fibrosis and tumor evaluation. Quant. Imaging Med. Surg. 2017, 7, 59–78. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, J.; Bai, X.; Liang, T. Evaluation of Intra-Tumoral Vascularization in Hepatocellular Carcinomas. Front. Med. 2020, 7, 584250. [Google Scholar] [CrossRef]
- Finkelstein, A.; Cao, X.; Liao, C.; Schifitto, G.; Zhong, J. Diffusion Encoding Methods in MRI: Perspectives and Challenges. Investig. Magn. Reson. Imaging 2022, 26, 208–219. [Google Scholar] [CrossRef]
- Kang, K.A.; Kim, Y.K.; Kim, E.; Jeong, W.K.; Choi, D.; Lee, W.J.; Jung, S.-H.; Baek, S.-Y. T2-Weighted Liver MRI Using the MultiVane Technique at 3T: Comparison with Conventional T2-Weighted MRI. Korean J. Radiol. 2015, 16, 1038–1046. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Washington, M.K.; Izzy, M.J.; Lu, M.; Yan, X.; Zu, Z.; Gore, J.C.; Xu, J. Distinguishing Hepatocellular Carcinoma from Cirrhotic Regenerative Nodules Using MR Cytometry. Cancers 2025, 17, 1204. https://doi.org/10.3390/cancers17071204
Jiang X, Washington MK, Izzy MJ, Lu M, Yan X, Zu Z, Gore JC, Xu J. Distinguishing Hepatocellular Carcinoma from Cirrhotic Regenerative Nodules Using MR Cytometry. Cancers. 2025; 17(7):1204. https://doi.org/10.3390/cancers17071204
Chicago/Turabian StyleJiang, Xiaoyu, Mary Kay Washington, Manhal J. Izzy, Ming Lu, Xinqiang Yan, Zhongliang Zu, John C. Gore, and Junzhong Xu. 2025. "Distinguishing Hepatocellular Carcinoma from Cirrhotic Regenerative Nodules Using MR Cytometry" Cancers 17, no. 7: 1204. https://doi.org/10.3390/cancers17071204
APA StyleJiang, X., Washington, M. K., Izzy, M. J., Lu, M., Yan, X., Zu, Z., Gore, J. C., & Xu, J. (2025). Distinguishing Hepatocellular Carcinoma from Cirrhotic Regenerative Nodules Using MR Cytometry. Cancers, 17(7), 1204. https://doi.org/10.3390/cancers17071204





