Simple Summary
This study compares the long-term survival of patients with stage IV lung adenocarcinoma treated with current therapies (checkpoint inhibitors or osimertinib for EGFR mutations) versus older treatments. The analysis included patients with EGFR, KRAS, or no mutations, divided into “current” and “historic” cohorts based on the timing of their mutation testing. Results showed that both current EGFR and no-mutation cohorts had significantly longer overall survival (OS) compared to historic cohorts. The improvement in survival was particularly notable for long-term survivors in the no-mutation group. KRAS patients had survival outcomes similar to those with no mutations, with about 20% reaching long-term survival. Overall, the findings suggest that newer treatments like checkpoint inhibitors and osimertinib have led to improved outcomes, aligning with phase III trial results and demonstrating their effectiveness in real-world practice.
Abstract
Background: Treatment of lung adenocarcinoma has changed and now includes checkpoint inhibitors (CPIs) or, in the case of an EGFR mutation, third-generation EGFR TKI osimertinib. Few data compare the long-term overall survival (OS) of current and historic subgroups. Methods: This real-world analysis (KOMPASS study) included stage IV lung-adenocarcinoma patients with either EGFR, KRAS, or no mutation. Patients were assigned to the “current” EGFR, KRAS, or no-mutation cohort if they had mutation testing using NGS (n = 199; median date of diagnosis 2021). If they had an EGFR PCR test only, they were assigned to the “historic” EGFR or no-mutation cohort (n = 127; median date of diagnosis 2014). Results: Both the current and the historic EGFR cohorts had significantly longer OS than the respective no-mutation cohorts (HR 0.58 and 0.60, respectively). The current no-mutation and EGFR cohorts had a strong trend to longer OS than the respective historic cohorts. In the no-mutation cohorts, the improvement was due to an increase in long-term survivors (HR 0.71), whereas in the EGFR mutation cohorts, the median OS was improved without long-term survivors (HR 0.70). The KRAS cohort showed OS like the no-mutation cohort, with a plateau of long-term survivors around 20%. Conclusions: A comparison of our data with that of the phase III trials KEYNOTE-189 and FLAURA suggests that the improved outcomes are due to the use of CPIs or osimertinib. The clinical trial results are well translated into real-world clinical practice with comparable OS. KRAS patients benefit from CPI treatment like no-mutation patients.
Keywords:
KRAS; G12D; EGFR; Exon 21; lung cancer; check point inhibitor; real-world data; overall survival 1. Introduction
Lung cancer is the second most common cause of cancer and remains the leading cause of cancer death [1,2]. Non-small cell lung cancer (NSCLC) accounts for 81% of lung cancers, with most patients presenting in advanced stages [3,4,5]. NSCLC, in particular lung adenocarcinoma, is characterized by molecularly defined subsets with targetable oncogenic drivers. In lung adenocarcinoma, mutations of the EGFR or KRAS gene represent the most common oncogenic drivers [6]. Common EGFR mutations (del exon 19 or exon 21 L858R) are associated with better overall survival (OS) compared to no-mutation lung adenocarcinoma [7,8]. KRAS mutations were found to be a negative prognostic parameter in NSCLC [7,8,9], whereas other studies did not find a difference in outcome after chemotherapy [10,11]. However, these data come from a time during which immunotherapy with checkpoint inhibitors (CPIs) was not widely available. The regular use of CPIs has been shown to be associated with improved OS in no-driver patients and KRAS patients but not in EGFR patients [12,13]. Treatment with the third-generation EGFR tyrosine-kinase inhibitor (TKI) osimertinib results in improved OS in EGFR del exon 19 patients but not in EGFR exon 21 L858R patients [14]. The regular use of CPIs or osimertinib may have changed the prognostic implications of EGFR or KRAS mutations compared to no-driver patients. Therefore, we provide data on trends in OS of unselected patients with stage IV lung adenocarcinoma and EGFR or KRAS mutation, or no-mutation in a current and historic real-world population.
Furthermore, data from randomized clinical trials of targeted therapies for EGFR or KRAS mutant NSCLC concentrate on patients with classical EGFR mutations or with KRAS G12C mutation. Following a pooled post-hoc analysis of the LUX-Lung trials and a more recent report, uncommon EGFR mutations are still generally treated with the second-generation EGFR TKI afatinib and are associated with poor survival [15,16,17]. Only recently, a retrospective analysis showed responsiveness to osimertinib [18]. Taken together, there is still a lack of data on the current survival of patients with uncommon EGFR mutations. Similarly, there are almost no data on the survival of patients with specific KRAS-nonG12C mutations [19]. However, in light of the development of pan-RAS inhibitors, which are currently reaching the early clinical trial stage [20,21], data on the prognostic significance of specific KRAS-nonG12C mutations are of special interest. To close these gaps, we provide data on the survival of patients with uncommon EGFR mutations and preliminary data on the survival of patients with various KRAS-nonG12C subtypes.
2. Materials and Methods
This retrospective real-world analysis was conducted as part of the KOMPASS study at an experienced lung cancer center certified by the German Cancer Society (DKG). Patients diagnosed with NSCLC and adenocarcinoma histology until 30 September 2024 were eligible if molecular pathology results for EGFR and ALK were available. All patients had histologically confirmed NSCLC with adenocarcinoma histology in the diagnostic biopsy, and complete tumor staging, including PET CT if clinically indicated, and contrast-enhanced MRI of the brain or, if MRI was contraindicated, contrast-enhanced CT at baseline. Staging was performed according to the IASLC 8th edition [22]. In earlier cases, molecular pathology was performed using (multiplex) polymerase-chain reaction (PCR) for EGFR Exon 18, 19, 20, and 21, and fluorescence in situ hybridization (FISH) for ALK translocations, not including KRAS testing. Between 2016 and 2020, molecular testing was gradually changed to DNA- and RNA-based next-generation sequencing (NGS) from tissue or peripheral blood (liquid biopsy) targeting a lung cancer panel. Patients who had an NGS test result available were assigned to the “current” cohort. Patients with a PCR result only were assigned to the “historic” cohort. Predictive EGFR mutations were grouped in exon 19 deletions, exon 21 L858R, and “uncommon” mutations (exon 18, exon 21 non-L858R, or complex mutations). For comparison with phase III clinical trial populations (KEYNOTE-189, FLAURA) [14,23], current and historic no-mutation* and EGFR* subpopulations were analyzed comprising only patients with good performance scores (ECOG 0-1) and common EGFR mutations (del exon 19 or exon 21 L858R).
Treatment was performed in accordance with national and international guidelines (Onkopedia [24], ESMO [25,26]). Each patient was discussed at least once in the multidisciplinary tumor board (MDT). If deemed necessary by the MDT, patients were additionally presented to the molecular tumor board (University of Heidelberg, Germany). The study was performed in accordance with the International Conference on Harmonization Guidelines on Good Clinical Practice and the Declaration of Helsinki. The KOMPASS study was approved by the ethics committee of Landesärztekammer Baden-Württemberg (F-2017-004, F-2019-092). Patients provided written informed consent. There was no funding.
The databank was locked on 28 February 2025. The original data will be made available as a data paper. For clarity and simplicity, some Kaplan–Meier plots contain more than two curves. For survival analysis, we performed Cox proportional hazard regression on pairwise Kaplan–Meier plots. Hazard ratios (HR), 95% confidence intervals (CI), and p-values were calculated using the log-rank (Mantel–Cox) test. Assuming non-parametric data, the significance of numeric values were calculated using the Mann–Whitney U-test. Kaplan–Meier plots and all statistical analyses were generated using GraphPad Prism8. The report is in line with the STROBE statement [27].
3. Results
3.1. Structure of Study Population
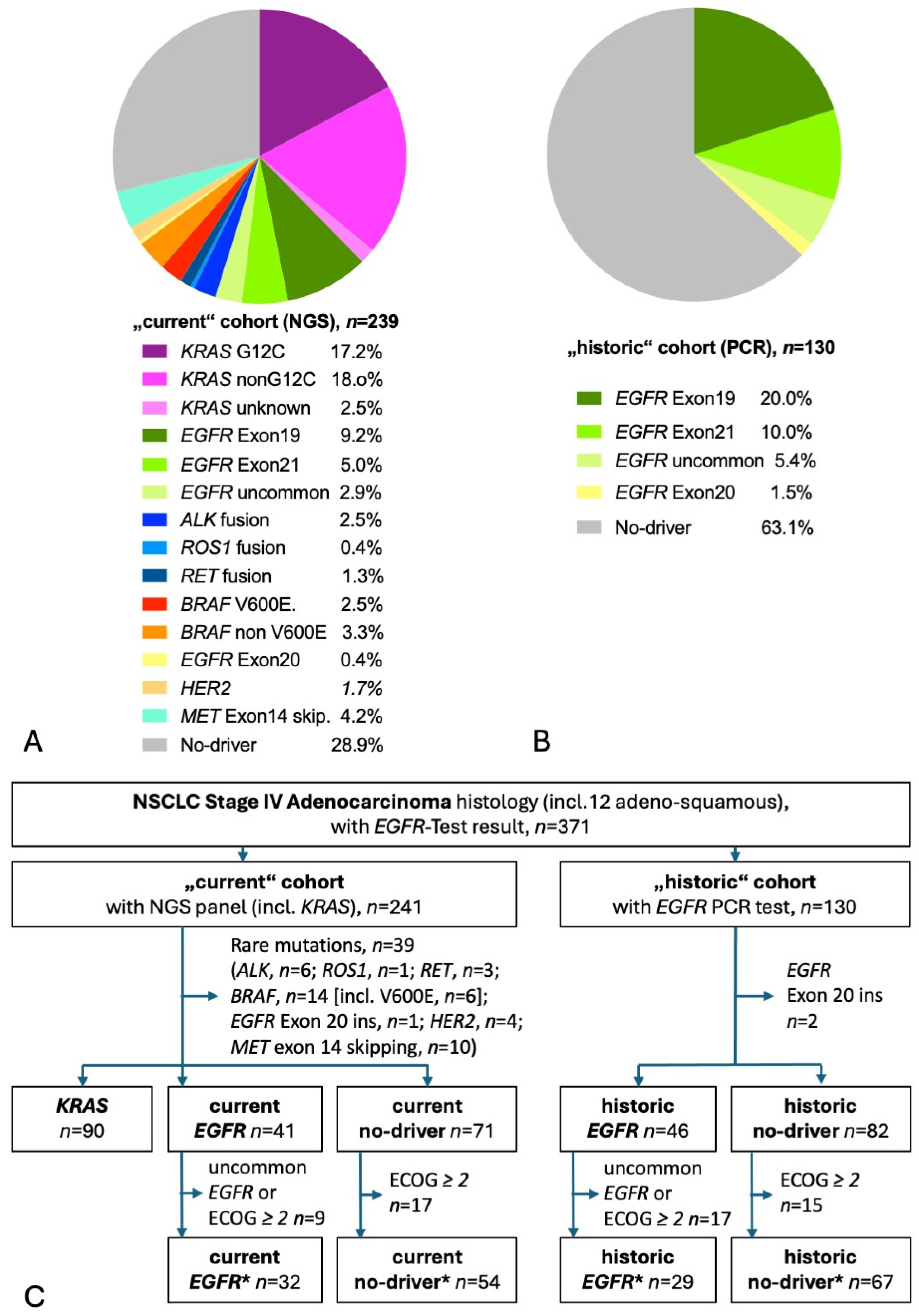
A total of 367 patients had molecular test results available, of those, 238 had used NGS (“current” cohort), and 129 had PCR results (“historic” cohort). Figure 1A shows the distribution of detected mutations in the current cohort. KRAS mutations were the most common mutations found in more than a third of tested patients. Almost half of KRAS patients had the targetable G12C mutation. The second most common were EGFR mutations, found in 17%. Rare mutations were found in every sixth patient and no-driver in almost 30%. Figure 1B shows the distribution of detected mutations in the historic cohort, with an EGFR mutation in exon 19, 21, or 18 detected in one-third of tested patients. Taking both current and historic EGFR patients together, 55% harbored the prognostically beneficial del exon 19 mutation, 29% showed the EGFR exon 21 L858R mutation, and 16% had an uncommon mutation [14,16,18]. The CONSORT diagram shows the selection process of patients for this analysis (Figure 1C) [28]. A total of 199 current patients had adenocarcinoma stage IV with either KRAS mutation (n = 90), EGFR mutation (n = 41), or no known driver (n = 68). A total of 127 historic patients had adenocarcinoma stage IV with either EGFR mutation (n = 46) or no known driver (n = 81). Table 1 gives baseline characteristics and survival. At the time of the database lock, there were 132 deaths (66%), with a median follow-up of living patients of 33.3 months in the current cohort and 117 deaths (92%) with a median follow-up of 87.8 months in the historic cohort. In the current cohort, one-quarter of the living patients had a follow-up of more than 5 years. In comparison with a general NSCLC population, the patients of both the current and historic cohorts had a high proportion of female patients and of never-smokers. As expected in a population enriched in women and non-smokers, the proportion of TTF-1-positive adenocarcinoma was high [29]. In line with the fourfold higher likelihood of an EGFR mutation in TTF1-positive adenocarcinoma, almost every EGFR mutation-positive histology was TTF1-positive adenocarcinoma [30]. A PD-L1 TPS was available for 86% of the current cohort and for 37% of the historic cohort. In line with previous reports, PD-L1 TPS was significantly higher in KRAS patients and no-driver patients compared to EGFR patients [31,32,33,34]. The most common treatment of EGFR patients was an EGFR TKI (100% and 91% in the current and historic cohort, respectively). Of note, only 22% of historic EGFR patients received a third-generation EGFR TKI (osimertinib) as opposed to 88% of current EGFR patients. The most common treatment of KRAS patients and no-driver patients was a CPI (88% and 76%, respectively). Since the historic no-driver patients were diagnosed at a time around 2014, when first-line CPIs were not approved, the historic cohort had little and, if any, later-line CPI treatment.
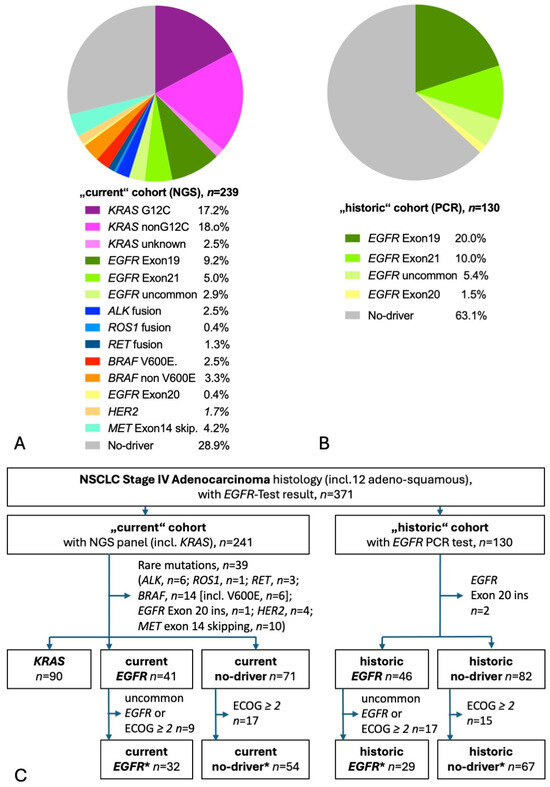
Figure 1.
Detected mutations and patient flow. (A) Pie diagram of patients with NGS molecular test results. Of the 238 NGS test results, 192 had used tissue only, 10 had NGS on liquid biopsy (blood) only, and 36 had NGS both on tissue and liquid biopsy. (B) Pie diagram of patients with EGFR-PCR test results. (C) CONSORT diagram. The “star” (*) cohorts contain only patients fulfilling the major inclusion criteria of the respective phase III studies KEYNOTE-189 and FLAURA.

Table 1.
Baseline characteristics, treatment, and follow-up of the current and historic cohorts.
The historic EGFR and no-driver cohorts were diagnosed at a similar time (median date of diagnosis 2014) and were diagnosed about 7 years earlier than the respective current cohorts (median date of diagnosis 2021). The current cohorts had a higher use of PET-CT for staging and a slightly higher proportion of stage IVA patients. Otherwise, the baseline characteristics were well-balanced. These considerations hold true for the no-mutation* cohorts and the EGFR* cohorts as well (Table S1). A high proportion of the current no-mutation* cohort received first-line CPIs (64%, most commonly pembrolizumab [60%]), whereas only a minority of the historic no-mutation* cohort received first-line CPIs (5%). Thus, the current and historic no-mutation* cohorts match with the arms of the KEYNOTE-189 trial [23]. All EGFR* patients received first-line EGFR TKI. For the current EGFR* cohort, this was mainly osimertinib (94%), and for the historic EGFR* cohort, this was mainly a first-generation EGFR TKI (79%). Thus, the current and historic EGFR* cohorts match well with the arms of the FLAURA trial [14].
3.2. Analysis of Overall Survival
3.2.1. Gene-Wise Analysis of Overall Survival
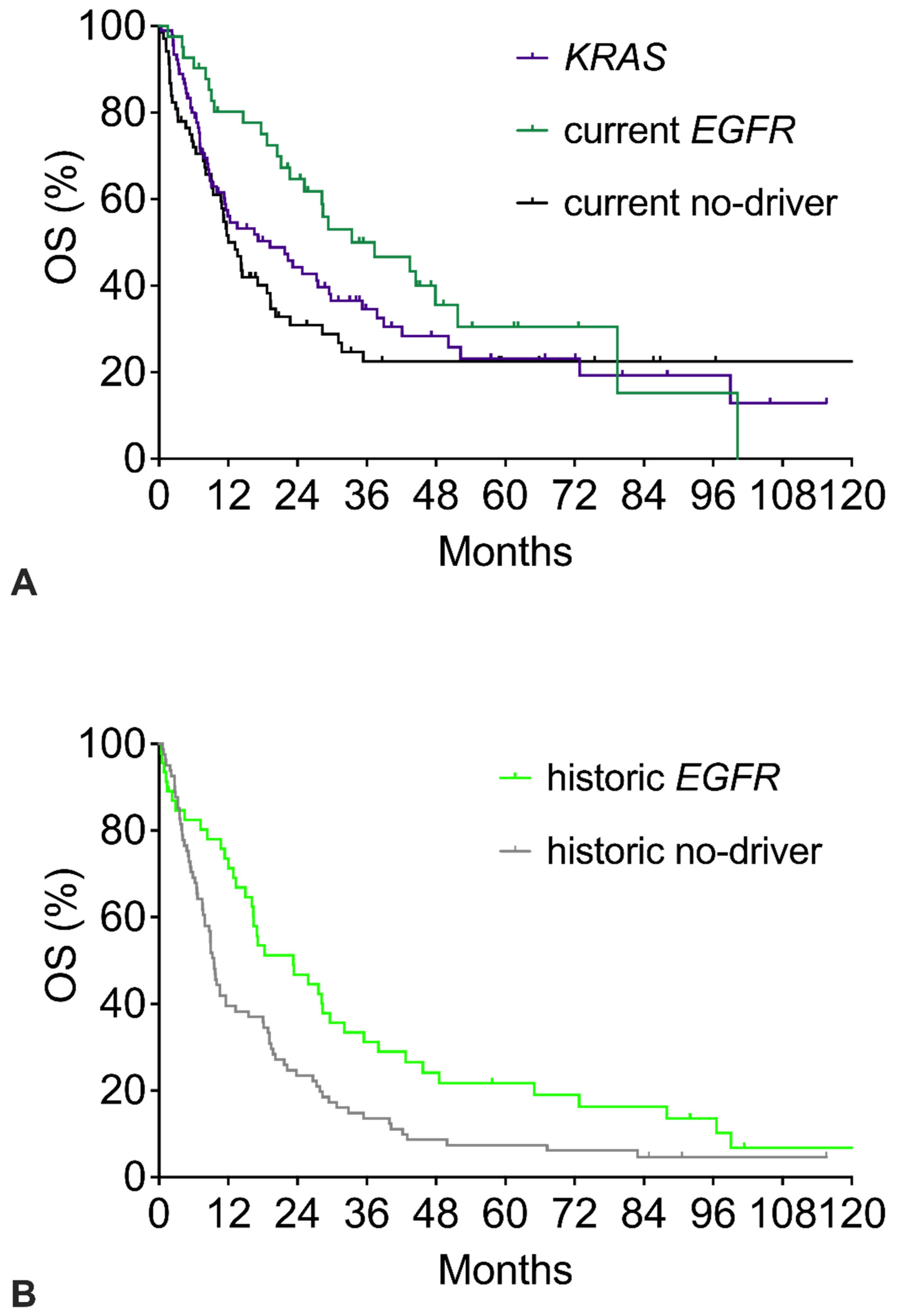
Figure 2 shows the survival of the three current cohorts (Figure 2A) and the two historic cohorts (Figure 2B). Compared to the respective no-driver cohorts, both the current and the historic EGFR cohorts had significantly better OS with similar hazard ratios around 0.59 (Table 2). However, beyond 6 years, the tail of the current EGFR Kaplan–Meier survival curve shows a late further decline, whereas the current no-driver curve remains stable at a plateau of 22%. In the historic cohorts, the tail of the current EGFR survival curve again shows a late further decline. The historic no-driver curve continuously declines to about 5% and does not show a plateau. The KRAS curve shows a course like the current no-driver cohort with a plateau of 20% long-term survivors (Figure 2A). OS showed a minor trend in favor of KRAS patients (HR 0.841 for KRAS vs. no-driver).
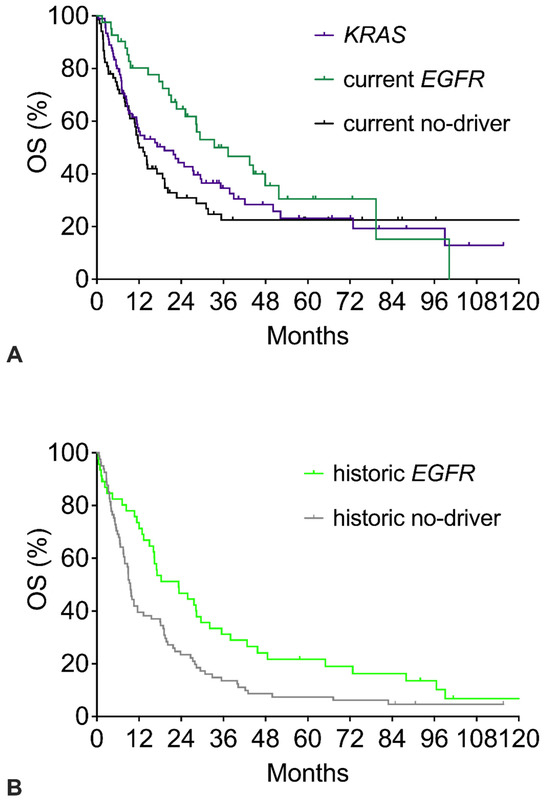
Figure 2.
Overall survival (OS) of patients with specific mutations: EGFR mutation, KRAS mutation, or no-driver patients. (A) OS of current no-driver patients, current EGFR patients, and KRAS patients. (B) OS of historic no-driver patients and historic EGFR patients. The corresponding numerical values and statistics are given in Table 2.

Table 2.
Summary of survival data in subgroups.
3.2.2. Trends in Overall Survival
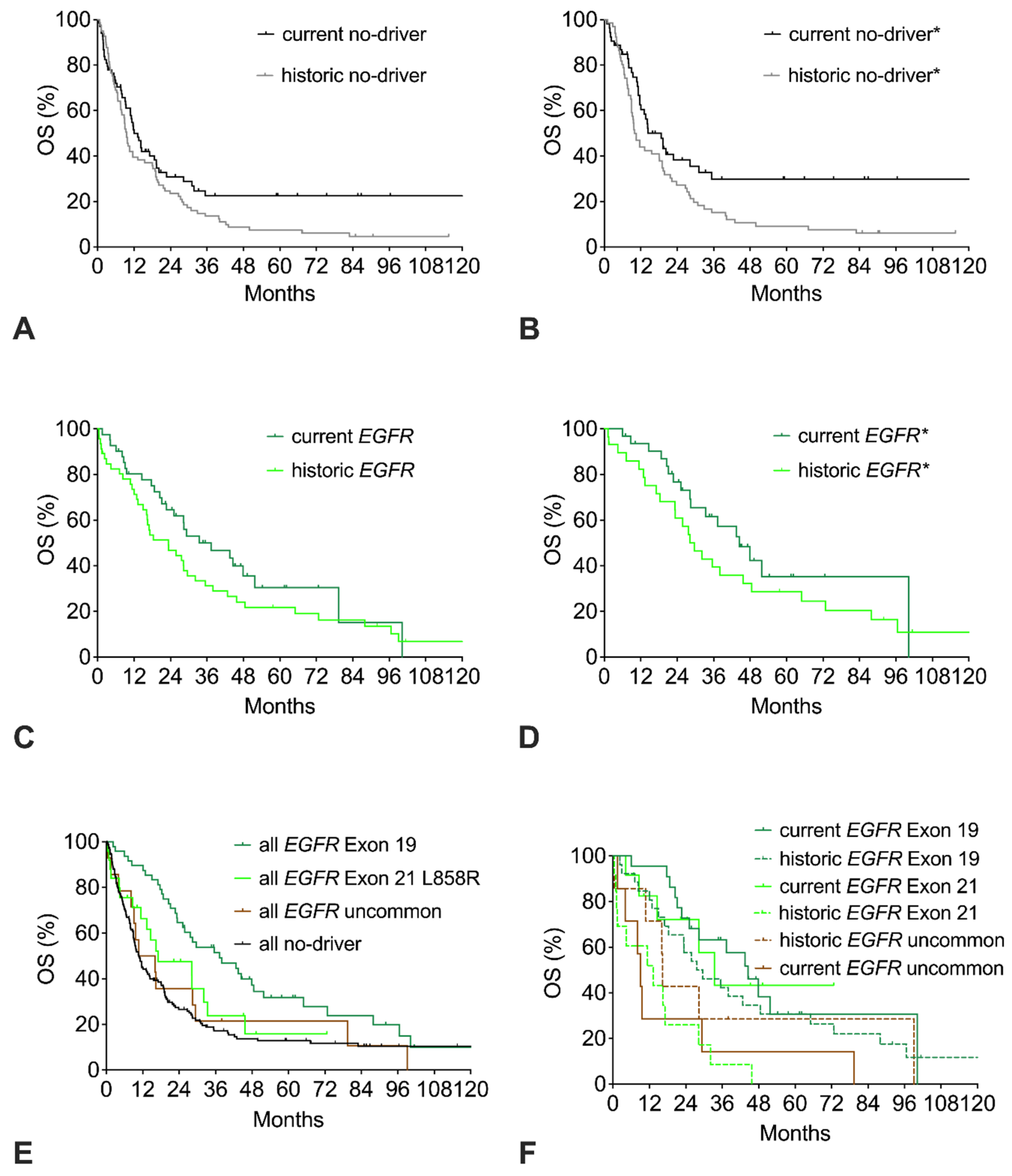
The survival curves of the current and the historic no-driver cohorts show a similar early decline with only 40% of patients alive at 18 months. However, beyond 18 months, the curves clearly separate, resulting in a strong trend to improved OS of the current cohort with a HR of 0.71 (p = 0.06; Figure 3A, Table 2). Restricting the analysis to good-performance patients does not lead to a relevant change in the historic cohort but results in an improvement of the current no-driver* cohort, with an elevation of the long-term plateau from 25% to 31% resulting in significantly improved OS with a HR of 0.60 (Figure 3B).
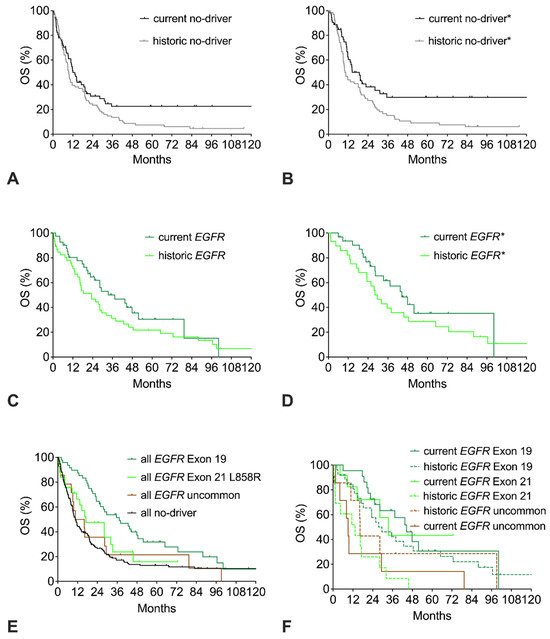
Figure 3.
Trends in overall survival (OS) of no-driver patients, EGFR patients, or EGFR mutation subtypes. (A) OS of current or historic no-driver patients. (B) OS of current or historic no-driver* patients (includes only ECOG 0-1 patients). (C) OS of current or historic EGFR patients. (D) OS of current or historic EGFR* patients (includes only patients with common EGFR mutations and ECOG 0-1). (E) OS of all EGFR patients with del Exon 19 mutation, Exon 21 L858R mutation, uncommon EGFR mutation, or no-driver patients (includes both current and historic cohorts). (F) OS of current and historic EGFR patients with del Exon 19 mutation, Exon 21 L858R mutation, or uncommon EGFR mutation.
3.2.3. Overall Survival of EGFR-Mutation Subtypes
Compared to the historic EGFR cohort, the current EGFR cohort has a trend to improved OS (Figure 3C, Table 2). Restricting the analysis of the EGFR cohorts to patients with common mutations and good performance score (ECOG 0-1) results in about six months longer median OS with a similar trend to improved OS in favor of the current cohort (EGFR* cohorts, Figure 3D). With respect to the specific mutation subtype, the EGFR del exon 19 cohort had significantly longer OS than the EGFR exon 21 L858R cohort or the uncommon EGFR cohort (Figure 3E). Compared to the no-driver cohort, only the EGFR exon 19 cohort had significantly longer OS. The EGFR exon 21 L858R cohort had a trend to improved survival, whereas the uncommon EGFR cohort had similar OS compared to the no-driver cohort. Both the current EGFR del exon 19 cohort and the current EGFR exon 21 cohort had improved survival compared to the respective historic cohorts (Figure 3F). In contrast, no improvement was seen for the uncommon EGFR cohorts.
3.2.4. Overall Survival of KRAS-Mutation Subtypes
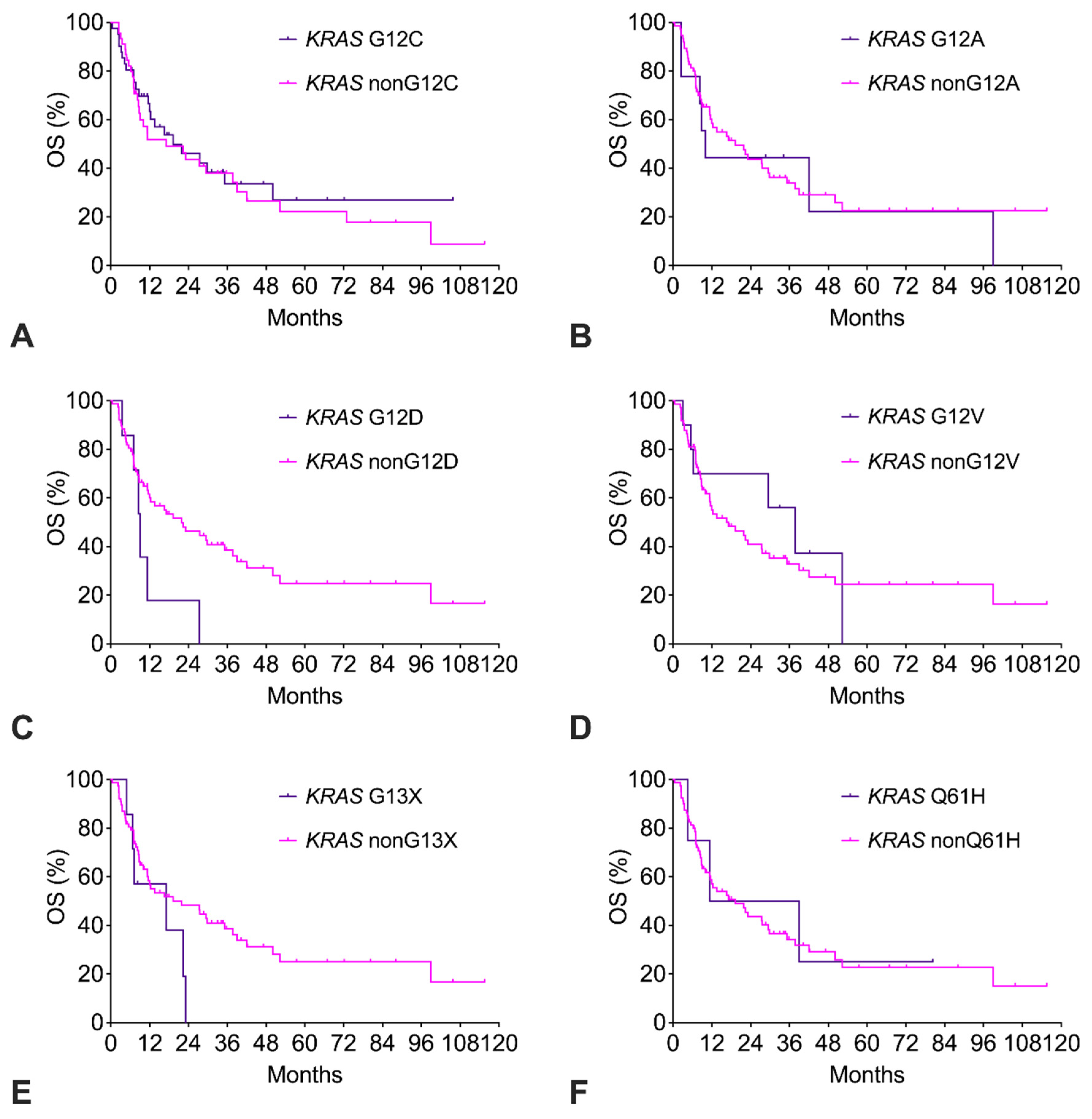
The KRAS G12C cohort had similar OS compared to the KRAS nonG12C cohort (Figure 4A). Of note, only 14% of KRAS G12C patients had received a G12C-targeted therapy (sotorasib, adagrasib). The exploratory survival analysis of the other KRAS subtypes with n ≥ 4 is shown in Figure 4B–F. For KRAS G12A, G12V, and Q61H, OS was like the remaining KRAS subtypes. In contrast, for KRAS G12D and KRAS G13X, there was a strong trend to impaired OS with a more than twofold increased risk of death (Table 2).
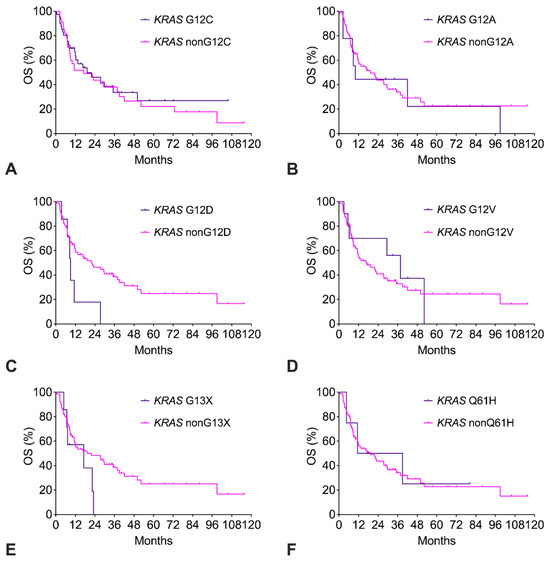
Figure 4.
(A–F). Overall survival (OS) of patients with KRAS mutation subtypes.
Subtypes with n ≥ 4 were analyzed. The corresponding numerical values and statistics are given in Table S2.
4. Discussion
The high proportion of females and never-smokers as well as the low PD-L1 TPS in no-driver patients implies a selection by the treating physician towards testing preferentially patients with a high likelihood of a driver mutation. This accounts for the high proportion of patients with a targetable driver mutation in our cohort exceeding the proportion of 50% to 66% generally reported for Western adenocarcinoma populations [35,36,37,38,39,40]. The distribution of detected mutations in our cohort is in line with these reports.
Compared to the respective historic cohorts, both the current EGFR cohort and the current no-driver cohort show clinically relevant improvements in OS with a different pattern of change in the Kaplan–Meier curves. The current EGFR cohort has a marked improvement in median OS with little change in long-term survival, whereas the no-driver cohort experiences little change in median OS but a marked improvement in long-term survival. Since these changes in OS occurred without a change in the lung cancer center setting, they imply an effect of different treatment changes on OS.
More than three-quarters of current no-driver patients received immuno-oncological therapy with CPIs, most commonly first line. In the historic cohort, only 5% received first-line CPIs, and 70% did not receive any CPI. The no-driver* cohorts comprised patients fulfilling the main inclusion criteria of the KEYNOTE-189 phase III trial comparing the pembrolizumab plus chemotherapy with chemotherapy alone [23]. OS of the current no-driver* cohort with a tail of 30% long-term survivors is comparable with OS of the KEYNOTE-189 pembrolizumab arm, whereas OS of the historic no-driver* resembles OS of the chemotherapy arm with no long-term survivors (Supplemental Table S3A) [41]. Taken together, the improved OS of current no-driver patients is likely to reflect the benefit of immuno-oncological therapy with CPIs [42]. Conversely, the lack of long-term survivors despite CPI therapy in every fourth current EGFR-mutation-positive patient is in line with the literature that these patients do not derive a long-term benefit from immuno-oncological therapy with CPIs [43].
The EGFR* cohorts comprised patients fulfilling the main inclusion criteria of the FLAURA phase III trial comparing the third-generation EGFR TKI osimertinib with the first-generation EGFR TKIs erlotinib or gefitinib (“comparator”) [14,44,45]. Like the FLAURA-verum patients, the current EGFR* patients mainly received first-line osimertinib, whereas most historic EGFR* patients received a first-line, first-generation EGFR TKI (Supplemental Table S3B). OS of the current EGFR* cohort is comparable with OS of the FLAURA osimertinib arm, whereas OS of the historic EGFR* resembles OS of the comparator arm. Our data suggest that the improvement in OS of EGFR-mutant patients is due to the switch of treatment from first-generation EGFR TKIs to third-generation EGFR TKI osimertinib following publication of the FLAURA trial. In contrast to a recent French and British real-world analysis, we find that real-world OS is at least comparable to OS in the FLAURA trial [46,47].
With respect to EGFR-mutation subtypes, our data provide another confirmation of the prognostically beneficial effect of the EGFR del Exon 19 mutation compared to other EGFR mutations in a real-world population with regular access to EGFR-TKI treatment [14,16,18]. The current cohorts of both common EGFR-mutation subtypes had improved OS compared to the respective historic cohorts. Since in both common EGFR-mutation subtypes, the mainstay of treatment was a first-generation EGFR TKI in the historic cohorts and changed to osimertinib in the current cohorts, our data suggest that both EGFR del exon 19 patients and EGFR exon 21 L858R patients benefit from osimertinib in terms of OS. In contrast, the FLAURA trial showed an OS benefit only for EGFR del exon 19 patients but not for EGFR exon 21 L858R patients; however, both EGFR subtypes had a benefit in progression-free survival [14,44]. A possible explanation may be that the improved tolerability of osimertinib compared to first-generation EGFR-TKIs leads to better compliance in the real-world setting.
In contrast to most trials on EGFR-TKIs, our EGFR cohort includes 15% of patients with an uncommon EGFR mutation. In line with the literature, these patients had worse OS compared to the common EGFR exon 19 and exon 21 mutations [15,48,49,50,51]. No improvement in survival was found in the uncommon EGFR cohorts. No change in treatment was observed with first- and second-generation EGFR TKIs remaining the mainstay of treatment for patients with uncommon EGFR mutations. This highlights the need for further research to improve the outcome in patients with uncommon EGFR mutations, which were found in about 3% of our population, slightly exceeding the frequency of ALK fusions with many specific targeted treatments available. Taken together, our data on all EGFR cohorts emphasize the need for the development of treatments focusing on long-term tumor control, e.g., by immune-combination strategies, which might make EGFR-tumor cells more “visible” to the immune system.
In line with previous registry reports, our data show high levels of PD-L1 expression in the KRAS cohort [34]. The survival curve of our KRAS cohort resembles that of the no-driver patients. In line with another report of a recent KRAS cohort with a documented high proportion of treatment with CPIs [52], our data do not support the previously reported negative prognostic effect of KRAS on OS [7,8,9]. Since our KRAS cohort has received a high rate of CPI treatment and since the survival curve shows a relevant tail of long-term survivors similar to the phase III CPI trials [41,53,54], our data suggest that patients with KRAS mutations generally benefit from CPI treatment. However, in line with our findings and a recent real-world report [55], KRAS G12D patients did not benefit from CPIs in the POSEIDON trial [56]. Our data suggest that KRAS G13X patients may also not respond to CPIs, but there are no corresponding reports in the literature. Taken together, our data on the KRAS cohort emphasize the need for further development of both KRAS G12C inhibitors and pan-RAS inhibitors into first-line therapy, ideally combined with first-line immunotherapy to achieve better medium OS driven by the KRAS-targeted treatment and better long-term OS driven by immunotherapy.
The limitations of our study include the intrinsic heterogeneity of patients, limited patient numbers in subgroups, and the variable follow-up period. Strengths of our study include a mature dataset of a current lung cancer population with homogeneous detailed clinical characterization, staging, and treatment in an expert lung cancer referral center, with the hard endpoint of OS and survival data beyond 5 years of follow-up. Our real-world data offer the opportunity to compare different patient populations in the same healthcare setting, which would require cross-trial comparisons of randomized clinical trials.
5. Conclusions
Our real-world study shows that OS in no-mutation patients and EGFR patients with common mutations improved to a similar extent as in the respective phase III clinical trials, likely due to the use of CPIs or osimertinib, respectively. Except for G12D and possibly G13X patients, KRAS patients benefit from CPIs as well.
Supplementary Materials
The following Supporting Information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers17071237/s1, Table S1: “Star” (*) Cohorts: Baseline characteristics, treatment, and follow-up of the current and historic cohorts*; Table S2: KRAS-Subtypes: Baseline characteristics, treatment, and follow-up of KRAS-subtype cohorts. Table S3: Comparison with Phase III Trial Results.
Author Contributions
Author contributions: M.F.: Conceptualization, methodology, recruitment and treatment of patients, validation, formal analysis, writing—original draft, visualization, supervision, project administration. S.F.: Recruitment and treatment of patients, review and editing. B.S.: Recruitment and treatment of patients, review and editing. H.S.: Radiological staging, review and editing. J.S.: Pathology analysis, writing—review and editing. C.L.: Conceptualization, methodology, validation, formal analysis, writing, visualization, supervision. P.C.: Conceptualization, methodology, validation, formal analysis, writing, visualization, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
Part of this work was funded by the German Cancer Consortium (DKTK).
Institutional Review Board Statement
Ethics approval and consent to participate: The study was performed in accordance with the International Conference on Harmonization Guidelines on Good Clinical Practice and the Declaration of Helsinki. The study was approved by the ethics committee of Landesärztekammer Baden-Württemberg (F-2017-004, F-2019-092). The study is registered on clinicaltrials.gov (NCT04926584).
Informed Consent Statement
All patients provided written informed consent before enrollment.
Data Availability Statement
The original data will be made available as a data paper.
Acknowledgments
The authors acknowledge A. Landmesser, K. Hubertus, and B. Blaich for meticulous documentation of the patient data, and A. Grammatikou for superb secretarial work during the study.
Conflicts of Interest
The authors declare the following competing interests: M. Faehling is prin-cipal investigator in phase 1b—3 clinical trials by AstraZeneca, AMGEN, BMS, MSD, and Roche, for which payments were made to his institution. He has received payment for advisory boards and speaker’s honoraria from AstraZeneca, AMGEN, BMS, MSD, and Roche. The other authors report no potential conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the title and the keywords. This change does not affect the scientific content of the article.
Abbreviations
| CPI | checkpoint inhibitor |
| MDT | multidisciplinary tumor board |
| NSCLC | non-small cell lung cancer |
| NGS | next-generation sequencing |
| OS | overall survival |
| PCR | polymerase-chain reaction |
| TKI | tyrosine-kinase inhibitor |
| TPS | tumor-proportion score |
References
- World Cancer Research Fund. International Lung Cancer Statistics. 2020. Available online: https://www.wcrf.org/cancer-trends/lung-cancer-statistics/ (accessed on 6 April 2024).
- WHO. Lung Cancer: Key Facts. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/lung-cancer#:~:text=globocan%202020%20estimates%20of%20cancer,deaths%20(18%25)%20in%202020 (accessed on 6 April 2024).
- Cancer.Net. Lung Cancer—Non-Small Cell. Available online: https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/statistics (accessed on 6 April 2024).
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef] [PubMed]
- Vachani, A.; Sequist, L.V.; Spira, A. AJRCCM: 100-Year Anniversary. The Shifting Landscape for Lung Cancer: Past, Present, and Future. Am. J. Respir. Crit. Care Med. 2017, 195, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Tan, D.S.W. Targeted Therapies for Lung Cancer Patients With Oncogenic Driver Molecular Alterations. J. Clin. Oncol. 2022, 40, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Sima, C.S.; Chaft, J.; Paik, P.K.; Pao, W.; Kris, M.G.; Ladanyi, M.; Riely, G.J. Association of KRAS and EGFR Mutations with Survival in Patients with Advanced Lung Adenocarcinomas. Cancer 2013, 119, 356–362. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Colombino, M.; Sini, M.C.; Manca, A.; Casula, M.; Palomba, G.; Pisano, M.; Doneddu, V.; Zinellu, A.; Santeufemia, D.; et al. Global Prognostic Impact of Driver Genetic Alterations in Patients with Lung Adenocarcinoma: A Real-Life Study. BMC Pulm. Med. 2022, 22, 32. [Google Scholar] [CrossRef]
- Goulding, R.E.; Chenoweth, M.; Carter, G.C.; Boye, M.E.; Sheffield, K.M.; John, W.J.; Leusch, M.S.; Muehlenbein, C.E.; Li, L.; Jen, M.-H.; et al. KRAS Mutation as a Prognostic Factor and Predictive Factor in Advanced/Metastatic Non-Small Cell Lung Cancer: A Systematic Literature Review and Meta-Analysis. Cancer Treat. Res. Commun. 2020, 24, 100200. [Google Scholar] [CrossRef]
- Mellema, W.W.; Dingemans, A.-M.C.; Thunnissen, E.; Snijders, P.J.F.; Derks, J.; Heideman, D.A.M.; Van Suylen, R.; Smit, E.F. KRAS Mutations in Advanced Nonsquamous Non-Small-Cell Lung Cancer Patients Treated with First-Line Platinum-Based Chemotherapy Have No Predictive Value. J. Thorac. Oncol. 2013, 8, 1190–1195. [Google Scholar] [CrossRef]
- Brady, A.K.; McNeill, J.D.; Judy, B.; Bauml, J.; Evans, T.L.; Cohen, R.B.; Langer, C.; Vachani, A.; Aggarwal, C. Survival Outcome According to KRAS Mutation Status in Newly Diagnosed Patients with Stage IV Non-Small Cell Lung Cancer Treated with Platinum Doublet Chemotherapy. Oncotarget 2015, 6, 30287–30294. [Google Scholar] [CrossRef]
- Lee, C.K.; Man, J.; Lord, S.; Links, M.; Gebski, V.; Mok, T.; Yang, J.C.-H. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J. Thorac. Oncol. 2017, 12, 403–407. [Google Scholar] [CrossRef]
- Lee, C.K.; Man, J.; Lord, S.; Cooper, W.; Links, M.; Gebski, V.; Herbst, R.S.; Gralla, R.J.; Mok, T.; Yang, J.C.-H. Clinical and Molecular Characteristics Associated With Survival Among Patients Treated With Checkpoint Inhibitors for Advanced Non-Small Cell Lung Carcinoma: A Systematic Review and Meta-Analysis. JAMA Oncol. 2018, 4, 210–216. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.-H.; Sequist, L.V.; Geater, S.L.; Tsai, C.-M.; Mok, T.S.K.; Schuler, M.; Yamamoto, N.; Yu, C.-J.; Ou, S.-H.I.; Zhou, C.; et al. Clinical Activity of Afatinib in Patients with Advanced Non-Small-Cell Lung Cancer Harbouring Uncommon EGFR Mutations: A Combined Post-Hoc Analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015, 16, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Yamanaka, T.; Kato, T.; Ikeda, S.; Horinouchi, H.; Ichihara, E.; Kanazu, M.; Takiguchi, Y.; Tanaka, K.; Goto, Y.; et al. Treatment Rationale and Design of a Phase III Study of Afatinib or Chemotherapy in Patients with Non-Small-Cell Lung Cancer Harboring Sensitizing Uncommon Epidermal Growth Factor Receptor Mutations (ACHILLES/TORG1834). Clin. Lung Cancer 2020, 21, e592–e596. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Tanaka, H.; Misumi, T.; Yoshioka, H.; Kurata, T.; Tokito, T.; Fukuhara, T.; Sato, Y.; Shiraishi, Y.; Kusuhara, S.; et al. LBA66 Afatinib versus Chemotherapy for Treatment-Naïve Non-Small Cell Lung Cancer with a Sensitizing Uncommon Epidermal Growth Factor Receptor Mutation: A Phase III Study (ACHILLES/TORG1834). Ann. Oncol. 2023, 34, S1310–S1311. [Google Scholar] [CrossRef]
- Priantti, J.N.; Fujiwara, Y.; Aquino de Moraes, F.C.; Michelon, I.; Castro, C.; Leighl, N.B.; Cavalcante, L.; Addeo, A.; Bar, J.; Horita, N.; et al. Safety and Efficacy of Osimertinib in Patients With Non-Small-Cell Lung Cancer and Uncommon Tumoral Epidermal Growth Factor Receptor Mutations: A Systematic Review and Single-Arm Meta-Analysis. JCO Precis. Oncol. 2024, 8, e2400331. [Google Scholar] [CrossRef]
- Mazzaschi, G.; Perrone, F.; Minari, R.; Verzè, M.; Azzoni, C.; Bottarelli, L.; Pluchino, M.; Armillotta, M.P.; Ubaldi, A.; Altimari, A.; et al. Therapeutic Outcomes and Clinical Features of Advanced Non-Small Cell Lung Cancer Carrying KRAS Mutations: A Multicenter Real-Life Retrospective Study. Clin. Lung Cancer 2022, 23, e478–e488. [Google Scholar] [CrossRef]
- Holderfield, M.; Lee, B.J.; Jiang, J.; Tomlinson, A.; Seamon, K.J.; Mira, A.; Patrucco, E.; Goodhart, G.; Dilly, J.; Gindin, Y.; et al. Concurrent Inhibition of Oncogenic and Wild-Type RAS-GTP for Cancer Therapy. Nature 2024, 629, 919–926. [Google Scholar] [CrossRef]
- Jiang, J.; Jiang, L.; Maldonato, B.J.; Wang, Y.; Holderfield, M.; Aronchik, I.; Winters, I.P.; Salman, Z.; Blaj, C.; Menard, M.; et al. Translational and Therapeutic Evaluation of RAS-GTP Inhibition by RMC-6236 in RAS-Driven Cancers. Cancer Discov. 2024, 14, 994–1017. [Google Scholar] [CrossRef]
- Brierley, J.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) TNM Classification of Malignant Tumours, 8th ed.; John Wiley & Sons, Inc.: Chichester, UK; Hoboken, NJ, USA, 2017; ISBN 978-1-119-26357-9. [Google Scholar]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- ONKOPEDIA. Lungenkarzinom, Nicht-Kleinzellig (NSCLC) 2025. Available online: https://www.onkopedia.com/de/onkopedia/guidelines/lungenkarzinom-nicht-kleinzellig-nsclc/@@guideline/html/index.html (accessed on 4 April 2025).
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. Oncogene-Addicted Metastatic Non-Small-Cell Lung Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023, 34, 339–357. [Google Scholar] [CrossRef]
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. Non-Oncogene-Addicted Metastatic Non-Small-Cell Lung Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023, 34, 358–376. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A. PAFS consensus group CONSORT 2010 Statement: Extension to Randomised Pilot and Feasibility Trials. Pilot Feasibility Stud. 2016, 2, 64. [Google Scholar] [CrossRef] [PubMed]
- Yatabe, Y.; Mitsudomi, T.; Takahashi, T. TTF-1 Expression in Pulmonary Adenocarcinomas. Am. J. Surg. Pathol. 2002, 26, 767–773. [Google Scholar] [CrossRef]
- Schilsky, J.B.; Ni, A.; Ahn, L.; Datta, S.; Travis, W.D.; Kris, M.G.; Chaft, J.E.; Rekhtman, N.; Hellmann, M.D. Prognostic Impact of TTF-1 Expression in Patients with Stage IV Lung Adenocarcinomas. Lung Cancer 2017, 108, 205–211. [Google Scholar] [CrossRef]
- Kerr, K.M.; Bibeau, F.; Thunnissen, E.; Botling, J.; Ryška, A.; Wolf, J.; Öhrling, K.; Burdon, P.; Malapelle, U.; Büttner, R. The Evolving Landscape of Biomarker Testing for Non-Small Cell Lung Cancer in Europe. Lung Cancer 2021, 154, 161–175. [Google Scholar] [CrossRef]
- Tsunoda, A.; Morikawa, K.; Inoue, T.; Miyazawa, T.; Hoshikawa, M.; Takagi, M.; Mineshita, M. A Prospective Observational Study to Assess PD-L1 Expression in Small Biopsy Samples for Non-Small-Cell Lung Cancer. BMC Cancer 2019, 19, 546. [Google Scholar] [CrossRef]
- Negrao, M.V.; Skoulidis, F.; Montesion, M.; Schulze, K.; Bara, I.; Shen, V.; Xu, H.; Hu, S.; Sui, D.; Elamin, Y.Y.; et al. Oncogene-Specific Differences in Tumor Mutational Burden, PD-L1 Expression, and Outcomes from Immunotherapy in Non-Small Cell Lung Cancer. J. Immunother. Cancer 2021, 9, e002891. [Google Scholar] [CrossRef]
- Thomas, Q.D.; Quantin, X.; Lemercier, P.; Chouaid, C.; Schneider, S.; Filleron, T.; Remon-Masip, J.; Perol, M.; Debieuvre, D.; Audigier-Valette, C.; et al. Clinical Characteristic and Survival Outcomes of Patients with Advanced NSCLC According to KRAS Mutational Status in the French Real-Life ESME Cohort. ESMO Open 2024, 9, 103473. [Google Scholar] [CrossRef]
- Tsao, A.S.; Scagliotti, G.V.; Bunn, P.A.; Carbone, D.P.; Warren, G.W.; Bai, C.; de Koning, H.J.; Yousaf-Khan, A.U.; McWilliams, A.; Tsao, M.S.; et al. Scientific Advances in Lung Cancer 2015. J. Thorac. Oncol. 2016, 11, 613–638. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Suda, K.; Wiens, J.; Bunn, P.A. New and Emerging Targeted Treatments in Advanced Non-Small-Cell Lung Cancer. Lancet 2016, 388, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Kung, H.-J.; Mack, P.C.; Gandara, D.R. Genotyping and Genomic Profiling of Non-Small-Cell Lung Cancer: Implications for Current and Future Therapies. J. Clin. Oncol. 2013, 31, 1039–1049. [Google Scholar] [CrossRef]
- Schwegler, C.; Kaufmann, D.; Pfeiffer, D.; Aebi, S.; Diebold, J.; Gautschi, O. Population-Level Effect of Molecular Testing and Targeted Therapy in Patients with Advanced Pulmonary Adenocarcinoma: A Prospective Cohort Study. Virchows. Arch. 2018, 472, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Jordan, E.J.; Kim, H.R.; Arcila, M.E.; Barron, D.; Chakravarty, D.; Gao, J.; Chang, M.T.; Ni, A.; Kundra, R.; Jonsson, P.; et al. Prospective Comprehensive Molecular Characterization of Lung Adenocarcinomas for Efficient Patient Matching to Approved and Emerging Therapies. Cancer Discov. 2017, 7, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Pakkala, S.; Ramalingam, S.S. Personalized Therapy for Lung Cancer: Striking a Moving Target. JCI Insight 2018, 3, e120858. [Google Scholar] [CrossRef]
- Garassino, M.C.; Gadgeel, S.; Speranza, G.; Felip, E.; Esteban, E.; Dómine, M.; Hochmair, M.J.; Powell, S.F.; Bischoff, H.G.; Peled, N.; et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J. Clin. Oncol. 2023, 41, 1992–1998. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. Immune Checkpoint Targeting in Cancer Therapy: Toward Combination Strategies with Curative Potential. Cell 2015, 161, 205–214. [Google Scholar] [CrossRef]
- Yang, J.C.-H.; Lee, D.H.; Lee, J.-S.; Fan, Y.; de Marinis, F.; Iwama, E.; Inoue, T.; Rodríguez-Cid, J.; Zhang, L.; Yang, C.-T.; et al. Phase III KEYNOTE-789 Study of Pemetrexed and Platinum With or Without.Pembrolizumab for Tyrosine Kinase Inhibitor–Resistant, EGFR-Mutant, Metastatic Nonsquamous Non-Small Cell Lung Cancer. J. Clin. Oncol. 2024, 42, 4029–4039. [Google Scholar] [CrossRef]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR -Mutated Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Yang, J.C.-H.; Lee, C.K.; Kurata, T.; Kim, D.-W.; John, T.; Nogami, N.; Ohe, Y.; Mann, H.; Rukazenkov, Y.; et al. Osimertinib As First-Line Treatment of EGFR Mutation–Positive Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 841–849. [Google Scholar] [CrossRef]
- Thomas, Q.D.; Girard, N.; Bosquet, L.; Cavaillon, S.; Filleron, T.; Eltaief, S.; Chouaid, C.; Lena, H.; Debieuvre, D.; Perol, M.; et al. Optimizing Treatment Strategies for Egfr-Mutated Non-Small-Cell Lung Cancer Treated with Osimertinib: Real-World Outcomes and Insights. Cancers 2024, 16, 3563. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Randulfe, I.; Scanlon, L.A.; Carter, M.; Moliner, L.; Cil, E.; Califano, R.; Summers, Y.; Blackhall, F.; Lindsay, C.R.; Lewis, J.; et al. First-Line Osimertinib Compared to Earlier Generation TKIs in Advanced EGFR-Mutant NSCLC: A Real-World Survival Analysis. Lung Cancer 2025, 200, 108084. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus Standard Chemotherapy as First-Line Treatment for European Patients with Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer (EURTAC): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Karachaliou, N.; Mayo-de las Casas, C.; Queralt, C.; de Aguirre, I.; Melloni, B.; Cardenal, F.; Garcia-Gomez, R.; Massuti, B.; Sánchez, J.M.; Porta, R.; et al. Association of EGFR L858R Mutation in Circulating Free DNA With Survival in the EURTAC Trial. JAMA Oncol. 2015, 1, 149–157. [Google Scholar] [CrossRef]
- Villaruz, L.C.; Wang, X.; Bertino, E.M.; Gu, L.; Antonia, S.J.; Burns, T.F.; Clarke, J.; Crawford, J.; Evans, T.L.; Friedland, D.M.; et al. A Single-Arm, Multicenter, Phase II Trial of Osimertinib in Patients with Epidermal Growth Factor Receptor Exon 18 G719X, Exon 20 S768I, or Exon 21 L861Q Mutations. ESMO Open 2023, 8, 101183. [Google Scholar] [CrossRef]
- Lee, V.H.F.; Tin, V.P.C.; Choy, T.; Lam, K.; Choi, C.; Chung, L.; Tsang, J.W.H.; Ho, P.P.Y.; Leung, D.K.C.; Ma, E.S.K.; et al. Association of Exon 19 and 21 EGFR Mutation Patterns with Treatment Outcome after First-Line Tyrosine Kinase Inhibitor in Metastatic Non-Small-Cell Lung Cancer. J. Thorac. Oncol. 2013, 8, 1148–1155. [Google Scholar] [CrossRef]
- Sebastian, M.; Eberhardt, W.E.E.; Hoffknecht, P.; Metzenmacher, M.; Wehler, T.; Kokowski, K.; Alt, J.; Schütte, W.; Büttner, R.; Heukamp, L.C.; et al. KRAS G12C-Mutated Advanced Non-Small Cell Lung Cancer: A Real-World Cohort from the German Prospective, Observational, Nation-Wide CRISP Registry (AIO-TRK-0315). Lung Cancer 2021, 154, 51–61. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic Non–Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50%. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef]
- Antonia, S.J.; Borghaei, H.; Ramalingam, S.S.; Horn, L.; De Castro Carpeño, J.; Pluzanski, A.; Burgio, M.A.; Garassino, M.; Chow, L.Q.M.; Gettinger, S.; et al. Four-Year Survival with Nivolumab in Patients with Previously Treated Advanced Non-Small-Cell Lung Cancer: A Pooled Analysis. Lancet Oncol. 2019, 20, 1395–1408. [Google Scholar] [CrossRef]
- Shahnam, A.; Davis, A.; Brown, L.J.; Sullivan, I.; Lin, K.; Ng, C.; Yeo, N.; Kong, B.Y.; Khoo, T.; Warburton, L.; et al. Real-World Outcomes of Non-Small Cell Lung Cancer Patients Harbouring KRAS G12C and KRAS G12D Mutations. Lung Cancer 2025, 201, 108421. [Google Scholar] [CrossRef]
- Peters, S.; Cho, B.C.; Luft, A.V.; Alatorre-Alexander, J.; Geater, S.L.; Laktionov, K.; Trukhin, D.; Kim, S.-W.; Ursol, G.M.; Hussein, M.; et al. Durvalumab With or Without Tremelimumab in Combination With Chemotherapy in First-Line Metastatic NSCLC: Five-Year Overall Survival Outcomes From the Phase 3 POSEIDON Trial. J. Thorac. Oncol. 2025, 20, 76–93. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).