Oral Cancer and Sleep Disturbances: A Narrative Review on Exploring the Bidirectional Relationship
Simple Summary
Abstract
1. Introduction
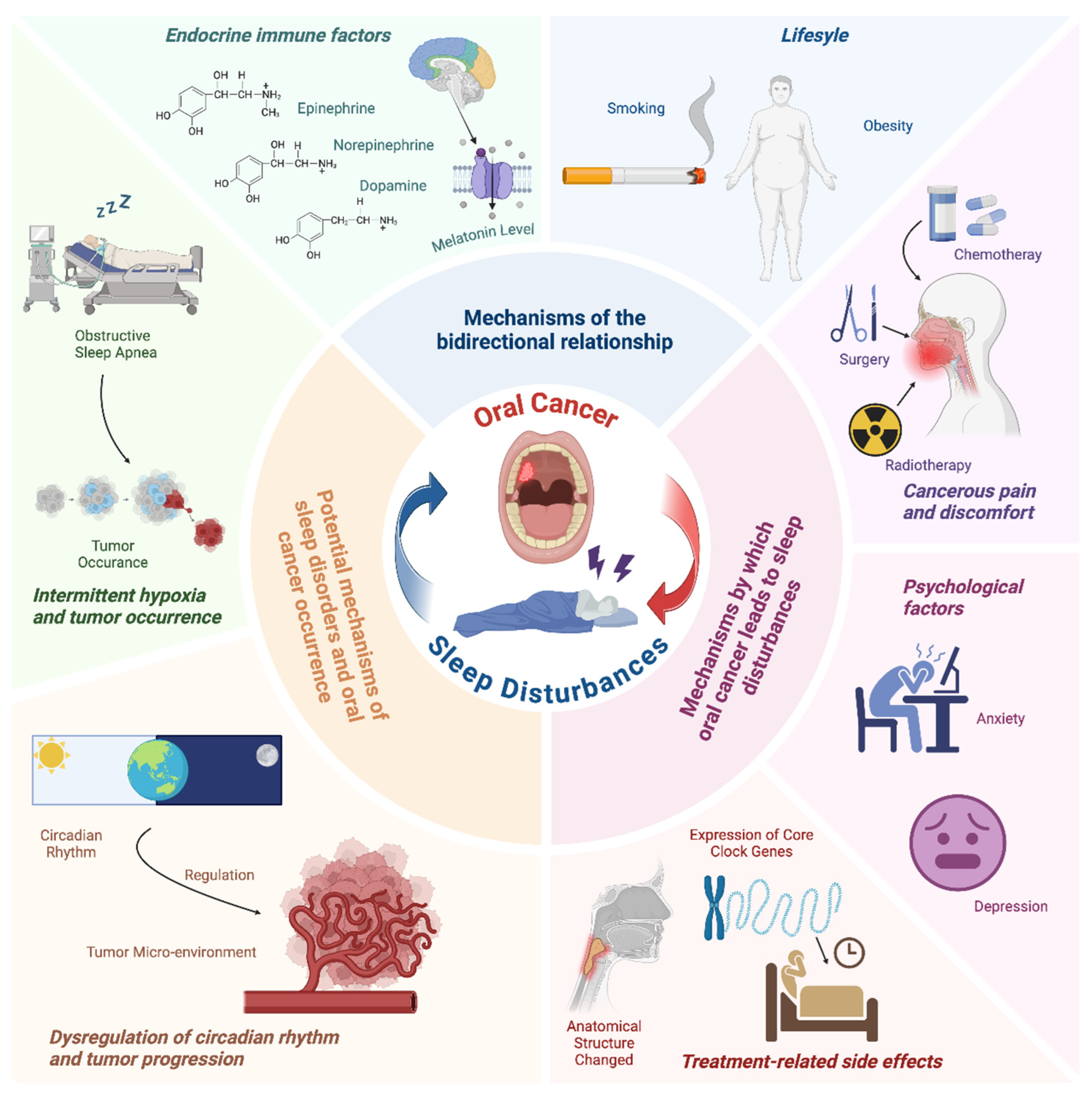
2. Mechanisms by Which Oral Cancer Leads to Sleep Disturbances
2.1. Tumor Size and Location
2.2. Cancerous Pain and Discomfort
2.3. Psychological Factors
2.4. Treatment-Related Side Effects
3. Potential Mechanisms Underlying the Correlation Between Sleep Disorders and Oral Cancer Occurrence
3.1. Intermittent Hypoxia and Tumor Occurrence
3.2. Dysregulation of Circadian Rhythm and Tumor Progression
4. Other Shared Underlying Mechanisms in This Bidirectional Relationship
4.1. Endocrine Immune Factors
4.2. Lifestyle
5. Intervention and Treatment Measures
5.1. Lifestyle Management and Psychobehavioral Therapy
5.2. Pain Management and OSA Treatment
6. Conclusions and Future Research Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Montero, P.H.; Patel, S.G. Cancer of the Oral Cavity. Surg. Oncol. Clin. N. Am. 2015, 24, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.E.; Ilaghi, M.; Mirzazadeh, Y.; Mosavi Jarrahi, A.; Nejadghaderi, S.A. Corrigendum: Global epidemiology and socioeconomic correlates of hypopharyngeal cancer in 2020 and its projection to 2040: Findings from GLOBOCAN 2020. Front. Oncol. 2024, 14, 1520064. [Google Scholar] [CrossRef]
- Gasparro, R.; Calabria, E.; Coppola, N.; Marenzi, G.; Sammartino, G.; Aria, M.; Mignogna, M.D.; Adamo, D. Sleep Disorders and Psychological Profile in Oral Cancer Survivors: A Case-Control Clinical Study. Cancers 2021, 13, 1855. [Google Scholar] [CrossRef]
- Santoso, A.M.M.; Jansen, F.; Lissenberg-Witte, B.I.; Baatenburg de Jong, R.J.; Langendijk, J.A.; Leemans, C.R.; Smit, J.H.; Takes, R.P.; Terhaard, C.H.J.; van Straten, A.; et al. Poor sleep quality among newly diagnosed head and neck cancer patients: Prevalence and associated factors. Support. Care Cancer 2021, 29, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Seifen, C.; Huppertz, T.; Matthias, C.; Gouveris, H. Obstructive Sleep Apnea in Patients with Head and Neck Cancer-More than Just a Comorbidity? Medicina 2021, 57, 1174. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.-H.; Chang, C.-C.; Lu, Y.-C.; Lin, C.-Y.; Lai, W.-S. Impact of free flap reconstruction on obstructive sleep apnea in patients with oral and oropharyngeal cancer. Asia-Pac. J. Oncol. Nurs. 2022, 9, 100136. [Google Scholar] [CrossRef]
- Irwin, M.R. Why Sleep Is Important for Health: A Psychoneuroimmunology Perspective. Annu. Rev. Psychol. 2015, 66, 143–172. [Google Scholar] [CrossRef]
- von Ruesten, A.; Weikert, C.; Fietze, I.; Boeing, H. Association of Sleep Duration with Chronic Diseases in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. PLoS ONE 2012, 7, e30972. [Google Scholar] [CrossRef]
- Iovoli, A.J.; Smith, K.; Yu, H.; Kluczynski, M.A.; Jungquist, C.R.; Ray, A.D.; Farrugia, M.K.; Gu, F.; Singh, A.K. Association of Insomnia and Obstructive Sleep Apnea with Worse Oral Mucositis and Quality of Life in Head and Neck Cancer Patients Undergoing Radiation Therapy. Cancers 2024, 16, 1335. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Zheng, X.; Xia, Y.; Fu, Y.; Li, X.; Qian, Y.; Zou, J.; Zhao, A.; Guan, J.; et al. Pediatric Obstructive Sleep Apnea is Associated With Changes in the Oral Microbiome and Urinary Metabolomics Profile: A Pilot Study. J. Clin. Sleep Med. 2018, 14, 1559–1567. [Google Scholar] [CrossRef]
- Murata, M. Inflammation and cancer. Environ. Health Prev. Med. 2018, 23, 50. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, Z.; Sun, L.; Fan, S.; Huang, Z.; Zhang, D.; Yang, Z.; Li, J.; Chen, W. Akt/Ezrin Tyr353/NF-κB pathway regulates EGF-induced EMT and metastasis in tongue squamous cell carcinoma. Br. J. Cancer 2014, 110, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Hsin, C.-H.; Chou, Y.-E.; Yang, S.-F.; Su, S.-C.; Chuang, Y.-T.; Lin, S.-H.; Lin, C.-W. MMP-11 promoted the oral cancer migration and Fak/Src activation. Oncotarget 2017, 8, 32783–32793. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Liu, J.-F.; Tsai, H.-C.; Tzeng, H.-E.; Hsieh, T.-H.; Wang, M.; Lin, Y.-F.; Lu, C.-C.; Lien, M.-Y.; Tang, C.-H. Interleukin-11/gp130 upregulates MMP-13 expression and cell migration in OSCC by activating PI3K/Akt and AP-1 signaling. J. Cell. Physiol. 2022, 237, 4551–4562. [Google Scholar] [CrossRef]
- Li, W.; Zeng, Q.; Wang, B.; Lv, C.; He, H.; Yang, X.; Cheng, B.; Tao, X. Oxidative stress promotes oral carcinogenesis via Thbs1-mediated M1-like tumor-associated macrophages polarization. Redox Biol. 2024, 76, 103335. [Google Scholar] [CrossRef] [PubMed]
- Santoso, A.M.M.; Jansen, F.; Lissenberg-Witte, B.I.; Baatenburg de Jong, R.J.; Langendijk, J.A.; Leemans, C.R.; Smit, J.H.; Takes, R.P.; Terhaard, C.H.J.; van Straten, A.; et al. Sleep quality trajectories from head and neck cancer diagnosis to six months after treatment. Oral Oncol. 2021, 115, 105211. [Google Scholar] [CrossRef]
- Friedman, M.; Landsberg, R.; Pryor, S.; Syed, Z.; Ibrahim, H.; Caldarelli, D.D. The occurrence of sleep-disordered breathing among patients with head and neck cancer. Laryngoscope 2001, 111 Pt 1, 1917–1919. [Google Scholar] [CrossRef]
- Bagan, J.; Sarrion, G.; Jimenez, Y. Oral cancer: Clinical features. Oral Oncol. 2010, 46, 414–417. [Google Scholar] [CrossRef]
- Payne, R.J.; Hier, M.P.; Kost, K.M.; Black, M.J.; Zeitouni, A.G.; Frenkiel, S.; Naor, N.; Kimoff, R.J. High prevalence of obstructive sleep apnea among patients with head and neck cancer. J. Otolaryngol. 2005, 34, 304–311. [Google Scholar] [CrossRef]
- Huppertz, T.; Horstmann, V.; Scharnow, C.; Ruckes, C.; Bahr, K.; Matthias, C.; Gouveris, H. OSA in patients with head and neck cancer is associated with cancer size and oncologic outcome. Eur. Arch. Otorhinolaryngol. 2021, 278, 2485–2491. [Google Scholar] [CrossRef]
- Viet, C.T.; Schmidt, B.L. Biologic Mechanisms of Oral Cancer Pain and Implications for Clinical Therapy. J. Dent. Res. 2012, 91, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.L. The Neurobiology of Cancer Pain. Neuroscientist 2014, 20, 546–562. [Google Scholar] [CrossRef] [PubMed]
- Scheff, N.N.; Ye, Y.; Bhattacharya, A.; MacRae, J.; Hickman, D.N.; Sharma, A.K.; Dolan, J.C.; Schmidt, B.L. Tumor necrosis factor alpha secreted from oral squamous cell carcinoma contributes to cancer pain and associated inflammation. Pain 2017, 158, 2396–2409. [Google Scholar] [CrossRef]
- Tu, N.H.; Inoue, K.; Chen, E.; Anderson, B.M.; Sawicki, C.M.; Scheff, N.N.; Tran, H.D.; Kim, D.H.; Alemu, R.G.; Yang, L.; et al. Cathepsin S Evokes PAR2-Dependent Pain in Oral Squamous Cell Carcinoma Patients and Preclinical Mouse Models. Cancers 2021, 13, 4697. [Google Scholar] [CrossRef]
- Grayson, M.; Arris, D.; Wu, P.; Merlo, J.; Ibrahim, T.; Fang-Mei, C.; Valenzuela, V.; Ganatra, S.; Ruparel, S. Oral squamous cell carcinoma-released brain-derived neurotrophic factor contributes to oral cancer pain by peripheral tropomyosin receptor kinase B activation. Pain 2022, 163, 496–507. [Google Scholar] [CrossRef]
- Karaman, S.; Karaman, T.; Dogru, S.; Onder, Y.; Citil, R.; Bulut, Y.E.; Tapar, H.; Sahin, A.; Arici, S.; Kaya, Z.; et al. Prevalence of sleep disturbance in chronic pain. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2475–2481. [Google Scholar]
- Kang, J.-H.; Lee, J.K. Does risk of obstructive sleep apnea have interaction with chronic facial pain? J. Korean Assoc. Oral Maxillofac. Surg. 2022, 48, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Lou, D.I.; Dietrich, M.S.; Deng, J.; Murphy, B.A. Mechanisms of pain and their manifestations in head and neck cancer: Importance of classifying pain subtypes. Head Neck 2021, 43, 3720–3729. [Google Scholar] [CrossRef]
- Terkawi, A.S.; Tsang, S.; Alshehri, A.S.; Mulafikh, D.S.; Alghulikah, A.A.; AlDhahri, S.F. The burden of chronic pain after major head and neck tumor therapy. Saudi J. Anaesth. 2017, 11 (Suppl. S1), S71–S79. [Google Scholar] [CrossRef]
- Zuo, X.; Chen, Y.; Zhu, Y.; Pan, D.; Rong, X.; Shen, Q.; Li, H.; Xu, Y.; Tang, Y. Radiation-induced Chronic Pain Plagues Head and Neck Cancer Survivors: A Cross-sectional Analysis From the Cohort in Radiotherapy-related Nervous System Complications. J. Pain 2024, 25, 104612. [Google Scholar] [CrossRef]
- Hernández, S.H.; Guía, V.G.J.; Núñez, J.M.; Ciuró, A.H.; Otero, A.N.; Mohedo, E.D.; Valenza, M.C. Widespread distribution and altered pain processing in head and neck cancer survivors at long-term after treatment. Support. Care Cancer 2023, 31, 394. [Google Scholar] [CrossRef] [PubMed]
- Schaller, A.; Larsson, B.; Lindblad, M.; Liedberg, G.M. Experiences of pain: A longitudinal, qualitative study of patients with head and neck cancer recently treated with radiotherapy. Pain Manag. Nurs. 2015, 16, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Bavia, P.F.; Khawaja, S.; Hernández-Nuño de la Rosa, M.F.; Tseng, L.A.; Keith, D.A. Association Between Pharmacotherapy and Sleep Quality in Patients with Chronic Orofacial and Chronic Body Pain: A Cross-Sectional Study. J. Pain Res. 2023, 16, 3433–3440. [Google Scholar] [CrossRef]
- Roehrs, T.; Roth, T. Sleep and pain: Interaction of two vital functions. Semin. Neurol. 2005, 25, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.; Hamada, A.; Hamada, Y.; Yanase, M.; Sakaki, M.; Someya, K.; Narita, M.; Kuzumaki, N.; Ikegami, D.; Sakai, H.; et al. Possible involvement of activated locus coeruleus-noradrenergic neurons in pain-related sleep disorders. Neurosci. Lett. 2015, 589, 200–206. [Google Scholar] [CrossRef]
- Fine, P.G. Long-term consequences of chronic pain: Mounting evidence for pain as a neurological disease and parallels with other chronic disease states. Pain Med. 2011, 12, 996–1004. [Google Scholar] [CrossRef]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef]
- Jimenez-Labaig, P.; Aymerich, C.; Braña, I.; Rullan, A.; Cacicedo, J.; González-Torres, M.Á.; Harrington, K.J.; Catalan, A. A comprehensive examination of mental health in patients with head and neck cancer: Systematic review and meta-analysis. JNCI Cancer Spectr. 2024, 8, pkae031. [Google Scholar] [CrossRef]
- Fischer, D.J.; Villines, D.; Kim, Y.O.; Epstein, J.B.; Wilkie, D.J. Anxiety, depression and pain: Differences by primary cancer. Support. Care Cancer 2010, 18, 801–810. [Google Scholar] [CrossRef]
- Geiss, C.; Hoogland, A.I.; Arredondo, B.; Rodriguez, Y.; Bryant, C.; Chung, C.H.; Patel, K.B.; Gonzalez, B.D.; Jim, H.S.L.; Kirtane, K.; et al. Psychosocial consequences of head and neck cancer symptom burden after chemoradiation: A mixed-method study. Support. Care Cancer 2024, 32, 254. [Google Scholar] [CrossRef]
- Pecorari, G.; Moglio, S.; Gamba, D.; Briguglio, M.; Cravero, E.; Sportoletti Baduel, E.; Riva, G. Sleep Quality in Head and Neck Cancer. Curr. Oncol. 2024, 31, 7000–7013. [Google Scholar] [CrossRef] [PubMed]
- Santoso, A.M.M.; Jansen, F.; Peeters, C.F.W.; Baatenburg de Jong, R.J.; Brakenhoff, R.H.; Langendijk, J.A.; Leemans, C.R.; Takes, R.P.; Terhaard, C.H.J.; van Straten, A.; et al. Psychoneurological Symptoms and Biomarkers of Stress and Inflammation in Newly Diagnosed Head and Neck Cancer Patients: A Network Analysis. Curr. Oncol. 2022, 29, 7109–7121. [Google Scholar] [CrossRef]
- Lin, Y.; Peng, G.; Bruner, D.W.; Miller, A.H.; Saba, N.F.; Higgins, K.A.; Shin, D.M.; Claussen, H.; Johnston, H.R.; Houser, M.C.; et al. Associations of differentially expressed genes with psychoneurological symptoms in patients with head and neck cancer: A longitudinal study. J. Psychosom. Res. 2023, 175, 111518. [Google Scholar] [CrossRef] [PubMed]
- Ei, H.; Sy, H.; Yc, L.; Cm, L.; Ck, L.; Hk, Y.; Cm, W. Probable Change of Sleep Parameters after Resection and Reconstruction Surgeries in Patients with Oral Cavity or Oropharyngeal Cancers. BioMed Res. Int. 2021, 2021, 7408497. [Google Scholar] [CrossRef]
- Ritschl, L.M.; Sackerer, V.; Pippich, K.; Zink, J.K.; Singer, H.; Grabenhorst, A.; Hedderich, D.M.; Wirth, M.H.; Wolff, K.-D.; Fichter, A.M.; et al. Impact of tumor localization and choice of microvascular flap on posterior airway changes following ablative surgery in primary oral squamous cell carcinoma: A monocentric cross-sectional study. Oral Oncol. 2024, 159, 107080. [Google Scholar] [CrossRef]
- Gilat, H.; Shpitzer, T.; Guttman, D.; Soudry, E.; Feinmesser, R.; Bachar, G. Obstructive sleep apnea after radial forearm free flap reconstruction of the oral tongue. Laryngoscope 2013, 123, 3223–3226. [Google Scholar] [CrossRef]
- Huyett, P.; Kim, S.; Johnson, J.T.; Soose, R.J. Obstructive sleep apnea in the irradiated head and neck cancer patient. Laryngoscope 2017, 127, 2673–2677. [Google Scholar] [CrossRef]
- Tawfik, G.M.; Mostafa, E.M.; Alshareef, A.; Hmeda, A.B.; Khaled, S.; Abdelwahed, K.A.; Mahran, S.A.; Agage, H.S.; Amer, A.E.; Emara, N.S.; et al. Association between radiotherapy and obstructive sleep apnea in head and neck cancer patients: A systematic review and meta-analysis. Auris Nasus Larynx 2021, 48, 1126–1134. [Google Scholar] [CrossRef]
- Faiz, S.A.; Balachandran, D.; Hessel, A.C.; Lei, X.; Beadle, B.M.; William, W.N.; Bashoura, L. Sleep-Related Breathing Disorders in Patients With Tumors in the Head and Neck Region. Oncologist 2014, 19, 1200–1206. [Google Scholar] [CrossRef]
- Inoshita, A.; Sata, N.; Ohba, S.; Suzuki, Y.; Ito, S.; Shiroshita, N.; Kawana, F.; Kasai, T.; Higo, R.; Ikeda, K.; et al. Impact of radiotherapy for head and neck cancer on obstructive sleep apnea: A prospective study. Ann. Palliat. Med. 2022, 11, 2631–2640. [Google Scholar] [CrossRef]
- Rose-Ped, A.M.; Bellm, L.A.; Epstein, J.B.; Trotti, A.; Gwede, C.; Fuchs, H.J. Complications of radiation therapy for head and neck cancers. The patient’s perspective. Cancer Nurs. 2002, 25, 461–467; quiz 468–469. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Suryavanshi, J.; Waindeskar, V.; Gupta, M.; Kaushal, A.; Kumar, H. Assessment of sleep characteristics using Fitbit Charge 4 in head and neck cancer patients undergoing palliative chemotherapy and radiotherapy: A prospective observational study. Palliat. Care Soc. Pract. 2024, 18, 26323524241283067. [Google Scholar] [CrossRef]
- Tapp, Z.M.; Ghosh, A.K.; Obrietan, K.H.; Pyter, L.M. Mechanistic insights into chemotherapy-induced circadian disruption using rodent models. Trends Neurosci. 2025; online now. [Google Scholar] [CrossRef]
- Fang, H.-F.; Miao, N.-F.; Chen, C.-D.; Sithole, T.; Chung, M.-H. Risk of Cancer in Patients with Insomnia, Parasomnia, and Obstructive Sleep Apnea: A Nationwide Nested Case-Control Study. J. Cancer 2015, 6, 1140–1147. [Google Scholar] [CrossRef]
- Isono, S. Obesity and obstructive sleep apnoea: Mechanisms for increased collapsibility of the passive pharyngeal airway. Respirology 2012, 17, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Moriondo, G.; Soccio, P.; Minoves, M.; Scioscia, G.; Tondo, P.; Foschino Barbaro, M.P.; Pépin, J.-L.; Briançon-Marjollet, A.; Lacedonia, D. Intermittent Hypoxia Mediates Cancer Development and Progression Through HIF-1 and miRNA Regulation. Arch. Bronconeumol. 2023, 59, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Saghari, S.; Bassiri, F.; Raesi, R.; Zarrabi, A.; Hushmandi, K.; Sethi, G.; Tergaonkar, V. NF-κB as a regulator of cancer metastasis and therapy response: A focus on epithelial-mesenchymal transition. J. Cell. Physiol. 2022, 237, 2770–2795. [Google Scholar] [CrossRef]
- Lavalle, S.; Masiello, E.; Iannella, G.; Magliulo, G.; Pace, A.; Lechien, J.R.; Calvo-Henriquez, C.; Cocuzza, S.; Parisi, F.M.; Favier, V.; et al. Unraveling the Complexities of Oxidative Stress and Inflammation Biomarkers in Obstructive Sleep Apnea Syndrome: A Comprehensive Review. Life 2024, 14, 425. [Google Scholar] [CrossRef]
- Janssen, H.L.; Haustermans, K.M.; Balm, A.J.; Begg, A.C. Hypoxia in head and neck cancer: How much, how important? Head Neck 2005, 27, 622–638. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, Z.; Chen, C.; Lu, G.; Wang, C.; Gao, S.; Shen, J.; Liu, J.; He, J.; Liang, W. Impact of obstructive sleep apnea on cancer risk: A systematic review and meta-analysis. Sleep Breath 2023, 27, 843–852. [Google Scholar] [CrossRef]
- Savvidis, C.; Kallistrou, E.; Kouroglou, E.; Dionysopoulou, S.; Gavriiloglou, G.; Ragia, D.; Tsiama, V.; Proikaki, S.; Belis, K.; Ilias, I. Circadian rhythm disruption and endocrine-related tumors. World J. Clin. Oncol. 2024, 15, 818–834. [Google Scholar] [CrossRef]
- Hsu, C.-M.; Lin, S.-F.; Lu, C.-T.; Lin, P.-M.; Yang, M.-Y. Altered expression of circadian clock genes in head and neck squamous cell carcinoma. Tumour Biol. 2012, 33, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Tang, H.; Yang, K. PER1 suppresses glycolysis and cell proliferation in oral squamous cell carcinoma via the PER1/RACK1/PI3K signaling complex. Cell Death Dis. 2021, 12, 276. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ao, Y.; Yang, K.; Tang, H.; Chen, D. Circadian clock gene Per2 plays an important role in cell proliferation, apoptosis and cell cycle progression in human oral squamous cell carcinoma. Oncol. Rep. 2016, 35, 3387–3394. [Google Scholar] [CrossRef]
- Wang, Y.; Narasimamurthy, R.; Qu, M.; Shi, N.; Guo, H.; Xue, Y.; Barker, N. Circadian regulation of cancer stem cells and the tumor microenvironment during metastasis. Nat. Cancer 2024, 5, 546–556. [Google Scholar] [CrossRef]
- Solomon, I.; Voiculescu, V.M.; Caruntu, C.; Lupu, M.; Popa, A.; Ilie, M.A.; Albulescu, R.; Caruntu, A.; Tanase, C.; Constantin, C.; et al. Neuroendocrine Factors and Head and Neck Squamous Cell Carcinoma: An Affair to Remember. Dis. Markers 2018, 2018, 9787831. [Google Scholar] [CrossRef] [PubMed]
- Wackerhage, H.; Christensen, J.F.; Ilmer, M.; von Luettichau, I.; Renz, B.W.; Schönfelder, M. Cancer catecholamine conundrum. Trends Cancer 2022, 8, 110–122. [Google Scholar] [CrossRef]
- Bastos, D.B.; Sarafim-Silva, B.A.M.; Sundefeld, M.L.M.M.; Ribeiro, A.A.; Brandão, J.D.P.; Biasoli, É.R.; Miyahara, G.I.; Casarini, D.E.; Bernabé, D.G. Circulating catecholamines are associated with biobehavioral factors and anxiety symptoms in head and neck cancer patients. PLoS ONE 2018, 13, e0202515. [Google Scholar] [CrossRef]
- Santos-Sousa, A.L.; Kayahara, G.M.; Bastos, D.B.; Sarafim-Silva, B.A.M.; Crivelini, M.M.; Valente, V.B.; Corrente, J.E.; Xavier-Júnior, J.C.C.; Miyahara, G.I.; Bernabé, D.G. Expression of β1- and β2-adrenergic receptors in oral squamous cell carcinoma and their association with psychological and clinical factors. Arch. Oral Biol. 2024, 162, 105939. [Google Scholar] [CrossRef]
- Salarić, I.; Karmelić, I.; Lovrić, J.; Baždarić, K.; Rožman, M.; Čvrljević, I.; Zajc, I.; Brajdić, D.; Macan, D. Salivary melatonin in oral squamous cell carcinoma patients. Sci. Rep. 2021, 11, 13201. [Google Scholar] [CrossRef]
- Nijakowski, K.; Surdacki, M.; Sobieszczańska, M. Salivary Melatonin Changes in Oncological Patients: A Systematic Review. Metabolites 2022, 12, 439. [Google Scholar] [CrossRef]
- Cutando, A.; Aneiros-Fernández, J.; López-Valverde, A.; Arias-Santiago, S.; Aneiros-Cachaza, J.; Reiter, R.J. A new perspective in Oral health: Potential importance and actions of melatonin receptors MT1, MT2, MT3, and RZR/ROR in the oral cavity. Arch. Oral Biol. 2011, 56, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Targhazeh, N.; Reiter, R.J.; Rahimi, M.; Qujeq, D.; Yousefi, T.; Shahavi, M.H.; Mir, S.M. Oncostatic activities of melatonin: Roles in cell cycle, apoptosis, and autophagy. Biochimie 2022, 202, 34–48. [Google Scholar] [CrossRef]
- Auld, F.; Maschauer, E.L.; Morrison, I.; Skene, D.J.; Riha, R.L. Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med. Rev. 2017, 34, 10–22. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Li, X.; Tao, Z.; Zhu, W.; Su, Y.; Choi, W.S. Melatonin and erastin emerge synergistic anti-tumor effects on oral squamous cell carcinoma by inducing apoptosis, ferroptosis, and inhibiting autophagy through promoting ROS. Cell. Mol. Biol. Lett. 2023, 28, 36. [Google Scholar] [CrossRef]
- Reiter, R.J.; De Almeida Chuffa, L.G.; Simão, V.A.; Martín Giménez, V.M.; De Las Heras, N.; Spandidos, D.A.; Manucha, W. Melatonin and vitamin D as potential synergistic adjuvants for cancer therapy (Review). Int. J. Oncol. 2024, 65, 114. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gao, Z.; Liu, H.; An, L.; Yang, T.; Zhang, B.; Liu, G.; Sun, D. Associations of lifestyle factors with oral cancer risk: An umbrella review. J. Stomatol. Oral Maxillofac. Surg. 2025; in press. [Google Scholar] [CrossRef]
- Mariano, L.C.; Warnakulasuriya, S.; Straif, K.; Monteiro, L. Secondhand smoke exposure and oral cancer risk: A systematic review and meta-analysis. Tob. Control. 2022, 31, 597–607. [Google Scholar] [CrossRef]
- Khowal, S.; Wajid, S. Role of Smoking-Mediated molecular events in the genesis of oral cancers. Toxicol. Mech. Methods 2019, 29, 665–685. [Google Scholar] [CrossRef]
- Jang, Y.S.; Nerobkova, N.; Hurh, K.; Park, E.-C.; Shin, J. Association between smoking and obstructive sleep apnea based on the STOP-Bang index. Sci. Rep. 2023, 13, 9085. [Google Scholar] [CrossRef]
- De Pasquale, G.; Mancin, S.; Matteucci, S.; Cattani, D.; Pastore, M.; Franzese, C.; Scorsetti, M.; Mazzoleni, B. Nutritional prehabilitation in head and neck cancer: A systematic review of literature. Clin. Nutr. ESPEN 2023, 58, 326–334. [Google Scholar] [CrossRef]
- Romano, F.; Muscogiuri, G.; Di Benedetto, E.; Zhukouskaya, V.V.; Barrea, L.; Savastano, S.; Colao, A.; Di Somma, C. Vitamin D and Sleep Regulation: Is there a Role for Vitamin D? Curr. Pharm. Des. 2020, 26, 2492–2496. [Google Scholar] [CrossRef]
- Wang, K.; Yu, X.-H.; Tang, Y.-J.; Tang, Y.-L.; Liang, X.-H. Obesity: An emerging driver of head and neck cancer. Life Sci. 2019, 233, 116687. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liu, J.; Wan, Q.; Wu, Y.; Wu, W.; Chen, Y. Chemerin promotes invasion of oral squamous cell carcinoma by stimulating IL-6 and TNF-α production via STAT3 activation. Mol. Biol. Rep. 2024, 51, 436. [Google Scholar] [CrossRef]
- Peng, J.; Hu, Q.; Chen, X.; Wang, C.; Zhang, J.; Ren, X.; Wang, Y.; Tao, X.; Li, H.; Song, M.; et al. Diet-induced obesity accelerates oral carcinogenesis by recruitment and functional enhancement of myeloid-derived suppressor cells. Cell Death Dis. 2021, 12, 946. [Google Scholar] [CrossRef]
- Li, B.; Wang, L.; Du, M.; He, H. FTO in oral diseases: Functions, mechanisms, and therapeutic potential. FASEB J. 2024, 38, e70115. [Google Scholar] [CrossRef] [PubMed]
- Almendros, I.; Martinez-Garcia, M.A.; Farré, R.; Gozal, D. Obesity, sleep apnea, and cancer. Int. J. Obes. 2020, 44, 1653–1667. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Miao, Y.; Tan, J.; Zhang, Q. Association of modifiable risk factors with obstructive sleep apnea: A Mendelian randomization study. Aging 2023, 15, 14039–14065. [Google Scholar] [CrossRef]
- Messineo, L.; Bakker, J.P.; Cronin, J.; Yee, J.; White, D.P. Obstructive sleep apnea and obesity: A review of epidemiology, pathophysiology and the effect of weight-loss treatments. Sleep Med. Rev. 2024, 78, 101996. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, X.; Wang, Z.; Zhou, K.; Yang, H.; Zhou, L.; Gou, X. Associations between dietary nutrient intake and sleep disorders in cancer survivors base on NHANES 2005 to 2018. Sci. Rep. 2024, 14, 26160. [Google Scholar] [CrossRef]
- Calver, L.; Tickle, A.; Moghaddam, N.; Biswas, S. The effect of psychological interventions on quality of life in patients with head and neck cancer: A systematic review and meta-analysis. Eur. J. Cancer Care 2018, 27, e12789. [Google Scholar] [CrossRef]
- Loh, E.-W.; Shih, H.-F.; Lin, C.-K.; Huang, T.-W. Effect of progressive muscle relaxation on postoperative pain, fatigue, and vital signs in patients with head and neck cancers: A randomized controlled trial. Patient Educ. Couns. 2022, 105, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.I.; Scherer, R.W.; Geigle, P.M.; Berlanstein, D.R.; Topaloglu, O.; Gotay, C.C.; Snyder, C. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst. Rev. 2012, 2012, CD007566. [Google Scholar] [CrossRef] [PubMed]
- Lynch, P.T.; Horani, S.; Lee, R.; Sumer, B.D.; Lee, S.C.; Mayo, H.G.; Rethorst, C.; Day, A.T. Effectiveness of physical activity interventions in improving objective and patient-reported outcomes in head and neck cancer survivors: A systematic review. Oral Oncol. 2021, 117, 105253. [Google Scholar] [CrossRef]
- Imani, F.; Mohebbi, S.; Mohseni, M.; Karimi, B.; Rahimi, S.; Dikafraz Shokooh, G.A. A Narrative Review on Pain Management in Head and Neck Cancer: Integrating Multimodal Analgesia and Interventional Procedures. Anesthesiol. Pain Med. 2024, 14, e146825. [Google Scholar] [CrossRef]
- Saxena, A.; Andley, M.; Gnanasekaran, N. Comparison of piroxicam and acetylsalicylic acid for pain in head and neck cancers: A double-blind study. Palliat. Med. 1994, 8, 223–229. [Google Scholar] [CrossRef]
- Inoshita, A.; Matsumoto, F.; Ohba, S.; Sata, N.; Matsuoka, R.; Suzuki, Y.; Ito, S.; Koiwai, H.; Shiroshita, N.; Kasai, T.; et al. Severe obstructive sleep apnea after concurrent chemoradiotherapy for laryngeal and hypopharyngeal cancer managed by CPAP. Auris Nasus Larynx 2022, 49, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Park, P.; Kim, J.; Song, Y.J.; Lim, J.H.; Cho, S.W.; Won, T.-B.; Han, D.H.; Kim, D.-Y.; Rhee, C.S.; Kim, H.J. Influencing factors on CPAP adherence and anatomic characteristics of upper airway in OSA subjects. Medicine 2017, 96, e8818. [Google Scholar] [CrossRef]
- Koka, V.; De Vito, A.; Roisman, G.; Petitjean, M.; Filograna Pignatelli, G.R.; Padovani, D.; Randerath, W. Orofacial Myofunctional Therapy in Obstructive Sleep Apnea Syndrome: A Pathophysiological Perspective. Medicina 2021, 57, 323. [Google Scholar] [CrossRef]
- Gambino, F.; Zammuto, M.M.; Virzì, A.; Conti, G.; Bonsignore, M.R. Treatment options in obstructive sleep apnea. Intern. Emerg. Med. 2022, 17, 971–978. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Jin, H.; Zhao, C.; Wang, W.; Li, W.-Y. Oral Cancer and Sleep Disturbances: A Narrative Review on Exploring the Bidirectional Relationship. Cancers 2025, 17, 1262. https://doi.org/10.3390/cancers17081262
Yang R, Jin H, Zhao C, Wang W, Li W-Y. Oral Cancer and Sleep Disturbances: A Narrative Review on Exploring the Bidirectional Relationship. Cancers. 2025; 17(8):1262. https://doi.org/10.3390/cancers17081262
Chicago/Turabian StyleYang, Runhua, Hongyu Jin, Chenyu Zhao, Wei Wang, and Wen-Yang Li. 2025. "Oral Cancer and Sleep Disturbances: A Narrative Review on Exploring the Bidirectional Relationship" Cancers 17, no. 8: 1262. https://doi.org/10.3390/cancers17081262
APA StyleYang, R., Jin, H., Zhao, C., Wang, W., & Li, W.-Y. (2025). Oral Cancer and Sleep Disturbances: A Narrative Review on Exploring the Bidirectional Relationship. Cancers, 17(8), 1262. https://doi.org/10.3390/cancers17081262





