The Influence of the Germline HSD3B1 Adrenal-Permissive Allele (c.1100 C) on the Somatic Alteration Landscape, the Transcriptome, and Immune Cell Infiltration in Prostate Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort and HSD3B1 Genotype Assignment
2.2. Whole-Exome Sequencing and Next-Generation Sequencing
2.3. Whole-Transcriptome Sequencing
2.4. Immune Signatures
2.5. Statistical Analysis
2.6. Tumor Mutation Burden and MSI-H/dMMR
3. Results
3.1. Prevalence of HSD3B1 Variants Based on Race, Treatment Status, and Tumor Biopsy Location
3.2. HSD3B1 C.1100 Genotypes Lack Association with AR and AR-Related Genes
3.3. HSD3B1 Variants Have Limited Impact on the Transcriptome but Regulate Hallmark Signaling Pathways
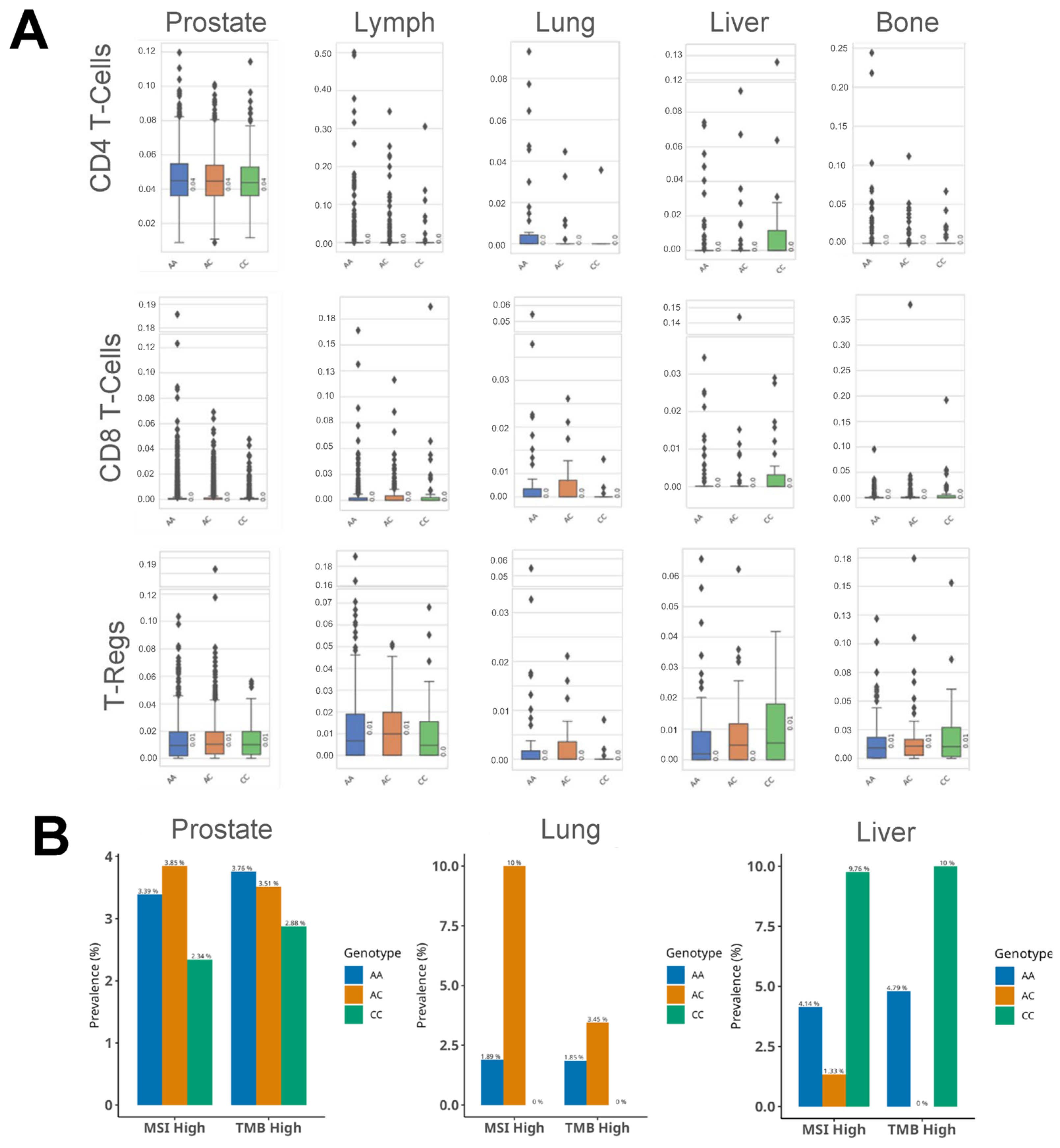
3.4. HSD3B1 Genotypes Are Associated with Differences in the Immune Microenvironment
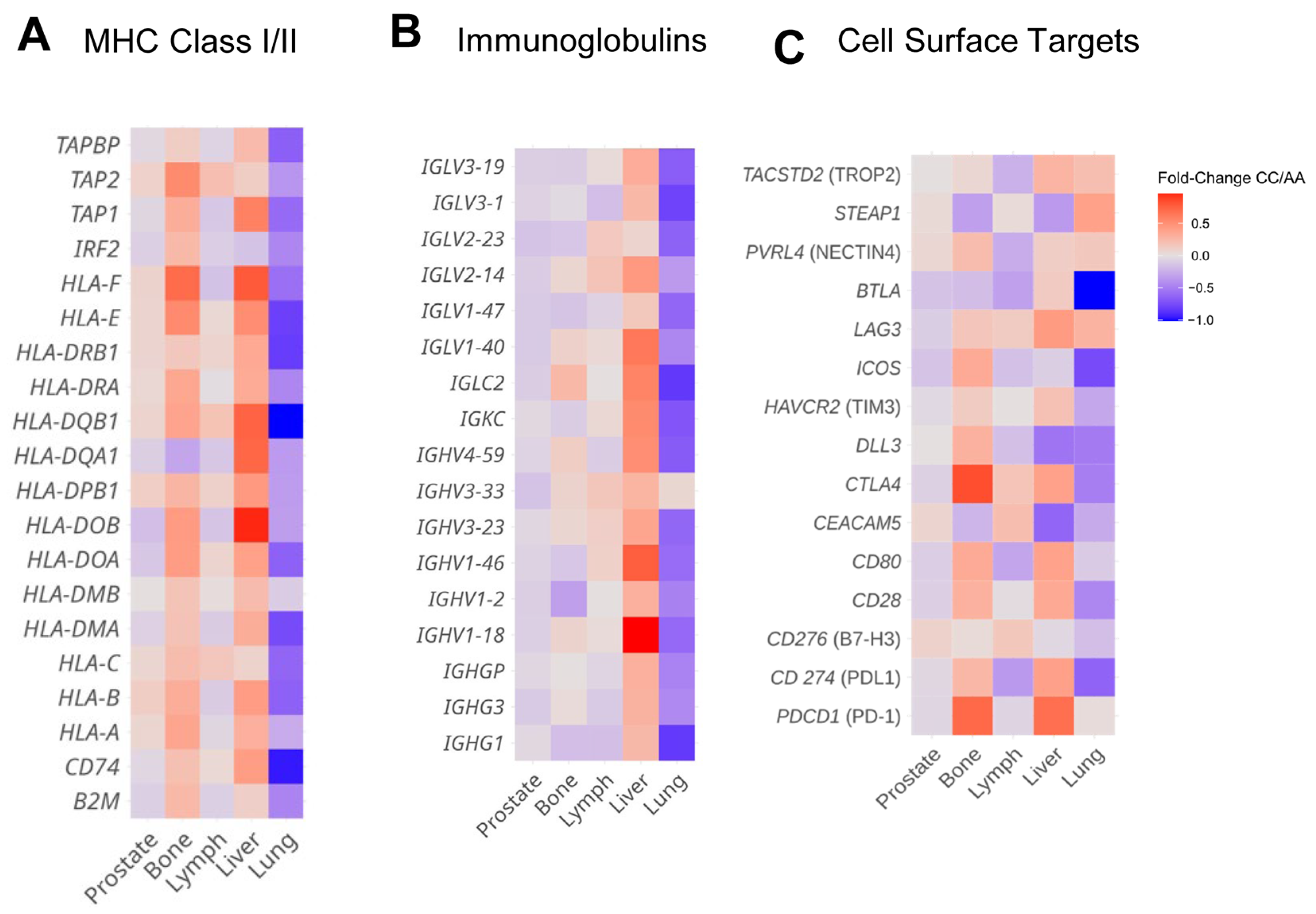
3.5. HSD3B1 Variants Are Associated with Divergent Expression of Immune Regulatory Features and Cell Surface Genes in a Tissue-Specific Manner
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PC | Prostate cancer |
| 3βHSD1 | 3β-Hydroxysteroid dehydrogenase type 1 |
| DHEA | Dehydroepiandrosterone |
| DHT | Dihydrotestosterone |
| mCRPC | Metastatic castration-resistant prostate cancer |
| AR | Androgen receptor |
| ADT | Androgen depravation therapy |
| OS | Overall survival |
| ICI | Immune checkpoint inhibitor |
| ER | Estrogen receptor |
References
- Chang, K.-H.; Li, R.; Kuri, B.; Lotan, Y.; Roehrborn, C.G.; Liu, J.; Vessella, R.; Nelson, P.S.; Kapur, P.; Guo, X.; et al. A Gain-of-Function Mutation in DHT Synthesis in Castration-Resistant Prostate Cancer. Cell 2013, 154, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- McManus, J.M.; Vargas, R.; Bazeley, P.S.; Schumacher, F.R.; Sharifi, N. Association Between Adrenal-Restrictive HSD3B1 Inheritance and Hormone-Independent Subtypes of Endometrial and Breast Cancer. JNCI Cancer Spectr. 2022, 6, pkac061. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, N.; Sharifi, N. HSD3B1 Genotypes Conferring Adrenal-Restrictive and Adrenal-Permissive Phenotypes in Prostate Cancer and Beyond. Endocrinology 2019, 160, 2180–2188. [Google Scholar] [CrossRef]
- Freitas, P.F.S.; Abdshah, A.; McKay, R.R.; Sharifi, N. HSD3B1, Prostate Cancer Mortality and Modifiable Outcomes. Nat. Rev. Urol. 2024. [Google Scholar] [CrossRef]
- Flanagan, M.R.; Doody, D.R.; Voutsinas, J.; Wu, Q.; Banda, K.; Sharifi, N.; Li, C.I.; Gadi, V.K. Association of HSD3B1 Genotype and Clinical Outcomes in Postmenopausal Estrogen-Receptor-Positive Breast Cancer. Ann. Surg. Oncol. 2022, 29, 7194–7201. [Google Scholar] [CrossRef]
- Desai, K.; McManus, J.M.; Sharifi, N. Hormonal Therapy for Prostate Cancer. Endocr. Rev. 2021, 42, 354–373. [Google Scholar] [CrossRef]
- Hearn, J.W.D.; AbuAli, G.; Reichard, C.A.; Reddy, C.A.; Magi-Galluzzi, C.; Chang, K.-H.; Carlson, R.; Rangel, L.; Reagan, K.; Davis, B.J.; et al. HSD3B1 and Resistance to Androgen-Deprivation Therapy in Prostate Cancer: A Retrospective, Multicohort Study. Lancet Oncol. 2016, 17, 1435–1444. [Google Scholar] [CrossRef]
- Hearn, J.W.D.; Sweeney, C.J.; Almassi, N.; Reichard, C.A.; Reddy, C.A.; Li, H.; Hobbs, B.; Jarrard, D.F.; Chen, Y.-H.; Dreicer, R.; et al. HSD3B1 Genotype and Clinical Outcomes in Metastatic Castration-Sensitive Prostate Cancer. JAMA Oncol. 2020, 6, e196496. [Google Scholar] [CrossRef]
- Chen, W.S.; Feng, E.; Aggarwal, R.; Foye, A.; Beer, T.M.; Alumkal, J.J.; Gleave, M.; Chi, K.N.; Reiter, R.E.; Rettig, M.B.; et al. Germline Polymorphisms Associated with Impaired Survival Outcomes and Somatic Tumor Alterations in Advanced Prostate Cancer. Prostate Cancer Prostatic Dis. 2020, 23, 316–323. [Google Scholar] [CrossRef]
- Finotello, F.; Mayer, C.; Plattner, C.; Laschober, G.; Rieder, D.; Hackl, H.; Krogsdam, A.; Loncova, Z.; Posch, W.; Wilflingseder, D.; et al. Molecular and Pharmacological Modulators of the Tumor Immune Contexture Revealed by Deconvolution of RNA-Seq Data. Genome Med. 2019, 11, 34. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Vanderwalde, A.; Spetzler, D.; Xiao, N.; Gatalica, Z.; Marshall, J. Microsatellite Instability Status Determined by Next-Generation Sequencing and Compared with PD-L1 and Tumor Mutational Burden in 11,348 Patients. Cancer Med. 2018, 7, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Rs1047303 RefSNP Report-dbSNP-NCBI. Available online: https://www.ncbi.nlm.nih.gov/snp/rs1047303?horizontal_tab=true (accessed on 18 February 2025).
- Beltran, H.; Prandi, D.; Mosquera, J.M.; Benelli, M.; Puca, L.; Cyrta, J.; Marotz, C.; Giannopoulou, E.; Chakravarthi, B.V.S.K.; Varambally, S.; et al. Divergent Clonal Evolution of Castration-Resistant Neuroendocrine Prostate Cancer. Nat. Med. 2016, 22, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-W.; Kim, K.-H.; Kim, E.-Y.; Lee, S.-Y.; Ko, J.-J.; Lee, K.-A. Associations among Sebox and Other MEGs and Its Effects on Early Embryogenesis. PLoS ONE 2015, 10, e0115050. [Google Scholar] [CrossRef]
- Mao, X.; Ye, Q.; Zhang, G.; Jiang, J.; Zhao, H.; Shao, Y.; Ye, Z.; Xuan, Z.; Huang, P. Identification of Differentially Methylated Genes as Diagnostic and Prognostic Biomarkers of Breast Cancer. World J. Surg. Oncol. 2021, 19, 29. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) Hallmark Gene Set Collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Chesner, L.N.; Polesso, F.; Graff, J.N.; Hawley, J.E.; Smith, A.K.; Lundberg, A.; Das, R.; Shenoy, T.; Sjöström, M.; Zhao, F.; et al. Androgen Receptor Inhibition Increases MHC Class I Expression and Improves Immune Response in Prostate Cancer. Cancer Discov. 2025, 15, 481–494. [Google Scholar] [CrossRef]
- Guan, X.; Polesso, F.; Wang, C.; Sehrawat, A.; Hawkins, R.M.; Murray, S.E.; Thomas, G.V.; Caruso, B.; Thompson, R.F.; Wood, M.A.; et al. Androgen Receptor Activity in T Cells Limits Checkpoint Blockade Efficacy. Nature 2022, 606, 791–796. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Park, S.H.; Goh, J.C.; Shin, S.J.; Lee, J.L.; Mehra, N.; McDermott, R.; Sala-Gonzalez, N.; Fong, P.C.; Greil, R.; et al. Pembrolizumab Plus Olaparib for Patients With Previously Treated and Biomarker-Unselected Metastatic Castration-Resistant Prostate Cancer: The Randomized, Open-Label, Phase III KEYLYNK-010 Trial. J. Clin. Oncol. 2023, 41, 3839–3850. [Google Scholar] [CrossRef]
- Bergom, H.E.; Sena, L.A.; Day, A.; Miller, B.; Miller, C.D.; Lozada, J.R.; Zorko, N.; Wang, J.; Shenderov, E.; Lobo, F.P.; et al. Divergent Immune Microenvironments in Two Tumor Nodules from a Patient with Mismatch Repair-Deficient Prostate Cancer. NPJ Genom. Med. 2024, 9, 7. [Google Scholar] [CrossRef]
- Ajkunic, A.; Sayar, E.; Roudier, M.P.; Patel, R.A.; Coleman, I.M.; De Sarkar, N.; Hanratty, B.; Adil, M.; Zhao, J.; Zaidi, S.; et al. Assessment of TROP2, CEACAM5 and DLL3 in Metastatic Prostate Cancer: Expression Landscape and Molecular Correlates. NPJ Precis. Oncol. 2024, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Geri, J.B.; Pao, W. Elucidating the Cell Surfaceome to Accelerate Cancer Drug Development. Cancer Discov. 2024, 14, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, A.; Zhang, M.; Aggarwal, R.; Li, H.; Zhang, L.; Foye, A.; Sjöström, M.; Chou, J.; Chang, K.; Moreno-Rodriguez, T.; et al. The Genomic and Epigenomic Landscape of Double-Negative Metastatic Prostate Cancer. Cancer Res. 2023, 83, 2763–2774. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, N.; Liu, Q.; Guo, J.; Pan, Q.; Cheng, B.; Xu, J.; Dong, B.; Yang, G.; Yang, B.; et al. Antiandrogen Treatment Induces Stromal Cell Reprogramming to Promote Castration Resistance in Prostate Cancer. Cancer Cell 2023, 41, 1345–1362.e9. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, C.; Chen, X.; Wang, J.; Liu, K.; Jing, N.; Xu, P.; Xi, X.; Sun, Y.; Ji, Z.; et al. IL-1β Is an Androgen-Responsive Target in Macrophages for Immunotherapy of Prostate Cancer. Adv. Sci. 2023, 10, e2206889. [Google Scholar] [CrossRef]
- Yang, C.; Jin, J.; Yang, Y.; Sun, H.; Wu, L.; Shen, M.; Hong, X.; Li, W.; Lu, L.; Cao, D.; et al. Androgen Receptor-Mediated CD8+ T Cell Stemness Programs Drive Sex Differences in Antitumor Immunity. Immunity 2022, 55, 1268–1283.e9. [Google Scholar] [CrossRef]
- Kwon, H.; Schafer, J.M.; Song, N.-J.; Kaneko, S.; Li, A.; Xiao, T.; Ma, A.; Allen, C.; Das, K.; Zhou, L.; et al. Androgen Conspires with the CD8+ T Cell Exhaustion Program and Contributes to Sex Bias in Cancer. Sci. Immunol. 2022, 7, eabq2630. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, D.; Gong, C.; Zhang, F.; He, J.; Zhang, W.; Zhao, Y.; Sun, J. Prognostic Role of Hormone Receptors in Endometrial Cancer: A Systematic Review and Meta-Analysis. World J. Surg. Oncol. 2015, 13, 208. [Google Scholar] [CrossRef]
- Rodriguez, A.C.; Blanchard, Z.; Maurer, K.A.; Gertz, J. Estrogen Signaling in Endometrial Cancer: A Key Oncogenic Pathway with Several Open Questions. Horm. Cancer 2019, 10, 51–63. [Google Scholar] [CrossRef]
- Hwang, J.; Nazari, S.; Elliott, A.; Vanderwalde, A.M.; Lange, C.A.; Geller, M.A.; Ryan, C.J.; Nabhan, C.; Sharifi, N.; Antonarakis, E.S. Association of Germline HSD3B1 (c.1100) Genotype with Tumoral and Clinical Outcomes in Breast and Endometrial Cancers. J. Clin. Oncol. 2024, 42, 10587. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kellen, S.; Makovec, A.; Miller, C.D.; Nazari, S.S.; Elliott, A.; Deacon, A.; John, E.; Vobugari, N.; Agarwal, N.; McKay, R.R.; et al. The Influence of the Germline HSD3B1 Adrenal-Permissive Allele (c.1100 C) on the Somatic Alteration Landscape, the Transcriptome, and Immune Cell Infiltration in Prostate Cancer. Cancers 2025, 17, 1270. https://doi.org/10.3390/cancers17081270
Kellen S, Makovec A, Miller CD, Nazari SS, Elliott A, Deacon A, John E, Vobugari N, Agarwal N, McKay RR, et al. The Influence of the Germline HSD3B1 Adrenal-Permissive Allele (c.1100 C) on the Somatic Alteration Landscape, the Transcriptome, and Immune Cell Infiltration in Prostate Cancer. Cancers. 2025; 17():1270. https://doi.org/10.3390/cancers17081270
Chicago/Turabian StyleKellen, Samuel, Allison Makovec, Carly D. Miller, Shayan S. Nazari, Andrew Elliott, Aiden Deacon, Emily John, Nikitha Vobugari, Neeraj Agarwal, Rana R. McKay, and et al. 2025. "The Influence of the Germline HSD3B1 Adrenal-Permissive Allele (c.1100 C) on the Somatic Alteration Landscape, the Transcriptome, and Immune Cell Infiltration in Prostate Cancer" Cancers 17, no. : 1270. https://doi.org/10.3390/cancers17081270
APA StyleKellen, S., Makovec, A., Miller, C. D., Nazari, S. S., Elliott, A., Deacon, A., John, E., Vobugari, N., Agarwal, N., McKay, R. R., Barata, P. C., Ryan, C. J., Sharifi, N., Hwang, J., & Antonarakis, E. S. (2025). The Influence of the Germline HSD3B1 Adrenal-Permissive Allele (c.1100 C) on the Somatic Alteration Landscape, the Transcriptome, and Immune Cell Infiltration in Prostate Cancer. Cancers, 17(), 1270. https://doi.org/10.3390/cancers17081270






