Role of Neutrophils in Anti-Tumor Activity: Characteristics and Mechanisms of Action
Simple Summary
Abstract
1. Neutrophils: An Overview of Functions, Development, and Polarization
2. Neutrophils and Tumors
2.1. Functions of Neutrophils in Tumors
2.2. Phenotypes of Neutrophils in Tumors
| Species | Tumor | Neutrophils Origin Tissue | Clusters | Signatures and/or Functions | Reference |
|---|---|---|---|---|---|
| Human | NSCLC | Tumor | TAN1-4;NAN1-2 | TAN1: CXCL8, CXCL1, CXCL2, ICAM1, and CD44; TAN2: HLA-DRA, CD74, and HLA-DPB1; TAN3:PLIN2, PLPP, MAP1, LC3B, and PLAU; TAN4: RPL10, RPS2, RPS18, RPL3, NAN1, S100A12, PAD14, PROK2, and MMP9; NAN2: similar to NAN1 cluster, and decreased expression of S100A12, MME, and PROK2. | [64] |
| NSCLC | Tumor | hN1–hN5 | hN1: MMP8, MMP9, S100A8, S100A 9, and ADAM8; hN2: IFIT1, IRF7, and RSAD2; hN3: CASS4; hN4: CTSC; hN5: CCL3, CSF1, CTSB, and IRAK2. | [67] | |
| 31 solid cancers | Tumor | 10 distinct clusters | S100A12+; HLA-DR+CD74; VEGFA+SPP1+; TXNIP+; CXCL8+IL1B+; CXCR2+; IFIT1+ISG15+; MMP9+; NFKBIZ+HIF1A+; ARG1+. | [65] | |
| Pancreatic ductal adenocarcinoma | Tumor | TNA0–TNN5 | TAN-0: no cluster-specific distinctive features; TAN-1: terminally differentiated pro-tumor subpopulation; TAN-2: inflammatory subpopulation; TAN-3: transitional stage subpopulation; TAN-4: expression of interferon-stimulated genes; TAN-5: undefined subpopulation of low-quality cells. | [68] | |
| Melanoma | Peripheral Blood | hNeP and Cneut1-5 | hNep: CD117+CD66b+CD38+ neutrophil progenitors; Cneut1: CD16dimCD62 Lbright band cell; Cneut2: terminally differentiated, mature neutrophils; Cneut3: CXCR4+CD49d+CD62 Llo aged neutrophils; Cneut4: no specific features; Cneut5: immature neutrophils; Cneut6: CD16dimCD62 Lbright band cells. | [69] | |
| Mouse | Pancreatic cancer model | Tumor | T1-3 | T1: CD101− dcTRAIL-R1−; T2: CD101+ dcTRAIL-R1−; T3: CD101+/− dcTRAIL-R1+. | [22] |
| Lewis lung cancer model | Tumor | PMN1-3 | PMN1: account for almost 95% neutrophils in control spleen; PMN2: Ngp, Ltf, Cd177, Anxa1, MMP8, S100A8, S100A9, Cebpe, Ltb4r1, and Cybb; PMN3:CCL4, CCL3, CXCL2, CXCL3, SPP1,IL1B, NFKBIA, SOCS3, MIF, KLF6, ATF3, PTGS2, and XBP1. | [66] | |
| NSCLC lung cancer model | Tumor | mN1-6 | mN1: MMP8, MMP9, S100A8, S100A9, and ADAM8; mN2: IFIT1, IRF7, and RSAD2; mN3: CXCL3; mN4: PALD1; mN5: CCL3, CSF1, CSTB, and IRAK2; mN6: FCNB and NGP. | [67] | |
| MMTV-PyMT transgenic mouse model of breast cancer | Tumor | C0, C2, C4, C5, C7, and C8 | C0: CAMP17 and LY6g; C2: IL1β and Arg2; C4, C5: CEBPE and RETNLG; C7, C8: Tuba1b and Cdc20. | [70] |
3. Potential Mechanisms of Neutrophil-Induced Tumor-Killing Activity
3.1. Neutrophils and Reactive Oxygen Species-Induced Tumor Killing
3.2. Neutrophils and Cytotoxic T Lymphocytes-Induced Tumor Killing
3.3. Neutrophils and Trogocytosis
3.4. Neutrophils and Cytotoxic Enzymes
3.4.1. Neutrophil Extracellular Traps
3.4.2. Neutrophil Elastase
3.5. Neutrophils and Apoptosis
3.6. Neutrophils and Trained Immunity
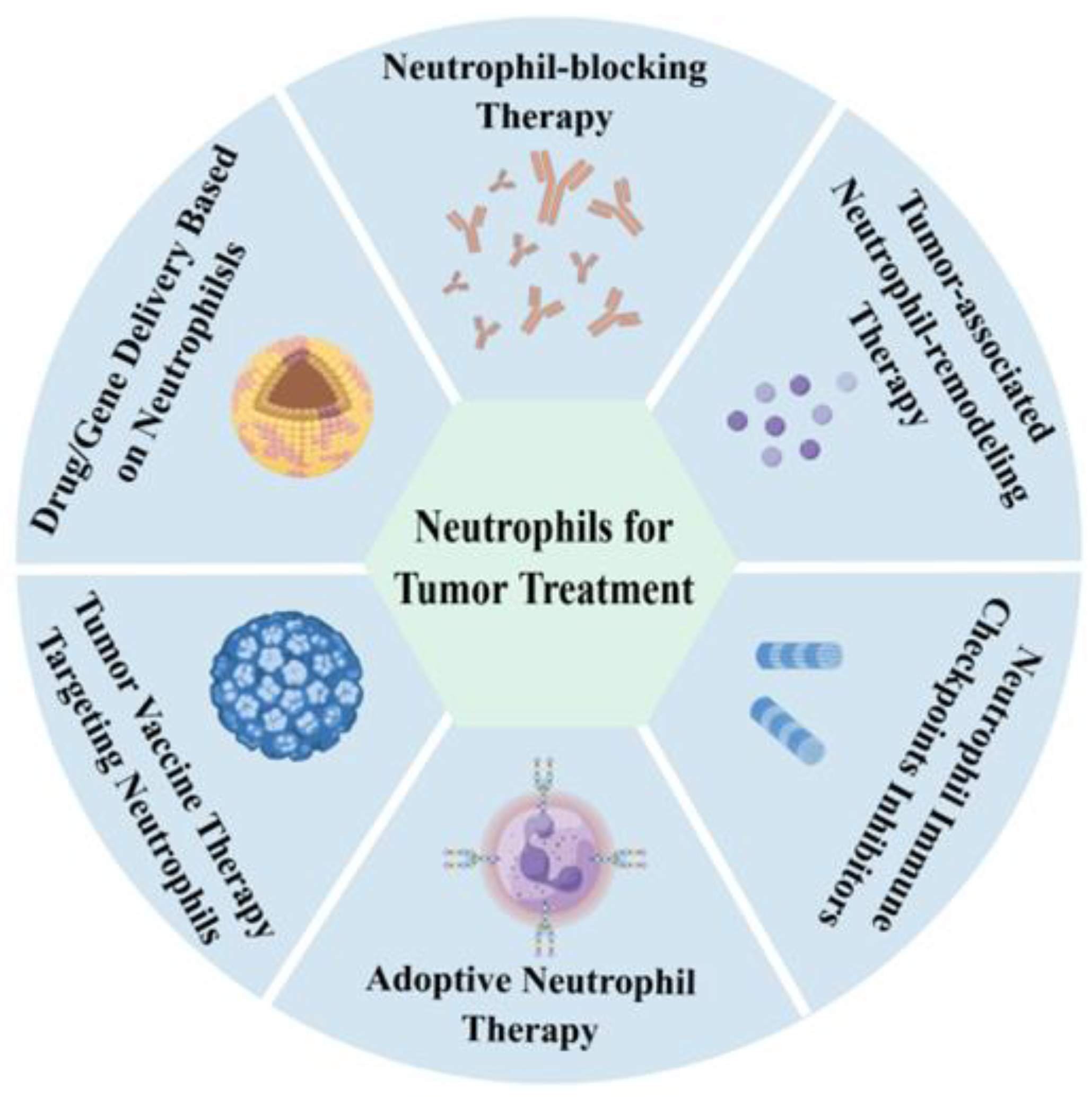
4. Neutrophils as a New Strategy in Tumor Treatment
- (1)
- Neutrophil-blocking Therapy: In the past, numerous studies have suggested that neutrophils promote tumor initiation, progression, and metastasis, making neutrophil-blocking therapy a targeted tumor strategy [92]. Chemokine (C-X-C motif) receptor 1 (CXCR1) and chemokine (C-X-C motif) receptor 2 (CXCR2), expressed on neutrophils, guide integrin activation, and neutrophil recruitment. Blocking CXCR1/CXCR2 signaling has been considered a potent strategy for neutrophil-targeted cancer therapy [93,94]. A phase 1b trial in patients with metastatic breast cancer found that combining reparixin with paclitaxel was safe [95]. However, the primary endpoint of extending progression-free survival was not met in the phase 2 trial [96]. There are no CXCR2-targeting drugs that have been approved for cancer treatment now; other CXCR2 antagonists are in various stages of clinical development [97]. NETs are seen as promising emerging targets in tumor therapy due to their ability to induce tumor cell metastasis [98]. Treatment could involve inhibiting the formation and/or activation of NETs in tumor tissues. However, clinical trials have not yet identified the best way to target NETs, possibly due to the lack of a good biomarker for NET treatment response.
- (2)
- Tumor-associated Neutrophil-remodeling Therapy: Due to the diversity and plasticity of neutrophils in tumors, several pathways can remodel tumor microenvironment neutrophils to achieve anti-tumor phenotypes. Inhibiting the TGF-β signaling pathway can induce anti-tumor TAN1 phenotypes and other anti-tumor immune cells. Neutralizing TGF-β increases neutrophil–chemoattractant production, leading to the recruitment and activation of anti-tumor neutrophil subsets. Currently, various clinical-stage TGF-β-targeted drugs include monoclonal antibodies, TGF-β R fusion proteins, antisense oligonucleotides, and TGF-β R kinase inhibitors [99]. The polarization of N1 TANs can also be induced by type 1 interferons. Interferon-α (IFN-α) was approved by the FDA in 1986 for treating hairy cell leukemia, malignant melanoma, and other cancers [100]. However, cytokines have complex mechanisms in the body and can easily cause adverse reactions after anti-tumor immune therapy administration. Future research into nano-mechanisms may improve cytokine therapy. Moreover, more studies are needed to explore the molecular mechanisms of neutrophil remodeling in the TME, providing more molecular targets and a theoretical basis for tumor-associated neutrophil-remodeling therapy.
- (3)
- Neutrophil Immune Checkpoints Inhibitors: Targeting programmed death 1 (PD1)/programmed cell death ligand 1 (PDL1) has proven effective in immunotherapy for various cancers. Studies have found that TAN2 also expresses high levels of PD-L1 [101], which can be effectively targeted. Additionally, besides TAN2, there are many G-MDSCs in the TME that exhibit immunosuppressive functions. Future research should explore more immune checkpoints for such cells.
- (4)
- Adoptive Neutrophil Therapy: Maharaj et al. [33] conducted a combined phase I/II open-label clinical trial with three patients with advanced, relapsed, or refractory solid tumors to test the antineoplastic efficacy of HLA-mismatched non-irradiated white cells (68–91% granulocytes) from young, healthy donors. Although neutrophil infusion did not alter patient outcomes, pathological examination revealed extensive tumor necrosis and leukocyte infiltration caused by granulocyte infusion. This may be due to the rapid remodeling of neutrophils by the tumor microenvironment post-infusion. LifT BioSciences has since identified immunomodulatory alpha neutrophils (IMANs) that differentiate into N1 neutrophils after infusion, exhibiting selective cancer cytotoxicity, granules, and cationic peptides with a positive net cell charge [102]. Preclinical data shows IMANs demonstrate stronger tumor tissue infiltration and effective pancreatic tumor killing compared to adoptive T-cell therapy. Chimeric antigen receptor (CAR) neutrophils present a more promising research direction, targeting tumors precisely. The company engineered human epidermal growth factor receptor 2 (HER2) CAR on induced pluripotent stem cell-derived IMANs, enhancing its cancer-killing ability fourfold. Both neutrophil therapies are currently applied for clinical trials, and we look forward to their clinical data. Despite their selection or modification, these immunomodulatory alpha neutrophils still face the challenge of being remodeled by the tumor microenvironment after entering tumors. Future research should focus on neutrophil phenotypes and the regulatory factors for phenotype conversion, providing a detailed theoretical basis for adoptive neutrophil therapy. Additionally, neutrophil research should focus more on the molecular mechanisms of neutrophil recognition of tumor cells and their anti-tumor effects to design more reasonable CAR neutrophils.
- (5)
- Tumor Vaccine Therapy Targeting Neutrophils: Recent studies have confirmed that HLA-DR+CD74+ antigen-presenting neutrophils can induce T-cell responses in tumor tissues, forming a “hot tumor” microenvironment favorable for anti-tumor immunity [65]. This provides a new research direction for tumor vaccine design, targeting HLA-DR+CD74+ antigen-presenting neutrophils to turn “cold” tumors into “hot” tumors, remodel the TME, and enhance the efficacy of tumor vaccine immunotherapy. Additionally, research has found that neutrophils can exert anti-tumor effects through trained immunity [51], suggesting a future direction for tumor vaccine design using this approach. However, how neutrophil recognition of tumor cell patterns and phenotypic changes lead to trained immunity remains unclear. Furthermore, whether neutrophils are involved in trained tumor immunotherapy is another future research direction.
- (6)
- Drug/Gene Delivery Based on Neutrophils: Neutrophils, as critical participants in the tumor microenvironment, respond to tumor signals via chemokines, such as CXCL8 and CXCL5, accumulating in large numbers at tumor sites [103]. This behavior provides a novel direction for drug and gene delivery research. Neutrophil-based delivery systems offer distinct advantages, including efficient phagocytic ability, specific chemotaxis, and rapid responsiveness [104,105]. The delivery methods based on neutrophils can be categorized as follows: (1) Ex vivo drug/gene loading and reinfusion: Neutrophils are cultured ex vivo to internalize nanodrugs before being reinfused into the body. For instance, Li et al. [106] developed a method utilizing bone marrow-tropic neutrophils (BMTNs) to deliver drugs to bone marrow, significantly improving therapeutic efficacy. (2) Engineered neutrophils: Bao et al. [107] designed CLTX-CAR structures containing T-cell or neutrophil-specific signaling domains and loaded chemotherapy drugs. This approach enabled CAR-neutrophils to deliver and release tumor-responsive nanodrugs non-invasively in gliomas, maintaining their anti-tumor N1 phenotype. (3) Biomimetic nanoparticles: Cell-membrane-coated nanoparticles (CNPs) are fabricated by extracting fragments from parental cell membranes, inheriting their characteristics. For example, Wang et al. [108] developed neutrophils and macrophage membrane coated-PLGA/RAPA nanoparticles loaded with rapamycin, which could autonomously cross the blood–brain barrier. These biomimetic nanoparticles combined macrophage-stimulated self-recurrence and neutrophil-mediated inflammatory chemotaxis, effectively enhancing glioma treatment. These studies reveal the potential of neutrophil-based drug delivery systems for precision cancer therapy.
5. Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ganesh, K.; Joshi, M.B. Neutrophil Sub-Types in Maintaining Immune Homeostasis During Steady State, Infections and Sterile Inflammation. Inflamm. Res. 2023, 72, 1175–1192. [Google Scholar] [CrossRef] [PubMed]
- Koenderman, L.; Tesselaar, K.; Vrisekoop, N. Human Neutrophil Kinetics: A Call to Revisit Old Evidence. Trends Immunol. 2022, 43, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Liew, P.X.; Kubes, P. The Neutrophil’s Role During Health and Disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef]
- Teng, T.S.; Ji, A.L.; Ji, X.Y.; Li, Y.Z. Neutrophils and Immunity: From Bactericidal Action to Being Conquered. J. Immunol. Res. 2017, 2017, 9671604. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Miralda, I.; Armstrong, C.L.; Uriarte, S.M.; Bagaitkar, J. The Roles of Nadph Oxidase in Modulating Neutrophil Effector Responses. Mol. Oral Microbiol. 2019, 34, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Galkina, S.I.; Golenkina, E.A.; Viryasova, G.M.; Romanova, Y.M.; Sud’ina, G.F. Nitric Oxide in Life and Death of Neutrophils. Curr. Med. Chem. 2019, 26, 5764–5780. [Google Scholar] [CrossRef]
- Maianski, N.A.; Maianski, A.N.; Kuijpers, T.W.; Roos, D. Apoptosis of Neutrophils. Acta Haematol. 2004, 111, 56–66. [Google Scholar] [CrossRef]
- Stegelmeier, A.A.; Darzianiazizi, M.; Hanada, K.; Sharif, S.; Wootton, S.K.; Bridle, B.W.; Karimi, K. Type I Interferon-Mediated Regulation of Antiviral Capabilities of Neutrophils. Int. J. Mol. Sci. 2021, 22, 4726. [Google Scholar] [CrossRef]
- Worley, M.J.; Fei, K.; Lopez-Denman, A.J.; Kelleher, A.D.; Kent, S.J.; Chung, A.W. Neutrophils Mediate Hiv-Specific Antibody-Dependent Phagocytosis and Adcc. J. Immunol. Methods 2018, 457, 41–52. [Google Scholar] [CrossRef]
- Cziupka, K.; Busemann, A.; Partecke, L.I.; Potschke, C.; Rath, M.; Traeger, T.; Koerner, P.; von Bernstorff, W.; Kessler, W.; Diedrich, S.; et al. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (Trail) Improves the Innate Immune Response and Enhances Survival in Murine Polymicrobial Sepsis. Crit. Care Med. 2010, 38, 2169–2174. [Google Scholar] [CrossRef]
- Jaillon, S.; Ponzetta, A.; Di Mitri, D.; Santoni, A.; Bonecchi, R.; Mantovani, A. Neutrophil Diversity and Plasticity in Tumour Progression and Therapy. Nat. Rev. Cancer 2020, 20, 485–503. [Google Scholar] [CrossRef] [PubMed]
- Akashi, K.; Traver, D.; Miyamoto, T.; Weissman, I.L. A Clonogenic Common Myeloid Progenitor That Gives Rise to All Myeloid Lineages. Nature 2000, 404, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Muthas, D.; Reznichenko, A.; Balendran, C.A.; Bottcher, G.; Clausen, I.G.; Karrman Mardh, C.; Ottosson, T.; Uddin, M.; MacDonald, T.T.; Danese, S.; et al. Neutrophils in Ulcerative Colitis: A Review of Selected Biomarkers and Their Potential Therapeutic Implications. Scand. J. Gastroenterol. 2017, 52, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Kwok, I.; Becht, E.; Xia, Y.; Ng, M.; Teh, Y.C.; Tan, L.; Evrard, M.; Li, J.L.Y.; Tran, H.T.N.; Tan, Y.; et al. Combinatorial Single-Cell Analyses of Granulocyte-Monocyte Progenitor Heterogeneity Reveals an Early Uni-Potent Neutrophil Progenitor. Immunity 2020, 53, 303–318.e5. [Google Scholar] [CrossRef]
- Xie, X.; Shi, Q.; Wu, P.; Zhang, X.; Kambara, H.; Su, J.; Yu, H.; Park, S.Y.; Guo, R.; Ren, Q.; et al. Single-Cell Transcriptome Profiling Reveals Neutrophil Heterogeneity in Homeostasis and Infection. Nat. Immunol. 2020, 21, 1119–1133. [Google Scholar] [CrossRef]
- Sionov, R.V. Leveling up the Controversial Role of Neutrophils in Cancer: When the Complexity Becomes Entangled. Cells 2021, 10, 2486. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Albelda, S.M. Tumor-Associated Neutrophils: Friend or Foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef]
- Jablonska, J.; Rist, M.; Lang, S.; Brandau, S. Neutrophils in the Tumor Microenvironment-Foes or Friends? HNO 2020, 68, 891–898. [Google Scholar] [CrossRef]
- Giese, M.A.; Hind, L.E.; Huttenlocher, A. Neutrophil Plasticity in the Tumor Microenvironment. Blood 2019, 133, 2159–2167. [Google Scholar] [CrossRef]
- Swierczak, A.; Mouchemore, K.A.; Hamilton, J.A.; Anderson, R.L. Neutrophils: Important Contributors to Tumor Progression and Metastasis. Cancer Metastasis Rev. 2015, 34, 735–751. [Google Scholar] [CrossRef]
- Antuamwine, B.B.; Bosnjakovic, R.; Hofmann-Vega, F.; Wang, X.; Theodosiou, T.; Iliopoulos, I.; Brandau, S. N1 Versus N2 and Pmn-Mdsc: A Critical Appraisal of Current Concepts on Tumor-Associated Neutrophils and New Directions for Human Oncology. Immunol. Rev. 2023, 314, 250–279. [Google Scholar] [CrossRef]
- Ng, M.S.F.; Kwok, I.; Tan, L.; Shi, C.; Cerezo-Wallis, D.; Tan, Y.; Leong, K.; Calvo, G.F.; Yang, K.; Zhang, Y.; et al. Deterministic Reprogramming of Neutrophils within Tumors. Science 2024, 383, eadf6493. [Google Scholar] [CrossRef]
- Rao, H.L.; Chen, J.W.; Li, M.; Xiao, Y.B.; Fu, J.; Zeng, Y.X.; Cai, M.Y.; Xie, D. Increased Intratumoral Neutrophil in Colorectal Carcinomas Correlates Closely with Malignant Phenotype and Predicts Patients’ Adverse Prognosis. PLoS ONE 2012, 7, e30806. [Google Scholar] [CrossRef]
- Droeser, R.A.; Hirt, C.; Eppenberger-Castori, S.; Zlobec, I.; Viehl, C.T.; Frey, D.M.; Nebiker, C.A.; Rosso, R.; Zuber, M.; Amicarella, F.; et al. High Myeloperoxidase Positive Cell Infiltration in Colorectal Cancer Is an Independent Favorable Prognostic Factor. PLoS ONE 2013, 8, e64814. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, T.; Clarke, M.; Steele, C.W.; Samuel, M.S.; Neumann, J.; Jung, A.; Huels, D.; Olson, M.F.; Das, S.; Nibbs, R.J.; et al. Inhibition of Cxcr2 Profoundly Suppresses Inflammation-Driven and Spontaneous Tumorigenesis. J. Clin. Investig. 2012, 122, 3127–3144. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.; Bridgeman, V.L.; Ombrato, L.; Karoutas, A.; Rabas, N.; Sewnath, C.A.N.; Vasquez, M.; Rodrigues, F.S.; Horswell, S.; Faull, P.; et al. Radiation Exposure Elicits a Neutrophil-Driven Response in Healthy Lung Tissue That Enhances Metastatic Colonization. Nat. Cancer 2022, 3, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Zucker, A.; Winter, A.; Lumley, D.; Karwowski, P.; Jung, M.K.; Kao, J. Prognostic Role of Baseline Neutrophil-to-Lymphocyte Ratio in Metastatic Solid Tumors. Mol. Clin. Oncol. 2020, 13, 25. [Google Scholar] [CrossRef]
- Bae, S.J.; Cha, Y.J.; Yoon, C.; Kim, D.; Lee, J.; Park, S.; Cha, C.; Kim, J.Y.; Ahn, S.G.; Park, H.S.; et al. Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer Patients Who Received Neoadjuvant Chemotherapy. Sci. Rep. 2020, 10, 13078. [Google Scholar] [CrossRef]
- Pointer, D.T., Jr.; Roife, D.; Powers, B.D.; Murimwa, G.; Elessawy, S.; Thompson, Z.J.; Schell, M.J.; Hodul, P.J.; Pimiento, J.M.; Fleming, J.B.; et al. Neutrophil to Lymphocyte Ratio, Not Platelet to Lymphocyte or Lymphocyte to Monocyte Ratio, Is Predictive of Patient Survival after Resection of Early-Stage Pancreatic Ductal Adenocarcinoma. BMC Cancer 2020, 20, 750. [Google Scholar] [CrossRef]
- Gungabeesoon, J.; Gort-Freitas, N.A.; Kiss, M.; Bolli, E.; Messemaker, M.; Siwicki, M.; Hicham, M.; Bill, R.; Koch, P.; Cianciaruso, C.; et al. A Neutrophil Response Linked to Tumor Control in Immunotherapy. Cell 2023, 186, 1448–1464.e20. [Google Scholar] [CrossRef]
- Yan, J.; Kloecker, G.; Fleming, C.; Bousamra, M.; Hansen, R.; Hu, X.; Ding, C.; Cai, Y.; Xiang, D.; Donninger, H.; et al. Human Polymorphonuclear Neutrophils Specifically Recognize and Kill Cancerous Cells. Oncoimmunology 2014, 3, e950163. [Google Scholar] [CrossRef]
- Huang, X.; Le, W.; Chen, Q.; Chen, J.; Zhu, Y.; Shi, D.; Chen, B.; Cui, Z. Suppression of the Innate Cancer-Killing Activity in Human Granulocytes by Stress Reaction as a Possible Mechanism for Affecting Cancer Development. Stress 2020, 23, 87–96. [Google Scholar] [CrossRef]
- Maharaj, D.; Vianna, P.G.; Ward, W.; Messina, A.J.; Rayborn, T.; Gouvea, J.V.; Hammer, R.D.; Cui, Z. Young Donor White Blood Cell Immunotherapy Induces Extensive Tumor Necrosis in Advanced-Stage Solid Tumors. Heliyon 2017, 3, e00438. [Google Scholar] [CrossRef] [PubMed]
- Granot, Z.; Henke, E.; Comen, E.A.; King, T.A.; Norton, L.; Benezra, R. Tumor Entrained Neutrophils Inhibit Seeding in the Premetastatic Lung. Cancer Cell 2011, 20, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, J.Y.; Michaeli, J.; Assi, S.; Mishalian, I.; Kisos, H.; Levy, L.; Damti, P.; Lumbroso, D.; Polyansky, L.; Sionov, R.V.; et al. Phenotypic Diversity and Plasticity in Circulating Neutrophil Subpopulations in Cancer. Cell Rep. 2015, 10, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Granot, Z. Neutrophils as a Therapeutic Target in Cancer. Front. Immunol. 2019, 10, 1710. [Google Scholar] [CrossRef]
- Hirschhorn, D.; Budhu, S.; Kraehenbuehl, L.; Gigoux, M.; Schroder, D.; Chow, A.; Ricca, J.M.; Gasmi, B.; De Henau, O.; Mangarin, L.M.B.; et al. T Cell Immunotherapies Engage Neutrophils to Eliminate Tumor Antigen Escape Variants. Cell 2023, 186, 1432–1447.e17. [Google Scholar] [CrossRef]
- Linde, I.L.; Prestwood, T.R.; Qiu, J.; Pilarowski, G.; Linde, M.H.; Zhang, X.; Shen, L.; Reticker-Flynn, N.E.; Chiu, D.K.; Sheu, L.Y.; et al. Neutrophil-Activating Therapy for the Treatment of Cancer. Cancer Cell 2023, 41, 356–372.e10. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of Tumor-Associated Neutrophil Phenotype by Tgf-Beta: “N1” Versus “N2” Tan. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef]
- Gershkovitz, M.; Fainsod-Levi, T.; Zelter, T.; Sionov, R.V.; Granot, Z. Trpm2 Modulates Neutrophil Attraction to Murine Tumor Cells by Regulating Cxcl2 Expression. Cancer Immunol. Immunother. 2019, 68, 33–43. [Google Scholar] [CrossRef]
- Rice, C.M.; Davies, L.C.; Subleski, J.J.; Maio, N.; Gonzalez-Cotto, M.; Andrews, C.; Patel, N.L.; Palmieri, E.M.; Weiss, J.M.; Lee, J.M.; et al. Tumour-Elicited Neutrophils Engage Mitochondrial Metabolism to Circumvent Nutrient Limitations and Maintain Immune Suppression. Nat. Commun. 2018, 9, 5099. [Google Scholar] [CrossRef]
- Patel, S.; Fu, S.; Mastio, J.; Dominguez, G.A.; Purohit, A.; Kossenkov, A.; Lin, C.; Alicea-Torres, K.; Sehgal, M.; Nefedova, Y.; et al. Unique Pattern of Neutrophil Migration and Function During Tumor Progression. Nat. Immunol. 2018, 19, 1236–1247. [Google Scholar] [CrossRef]
- Ponzetta, A.; Carriero, R.; Carnevale, S.; Barbagallo, M.; Molgora, M.; Perucchini, C.; Magrini, E.; Gianni, F.; Kunderfranco, P.; Polentarutti, N.; et al. Neutrophils Driving Unconventional T Cells Mediate Resistance against Murine Sarcomas and Selected Human Tumors. Cell 2019, 178, 346–360.e24. [Google Scholar] [CrossRef] [PubMed]
- Matlung, H.L.; Babes, L.; Zhao, X.W.; van Houdt, M.; Treffers, L.W.; van Rees, D.J.; Franke, K.; Schornagel, K.; Verkuijlen, P.; Janssen, H.; et al. Neutrophils Kill Antibody-Opsonized Cancer Cells by Trogoptosis. Cell Rep. 2018, 23, 3946–3959.e6. [Google Scholar] [CrossRef] [PubMed]
- Zha, C.; Meng, X.; Li, L.; Mi, S.; Qian, D.; Li, Z.; Wu, P.; Hu, S.; Zhao, S.; Cai, J.; et al. Neutrophil Extracellular Traps Mediate the Crosstalk between Glioma Progression and the Tumor Microenvironment Via the Hmgb1/Rage/Il-8 Axis. Cancer Biol. Med. 2020, 17, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Boone, B.A.; Orlichenko, L.; Schapiro, N.E.; Loughran, P.; Gianfrate, G.C.; Ellis, J.T.; Singhi, A.D.; Kang, R.; Tang, D.; Lotze, M.T.; et al. The Receptor for Advanced Glycation End Products (Rage) Enhances Autophagy and Neutrophil Extracellular Traps in Pancreatic Cancer. Cancer Gene Ther. 2015, 22, 326–334. [Google Scholar] [CrossRef]
- Munir, H.; Jones, J.O.; Janowitz, T.; Hoffmann, M.; Euler, M.; Martins, C.P.; Welsh, S.J.; Shields, J.D. Stromal-Driven and Amyloid Beta-Dependent Induction of Neutrophil Extracellular Traps Modulates Tumor Growth. Nat. Commun. 2021, 12, 683. [Google Scholar] [CrossRef]
- Martins-Cardoso, K.; Almeida, V.H.; Bagri, K.M.; Rossi, M.I.D.; Mermelstein, C.S.; Konig, S.; Monteiro, R.Q. Neutrophil Extracellular Traps (Nets) Promote Pro-Metastatic Phenotype in Human Breast Cancer Cells through Epithelial-Mesenchymal Transition. Cancers 2020, 12, 1542. [Google Scholar] [CrossRef]
- Yang, R.; Zhong, L.; Yang, X.Q.; Jiang, K.L.; Li, L.; Song, H.; Liu, B.Z. Neutrophil Elastase Enhances the Proliferation and Decreases Apoptosis of Leukemia Cells Via Activation of Pi3k/Akt Signaling. Mol. Med. Rep. 2016, 13, 4175–4182. [Google Scholar] [CrossRef]
- Cui, C.; Chakraborty, K.; Tang, X.A.; Zhou, G.; Schoenfelt, K.Q.; Becker, K.M.; Hoffman, A.; Chang, Y.F.; Blank, A.; Reardon, C.A.; et al. Neutrophil Elastase Selectively Kills Cancer Cells and Attenuates Tumorigenesis. Cell 2021, 184, 3163–3177.e21. [Google Scholar] [CrossRef]
- Kalafati, L.; Kourtzelis, I.; Schulte-Schrepping, J.; Li, X.; Hatzioannou, A.; Grinenko, T.; Hagag, E.; Sinha, A.; Has, C.; Dietz, S.; et al. Innate Immune Training of Granulopoiesis Promotes Anti-Tumor Activity. Cell 2020, 183, 771–785.e12. [Google Scholar] [CrossRef] [PubMed]
- Shaul, M.E.; Fridlender, Z.G. Neutrophils as Active Regulators of the Immune System in the Tumor Microenvironment. J. Leukoc. Biol. 2017, 102, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Bergenfelz, C.; Leandersson, K. The Generation and Identity of Human Myeloid-Derived Suppressor Cells. Front. Oncol. 2020, 10, 109. [Google Scholar] [CrossRef]
- Moses, K.; Brandau, S. Human Neutrophils: Their Role in Cancer and Relation to Myeloid-Derived Suppressor Cells. Semin Immunol. 2016, 28, 187–196. [Google Scholar] [CrossRef]
- Sionov, R.V.; Fridlender, Z.G.; Granot, Z. The Multifaceted Roles Neutrophils Play in the Tumor Microenvironment. Cancer Microenviron. 2015, 8, 125–158. [Google Scholar] [CrossRef]
- Mukaida, N.; Sasaki, S.I.; Baba, T. Two-Faced Roles of Tumor-Associated Neutrophils in Cancer Development and Progression. Int. J. Mol. Sci. 2020, 21, 3457. [Google Scholar] [CrossRef]
- Mouchemore, K.A.; Anderson, R.L.; Hamilton, J.A. Neutrophils, G-Csf and Their Contribution to Breast Cancer Metastasis. FEBS J. 2018, 285, 665–679. [Google Scholar] [CrossRef]
- Andzinski, L.; Kasnitz, N.; Stahnke, S.; Wu, C.F.; Gereke, M.; von Kockritz-Blickwede, M.; Schilling, B.; Brandau, S.; Weiss, S.; Jablonska, J. Type I Ifns Induce Anti-Tumor Polarization of Tumor Associated Neutrophils in Mice and Human. Int. J. Cancer 2016, 138, 1982–1993. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Shi, L.; Venkataramani, M.; Abdelaal, A.M.; Culpepper, C.; Kidder, K.; Liang, H.; Zen, K.; Liu, Y. Tumor Conditions Induce Bone Marrow Expansion of Granulocytic, but Not Monocytic, Immunosuppressive Leukocytes with Increased Cxcr2 Expression in Mice. Eur. J. Immunol. 2018, 48, 532–542. [Google Scholar] [CrossRef]
- Shaul, M.E.; Levy, L.; Sun, J.; Mishalian, I.; Singhal, S.; Kapoor, V.; Horng, W.; Fridlender, G.; Albelda, S.M.; Fridlender, Z.G. Tumor-Associated Neutrophils Display a Distinct N1 Profile Following Tgfbeta Modulation: A Transcriptomics Analysis of Pro- Vs. Antitumor Tans. Oncoimmunology 2016, 5, e1232221. [Google Scholar] [CrossRef]
- Kim, J.; Bae, J.S. Tumor-Associated Macrophages and Neutrophils in Tumor Microenvironment. Mediators Inflamm. 2016, 2016, 6058147. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Q.; Lu, L.; Xu, C.; Li, J.; Zha, J.; Ma, F.; Luo, H.R.; Hsu, A.Y. Heterogeneity of Neutrophils in Cancer: One Size Does Not Fit All. Cancer Biol. Med. 2022, 19, 1629–1648. [Google Scholar] [CrossRef]
- Ng, M.; Cerezo-Wallis, D.; Ng, L.G.; Hidalgo, A. Adaptations of Neutrophils in Cancer. Immunity 2025, 58, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Salcher, S.; Sturm, G.; Horvath, L.; Untergasser, G.; Kuempers, C.; Fotakis, G.; Panizzolo, E.; Martowicz, A.; Trebo, M.; Pall, G.; et al. High-Resolution Single-Cell Atlas Reveals Diversity and Plasticity of Tissue-Resident Neutrophils in Non-Small Cell Lung Cancer. Cancer Cell 2022, 40, 1503–1520.e8. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ma, J.; Yang, X.; Nan, F.; Zhang, T.; Ji, S.; Rao, D.; Feng, H.; Gao, K.; Gu, X.; et al. Neutrophil Profiling Illuminates Anti-Tumor Antigen-Presenting Potency. Cell 2024, 187, 1422–1439.e24. [Google Scholar] [CrossRef]
- Veglia, F.; Hashimoto, A.; Dweep, H.; Sanseviero, E.; De Leo, A.; Tcyganov, E.; Kossenkov, A.; Mulligan, C.; Nam, B.; Masters, G.; et al. Analysis of Classical Neutrophils and Polymorphonuclear Myeloid-Derived Suppressor Cells in Cancer Patients and Tumor-Bearing Mice. J. Exp. Med. 2021, 218, e20201803. [Google Scholar] [CrossRef]
- Zilionis, R.; Engblom, C.; Pfirschke, C.; Savova, V.; Zemmour, D.; Saatcioglu, H.D.; Krishnan, I.; Maroni, G.; Meyerovitz, C.V.; Kerwin, C.M.; et al. Single-Cell Transcriptomics of Human and Mouse Lung Cancers Reveals Conserved Myeloid Populations across Individuals and Species. Immunity 2019, 50, 1317–1334.e10. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Dai, Y.; Tang, X.; Yin, T.; Wang, C.; Wang, T.; Dong, L.; Shi, M.; Qin, J.; et al. Single-Cell Rna-Seq Analysis Reveals Bhlhe40-Driven Pro-Tumour Neutrophils with Hyperactivated Glycolysis in Pancreatic Tumour Microenvironment. Gut 2023, 72, 958–971. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Eggert, T.; Araujo, D.J.; Vijayanand, P.; Ottensmeier, C.H.; Hedrick, C.C. Cytof Mass Cytometry Reveals Phenotypically Distinct Human Blood Neutrophil Populations Differentially Correlated with Melanoma Stage. J. Immunother. Cancer 2020, 8, e000473. [Google Scholar] [CrossRef]
- Alshetaiwi, H.; Pervolarakis, N.; McIntyre, L.L.; Ma, D.; Nguyen, Q.; Rath, J.A.; Nee, K.; Hernandez, G.; Evans, K.; Torosian, L.; et al. Defining the Emergence of Myeloid-Derived Suppressor Cells in Breast Cancer Using Single-Cell Transcriptomics. Sci. Immunol. 2020, 5, eaay6017. [Google Scholar] [CrossRef]
- Sionov, R.V.; Fainsod-Levi, T.; Zelter, T.; Polyansky, L.; Pham, C.T.; Granot, Z. Neutrophil Cathepsin G and Tumor Cell Rage Facilitate Neutrophil Anti-Tumor Cytotoxicity. Oncoimmunology 2019, 8, e1624129. [Google Scholar] [CrossRef]
- Knowles, H.; Li, Y.; Perraud, A.L. The Trpm2 Ion Channel, an Oxidative Stress and Metabolic Sensor Regulating Innate Immunity and Inflammation. Immunol. Res. 2013, 55, 241–248. [Google Scholar] [CrossRef]
- Khanna, S.; Graef, S.; Mussai, F.; Thomas, A.; Wali, N.; Yenidunya, B.G.; Yuan, C.; Morrow, B.; Zhang, J.; Korangy, F.; et al. Tumor-Derived Gm-Csf Promotes Granulocyte Immunosuppression in Mesothelioma Patients. Clin. Cancer Res. 2018, 24, 2859–2872. [Google Scholar] [CrossRef] [PubMed]
- Valgardsdottir, R.; Cattaneo, I.; Klein, C.; Introna, M.; Figliuzzi, M.; Golay, J. Human Neutrophils Mediate Trogocytosis Rather Than Phagocytosis of Cll B Cells Opsonized with Anti-Cd20 Antibodies. Blood 2017, 129, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, H.; Onuma, A.E.; Tsung, A. Neutrophil Elastase and Neutrophil Extracellular Traps in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1263, 13–23. [Google Scholar]
- Masucci, M.T.; Minopoli, M.; Del Vecchio, S.; Carriero, M.V. The Emerging Role of Neutrophil Extracellular Traps (Nets) in Tumor Progression and Metastasis. Front. Immunol. 2020, 11, 1749. [Google Scholar] [CrossRef] [PubMed]
- Najmeh, S.; Cools-Lartigue, J.; Rayes, R.F.; Gowing, S.; Vourtzoumis, P.; Bourdeau, F.; Giannias, B.; Berube, J.; Rousseau, S.; Ferri, L.E.; et al. Neutrophil Extracellular Traps Sequester Circulating Tumor Cells Via Beta1-Integrin Mediated Interactions. Int. J. Cancer 2017, 140, 2321–2330. [Google Scholar] [CrossRef]
- Baumann, M.; Pham, C.T.; Benarafa, C. Serpinb1 Is Critical for Neutrophil Survival through Cell-Autonomous Inhibition of Cathepsin G. Blood 2013, 121, 3900–3907. [Google Scholar] [CrossRef]
- DiCamillo, S.J.; Yang, S.; Panchenko, M.V.; Toselli, P.A.; Naggar, E.F.; Rich, C.B.; Stone, P.J.; Nugent, M.A.; Panchenko, M.P. Neutrophil Elastase-Initiated Egfr/Mek/Erk Signaling Counteracts Stabilizing Effect of Autocrine Tgf-Beta on Tropoelastin Mrna in Lung Fibroblasts. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L232–L243. [Google Scholar] [CrossRef]
- Wada, Y.; Yoshida, K.; Tsutani, Y.; Shigematsu, H.; Oeda, M.; Sanada, Y.; Suzuki, T.; Mizuiri, H.; Hamai, Y.; Tanabe, K.; et al. Neutrophil Elastase Induces Cell Proliferation and Migration by the Release of Tgf-A, Pdgf and Vegf in Esophageal Cell Lines. Oncol. Rep. 2007, 17, 161–167. [Google Scholar] [CrossRef]
- Singh, D.; Tewari, M.; Singh, S.; Narayan, G. Revisiting the Role of Trail/Trail-R in Cancer Biology and Therapy. Future Oncol. 2021, 17, 581–596. [Google Scholar] [CrossRef]
- Yuan, X.; Gajan, A.; Chu, Q.; Xiong, H.; Wu, K.; Wu, G.S. Developing Trail/Trail Death Receptor-Based Cancer Therapies. Cancer Metastasis Rev. 2018, 37, 733–748. [Google Scholar] [CrossRef]
- Koga, Y.; Matsuzaki, A.; Suminoe, A.; Hattori, H.; Hara, T. Neutrophil-Derived Tnf-Related Apoptosis-Inducing Ligand (Trail): A Novel Mechanism of Antitumor Effect by Neutrophils. Cancer Res. 2004, 64, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Kemp, T.J.; Ludwig, A.T.; Earel, J.K.; Moore, J.M.; Vanoosten, R.L.; Moses, B.; Leidal, K.; Nauseef, W.M.; Griffith, T.S. Neutrophil Stimulation with Mycobacterium Bovis Bacillus Calmette-Guerin (Bcg) Results in the Release of Functional Soluble Trail/Apo-2l. Blood 2005, 106, 3474–3482. [Google Scholar] [CrossRef]
- Chen, C.L.; Wang, Y.; Huang, C.Y.; Zhou, Z.Q.; Zhao, J.J.; Zhang, X.F.; Pan, Q.Z.; Wu, J.X.; Weng, D.S.; Tang, Y.; et al. Il-17 Induces Antitumor Immunity by Promoting Beneficial Neutrophil Recruitment and Activation in Esophageal Squamous Cell Carcinoma. Oncoimmunology 2017, 7, e1373234. [Google Scholar] [CrossRef] [PubMed]
- Divangahi, M.; Aaby, P.; Khader, S.A.; Barreiro, L.B.; Bekkering, S.; Chavakis, T.; van Crevel, R.; Curtis, N.; DiNardo, A.R.; Dominguez-Andres, J.; et al. Trained Immunity, Tolerance, Priming and Differentiation: Distinct Immunological Processes. Nat. Immunol. 2021, 22, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G. “Training Innate Immunity: The Changing Concept of Immunological Memory in Innate Host Defence. Eur. J. Clin. Invest. 2013, 43, 881–884. [Google Scholar] [CrossRef]
- Xing, Z.; Afkhami, S.; Bavananthasivam, J.; Fritz, D.K.; D’Agostino, M.R.; Vaseghi-Shanjani, M.; Yao, Y.; Jeyanathan, M. Innate Immune Memory of Tissue-Resident Macrophages and Trained Innate Immunity: Re-Vamping Vaccine Concept and Strategies. J. Leukoc. Biol. 2020, 108, 825–834. [Google Scholar] [CrossRef]
- Cirovic, B.; de Bree, L.C.J.; Groh, L.; Blok, B.A.; Chan, J.; van der Velden, W.; Bremmers, M.E.J.; van Crevel, R.; Handler, K.; Picelli, S.; et al. Bcg Vaccination in Humans Elicits Trained Immunity Via the Hematopoietic Progenitor Compartment. Cell Host Microbe. 2020, 28, 322–334.e5. [Google Scholar] [CrossRef]
- Suttmann, H.; Riemensberger, J.; Bentien, G.; Schmaltz, D.; Stockle, M.; Jocham, D.; Bohle, A.; Brandau, S. Neutrophil Granulocytes Are Required for Effective Bacillus Calmette-Guerin Immunotherapy of Bladder Cancer and Orchestrate Local Immune Responses. Cancer Res. 2006, 66, 8250–8257. [Google Scholar] [CrossRef]
- Mitroulis, I.; Ruppova, K.; Wang, B.; Chen, L.S.; Grzybek, M.; Grinenko, T.; Eugster, A.; Troullinaki, M.; Palladini, A.; Kourtzelis, I.; et al. Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell 2018, 172, 147–161.e12. [Google Scholar] [CrossRef] [PubMed]
- Que, H.; Fu, Q.; Lan, T.; Tian, X.; Wei, X. Tumor-Associated Neutrophils and Neutrophil-Targeted Cancer Therapies. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188762. [Google Scholar] [CrossRef] [PubMed]
- Teijeira, A.; Garasa, S.; Gato, M.; Alfaro, C.; Migueliz, I.; Cirella, A.; de Andrea, C.; Ochoa, M.C.; Otano, I.; Etxeberria, I.; et al. Cxcr1 and Cxcr2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps That Interfere with Immune Cytotoxicity. Immunity 2020, 52, 856–871.e8. [Google Scholar] [CrossRef]
- Cheng, Y.; Mo, F.; Li, Q.; Han, X.; Shi, H.; Chen, S.; Wei, Y.; Wei, X. Targeting Cxcr2 Inhibits the Progression of Lung Cancer and Promotes Therapeutic Effect of Cisplatin. Mol. Cancer 2021, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Schott, A.F.; Goldstein, L.J.; Cristofanilli, M.; Ruffini, P.A.; McCanna, S.; Reuben, J.M.; Perez, R.P.; Kato, G.; Wicha, M. Phase Ib Pilot Study to Evaluate Reparixin in Combination with Weekly Paclitaxel in Patients with Her-2-Negative Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 5358–5365. [Google Scholar] [CrossRef]
- Goldstein, L.J.; Mansutti, M.; Levy, C.; Chang, J.C.; Henry, S.; Fernandez-Perez, I.; Prausova, J.; Staroslawska, E.; Viale, G.; Butler, B.; et al. A Randomized, Placebo-Controlled Phase 2 Study of Paclitaxel in Combination with Reparixin Compared to Paclitaxel Alone as Front-Line Therapy for Metastatic Triple-Negative Breast Cancer (Frida). Breast Cancer Res. Treat. 2021, 190, 265–275. [Google Scholar] [CrossRef]
- Xie, Y.; Kuang, W.; Wang, D.; Yuan, K.; Yang, P. Expanding Role of Cxcr2 and Therapeutic Potential of Cxcr2 Antagonists in Inflammatory Diseases and Cancers. Eur. J. Med. Chem. 2023, 250, 115175. [Google Scholar] [CrossRef]
- Mousset, A.; Bellone, L.; Gaggioli, C.; Albrengues, J. Netscape or Nethance: Tailoring Anti-Cancer Therapy. Trends Cancer 2024, 10, 655–667. [Google Scholar] [CrossRef]
- Peng, D.; Fu, M.; Wang, M.; Wei, Y.; Wei, X. Targeting Tgf-Beta Signal Transduction for Fibrosis and Cancer Therapy. Mol. Cancer 2022, 21, 104. [Google Scholar] [CrossRef]
- Tarhini, A.A.; Gogas, H.; Kirkwood, J.M. Ifn-Alpha in the Treatment of Melanoma. J. Immunol. 2012, 189, 3789–3793. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, H.; Deng, Y.; Tai, Y.; Zeng, K.; Zhang, Y.; Liu, W.; Zhang, Q.; Yang, Y. Cancer-Associated Fibroblasts Induce Pdl1+ Neutrophils through the Il6-Stat3 Pathway That Foster Immune Suppression in Hepatocellular Carcinoma. Cell Death Dis. 2018, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- McGinley, A.; Florence, S.; Woodward, M.J.; Patel, D.; Willis, A.; Blyth, A.; Quintas-Cardama, A.; Polyakova, O. Immunomodulatory Alpha Neutrophils: The First-in-Class Neutrophil Progenitor-Based Allogeneic Immuno-Cell Therapy with Cytotoxic and Immunomodulatory Functionality for the Treatment of Solid Tumours. J. Clin. Oncol. 2024, 42 (Suppl. 16), e14523. [Google Scholar] [CrossRef]
- Bonecchi, R.; Mantovani, A.; Jaillon, S. Chemokines as Regulators of Neutrophils: Focus on Tumors, Therapeutic Targeting, and Immunotherapy. Cancers 2022, 14, 680. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, J.; Wang, X.; Ji, C.; Yu, D.; Wang, M.; Pan, J.; Santos, H.A.; Zhang, H.; Zhang, X. Engineering and Targeting Neutrophils for Cancer Therapy. Adv. Mater 2024, 36, e2310318. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, D.; Zou, J.; Li, X.; Guo, X.D.; Tang, Y.; Liu, C.; Chen, W.; Kong, N.; Zhang, C.Y.; et al. Living Leukocyte-Based Drug Delivery Systems. Adv. Mater 2023, 35, e2207787. [Google Scholar] [CrossRef]
- Luo, Z.; Lu, Y.; Shi, Y.; Jiang, M.; Shan, X.; Li, X.; Zhang, J.; Qin, B.; Liu, X.; Guo, X.; et al. Neutrophil Hitchhiking for Drug Delivery to the Bone Marrow. Nat. Nanotechnol. 2023, 18, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Cai, X.; Syahirah, R.; Yao, Y.; Xu, Y.; Jin, G.; Bhute, V.J.; Torregrosa-Allen, S.; Elzey, B.D.; Won, Y.Y.; et al. Car-Neutrophil Mediated Delivery of Tumor-Microenvironment Responsive Nanodrugs for Glioblastoma Chemo-Immunotherapy. Nat. Commun. 2023, 14, 2266. [Google Scholar] [CrossRef]
- Yin, Y.; Tang, W.; Ma, X.; Tang, L.; Zhang, Y.; Yang, M.; Hu, F.; Li, G.; Wang, Y. Biomimetic Neutrophil and Macrophage Dual Membrane-Coated Nanoplatform with Orchestrated Tumor-Microenvironment Responsive Capability Promotes Therapeutic Efficacy against Glioma. Chem. Eng. J. 2022, 433, 133848. [Google Scholar] [CrossRef]
- Rudloff, M.W.; Zumbo, P.; Favret, N.R.; Roetman, J.J.; Roman, C.R.D.; Erwin, M.M.; Murray, K.A.; Jonnakuti, S.T.; Dundar, F.; Betel, D.; et al. Hallmarks of Cd8(+) T Cell Dysfunction Are Established within Hours of Tumor Antigen Encounter before Cell Division. Nat. Immunol. 2023, 24, 1527–1539. [Google Scholar] [CrossRef]
- Kirschenbaum, D.; Xie, K.; Ingelfinger, F.; Katzenelenbogen, Y.; Abadie, K.; Look, T.; Sheban, F.; Phan, T.S.; Li, B.; Zwicky, P.; et al. Time-Resolved Single-Cell Transcriptomics Defines Immune Trajectories in Glioblastoma. Cell 2024, 187, 149–165.e23. [Google Scholar] [CrossRef]
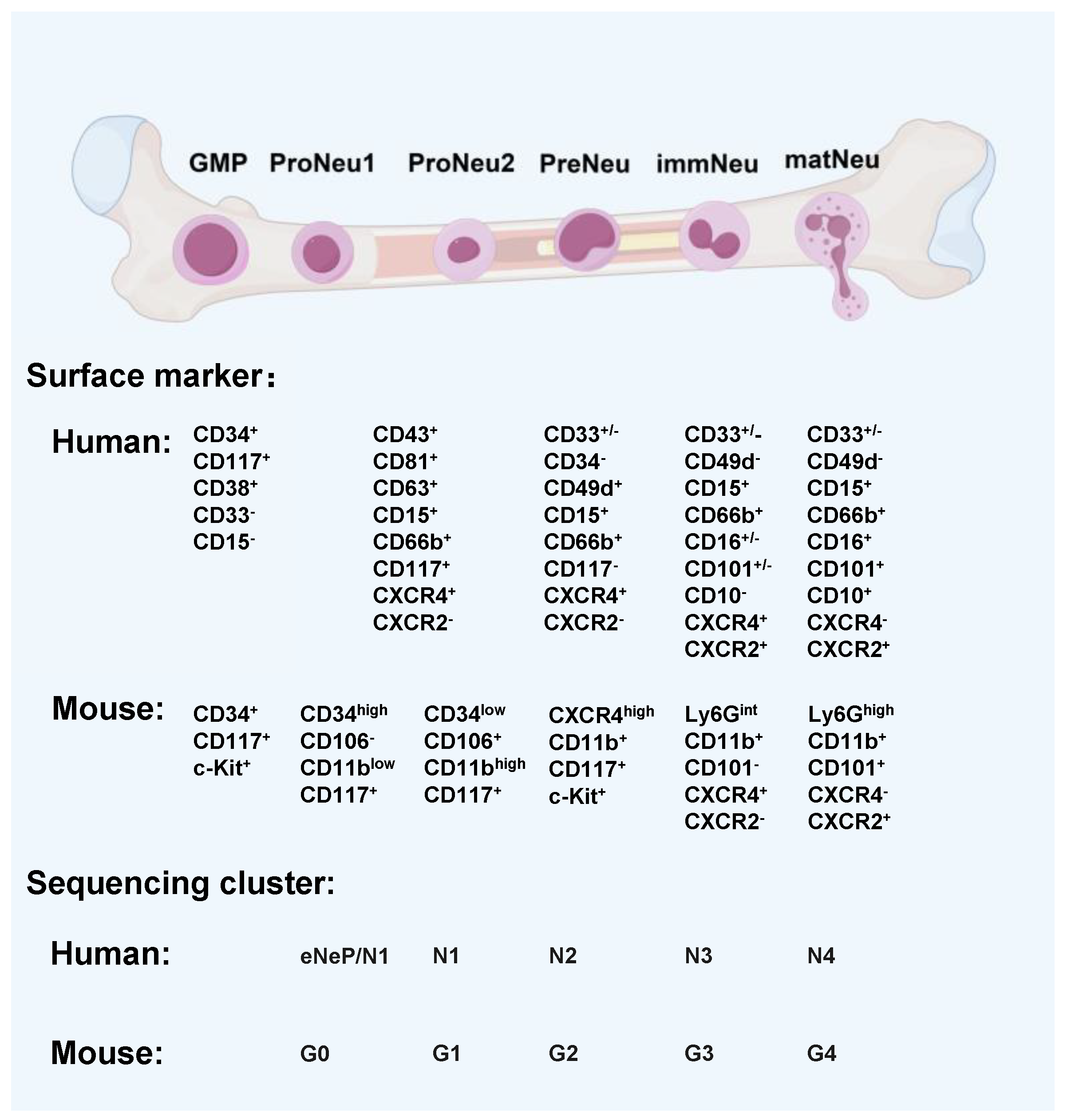

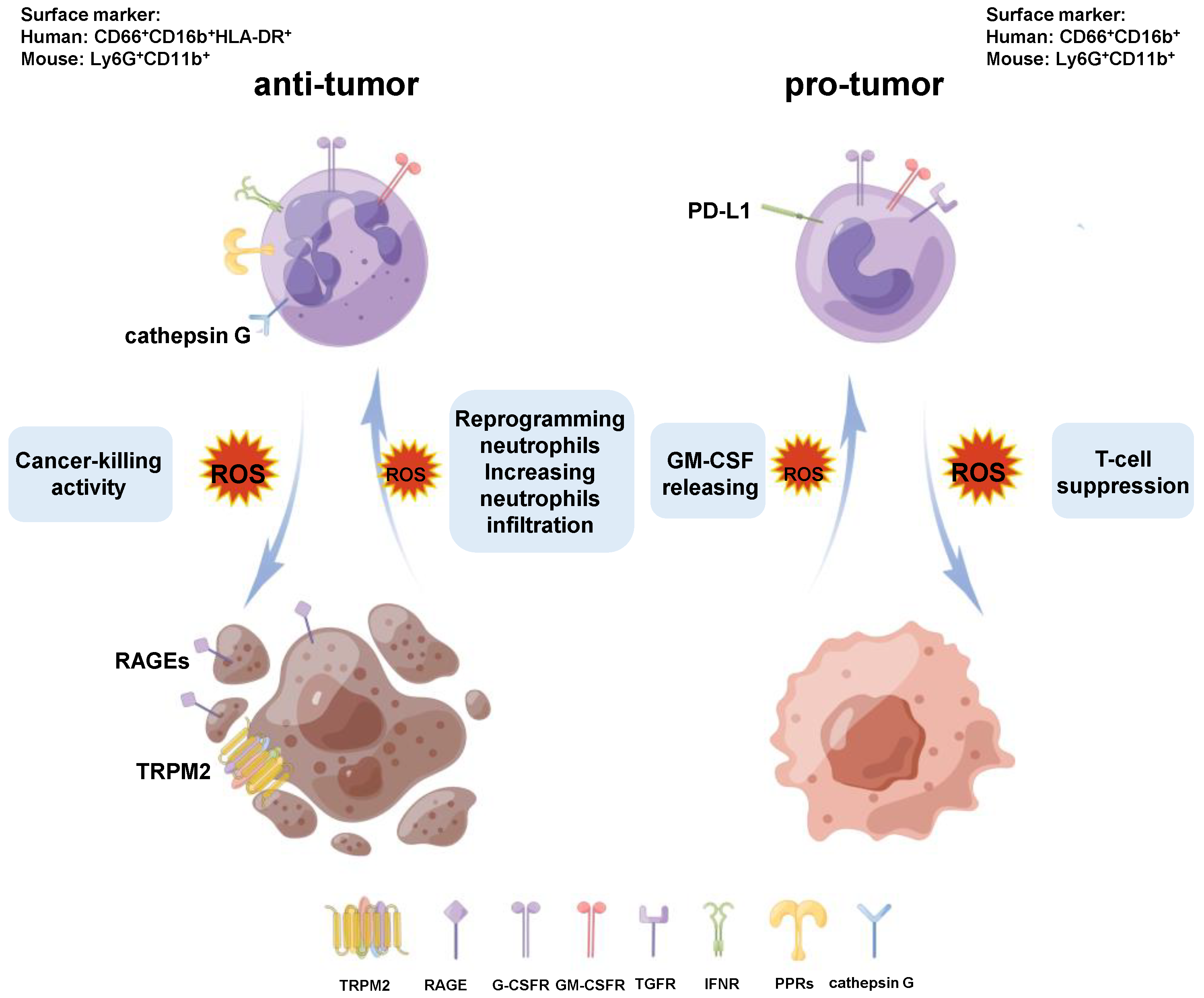
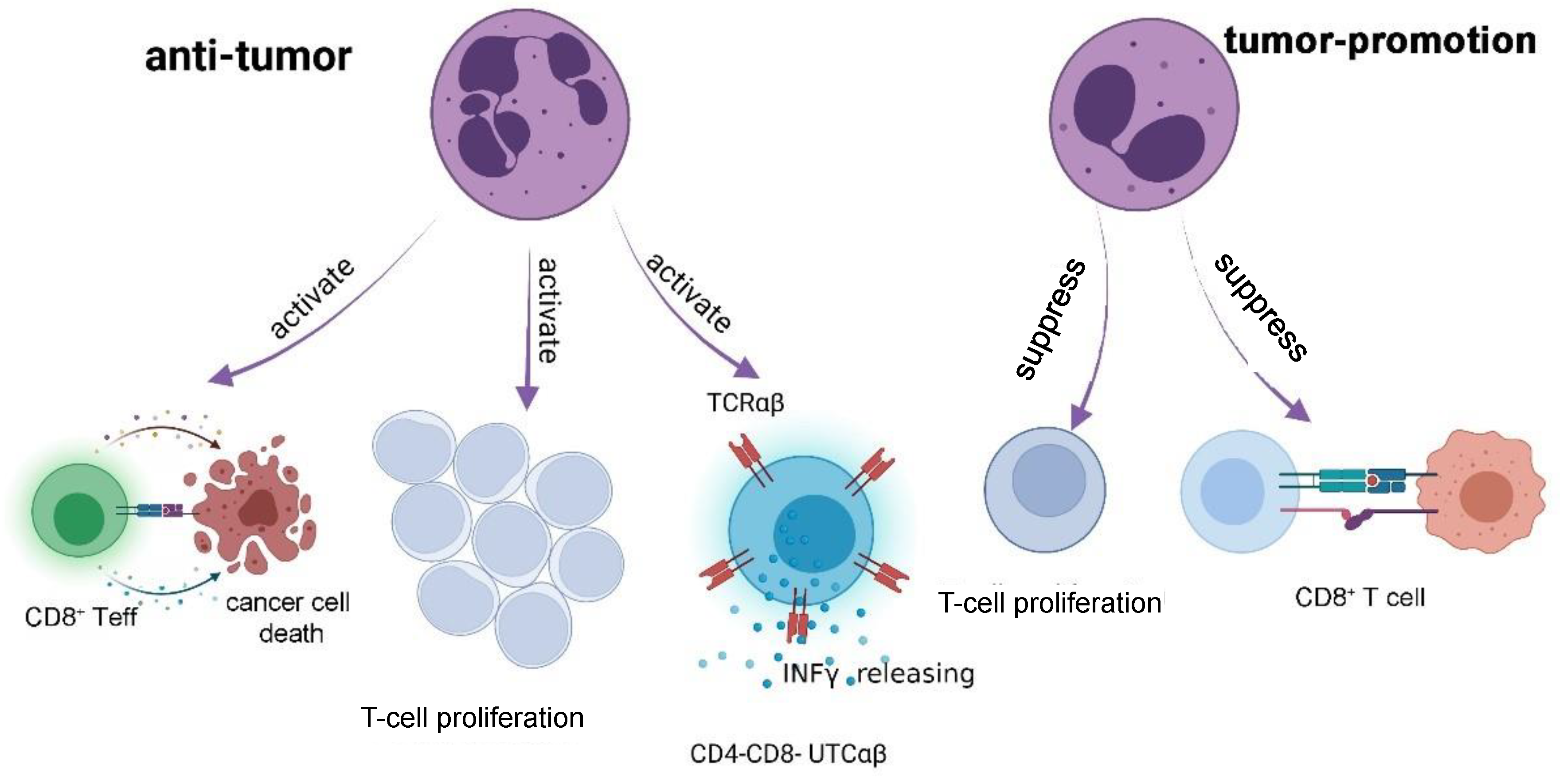
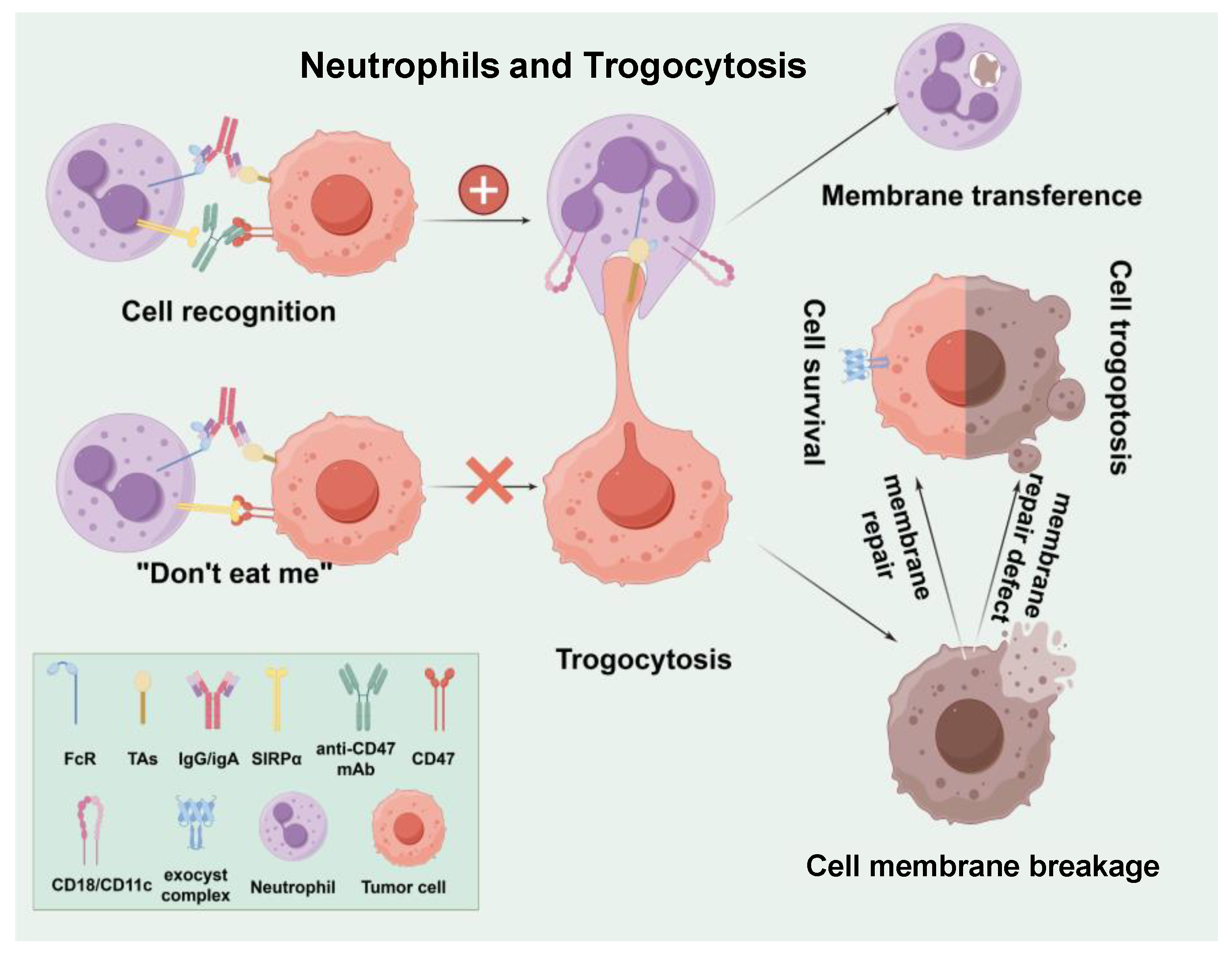
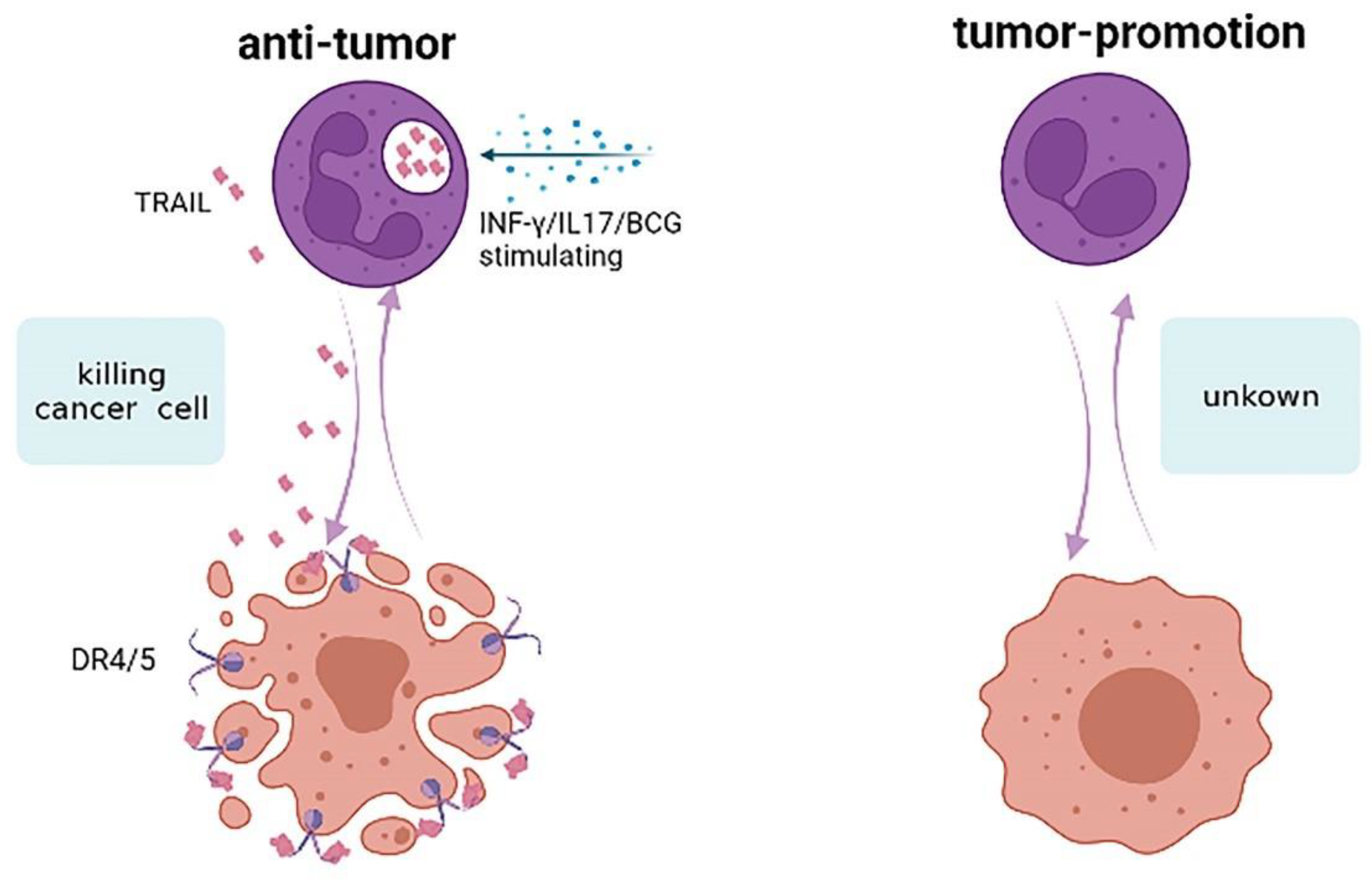
| Tumor Source | Neutrophil Source | Effect of Neutrophils in Tumor | Potential Mechanism | Reference |
|---|---|---|---|---|
| intestinal adenocarcinoma | neutrophils in spontaneous and inflammation-driven neoplasia (mice) | tumor promotion | CXCR2-MPO+ | [24] |
| lung metastasis | radiation-stimulated neutrophils (mice) | tumor promotion | degranulation | [26] |
| ovarian cancer, cervical cancer, lung cancer (cell line) | discrete neutrophils (healthy human donors) | tumor suppression | ROS-induced tumor killing | [31] |
| lung cancer | discrete neutrophils (healthy human donors) | tumor suppression | ROS-induced tumor killing | [32] |
| breast cancer | discrete high-density neutrophils (lung cancer mice) | tumor suppression | ROS-induced tumor killing | [35] |
| breast cancer | discrete low-density neutrophil (lung cancer mice) | no significant tumor suppression | none | [35] |
| mesothelioma | TGF-β blockade stimulated neutrophil (mice) | tumor suppression | ROS-induced tumor killing, CTL-induced tumor killing | [39] |
| breast cancer, Lewis lung carcinoma | discrete high-density neutrophils (mice) | tumor suppression | ROS-induced tumor killing (RAGE/cathepsin G-mediated cytotoxicity) | [16] |
| breast cancer, Lewis lung carcinoma | neutrophils (mice) | tumor suppression | ROS-induced tumor killing | [40] |
| breast cancer | neutrophils (mice) | tumor promotion | oxidative mitochondrial metabolism, T-cell suppression | [41] |
| Lewis lung carcinoma | discrete neutrophils (mice with early-stage cancer) | tumor suppression | CTL-induced tumor killing | [42] |
| Lewis lung carcinoma | discrete neutrophils (mice with late-stage cancer) | tumor promotion | T-cell suppression | [42] |
| 3-MCA sarcomagenesis | neutrophils (mice) | tumor suppression | UTCαβ induced tumor suppression | [43] |
| breast cancer | discrete neutrophils (unknown) | tumor suppression | trogocytosis | [44] |
| glioma | neutrophils (mice) | tumor promotion | NETs produced by neutrophils | [45] |
| pancreatic ductal adenocarcinoma | neutrophils (mice) | tumor promotion | NETs produced by neutrophils | [46] |
| pancreatic, lung, or skin tumors | neutrophils (mice) | tumor promotion | NETs produced by neutrophils | [47] |
| breast cancer (cell line) | discrete neutrophil stimulated phorbol 12-myristate 13-acetate (healthy human donors) | tumor promotion | NETs produced by neutrophils | [48] |
| leukemia (cell line) | NB4 acute pro-myelocytic leukemia cells containing NE gene | tumor promotion | NE produced by acute pro-myelocytic leukemia cells | [49] |
| thirty-five different human or murine cancers (cell line) | discrete neutrophils (healthy mice and human donors) | tumor suppression | NE produced by neutrophils; CTL-induced tumor killing | [50] |
| lung carcinoma | discrete neutrophils (healthy mice) | tumor suppression | NE produced by neutrophils; CTL-induced tumor killing | [50] |
| melanoma | β-glucan stimulated neutrophils (mice) | tumor suppression | trained immunity | [51] |
| lung carcinoma | neutrophils after immunotherapy (mice and human) | tumor suppression | Interferon gene | [30] |
| melanoma | neutrophils after immunotherapy (mice) | tumor eradication | iNOS-dependent mechanism | [37] |
| melanoma and metastatic seeding | neutrophils after combination activated (mice and human) | tumor eradication | ROS-induced tumor killing, CTL-induced tumor killing | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Chen, B.; Zhao, H. Role of Neutrophils in Anti-Tumor Activity: Characteristics and Mechanisms of Action. Cancers 2025, 17, 1298. https://doi.org/10.3390/cancers17081298
Chen X, Chen B, Zhao H. Role of Neutrophils in Anti-Tumor Activity: Characteristics and Mechanisms of Action. Cancers. 2025; 17(8):1298. https://doi.org/10.3390/cancers17081298
Chicago/Turabian StyleChen, Xin, Bingdi Chen, and Huadong Zhao. 2025. "Role of Neutrophils in Anti-Tumor Activity: Characteristics and Mechanisms of Action" Cancers 17, no. 8: 1298. https://doi.org/10.3390/cancers17081298
APA StyleChen, X., Chen, B., & Zhao, H. (2025). Role of Neutrophils in Anti-Tumor Activity: Characteristics and Mechanisms of Action. Cancers, 17(8), 1298. https://doi.org/10.3390/cancers17081298





