Current and Future Applications of 5-Aminolevulinic Acid in Neurosurgical Oncology
Simple Summary
Abstract
1. Introduction
2. Current Neurosurgical Applications of 5-ALA
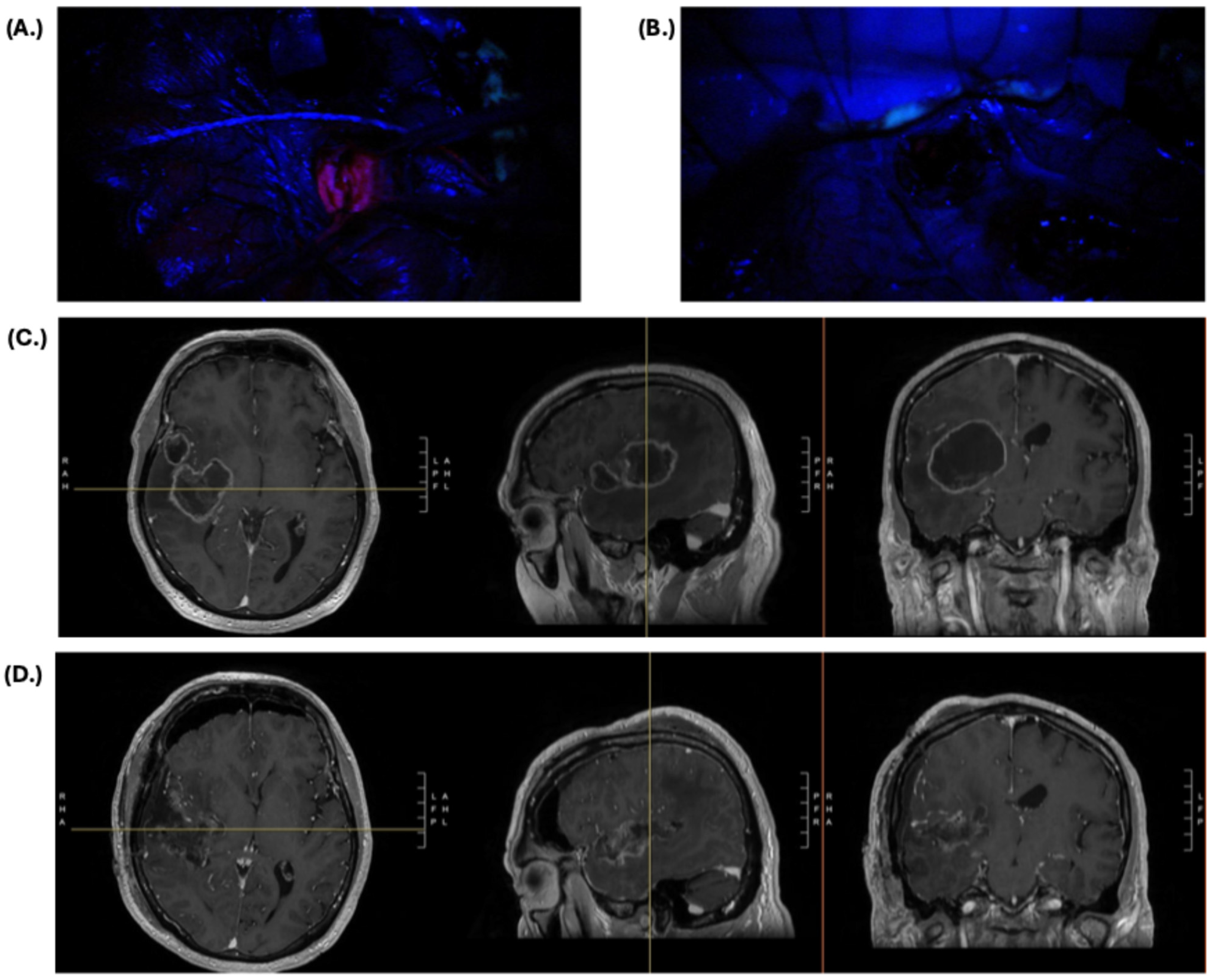
2.1. Primary and Recurrent High-Grade Glioma
2.2. Low-Grade Glioma
2.3. Meningioma
3. Patient Considerations for 5-ALA
3.1. Preoperative
3.2. Intraoperative
4. Limitations and Future Neurosurgical Applications of 5-ALA
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yoo, H.; Kim, Y.Z.; Nam, B.H.; Shin, S.H.; Yang, H.S.; Lee, J.S.; Zo, J.I.; Lee, S.H. Reduced Local Recurrence of a Single Brain Metastasis through Microscopic Total Resection. J. Neurosurg. 2009, 110, 730–736. [Google Scholar] [CrossRef]
- Sanai, N.; Polley, M.-Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An Extent of Resection Threshold for Newly Diagnosed Glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bloch, O.; Han, S.J.; Cha, S.; Sun, M.Z.; Aghi, M.K.; McDermott, M.W.; Berger, M.S.; Parsa, A.T. Impact of Extent of Resection for Recurrent Glioblastoma on Overall Survival: Clinical Article. J. Neurosurg. 2012, 117, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.L.; Marciscano, A.E.; Niemierko, A.; Kim, D.W.; Stemmer-Rachamimov, A.O.; Curry, W.T.; Barker, F.G., 2nd; Martuza, R.L.; Loeffler, J.S.; Oh, K.S.; et al. Imaging and Extent of Surgical Resection Predict Risk of Meningioma Recurrence Better than WHO Histopathological Grade. Neuro-Oncol. 2016, 18, 863–872. [Google Scholar] [CrossRef]
- Hatoum, R.; Chen, J.-S.; Lavergne, P.; Shlobin, N.A.; Wang, A.; Elkaim, L.M.; Dodin, P.; Couturier, C.P.; Ibrahim, G.M.; Fallah, A.; et al. Extent of Tumor Resection and Survival in Pediatric Patients with High-Grade Gliomas: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2022, 5, e2226551. [Google Scholar] [CrossRef]
- Rao, J.S. Molecular Mechanisms of Glioma Invasiveness: The Role of Proteases. Nat. Rev. Cancer 2003, 3, 489–501. [Google Scholar] [CrossRef]
- Albert, F.K.; Forsting, M.; Sartor, K.; Adams, H.P.; Kunze, S. Early Postoperative Magnetic Resonance Imaging after Resection of Malignant Glioma: Objective Evaluation of Residual Tumor and Its Influence on Regrowth and Prognosis. Neurosurgery 1994, 34, 45–60; discussion 60–61. [Google Scholar] [CrossRef] [PubMed]
- Yamahara, T.; Numa, Y.; Oishi, T.; Kawaguchi, T.; Seno, T.; Asai, A.; Kawamoto, K. Morphological and Flow Cytometric Analysis of Cell Infiltration in Glioblastoma: A Comparison of Autopsy Brain and Neuroimaging. Brain Tumor Pathol. 2010, 27, 81–87. [Google Scholar] [CrossRef]
- Barajas, R.F., Jr.; Phillips, J.J.; Parvataneni, R.; Molinaro, A.; Essock-Burns, E.; Bourne, G.; Parsa, A.T.; Aghi, M.K.; McDermott, M.W.; Berger, M.S.; et al. Regional Variation in Histopathologic Features of Tumor Specimens from Treatment-Naive Glioblastoma Correlates with Anatomic and Physiologic MR Imaging. Neuro-Oncol. 2012, 14, 942–954. [Google Scholar] [CrossRef]
- Eidel, O.; Burth, S.; Neumann, J.-O.; Kieslich, P.J.; Sahm, F.; Jungk, C.; Kickingereder, P.; Bickelhaupt, S.; Mundiyanapurath, S.; Bäumer, P.; et al. Tumor Infiltration in Enhancing and Non-Enhancing Parts of Glioblastoma: A Correlation with Histopathology. PLoS ONE 2017, 12, e0169292. [Google Scholar] [CrossRef]
- Haddad, A.F.; Young, J.S.; Morshed, R.A.; Berger, M.S. FLAIRectomy: Resecting beyond the Contrast Margin for Glioblastoma. Brain Sci. 2022, 12, 544. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.V.; Magaut, C.R.; Storevik, S.; Geraldo, L.H.; Mathivet, T.; Latif, M.A.; Rudewicz, J.; Guyon, J.; Gambaretti, M.; Haukas, F.; et al. TGF-β Promotes Microtube Formation in Glioblastoma through Thrombospondin 1. Neuro-Oncol. 2022, 24, 541–553. [Google Scholar] [CrossRef]
- Venkataramani, V.; Yang, Y.; Schubert, M.C.; Reyhan, E.; Tetzlaff, S.K.; Wißmann, N.; Botz, M.; Soyka, S.J.; Beretta, C.A.; Pramatarov, R.L.; et al. Glioblastoma Hijacks Neuronal Mechanisms for Brain Invasion. Cell 2022, 185, 2899–2917.e31. [Google Scholar] [CrossRef]
- Mathur, R.; Wang, Q.; Schupp, P.G.; Nikolic, A.; Hilz, S.; Hong, C.; Grishanina, N.R.; Kwok, D.; Stevers, N.O.; Jin, Q.; et al. Glioblastoma Evolution and Heterogeneity from a 3D Whole-Tumor Perspective. Cell 2024, 187, 446–463.e16. [Google Scholar] [CrossRef]
- Molinaro, A.M.; Hervey-Jumper, S.; Morshed, R.A.; Young, J.; Han, S.J.; Chunduru, P.; Zhang, Y.; Phillips, J.J.; Shai, A.; Lafontaine, M.; et al. Association of Maximal Extent of Resection of Contrast-Enhanced and Non-Contrast-Enhanced Tumor with Survival within Molecular Subgroups of Patients with Newly Diagnosed Glioblastoma. JAMA Oncol. 2020, 6, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Suki, D.; Hess, K.; Sawaya, R. The Influence of Maximum Safe Resection of Glioblastoma on Survival in 1229 Patients: Can We Do Better than Gross-Total Resection? J. Neurosurg. 2016, 124, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Incekara, F.; Koene, S.; Vincent, A.J.P.E.; van den Bent, M.J.; Smits, M. Association between Supratotal Glioblastoma Resection and Patient Survival: A Systematic Review and Meta-Analysis. World Neurosurg. 2019, 127, 617–624.e2. [Google Scholar] [CrossRef]
- Jackson, C.; Choi, J.; Khalafallah, A.M.; Price, C.; Bettegowda, C.; Lim, M.; Gallia, G.; Weingart, J.; Brem, H.; Mukherjee, D. A Systematic Review and Meta-Analysis of Supratotal versus Gross Total Resection for Glioblastoma. J. Neurooncol. 2020, 148, 419–431. [Google Scholar] [CrossRef]
- Hirono, S.; Ozaki, K.; Kobayashi, M.; Hara, A.; Yamaki, T.; Matsutani, T.; Iwadate, Y. Oncological and Functional Outcomes of Supratotal Resection of IDH1 Wild-Type Glioblastoma Based on 11C-Methionine PET: A Retrospective, Single-Center Study. Sci. Rep. 2021, 11, 14554. [Google Scholar] [CrossRef]
- Tabor, J.K.; Bonda, D.; LeMonda, B.C.; D’Amico, R.S. Neuropsychological Outcomes Following Supratotal Resection for High-Grade Glioma: A Review. J. Neurooncol. 2021, 152, 429–437. [Google Scholar] [CrossRef]
- Yoo, J.; Yoon, S.-J.; Kim, K.H.; Jung, I.-H.; Lim, S.H.; Kim, W.; Yoon, H.I.; Kim, S.H.; Sung, K.S.; Roh, T.H.; et al. Patterns of Recurrence According to the Extent of Resection in Patients with IDH-Wild-Type Glioblastoma: A Retrospective Study. J. Neurosurg. 2022, 137, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Massaad, E.; Smith, W.J.; Bradley, J.; Esposito, E.; Gupta, M.; Burns, E.; Burns, R.; Velarde, J.K.; Berglar, I.K.; Gupta, R.; et al. Radical Surgical Resection with Molecular Margins Is Associated with Improved Survival in IDH Wild-Type Glioblastoma. Neuro-Oncol. 2024, 26, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- McGirt, M.J.; Mukherjee, D.; Chaichana, K.L.; Than, K.D.; Weingart, J.D.; Quinones-Hinojosa, A. Association of Surgically Acquired Motor and Language Deficits on Overall Survival after Resection of Glioblastoma Multiforme. Neurosurgery 2009, 65, 463–469; discussion 469–470. [Google Scholar] [CrossRef]
- Rahman, M.; Abbatematteo, J.; De Leo, E.K.; Kubilis, P.S.; Vaziri, S.; Bova, F.; Sayour, E.; Mitchell, D.; Quinones-Hinojosa, A. The Effects of New or Worsened Postoperative Neurological Deficits on Survival of Patients with Glioblastoma. J. Neurosurg. 2017, 127, 123–131. [Google Scholar] [CrossRef]
- Roder, C.; Stummer, W.; Coburger, J.; Scherer, M.; Haas, P.; von der Brelie, C.; Kamp, M.A.; Löhr, M.; Hamisch, C.A.; Skardelly, M.; et al. Intraoperative MRI-Guided Resection Is Not Superior to 5-Aminolevulinic Acid Guidance in Newly Diagnosed Glioblastoma: A Prospective Controlled Multicenter Clinical Trial. J. Clin. Oncol. 2023, 41, 5512–5523. [Google Scholar] [CrossRef] [PubMed]
- Krieg, R.C.; Messmann, H.; Rauch, J.; Seeger, S.; Knuechel, R. Metabolic Characterization of Tumor Cell-Specific Protoporphyrin IX Accumulation after Exposure to 5-Aminolevulinic Acid in Human Colonic Cells. Photochem. Photobiol. 2002, 76, 518–525. [Google Scholar] [CrossRef]
- Ohgari, Y.; Nakayasu, Y.; Kitajima, S.; Sawamoto, M.; Mori, H.; Shimokawa, O.; Matsui, H.; Taketani, S. Mechanisms Involved in Delta-Aminolevulinic Acid (ALA)-Induced Photosensitivity of Tumor Cells: Relation of Ferrochelatase and Uptake of ALA to the Accumulation of Protoporphyrin. Biochem. Pharmacol. 2005, 71, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Krieg, R.C.; Fickweiler, S.; Wolfbeis, O.S.; Knuechel, R. Cell-Type Specific Protoporphyrin IX Metabolism in Human Bladder Cancer in Vitro. Photochem. Photobiol. 2007, 72, 226–233. [Google Scholar] [CrossRef]
- Jin, Y.; Bin, Z.Q.; Qiang, H.; Liang, C.; Hua, C.; Jun, D.; Dong, W.A.; Qing, L. ABCG2 Is Related with the Grade of Glioma and Resistance to Mitoxantone, a Chemotherapeutic Drug for Glioma. J. Cancer Res. Clin. Oncol. 2009, 135, 1369–1376. [Google Scholar] [CrossRef]
- Kobuchi, H.; Moriya, K.; Ogino, T.; Fujita, H.; Inoue, K.; Shuin, T.; Yasuda, T.; Utsumi, K.; Utsumi, T. Mitochondrial Localization of ABC Transporter ABCG2 and Its Function in 5-Aminolevulinic Acid-Mediated Protoporphyrin IX Accumulation. PLoS ONE 2012, 7, e50082. [Google Scholar] [CrossRef]
- Stepp, H.; Stummer, W. 5-ALA in the Management of Malignant Glioma. Lasers Surg. Med. 2018, 50, 399–419. [Google Scholar] [CrossRef]
- Gautheron, A.; Bernstock, J.D.; Picart, T.; Guyotat, J.; Valdés, P.A.; Montcel, B. 5-ALA Induced PpIX Fluorescence Spectroscopy in Neurosurgery: A Review. Front. Neurosci. 2024, 18, 1310282. [Google Scholar] [CrossRef]
- Stummer, W.; Stocker, S.; Novotny, A.; Heimann, A.; Sauer, O.; Kempski, O.; Plesnila, N.; Wietzorrek, J.; Reulen, H.J. In Vitro and in Vivo Porphyrin Accumulation by C6 Glioma Cells after Exposure to 5-Aminolevulinic Acid. J. Photochem. Photobiol. B 1998, 45, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J. ALA-Glioma Study Group Fluorescence-Guided Surgery with 5-Aminolevulinic Acid for Resection of Malignant Glioma: A Randomised Controlled Multicentre Phase III Trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Nakayama, N.; Ohe, N.; Miwa, K.; Shinoda, J.; Iwama, T. Pathological Analysis of the Surgical Margins of Resected Glioblastomas Excised Using Photodynamic Visualization with Both 5-Aminolevulinic Acid and Fluorescein Sodium. J. Neurooncol. 2017, 133, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, L.C.; Krabbenhøft, M.G.; Hansen, R.W.; Mikic, N.; Pedersen, C.B.; Poulsen, F.R.; Korshoej, A.R. Effect of 5-Aminolevulinic Acid and Sodium Fluorescein on the Extent of Resection in High-Grade Gliomas and Brain Metastasis. Cancers 2022, 14, 617. [Google Scholar] [CrossRef]
- Eljamel, S. 5-ALA Fluorescence Image Guided Resection of Glioblastoma Multiforme: A Meta-Analysis of the Literature. Int. J. Mol. Sci. 2015, 16, 10443–10456. [Google Scholar] [CrossRef]
- Gandhi, S.; Tayebi Meybodi, A.; Belykh, E.; Cavallo, C.; Zhao, X.; Syed, M.P.; Borba Moreira, L.; Lawton, M.T.; Nakaji, P.; Preul, M.C. Survival Outcomes among Patients with High-Grade Glioma Treated with 5-Aminolevulinic Acid-Guided Surgery: A Systematic Review and Meta-Analysis. Front. Oncol. 2019, 9, 620. [Google Scholar] [CrossRef]
- Baig Mirza, A.; Christodoulides, I.; Lavrador, J.P.; Giamouriadis, A.; Vastani, A.; Boardman, T.; Ahmed, R.; Norman, I.; Murphy, C.; Devi, S.; et al. 5-Aminolevulinic Acid-Guided Resection Improves the Overall Survival of Patients with Glioblastoma-a Comparative Cohort Study of 343 Patients. Neurooncol. Adv. 2021, 3, vdab047. [Google Scholar] [CrossRef]
- Picart, T.; Pallud, J.; Berthiller, J.; Dumot, C.; Berhouma, M.; Ducray, F.; Armoiry, X.; Margier, J.; Guerre, P.; Varlet, P.; et al. Use of 5-ALA Fluorescence-Guided Surgery versus White-Light Conventional Microsurgery for the Resection of Newly Diagnosed Glioblastomas (RESECT Study): A French Multicenter Randomized Phase III Study. J. Neurosurg. 2024, 140, 987–1000. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, J.; Wang, C.; Liu, H.; Dong, X.; Shi, C.; Shi, C.; Liu, Y.; Teng, L.; Han, D.; et al. Intraoperative Fluorescence-Guided Resection of High-Grade Malignant Gliomas Using 5-Aminolevulinic Acid-Induced Porphyrins: A Systematic Review and Meta-Analysis of Prospective Studies. PLoS ONE 2013, 8, e63682. [Google Scholar] [CrossRef]
- Schupper, A.J.; Baron, R.B.; Cheung, W.; Rodriguez, J.; Kalkanis, S.N.; Chohan, M.O.; Andersen, B.J.; Chamoun, R.; Nahed, B.V.; Zacharia, B.E.; et al. 5-Aminolevulinic Acid for Enhanced Surgical Visualization of High-Grade Gliomas: A Prospective, Multicenter Study. J. Neurosurg. 2022, 136, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Eatz, T.A.; Eichberg, D.G.; Lu, V.M.; Di, L.; Komotar, R.J.; Ivan, M.E. Intraoperative 5-ALA Fluorescence-Guided Resection of High-Grade Glioma Leads to Greater Extent of Resection with Better Outcomes: A Systematic Review. J. Neurooncol. 2022, 156, 233–256. [Google Scholar] [CrossRef] [PubMed]
- Chohan, M.O.; Berger, M.S. 5-Aminolevulinic Acid Fluorescence Guided Surgery for Recurrent High-Grade Gliomas. J. Neurooncol. 2019, 141, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, A.; Thurm, H.; Zountsas, B.; Pietsch, T.; Lanfermann, H.; Pichlmeier, U.; Mehdorn, M. 5-ALA Recurrent Glioma Study Group Five-Aminolevulinic Acid for Fluorescence-Guided Resection of Recurrent Malignant Gliomas: A Phase II Study. Neurosurgery 2009, 65, 1070–1076; discussion 1076–1077. [Google Scholar] [CrossRef]
- Hickmann, A.-K.; Nadji-Ohl, M.; Hopf, N.J. Feasibility of Fluorescence-Guided Resection of Recurrent Gliomas Using Five-Aminolevulinic Acid: Retrospective Analysis of Surgical and Neurological Outcome in 58 Patients. J. Neurooncol. 2015, 122, 151–160. [Google Scholar] [CrossRef]
- Ringel, F.; Pape, H.; Sabel, M.; Krex, D.; Bock, H.C.; Misch, M.; Weyerbrock, A.; Westermaier, T.; Senft, C.; Schucht, P.; et al. Clinical Benefit from Resection of Recurrent Glioblastomas: Results of a Multicenter Study Including 503 Patients with Recurrent Glioblastomas Undergoing Surgical Resection. Neuro-Oncol. 2016, 18, 96–104. [Google Scholar] [CrossRef]
- da Silva, E.B., Jr.; Vasquez, M.W.M.; de Almeida Teixeira, B.C.; Neto, M.C.; Sprenger, F.; Filho, J.L.N.; Almeida-Lopes, L.; Ramina, R. Association of 5-Aminolevulinic Acid Fluorescence Guided Resection with Photodynamic Therapy in Recurrent Glioblastoma: A Matched Cohort Study. Acta Neurochir. 2024, 166, 212. [Google Scholar] [CrossRef]
- Beck, T.J.; Kreth, F.W.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Stepp, H.; Stummer, W.; Baumgartner, R. Interstitial Photodynamic Therapy of Nonresectable Malignant Glioma Recurrences Using 5-Aminolevulinic Acid Induced Protoporphyrin IX. Lasers Surg. Med. 2007, 39, 386–393. [Google Scholar] [CrossRef]
- Eljamel, M.S.; Goodman, C.; Moseley, H. ALA and Photofrin Fluorescence-Guided Resection and Repetitive PDT in Glioblastoma Multiforme: A Single Centre Phase III Randomised Controlled Trial. Lasers Med. Sci. 2008, 23, 361–367. [Google Scholar] [CrossRef]
- Stummer, W.; Gerwing, M.; Bilgin, S.S.; Thomas, C.; Villanueva-Meyer, J.; Agarwal, V.; Stögbauer, L.; Schroeteler, J.; Müther, M. Sonodynamic Therapy with a Single Neoadjuvant, Diffuse Delivery of Low-Intensity Ultrasound with 5-ALA in Treatment Naïve Glioblastoma Results in Tumor-Specific Cytotoxic Edema and Increased Apoptosis. J. Neurooncol. 2025, 172, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.W.; Valdés, P.A.; Harris, B.T.; Fontaine, K.M.; Hartov, A.; Fan, X.; Ji, S.; Lollis, S.S.; Pogue, B.W.; Leblond, F.; et al. Coregistered Fluorescence-Enhanced Tumor Resection of Malignant Glioma: Relationships between δ-Aminolevulinic Acid–Induced Protoporphyrin IX Fluorescence, Magnetic Resonance Imaging Enhancement, and Neuropathological Parameters. J. Neurosurg. 2011, 114, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Jaber, M.; Wölfer, J.; Ewelt, C.; Holling, M.; Hasselblatt, M.; Niederstadt, T.; Zoubi, T.; Weckesser, M.; Stummer, W. The Value of 5-Aminolevulinic Acid in Low-Grade Gliomas and High-Grade Gliomas Lacking Glioblastoma Imaging Features: An Analysis Based on Fluorescence, Magnetic Resonance Imaging, 18F-Fluoroethyl Tyrosine Positron Emission Tomography, and Tumor Molecular Factors. Neurosurgery 2016, 78, 401–411; discussion 411. [Google Scholar]
- Kiesel, B.; Mischkulnig, M.; Woehrer, A.; Martinez-Moreno, M.; Millesi, M.; Mallouhi, A.; Czech, T.; Preusser, M.; Hainfellner, J.A.; Wolfsberger, S.; et al. Systematic Histopathological Analysis of Different 5-Aminolevulinic Acid–Induced Fluorescence Levels in Newly Diagnosed Glioblastomas. J. Neurosurg. 2018, 129, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Traylor, J.I.; Pernik, M.N.; Sternisha, A.C.; McBrayer, S.K.; Abdullah, K.G. Molecular and Metabolic Mechanisms Underlying Selective 5-Aminolevulinic Acid-Induced Fluorescence in Gliomas. Cancers 2021, 13, 580. [Google Scholar] [CrossRef]
- McCracken, D.J.; Schupper, A.J.; Lakomkin, N.; Malcolm, J.; Painton Bray, D.; Hadjipanayis, C.G. Turning on the Light for Brain Tumor Surgery: A 5-Aminolevulinic Acid Story. Neuro-Oncol. 2022, 24, S52–S61. [Google Scholar] [CrossRef]
- Hosmann, A.; Jaber, M.; Roetzer-Pejrimovsky, T.; Timelthaler, G.; Borkovec, M.; Kiesel, B.; Wadiura, L.I.; Millesi, M.; Mercea, P.A.; Phillips, J.; et al. CD34 Microvascularity in Low-Grade Glioma: Correlation with 5-Aminolevulinic Acid Fluorescence and Patient Prognosis in a Multicenter Study at Three Specialized Centers. J. Neurosurg. 2023, 138, 1281–1290. [Google Scholar] [CrossRef]
- Jaber, M.; Ewelt, C.; Wölfer, J.; Brokinkel, B.; Thomas, C.; Hasselblatt, M.; Grauer, O.; Stummer, W. Is Visible Aminolevulinic Acid-Induced Fluorescence an Independent Biomarker for Prognosis in Histologically Confirmed (World Health Organization 2016) Low-Grade Gliomas? Neurosurgery 2019, 84, 1214–1224. [Google Scholar] [CrossRef]
- Hosmann, A.; Millesi, M.; Wadiura, L.I.; Kiesel, B.; Mercea, P.A.; Mischkulnig, M.; Borkovec, M.; Furtner, J.; Roetzer, T.; Wolfsberger, S.; et al. 5-ALA Fluorescence Is a Powerful Prognostic Marker during Surgery of Low-Grade Gliomas (WHO Grade II)-Experience at Two Specialized Centers. Cancers 2021, 13, 2540. [Google Scholar] [CrossRef]
- Widhalm, G.; Wolfsberger, S.; Minchev, G.; Woehrer, A.; Krssak, M.; Czech, T.; Prayer, D.; Asenbaum, S.; Hainfellner, J.A.; Knosp, E. 5-Aminolevulinic Acid Is a Promising Marker for Detection of Anaplastic Foci in Diffusely Infiltrating Gliomas with Nonsignificant Contrast Enhancement. Cancer 2010, 116, 1545–1552. [Google Scholar] [CrossRef]
- Widhalm, G.; Kiesel, B.; Woehrer, A.; Traub-Weidinger, T.; Preusser, M.; Marosi, C.; Prayer, D.; Hainfellner, J.A.; Knosp, E.; Wolfsberger, S. 5-Aminolevulinic Acid Induced Fluorescence Is a Powerful Intraoperative Marker for Precise Histopathological Grading of Gliomas with Non-Significant Contrast-Enhancement. PLoS ONE 2013, 8, e76988. [Google Scholar] [CrossRef] [PubMed]
- Widhalm, G.; Olson, J.; Weller, J.; Bravo, J.; Han, S.J.; Phillips, J.; Hervey-Jumper, S.L.; Chang, S.M.; Roberts, D.W.; Berger, M.S. The Value of Visible 5-ALA Fluorescence and Quantitative Protoporphyrin IX Analysis for Improved Surgery of Suspected Low-Grade Gliomas. J. Neurosurg. 2020, 133, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Valdés, P.A.; Jacobs, V.; Harris, B.T.; Wilson, B.C.; Leblond, F.; Paulsen, K.D.; Roberts, D.W. Quantitative Fluorescence Using 5-Aminolevulinic Acid-Induced Protoporphyrin IX Biomarker as a Surgical Adjunct in Low-Grade Glioma Surgery. J. Neurosurg. 2015, 123, 771–780. [Google Scholar] [CrossRef]
- Kajimoto, Y.; Kuroiwa, T.; Miyatake, S.-I.; Ichioka, T.; Miyashita, M.; Tanaka, H.; Tsuji, M. Use of 5-Aminolevulinic Acid in Fluorescence-Guided Resection of Meningioma with High Risk of Recurrence. Case Report. J. Neurosurg. 2007, 106, 1070–1074. [Google Scholar] [CrossRef]
- Coluccia, D.; Fandino, J.; Fujioka, M.; Cordovi, S.; Muroi, C.; Landolt, H. Intraoperative 5-Aminolevulinic-Acid-Induced Fluorescence in Meningiomas. Acta Neurochir. 2010, 152, 1711–1719. [Google Scholar] [CrossRef]
- Millesi, M.; Kiesel, B.; Mischkulnig, M.; Martínez-Moreno, M.; Wöhrer, A.; Wolfsberger, S.; Knosp, E.; Widhalm, G. Analysis of the Surgical Benefits of 5-ALA-Induced Fluorescence in Intracranial Meningiomas: Experience in 204 Meningiomas. J. Neurosurg. 2016, 125, 1408–1419. [Google Scholar] [CrossRef]
- Wadiura, L.I.; Millesi, M.; Makolli, J.; Wais, J.; Kiesel, B.; Mischkulnig, M.; Mercea, P.A.; Roetzer, T.; Knosp, E.; Rössler, K.; et al. High Diagnostic Accuracy of Visible 5-ALA Fluorescence in Meningioma Surgery According to Histopathological Analysis of Tumor Bulk and Peritumoral Tissue. Lasers Surg. Med. 2021, 53, 300–308. [Google Scholar] [CrossRef]
- Morofuji, Y.; Matsuo, T.; Hayashi, Y.; Suyama, K.; Nagata, I. Usefulness of Intraoperative Photodynamic Diagnosis Using 5-Aminolevulinic Acid for Meningiomas with Cranial Invasion: Technical Case Report. Neurosurgery 2008, 62, 102–103; discussion 103–104. [Google Scholar] [CrossRef]
- Spille, D.C.; Bunk, E.C.; Thomas, C.; Özdemir, Z.; Wagner, A.; Akkurt, B.H.; Mannil, M.; Paulus, W.; Grauer, O.M.; Stummer, W.; et al. Protoporphyrin IX (PpIX) Fluorescence during Meningioma Surgery: Correlations with Histological Findings and Expression of Heme Pathway Molecules. Cancers 2023, 15, 304. [Google Scholar] [CrossRef] [PubMed]
- Valdés, P.A.; Leblond, F.; Kim, A.; Harris, B.T.; Wilson, B.C.; Fan, X.; Tosteson, T.D.; Hartov, A.; Ji, S.; Erkmen, K.; et al. Quantitative Fluorescence in Intracranial Tumor: Implications for ALA-Induced PpIX as an Intraoperative Biomarker. J. Neurosurg. 2011, 115, 11–17. [Google Scholar] [CrossRef]
- Valdes, P.A.; Bekelis, K.; Harris, B.T.; Wilson, B.C.; Leblond, F.; Kim, A.; Simmons, N.E.; Erkmen, K.; Paulsen, K.D.; Roberts, D.W. 5-Aminolevulinic Acid-Induced Protoporphyrin IX Fluorescence in Meningioma. Oper. Neurosurg. 2014, 10, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Knipps, J.; Beseoglu, K.; Kamp, M.; Fischer, I.; Felsberg, J.; Neumann, L.M.; Steiger, H.-J.; Cornelius, J.F. Fluorescence Behavior and Dural Infiltration of Meningioma Analyzed by 5-Aminolevulinic Acid-Based Fluorescence: Operating Microscope versus Mini-Spectrometer. World Neurosurg. 2017, 108, 118–127. [Google Scholar] [CrossRef]
- Kaneko, S.; Brokinkel, B.; Suero Molina, E.; Warneke, N.; Holling, M.; Bunk, E.C.; Hess, K.; Senner, V.; Paulus, W.; Stummer, W. Real-Time in Vivo Kinetics of Protoporphyrin IX after Administration of 5-Aminolevulinic Acid in Meningiomas and Comparative Analyses with Glioblastomas. Acta Neurochir. 2020, 162, 2197–2202. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Stepp, H.; Wiestler, O.D.; Pichlmeier, U. Randomized, Prospective Double-Blinded Study Comparing 3 Different Doses of 5-Aminolevulinic Acid for Fluorescence-Guided Resections of Malignant Gliomas. Neurosurgery 2017, 81, 230–239. [Google Scholar] [CrossRef]
- Suero Molina, E.; Black, D.; Kaneko, S.; Müther, M.; Stummer, W. Double Dose of 5-Aminolevulinic Acid and Its Effect on Protoporphyrin IX Accumulation in Low-Grade Glioma. J. Neurosurg. 2022, 137, 943–952. [Google Scholar] [CrossRef]
- Bunk, E.C.; Wagner, A.; Stummer, W.; Senner, V.; Brokinkel, B. 5-ALA Kinetics in Meningiomas: Analysis of Tumor Fluorescence and PpIX Metabolism in Vitro and Comparative Analyses with High-Grade Gliomas. J. Neurooncol. 2021, 152, 37–46. [Google Scholar] [CrossRef]
- Kaneko, S.; Suero Molina, E.; Ewelt, C.; Warneke, N.; Stummer, W. Fluorescence-Based Measurement of Real-Time Kinetics of Protoporphyrin IX after 5-Aminolevulinic Acid Administration in Human in Situ Malignant Gliomas. Neurosurgery 2019, 85, E739–E746. [Google Scholar] [CrossRef]
- Teixidor, P.; Arráez, M.Á.; Villalba, G.; Garcia, R.; Tardáguila, M.; González, J.J.; Rimbau, J.; Vidal, X.; Montané, E. Safety and Efficacy of 5-Aminolevulinic Acid for High Grade Glioma in Usual Clinical Practice: A Prospective Cohort Study. PLoS ONE 2016, 11, e0149244. [Google Scholar] [CrossRef]
- Fatima, B.; Licatino, L.K.; Abcejo, A.S. Keeping Patients in the Dark: Perioperative Anesthetic Considerations for Patients Receiving 5-Aminolevulinic Acid for Glioma Resection. Curr. Opin. Anaesthesiol. 2024, 37, 446–452. [Google Scholar] [CrossRef]
- Elliott, J.T.; Wirth, D.J.; Davis, S.C.; Olson, J.D.; Simmons, N.E.; Ryken, T.C.; Paulsen, K.D.; Roberts, D.W. Improving the Usability of 5-Aminolevulinic Acid Fluorescence-Guided Surgery by Adding an Optimized Secondary Light Source. World Neurosurg. 2021, 149, 195–203.e4. [Google Scholar] [CrossRef]
- Suero Molina, E.; Stögbauer, L.; Jeibmann, A.; Warneke, N.; Stummer, W. Validating a New Generation Filter System for Visualizing 5-ALA-Induced PpIX Fluorescence in Malignant Glioma Surgery: A Proof of Principle Study. Acta Neurochir. 2020, 162, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Henderson, F., Jr.; Belykh, E.; Ramos, A.D.; Schwartz, T.H. Qualitative Head-to-Head Comparison of Headlamp and Microscope for Visualizing 5-ALA Fluorescence during Resection of Glioblastoma. Neurosurg. Focus. Video 2022, 6, V7. [Google Scholar] [CrossRef]
- Morshed, R.A.; Han, S.J.; Lau, D.; Berger, M.S. Wavelength-Specific Lighted Suction Instrument for 5-Aminolevulinic Acid Fluorescence-Guided Resection of Deep-Seated Malignant Glioma: Technical Note. J. Neurosurg. 2018, 128, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Ritz, R.; Feigl, G.C.; Schuhmann, M.U.; Ehrhardt, A.; Danz, S.; Noell, S.; Bornemann, A.; Tatagiba, M.S. Use of 5-ALA Fluorescence Guided Endoscopic Biopsy of a Deep-Seated Primary Malignant Brain Tumor. J. Neurosurg. 2011, 114, 1410–1413. [Google Scholar] [CrossRef] [PubMed]
- Rapp, M.; Kamp, M.; Steiger, H.-J.; Sabel, M. Endoscopic-Assisted Visualization of 5-Aminolevulinic Acid-Induced Fluorescence in Malignant Glioma Surgery: A Technical Note. World Neurosurg. 2014, 82, e277–e279. [Google Scholar] [CrossRef]
- Marbacher, S.; Klinger, E.; Schwyzer, L.; Fischer, I.; Nevzati, E.; Diepers, M.; Roelcke, U.; Fathi, A.-R.; Coluccia, D.; Fandino, J. Use of Fluorescence to Guide Resection or Biopsy of Primary Brain Tumors and Brain Metastases. Neurosurg. Focus. 2014, 36, E10. [Google Scholar] [CrossRef]
- Goryaynov, S.A.; Okhlopkov, V.A.; Golbin, D.A.; Chernyshov, K.A.; Svistov, D.V.; Martynov, B.V.; Kim, A.V.; Byvaltsev, V.A.; Pavlova, G.V.; Batalov, A.; et al. Fluorescence Diagnosis in Neurooncology: Retrospective Analysis of 653 Cases. Front. Oncol. 2019, 9, 830. [Google Scholar] [CrossRef]
- Bianconi, A.; Bonada, M.; Zeppa, P.; Colonna, S.; Tartara, F.; Melcarne, A.; Garbossa, D.; Cofano, F. How Reliable Is Fluorescence-Guided Surgery in Low-Grade Gliomas? A Systematic Review Concerning Different Fluorophores. Cancers 2023, 15, 4130. [Google Scholar] [CrossRef]
- Lau, D.; Hervey-Jumper, S.L.; Chang, S.; Molinaro, A.M.; McDermott, M.W.; Phillips, J.J.; Berger, M.S. A Prospective Phase II Clinical Trial of 5-Aminolevulinic Acid to Assess the Correlation of Intraoperative Fluorescence Intensity and Degree of Histologic Cellularity during Resection of High-Grade Gliomas. J. Neurosurg. 2016, 124, 1300–1309. [Google Scholar] [CrossRef]
- Blake, E.; Curnow, A. The Hydroxypyridinone Iron Chelator CP94 Can Enhance PpIX-Induced PDT of Cultured Human Glioma Cells. Photochem. Photobiol. 2010, 86, 1154–1160. [Google Scholar] [CrossRef]
- Teng, L.; Nakada, M.; Zhao, S.-G.; Endo, Y.; Furuyama, N.; Nambu, E.; Pyko, I.V.; Hayashi, Y.; Hamada, J.-I. Silencing of Ferrochelatase Enhances 5-Aminolevulinic Acid-Based Fluorescence and Photodynamic Therapy Efficacy. Br. J. Cancer 2011, 104, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-G.; Chen, X.-F.; Wang, L.-G.; Yang, G.; Han, D.-Y.; Teng, L.; Yang, M.-C.; Wang, D.-Y.; Shi, C.; Liu, Y.-H.; et al. Increased Expression of ABCB6 Enhances Protoporphyrin IX Accumulation and Photodynamic Effect in Human Glioma. Ann. Surg. Oncol. 2013, 20, 4379–4388. [Google Scholar] [CrossRef]
- Chen, X.; Wang, C.; Teng, L.; Liu, Y.; Chen, X.; Yang, G.; Wang, L.; Liu, H.; Liu, Z.; Zhang, D.; et al. Calcitriol Enhances 5-Aminolevulinic Acid-Induced Fluorescence and the Effect of Photodynamic Therapy in Human Glioma. Acta Oncol. 2014, 53, 405–413. [Google Scholar] [CrossRef]
- Schucht, P.; Beck, J.; Abu-Isa, J.; Andereggen, L.; Murek, M.; Seidel, K.; Stieglitz, L.; Raabe, A. Gross Total Resection Rates in Contemporary Glioblastoma Surgery. Neurosurgery 2012, 71, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Pastor, J.; Vega-Zelaya, L.; Pulido, P.; Garnés-Camarena, O.; Abreu, A.; Sola, R.G. Role of Intraoperative Neurophysiological Monitoring during Fluorescence-Guided Resection Surgery. Acta Neurochir. (Wien) 2013, 155, 2201–2213. [Google Scholar] [CrossRef] [PubMed]
- Della Puppa, A.; De Pellegrin, S.; d’Avella, E.; Gioffrè, G.; Rossetto, M.; Gerardi, A.; Lombardi, G.; Manara, R.; Munari, M.; Saladini, M.; et al. 5-Aminolevulinic Acid (5-ALA) Fluorescence Guided Surgery of High-Grade Gliomas in Eloquent Areas Assisted by Functional Mapping. Our Experience and Review of the Literature. Acta Neurochir. 2013, 155, 965–972; discussion 972. [Google Scholar] [CrossRef]
- Peters, D.R.; Halimi, F.; Ozduman, K.; Levivier, M.; Conti, A.; Reyns, N.; Tuleasca, C. Resection of the Contrast-Enhancing Tumor in Diffuse Gliomas Bordering Eloquent Areas Using Electrophysiology and 5-ALA Fluorescence: Evaluation of Resection Rates and Neurological Outcome-a Systematic Review and Meta-Analysis. Neurosurg. Rev. 2023, 46, 185. [Google Scholar] [CrossRef]
- Coburger, J.; Hagel, V.; Wirtz, C.R.; König, R. Surgery for Glioblastoma: Impact of the Combined Use of 5-Aminolevulinic Acid and Intraoperative MRI on Extent of Resection and Survival. PLoS ONE 2015, 10, e0131872. [Google Scholar] [CrossRef]
- Gessler, F.; Forster, M.-T.; Duetzmann, S.; Mittelbronn, M.; Hattingen, E.; Franz, K.; Seifert, V.; Senft, C. Combination of Intraoperative Magnetic Resonance Imaging and Intraoperative Fluorescence to Enhance the Resection of Contrast Enhancing Gliomas. Neurosurgery 2015, 77, 16–22; discussion 22. [Google Scholar] [CrossRef]
- Coburger, J.; Engelke, J.; Scheuerle, A.; Thal, D.R.; Hlavac, M.; Wirtz, C.R.; König, R. Tumor Detection with 5-Aminolevulinic Acid Fluorescence and Gd-DTPA-Enhanced Intraoperative MRI at the Border of Contrast-Enhancing Lesions: A Prospective Study Based on Histopathological Assessment. Neurosurg. Focus. 2014, 36, E3. [Google Scholar] [CrossRef]
- Liu, Z.; Mela, A.; Argenziano, M.G.; Banu, M.A.; Furnari, J.; Kotidis, C.; Sperring, C.P.; Humala, N.; Mahajan, A.; Bruce, J.N.; et al. Single-Cell Analysis of 5-Aminolevulinic Acid Intraoperative Labeling Specificity for Glioblastoma. J. Neurosurg. 2024, 140, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Utsuki, S.; Oka, H.; Sato, S.; Shimizu, S.; Suzuki, S.; Tanizaki, Y.; Kondo, K.; Miyajima, Y.; Fujii, K. Histological Examination of False Positive Tissue Resection Using 5-Aminolevulinic Acid-Induced Fluorescence Guidance. Neurol. Med. Chir. 2007, 47, 210–213; discussion 213–214. [Google Scholar] [CrossRef] [PubMed]
- La Rocca, G.; Sabatino, G.; Menna, G.; Altieri, R.; Ius, T.; Marchese, E.; Olivi, A.; Barresi, V.; Della Pepa, G.M. 5-Aminolevulinic Acid False Positives in Cerebral Neuro-Oncology: Not All That Is Fluorescent Is Tumor. A Case-Based Update and Literature Review. World Neurosurg. 2020, 137, 187–193. [Google Scholar] [CrossRef]
- Olivo, M.; Wilson, B.C. Mapping ALA-Induced PPIX Fluorescence in Normal Brain and Brain Tumour Using Confocal Fluorescence Microscopy. Int. J. Oncol. 2004, 25, 37–45. [Google Scholar] [CrossRef]
- Dietze, A.; Berg, K. ALA-Induced Porphyrin Formation and Fluorescence in Synovitis Tissue In-Vitro and in Vivo Studies. Photodiagnosis Photodyn. Ther. 2005, 2, 299–307. [Google Scholar] [CrossRef]
- Ando, T.; Kobayashi, E.; Liao, H.; Maruyama, T.; Muragaki, Y.; Iseki, H.; Kubo, O.; Sakuma, I. Precise Comparison of Protoporphyrin IX Fluorescence Spectra with Pathological Results for Brain Tumor Tissue Identification. Brain Tumor Pathol. 2011, 28, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kantelhardt, S.R.; Diddens, H.; Leppert, J.; Rohde, V.; Hüttmann, G.; Giese, A. Multiphoton Excitation Fluorescence Microscopy of 5-Aminolevulinic Acid Induced Fluorescence in Experimental Gliomas. Lasers Surg. Med. 2008, 40, 273–281. [Google Scholar] [CrossRef]
- Mansi, M.; Howley, R.; Chandratre, S.; Chen, B. Inhibition of ABCG2 Transporter by Lapatinib Enhances 5-Aminolevulinic Acid-Mediated Protoporphyrin IX Fluorescence and Photodynamic Therapy Response in Human Glioma Cell Lines. Biochem. Pharmacol. 2022, 200, 115031. [Google Scholar] [CrossRef]
- Hollon, T.C.; Pandian, B.; Urias, E.; Save, A.V.; Adapa, A.R.; Srinivasan, S.; Jairath, N.K.; Farooq, Z.; Marie, T.; Al-Holou, W.N.; et al. Rapid, Label-Free Detection of Diffuse Glioma Recurrence Using Intraoperative Stimulated Raman Histology and Deep Neural Networks. Neuro-Oncol. 2021, 23, 144–155. [Google Scholar] [CrossRef]
- Herta, J.; Cho, A.; Roetzer-Pejrimovsky, T.; Höftberger, R.; Marik, W.; Kronreif, G.; Peilnsteiner, T.; Rössler, K.; Wolfsberger, S. Optimizing Maximum Resection of Glioblastoma: Raman Spectroscopy versus 5-Aminolevulinic Acid. J. Neurosurg. 2023, 139, 334–343. [Google Scholar] [CrossRef]
- Nasir-Moin, M.; Wadiura, L.I.; Sacalean, V.; Juros, D.; Movahed-Ezazi, M.; Lock, E.K.; Smith, A.; Lee, M.; Weiss, H.; Müther, M.; et al. Localization of Protoporphyrin IX during Glioma-Resection Surgery via Paired Stimulated Raman Histology and Fluorescence Microscopy. Nat. Biomed. Eng. 2024, 8, 672–688. [Google Scholar] [CrossRef] [PubMed]
- Kamp, M.A.; Grosser, P.; Felsberg, J.; Slotty, P.J.; Steiger, H.-J.; Reifenberger, G.; Sabel, M. 5-Aminolevulinic Acid (5-ALA)-Induced Fluorescence in Intracerebral Metastases: A Retrospective Study. Acta Neurochir. 2012, 154, 223–228; discussion 228. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.; Rohde, V.; Wolfert, C.; Hernandez-Duran, S.; Fiss, I.; Bleckmann, A.; Freer, A.B.; Mielke, D.; Schatlo, B. Survival after Resection of Brain Metastases with White Light Microscopy versus Fluorescence-Guidance: A Matched Cohort Analysis of the Metastasys Study Data. Oncotarget 2020, 11, 3026–3034. [Google Scholar] [CrossRef] [PubMed]
- Marhold, F.; Mercea, P.A.; Scheichel, F.; Berghoff, A.S.; Heicappell, P.; Kiesel, B.; Mischkulnig, M.; Borkovec, M.; Wolfsberger, S.; Woehrer, A.; et al. Detailed Analysis of 5-Aminolevulinic Acid Induced Fluorescence in Different Brain Metastases at Two Specialized Neurosurgical Centers: Experience in 157 Cases. J. Neurosurg. 2020, 133, 1032–1043. [Google Scholar] [CrossRef]
- Mercea, P.A.; Mischkulnig, M.; Kiesel, B.; Wadiura, L.I.; Roetzer, T.; Prihoda, R.; Heicappell, P.; Kreminger, J.; Furtner, J.; Woehrer, A.; et al. Prognostic Value of 5-ALA Fluorescence, Tumor Cell Infiltration and Angiogenesis in the Peritumoral Brain Tissue of Brain Metastases. Cancers 2021, 13, 603. [Google Scholar] [CrossRef]
- Mischkulnig, M.; Roetzer-Pejrimovsky, T.; Lötsch-Gojo, D.; Kastner, N.; Bruckner, K.; Prihoda, R.; Lang, A.; Martinez-Moreno, M.; Furtner, J.; Berghoff, A.; et al. Heme Biosynthesis Factors and 5-ALA Induced Fluorescence: Analysis of MRNA and Protein Expression in Fluorescing and Non-Fluorescing Gliomas. Front. Med. 2022, 9, 907442. [Google Scholar] [CrossRef]
| Fluorophore | Characteristics | Applications | Advantages |
|---|---|---|---|
| 5-ALA | Excitation: 375–440 nm Emission: 630–720 nm Administration: Oral Half-Life: 1–3 h Latency Period: 7–9 h |
|
|
| Fluorescein | Excitation: 460–500 nm Emission: 540–690 nm Administration: IV Half-Life: 23.5 min Latency Period: 2–4 h |
|
|
| ICG | Excitation: 750–800 nm Emission: 700–850 nm Administration: IV Half-Life: 3–4 min Latency Period: Seconds |
|
|
| High-Grade Glioma | Low-Grade Glioma | Meningioma | |
|---|---|---|---|
| Rates of 5-ALA Fluorescence | 83–96% [56,86,87] | 22–56% [58,86,87,88] | 77–94% [56,86,87] |
| Patient Factors | Preoperatively evaluate for
| ||
| 5-ALA Dose | 20 mg/kg | At least 20 mg/kg, but consider up to 40 mg/kg for maximal fluorescent intensity | 20 mg/kg |
| Timing * | 3–5 h prior to anesthesia induction | 3–5 h prior to anesthesia induction | No more than 3–5 h prior to anesthesia induction and as low as 1–3 h prior can be considered depending on anticipated time to dural incision |
| Tumor-Specific Intraoperative Adjuncts Ψ | Primary or recurrent:
| Primary or recurrent:
| Raman spectroscopy for distinguishing between pathologic dural tail and dura versus healthy dura |
| Postoperative Precautions |
| ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-S.; Young, J.S.; Berger, M.S. Current and Future Applications of 5-Aminolevulinic Acid in Neurosurgical Oncology. Cancers 2025, 17, 1332. https://doi.org/10.3390/cancers17081332
Chen J-S, Young JS, Berger MS. Current and Future Applications of 5-Aminolevulinic Acid in Neurosurgical Oncology. Cancers. 2025; 17(8):1332. https://doi.org/10.3390/cancers17081332
Chicago/Turabian StyleChen, Jia-Shu, Jacob S. Young, and Mitchel S. Berger. 2025. "Current and Future Applications of 5-Aminolevulinic Acid in Neurosurgical Oncology" Cancers 17, no. 8: 1332. https://doi.org/10.3390/cancers17081332
APA StyleChen, J.-S., Young, J. S., & Berger, M. S. (2025). Current and Future Applications of 5-Aminolevulinic Acid in Neurosurgical Oncology. Cancers, 17(8), 1332. https://doi.org/10.3390/cancers17081332





