Learning Curves in Robotic Urological Oncological Surgery: Has Anything Changed During the Last Five Years?
Simple Summary
Abstract
1. Introduction
2. Material and Methods
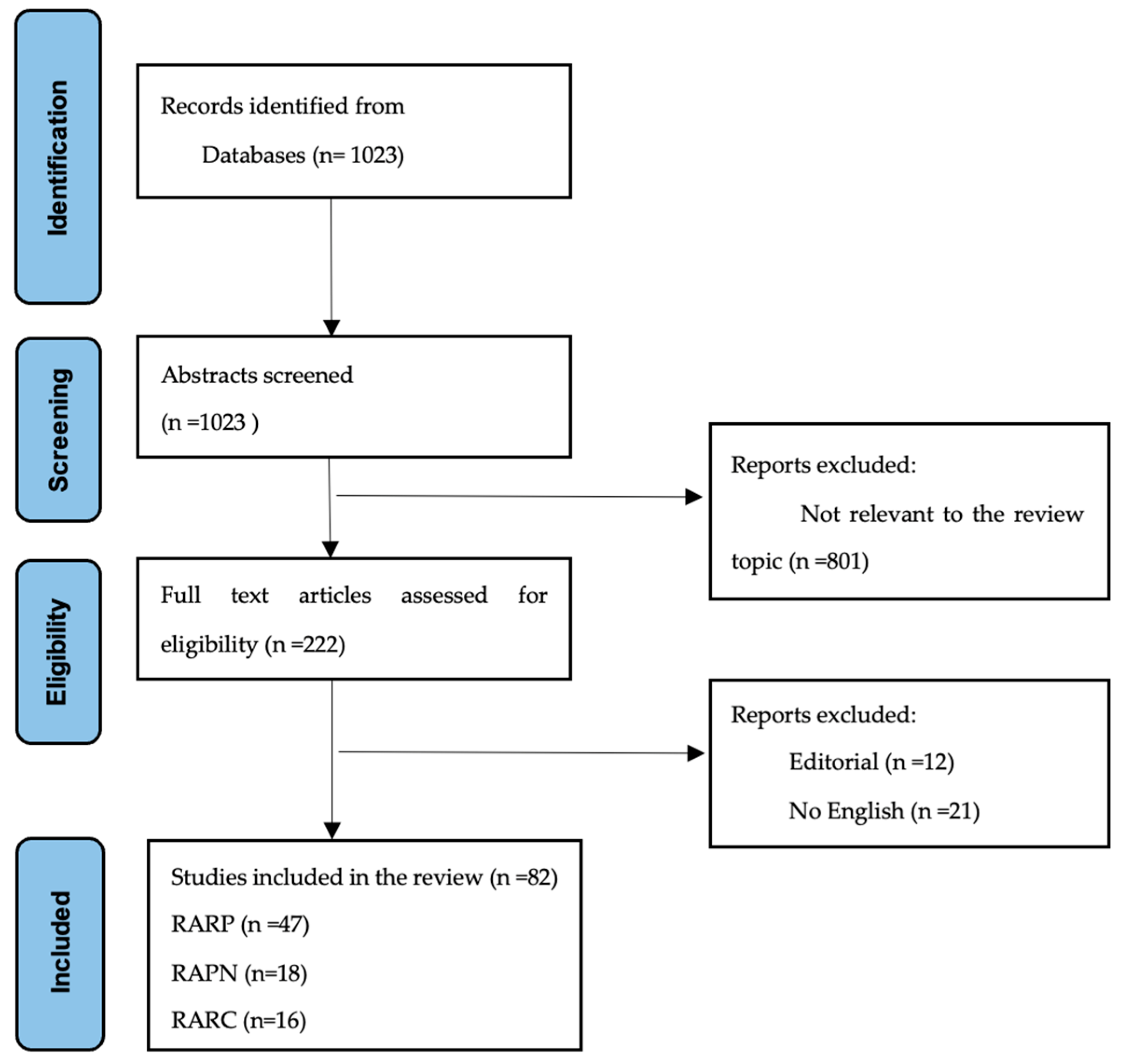
3. Results
3.1. Robotic-Assisted Radical Prostatectomy
3.2. Robotic-Assisted Partial Nephrectomy
3.3. Robotic-Assisted Radical Cystectomy
3.4. Comparing Different Procedure Plateau Case Numbers of the Last Five Years to the Ones of Previous Years
3.5. Risk of Bias Assessment of Included Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abboudi, H.; Khan, M.S.; Guru, K.A.; Froghi, S.; de Win, G.; Van Poppel, H.; Dasgupta, P.; Ahmed, K. Learning curves for urological procedures: A systematic review. BJU Int. 2013, 114, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Kassite, I.; Bejan-Angoulvant, T.; Lardy, H.; Binet, A. A systematic review of the learning curve in robotic surgery: Range and heterogeneity. Surg. Endosc. 2018, 33, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Soomro, N.A.; Hashimoto, D.A.; Porteous, A.J.; Ridley, C.J.A.; Marsh, W.J.; Ditto, R.; Roy, S. Systematic review of learning curves in robot-assisted surgery. BJS Open 2020, 4, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Grivas, N.; Zachos, I.; Georgiadis, G.; Karavitakis, M.; Tzortzis, V.; Mamoulakis, C. Learning curves in laparoscopic and robot-assisted prostate surgery: A systematic search and review. World J. Urol. 2021, 40, 929–949. [Google Scholar] [CrossRef]
- Larcher, A.; Muttin, F.; Peyronnet, B.; De Naeyer, G.; Khene, Z.-E.; Dell’oglio, P.; Ferreiro, C.; Schatteman, P.; Capitanio, U.; D’hondt, F.; et al. The Learning Curve for Robot-assisted Partial Nephrectomy: Impact of Surgical Experience on Perioperative Outcomes. Eur. Urol. 2019, 75, 253–256. [Google Scholar] [CrossRef]
- Montorsi, F.; Bandini, M.; Briganti, A.; Dasgupta, P.; Gallina, A.; Gallucci, M.; Gill, I.S.; Guru, K.A.; Hemal, A.; Menon, M.; et al. Re-establishing the Role of Robot-assisted Radical Cystectomy After the 2020 EAU Muscle-invasive and Metastatic Bladder Cancer Guideline Panel Recommendations. Eur. Urol. 2020, 78, 489–491. [Google Scholar] [CrossRef]
- Morozov, A.; Babaevskaya, D.; Taratkin, M.S.; Inoyatov, J.; Laukhtina, E.; Moschini, M.; Singla, N.; Rivas, J.G.; Teoh, J.Y.-C.; Glybochko, P.V.; et al. Systematic Review: The Learning Curve for Robot-Assisted Radical Cystectomy—What Do We Know? J. Endourol. 2022, 36, 770–784. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Patel, V.R.; Tully, A.; Holmes, R.; Lindsay, J. Robotic Radical Prostatectomy in the Community Setting—The Learning Curve and Beyond: Initial 200 Cases. J. Urol. 2005, 174, 269–272. [Google Scholar] [CrossRef]
- Atug, F.; Castle, E.P.; Srivastav, S.K.; Burgess, S.V.; Thomas, R.; Davis, R. Positive Surgical Margins in Robotic-Assisted Radical Prostatectomy: Impact of Learning Curve on Oncologic Outcomes. Eur. Urol. 2006, 49, 866–872. [Google Scholar] [CrossRef]
- Raman, J.D.; Dong, S.; Levinson, A.; Samadi, D.; Scherr, D.S. Robotic Radical Prostatectomy: Operative Technique, Outcomes, and Learning Curve. JSLS J. Soc. Laparoendosc. Surg. 2007, 11, 1–7. [Google Scholar]
- Samadi, D.; Levinson, A.; Hakimi, A.; Shabsigh, R.; Benson, M.C. From proficiency to expert, when does the learning curve for robotic-assisted prostatectomies plateau? The Columbia University experience. World J. Urol. 2006, 25, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Zorn, K.C.; Orvieto, M.A.; Gong, E.M.; Mikhail, A.A.; Gofrit, O.N.; Zagaja, G.P.; Shalhav, A.L. Robotic Radical Prostatectomy Learning Curve of a Fellowship-Trained Laparoscopic Surgeon. J. Endourol. 2007, 21, 441–447. [Google Scholar] [CrossRef]
- Artibani, W.; Fracalanza, S.; Cavalleri, S.; Iafrate, M.; Aragona, M.; Novara, G.; Gardiman, M.; Ficarra, V. Learning Curve and Preliminary Experience with da Vinci-Assisted Laparoscopic Radical Prostatectomy. Urol. Int. 2008, 80, 237–244. [Google Scholar] [CrossRef]
- Ou, Y.-C.; Yang, C.-R.; Wang, J.; Cheng, C.-L.; Patel, V.R. Robotic-assisted radical prostatectomy by a single surgeon in Taiwan: Experience with the initial 30 cases. J. Robot. Surg. 2008, 2, 173–179. [Google Scholar] [CrossRef]
- Pardalidis, N.P.; Andriopoulos, N.A.; Tsiga, A.; Giannakou, N.; Kosmaoglou, E. Robotic radical prostatectomy in Greece: The learning curve and beyond. The initial 40 cases. J. Robot. Surg. 2008, 2, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, J.; Castellucci, S.; Cathelineau, X.; Harmon, J.; Rozet, F.; Barret, E.; Vallancien, G. Robot-Assisted Laparoscopic Prostatectomy: A Single-Institutions Learning Curve. Urology 2009, 73, 127–133. [Google Scholar] [CrossRef]
- Ko, Y.H.; Ban, J.H.; Kang, S.H.; Park, H.S.; Lee, J.G.; Yoon, D.K.; Kim, J.J.; Cheon, J.; Patel, V.R. Does robot-assisted laparoscopic radical prostatectomy enable to obtain adequate oncological and functional outcomes during the learning curve? From the Korean experience. Asian J. Androl. 2009, 11, 167–175. [Google Scholar] [CrossRef]
- Tsao, A.K.; Smaldone, M.D.; Averch, T.D.; Jackman, S.V. Robot-Assisted Laparoscopic Prostatectomy: The First 100 Patients—Improving Patient Safety and Outcomes. J. Endourol. 2009, 23, 481–484. [Google Scholar] [CrossRef]
- Doumerc, N.; Yuen, C.; Savdie, R.; Rahman, B.; Benito, R.P.; Stricker, P. Robot-assisted laparoscopic prostatectomy: Analysis of an experienced open surgeon’s learning curve after 300 procedures. J. Robot. Surg. 2010, 3, 229–234. [Google Scholar] [CrossRef]
- Hong, Y.M.; Sutherland, D.E.; Linder, B.; Engel, J.D. “Learning Curve” May Not Be Enough: Assessing the Oncological Experience Curve for Robotic Radical Prostatectomy. J. Endourol. 2010, 24, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.C.; Yang, C.R.; Wang, J.; Cheng, C.L.; Patel, V.R. Robotic-assisted laparoscopic radical prostatectomy: Learning curve of first 100 cases. Int. J. Urol. 2010, 17, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Ploussard, G.; Xylinas, E.; Salomon, L.; Vordos, D.; Hoznek, A.; Abbou, C.; De La Taille, A. Robot-assisted extraperitoneal laparoscopic radical prostatectomy: Experience in a high-volume laparoscopy reference centre. BJU Int. 2010, 105, 1155–1160. [Google Scholar] [CrossRef]
- Gumus, E.; Boylu, U.; Turan, T.; Onol, F.F. The Learning Curve of Robot-Assisted Radical Prostatectomy. J. Endourol. 2011, 25, 1633–1637. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.-C.; Yang, C.-R.; Wang, J.; Cheng, C.-L.; Patel, V.R. Learning Curve of Robotic-assisted Radical Prostatectomy With 60 Initial Cases by a Single Surgeon. Asian J. Surg. 2011, 34, 74–80. [Google Scholar] [CrossRef]
- Ou, Y.; Yang, C.; Wang, J.; Yang, C.; Cheng, C.; Patel, V.R.; Tewari, A.K. The learning curve for reducing complications of robotic-assisted laparoscopic radical prostatectomy by a single surgeon. BJU Int. 2010, 108, 420–425. [Google Scholar] [CrossRef]
- Sharma, N.L.; Papadopoulos, A.; Lee, D.; McLoughlin, J.; Vowler, S.L.; Baumert, H.; Warren, A.Y.; Patil, V.; Shah, N.; Neal, D.E. First 500 cases of robotic-assisted laparoscopic radical prostatectomy from a single UK centre: Learning curves of two surgeons. BJU Int. 2010, 108, 739–747. [Google Scholar] [CrossRef]
- Lebeau, T.; Rouprêt, M.; Ferhi, K.; Chartier-Kastler, E.; Bitker, M.; Richard, F.; Vaessen, C. The role of a well-trained team on the early learning curve of robot-assisted laparoscopic procedures: The example of radical prostatectomy. Int. J. Med. Robot. Comput. Assist. Surg. 2011, 8, 67–72. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yoshioka, K.; Gondo, T.; Kamoda, N.; Satake, N.; Ozu, C.; Horiguchi, Y.; Namiki, K.; Nakashima, J.; Tachibana, M. Learning Curve and Perioperative Outcomes of Robot-Assisted Radical Prostatectomy in 200 Initial Japanese Cases by a Single Surgeon. J. Endourol. 2013, 27, 1218–1223. [Google Scholar] [CrossRef]
- Seo, D.Y.; Cho, H.J.; Cho, J.M.; Kang, J.Y.; Yoo, T.K. Experience With Robot-Assisted Laparoscopic Radical Prostatectomy at a Secondary Training Hospital: Operation Time, Treatment Outcomes, and Complications With the Accumulation of Experience. Korean J. Urol. 2013, 54, 522–526. [Google Scholar] [CrossRef]
- Vasdev, N.; Bishop, C.; Kass-Iliyya, A.; Hamid, S.; McNicholas, T.A.; Prasad, V.; Mohan-S, G.; Lane, T.; Boustead, G.; Adshead, J.M. Developing a Robotic Prostatectomy Service and a Robotic Fellowship Programme—Defining the Learning Curve. Curr. Urol. 2014, 7, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.W.; Kreaden, U.S.; Gabbert, J.; Thomas, R. Learning Curve Assessment of Robot-Assisted Radical Prostatectomy Compared with Open-Surgery Controls from the Premier Perspective Database. J. Endourol. 2014, 28, 560–566. [Google Scholar] [CrossRef]
- Di Pierro, G.B.; Wirth, J.G.; Ferrari, M.; Danuser, H.; Mattei, A. Impact of a Single-surgeon Learning Curve on Complications, Positioning Injuries, and Renal Function in Patients Undergoing Robot-assisted Radical Prostatectomy and Extended Pelvic Lymph Node Dissection. Urology 2014, 84, 1106–1111. [Google Scholar] [CrossRef]
- Ou, Y.-C.; Yang, C.-K.; Chang, K.-S.; Wang, J.; Hung, S.-W.; Tung, M.-C.; Tewari, A.K.; Patel, V.R. The surgical learning curve for robotic-assisted laparoscopic radical prostatectomy: Experience of a single surgeon with 500 cases in Taiwan, China. Asian J. Androl. 2014, 16, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Good, D.W.; Stewart, G.D.; Laird, A.; Stolzenburg, J.-U.; Cahill, D.; McNeill, S.A. A Critical Analysis of the Learning Curve and Postlearning Curve Outcomes of Two Experience- and Volume-Matched Surgeons for Laparoscopic and Robot-Assisted Radical Prostatectomy. J. Endourol. 2015, 29, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Sumitomo, M.; Kanao, K.; Kato, Y.; Yoshizawa, T.; Watanabe, M.; Zennami, K.; Nakamura, K. Comparative investigation on clinical outcomes of robot-assisted radical prostatectomy between experienced open prostatic surgeons and novice open surgeons in a laparoscopically naïve center with a limited caseload. Int. J. Urol. 2015, 22, 469–474. [Google Scholar] [CrossRef]
- Chang, Y.; Qu, M.; Wang, L.; Yang, B.; Chen, R.; Zhu, F.; Wang, H.; Wang, Y.; Lu, X.; Ma, C.; et al. Robotic-assisted Laparoscopic Radical Prostatectomy From a Single Chinese Center: A Learning Curve Analysis. Urology 2016, 93, 104–111. [Google Scholar] [CrossRef]
- Adili, A.F.; Di Giovanni, J.; Kolesar, E.; Wong, N.C.; Hoogenes, J.; Dason, S.; Shayegan, B. Positive surgical margin rates during the robot-assisted laparoscopic radical prostatectomy learning curve of an experienced laparoscopic surgeon. Can. Urol. Assoc. J. 2017, 11, E409–E413. [Google Scholar] [CrossRef]
- Fossati, N.; Di Trapani, E.; Gandaglia, G.; Dell’Oglio, P.; Umari, P.; Buffi, N.M.; Guazzoni, G.; Mottrie, A.; Gaboardi, F.; Montorsi, F.; et al. Assessing the Impact of Surgeon Experience on Urinary Continence Recovery After Robot-Assisted Radical Prostatectomy: Results of Four High-Volume Surgeons. J. Endourol. 2017, 31, 872–877. [Google Scholar] [CrossRef]
- Wang, L.; Diaz, M.; Stricker, H.; Peabody, J.O.; Menon, M.; Rogers, C.G. Adding a newly trained surgeon into a high-volume robotic prostatectomy group: Are outcomes compromised? J. Robot. Surg. 2016, 11, 69–74. [Google Scholar] [CrossRef]
- Islamoglu, E.; Karamik, K.; Ozsoy, C.; Tokgoz, H.; Ates, M.; Savas, M. The Learning Curve Does Not Affect Positive Surgical Margin Status in Robot-Assisted Laparoscopic Prostatectomy. Urol. J. 2018, 15, 333–338. [Google Scholar] [PubMed]
- Jaulim, A.; Srinivasan, A.; Hori, S.; Kumar, N.; Warren, A.; Shah, N.; Gnanapragasam, V. A comparison of operative and margin outcomes from surgeon learning curves in robot assisted radical prostatectomy in a changing referral practice. Ind. Mark. Manag. 2018, 100, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Lott, F.M.; Siqueira, D.; Argolo, H.; Nóbrega, B.L.; Campos, F.S.; Favorito, L.A. Analysis of the Learning Curve of Surgeons without Previous Experience in Laparoscopy to Perform Robot-Assisted Radical Prostatectomy. Adv. Urol. 2018, 2018, 9073807. [Google Scholar] [CrossRef]
- Schiavina, R.; Borghesi, M.; Dababneh, H.; Rossi, M.S.; Pultrone, C.V.; Vagnoni, V.; Chessa, F.; Bianchi, L.; Porreca, A.; Mottrie, A.; et al. The impact of a structured intensive modular training in the learning curve of robot assisted radical prostatectomy. Arch. Ital. Urol. E Androl. 2018, 90, 1–7. [Google Scholar] [CrossRef]
- Bravi, C.A.; Tin, A.; Vertosick, E.; Mazzone, E.; Martini, A.; Dell’Oglio, P.; Stabile, A.; Gandaglia, G.; Fossati, N.; Suardi, N.; et al. The Impact of Experience on the Risk of Surgical Margins and Biochemical Recurrence after Robot-Assisted Radical Prostatectomy: A Learning Curve Study. J. Urol. 2019, 202, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Ucar, M.; Varol, A.T.; Gülkesen, K.H.; E Caylan, A.; Kutlu, Ö.; Güntekin, E. Does The Learning Curve Affect the Surgical, Functional, and Oncologic Outcomes in Bilateral Nerve-Sparing Robot Assisted Laparoscopic Prostatectomy? Cureus 2019, 11, e5274. [Google Scholar] [CrossRef]
- Chen, H.; Lian, B.; Dong, Z.; Wang, Y.; Qu, M.; Zhu, F.; Sun, Y.; Gao, X. Experience of one single surgeon with the first 500 robot-assisted laparoscopic prostatectomy cases in mainland China. Asian J. Urol. 2020, 7, 170–176. [Google Scholar] [CrossRef]
- Song, W.; Lee, S.W.; Chung, J.H.; Kang, M.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Lee, H.M.; Jeon, S.S. Relationship between robotic-assisted radical prostatectomy and retropubic radical prostatectomy in the learning curve of a single surgeon as a novice in radical prostatectomy: A retrospective cohort study. Int. J. Surg. 2020, 81, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Baunacke, M.; Azawia, A.; Huber, J.; Groeben, C.; Thomas, C.; Borkowetz, A. Robotic radical prostatectomy: Difficult to start, fast to improve? Influence of surgical experience in robotic and open radical prostatectomy. World J. Urol. 2021, 39, 4311–4317. [Google Scholar] [CrossRef]
- Ambinder, D.; Wang, S.; Siddiqui, M.M. Determining the component-based operative time learning curve for robotic-assisted radical prostatectomy. Curr. Urol. 2022, 16, 240–245. [Google Scholar] [CrossRef]
- Bock, D.; Nyberg, M.; Lantz, A.; Carlsson, S.V.; Sjoberg, D.D.; Carlsson, S.; Stranne, J.; Steineck, G.; Wiklund, P.; Haglind, E.; et al. Learning curve for robot-assisted laparoscopic radical prostatectomy in a large prospective multicentre study. Scand. J. Urol. 2022, 56, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Gandi, C.; Totaro, A.; Bientinesi, R.; Marino, F.; Pierconti, F.; Martini, M.; Russo, A.; Racioppi, M.; Bassi, P.; Sacco, E. A multi-surgeon learning curve analysis of overall and site-specific positive surgical margins after RARP and implications for training. J. Robot. Surg. 2022, 16, 1451–1461. [Google Scholar] [CrossRef]
- Hashine, K.; Tada, K.; Minato, R.; Sawada, Y.; Matsumura, M. Patient-reported outcomes after robot-assisted radical prostatectomy and institutional learning curve for functional outcomes. Urol. Ann. 2022, 15, 60–67. [Google Scholar] [CrossRef]
- Perera, S.; Fernando, N.; O’Brien, J.; Murphy, D.; Lawrentschuk, N. Robotic-assisted radical prostatectomy: Learning curves and outcomes from an Australian perspective. Prostate Int. 2022, 11, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Carlos, A.-F.; Dario, V.-M.; Popescu, R.-I.; Mariela, C.; Venancio, C.-A. Robot-Assisted Radical Prostatectomy (RARP) Trifecta Learning Curve for Surgeons with Previous Experience in Laparoscopy. Medicina 2024, 60, 1032. [Google Scholar] [CrossRef] [PubMed]
- Haseebuddin, M.; Benway, B.M.; Cabello, J.M.; Bhayani, S.B. Robot-Assisted Partial Nephrectomy: Evaluation of Learning Curve for an Experienced Renal Surgeon. J. Endourol. 2010, 24, 57–61. [Google Scholar] [CrossRef]
- Mottrie, A.; De Naeyer, G.; Schatteman, P.; Carpentier, P.; Sangalli, M.; Ficarra, V. Impact of the Learning Curve on Perioperative Outcomes in Patients Who Underwent Robotic Partial Nephrectomy for Parenchymal Renal Tumours. Eur. Urol. 2010, 58, 127–133. [Google Scholar] [CrossRef]
- Lavery, H.J.; Small, A.C.; Samadi, D.B.; Palese, M.A. Transition From Laparoscopic to Robotic Partial Nephrectomy: The Learning Curve for an Experienced Laparoscopic Surgeon. JSLS J. Soc. Laparosc. Robot. Surg. 2011, 15, 291–297. [Google Scholar] [CrossRef]
- Pierorazio, P.M.; Patel, H.D.; Feng, T.; Yohannan, J.; Hyams, E.S.; Allaf, M.E. Robotic-assisted Versus Traditional Laparoscopic Partial Nephrectomy: Comparison of Outcomes and Evaluation of Learning Curve. Urology 2011, 78, 813–819. [Google Scholar] [CrossRef]
- Tobis, S.; Venigalla, S.; Knopf, J.K.; Scosyrev, E.; Erturk, E.N.; Golijanin, D.J.; Joseph, J.V.; Rashid, H.; Wu, G. Robot-assisted partial nephrectomy: Analysis of the first 100 cases from a single institution. J. Robot. Surg. 2011, 6, 139–147. [Google Scholar] [CrossRef]
- Yuh, B.; Muldrew, S.; Menchaca, A.; Yip, W.; Lau, C.; Wilson, T.; Josephson, D. Integrating robotic partial nephrectomy to an existing robotic surgery program. Can. J. Urol. 2012, 19, 6193–6200. [Google Scholar]
- Sundaram, C.P.; Dube, H.; Bahler, C.D. The learning curve and factors affecting warm ischemia time during robot-assisted partial nephrectomy. Indian J. Urol. 2015, 31, 223–228. [Google Scholar] [CrossRef]
- Hanzly, M.; Frederick, A.; Creighton, T.; Atwood, K.; Mehedint, D.; Kauffman, E.C.; Kim, H.L.; Schwaab, T. Learning Curves for Robot-Assisted and Laparoscopic Partial Nephrectomy. J. Endourol. 2015, 29, 297–303. [Google Scholar] [CrossRef]
- Xie, Y.; Ma, X.; Gu, L.; Li, H.; Lv, X.; Gao, Y.; Yao, Y.; Chen, L.; Zhang, Y.; Zhang, X. Associating the learning curve and tumor anatomical complexity with the margins, ischemia, and complications rate after robot-assisted partial nephrectomy. Int. J. Surg. 2016, 36, 219–224. [Google Scholar] [CrossRef]
- Dias, B.H.; Ali, M.S.; Dubey, S.; Krishnaswamy, S.A.; Rao, A.R.; Dubey, D. Impact of learning curve on the perioperative outcomes following robot-assisted partial nephrectomy for renal tumors. Indian J. Urol. 2018, 34, 62–67. [Google Scholar] [CrossRef]
- Omidele, O.O.; Davoudzadeh, N.; Palese, M. Trifecta Outcomes to Assess Learning Curve of Robotic Partial Nephrectomy. JSLS J. Soc. Laparosc. Robot. Surg. 2018, 22, e2017.00064. [Google Scholar] [CrossRef]
- Bajalia, E.M.; Parikh, K.A.; Haehn, D.A.; Kahn, A.E.; Ball, C.T.; Thiel, D.D. Assessment of Advanced Perioperative Outcomes to Identify the True Learning Curve of Robotic-assisted Partial Nephrectomy. Urology 2020, 144, 136–141. [Google Scholar] [CrossRef]
- Castilho, T.M.L.; Lemos, G.C.; Cha, J.D.; Colombo, J.R.; Claros, O.R.; Lemos, M.B.; Carneiro, A. Transition from open partial nephrectomy directly to robotic surgery: Experience of a single surgeon to achieve “TRIFECTA”. Int. Braz J Urol 2020, 46, 814–821. [Google Scholar] [CrossRef]
- Motoyama, D.; Matsushita, Y.; Watanabe, H.; Tamura, K.; Suzuki, T.; Ito, T.; Sugiyama, T.; Otsuka, A.; Miyake, H. Initial learning curve for robot-assisted partial nephrectomy performed by a single experienced robotic surgeon. Asian J. Endosc. Surg. 2020, 13, 59–64. [Google Scholar] [CrossRef]
- Fiorello, N.; Di Benedetto, A.; Summonti, D.; Mogorovich, A.; Sepich, C.A. Learning curve in robot-assisted partial nephrectomy: Comparison between an expert surgeon and a team in training in single-center experiences. Cent. Eur. J. Urol. 2021, 74, 523–527. [Google Scholar] [CrossRef]
- Zeuschner, P.; Greguletz, L.; Meyer, I.; Linxweiler, J.; Janssen, M.; Wagenpfeil, G.; Wagenpfeil, S.; Siemer, S.; Stöckle, M.; Saar, M. Open versus robot-assisted partial nephrectomy: A longitudinal comparison of 880 patients over 10 years. Int. J. Med. Robot. Comput. Assist. Surg. 2020, 17, 1–8. [Google Scholar] [CrossRef]
- Al-Nader, M.; Radtke, J.P.; Püllen, L.; Darr, C.; Kesch, C.; Hess, J.; Krafft, U.; Hadaschik, B.A.; Harke, N.; Mahmoud, O. Cumulative sum analysis (CUSUM) for evaluating learning curve (LC) of robotic-assisted laparoscopic partial nephrectomy (RALPN). J. Robot. Surg. 2023, 17, 2089–2098. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, B.; Liu, H.; Jia, G.; Tao, B.; Zhang, H.; Wang, C. How many cases are required to achieving early proficiency in purely off-clamp robot-assisted partial nephrectomy? Front. Surg. 2024, 10, 1309522. [Google Scholar] [CrossRef]
- Pruthi, R.S.; Smith, A.; Wallen, E.M. Evaluating the Learning Curve for Robot-Assisted Laparoscopic Radical Cystectomy. J. Endourol. 2008, 22, 2469–2474. [Google Scholar] [CrossRef]
- Guru, K.A.; Perlmutter, A.E.; Butt, Z.M.; Piacente, P.; Wilding, G.E.; Tan, W.; Kim, H.L.; Mohler, J.L. The Learning Curve for Robot-Assisted Radical Cystectomy. JSLS J. Soc. Laparosc. Robot. Surg. 2009, 13, 509–514. [Google Scholar] [CrossRef]
- Hayn, M.H.; Hussain, A.; Mansour, A.M.; Andrews, P.E.; Carpentier, P.; Castle, E.; Dasgupta, P.; Rimington, P.; Thomas, R.; Khan, S.; et al. The Learning Curve of Robot-Assisted Radical Cystectomy: Results from the International Robotic Cystectomy Consortium. Eur. Urol. 2010, 58, 197–202. [Google Scholar] [CrossRef]
- Hayn, M.H.; Hellenthal, N.J.; Seixas-Mikelus, S.A.; Mansour, A.M.; Stegemann, A.; Hussain, A.; Guru, K.A. Is patient outcome compromised during the initial experience with robot-assisted radical cystectomy? Results of 164 consecutive cases. BJU Int. 2010, 108, 882–887. [Google Scholar] [CrossRef]
- Richards, K.A.; Kader, K.; Pettus, J.A.; Smith, J.J.; Hemal, A.K. Does Initial Learning Curve Compromise Outcomes for Robot-Assisted Radical Cystectomy? A Critical Evaluation of the First 60 Cases While Establishing a Robotics Program. J. Endourol. 2011, 25, 1553–1558. [Google Scholar] [CrossRef]
- Collins, J.W.; Tyritzis, S.; Nyberg, T.; Schumacher, M.C.; Laurin, O.; Adding, C.; Jonsson, M.; Khazaeli, D.; Steineck, G.; Wiklund, P.; et al. Robot-assisted radical cystectomy (RARC) with intracorporeal neobladder—What is the effect of the learning curve on outcomes? BJU Int. 2013, 113, 100–107. [Google Scholar] [CrossRef]
- Desai, M.M.; Gill, I.S.; Abreu, A.L.d.C.; Hosseini, A.; Nyberg, T.; Adding, C.; Laurin, O.; Collins, J.; Miranda, G.; Goh, A.C.; et al. Robotic Intracorporeal Orthotopic Neobladder during Radical Cystectomy in 132 Patients. J. Urol. 2014, 192, 1734–1740. [Google Scholar] [CrossRef]
- Honore, M.; Roberts, M.J.; Morton, A.; Teloken, P.E.; Navaratnam, A.; Coughlin, G.D. Outcomes and learning curve for robotic-assisted radical cystectomy: An Australian experience. ANZ J. Surg. 2019, 89, 1593–1598. [Google Scholar] [CrossRef]
- Porreca, A.; Bianchi, F.M.; Romagnoli, D.; D’agostino, D.; Corsi, P.; Giampaoli, M.; Salvaggio, A.; Bianchi, L.; Schiavina, R.; Brunocilla, E.; et al. Robot-assisted radical cystectomy with totally intracorporeal urinary diversion: Surgical and early functional outcomes through the learning curve in a single high-volume center. J. Robot. Surg. 2019, 14, 261–269. [Google Scholar] [CrossRef]
- Lombardo, R.; Mastroianni, R.; Tuderti, G.; Ferriero, M.; Brassetti, A.; Anceschi, U.; Guaglianone, S.; De Nunzio, C.; Cicione, A.; Tubaro, A.; et al. Benchmarking PASADENA Consensus along the Learning Curve of Robotic Radical Cystectomy with Intracorporeal Neobladder: CUSUM Based Assessment. J. Clin. Med. 2021, 10, 5969. [Google Scholar] [CrossRef]
- Tuderti, G.; Mastroianni, R.; Brassetti, A.; Bove, A.M.; Misuraca, L.; Anceschi, U.; Ferriero, M.; Gallucci, M.; Simone, G. Robot-assisted radical cystectomy with intracorporeal neobladder: Impact of learning curve and long-term assessment of functional outcomes. Minerva Urol. Nephrol. 2022, 73, 754–762. [Google Scholar] [CrossRef]
- López-Molina, C.; Carrion, A.; Campistol, M.; Piñero, A.; Lozano, F.; Salvador, C.; Raventós, C.; Trilla, E. Evaluating the impact of the learning curve on the perioperative outcomes of robot-assisted radical cystectomy with intracorporeal urinary diversion. Actas Urol. Esp. 2021, 46, 57–62. [Google Scholar] [CrossRef]
- Wijburg, C.J.; Hannink, G.; Michels, C.T.; Weijerman, P.C.; Issa, R.; Tay, A.; Decaestecker, K.; Wiklund, P.; Hosseini, A.; Sridhar, A.; et al. Learning Curve Analysis for Intracorporeal Robot-assisted Radical Cystectomy: Results from the EAU Robotic Urology Section Scientific Working Group. Eur. Urol. Open Sci. 2022, 39, 55–61. [Google Scholar] [CrossRef]
- Achermann, C.; Sauer, A.; Cattaneo, M.; Walz, J.; Wyler, S.F.; Kwiatkowski, M.; Prause, L.W. Retrospective Evaluation of a Single Surgeon’s Learning Curve of Robot-Assisted Radical Cystectomy with Intracorporeal Urinary Diversion via Ileal Conduit. Cancers 2023, 15, 3799. [Google Scholar] [CrossRef]
- Diamand, R.; D’Hondt, F.; Mjaess, G.; Jabbour, T.; Dell’Oglio, P.; Larcher, A.; Moschini, M.; Quackels, T.; Peltier, A.; Assenmacher, G.; et al. Teaching robotic cystectomy: Prospective pilot clinical validation of the ERUS training curriculum. BJU Int. 2023, 132, 84–91. [Google Scholar] [CrossRef]
- Tuderti, G.; Mastroianni, R.; Anceschi, U.; Bove, A.M.; Brassetti, A.; Ferriero, M.; Misuraca, L.; Flammia, R.S.; Proietti, F.; D’Annunzio, S.; et al. Learning curve for intracorporeal robotic Padua ileal bladder: 10-year functional assessment from a high-volume single-centre series. BJU Int. 2024, 134, 103–109. [Google Scholar] [CrossRef]
- Collins, J.W.; Ghazi, A.; Stoyanov, D.; Hung, A.; Coleman, M.; Cecil, T.; Ericsson, A.; Anvari, M.; Wang, Y.; Beaulieu, Y.; et al. Utilising an Accelerated Delphi Process to Develop Guidance and Protocols for Telepresence Applications in Remote Robotic Surgery Training. Eur. Urol. Open Sci. 2020, 22, 23–33. [Google Scholar] [CrossRef]
- Carneiro, A.; Claros, O.R.; Cha, J.D.; Kayano, P.P.; Apezzato, M.; Wagner, A.A.; Lemos, G.C. Can remote assistance for robotic surgery improve surgical performance in simulation training? A prospective clinical trial of urology residents using a simulator in South America. Int. Braz. J. Urol. 2022, 48, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Chen, G.; Li, L.; Chen, Z.; Yang, K.; Wang, X. Remote teaching system for robotic surgery and its validation: Results of a randomized controlled study. Surg. Endosc. 2023, 37, 9190–9200. [Google Scholar] [CrossRef]
- Sinha, A.; West, A.; Vasdev, N.; Sooriakumaran, P.; Rane, A.; Dasgupta, P.; McKirdy, M. Current practises and the future of robotic surgical training. Surg. 2023, 21, 314–322. [Google Scholar] [CrossRef]
- Farinha, R.; Breda, A.; Porter, J.; Mottrie, A.; Van Cleynenbreugel, B.; Sloten, J.V.; Mottaran, A.; Gallagher, A.G. International Expert Consensus on Metric-based Characterization of Robot-assisted Partial Nephrectomy. Eur. Urol. Focus 2022, 9, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.M.; Larkins, K.; To, G.; Burke, G.; Heriot, A.; Warrier, S.; Mohan, H. What are clinically relevant performance metrics in robotic surgery? A systematic review of the literature. J. Robot. Surg. 2022, 17, 335–350. [Google Scholar] [CrossRef]
- Anceschi, U.; Morelli, M.; Flammia, R.S.; Brassetti, A.; Dell’Oglio, P.; Galfano, A.; Tappero, S.; Vecchio, E.; Martiriggiano, M.; Luciani, L.G.; et al. Predictors of trainees’ proficiency during the learning curve of robot-assisted radical prostatectomy at high-volume institutions: Results from a multicentric series. Cent. Eur. J. Urol. 2023, 76, 38–43. [Google Scholar] [CrossRef]
- Lambert, E.; Allaeys, C.; Berquin, C.; De Visschere, P.; Verbeke, S.; Vanneste, B.; Fonteyne, V.; Van Praet, C.; Lumen, N. Is It Safe to Switch from a Standard Anterior to Retzius-Sparing Approach in Robot-Assisted Radical Prostatectomy? Curr. Oncol. 2023, 30, 3447–3460. [Google Scholar] [CrossRef] [PubMed]
- Galfano, A.; Secco, S.; Olivero, A.; Bocciardi, A.M.; Dell’oglio, P. The spread of retzius-sparing robotic prostatectomy: An update after 10 years. Curr. Opin. Urol. 2023, 33, 367–374. [Google Scholar] [CrossRef]
- Anceschi, U.; Flammia, R.S.; Tufano, A.; Morelli, M.; Galfano, A.; Luciani, L.G.; Misuraca, L.; Dell’oglio, P.; Tuderti, G.; Brassetti, A.; et al. Proficiency score as a predictor of early trifecta achievement during the learning curve of robot-assisted radical prostatectomy for high-risk prostate cancer: Results of a multicentric series. Curr. Urol. 2023, 18, 110–114. [Google Scholar] [CrossRef]
- Prata, F.; Basile, S.; Tedesco, F.; Ragusa, A.; Pira, M.; Iannuzzi, A.; Fantozzi, M.; Civitella, A.; Scarpa, R.M.; Papalia, R. Skill Transfer from Laparoscopic Partial Nephrectomy to the Hugo™ RAS System: A Novel Proficiency Score to Assess Surgical Quality during the Learning Curve. J. Clin. Med. 2024, 13, 2226. [Google Scholar] [CrossRef]
- Xiong, S.; Jiang, M.; Jiang, Y.; Hu, B.; Chen, R.; Yao, Z.; Deng, W.; Wan, X.; Liu, X.; Chen, L.; et al. Partial Nephrectomy Versus Radical Nephrectomy for Endophytic Renal Tumors: Comparison of Operative, Functional, and Oncological Outcomes by Propensity Score Matching Analysis. Front. Oncol. 2022, 12, 916018. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Number (Patients) | Number (Surgeons) | Prior Experience | Main Peri-Operative Outcomes | Main Oncological Outcomes | Safety Outcomes | Main Functional Outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Operative Time | Estimated Blood Loss | Length of Stay | PSM Rate | BCR Rate | Outcomes | Continence Rate | Potency Rate | ||||
| Chen (2020) [47] | 500 | 1 | Open radical prostatectomy | First plateau 200 cases; second decrease after 400 cases | First plateau 200 cases; second decrease after 400 cases | Plateau after 200 cases. | No statistically significant differences between groups | N/A | N/A | N/A | N/A |
| Song (2020) [48] | 480 | 1 | Novice in RARP | Plateau reached at 200th case (35 month) | Plateau reached at 230th case (37 months) | N/A | N/A | N/A | N/A | N/A | N/A |
| Baunacke (2021) [49] | 703 | 3 | Open radical prostatectomy | <100 cases: 233.7 min >100 cases: 184.1 min | <100 cases: 888.4 mL >100 cases: 604.2 mL | N/A | <100 cases: 21% >100 cases: 15% | N/A | N/A | N/A | N/A |
| Ambinder (2022) [50] | 120 | 1 | Fellowship program | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Bock (2022) [51] | 2672 | 25 | N/A | N/A | N/A | N/A | <75 cases 21% >300 cases 24% | <75 cases 11% >300 cases 13% | N/A | 3-month: <75 cases 54% >300 cases 44% 24-month: <75 cases 21% >300 cases 16% | 3-month: <75 cases 20% >300 cases 36% 24-month: <75 cases 38% >300 cases 53% |
| Gandi (2022) [52] | 761 | 3 | N/A | N/A | N/A | N/A | Surgeon A (mentor) 153 cases Surgeon B: 12 cases Surgeon C 31 cases | N/A | N/A | N/A | N/A |
| Hashine (2023) [53] | 319 | 7 | N/A | N/A | N/A | N/A | Not significant difference between groups (0–100, 100–200, >100) | Not significant difference between groups (0–100, 100–200, >100) | N/A | Better results after 200 cases | Better results after 200 cases |
| Perera (2023) [54] | 3969- 556 operated by surgeons who performed <50 RARP-general cohort surgeons | 53 | N/A | Mean operative time: 266 min for general cohort surgeons 240 min for surgeons in high volume centers | Mean estimate blood loss: 361 mL for general cohort surgeons 302 mL for surgeons in high volume centers | N/A | 14.4% for general cohort surgeons 6.1% for surgeons in high volume centers | N/A | N/A | N/A | N/A |
| Carlos (2024) [55] | 146 | 3 | Laparoscopy | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Author (Year) | Number (Patients) | Number (Surgeons) | Prior Experience | Main Peri-Operative Outcomes | Trifecta | Safety Outcomes | Warm Ischemia Time | ||
|---|---|---|---|---|---|---|---|---|---|
| Operative Time | Estimated Blood Loss | Length of Stay | |||||||
| Bajalia (2020) [67] | 406 | 1 | N/A | 1–50 cases: 223 min 51–100 cases: 204 min 101–150 cases: 202 min 151–200 cases: 201 min 201–250 Cases: 196 min 251–300 cases: 188 min 301–350 cases: 194 min 351–400 cases: 197 min >400 cases: 186 min | N/A | N/A | 1–50 cases: 63% 51–100 cases: 82% 101–150 cases: 66% 151–200 cases: 67% 201–250 cases: 54% 251–300 cases: 71% 301–350 cases: 84% 351–400 cases: 74% >400 cases: 72% Plateau 77 cases | High grade complication: 1–50 cases: 8% 51–100 cases: 4% 101–150 cases: 9% 151–200 cases: 10% 201–250 cases: 8% 251–300 cases:2% 301–350 cases: 6% 351–400 cases: 6% >400 cases: 6% | 1–50 cases: 16.5 min 51–100 cases: 13.4 min 101–150 cases: 16.7 min 151–200 cases: 17.1 min 201–250 cases: 19.6 min 251–300 cases: 16.7 min 301–350 cases: 18.2 min 351–400 cases: 16.6 min >400 cases: 17.7 min |
| Castilho (2020) [68] | 101 | 1 | N/A | 1–50 cases: 114 min 51–100 cases: 120 min | 1–50 cases: 295 mL 51–100 cases: 375 ml | N/A | 1–50 cases: 58% 51–100 cases: 87.8% | Complication rate:1–50 cases:18% 51–100 cases: 8% | 1–50 cases: 17.3 min 51–100 cases: 11.7 min |
| Motoyama (2020) [69] | 65 | 1 | RARP | 0–13 cases: 140–150 min 14–26 cases: 130–140 min 27–39 cases: 110–120 min 40–52 cases: 120–130 min 53–65 cases: 110–120 min | Median estimate blood loss: 50 mL | Median length of stay: 9 days | N/A | N/A | 0–13 cases: 19 min 14–26 cases: 16 min 27–39 cases: 17 min 40–52 cases: 15 min 53–65 cases: 13 min |
| Fiorello (2021) [70] | 172 experts (44–55-45_ training surgeons | 4 1 expert 3 training surgeons | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Zeuschner (2021) [71] | 500 | N/A | N/A | 1–143 cases: 172 min 144–500 cases: 152 min | 1–143 cases: 220 mL 144–500 cases: 200 mL | 1–143 cases: 7 days 144–500 cases: 6 days | 1–143 cases: 53.8% 144–500 cases: 60.8% | Complication rate: 1–143 cases: 30.1% 144–500 cases: 22.1% | 1–143 cases: 18 min 144–500 cases: 13 min |
| Al-Nader (2023) [72] | 127 | 2 | N/A | 1–18 case: 242 min 19–38 case: 208 min >39 cases: 109 min | N/A | N/A | N/A | N/A | N/A |
| Zhang (2023) [73] | 50 | 1 | N/A | 1–24 cases: 133.5 min 25–50 cases: 115.31 min | 1–24 cases: 117.92 mL 25–50 cases: 120.38 mL | 1–24 cases: 5.33 days 25–50 cases: 4.3 days | 1–24 cases: 22/24 (91.7%) 25–50 cases: 21/26 (81.8%) | Complication rate: 1–24 cases: CD < 2: 95.8% CD ≥ 2: 93.1% 25–50 cases: CD < 2: 4.2% CD ≥ 2: 6.9% | N/A |
| Author (Year) | Number (Patients) | Number (Surgeons) | Prior Experience | Main Peri-Operative Outcomes | PSM Rate | Safety Outcomes | Lymph Node Yield | ||
|---|---|---|---|---|---|---|---|---|---|
| Operative Time | Estimated Blood Loss | Length of Stay | |||||||
| Lombardo (2021) [83] | 100 | 1 | N/A | Plateau reached at 20th case; 0–10 cases: 640 min | <40 cases: drop of Hb >2 g/dL over 30% >40 cases: drop of Hb >2 g/dl under 30% | Plateau reached at 40th case | PSM do not change significantly along the LC | Complication rate: 0–20 cases: 35% 20–40 cases: 20% 40–60 cases: 15% 60–80 cases: 5% 80–100 cases: 0% | Number of LNs did not change along the LC |
| Tuderti (2021) [84] | 137 | 1 | N/A | 0–45 cases: 337.6 min 46–90 cases: 339.1 min 91–137 cases: 282.5 min | Number of intraoperative transfusions: 0–45 cases: 2 46–90 cases: 2 91–137 cases: 3 | 0–45 cases: 17.5 46–90 cases: 12.3 91–137 cases: 11.9 | 0–45 cases: 2 46–90 cases: 1 91–137 cases: 1 | 0–45 cases: 68.9% 46–90 cases: 28.2% 91–137 cases: 17.4% | 0–45 cases: 28.9 46–90 cases: 29.2 91–137 cases: 29.3 |
| Lopez Molina (2022) [85] | 62 | 3 | N/A | 0–20 cases: 398.5 min 21–40 cases: 315.3 min 41–62 cases: 337.4 min | Number of intraoperative transfusions: 0–20 cases: 2 21–40 cases: 0 41–62 cases: 1 | 0–20 cases: 10 days 21–40 cases: 9 days 41–62 cases: 11.5 days | 0–20 cases: 0 21–40 cases: 1 41–62 cases: 1 | Complication rate: 0–20 cases: 75% (15) 21–40 cases: 75% (15) 41–62 cases: 81.8% (18) | 0–20 cases: 20 21–40 cases: 17 41–62 cases: 15.5 |
| Wijburg (2022) [86] | 2186 | N/A | N/A | Plateau reached after 75 cases (321 min) | Plateau reached after 88 cases (292 mL) | Plateau reached after 198 cases (9.5 days) | N/A | Plateau reached after 97 cases (48%) | N/A |
| Achermann (2023) [87] | 53 | 1 | N/A | 0–14 cases: 415 min 15–27 cases: 390 min 28–40 cases: 445 min 41–53 cases: 361 min | 0–14 cases: 400 mL 15–27 cases: 300 mL 28–40 cases: 300 mL 41–53 cases: 200 mL | 0–14 cases: 16 days 15–27 cases: 16 days 28–40 cases: 22 days 41–53 cases: 16 days | N/A | Complication rate overall: 0–14 cases: 79% 15–27 cases: 69% 28–40 cases: 85% 41–53 cases: 38% | 0–14 cases: 19 15–27 cases: 29 28–40 cases: 19 41–53 cases: 23 |
| Diamand(2023) [88] | N/A | 1 | Erus curriculum | N/A | N/A | N/A | N/A | N/A | |
| Tuderti (2024) [89] | 200 | 2 | N/A | 0–66 cases: 342 min 67–133 cases: 316 min 134–200 cases: 319 min | N/A | 0–66 cases: 14.9 days 67–133 cases: 11.1 days 134–200 cases: 6.8 days | N/A | N/A | N/A |
| Study (Author, Year) | Selection Bias | Comparability (Confounding) | Outcome Assessment | Reporting Bias | Overall RoB |
|---|---|---|---|---|---|
| RARP—Recent (Last 5 Years) | |||||
| Chen (2020) [47] | Moderate | High | Moderate | Moderate | Moderate |
| Song (2020) [48] | Moderate | High | Moderate | Moderate | Moderate |
| Baunacke (2021) [49] | Moderate | Moderate | Moderate | Moderate | Moderate |
| Ambinder (2022) [50] | Moderate | High | Low | Moderate | Moderate |
| Bock (2022) [51] | Low | High | Moderate | Moderate | Moderate |
| Gandi (2022) [52] | Moderate | Moderate | Low | Moderate | Moderate |
| Hashine (2023) [53] | Moderate | High | Moderate | Moderate | Moderate |
| Perera (2022) [54] | Moderate | High | Moderate | Moderate | High |
| Carlos (2024) [55] | Moderate | High | Moderate | Moderate | High |
| RAPN—Recent (Last 5 Years) | |||||
| Bajalia (2020) [67] | Moderate | High | Moderate | Moderate | High |
| Castilho (2020) [68] | Moderate | High | Moderate | Moderate | High |
| Motoyama (2020) [69] | Moderate | High | Moderate | Moderate | High |
| Fiorello (2021) [70] | Moderate | High | Moderate | Moderate | High |
| Zeuschner (2021) [71] | Moderate | Moderate | Moderate | Moderate | Moderate |
| Al-Nader (2023) [72] | Moderate | High | Moderate | Moderate | High |
| Zhang (2023) [73] | Moderate | High | Moderate | Moderate | High |
| RARC—Recent (Last 5 Years) | |||||
| Porreca (2020) [82] | Moderate | High | Moderate | Moderate | High |
| Tuderti (2020) [84] | Moderate | High | Moderate | Moderate | High |
| Lombardo (2021) [83] | Moderate | High | Moderate | Moderate | Moderate |
| López-Molina (2021) [85] | Moderate | High | Moderate | Moderate | High |
| Wijburg (2022) [86] | Moderate | Moderate | Moderate | Moderate | Moderate |
| Achermann (2023) [87] | Moderate | High | Moderate | Moderate | High |
| Diamand (2023) [88] | Moderate | Moderate | Moderate | Moderate | Moderate |
| Tuderti (2024) [89] | Moderate | High | Moderate | Moderate | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokas, T.; Mavridis, C.; Bouchalakis, A.; Nakou, C.M.; Mamoulakis, C. Learning Curves in Robotic Urological Oncological Surgery: Has Anything Changed During the Last Five Years? Cancers 2025, 17, 1334. https://doi.org/10.3390/cancers17081334
Tokas T, Mavridis C, Bouchalakis A, Nakou CM, Mamoulakis C. Learning Curves in Robotic Urological Oncological Surgery: Has Anything Changed During the Last Five Years? Cancers. 2025; 17(8):1334. https://doi.org/10.3390/cancers17081334
Chicago/Turabian StyleTokas, Theodoros, Charalampos Mavridis, Athanasios Bouchalakis, Chrisoula Maria Nakou, and Charalampos Mamoulakis. 2025. "Learning Curves in Robotic Urological Oncological Surgery: Has Anything Changed During the Last Five Years?" Cancers 17, no. 8: 1334. https://doi.org/10.3390/cancers17081334
APA StyleTokas, T., Mavridis, C., Bouchalakis, A., Nakou, C. M., & Mamoulakis, C. (2025). Learning Curves in Robotic Urological Oncological Surgery: Has Anything Changed During the Last Five Years? Cancers, 17(8), 1334. https://doi.org/10.3390/cancers17081334







