Advancing Esophageal Cancer Staging and Restaging: The Role of MRI in Precision Diagnosis
Simple Summary
Abstract
1. Introduction
2. MRI Sequences in Esophageal Cancer Staging
- T2WI multi-shot turbo spin-echo (msTSE) imaging can accurately depict the internal structure of the esophageal wall, with clear distinction between the layers and surrounding structures assisting in local staging [8,9,11]. Optimization with automatic respiratory motion compensation and diaphragm position monitoring can be applied—the end-exhalation phase is the most stable part during the respiratory cycle. Fat saturation is not routinely used, due to the lack of visibility of fat infiltration, but can help delineate tumor volume when needed [12].
- T1WI Volumetric Interpolated Breath-hold Examination (VIBE) sequence is a type of 3D Gradient Echo (GRE) used for its improved image quality, resistance to motion, and high resolution. With contrast injection, it helps to differentiate the different layers of the esophageal wall, particularly at the delayed phase [11,13,14,15]. To make it easier for patients, some free-breathing sequences have been developed. The free-breathing radial VIBE sequence and star-VIBE are fat-suppressed T1WI sequences that are less sensitive to motion.
- DWI highlights microstructural changes in tumoral tissue, aiding in the detection and extension of tumors, particularly in the esophagogastric junction [16]. There is no consensus on the optimal b-value for esophagus MRI, with values ranging from 600 to 1000. Giganti et al. used DWI with b600 at 1.5T, achieving high specificity for T staging and similar sensitivity to EUS for the N stage [16]. Chapellier et al. used b800 at 3.0T for the NAT response [17], while Shuto et al. used b1000 for lymph nodes’ assessment [18].
- ADC derived from DWI can predict the response to chemoradiotherapy (1.5 T, B0-b1000) [19]. The application of intra-voxel incoherent motion (IVIM)-DWI quantifies both water molecule diffusion and blood perfusion, with studies showing lower blood perfusion in esophagus tumors compare to the normal esophagus, probably due to a faster tumor cell proliferation than vascularization [20]. Whole-body DWI with background body signal suppression (DWIBS) has also been explored for locoregional and distant evaluation [21].
- DCE-MRI assesses microcirculation and vascular density through perfusion measurement. It can also provide quantitive information about tumor angiogenesis and vascular permeability using some mathematic constant (Ktrans, Kep, and Ve, respectively, corresponding to endothelial transfer constant, reflux rate, and fractional extravascular extracellular space volume). DCE-MRI can distinguish a normal esophagus from a malignant tumor as well as early versus advanced tumoral stages [20,22]. It is also under investigation as a means to differentiate histological types and to evaluate treatment response [15,23,24].
- Kinetic sequences adapted from cardiac MRI—steady state free precession (SSFP) in sagittal and perpendicular plans relative to the tumor—have been studied to evaluate local tumor invasiveness, taking advantage of the natural motion of thoracic structures, using cardiac gating to limit artefacts [25,26]. Cine MR sequences showed a slightly better performance in distinguishing T1–T3 from T4 with a sensitivity, specificity, and AUC of 76.5%, 83.8%, and 0.801 (0.681–0.921), and an increased in T staging confidence [26].
- T2WI TSE Dark Blood is a high-resolution sequence derived from cardiac MRI. It can be focused on the esophagus lesion, with a small FOV allowing better visibility of local invasiveness. However, this sequence is sensitive to motion and requires breath holding and cardiac gating.
- T2WI short inversion time inversion-recovery (STIR) turbo spin echo (TSE) is a quick sequence with fat saturation, and is studied for lymph node detection using their T2 signal in comparison with a normal esophagus signal to differentiate malignant from benign lymph nodes [27].
- T2* WI can reflect blood oxygenation and has the potential for quantifying tumor neoangiogenesis, which increases with higher T stages [28]. However, this sequence is highly sensitive to the presence of air, limiting its use.
3. The Role of MRI in Initial Staging of Esophageal Cancer
3.1. T Staging
3.2. N Staging
3.3. M Staging
4. The Role of MRI in Restaging and Monitoring Treatment Response
- (1)
- no assessable disease, so patients might benefit from an active surveillance approach or esophagectomy;
- (2)
- persistent locoregional disease with no distant metastasis, so patients might require subsequent surgery;
- (3)
- evidence of distant spread, so patients should receive systemic treatment.
4.1. T Restaging
4.2. N and M Restaging
5. Future Directions and Research Priorities
6. Integrating Radiomics with Genomics and Clinical Data
- The use of MRI to assess treatment response is under investigation in multiple centers. The RELAY study (United States, 2020–2025, NCT04188535) investigates the feasibility and utility of using serial MRI to assess treatment response during and after radiation therapy for patients with advanced EC but also with glioblastoma, prostate cancer, vulvar cancer, or pediatric glioma. A Chinese study (2016–2025, NCT02988921) aims to explore the value of MRI for the prediction of tumor response to CRT and accurate target volume delineation as compared to CT simulation for patients with unresectable or potentially resectable EC.
- Personalized patient management is under investigation in the PIONEER study (United States, 2021–2025, NCT04846309) where MRI and 18F-fluoromisonidazole (FMISO)-PET are being used to evaluate tumor hypoxia. Patient with tumor hypoxia will receive a higher dose of radiation therapy while those without hypoxic tumor will be treated with the standard-of-care radiation regimen.
- PET/MRI is being investigated in multiple clinical trials: the Escape study (Italy, 2020–2025, NCT04359732) aims to investigate the potential role of PET/MRI as a predictor of response to NAT in EC. The FAZA study (Canada, 2020–2024, NCT04560036) is currently investigating the use of PET/MRI with a specific radiotracer, 18F-Fluoroazomycin Arabinoside (FAZA), before and after standard of care platinum-based chemotherapy for patients with metastasized EC, with the goal of improving the treatment response assessment.
- MRI, as a guide for radiotherapy, is currently being investigated in a German study (2018–2027, NCT04172753) using the 1.5 T hybrid magnetic resonance imaging—linear accelerator system (MR-Linac) to delivered radiotherapy with high precision for EC.
- Ongoing research is exploring how combining MRI with CT can improve overall staging accuracy and to assess the response after NAT (China, 2016–2025, NCT02988921).
7. Clinical Implications: How Advancements in MRI and Multi-Modal Approaches Can Improve Patient Management
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADC | Apparent diffusion coefficient |
| AJCC | American Joint Committee on Cancer |
| CT | Computed tomography |
| CE-CT | Contrast-enhanced computed tomography |
| CR | Complete clinical response |
| nCR | near-complete response |
| CRT | Chemoradiotherapy |
| DB | Dark blood |
| DCE | Dynamic contrast-enhanced magnetic resonance imaging |
| DWI | Diffusion-weighted imaging |
| EC | Esophageal cancer |
| EUS | Endoscopic ultrasound |
| FB | Free breathing |
| FDG PET/CT | Positron emission tomography with [18F]-fluorodexoxyglucose computed tomography |
| GRE | Gradient echo |
| IVIM-DWI | intra-voxel incoherent motion-diffusion-weighted imaging |
| MRI | Magnetic resonance imaging |
| MR-TRG | Magnetic resonance tumor regression grade |
| NAT | Neoadjuvant treatment |
| pCR | pathological complete response |
| pTRG | pathologic tumor regression grade |
| RECIST: | Response evaluation criteria in solid tumors |
| TE | Time to echo |
| TR | Time repetition |
| Ms TSE | Multi-shot turbo spin echo |
| TNM | Tumor, node, metastasis |
| SSFP | steady state free precession |
| STIR | short inversion time inversion recovery |
| UICC | Union for International Cancer Control |
| VIBE | Volume interpolated breath-hold examination |
| WI | Weighted imaging |
| ypTNM | pathological classification of malignant tumors after neoadjuvant treatment |
References
- Talasila, P.; Hedge, S.G.; Periasamy, K.; Nagaraj, S.S.; Singh, H.; Singh, H.; Gupta, P. Imaging in Esophageal Cancer: A Comprehensive Review. Indian. J. Radiol. Imaging 2024, 35, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Riddell, A.M.; Davies, D.C.; Allum, W.H.; Wotherspoon, A.C.; Richardson, C.; Brown, G. High-Resolution MRI in Evaluation of the Surgical Anatomy of the Esophagus and Posterior Mediastinum. Am. J. Roentgenol. 2007, 188, W37–W43. [Google Scholar] [CrossRef] [PubMed]
- Raptis, C.A.; Goldstein, A.; Henry, T.S.; Porter, K.K.; Catenacci, D.; Kelly, A.M.; Kuzniewski, C.T.; Lai, A.R.; Lee, E.; Long, J.M.; et al. ACR Appropriateness Criteria® Staging and Follow-Up of Esophageal Cancer. J. Am. Coll. Radiol. 2022, 19, S462–S472. [Google Scholar] [CrossRef]
- Wang, Z.; Chu, F.; Bai, B.; Lu, S.; Zhang, H.; Jia, Z.; Zhao, K.; Zhang, Y.; Zheng, Y.; Xia, Q.; et al. MR Imaging Characteristics of Different Pathologic Subtypes of Esophageal Carcinoma. Eur. Radiol. 2023, 33, 9233–9243. [Google Scholar] [CrossRef]
- Zhang, F.; Qu, J.; Zhang, H.; Liu, H.; Qin, J.; Ding, Z.; Li, Y.; Ma, J.; Zhang, Z.; Wang, Z.; et al. Preoperative T Staging of Potentially Resectable Esophageal Cancer: A Comparison between Free-Breathing Radial VIBE and Breath-Hold Cartesian VIBE, with Histopathological Correlation. Transl. Oncol. 2017, 10, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, F.; Ni, L.; Li, X. Meta-Analysis of MRI in Predicting Early Response to Radiotherapy and Chemotherapy in Esophageal Cancer. Acad. Radiol. 2024, 32, 798–812. [Google Scholar] [CrossRef]
- Lee, S.L.; Yadav, P.; Starekova, J.; Christensen, L.; Chandereng, T.; Chappell, R.; Reeder, S.B.; Bassetti, M.F. Diagnostic Performance of MRI for Esophageal Carcinoma: A Systematic Review and Meta-Analysis. Radiology 2021, 299, 583–594. [Google Scholar] [CrossRef]
- Riddell, A.M.; Richardson, C.; Scurr, E.; Brown, G. The Development and Optimization of High Spatial Resolution MRI for Imaging the Oesophagus Using an External Surface Coil. BJR 2006, 79, 873–879. [Google Scholar] [CrossRef]
- Riddell, A.M.; Allum, W.H.; Thompson, J.N.; Wotherspoon, A.C.; Richardson, C.; Brown, G. The Appearances of Oesophageal Carcinoma Demonstrated on High-Resolution, T2-Weighted MRI, with Histopathological Correlation. Eur. Radiol. 2007, 17, 391–399. [Google Scholar] [CrossRef]
- Preibisch, C.; Wallenhorst, T.; Heidemann, R.; Zanella, F.E.; Lanfermann, H. Comparison of Parallel Acquisition Techniques Generalized Autocalibrating Partially Parallel Acquisitions (GRAPPA) and Modified Sensitivity Encoding (mSENSE) in Functional MRI (fMRI) at 3T. Magn. Reson. Imaging 2008, 27, 590–598. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Qin, J.; Zhang, H.; Liu, W.; Zhao, Y.; Lu, Y.; Yan, X.; Zhang, Z.; Zhang, T.; et al. A Prospective Analysis of the Diagnostic Accuracy of 3 T MRI, CT and Endoscopic Ultrasound for Preoperative T Staging of Potentially Resectable Esophageal Cancer. Cancer Imaging 2020, 20, 64. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Chen, T.; Wu, L.; Ou, J.; Li, R.; Zhang, X.; Yang, J.; Cao, J. Is There Association of Gross Tumor Volume of Adenocarcinoma of Oesophagogastric Junction Measured on Magnetic Resonance Imaging with N Stage? Eur. J. Radiol. 2019, 110, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhang, H.; Wang, Z.; Zhang, F.; Liu, H.; Ding, Z.; Li, Y.; Ma, J.; Zhang, Z.; Zhang, S.; et al. Comparison between Free-Breathing Radial VIBE on 3-T MRI and Endoscopic Ultrasound for Preoperative T Staging of Resectable Oesophageal Cancer, with Histopathological Correlation. Eur. Radiol. 2018, 28, 780–787. [Google Scholar] [CrossRef]
- Block, K.T.; Chandarana, H.; Milla, S.; Bruno, M.; Mulholland, T.; Fatterpekar, G.; Hagiwara, M.; Grimm, R.; Geppert, C.; Kiefer, B.; et al. Towards Routine Clinical Use of Radial Stack-of-Stars 3D Gradient-Echo Sequences for Reducing Motion Sensitivity. J. Korean Soc. Magn. Reson. Med. 2014, 18, 87–106. [Google Scholar] [CrossRef]
- Deng, H.-P.; Li, X.-M.; Yang, L.; Wang, Y.; Wang, S.-Y.; Zhou, P.; Lu, Y.-J.; Ren, J.; Wang, M. DCE-MRI of Esophageal Carcinoma Using Star-VIBE Compared with Conventional 3D-VIBE. Sci. Rep. 2021, 11, 24091. [Google Scholar] [CrossRef] [PubMed]
- Giganti, F.; Ambrosi, A.; Petrone, M.C.; Canevari, C.; Chiari, D.; Salerno, A.; Arcidiacono, P.G.; Nicoletti, R.; Albarello, L.; Mazza, E.; et al. Prospective Comparison of MR with Diffusion-Weighted Imaging, Endoscopic Ultrasound, MDCT and Positron Emission Tomography-CT in the Pre-Operative Staging of Oesophageal Cancer: Results from a Pilot Study. BJR 2016, 89, 20160087. [Google Scholar] [CrossRef] [PubMed]
- Chapellier, P.; Fasquelle, F.; Saglietti, C.; Kinj, R.; Mantziari, S.; Schäfer, M.; Haefliger, L.; Jreige, M.; Vietti Violi, N.; Sempoux, C.; et al. Prospective Evaluation of MR-TRG (Tumor Regression Grade) in Esophageal Cancer after Neo-Adjuvant Therapy: Preliminary Results. Eur. J. Radiol. 2024, 171, 111263. [Google Scholar] [CrossRef]
- Shuto, K.; Kono, T.; Shiratori, T.; Akutsu, Y.; Uesato, M.; Mori, M.; Narushima, K.; Imanishi, S.; Nabeya, Y.; Yanagawa, N.; et al. Diagnostic Performance of Diffusion-Weighted Magnetic Resonance Imaging in Assessing Lymph Node Metastasis of Esophageal Cancer Compared with PET. Esophagus 2020, 17, 239–249. [Google Scholar] [CrossRef]
- Aoyagi, T.; Shuto, K.; Okazumi, S.; Shimada, H.; Kazama, T.; Matsubara, H. Apparent Diffusion Coefficient Values Measured by Diffusion-Weighted Imaging Predict Chemoradiotherapeutic Effect for Advanced Esophageal Cancer. Dig. Surg. 2011, 28, 252–257. [Google Scholar] [CrossRef]
- Lei, J.; Tian, Y. Preliminary Study of IVIM-DWI and DCE-MRI in Early Diagnosis of Esophageal Cancer. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3345–3350. [Google Scholar]
- Sakurada, A.; Takahara, T.; Kwee, T.C.; Yamashita, T.; Nasu, S.; Horie, T.; Van Cauteren, M.; Imai, Y. Diagnostic Performance of Diffusion-Weighted Magnetic Resonance Imaging in Esophageal Cancer. Eur. Radiol. 2009, 19, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, R.; Chen, T.; Ou, J.; Zhang, X.; Chen, F.; Wu, L.; Jiang, Y.; Laws, M.; Shah, K.; et al. Whole-Tumour Histogram Analysis of Pharmacokinetic Parameters from Dynamic Contrast-Enhanced MRI in Resectable Oesophageal Squamous Cell Carcinoma Can Predict T-Stage and Regional Lymph Node Metastasis. Eur. J. Radiol. 2019, 112, 112–120. [Google Scholar] [CrossRef]
- Oberholzer, K.; Pohlmann, A.; Schreiber, W.; Mildenberger, P.; Kunz, P.; Schmidberger, H.; Junginger, T.; Düber, C. Assessment of Tumor Microcirculation with Dynamic Contrast-Enhanced MRI in Patients with Esophageal Cancer: Initial Experience. J. Magn. Reson. Imaging 2008, 27, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.Y.; Li, X.; Jerosch-Herold, M.; Priest, R.A.; Enestvedt, C.K.; Xu, J.; Springer, C.S.; Jobe, B.A. The Evaluation of Esophageal Adenocarcinoma Using Dynamic Contrast-Enhanced Magnetic Resonance Imaging. J. Gastrointest. Surg. 2008, 12, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Umeoka, S.; Saga, T.; Watanabe, G.; Tamai, K.; Kobayashi, A.; Hiraga, A.; Shimada, Y.; Togashi, K. Evaluation of Esophageal Peristalsis in Patients with Esophageal Tumors: Initial Experience with Cine MR Imaging. Magn. Reson. Med. Sci. 2005, 4, 109–114. [Google Scholar] [CrossRef]
- Haefliger, L.; Jreige, M.; Du Pasquier, C.; Ledoux, J.-B.; Wagner, D.; Mantziari, S.; Shäfer, M.; Vietti Violi, N.; Dromain, C. Esophageal Cancer T-Staging on MRI: A Preliminary Study Using Cine and Static MR Sequences. Eur. J. Radiol. 2023, 166, 111001. [Google Scholar] [CrossRef]
- Alper, F.; Turkyilmaz, A.; Kurtcan, S.; Aydin, Y.; Onbas, O.; Acemoglu, H.; Eroglu, A. Effectiveness of the STIR Turbo Spin–Echo Sequence MR Imaging in Evaluation of Lymphadenopathy in Esophageal Cancer. Eur. J. Radiol. 2011, 80, 625–628. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, X.; Yang, Z.; Huang, Y.; Chen, T.; Chen, Y.; Chen, F.; Zeng, N.; Li, R.; Hu, J. The Blood Oxygenation T2* Values of Resectable Esophageal Squamous Cell Carcinomas as Measured by 3T Magnetic Resonance Imaging: Association with Tumor Stage. Korean J. Radiol. 2017, 18, 674. [Google Scholar] [CrossRef]
- Rice, T.W.; Patil, D.T.; Blackstone, E.H. 8th Edition AJCC/UICC Staging of Cancers of the Esophagus and Esophagogastric Junction: Application to Clinical Practice. Ann. Cardiothorac. Surg. 2017, 6, 119–130. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, S.; Shi, D.; Dou, S.; Sun, T.; Ning, P.; Zhao, C.; Li, Z.; Li, X.; Gao, F.; et al. Oesophageal Carcinoma: Comparison of Ex Vivo High-Resolution 3.0 T MR Imaging with Histopathological Findings. Sci. Rep. 2016, 6, 35109. [Google Scholar] [CrossRef]
- De Cobelli, F.; Palumbo, D.; Albarello, L.; Rosati, R.; Giganti, F. Esophagus and Stomach. Magn. Reson. Imaging Clin. North Am. 2020, 28, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-F.; Wang, B.-Z.; Feng, J.-L.; Cheng, W.-R.; Liu, G.-R.; Xu, X.-H.; Zheng, Z.-C. Preoperative TN Staging of Esophageal Cancer: Comparison of Miniprobe Ultrasonography, Spiral CT and MRI. World J. Gastroenterol. 2003, 9, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.; Harmon, M.; Johnston, C.; Fagan, A.J.; Claxton, Z.; Ravi, N.; O’’Toole, D.; Muldoon, C.; Keogan, M.; Reynolds, J.V.; et al. Whole Body MRI in the Staging of Esophageal Cancer—A Prospective Comparison with Whole Body 18F-FDG PET-CT. Dig. Surg. 2015, 32, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ou, J.; Chen, T.; Li, R.; Zhang, X.; Chen, Y.; Jiang, Y.; Yang, J.; Cao, J. Tumour Volume of Resectable Oesophageal Squamous Cell Carcinoma Measured with MRI Correlates Well with T Category and Lymphatic Metastasis. Eur. Radiol. 2018, 28, 4757–4765. [Google Scholar] [CrossRef]
- Wang, F.; Guo, R.; Zhang, Y.; Yu, B.; Meng, X.; Kong, H.; Yang, Y.; Yang, Z.; Li, N. Value of 18F-FDG PET/MRI in the Preoperative Assessment of Resectable Esophageal Squamous Cell Carcinoma: A Comparison With 18F-FDG PET/CT, MRI, and Contrast-Enhanced CT. Front. Oncol. 2022, 12, 844702. [Google Scholar] [CrossRef]
- Gao, Z.; Hua, B.; Ge, X.; Liu, J.; Xue, L.; Zhen, F.; Luo, J. Comparison Between Size and Stage of Preoperative Tumor Defined by Preoperative Magnetic Resonance Imaging and Postoperative Specimens After Radical Resection of Esophageal Cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819876263. [Google Scholar] [CrossRef]
- Van Rossum, P.S.N.; Van Lier, A.L.H.M.W.; Lips, I.M.; Meijer, G.J.; Reerink, O.; Van Vulpen, M.; Lam, M.G.E.H.; Van Hillegersberg, R.; Ruurda, J.P. Imaging of Oesophageal Cancer with FDG-PET/CT and MRI. Clin. Radiol. 2015, 70, 81–95. [Google Scholar] [CrossRef]
- Zhao, K.; Chu, F.; Wang, Z.; Zhang, H.; Lu, S.; Jia, Z.; Zheng, Y.; Xia, Q.; Kamel, I.R.; Li, H.; et al. Aorta and Tracheobronchial Invasion in Esophageal Cancer: Comparing Diagnostic Performance of 3.0-T MRI and CT. Eur. Radiol. 2023, 33, 4962–4972. [Google Scholar] [CrossRef]
- Kelly, S. A Systematic Review of the Staging Performance of Endoscopic Ultrasound in Gastro-Oesophageal Carcinoma. Gut 2001, 49, 534–539. [Google Scholar] [CrossRef]
- Van Westreenen, H.L.; Westerterp, M.; Bossuyt, P.M.M.; Pruim, J.; Sloof, G.W.; Van Lanschot, J.J.B.; Groen, H.; Plukker, J.T.M. Systematic Review of the Staging Performance of18 F-Fluorodeoxyglucose Positron Emission Tomography in Esophageal Cancer. JCO 2004, 22, 3805–3812. [Google Scholar] [CrossRef]
- Kwee, R.M.; Kwee, T.C. Imaging in Local Staging of Gastric Cancer: A Systematic Review. J. Clin. Oncol. 2007, 25, 2107–2116. [Google Scholar] [CrossRef] [PubMed]
- van Rossum, P.S.N.; van Hillegersberg, R.; Lever, F.M.; Lips, I.M.; van Lier, A.L.H.M.W.; Meijer, G.J.; van Leeuwen, M.S.; van Vulpen, M.; Ruurda, J.P. Imaging Strategies in the Management of Oesophageal Cancer: What’s the Role of MRI? Eur. Radiol. 2013, 23, 1753–1765. [Google Scholar] [CrossRef]
- Bruzzi, J.F.; Munden, R.F.; Truong, M.T.; Marom, E.M.; Sabloff, B.S.; Gladish, G.W.; Iyer, R.B.; Pan, T.-S.; Macapinlac, H.A.; Erasmus, J.J. PET/CT of Esophageal Cancer: Its Role in Clinical Management. RadioGraphics 2007, 27, 1635–1652. [Google Scholar] [CrossRef]
- Floriani, I.; Torri, V.; Rulli, E.; Garavaglia, D.; Compagnoni, A.; Salvolini, L.; Giovagnoni, A. Performance of Imaging Modalities in Diagnosis of Liver Metastases from Colorectal Cancer: A Systematic Review and Meta-Analysis. J. Magn. Reson. Imaging 2010, 31, 19–31. [Google Scholar] [CrossRef]
- Taylor, S.A.; Mallett, S.; Beare, S.; Bhatnagar, G.; Blunt, D.; Boavida, P.; Bridgewater, J.; Clarke, C.S.; Duggan, M.; Ellis, S.; et al. Diagnostic Accuracy of Whole-Body MRI versus Standard Imaging Pathways for Metastatic Disease in Newly Diagnosed Colorectal Cancer: The Prospective Streamline C Trial. Lancet Gastroenterol. Hepatol. 2019, 4, 529–537. [Google Scholar] [CrossRef]
- Vulasala, S.S.; Virarkar, M.; Karbasian, N.; Calimano-Ramirez, L.F.; Daoud, T.; Amini, B.; Bhosale, P.; Javadi, S. Whole-Body MRI in Oncology: A Comprehensive Review. Clin. Imaging 2024, 108, 110099. [Google Scholar] [CrossRef]
- Obermannová, R.; Alsina, M.; Cervantes, A.; Leong, T.; Lordick, F.; Nilsson, M.; van Grieken, N.C.T.; Vogel, A.; Smyth, E.C. Oesophageal Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022, 33, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- van Hagen, P.; Hulshof, M.C.C.M.; van Lanschot, J.J.B.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; Richel, D.J.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. Available online: https://www.nejm.org/doi/10.1056/NEJMoa1112088 (accessed on 27 March 2021).
- Reynolds, J.V.; Preston, S.R.; O’Neill, B.; Lowery, M.A.; Baeksgaard, L.; Crosby, T.; Cunningham, M.; Cuffe, S.; Griffiths, G.O.; Parker, I.; et al. Trimodality Therapy versus Perioperative Chemotherapy in the Management of Locally Advanced Adenocarcinoma of the Oesophagus and Oesophagogastric Junction (Neo-AEGIS): An Open-Label, Randomised, Phase 3 Trial. Lancet Gastroenterol. Hepatol. 2023, 8, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.; van Lanschot, J.J.B.; Hulshof, M.C.C.M.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant Chemoradiotherapy plus Surgery versus Surgery Alone for Oesophageal or Junctional Cancer (CROSS): Long-Term Results of a Randomised Controlled Trial. Lancet Oncol. 2015, 16, 1090–1098. [Google Scholar] [CrossRef]
- Noordman, B.J.; Spaander, M.C.W.; Valkema, R.; Wijnhoven, B.P.L.; van Berge Henegouwen, M.I.; Shapiro, J.; Biermann, K.; van der Gaast, A.; van Hillegersberg, R.; Hulshof, M.C.C.M.; et al. Detection of Residual Disease after Neoadjuvant Chemoradiotherapy for Oesophageal Cancer (preSANO): A Prospective Multicentre, Diagnostic Cohort Study. Lancet Oncol. 2018, 19, 965–974. [Google Scholar] [CrossRef]
- Defize, I.L.; Van Hillegersberg, R.; Mook, S.; Meijer, G.J.; Lin, S.H.; Ruurda, J.P.; Van Rossum, P.S.N. Restaging after Chemoradiotherapy for Locally Advanced Esophageal Cancer. Ann. Transl. Med. 2019, 7, S288. [Google Scholar] [CrossRef]
- Beseth, B.D.; Bedford, R.; Isacoff, W.H.; Holmes, E.C.; Cameron, R.B. Endoscopic Ultrasound Does Not Accurately Assess Pathologic Stage of Esophageal Cancer after Neoadjuvant Chemoradiotherapy. Am. Surg. 2000, 66, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, A.; Meyer, P.; Schnider, A.; Komminoth, P.; Schmid, M.; Lombriser, N.; Weishaupt, D. Accuracy of Multidetector-Row CT for Restaging after Neoadjuvant Treatment in Patients with Oesophageal Cancer. Eur. Radiol. 2013, 23, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Chen, T.; Han, J.; Ye, P.; Hu, J. Staging Accuracy of Endoscopic Ultrasound for Esophageal Cancer after Neoadjuvant Chemotherapy: A Meta-Analysis and Systematic Review. Dis. Esophagus 2015, 28, 757–771. [Google Scholar] [CrossRef]
- Valkema, M.J.; Noordman, B.J.; Wijnhoven, B.P.L.; Spaander, M.C.W.; Biermann, K.; Lagarde, S.M.; Bennink, R.J.; Schreurs, W.M.J.; Roef, M.J.; Hobbelink, M.G.G.; et al. Accuracy of18 F-FDG PET/CT in Predicting Residual Disease After Neoadjuvant Chemoradiotherapy for Esophageal Cancer. J. Nucl. Med. 2019, 60, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- De Gouw, D.J.J.M.; Klarenbeek, B.R.; Driessen, M.; Bouwense, S.A.W.; Van Workum, F.; Fütterer, J.J.; Rovers, M.M.; Ten Broek, R.P.G.; Rosman, C. Detecting Pathological Complete Response in Esophageal Cancer after Neoadjuvant Therapy Based on Imaging Techniques: A Diagnostic Systematic Review and Meta-Analysis. J. Thorac. Oncol. 2019, 14, 1156–1171. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Z.-Y.; Wu, H.-W.; Zhang, C.-P.; Hu, B.; Rong, L.; Chen, H.-Y.; Xie, H.-Y.; Wang, Y.-M.; Lin, H.-P.; et al. Diffusion-Weighted MRI and 18F-FDG PET/CT in Assessing the Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Esophageal Squamous Cell Carcinoma. Radiat. Oncol. 2021, 16, 132. [Google Scholar] [CrossRef]
- Vollenbrock, S.E.; Voncken, F.E.M.; Van Dieren, J.M.; Lambregts, D.M.J.; Maas, M.; Meijer, G.J.; Goense, L.; Mook, S.; Hartemink, K.J.; Snaebjornsson, P.; et al. Diagnostic Performance of MRI for Assessment of Response to Neoadjuvant Chemoradiotherapy in Oesophageal Cancer. Br. J. Surg. 2019, 106, 596–605. [Google Scholar] [CrossRef]
- Fiorino, C.; Palumbo, D.; Mori, M.; Palazzo, G.; Pellegrini, A.E.; Albarello, L.; Belardo, A.; Canevari, C.; Cossu, A.; Damascelli, A.; et al. Early Regression Index (ERI) on MR Images as Response Predictor in Esophageal Cancer Treated with Neoadjuvant Chemo-Radiotherapy: Interim Analysis of the Prospective ESCAPE Trial. Radiother. Oncol. 2024, 194, 110160. [Google Scholar] [CrossRef]
- Borggreve, A.S.; Heethuis, S.E.; Boekhoff, M.R.; Goense, L.; van Rossum, P.S.N.; Brosens, L.A.A.; van Lier, A.L.H.M.W.; van Hillegersberg, R.; Lagendijk, J.J.W.; Mook, S.; et al. Optimal Timing for Prediction of Pathologic Complete Response to Neoadjuvant Chemoradiotherapy with Diffusion-Weighted MRI in Patients with Esophageal Cancer. Eur. Radiol. 2020, 30, 1896–1907. [Google Scholar] [CrossRef]
- Elsherif, S.B.; Andreou, S.; Virarkar, M.; Soule, E.; Gopireddy, D.R.; Bhosale, P.R.; Lall, C. Role of Precision Imaging in Esophageal Cancer. J. Thorac. Dis. 2020, 12, 5159–5176. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, S.; Shuto, K.; Aoyagi, T.; Kono, T.; Saito, H.; Matsubara, H. Diffusion-Weighted Magnetic Resonance Imaging for Predicting and Detecting the Early Response to Chemoradiotherapy of Advanced Esophageal Squamous Cell Carcinoma. Dig. Surg. 2013, 30, 240–248. [Google Scholar] [CrossRef]
- Cheng, B.; Yu, J. Predictive Value of Diffusion-Weighted MR Imaging in Early Response to Chemoradiotherapy of Esophageal Cancer: A Meta-Analysis. Dis. Esophagus 2019, 32, doy065. [Google Scholar] [CrossRef]
- Heethuis, S.E.; Goense, L.; Van Rossum, P.S.N.; Borggreve, A.S.; Mook, S.; Voncken, F.E.M.; Bartels-Rutten, A.; Aleman, B.M.P.; Van Hillegersberg, R.; Ruurda, J.P.; et al. DW-MRI and DCE-MRI Are of Complementary Value in Predicting Pathologic Response to Neoadjuvant Chemoradiotherapy for Esophageal Cancer. Acta Oncologica 2018, 57, 1201–1208. [Google Scholar] [CrossRef]
- Langer, R.; Becker, K. Tumor Regression Grading of Gastrointestinal Cancers after Neoadjuvant Therapy. Virchows Arch. 2018, 472, 175–186. [Google Scholar] [CrossRef]
- Patel, U.B.; Taylor, F.; Blomqvist, L.; George, C.; Evans, H.; Tekkis, P.; Quirke, P.; Sebag-Montefiore, D.; Moran, B.; Heald, R.; et al. Magnetic Resonance Imaging-Detected Tumor Response for Locally Advanced Rectal Cancer Predicts Survival Outcomes: MERCURY Experience. J. Clin. Oncol. 2011, 29, 3753–3760. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhang, Y.; Lu, S.; Xing, W.; Zheng, Y.; Sun, H.; Gao, Q.; Xia, Q.; Wang, Z.; Zhang, H.; et al. Quantitative RECIST Derived from Multiparametric MRI in Evaluating Response of Esophageal Squamous Cell Carcinoma to Neoadjuvant Therapy. Eur. Radiol. 2022, 32, 7295–7306. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, E.P.M.; Heijenbrok-Kal, M.H.; Hunink, M.G.M.; Kuipers, E.J.; Siersema, P.D. Staging Investigations for Oesophageal Cancer: A Meta-Analysis. Br. J. Cancer 2008, 98, 547–557. [Google Scholar] [CrossRef]
- Goense, L.; Ruurda, J.P.; Carter, B.W.; Fang, P.; Ho, L.; Meijer, G.J.; van Hillegersberg, R.; Hofstetter, W.L.; Lin, S.H. Prediction and Diagnosis of Interval Metastasis after Neoadjuvant Chemoradiotherapy for Oesophageal Cancer Using 18F-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1742–1751. [Google Scholar] [CrossRef]
- Kantarcı, M.; Polat, P.; Alper, F.; Eroǧlu, A.; Eren, S.; Okur, A.; Onbaş, O. Comparison of CT and MRI for the Diagnosis Recurrent Esophageal Carcinoma after Operation. Dis. Esophagus 2004, 17, 32–37. [Google Scholar] [CrossRef]
- Shuto, K.; Saito, H.; Ohira, G.; Natsume, T.; Kono, T.; Tohma, T.; Sato, A.; Ota, T.; Akutsu, Y.; Aoyagi, T.; et al. Diffusion-weighted MR imaging for postoperative nodal recurrence of esophageal squamous cell cancer in comparison with FDG-PET. Gan Kagaku Ryoho 2009, 36, 2468–2470. [Google Scholar]
- Vollenbrock, S.E.; Voncken, F.E.M.; Lambregts, D.M.J.; Maas, M.; Donswijk, M.L.; Vegt, E.; ter Beek, L.C.; van Dieren, J.M.; van Sandick, J.W.; Aleman, B.M.P.; et al. Clinical Response Assessment on DW-MRI Compared with FDG-PET/CT after Neoadjuvant Chemoradiotherapy in Patients with Oesophageal Cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, M.; Albadawy, M. AI in Diagnostic Imaging: Revolutionising Accuracy and Efficiency. Comput. Methods Programs Biomed. Update 2024, 5, 100146. [Google Scholar] [CrossRef]
- Ma, D.; Zhou, T.; Chen, J.; Chen, J. Radiomics Diagnostic Performance for Predicting Lymph Node Metastasis in Esophageal Cancer: A Systematic Review and Meta-Analysis. BMC Med. Imaging 2024, 24, 144. [Google Scholar] [CrossRef]
- Heethuis, S.E.; Van Rossum, P.S.N.; Lips, I.M.; Goense, L.; Voncken, F.E.; Reerink, O.; Van Hillegersberg, R.; Ruurda, J.P.; Philippens, M.E.; Van Vulpen, M.; et al. Dynamic Contrast-Enhanced MRI for Treatment Response Assessment in Patients with Oesophageal Cancer Receiving Neoadjuvant Chemoradiotherapy. Radiother. Oncol. 2016, 120, 128–135. [Google Scholar] [CrossRef]
- Lo Gullo, R.; Daimiel, I.; Morris, E.A.; Pinker, K. Combining Molecular and Imaging Metrics in Cancer: Radiogenomics. Insights Into Imaging 2020, 11, 1. [Google Scholar] [CrossRef]


| T2WI TSE Blade | T2WI TSE Blade | DWI Chest | DWI Liver | T1WI Dynamic VIBE Dixon | OPTIONAL T2WI TSE DB | |
|---|---|---|---|---|---|---|
| Plane | Axial | Sagittal | Axial | Axial | Axial | Axial |
| Volume | Chest and liver 2 boxes | Chest | Chest | Liver | Chest and liver 2 boxes | Perpendicular to Lesion |
| Repetition Time TR (ms) | 2290 | 2020 | 3500 | 2500 | 4.5 | 550 (1) |
| Echo Time TE (ms) | 68 | 89 | 59 | 59 | 1.35–2.58 | 69 |
| Slice Thickness (mm) | 5 | 3 | 5 | 5 | 2.3 | 3 |
| Field of View (mm) | 371 × 371 | 400 × 400 | 380 × 261 | 380 × 261 | 350 × 273 | 230 × 187 |
| Phase Encoding | x (Radial) | x (Radial) | A > P | A > P | A > P | A > P |
| Matrix Size | 320 | 320 | 134 × 134 | 134 × 134 | 320 × 240 | 256 × 230 |
| Voxel Size (mm) | 1.2 × 1.2 × 5 | 1.3 × 1.3 × 3 | 1.4 × 1.4 × 5 (interpolated) | 1.4 × 1.4 × 5 (interpolated) | 1.1 × 1.1 × 2.3 | 0.9 × 0.9 × 3 |
| Number of Slices | 45 | 30 | 40 | 28 | 88 | 25 |
| Distance Factor (mm) | 1 | 0.3 | 1 | 1 | 0.46 | 0,3 |
| Flip Angle (degree) | 100 | 120 | x | x | 9 | 180 |
| Acceleration Factors | Grappa 3 | Grappa 2 | SMS 4 | SMS 4 | Caipirinha 4 | Grappa 2 |
| Turbo Factors (or EPI Factor) | 35 | 31 | 92 | 92 | x | 17 |
| Bandwidth (Hz/Px) | 781 | 1563 | 2332 | 2332 | 1040 | 849 |
| Nex | 1 | 1 | 1 | 1 | 1 | 1 |
| Acquisition Time (min) | 1 min 23 s | 1 min 51 s | 1 min 43 s | 1 min 14 s | 18 sec/phase | 8 min 34 s (1) |
| Number of Apnea | 4 | 4 | FB | FB | 4 | 13–25 (1) |
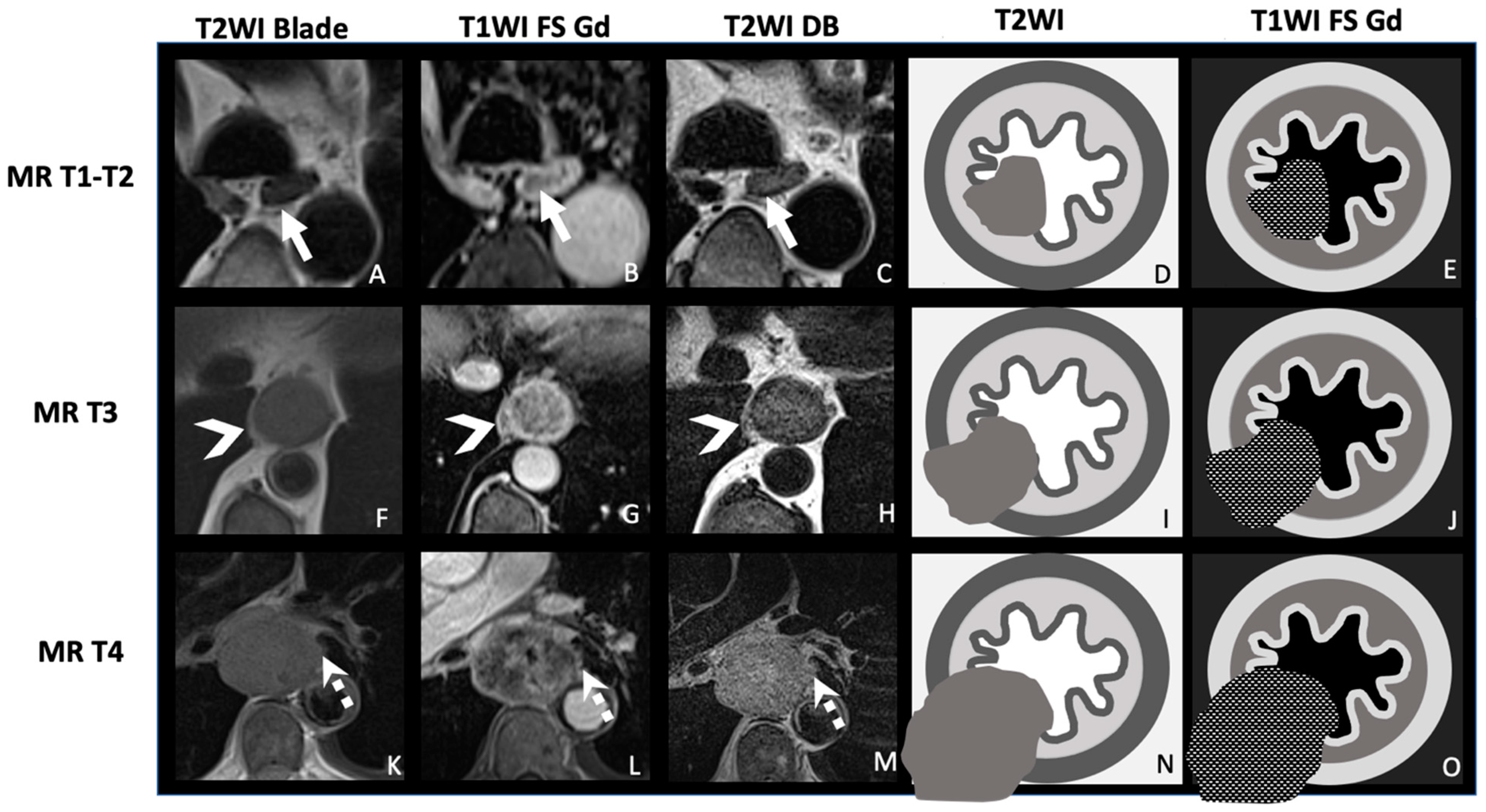
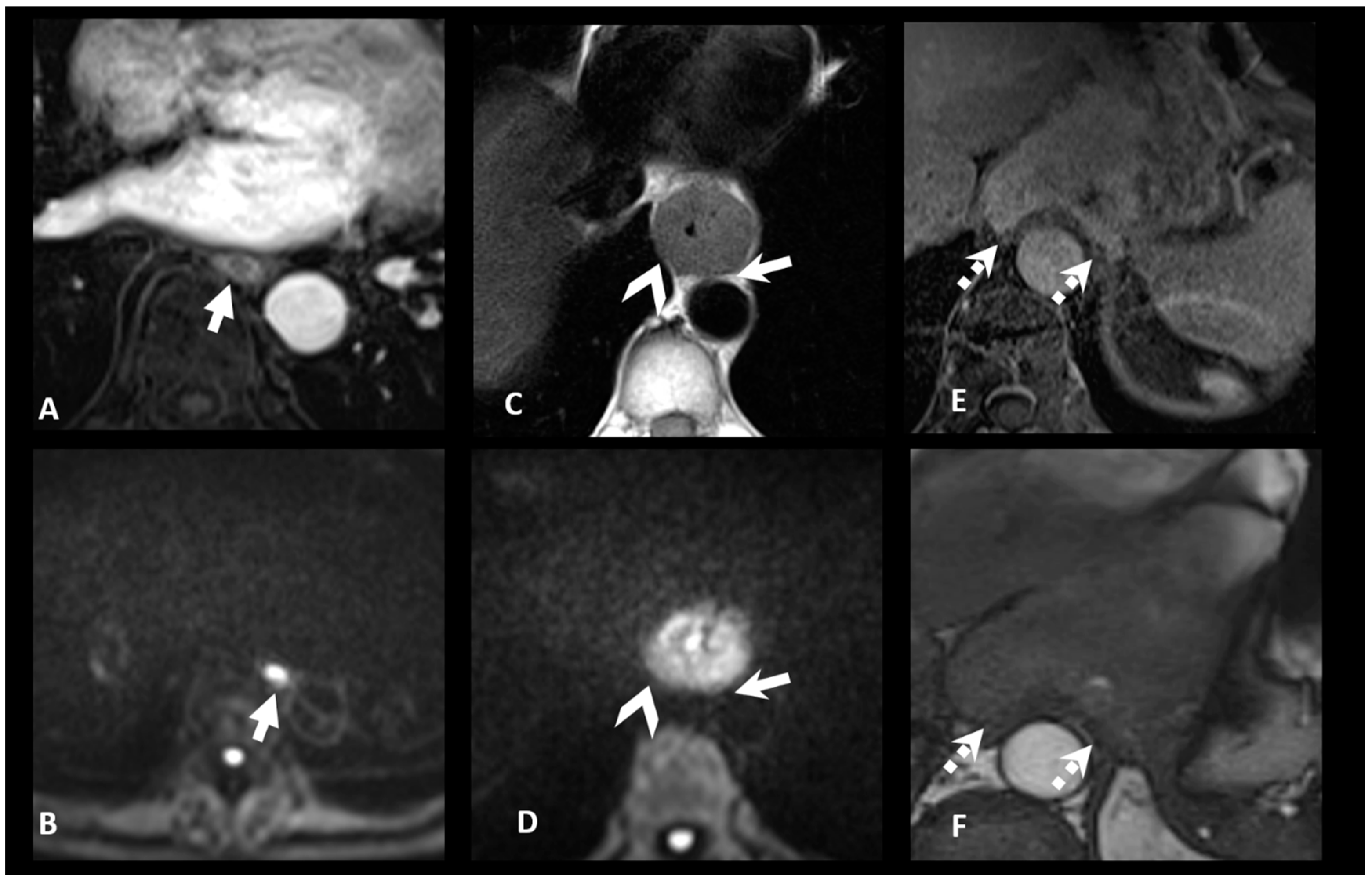
| TNM Histo-Pathologi Definition (According to AJCC/UICC 8th Edition) | MRI TNM Staging |
|---|---|
| T1: Tumor invades the submucosa | MR-T1-T2: Tumor signal intensity is confined to esophageal wall without extension to peri-esophagus fat |
| T2: Tumor invades the muscularis propria | |
| T3: Tumor invades adventitia | MR-T3: Tumor invades the peri-esophagus fat tissue |
T4: Tumor invades adjacent organ
| MR-T4: Tumor invades adjacent organs
|
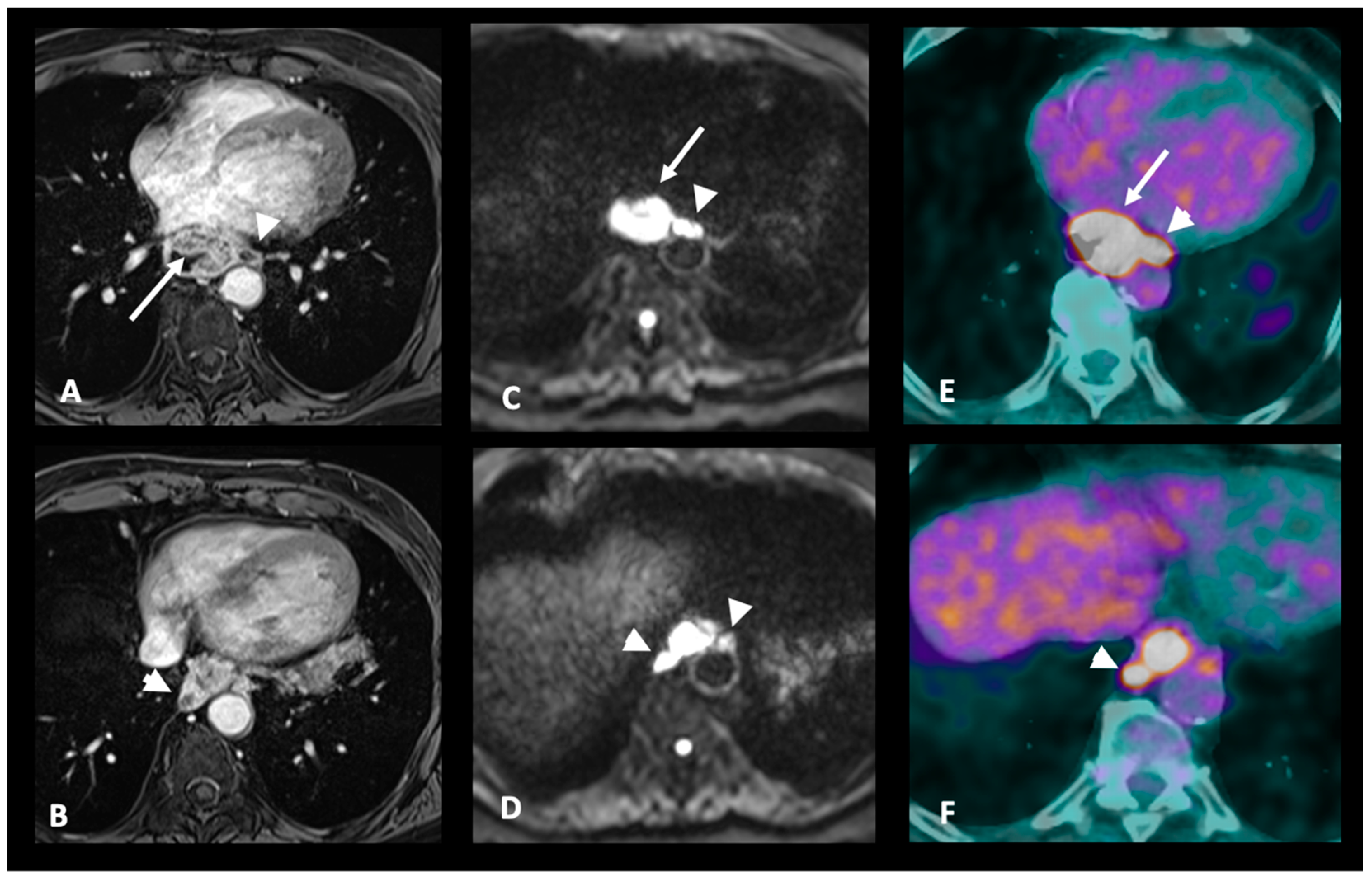
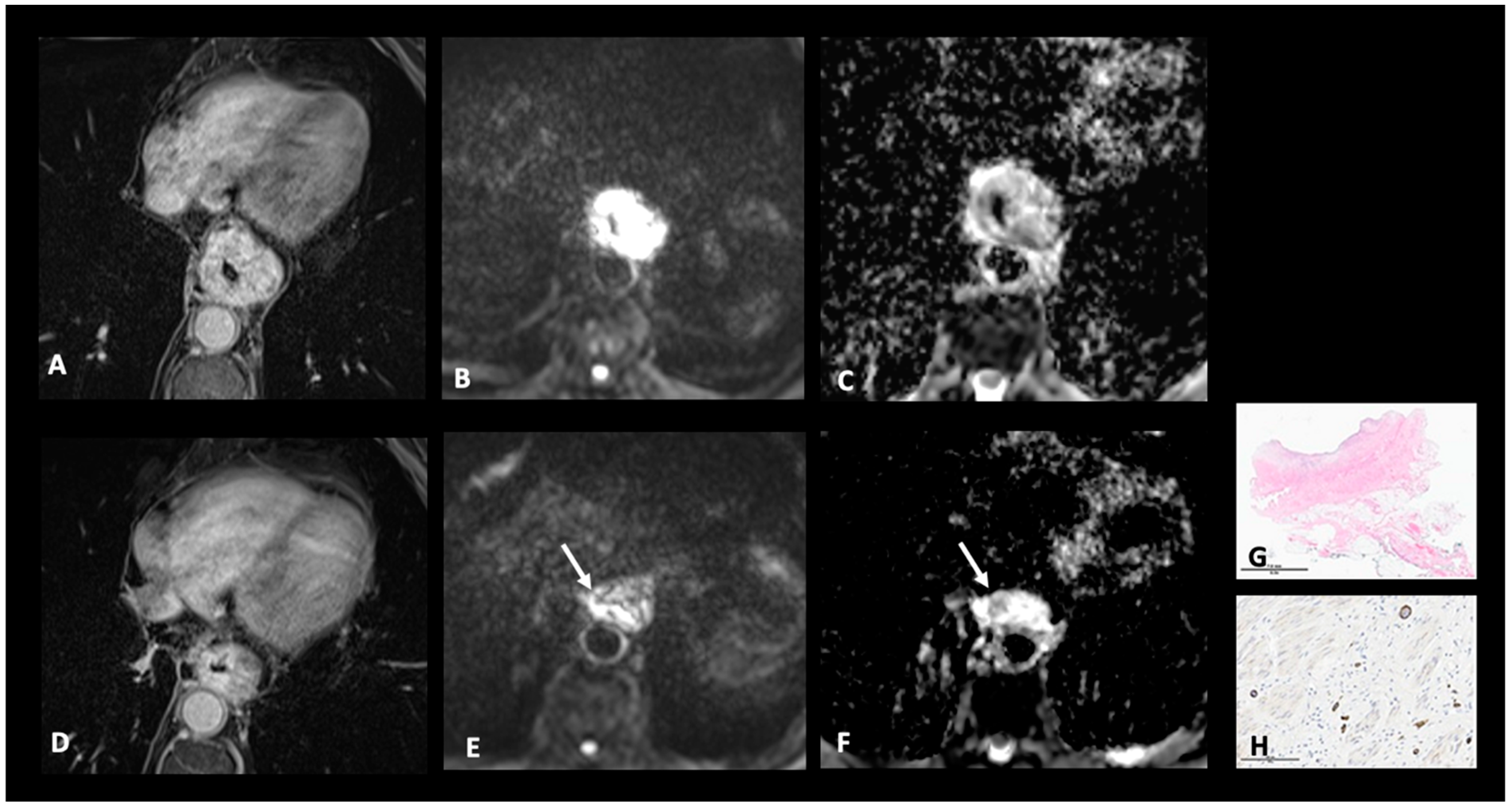
| N+: Regional lymph nodes metastasis | MRI-N+: Round, Intermediate to high T2 signal, heterogeneous T1 Gd, >5 mm small axe |
| M+: Distant metastases | MRI-M+: Distant lymph node (sus-clavicular e.g.), liver metastases or other organ metastases |
| First Author, Year | No. of Patients | MRI | Sequence | T Staging | N Staging | M Staging | |||
|---|---|---|---|---|---|---|---|---|---|
| Sens % | Spe % | Sens % | Spe % | Sens % | Spe % | ||||
| Ling-Fei Wu et al., 2003 [32] | 86 | 0.15 T | T1WI and T2WI | 40–55 | 55–63 | NA | NA | NA | NA |
| Sakurada et al., 2009 [21] | 24 | 1.5 T | DWIBS (b0-1000), T2WI | 33–100 | Not provided | >5 mm 77.8 | 55.6 | NA | NA |
| Malik et al., 2015 [33] | 49 | 3 T | DWIBS (b0-1000), T2WI | 59–98 | 92–100 | 30 | 100 | ||
| Lei et al., 2015 [20] | 30 | 3 T | IVIM-DWI (b0-1500), DCE MRI | 90 | 95 | NA | NA | 67 | 91 |
| Wei et al., 2016 [30] | 61 | 3 T | T2WI | 71–100 | 98–100 | NA | NA | NA | NA |
| Giganti et al., 2016 [16] | 18 | 1.5 T | T2WI, DWI (b0-600) | 67 | 83 | >10 mm 100 | 57 | NA | NA |
| Qu et al., 2018 [13] | 43 | 3 T | T1WI-CE-(VIBE) | 73–100 | 85–100 | NA | NA | NA | NA |
| Wu et al., 2018 [34] | 60 | 3 T | T2WI, CE-T1WI, DWI (b-values 0-800) | 84–100 | 100 | NA | NA | NA | NA |
| Chen et al., 2019 [22] | 42 | 3 T | DCE-MRI | 86.2 (Ve) | 61.5 | 77.8 (Kep) | 59.7 | NA | NA |
| Guo et al., 2020 [11] | 74 | 3 T | T2WI msTSE, DWI (b0-700), T1WI 3D GRE | 96–98 | 93 | NA | NA | NA | NA |
| Shuto et al., 2020 [18] | 76 | 1.5 T | DWI (b0-1000) | NA | NA | >10 mm; ADC < 1.2 s/mm2 67 | 98 | NA | NA |
| Wang et al., 2022 [35] | 35 | 3 T | T2IW, DWI (b0-800) | 85.7 | 77.1 | 47.8 | 91.5 | NA | NA |
| Haefliger et al., 2023 [26] | 53 | 3 T | T2WI, DWI (b0-800), T1WI-CE, Cine | 95.8 | 75 | NA | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haefliger, L.; Chapellier, P.; Vietti Violi, N.; Ledoux, J.-B.; Mantziari, S.; Schäfer, M.; Dromain, C. Advancing Esophageal Cancer Staging and Restaging: The Role of MRI in Precision Diagnosis. Cancers 2025, 17, 1351. https://doi.org/10.3390/cancers17081351
Haefliger L, Chapellier P, Vietti Violi N, Ledoux J-B, Mantziari S, Schäfer M, Dromain C. Advancing Esophageal Cancer Staging and Restaging: The Role of MRI in Precision Diagnosis. Cancers. 2025; 17():1351. https://doi.org/10.3390/cancers17081351
Chicago/Turabian StyleHaefliger, Laura, Pauline Chapellier, Naik Vietti Violi, Jean-Baptiste Ledoux, Styliani Mantziari, Markus Schäfer, and Clarisse Dromain. 2025. "Advancing Esophageal Cancer Staging and Restaging: The Role of MRI in Precision Diagnosis" Cancers 17, no. : 1351. https://doi.org/10.3390/cancers17081351
APA StyleHaefliger, L., Chapellier, P., Vietti Violi, N., Ledoux, J.-B., Mantziari, S., Schäfer, M., & Dromain, C. (2025). Advancing Esophageal Cancer Staging and Restaging: The Role of MRI in Precision Diagnosis. Cancers, 17(), 1351. https://doi.org/10.3390/cancers17081351







