The Effect of Staging Intervals on Progression-Free Survival in Registration Studies of Oncologic Drugs: A Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Study Selection
2.3. Data Extraction
2.4. Data Synthesis
2.5. Statistical Analyses
3. Results
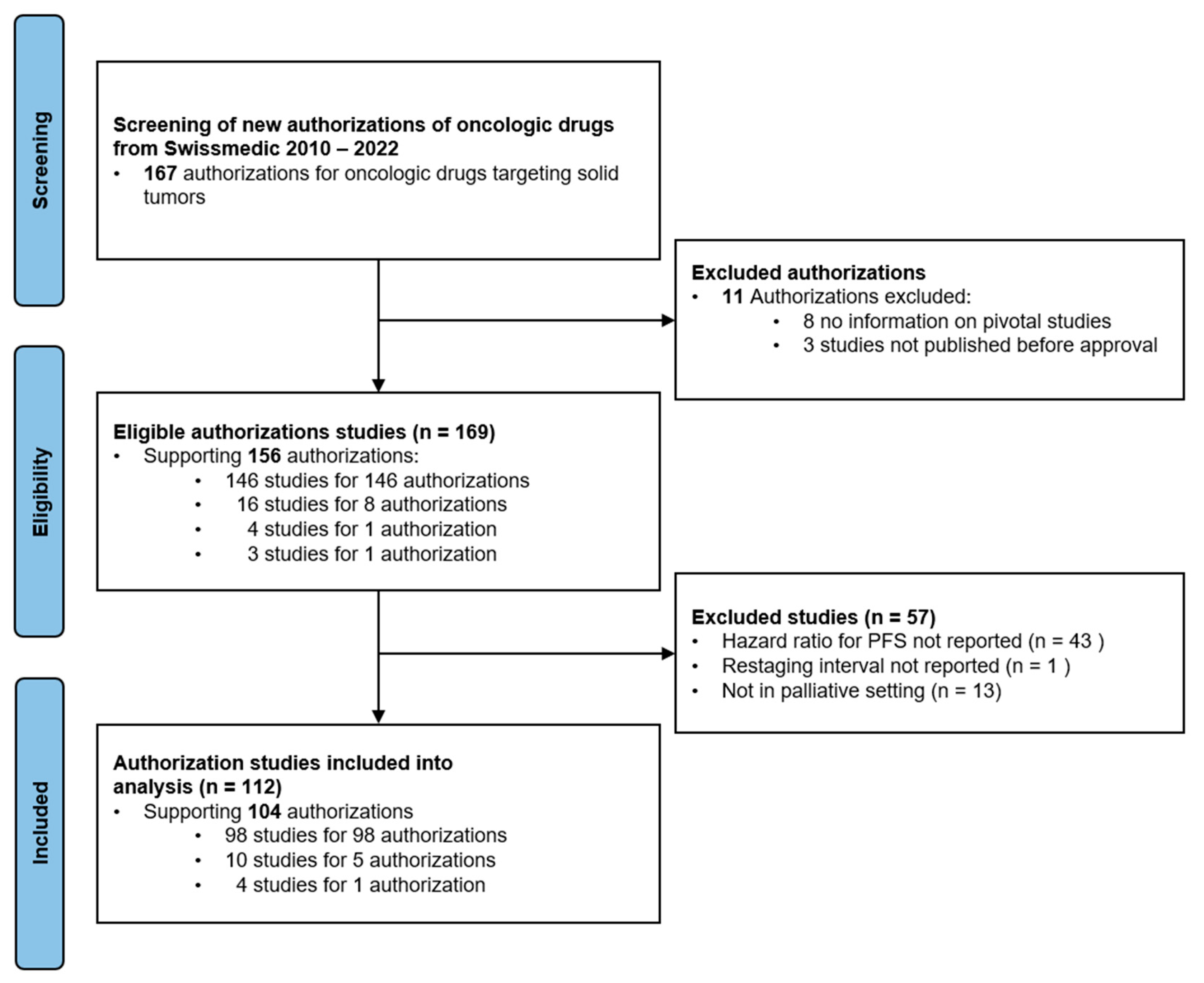
3.1. Included Studies
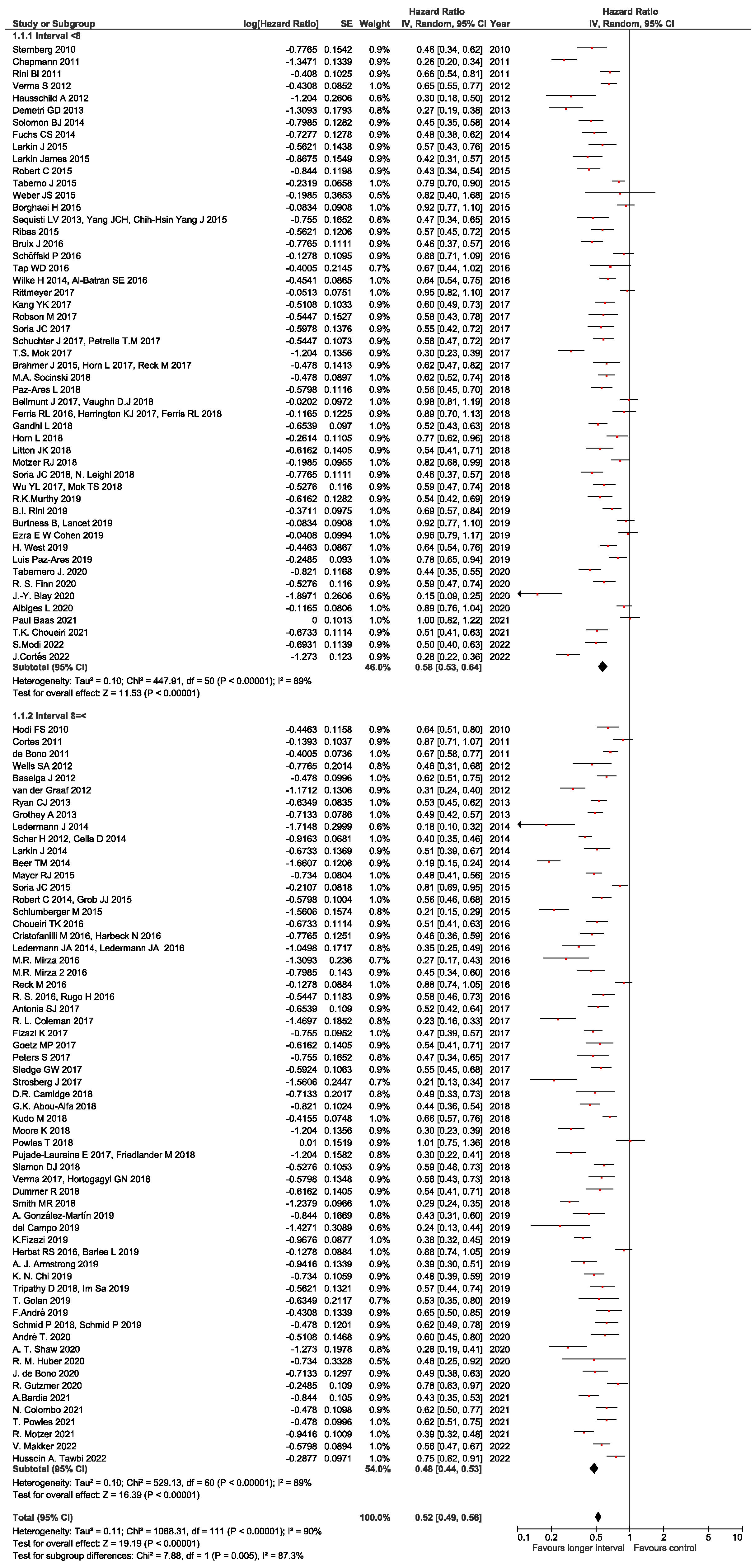
3.2. Staging Intervals
3.3. Subgroup Analyses
3.4. Sensitivity Analyses
3.5. Meta-Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PFS | Progression-Free-Survival |
| OS | Overall Survival |
| HR | Hazard Ratio |
| CI | Confidence Interval |
References
- Scott, E.C.; Baines, A.C.; Gong, Y.; Moore, R.; Pamuk, G.E.; Saber, H.; Subedee, A.; Thompson, M.D.; Xiao, W.; Pazdur, R.; et al. Trends in the Approval of Cancer Therapies by the FDA in the Twenty-First Century. Nat. Rev. Drug Discov. 2023, 22, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Del Paggio, J.C.; Berry, J.S.; Hopman, W.M.; Eisenhauer, E.A.; Prasad, V.; Gyawali, B.; Booth, C.M. Evolution of the Randomized Clinical Trial in the Era of Precision Oncology. JAMA Oncol. 2021, 7, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Pazdur, R. Endpoints for Assessing Drug Activity in Clinical Trials. Oncol. 2008, 13, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Prasad, V. Strength of Validation for Surrogate End Points Used in the US Food and Drug Administration’s Approval of Oncology Drugs. Mayo Clin. Proc. 2016, 91, 713–725. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Delgado, A.; Guddati, A.K. Clinical Endpoints in Oncology—A Primer. Am. J. Cancer Res. 2021, 11, 1121–1131. [Google Scholar]
- Panageas, K.S.; Ben-Porat, L.; Dickler, M.N.; Chapman, P.B.; Schrag, D. When You Look Matters: The Effect of Assessment Schedule on Progression-Free Survival. JNCI J. Natl. Cancer Inst. 2007, 99, 428–432. [Google Scholar] [CrossRef]
- Chen, E.Y.; Joshi, S.K.; Tran, A.; Prasad, V. Estimation of Study Time Reduction Using Surrogate End Points Rather Than Overall Survival in Oncology Clinical Trials. JAMA Intern. Med. 2019, 179, 642–647. [Google Scholar] [CrossRef]
- Tannock, I.F.; Amir, E.; Booth, C.M.; Niraula, S.; Ocana, A.; Seruga, B.; Templeton, A.J.; Vera-Badillo, F. Relevance of Randomised Controlled Trials in Oncology. Lancet Oncol. 2016, 17, e560–e567. [Google Scholar] [CrossRef]
- Dabush, D.R.; Shepshelovich, D.; Shochat, T.; Tibau, A.; Amir, E.; Goldvaser, H. The Impact of Radiological Assessment Schedules on Progression-Free Survival in Metastatic Breast Cancer: A Systemic Review and Meta-Analysis. Cancer Treat. Rev. 2021, 100, 102293. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Swissmedic 2019, Copyright© Swissmedic Journal. Available online: https://www.swissmedic.ch/swissmedic/de/home/ueber-uns/publikationen/swissmedic-journal.html (accessed on 17 February 2024).
- Compendium.Ch. Available online: https://compendium.ch/ (accessed on 17 February 2024).
- Adam, R.; Tibau, A.; Valiente, C.M.; Šeruga, B.; Ocaña, A.; Amir, E.; Templeton, A.J. Clinical Benefit of Cancer Drugs Approved in Switzerland 2010–2019. PLoS ONE 2022, 17, e0268545. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.S.; Borkar, N. Sensitivity and Subgroup Analysis in a Meta-Analysis—What We Should Know? Indian J. Anaesth. 2024, 68, 922–924. [Google Scholar] [CrossRef] [PubMed]
- Review Manager (RevMan). Available online: https://test-training.cochrane.org/online-learning/core-software-cochrane-reviews/review-manager-revman (accessed on 25 March 2025).
- Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://training.cochrane.org/handbook (accessed on 17 February 2024).
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. (Eds.) Chapter 10: Analysing Data and Undertaking Meta-Analyses. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (Updated August 2023); Cochrane Training: Cochrane, AB, Canada, 2023; Available online: https://training.cochrane.org/handbook/current/chapter-10 (accessed on 18 February 2024).
- 2 Identifying and Measuring Heterogeneity. Available online: https://handbook-5-1.cochrane.org/chapter_9/9_5_2_identifying_and_measuring_heterogeneity.htm (accessed on 25 March 2025).
- IBM SPSS Software. Available online: https://www.ibm.com/spss (accessed on 25 March 2025).
- PRISMA 2020 Flow Diagram—PRISMA Statement. Available online: https://www.prisma-statement.org/prisma-2020-flow-diagram (accessed on 25 March 2025).
- Burnand, B.; Kernan, W.N.; Feinstein, A.R. Indexes and Boundaries for “Quantitative Significance” in Statistical Decisions. J. Clin. Epidemiol. 1990, 43, 1273–1284. [Google Scholar] [CrossRef]
- Tumor Grade—NCI. Available online: https://www.cancer.gov/about-cancer/diagnosis-staging/diagnosis/tumor-grade (accessed on 3 April 2025).
- Joensuu, H.; Reichardt, P.; Eriksson, M.; Sundby Hall, K.; Vehtari, A. Gastrointestinal Stromal Tumor: A Method for Optimizing the Timing of CT Scans in the Follow-Up of Cancer Patients. Radiology 2014, 271, 96–106. [Google Scholar] [CrossRef]
- Ji, S.Y.; Lee, J.; Lee, J.H.; Lee, S.-T.; Won, J.K.; Kim, J.W.; Kim, Y.H.; Kim, T.M.; Choi, S.H.; Park, S.-H.; et al. Radiological Assessment Schedule for High-Grade Glioma Patients During the Surveillance Period Using Parametric Modeling. Neuro Oncol. 2021, 23, 837–847. [Google Scholar] [CrossRef]
- Mohammed, N.; Kestin, L.L.; Grills, I.S.; Battu, M.; Fitch, D.L.; Wong, C.-Y.O.; Margolis, J.H.; Chmielewski, G.W.; Welsh, R.J. Rapid Disease Progression with Delay in Treatment of Non-Small-Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 466–472. [Google Scholar] [CrossRef]
- Modi, N.D.; Swain, S.M.; Buyse, M.; Kuderer, N.M.; Rowland, A.; Rockhold, F.W.; Sorich, M.J.; Hopkins, A.M. Clinical Study Report and Individual Participant Data Transparency for US Food and Drug Administration-Approved Anticancer Drugs: A Call for Systematic Data Availability. J. Clin. Oncol. 2024, 42, 3773–3777. [Google Scholar] [CrossRef]
- Thomas, D.; Radji, S.; Benedetti, A. Systematic Review of Methods for Individual Patient Data Meta-Analysis with Binary Outcomes. BMC Med. Res. Methodol. 2014, 14, 79. [Google Scholar] [CrossRef]
- Case, L.D.; Ambrosius, W.T. Power and Sample Size. Methods Mol. Biol. 2007, 404, 377–408. [Google Scholar] [CrossRef]
- Pavic, M.; Wolfer, A.; Li, Q.; Gircys, A.; Juritz, S.; Wicki, A.; Cerny, T.; Rohr, U.-P. Regulatory Challenges: Is a Surrogate End Point Instead of Overall Survival Enough for Regulatory Approval of (Neo) Adjuvant Cancer Treatment? The Swissmedic Perspective. J. Clin. Oncol. 2023, 41, 4973–4975. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.J.; Kesselheim, A.S.; Tibau, A.; Lee, C.C.; Vokinger, K.N. Clinical Benefit and Expedited Approval of Cancer Drugs in the United States, European Union, Switzerland, Japan, Canada, and Australia. JCO Oncol. Pr. 2022, 18, e1522–e1532. [Google Scholar] [CrossRef] [PubMed]
- Rohr, U.; Iovino, M.; Rudofsky, L.; Li, Q.; Juritz, S.; Gircys, A.; Wildner, O.; Bujar, M.; Bolte, C.; Dalla Torre di Sanguinetto, S.; et al. A Decade Comparison of Regulatory Decision Patterns for Oncology Products to All Other Non-Oncology Products Among Swissmedic, European Medicines Agency, and US Food and Drug Administration. Clin. Transl. Sci. 2023, 16, 1569–1581. [Google Scholar] [CrossRef]
- Trikalinos, T.A.; Hoaglin, D.C.; Schmid, C.H. An Empirical Comparison of Univariate and Multivariate Meta-Analyses for Categorical Outcomes. Stat. Med. 2014, 33, 1441–1459. [Google Scholar] [CrossRef]
- Matsui, S.; Le-Rademacher, J.; Mandrekar, S.J. Statistical Models in Clinical Studies. J. Thorac. Oncol. 2021, 16, 734–739. [Google Scholar] [CrossRef]
| Total (n = 112) | Restaging Interval < 8 weeks (n = 51) | Restaging Interval ≥ 8 weeks (n = 61) | |
|---|---|---|---|
| Sample Size (Median, IQR) | 531 (342–713) | 537 (351–658) | 525 (341–762) |
| Primary Outcome | |||
| PFS, n (%) | 55 (49%) | 18 (16%) | 37 (3%) |
| OS, n (%) | 25 (22%) | 14 (12%) | 11 (10%) |
| PFS and OS n (%) | 25 (22%) | 15 (13%) | 10 (9%) |
| Others n (%) | 7 (6%) | 4 (3%) | 3 (3%) |
| Trial Phase | |||
| Phase 3 Trial, n (%) | 105 (93%) | 48 (43%) | 57 (50%) |
| Phase 2, n (%) | 6 (5%) | 3 (3%) | 3 (2%) |
| Phase 2 and 3 (%) | 1 (1%) | - | 1 (1%) |
| Randomized, n (%) | 112 (100%) | 51 (46%) | 61 (54%) |
| Blinded, n (%) | 58 (51%) | 17 (15%) | 41 (36%) |
| Setting | |||
| Firstline, n (%) | 37 (33%) | 22 (20%) | 15 (13%) |
| Other, n (%) | 75 (66%) | 29 (25%) | 46 (41%) |
| Drug class | |||
| Chemotherapy, n (%) | 4 (4%) | 1 (1%) | 3 (3%) |
| Endocrine Therapy, n (%) | 9 (8%) | - | 9 (8%) |
| Immunotherapy, n (%) | 38 (34%) | 26 (23%) | 12 (11%) |
| Small Molecule, n (%) | 50 (44%) | 17 (15%) | 33 (29%) |
| Antibody Therapy, n (%) | 10 (9%) | 7 (6%) | 3 (3%) |
| Radiotherapeutics, n (%) | 1 (1%) | - | 1 (1%) |
| Indication Group | |||
| Melanoma, n (%) | 14 (12%) | 8 (7%) | 6 (5%) |
| Breast, n (%) | 18 (16%) | 6 (5%) | 12 (11%) |
| GI (incl. HCC), n (%) | 13 (12%) | 7 (6%) | 6 (6%) |
| Lung, n (%) | 23 (20%) | 15 (13%) | 8 (7%) |
| Ovarian, n (%) | 9 (8%) | - | 9 (8%) |
| Prostate, n (%) | 10 (9%) | - | 10 (9%) |
| Renal, n (%) | 8 (7%) | 6 (5%) | 2 (2%) |
| Urothelial, n (%) | 3 (3%) | 1 (1%) | 2 (2%) |
| Sarcoma and GIST, n (%) | 5 (4%) | 4 (3%) | 1 (1%) |
| Other, n (%) | 9 (8%) | 4 (4%) | 5 (4%) |
| Subgroup | PFS HR (CI 95%) Restaging Interval < 8 Weeks | PFS HR (CI 95%) Restaging Interval ≥ 8 Weeks | p Value for the Subgroup Difference | Heterogeneity I2 |
|---|---|---|---|---|
| All (n = 112) | 0.58 (0.53, 0.63), (n = 51) | 0.48 (0.44, 0.52), (n = 61) | 0.004 | 90% |
| Drug Class (n = 98) | ||||
| Immunotherapy (n = 38) | 0.70 (0.63, 0.77), (n = 26) | 0.66 (0.57, 0.77), (n = 12) | 0.62 | 83% |
| Small Molecules (n = 50) | 0.43 (0.37, 0.50), (n = 17) | 0.45 (0.41, 0.51), (n = 33) | 0.63 | 81% |
| Antibodies (n = 10) | 0.55 (0.43, 0.70), (n = 7) | 0.55 (0.43, 0.70), (n = 3) | 1.00 | 88% |
| Indication Group (n = 76) | ||||
| Breast (n = 18) | 0.50 (0.39, 0.64), (n = 6) | 0.58 (0.52, 0.65), (n = 12) | 0.28 | 74% |
| Lung (n = 23) | 0.60 (0.51, 0.69), (n = 15) | 0.59 (0.46, 0.75), (n = 8) | 0.93 | 87% |
| GI (incl. HCC) (n = 13) | 0.57 (0.48, 0.68), (n = 7) | 0.53 (0.46, 0.61), (n = 6) | 0.52 | 79% |
| Melanoma (n = 14) | 0.46 (0.36, 0.57), (n = 8) | 0.63 (0.55, 0.73), (n = 6) | 0.02 | 81% |
| Kidney (n = 8) | 0.67 (0.55, 0.81), (n = 6) | 0.44 (0.34, 0.58), (n = 2) | 0.01 | 89% |
| Sarcoma and GIST (n = 5) | 0.40 (0.1, 0.88), (n = 4) | 0.31 (0.24, 0.40), (n = 1) | 0.55 | 95% |
| Trial Phase | ||||
| Phase 3 (n = 105) | 0.58 (0.52, 0.64), (n = 48) | 0.49 (0.44, 0.53), (n = 57) | 0.01 | 90% |
| Phase 2 (n = 7) | 0.57 (0.49, 0.67), (n = 3) | 0.40 (0.21, 0.74), (n = 4) | 0.27 | 81% |
| Primary Outcome | ||||
| PFS (n = 55) | 0.45 (0.39, 0.51), (n = 18) | 0.45 (0.40, 0.51), (n = 37) | 0.95 | 85% |
| OS (n = 25) | 0.72 (0.63, 0.83), (n = 14) | 0.58 (0.50, 0.67), (n = 11) | 0.03 | 90% |
| PFS and OS (n = 25) | 0.60 (0.52, 0.70), (n = 15) | 0.52 (0.41, 0.65), (n = 10) | 0.26 | 89% |
| Others (n = 7) | 0.71 (0.50, 1.01), (n = 4) | 0.35 (0.27, 0.44), (n = 3) | 0.001 | 95% |
| Staging Interval (weeks) | HR for PFS 95% Cl | p for Subgroup Difference | Heterogeneity I2 |
|---|---|---|---|
| <9 (n = 90) vs. ≥9 (n = 22) | 0.54 (0.50, 0.58) vs. 0.45 (0.38, 0.54) | 0.06 | 90% |
| <12 (n = 96) vs. ≥12 (n = 16) | 0.55 (0.52, 0.59) vs. 0.38 (0.32, 0.44) | 0.0001 | 90% |
| <8 (n = 48) vs. ≥8 (n = 57) without <6 and >12 | 0.60 (0.55, 0.66) vs. 0.49 (0.45, 0.54) | 0.002 | 89% |
| ≤6 (n = 51) vs. >6 to <12 (n = 45) vs. ≥12 (n = 16) | 0.58 (0.53, 0.64) vs. 0.52 (0.49, 0.58) vs. 0.38 (0.32, 0.44) | <0.001 | 90% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuellig, J.A.; Adam, R.; Udry, F.; Tibau, A.; Šeruga, B.; Ocaña, A.; Amir, E.; Templeton, A.J. The Effect of Staging Intervals on Progression-Free Survival in Registration Studies of Oncologic Drugs: A Meta-Analysis. Cancers 2025, 17, 1359. https://doi.org/10.3390/cancers17081359
Zuellig JA, Adam R, Udry F, Tibau A, Šeruga B, Ocaña A, Amir E, Templeton AJ. The Effect of Staging Intervals on Progression-Free Survival in Registration Studies of Oncologic Drugs: A Meta-Analysis. Cancers. 2025; 17():1359. https://doi.org/10.3390/cancers17081359
Chicago/Turabian StyleZuellig, Jonas A., Roman Adam, Filomena Udry, Ariadna Tibau, Bostjan Šeruga, Alberto Ocaña, Eitan Amir, and Arnoud J. Templeton. 2025. "The Effect of Staging Intervals on Progression-Free Survival in Registration Studies of Oncologic Drugs: A Meta-Analysis" Cancers 17, no. : 1359. https://doi.org/10.3390/cancers17081359
APA StyleZuellig, J. A., Adam, R., Udry, F., Tibau, A., Šeruga, B., Ocaña, A., Amir, E., & Templeton, A. J. (2025). The Effect of Staging Intervals on Progression-Free Survival in Registration Studies of Oncologic Drugs: A Meta-Analysis. Cancers, 17(), 1359. https://doi.org/10.3390/cancers17081359






