Microvascular Cortical Dynamics in Minimal Invasive Deep-Seated Brain Tumour Surgery
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- (a)
- Delay: the time interval from 0 to 50% of maximum fluorescence intensity.
- (b)
- Speed: velocity increase in fluorescence intensity during an observation period.
- (c)
- Time to Peak (TtP): from the appearance of fluorescence to maximum fluorescence intensity.
- (d)
- Rise Time (RT): time during which fluorescence intensity rises from 10 to 90% of its peak.
- (e)
- Maximal Fluorescence: maximal intensity measured in arbitrary intensity units.
- (f)
- Cerebral Blood Flow Index (CBFI): ratio of maximum fluorescence intensity to rise time.
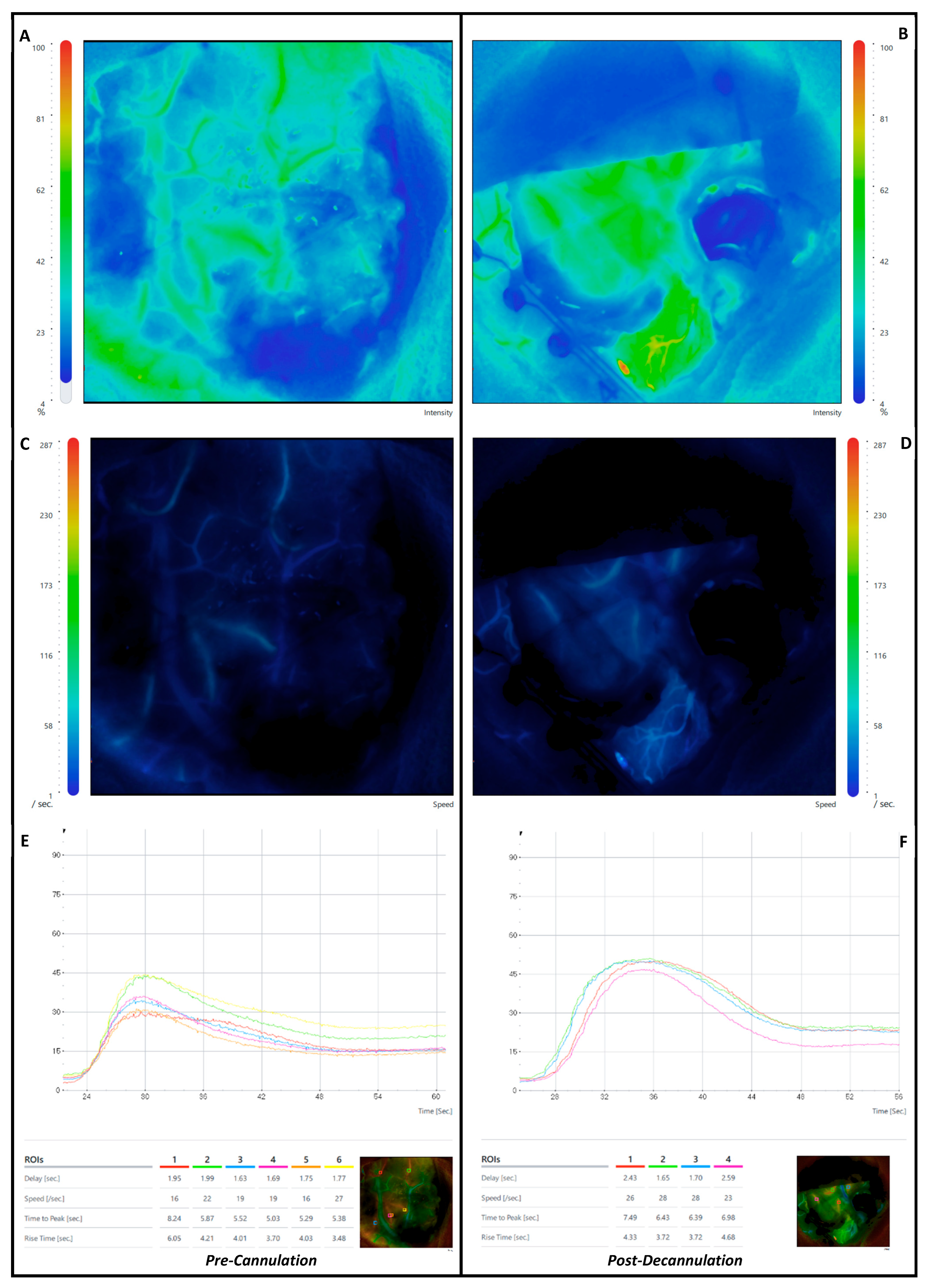
3. Results
3.1. Neurological Outcome
3.1.1. Region-of-Interest-Based (ROI)
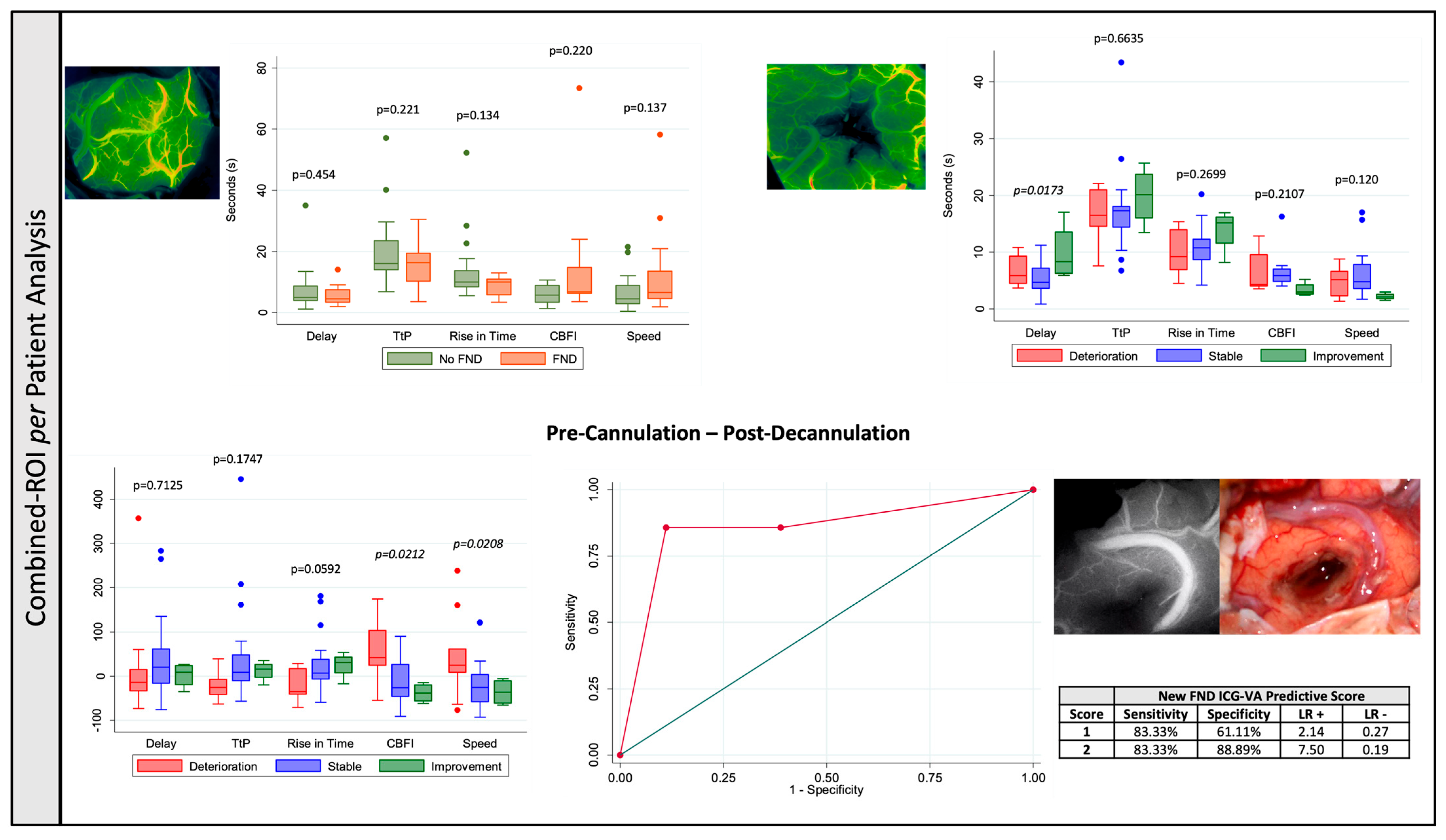
3.1.2. Combined-ROI per Patient Analysis
4. Discussion
5. Limitations and Strengths
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerritsen, J.K.W.; Zwarthoed, R.H.; Kilgallon, J.L.; Nawabi, N.L.; Versyck, G.; Jessurun, C.A.C.; Pruijn, K.P.; Fisher, F.L.; Larivière, E.; Solie, L.; et al. Impact of maximal extent of resection on postoperative deficits, patient functioning, and survival within clinically important glioblastoma subgroups. Neuro. Oncol. 2023, 25, 958–972. [Google Scholar] [CrossRef] [PubMed]
- Karschnia, P.; Young, J.S.; Dono, A.; Häni, L.; Sciortino, T.; Bruno, F.; Juenger, S.T.; Teske, N.; Morshed, R.A.; Haddad, A.F.; et al. Prognostic validation of a new classification system for extent of resection in glioblastoma: A report of the RANO resect group. Neuro. Oncol. 2023, 25, 940–954. [Google Scholar] [CrossRef]
- Peters, D.R.; Halimi, F.; Ozduman, K.; Levivier, M.; Conti, A.; Reyns, N.; Tuleasca, C. Resection of the contrast-enhancing tumor in diffuse gliomas bordering eloquent areas using electrophysiology and 5-ALA fluorescence: Evaluation of resection rates and neurological outcome—A systematic review and meta-analysis. Neurosurg. Rev. 2023, 46, 185. [Google Scholar] [CrossRef] [PubMed]
- Abdala-Vargas, N.J.; Umana, G.E.; Patiño-Gomez, J.G.; Ordoñez-Rubiano, E.; Cifuentes-Lobelo, H.A.; Palmisciano, P.; Ferini, G.; Viola, A.; Zagardo, V.; Casanova-Martínez, D.; et al. Standardization of Strategies to Perform a Parafascicular Tubular Approach for the Resection of Brain Tumors in Eloquent Areas. Brain Sci. 2023, 13, 498. [Google Scholar] [CrossRef] [PubMed]
- Lavrador, J.P.; Oviedova, A.; Pereira, N.; Patel, S.; Rajwani, K.M.; Sekhon, P.; Gullan, R.; Ashkan, K.; Vergani, F.; Bhangoo, R. Minimally invasive approach to a deep-seated motor eloquent brain tumour: A technical note. J. Surg. Case Rep. 2022, 2022, rjab611. [Google Scholar] [CrossRef]
- Roca, E.; Ramorino, G. Brain retraction injury: Systematic literature review. Neurosurg. Rev. 2023, 46, 257. [Google Scholar] [CrossRef]
- Assina, R.; Rubino, S.; Sarris, C.E.; Gandhi, C.D.; Prestigiacomo, C.J. The history of brain retractors throughout the development of neurological surgery. Neurosurg. Focus 2014, 36, E8. [Google Scholar] [CrossRef]
- Recinos, P.F.; Raza, S.M.; Jallo, G.I.; Recinos, V.R. Use of a minimally invasive tubular retraction system for deep-seated tumors in pediatric patients: Technical note. J. Neurosurg. Pediatr. 2011, 7, 516–521. [Google Scholar] [CrossRef]
- Rakovec, M.; Camp, S.; Day, D.; Chakravarti, S.; Parker, M.; Porras, J.L.; Jackson, C.M.; Huang, J.; Bettegowda, C.; Lim, M.; et al. Use of tubular retractors to access deep brain lesions: A case series. J. Clin. Neurosci. 2023, 114, 64–69. [Google Scholar] [CrossRef]
- Sangha, M.S.; Rajwani, K.M.; Price, S.A.; Wren, H.; Pescador, A.M.; Gullan, R.; Ashkan, K.; Vergani, F.; Bhangoo, R.; Lavrador, J.P. Awake minimally invasive parafascicular approach to a language eloquent brain tumour-surgical video. J. Surg. Case Rep. 2023, 10, rjad519. [Google Scholar] [CrossRef]
- Shapiro, S.Z.; Sabacinski, K.A.; Mansour, S.A.; Echeverry, N.B.; Shah, S.S.; Stein, A.A.; Snelling, B.M. Use of Vycor Tubular Retractors in the Management of Deep Brain Lesions: A Review of Current Studies. World Neurosurg. 2020, 133, 283–290. [Google Scholar] [CrossRef]
- Gallagher, M.J.; Lavrador, J.P.; Coelho, P.; Mirallave-Pescador, A.; Bleil, C.; Gullan, R.; Ashkan, K.; Vergani, F.; Bhangoo, R. Continuous Microdebrider-Based Dynamic Subcortical Motor Mapping: A Technical Advance in Tubular Retractor–Assisted Surgery. Oper. Neurosurg. 2022, 23, 217–224. [Google Scholar] [CrossRef]
- Raabe, A.; Beck, J.; Gerlach, R.; Zimmermann, M.; Seifert, V. Near-infrared indocyanine green video angiography: A new method for intraoperative assessment of vascular flow. Neurosurgery 2003, 52, 132–139. [Google Scholar] [PubMed]
- Reinhart, M.B.; Huntington, C.R.; Blair, L.J.; Heniford, B.T.; Augenstein, V.A. Indocyanine Green: Historical Context, Current Applications, and Future Considerations. Surg. Innov. 2016, 23, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Z.; Jiang, F.; Yang, X.; Tan, X.; Chen, Z.; Liu, Y.; Zhu, Y.; Wang, Z.; Chen, G. Utility of indocyanine green videoangiography with FLOW 800 analysis in brain tumour resection as a venous protection technique. BMC Surg. 2022, 22, 126. [Google Scholar] [CrossRef] [PubMed]
- Kamp, M.A.; Slotty, P.; Turowski, B.; Etminan, N.; Steiger, H.J.; Hänggi, D.; Stummer, W. Microscope-integrated quantitative analysis of intraoperative indocyanine green fluorescence angiography for blood flow assessment: First experience in 30 patients. Neurosurgery 2012, 70, 65–74. [Google Scholar] [CrossRef]
- Van Den Hoven, P.; Osterkamp, J.; Nerup, N.; Svendsen, M.B.S.; Vahrmeijer, A.; Van Der Vorst, J.R.; Achiam, M.P. Quantitative perfusion assessment using indocyanine green during surgery—Current applications and recommendations for future use. Langenbeck’s Arch. Surg. 2023, 408, 67. [Google Scholar] [CrossRef]
- Martirosyan, N.L.; Skoch, J.; Watson, J.R.; Lemole, G.M.; Jr Romanowski, M.; Anton, R. Integration of indocyanine green videoangiography with operative microscope: Augmented reality for interactive assessment of vascular structures and blood flow. Oper. Neurosurg. 2015, 11, 252–257. [Google Scholar] [CrossRef]
- Shah, K.J.; Cohen-Gadol, A.A. The Application of FLOW 800 ICG Videoangiography Color Maps for Neurovascular Surgery and Intraoperative Decision Making. World Neurosurg. 2019, 122, e186–e197. [Google Scholar] [CrossRef]
- Raabe, A.; Nakaji, P.; Beck, J.; Kim, L.J.; Hsu, F.P.; Kamerman, J.D.; Seifert, V.; Spetzler, R.F. Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green videoangiography during aneurysm surgery. J. Neurosurg. 2005, 103, 982–989. [Google Scholar] [CrossRef]
- Goertz, L.; Hof, M.; Timmer, M.; Schulte, A.P.; Kabbasch, C.; Krischek, B.; Stavrinou, P.; Reiner, M.; Goldbrunner, R.; Brinker, G. Application of Intraoperative FLOW 800 Indocyanine Green Videoangiography Color-Coded Maps for Microsurgical Clipping of Intracranial Aneurysms. World Neurosurg. 2019, 131, e192–e200. [Google Scholar] [CrossRef]
- Norat, P.; Soldozy, S.; Elsarrag, M.; Sokolowski, J.; Yaǧmurlu, K.; Park, M.S.; Tvrdik, P.; Kalani, M.Y.S. Application of indocyanine green videoangiography in aneurysm surgery: Evidence, techniques, practical tips. Front. Surg. 2019, 6, 34. [Google Scholar] [CrossRef]
- Kato, N.; Prinz, V.; Dengler, J.; Vajkoczy, P. Blood Flow Assessment of Arteriovenous Malformations Using Intraoperative Indocyanine Green Videoangiography. Stroke Res. Treat. 2019, 2019, 7292304. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.H.; Morone, P.J.; Tomlinson, S.B.; Cohen-Gadol, A.A. Application of Indocyanine Green During Arteriovenous Malformation Surgery: Evidence, Techniques, and Practical Pearls. Front. Surg. 2019, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Hänggi, D.; Etminan, N.; Steiger, H.J. The impact of microscope-integrated intraoperative near-infrared indocyanine green videoangiography on surgery of arteriovenous malformations and dural arteriovenous fistulae. Neurosurgery 2010, 67, 1094–1103. [Google Scholar] [CrossRef]
- Rustemi, O.; Scienza, R.; Della Puppa, A. ICG-VA application in subtemporal transtentorial treatment of a Cognard V dural arteriovenous fistula. Neurosurg. Focus 2016, 40 (Suppl. S1), 1. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, C.; Gandhi, S.; Zhao, X.; Belykh, E.; Valli, D.; Nakaji, P.; Preul, M.C.; Lawton, M.T. Applications of Microscope-Integrated Indocyanine Green Videoangiography in Cerebral Revascularization Procedures. Front. Surg. 2019, 6, 59. [Google Scholar] [CrossRef]
- Rennert, R.C.; Strickland, B.A.; Ravina, K.; Bakhsheshian, J.; Fredrickson, V.; Carey, J.; Russin, J.J. Intraoperative assessment of cortical perfusion after intracranial-to-intracranial and extracranial-to-intracranial bypass for complex cerebral aneurysms using Flow 800. Oper. Neurosurg. 2019, 16, 583–592. [Google Scholar] [CrossRef]
- Ferroli, P.; Acerbi, F.; Albanese, E.; Tringali, G.; Broggi, M.; Franzini, A.; Broggi, G. Application of intraoperative indocyanine green angiography for CNS tumors: Results on the first 100 cases. Acta Neurochir. Suppl. 2011, 109, 251–257. [Google Scholar]
- Acerbi, F.; Vetrano, I.G.; Sattin, T.; de Laurentis, C.; Bosio, L.; Rossini, Z.; Broggi, M.; Schiariti, M.; Ferroli, P. The role of indocyanine green videoangiography with FLOW 800 analysis for the surgical management of central nervous system tumors: An update. Neurosurg. Focus 2018, 44, E6. [Google Scholar] [CrossRef]
- Cho, S.S.; Salinas, R.; Lee, J.Y.K. Indocyanine-green for fluorescence-guided surgery of brain tumors: Evidence, techniques, and practical experience. Front. Surg. 2019, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.W.; Huang, V.; Arguelles, G.R.; Zhou, C.; Cho, S.S.; Harmsen, S.; Lee, J.Y.K. Applications of indocyanine green in brain tumor surgery: Review of clinical evidence and emerging technologies. Neurosurg. Focus 2021, 50, E4. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Yamamoto, Y.; Miyamoto, T.; Sogabe, S.; Fujihara, T.; Nakajima, K.; Mizobuchi, Y.; Kanematsu, Y.; Takagi, Y. Efficacy of Intra-arterial Indocyanine Green Videoangiography in Hemangioblastoma Surgery: A Case Report. NMC Case Rep. J. 2021, 8, 295–300. [Google Scholar] [CrossRef]
- Kim, J.H.; Moon, K.S.; Jung, J.H.; Jang, W.Y.; Jung, T.Y.; Kim, I.Y.; Lee, K.H.; Jung, S. Importance of collateral venous circulation on indocyanine green videoangiography in intracranial meningioma resection: Direct evidence for venous compression theory in peritumoral edema formation. J. Neurosurg. 2020, 132, 1715–1723. [Google Scholar] [CrossRef]
- Acerbi, F.; Cavallo, C.; Ferroli, P. Intraoperative assessment of blood flow with quantitative indocyanine green videoangiography: The role for diagnosis of regional cerebral hypoperfusion. Neurosurgery 2016, 78, E310–E312. [Google Scholar] [CrossRef]
- Katirji, M.B. Aids to the Examination of the Peripheral Nervous System. Neurology 1988, 38, 1663. [Google Scholar] [CrossRef]
- Syder, D.; Body, R.; Parker, M.; Boddy, M. Sheffield Screening Test for Acquired Language Disorders: Manual. Trials 1993, 14, 78–79. [Google Scholar]
- Staehr, C.; Giblin, J.T.; Gutiérrez-Jiménez, E.; Guldbrandsen, H.Ø.; Tang, J.; Sandow, S.L.; Boas, D.A.; Matchkov, V.V. Neurovascular Uncoupling Is Linked to Microcirculatory Dysfunction in Regions Outside the Ischemic Core Following Ischemic Stroke. J. Am. Heart Assoc. 2023, 12, e029527. [Google Scholar] [CrossRef] [PubMed]
- Rolfes, L.; Riek-Burchardt, M.; Pawlitzki, M.; Minnerup, J.; Bock, S.; Schmidt, M.; Meuth, S.G.; Gunzer, M.; Neumann, J. Neutrophil granulocytes promote flow stagnation due to dynamic capillary stalls following experimental stroke. Brain. Behav. Immun. 2021, 93, 322–330. [Google Scholar] [CrossRef]
- Shih, A.Y.; Rühlmann, C.; Blinder, P.; Devor, A.; Drew, P.J.; Friedman, B.; Knutsen, P.M.; Lyden, P.D.; Mateo, C.; Mellander, L.; et al. Robust and fragile aspects of cortical blood flow in relation to the underlying angioarchitecture. Microcirculation 2015, 22, 204–218. [Google Scholar] [CrossRef]
- Blinder, P.; Tsai, P.S.; Kaufhold, J.P.; Knutsen, P.M.; Suhl, H.; Kleinfeld, D. The cortical angiome: An interconnected vascular network with noncolumnar patterns of blood flow. Nat. Neurosci. 2013, 16, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Kisler, K.; Nelson, A.R.; Rege, S.V.; Ramanathan, A.; Wang, Y.; Ahuja, A.; Lazic, D.; Tsai, P.S.; Zhao, Z.; Zhou, Y.; et al. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat. Neurosci. 2017, 20, 406–416. [Google Scholar] [CrossRef]
- Østergaard, L.; Jespersen, S.N.; Mouridsen, K.; Mikkelsen, I.K.; Jonsdottír, K.Ý.; Tietze, A.; Blicher, J.U.; Aamand, R.; Hjort, N.; Iversen, N.K.; et al. The role of the cerebral capillaries in acute ischemic stroke: The extended penumbra model. J. Cereb. Blood Flow Metab. 2013, 33, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Ayata, C.; Lauritzen, M. Spreading depression, spreading depolarizations, and the cerebral vasculature. Physiol. Rev. 2015, 95, 953–993. [Google Scholar] [CrossRef] [PubMed]
- Piilgaard, H.; Lauritzen, M. Persistent increase in oxygen consumption and impaired neurovascular coupling after spreading depression in rat neocortex. J. Cereb. Blood Flow Metab. 2009, 29, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Dreier, J.P.; Woitzik, J.; Fabricius, M.; Bhatia, R.; Major, S.; Drenckhahn, C.; Lehmann, T.N.; Sarrafzadeh, A.; Willumsen, L.; Hartings, J.A.; et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain 2006, 129, 3224–3237. [Google Scholar] [CrossRef]
- Colpitts, K.; Desai, M.J.; Kogan, M.; Shuttleworth, C.W.; Carlson, A.P. Brain Tsunamis in Human High-Grade Glioma: Preliminary Observations. Brain Sci. 2022, 12, 710. [Google Scholar] [CrossRef]
- Hatcher, A.; Yu, K.; Meyer, J.; Aiba, I.; Deneen, B.; Noebels, J.L. Pathogenesis of peritumoral hyperexcitability in an immunocompetent CRISPR-based glioblastoma model. J. Clin. Investig. 2020, 130, 2286–2300. [Google Scholar] [CrossRef]
- von Bornstädt, D.; Houben, T.; Seidel, J.L.; Zheng, Y.; Dilekoz, E.; Qin, T.; Sandow, N.; Kura, S.; Eikermann-Haerter, K.; Endres, M. Supply-demand mismatch transients in susceptible peri-infarct hot zones explain the origins of spreading injury depolarizations. Neuron 2015, 85, 1117–1131. [Google Scholar] [CrossRef]
- Li, P.; Ma, S.; Ma, X.; Ding, D.; Zhu, X.; Zhang, H.; Liu, J.; Mu, J.; Zhang, M. Reversal of neurovascular decoupling and cognitive impairment in patients with end-stage renal disease during a hemodialysis session: Evidence from a comprehensive fMRI analysis. Hum. Brain Mapp. 2023, 44, 989–1001. [Google Scholar] [CrossRef]
- Griffiths, E.; Jayamohan, J.; Budday, S. A comparison of brain retraction mechanisms using finite element analysis and the effects of regionally heterogeneous material properties. Biomech. Model. Mechanobiol. 2024, 23, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Eliyas, J.K.; Glynn, R.; Kulwin, C.G.; Rovin, R.; Young, R.; Alzate, J.; Pradilla, G.; Shah, M.V.; Kassam, A.; Ciric, I.; et al. Minimally Invasive Transsulcal Resection of Intraventricular and Periventricular Lesions Through a Tubular Retractor System: Multicentric Experience and Results. World Neurosurg. 2016, 90, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Prinz, V.; Hecht, N.; Kato, N.; Vajkoczy, P. FLOW 800 allows visualization of hemodynamic changes after extracranial-to-intracranial bypass surgery but not assessment of quantitative perfusion or flow. Neurosurgery 2014, 10 (Suppl. S2), 231–239. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Welker, K.M.; Black, D.F.; Little, J.T.; DeLone, D.R.; Messina, S.A.; Passe, T.J.; Bettegowda, C.; Pillai, J.J. Detection and Mitigation of Neurovascular Uncoupling in Brain Gliomas. Cancers 2023, 15, 4473. [Google Scholar] [CrossRef]

| Demographics, Clinical and Neuropathology | |
|---|---|
| Age (y) | 54.94 ± 15.32 |
| Sex Male Female | 20 (57.14%) 15 (42.86%) |
| Location Frontal Temporal Intraventricular Cerebellum Cingulate Parietal | 12 (34.29%) 8 (22.86%) 6 (17.14%) 5 (14.29%) 3 (8.57%) 1 (2.86%) |
| Laterality Right Left | 14 (40.00%) 21 (60.00%) |
| Neurological Examination Preoperative Neurological Deficit Postoperative Neurological Deficit Deterioration Stable Improvement Permanent Neurological Deficit (Preop + Post-op New Deficit) | 13 (37.14%) 17 (48.57%) 10 (28.57%) 21 (60.00%) 4 (11.43%) 9 (25.71%) |
| WHO Grade * 1 2 3 4 | 6 (17.14%) 2 (5.71%) - 18 (51.43%) |
| Histology Glioblastoma Metastasis Meningioma Extraventricular Anaplastic Ependymomma Subependymmal Giant Astrocytoma WHO Grade 2 Astrocytoma WHO Grade 2 Central Neurocytoma | 18 (51.43%) 9 (25.71%) 2 (5.71%) 1 (2.86%) 1 (2.86%) 2 (5.71%) 2 (5.71%) |
| Surgery | |
| Number of Regions-of-Interest Mean per patient | 144 4.11 ± 1.57 |
| Time of Brain Cannulation (min) | 147.09 ± 71.91 |
| Access Superior Frontal Sulcus Intraparietal Sulcus Superior Temporal Sulcus Longitudinal Sulcus | 17 (48.57%) 7 (20.00%) 5 (14.29%) 6 (17.14%) |
| Length Tubular Retractor (mm) 50 60 75 | 2 (5.71%) 25 (71.43%) 8 (22.86%) |
| Imaging | |
| Depth of Lesion (mm) | 3.72 ± 2.30 |
| Preoperative Volume (cc) | 29.92 ± 24.40 |
| Residual Tumour Volume (cc) ** | 4.00 ± 9.94 |
| Extent of Resection (%) ** GTR Near Total Resection Subtotal Partial Biopsy | 88.27 ± 18.71 14 (40%) 4 (11.43%) 9 (25.71%) 5 (14.29%) 3 (8.57%) |
| Total Restriction to Diffusion (cc) | 12.46 ± 20.72 |
| Along-the-Path Restriction to Diffusion (cc) | 5.16 ± 6.76 |
| Pre-Cannulation in ICG-VA Properties (s) | Focal Neurological Deficit (FND) | |||
|---|---|---|---|---|
| No FND | FND | p value | ||
| Delay | 7.04 ± 5.95 | 5.07 ± 3.58 | 0.0147 | |
| Speed | 70.15 ± 58.82 | 137.49 ± 156.53 | 0.0044 | |
| Time to Peak | 20.27 ± 9.98 | 15.08 ± 7.33 | 0.0005 | |
| Rise in Time | 13.35 ± 8.41 | 8.07 ± 3.59 | <0.0001 | |
| Cerebral Blood Flow Index | 6.14 ± 3.18 | 15.07 ± 20.81 | 0.0085 | |
| Post-Decannulation in ICG-VA properties (s) | Overall Neurology | |||
| Deterioration | Stable | Improvement | p value | |
| Delay | 8.73 ± 7.35 | 4.72 ± 3.51 | 9.36 ± 3.23 | 0.0001 |
| Speed | 49.98 ± 32.04 | 123.93 ± 128.86 | 37.93 ± 11.30 | 0.0001 |
| Time to Peak | 21.61 ± 11.00 | 17.05 ± 8.99 | 17.53 ± 3.27 | 0.1315 |
| Rise in Time | 14.69 ± 10.31 | 10.05 ± 5.92 | 10.8 ± 1.71 | 0.0016 |
| Cerebral Blood Flow Index | 5.53 ± 3.16 | 11.94 ± 16.92 | 5.78 ± 1.37 | 0.0057 |
| Post–Pre-Cannulation Difference in ICG-VA properties (%) | Overall Neurology | |||
| Deterioration | Stable | Improvement | p value | |
| Delay | 140.77 ± 816.54 | 152.97 ± 458.70 | −2.93 ± 34.84 | 0.6351 |
| Speed | 43.12 ± 80.60 | −14.51 ± 57.80 | −36.93 ± 31.33 | <0.0001 |
| Time to Peak | −20.16 ± 27.32 | 34.73 ± 143.83 | 14.20 ± 32.31 | 0.0406 |
| Rise in Time | −21.97 ± 32.35 | 57.33 ± 219.90 | 26.53 ± 39.13 | 0.0552 |
| Cerebral Blood Flow Index | 50.40 ± 88.17 | −2.70 ± 67.54 | −38.98 ± 26.17 | 0.0005 |
| Pre- Cannulation in ICG-VA Properties (s) | Focal Neurological Deficit (FND) | |||
|---|---|---|---|---|
| No FND | FND | p value | ||
| Delay | 7.26 ± 7.16 | 5.57 ± 3.47 | 0.3602 | |
| Speed | 65.47 ± 54.40 | 131.83 ± 158.19 | 0.1658 | |
| Time to Peak | 19.52 ± 11.43 | 15.00 ± 7.40 | 0.1649 | |
| Rise in Time | 13.43 ± 10.33 | 8.70 ± 3.09 | 0.0565 | |
| Cerebral Blood Flow Index | 6.09 ± 2.91 | 15.25 ± 21.33 | 0.2089 | |
| Post-Decannulation in ICG-VA properties (s) | Overall Neurology | |||
| Deterioration | Stable | Improvement | p value | |
| Delay | 9.70 ± 9.59 | 4.58 ± 2.93 | 9.49 ± 3.15 | 0.0173 |
| Speed | 52.2 ± 39.22 | 118.05 ± 130.88 | 38.23 ± 12.75 | 0.1200 |
| Time to Peak | 23.01 ± 13.52 | 15.40 ± 8.68 | 17.74 ± 2.74 | 0.6625 |
| Rise in Time | 16.35 ± 13.72 | 9.61 ± 5.20 | 11.04 ± 1.40 | 0.2669 |
| Cerebral Blood Flow Index | 5.09 ± 3.26 | 13.15 ± 18.14 | 5.73 ± 1.39 | 0.2107 |
| Post–Pre-Cannulation Difference in ICG-VA properties (%) | Overall Neurology | |||
| Deterioration | Stable | Improvement | p value | |
| Delay | 20.95 ± 123.94 | 42.54 ± 96.08 | 2.20 ± 28.21 | 0.7125 |
| Speed | 45.33 ± 94.28 | −21.31 ± 46.96 | −36.09 ± 30.28 | 0.0208 |
| Time to Peak | −18.98 ± 29.95 | 45.78 ± 110.03 | 11.81 ± 23.27 | 0.1747 |
| Rise in Time | −21.61 ± 33.65 | 27.71 ± 61.30 | 24.88 ± 30.27 | 0.0592 |
| Cerebral Blood Flow Index | 55.17 ± 77.45 | −14.13 ± 47.43 | −38.39 ± 22.19 | 0.0212 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavrador, J.P.; Wroe-Wright, O.; Marchi, F.; Elhag, A.; O’Keeffe, A.; De La Fuente, P.; Soumpasis, C.; Cardia, A.; Mirallave-Pescador, A.; Díaz-Baamonde, A.; et al. Microvascular Cortical Dynamics in Minimal Invasive Deep-Seated Brain Tumour Surgery. Cancers 2025, 17, 1392. https://doi.org/10.3390/cancers17091392
Lavrador JP, Wroe-Wright O, Marchi F, Elhag A, O’Keeffe A, De La Fuente P, Soumpasis C, Cardia A, Mirallave-Pescador A, Díaz-Baamonde A, et al. Microvascular Cortical Dynamics in Minimal Invasive Deep-Seated Brain Tumour Surgery. Cancers. 2025; 17(9):1392. https://doi.org/10.3390/cancers17091392
Chicago/Turabian StyleLavrador, José Pedro, Oliver Wroe-Wright, Francesco Marchi, Ali Elhag, Andrew O’Keeffe, Pablo De La Fuente, Christos Soumpasis, Andrea Cardia, Ana Mirallave-Pescador, Alba Díaz-Baamonde, and et al. 2025. "Microvascular Cortical Dynamics in Minimal Invasive Deep-Seated Brain Tumour Surgery" Cancers 17, no. 9: 1392. https://doi.org/10.3390/cancers17091392
APA StyleLavrador, J. P., Wroe-Wright, O., Marchi, F., Elhag, A., O’Keeffe, A., De La Fuente, P., Soumpasis, C., Cardia, A., Mirallave-Pescador, A., Díaz-Baamonde, A., Mosquera, J. S., Coiteiro, D., Jewell, S., Strong, A., Gullan, R., Ashkan, K., Vergani, F., Vasan, A. K., & Bhangoo, R. (2025). Microvascular Cortical Dynamics in Minimal Invasive Deep-Seated Brain Tumour Surgery. Cancers, 17(9), 1392. https://doi.org/10.3390/cancers17091392