Simple Summary
Ovarian cancer treatment typically involves surgery and chemotherapy, but older women often receive non-standard therapies despite limited evidence linking age to prognosis. In this study, we analyzed data from patients treated at the Hospital del Mar to understand how age influences treatment decisions and survival outcomes. We found that women aged 70 or older were more frequently diagnosed at advanced stages, received different treatment approaches, and had lower rates of complete tumor removal compared to younger patients. While disease-free survival was similar between age groups, overall survival was worse in older women. These findings highlight the need for treatment strategies that consider the specific needs of elderly patients to improve their outcomes.
Abstract
Background: Ovarian cancer, particularly in advanced stages, requires cytoreductive surgery followed by chemotherapy. A significant proportion of patients are elderly, yet older women are often treated with non-standard regimens despite a lack of consistent evidence linking age to prognosis. The aim of this study is to assess age-specific differences in treatment and survival outcomes for ovarian cancer in women aged 70 years or older. Methods: This retrospective study included ovarian cancer patients treated at the Hospital del Mar, Barcelona, between 2016 and 2022. Patients were stratified into two groups: <70 and ≥70 years. Clinical and pathological data were analyzed, and hazard ratios (HR) for overall survival (OS) and disease-free survival (DFS) were calculated using Cox proportional hazards regression models. Multivariate analysis was performed to compare outcomes. Results: A total of 110 patients were included (73 <70 years, 37 ≥70 years). Among the older group, 80.5% were diagnosed at advanced stages (III–IV), compared to 63% in the younger group (p = 0.012). Patients aged ≥70 were more likely to undergo interval surgery (p = 0.053) and receive non-standard treatment (p = 0.023). Complete cytoreduction was achieved in 95.8% of younger patients versus 81.3% of older patients (p = 0.024). Age ≥70 did not significantly impact DFS (p = 0.091), but OS was significantly worse in the older group (44.4% vs. 67.2%, p = 0.014). Conclusions: Older women (≥70 years) with ovarian cancer are more likely to be diagnosed at advanced stages, receive non-standard treatment, and achieve suboptimal cytoreduction compared to younger patients. While DFS was similar across age groups, older age was associated with worse OS, highlighting the need for age-tailored treatment strategies.
1. Introduction
Ovarian cancer is a heterogeneous disease that stands as the second most common gynecological tumor, with an incidence of 11.8 per 100,000 women [1,2]. The risk of developing the disease increases with age, with over half occurring in patients aged 65 or older [3,4]. As it often presents with non-specific symptoms, up to 70% of cases are diagnosed in advanced stages [1,5]. Consequently, ovarian cancer has the highest mortality rate among gynecological cancers, as this late-stage diagnosis significantly hinders effective treatment [5,6].
The standard treatment for advanced-stage ovarian cancer usually consists of complete cytoreductive surgery followed by six cycles of adjuvant chemotherapy, with platinum and taxane-based drugs [1,5]. Complete cytoreduction means total resection of any evidence of macroscopic intra-abdominal tumor, which often implies extended surgery with retroperitoneal, digestive, urological and peritoneal resections [5,7]. Primary surgery for ovarian cancer is considered the gold-standard treatment, with a 50% survival rate at 5 years when complete cytoreduction is accomplished [8]. Patient selection for primary surgery mainly considers the patient’s basal physical state and tumoral burden. However, especially in older patients, frailty and overall health can define surgical comorbidity and determine the choice of alternative treatments [6,9]. When complete cytoreduction is deemed unattainable or too risky based on the patient’s condition, neoadjuvant chemotherapy is administered prior to interval surgery [5]. Studies like EORTC and CHORUS have demonstrated the effectiveness of neoadjuvant chemotherapy in selected cases initially deemed non-cytoreductive [10,11,12]. Therefore, personalized treatments are essential to ensure each patient receives the optimal care, maximizing both survival rates and quality of life.
The hypothesis of the study is that elderly ovarian cancer patients undergo non-standard treatment more often compared to younger patients. The study aimed to categorize ovarian cancer in older women in terms of tumor characteristics, treatment approaches, and oncological outcomes and assess age-specific survival differences, considering overall survival (OS) and disease-free survival (DFS).
2. Materials and Methods
2.1. Study Design and Participants
This single institution, observational longitudinal retrospective study was conducted in the Hospital del Mar, Barcelona, between January 2016 and December 2022. Women newly diagnosed of ovarian cancer with medical registers and necessary data available were included.
2.2. Data Collection and Outcomes
Demographic, clinical, tumor-related, and treatment-related data were obtained from the electronic medical records from the Hospital del Mar. For the purpose of the study, patients were categorized into two groups based on their age at diagnosis: <70 or ≥70 years old, following geriatric recommendations about screening frailty on older age [13]. To analyze patient comorbidity, the Charlson Index was calculated using an online calculator from SAMIUC (Sociedad Andaluza de Medicina Intensiva y Unidades Coronarias) [14]. Tumor stage was defined according to the 2014 International Federation of Gynecology and Obstetrics (FIGO) staging system [15]. The type of surgery was categorized as “primary” when cytoreductive surgery was performed before adjuvant chemotherapy or if no chemotherapy was administered, “interval” when surgery was performed after two or four cycles of neoadjuvant chemotherapy and after surgery chemotherapy continued to complete six cycles, and “rescue” when surgery was performed after six cycles of neoadjuvant chemotherapy. Additionally, based on residual macroscopic tumor, surgery was considered complete (R0) and incomplete (R1) cytoreduction [7]. In summary, treatment approaches were classified into two categories: “standard” treatment, which included primary or interval debulking surgery. “Non-standard” treatment encompassed any therapeutic strategy that did not involve cytoreductive surgery, such as exclusive chemotherapy, palliative surgery, or other non-curative interventions. Maintenance treatment was defined as any type of treatment administered after the finalization of the main treatment, considering tumor characteristics, preferences, age-specific toxicity rates, and patient’s needs according to oncological guidelines including PARP Inhibitors or Antiangiogenic Therapy [16]. To analyze the complexity of the surgeries performed, the Aletti Surgical Complexity Scoring System was used, and the score obtained was divided into three groups: low risk (≤3), intermediate risk (4–7), and high risk (≥8) [17]. Regarding surgery complications, the 2004 Clavien Dindo classification was used [18]. The follow-up period was defined as the time between first treatment and disease’s recurrence (DFS), any cause of death (OS), or last medical appointment.
2.3. Ethical Considerations
The study was approved by the ethics committee of the Hospital del Mar number CEIm 2023/11249 and was carried out in compliance with the guidelines of the Declaration of Helsinki, Fortaleza, Brazil, 2013. A waiver of informed consent was obtained from the institutional ethical review board from the home institution.
2.4. Statistical Analysis
The variables were described with relative and absolute frequencies for categorical variables, and mean, quartiles or standard deviation for continuous variables. A chi-square test was used to study differences between groups for categorical variables. Fisher’s exact test was used when the frequency of any cell in the contingency table was lower than five. For continuous variables, the non-parametric Kruskal–Wallis test was used. Overall survival (OS) and disease-free survival (DFS) were studied using survival analysis with Kaplan–Meier curves and log-rank tests to determine differences between age groups. Univariate Cox proportional hazards regression models were used to identify potential associated variables. Multivariate analysis was conducted using a Cox proportional hazard regression model, considering age as the primary factor. Other significant variables, identified through univariate analysis and based on their clinical relevance, were included as covariates in the model. Results were expressed as hazard ratios (HR) with corresponding 95% confidence intervals. All p-values lower than the significance level of 0.05 were considered significant. Analyses were performed in R version 4.2.2.
3. Results
During the study period, a total of 110 patients were included with a mean age of 62.9 (SD ± 14.03) years at time of diagnosis. Of the total of patients included, 73 (66.4%) were younger than 70 years old and 37 (33.6%) were 70 or older. Compared with patients under 70, older patients had a higher Comorbidity Charlson Index (p < 0.001) and a higher ASA score (p = 0.013). Concerning tumoral characteristics, 80.5% of older patients were diagnosed at advanced stages (III–IV), compared to 63% in <70 patients (p = 0.012). There were no differences when considering histological type of tumor or presence of tumoral ascites at the time of diagnosis (Table 1).

Table 1.
Descriptive characteristics of ovarian cancer patients according to diagnostic age.
Regarding treatment type, 80.9% of patients of our cohort received standard treatment while 19.1% received non-standard treatment. Patients ≥70 were more likely to receive non-standard treatment compared to younger patients (32.4% vs. 12.3%. p = 0.023). Out of all patients who underwent surgery, 89.3% received either primary or interval surgery. When comparing by age, 93.0% of patients under 70 underwent primary or interval surgery, while this rate was 81.2% among older patients. Specifically, older patients were more likely to undergo interval surgery (31.2%) as well as rescue surgery (18.8%) compared to patients under 70 (19.7% and 7%, consecutively, p = 0.053). Complete cytoreduction was achieved in 95.8% of patients <70 compared to 81.3% in older patients (p = 0.024). However, when considering the Aletti Score, duration of surgery, and surgery complications (Clavien Dindo Classification), there were no significant differences when comparing age groups. Regarding chemotherapy variables, 80% of patients of our cohort received chemotherapy, without differences when considering chemotherapy administration, completeness of cycles, or chemotherapy complications between age groups. At the time of the last follow-up, 75 (68.2%) patients included were alive; particularly, 56 (76.7%) patients under 70 years old compared to 19 (51.4%) patients older (p = 0.006). After a median overall follow-up of 21.6 months (IQR: 13.8–32.5), 54 out of 110 patients (56.3%) experienced relapse. Among patients aged 70 years or older, 20 (71.4%) relapsed, compared to 34 (50%) of those younger than 70 (p = 0.054) (Table 2).

Table 2.
Treatment characteristics and outcomes of ovarian cancer patients included in the study.
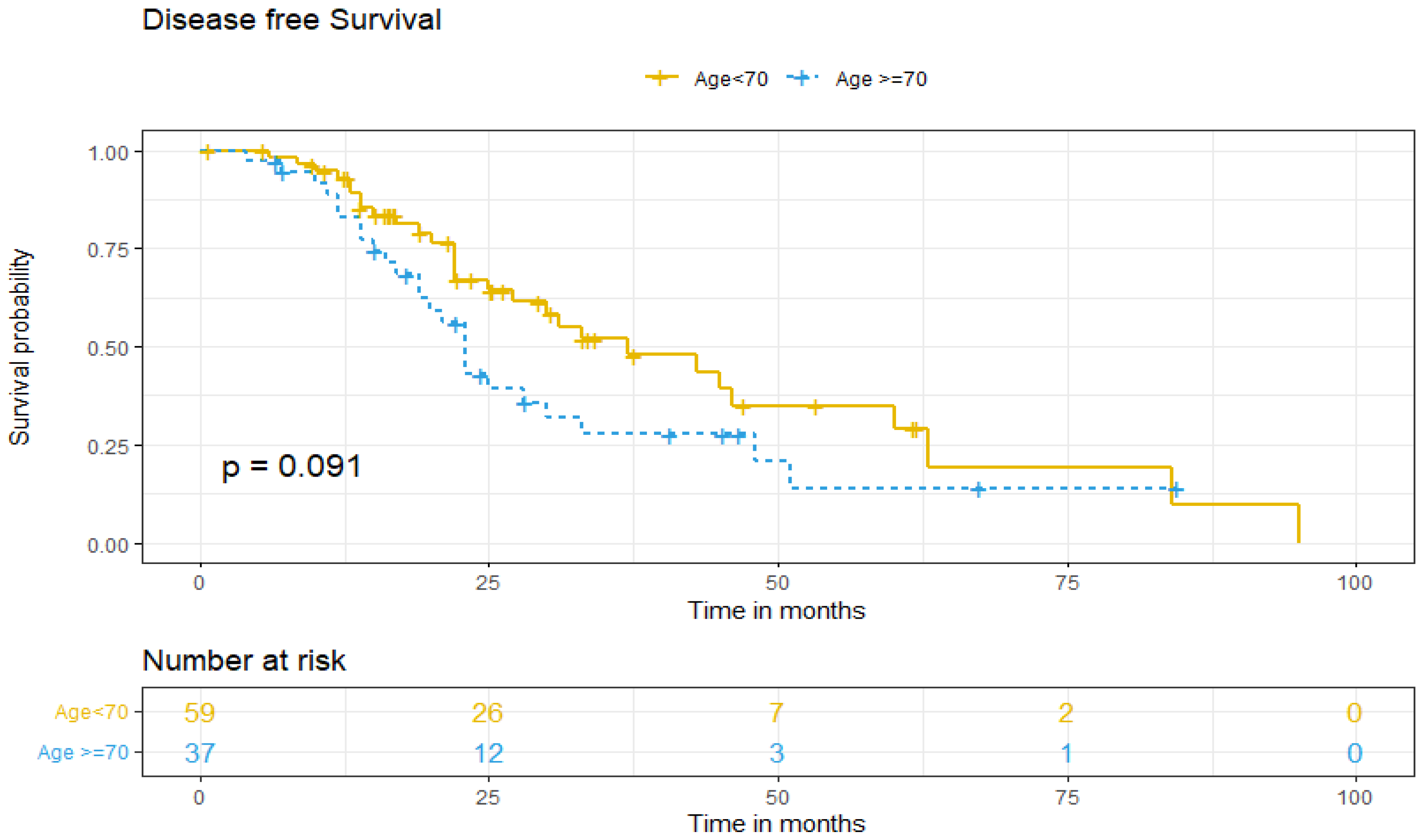
Regarding DFS between both age groups, five-year DFS was 13% for older patients and 37% for patients under 70 (p = 0.091) (Figure 1). DFS was adjusted for patient-related factors that could potentially affect survival. Univariate and multivariate analysis showed that age ≥70 (aHR = 1.24. 95% IC, 0.55–2.78, p > 0.05) and non-standard treatment (aHR = 0.58. 95% IC, 0.24–1.4, p > 0.05) did not independently predict worse DFS of ovarian cancer patients. However, being diagnosed at higher stages (III–IV) increases the risk of recurrence by more than three times (aHR 3.44; 95% IC, 1.50–7.87, p = 0.003), and the presence of tumoral ascites doubles it (aHR 2.23; 95% IC, 1.15–4.35, p = 0.018), as independent predictive factors (Table 3).
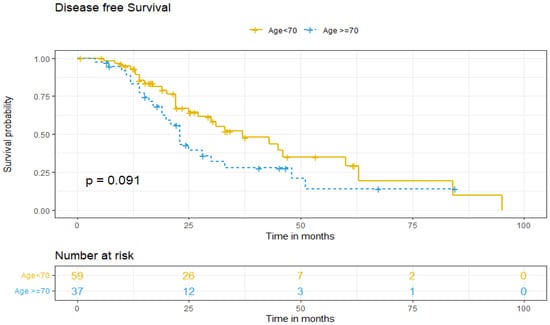
Figure 1.
Disease-free survival according to age group.

Table 3.
DFS. Univariate and multivariate analysis results.
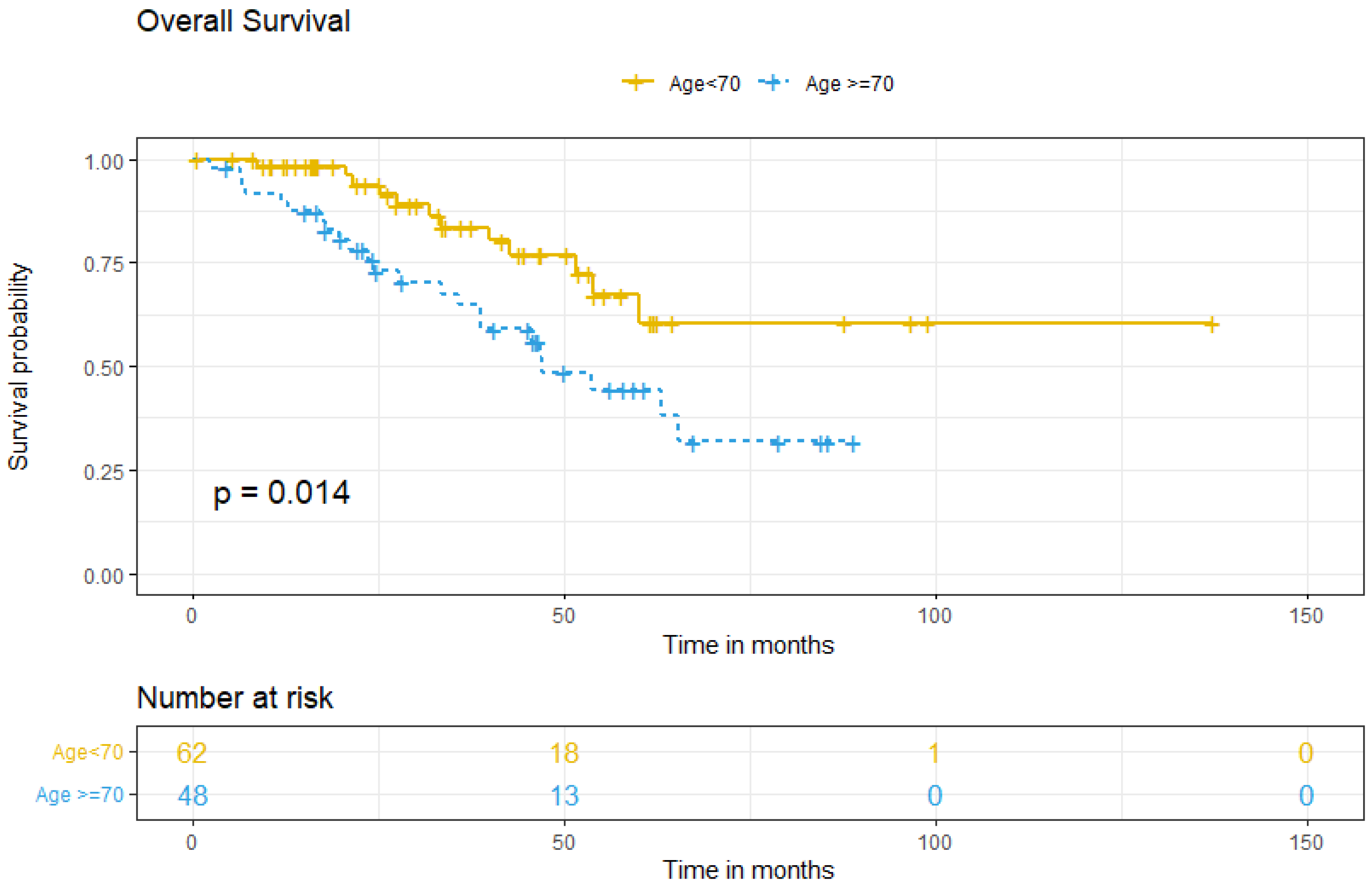
Survival analysis between groups showed that the five-year OS for patients <70 was 67.2%, compared to 44.4% for older patients (p = 0.014) (Figure 2). Multivariate analysis for OS showed that ≥70, comorbidity, FIGO stage at diagnosis, tumoral ascites, and treatment type did not independently predict worse OS (Table 4).
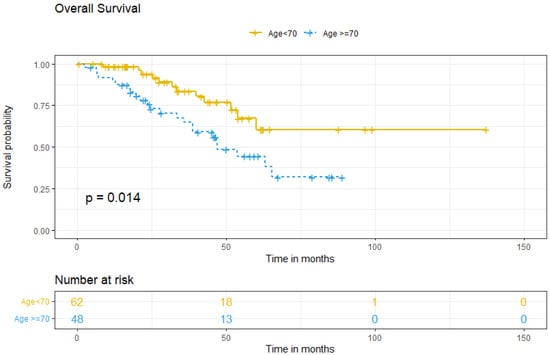
Figure 2.
Overall survival according to age group.

Table 4.
OS. Univariate and multivariate analysis results.
4. Discussion
To our knowledge, this study represents one of the few investigations that assesses specific differences among ovarian cancer patients in terms of age. Our study showed that older patients diagnosed with ovarian cancer are less likely to receive standard treatment in the form of surgery (primary or interval) plus chemotherapy, which could explain their worse OS when compared to younger patients. However, there is no consistent evidence evaluating the impact of older age in survival for ovarian cancer [3,9].
Among ovarian cancer patients, there is a significant portion of elderly women, of whom treatment is controversial [3,4]. Some studies indicate that older patients with ovarian cancer tend to receive less aggressive treatment due to concerns about increased surgical risks and comorbidities, even though some guidelines consider frailty as eligibility criteria to neoadjuvant chemotherapy [9]. In this line, recent studies suggest that older patients could benefit from geriatric screening tests, with the goal of identifying patients with age-related problems not typically identified by a routine history and physical examination, like nutritional issues or functional impairments, who could benefit from geriatric interventions and optimization programs, such as prehabilitation, and on the other hand, can help select an appropriate cancer treatment [13,19]. Therefore, relying solely on age to exclude patients from standard treatment might overlook many individuals, potentially impacting their prognosis. Hence, it’s crucial to investigate whether older patients are indeed more frequently excluded from standard treatment and whether OS and DFS vary by age. As it was expected, our findings reveal that older women had more comorbidities, a higher ASA score at diagnosis, and were more likely to be diagnosed at more advanced stages (FIGO III–IV). This higher rate of advanced stage diagnosis could be explained by the unspecific symptoms that characterize ovarian cancer being dismissed or attributed to another disease more often in older women, thus delaying the diagnosis [1].
Regarding treatment, our study showed that older women more frequently underwent neoadjuvant chemotherapy followed by interval or rescue surgery. This approach is considered when a patient is initially not suitable for primary surgery due to factors like tumor extension or patient characteristics, as neoadjuvant chemotherapy aims to reduce tumor extension or minimize the extent of surgical resection, enabling these patients to better tolerate the subsequent surgery [5]. Despite this, older patients were less likely to achieve complete cytoreduction. Nevertheless, it is important to note that there were no significant differences between age groups when considering surgery complications. Hence, it could be inferred that if older patients were selected for cytoreductive surgery based on their general health status, they could fare as well as younger patients when it comes to surgery complications. Other studies support this theory, suggesting that underlying comorbidities rather than age alone are responsible for increased surgical morbidity and mortality in older patients [9]. On the other hand, it could also be suggested that surgery approach in older patients is less radical, which could lead to less surgical complications, even though the Aletti score showed no significant differences between age groups.
Lastly, the final aim of our study was to evaluate the impact of age and oncological management in survival. The study showed that despite older patients being diagnosed at later stages and undergoing more non-standard treatment, there were no differences in DFS between age groups. This could be explained by the natural history and progression of the disease and the fact that recurrence generally occurs at a high rate, especially between the first 12 to 24 months [20]. On the other hand, OS was lower in women aged 70 or older, possibly due to older patients receiving non-standard treatment more frequently and having a lower rate of complete cytoreduction. Both of these factors are known to impact prognosis according to published literature, although treatment type was not shown to be independent in our multivariate analysis [21,22].
The study does present some limitations. Most notably, it being a non-randomized control trial study, with common biases of observational studies. Another limitation is the sample size, which was relatively small, which limits the generalizability of findings and could impair the results, especially in the case of multivariate analysis. However, the patients included were treated by the same multidisciplinary team at the Hospital del Mar, following the same internationally accepted treatment guidelines for ovarian cancer. Secondly, as it consisted of a retrospective study, it is prone to selection and information bias, as well, data collection could have been conditioned. In fact, some variables were not available for every patient, and to reduce heterogeneity, some data were pooled into categories. This could affect the results when considering alternative explanations. As strengths, this study enhances the current understanding of ovarian cancer management in elderly patients, contributing to existing knowledge, and may help optimize treatment strategy in the future, potentially leading to improved patient outcomes.
5. Conclusions
In conclusion, considering the results of our study, we can affirm that women 70 or older are more likely to be diagnosed at later stages and treated less radically than younger women, with no age specific differences for DFS. Moreover, even though our results showed that women 70 or older had more comorbidities and a higher ASA classification at the time of diagnosis, it is important to consider that this only reflects a tendency in the group. Therefore, as age did not show to be an independent factor for DFS, the choice of treatment should not be based on age only, but should be individualized and consider the overall state of the patient, in order to achieve the best outcome possible when considering patient characteristics.
To expand knowledge on the matter and confirm our findings, future research should include larger multicentric prospective studies. Moreover, it would be ideal to create a guideline in order to select older patients suited for standard treatment better, instead of basing the decision mainly on age, and to finally be able to provide individualized treatment, achieving the best outcome considering patient characteristics.
Author Contributions
Conceptualization, G.M. and E.M.; methodology, A.V.; software, G.P.; validation, M.A., R.A.S.-G. and J.M.S.-S.; formal analysis, A.V.; investigation, G.P.; resources, G.P.; data curation, B.F.; writing—original draft preparation, G.P.; writing—review and editing, E.M.; visualization, E.M.; supervision, G.M.; project administration, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the institutional ethics committee Comitè d’Ètica de la Investigació amb medicaments del Parc de Salut MAR (Institutional Review Board Project No. 2023/11249) in April 2024.
Informed Consent Statement
The requirement for informed consent was waived because no intervention or changes in practical clinics were performed according to these study results.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| OS | overall survival |
| HR | hazard ratio |
| DFS | disease-free survival |
| SAMIUC | Sociedad Andaluza de Medicina Intensiva y Unidades Coronarias |
| FIGO | International Federation of Gynecology and Obstetrics |
| SD | standard deviation |
| aHR | average hazard ratio |
| ASA | American Society of Anesthesiologists |
References
- Roett, M.A.; Evans, P. Ovarian Cancer: An Overview. Am. Fam. Physician 2009, 80, 609–616. [Google Scholar] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.K.; Nayak, B. Management of Ovarian Cancer in Elderly. Rev. Recent Clin. Trials 2015, 10, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Ovarian Cancer Incidence Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/ovarian-cancer/incidence (accessed on 23 September 2023).
- Kuroki, L.; Guntupalli, S.R. Treatment of Epithelial Ovarian Cancer. BMJ 2020, 371, m3773. [Google Scholar] [CrossRef] [PubMed]
- Orr, B.; Edwards, R.P. Diagnosis and Treatment of Ovarian Cancer. Hematol. Oncol. Clin. N. Am. 2018, 32, 943–964. [Google Scholar] [CrossRef] [PubMed]
- Shih, K.K.; Chi, D.S. Maximal Cytoreductive Effort in Epithelial Ovarian Cancer Surgery. J. Gynecol. Oncol. 2010, 21, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Cancer of the Ovary—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/ovary.html (accessed on 23 September 2023).
- Park, S.J.; Mun, J.; Yim, G.W.; Lee, M.; Chung, H.H.; Kim, J.W.; Park, N.H.; Song, Y.S.; Kim, H.S. Is Increased Chronological Age a Contraindication to Debulking Surgery for Elderly Patients with Advanced Ovarian Cancer? J. Obstet. Gynaecol. 2022, 42, 3254–3259. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, S.; Hook, J.; Nankivell, M.; Jayson, G.C.; Kitchener, H.; Lopes, T.; Luesley, D.; Perren, T.; Bannoo, S.; Mascarenhas, M.; et al. Primary Chemotherapy versus Primary Surgery for Newly Diagnosed Advanced Ovarian Cancer (CHORUS): An Open-Label, Randomised, Controlled, Non-Inferiority Trial. Lancet Lond. Engl. 2015, 386, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; De Wever, I.; Tjalma, W.; Van Gramberen, M.; Decloedt, J.; van Dam, P. Neoadjuvant Chemotherapy or Primary Debulking Surgery in Advanced Ovarian Carcinoma: A Retrospective Analysis of 285 Patients. Gynecol. Oncol. 1998, 71, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Coens, C.; Nankivell, M.; Kristensen, G.B.; Parmar, M.K.B.; Ehlen, T.; Jayson, G.C.; Johnson, N.; Swart, A.M.; Verheijen, R.; et al. Neoadjuvant Chemotherapy versus Debulking Surgery in Advanced Tubo-Ovarian Cancers: Pooled Analysis of Individual Patient Data from the EORTC 55971 and CHORUS Trials. Lancet Oncol. 2018, 19, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Wildiers, H.; Heeren, P.; Puts, M.; Topinkova, E.; Janssen-Heijnen, M.L.G.; Extermann, M.; Falandry, C.; Artz, A.; Brain, E.; Colloca, G.; et al. International Society of Geriatric Oncology Consensus on Geriatric Assessment in Older Patients with Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 2595–2603. [Google Scholar] [CrossRef] [PubMed]
- Rubiales, E. Indice de Comorbilidad de Charlson (CCI). SAMIUC. Available online: https://www.samiuc.es/indice-de-comorbilidad-de-charlson-cci/ (accessed on 23 September 2023).
- Prat, J.; FIGO Committee on Gynecologic Oncology. Staging Classification for Cancer of the Ovary, Fallopian Tube, and Peritoneum. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2014, 124, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gogineni, V.; Morand, S.; Staats, H.; Royfman, R.; Devanaboyina, M.; Einloth, K.; Dever, D.; Stanbery, L.; Aaron, P.; Manning, L.; et al. Current Ovarian Cancer Maintenance Strategies and Promising New Developments. J. Cancer 2021, 12, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Aletti, G.D.; Santillan, A.; Eisenhauer, E.L.; Hu, J.; Aletti, G.; Podratz, K.C.; Bristow, R.E.; Chi, D.S.; Cliby, W.A. A New Frontier for Quality of Care in Gynecologic Oncology Surgery: Multi-Institutional Assessment of Short-Term Outcomes for Ovarian Cancer Using a Risk-Adjusted Model. Gynecol. Oncol. 2007, 107, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Miralpeix, E.; Sole-Sedeno, J.-M.; Rodriguez-Cosmen, C.; Taus, A.; Muns, M.-D.; Fabregó, B.; Mancebo, G. Impact of Prehabilitation during Neoadjuvant Chemotherapy and Interval Cytoreductive Surgery on Ovarian Cancer Patients: A Pilot Study. World J. Surg. Oncol. 2022, 20, 46. [Google Scholar] [CrossRef] [PubMed]
- Salani, R.; Backes, F.J.; Fung, M.F.K.; Holschneider, C.H.; Parker, L.P.; Bristow, R.E.; Goff, B.A. Posttreatment Surveillance and Diagnosis of Recurrence in Women with Gynecologic Malignancies: Society of Gynecologic Oncologists Recommendations. Am. J. Obstet. Gynecol. 2011, 204, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Polterauer, S.; Vergote, I.; Concin, N.; Braicu, I.; Chekerov, R.; Mahner, S.; Woelber, L.; Cadron, I.; Van Gorp, T.; Zeillinger, R.; et al. Prognostic Value of Residual Tumor Size in Patients with Epithelial Ovarian Cancer FIGO Stages IIA-IV. Int. J. Gynecol. Cancer 2012, 22, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Della Pepa, C.; Tonini, G.; Pisano, C.; Di Napoli, M.; Cecere, S.C.; Tambaro, R.; Facchini, G.; Pignata, S. Ovarian Cancer Standard of Care: Are There Real Alternatives? Chin. J. Cancer 2015, 34, 17–27. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).