Outcomes for Medicaid Patients with Colorectal Cancer Are Improved in Affluent Neighborhoods, but Disparities Persist
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Groups and Outcomes
2.3. Statistical Analysis
3. Results
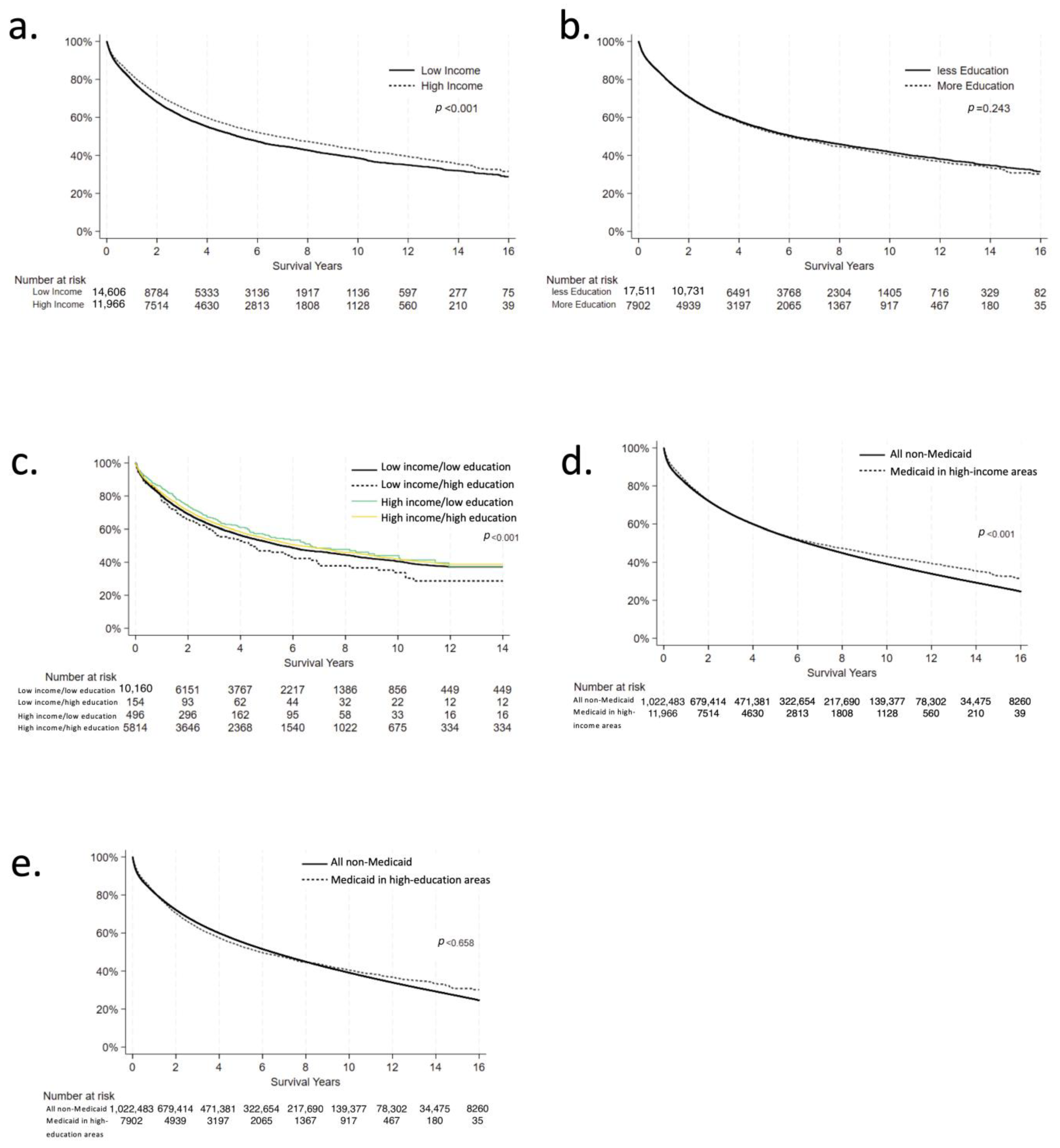
3.1. Income Analysis
3.2. Education Analysis
3.3. Combined Variable Analysis
3.4. Medicaid vs. Non-Medicaid Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Issaka, R.B.; Chan, A.T.; Gupta, S. AGA Clinical Practice Update on Risk Stratification for Colorectal Cancer Screening and Post-Polypectomy Surveillance: Expert Review. Gastroenterology 2023, 165, 1280–1291. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Adam, M.; Chang, G.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.A.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Colon Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024, 22, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Adam, M.; Chang, G.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.A.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. NCCN Guidelines® Insights: Rectal Cancer, Version 3.2024. J. Natl. Compr. Cancer Netw. 2024, 22, 366–375. [Google Scholar] [CrossRef]
- Turrell, G.; Kavanagh, A.; Draper, G.; Subramanian, S.V. Do places affect the probability of death in Australia? A multilevel study of area-level disadvantage, individual-level socioeconomic position and all-cause mortality, 1998–2000. J. Epidemiol. Community Health 2007, 61, 13–19. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Sundquist, J.; Zöller, B.; Sundquist, K. Neighborhood Deprivation and Lung Cancer Incidence and Mortality: A Multilevel Analysis from Sweden. J. Thorac. Oncol. 2015, 10, 256–263. [Google Scholar] [CrossRef]
- Goel, N.; Hernandez, A.; Thompson, C.; Choi, S.; Westrick, A.; Stoler, J.; Antoni, M.H.; Rojas, K.; Kesmodel, S.; Figueroa, M.E.; et al. Neighborhood Disadvantage and Breast Cancer–Specific Survival. JAMA Netw. Open 2023, 6, e238908. [Google Scholar] [CrossRef]
- Bentley, R.; Kavanagh, A.M.; Subramanian, S.V.; Turrell, G. Area disadvantage, individual socio-economic position, and premature cancer mortality in Australia 1998 to 2000: A multilevel analysis. Cancer Causes Control 2007, 19, 183–193. [Google Scholar] [CrossRef]
- van Loon, A.; Brandt, P.v.D.; Golbohm, R. Socioeconomic status and colon cancer incidence: A prospective cohort study. Br. J. Cancer 1995, 71, 882–887. [Google Scholar] [CrossRef]
- Kim, D.; Masyn, K.E.; Kawachi, I.; Laden, F.; Colditz, G.A. Neighborhood socioeconomic status and behavioral pathways to risks of colon and rectal cancer in women. Cancer 2010, 116, 4187–4196. [Google Scholar] [CrossRef]
- Doubeni, C.A.; Laiyemo, A.O.; Major, J.M.; Schootman, M.; Lian, M.; Park, Y.; Graubard, B.I.; Hollenbeck, A.R.; Sinha, R. Socioeconomic status and the risk of colorectal cancer. Cancer 2012, 118, 3636–3644. [Google Scholar] [CrossRef]
- Zhang, D.; Matthews, C.E.; Powell-Wiley, T.M.; Xiao, Q. Ten-year change in neighborhood socioeconomic status and colorectal cancer. Cancer 2018, 125, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, K.L.; Crossley-May, H.; Vigneau, F.D.; Brown, K.; Banerjee, M. Race, socioeconomic status and stage at diagnosis for five common malignancies. Cancer Causes Control 2003, 14, 761–766. [Google Scholar] [CrossRef] [PubMed]
- On behalf of Danish Colorectal Cancer Group; Frederiksen, B.L.; Osler, M.; Harling, H.; Jørgensen, T. Social inequalities in stage at diagnosis of rectal but not in colonic cancer: A nationwide study. Br. J. Cancer 2008, 98, 668–673. [Google Scholar] [CrossRef]
- Salem, M.E.; Puccini, A.; Trufan, S.J.; Sha, W.; Kadakia, K.C.; Hartley, M.L.; Musselwhite, L.W.; Symanowski, J.T.; Hwang, J.J.; Raghavan, D. Impact of Sociodemographic Disparities and Insurance Status on Survival of Patients with Early-Onset Colorectal Cancer. Oncologist 2021, 26, e1730–e1741. [Google Scholar] [CrossRef] [PubMed]
- Ko, T.M.; Laraia, K.N.; Alexander, H.R.; Ecker, B.L.; Grandhi, M.S.; Kennedy, T.J.; In, H.; Langan, R.C.; Pitt, H.A.; Stroup, A.M.; et al. Low neighborhood socioeconomic status is associated with poor outcomes in young adults with colorectal cancer. Surgery 2024, 176, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.; Yousef, A.; Chowdhury, S.; Fanaeian, M.M.; Knafl, M.; Peterson, J.; Zeineddine, M.; Alfaro, K.; Zeineddine, F.; Goldstein, D.; et al. Molecular, Socioeconomic, and Clinical Factors Affecting Racial and Ethnic Disparities in Colorectal Cancer Survival. JAMA Oncol. 2024, 10, 1519–1529. [Google Scholar] [CrossRef]
- Jansen, L.; Eberle, A.; Emrich, K.; Gondos, A.; Holleczek, B.; Kajüter, H.; Maier, W.; Nennecke, A.; Pritzkuleit, R.; Brenner, H.; et al. Socioeconomic deprivation and cancer survival in Germany: An ecological analysis in 200 districts in Germany. Int. J. Cancer 2013, 134, 2951–2960. [Google Scholar] [CrossRef]
- Tham, N.L.; Skandarajah, A.; Hayes, I.P. Socioeconomic disadvantage and its impact on colorectal cancer in Australia: A scoping review. ANZ J. Surg. 2022, 92, 2808–2815. [Google Scholar] [CrossRef] [PubMed]
- Blair, A.; Datta, G.D. Associations between area-level deprivation, rural residence, physician density, screening policy and late-stage colorectal cancer in Canada. Cancer Epidemiol. 2020, 64, 101654. [Google Scholar] [CrossRef] [PubMed]
- Renna Junior, N.L.; Silva, G.d.A.e. Socioeconomic status and cancer survival in Brazil: Analysis of population data from the municipalities of Aracaju and Curitiba, 1996–2012. Cancer Epidemiol. 2023, 85, 102394. [Google Scholar] [CrossRef]
- Kaneko, N.; Nishino, Y.; Ito, Y.; Nakaya, T.; Kanemura, S. Association of Socioeconomic Status Assessed by Areal Deprivation With Cancer Incidence and Detection by Screening in Miyagi, Japan Between 2005 and 2010. J. Epidemiol. 2023, 33, 521–530. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.; Kim, N.; Nari, F.; Bae, S.; Lee, H.J.; Lee, M.; Jun, J.K.; Choi, K.S.; Suh, M. Socioeconomic Disparities in Six Common Cancer Survival Rates in South Korea: Population-Wide Retrospective Cohort Study. JMIR Public Health Surveill. 2024, 10, e55011. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.; Blakely, T.; Sarfati, D.; Fawcett, J.; Peace, J. Trends in colorectal cancer mortality by ethnicity and socio-economic position in New Zealand, 1981–99: One country, many stories. Aust. N. Z. J. Public Health 2006, 30, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, R.; Halkjær, J.; Rottmann, N.; Hansen, L.; Holten, I. Social inequality and incidence of and survival from cancers of the colon and rectum in a population-based study in Denmark, 1994–2003. Eur. J. Cancer 2008, 44, 1978–1988. [Google Scholar] [CrossRef]
- GlobalSurg, C. National Institute for Health Research Global Health Research Unit on Global S. Global variation in postoperative mortality and complications after cancer surgery: A multicentre, prospective cohort study in 82 countries. Lancet 2021, 397, 387–397. [Google Scholar]
- Du, X.L.; Fang, S.; Vernon, S.W.; El-Serag, H.; Shih, Y.T.; Davila, J.; Rasmus, M.L. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer 2007, 110, 660–669. [Google Scholar] [CrossRef]
- Niu, X.; Pawlish, K.S.; Roche, L.M. Cancer Survival Disparities by Race/Ethnicity and Socioeconomic Status in New Jersey. J. Health Care Poor Underserved 2010, 21, 144–160. [Google Scholar] [CrossRef]
- Hines, R.; Markossian, T.; Johnson, A.; Dong, F.; Bayakly, R. Geographic Residency Status and Census Tract Socioeconomic Status as Determinants of Colorectal Cancer Outcomes. Am. J. Public Health 2014, 104, e63–e71. [Google Scholar] [CrossRef]
- Tannenbaum, S.L.; Hernandez, M.; Zheng, D.D.; Sussman, D.A.; Lee, D.J. Individual- and Neighborhood-Level Predictors of Mortality in Florida Colorectal Cancer Patients. PLoS ONE 2014, 9, e106322. [Google Scholar] [CrossRef] [PubMed]
- A Hastert, T.; A A Beresford, S.; Sheppard, L.; White, E. Disparities in cancer incidence and mortality by area-level socioeconomic status: A multilevel analysis. J. Epidemiol. Community Health 2014, 69, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Hu, F.-H.; Jia, Y.-J.; Zhao, D.-Y.; Zhang, W.-Q.; Tang, W.; Hu, S.-Q.; Ge, M.-W.; Du, W.; Shen, W.-Q.; et al. Socioeconomic status on survival outcomes in patients with colorectal cancer: A cross-sectional study. J. Cancer Res. Clin. Oncol. 2023, 149, 15641–15655. [Google Scholar] [CrossRef] [PubMed]
- Habermann, E.B.; Day, C.N.; EPalis, B.; Plichta, J.K.M.F.; Wasif, N.M.; Weigel, R.J.M.; Boughey, J.C.M. American College of Surgeons Cancer Program Annual Report from 2021 Participant User File. J. Am. Coll. Surg. 2024, 240, 95–110. [Google Scholar] [CrossRef]
- Donohue, J.M.; Cole, E.S.; James, C.V.; Jarlenski, M.; Michener, J.D.; Roberts, E.T. The US Medicaid Program. JAMA 2022, 328, 1085–1099. [Google Scholar] [CrossRef]
- Lewis, V.A.; Maddox, K.J.; Austin, A.M.; Gottlieb, D.J.; Bynum, J.P. Developing and Validating a Measure to Estimate Poverty in Medicare Administrative Data. Med. Care 2019, 57, 601–607. [Google Scholar] [CrossRef]
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef]
- Goulart, B.H.; Reyes, C.M.; Fedorenko, C.R.; Mummy, D.G.; Satram-Hoang, S.; Koepl, L.M.; Blough, D.K.; Ramsey, S.D. Referral and Treatment Patterns Among Patients with Stages III and IV Non–Small-Cell Lung Cancer. J. Oncol. Pract. 2013, 9, 42–50. [Google Scholar] [CrossRef]
- Doubeni, C.A.; Laiyemo, A.O.; Reed, G.; Field, T.S.; Fletcher, R.H. Socioeconomic and Racial Patterns of Colorectal Cancer Screening among Medicare Enrollees in 2000 to 2005. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2170–2175. [Google Scholar] [CrossRef]
- Lian, M.; Schootman, M.; Yun, S. Geographic variation and effect of area-level poverty rate on colorectal cancer screening. BMC Public Health 2008, 8, 358. [Google Scholar] [CrossRef]
- Khan, M.M.M.; Munir, M.M.; Woldesenbet, S.; Endo, Y.; Khalil, M.; Tsilimigras, D.; Harzman, A.; Huang, E.; Kalady, M.; Pawlik, T.M. Association of COVID-19 Pandemic with Colorectal Cancer Screening: Impact of Race/Ethnicity and Social Vulnerability. Ann. Surg. Oncol. 2024, 31, 3222–3232. [Google Scholar] [CrossRef] [PubMed]
- Platz, E.A.; Willett, W.C.; Colditz, G.A.; Rimm, E.B.; Spiegelman, D.; Giovannucci, E. Proportion of colon cancer risk that might be preventable in a cohort of middle-aged US men. Cancer Causes Control 2000, 11, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Pischon, T.; Jenab, M.; Bueno-De-Mesquita, H.B.; Fedirko, V.; Norat, T.; Romaguera, D.; Knüppel, S.; Boutron-Ruault, M.-C.; Dossus, L.; et al. Combined impact of healthy lifestyle factors on colorectal cancer: A large European cohort study. BMC Med. 2014, 12, 168. [Google Scholar] [CrossRef] [PubMed]
- Botteri, E.; Peveri, G.; Berstad, P.; Bagnardi, V.; Chen, S.L.; Sandanger, T.M.; Hoff, G.; Dahm, C.C.; Antoniussen, C.S.; Tjønneland, A.; et al. Changes in Lifestyle and Risk of Colorectal Cancer in the European Prospective Investigation into Cancer and Nutrition. Am. J. Gastroenterol. 2022, 118, 702–711. [Google Scholar] [CrossRef]
- Torres Stone, R.A.; EWaring, M.; Cutrona, S.L.; IKiefe, C.; Allison, J.; ADoubeni, C. The association of dietary quality with colorectal cancer among normal weight, overweight and obese men and women: A prospective longitudinal study in the USA. BMJ Open 2017, 7, e015619. [Google Scholar] [CrossRef]
| Low Income | High Income | p-Value | |
|---|---|---|---|
| 15,730 (54.7%) | 13,046 (45.3%) | ||
| Age at Diagnosis † | 55.615 (11.974) | 57.660 (14.012) | <0.001 |
| Sex | |||
| Male | 7873 (50.1%) | 6428 (49.3%) | 0.188 |
| Female | 7857 (49.9%) | 6618 (50.7%) | |
| Race | |||
| White | 8046 (51.2%) | 8884 (68.1%) | <0.001 |
| Black | 6607 (42.0%) | 1940 (14.9%) | |
| Asian | 422 (2.7%) | 1508 (11.6%) | |
| Other | 655 (4.2%) | 714 (5.5%) | |
| Ethnicity | |||
| Non-Hispanic | 12,716 (83.7%) | 11,031 (87.4%) | <0.001 |
| Hispanic | 2485 (16.3%) | 1584 (12.6%) | |
| Charlson-Deyo Score † | 0.454 (0.800) | 0.363 (0.725) | <0.001 |
| Facility Type | |||
| Community Cancer Program | 1262 (8.8%) | 1155 (9.8%) | <0.001 |
| Comprehensive Community Cancer Program | 4403 (30.8%) | 4010 (34.0%) | |
| Academic/Research Program | 6292 (44.0%) | 4321 (36.6%) | |
| Integrated Network Cancer Program | 2343 (16.4%) | 2315 (19.6%) | |
| Clinical Stage | |||
| 0 | 653 (8.7%) | 502 (8.2%) | 0.001 |
| 1 | 1224 (16.2%) | 1139 (18.7%) | |
| 2 | 1003 (13.3%) | 836 (13.7%) | |
| 3 | 808 (10.7%) | 675 (11.1%) | |
| 4 | 3851 (51.1%) | 2948 (48.3%) | |
| Received Chemotherapy | |||
| No | 7992 (50.8%) | 6838 (52.4%) | 0.023 |
| Yes | 7199 (45.8%) | 5764 (44.2%) | |
| Unknown | 539 (3.4%) | 444 (3.4%) | |
| Received Immunotherapy | |||
| No | 14,589 (92.8%) | 12,277 (94.1%) | <0.001 |
| Yes | 1016 (6.5%) | 664 (5.1%) | |
| Unknown | 124 (0.8%) | 105 (0.8%) | |
| Received Surgery | |||
| No | 3019 (19.3%) | 2304 (17.7%) | <0.001 |
| Yes | 12,661 (80.7%) | 10,715 (82.3%) |
| Estimate (Ref: Low-Income) | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| Time to treatment initiation † | −1.847 | −3.342, −0.353 | 0.015 |
| Time to surgery † | −1.390 | −4.014, 1.233 | 0.299 |
| 30-day postoperative mortality ‡ | 0.730 | 0.573, 1.098 | 0.163 |
| 90-day postoperative mortality ‡ | 0.863 | 0.684, 1.088 | 0.211 |
| Overall survival § | 0.810 | 0.768, 0.855 | <0.001 |
| Less Education | More Education | p-Value | |
|---|---|---|---|
| 19,073 (69.5%) | 8375 (30.5%) | ||
| Age at Diagnosis † | 55.613 (12.517) | 57.500 (13.976) | <0.001 |
| Sex | |||
| Male | 9510 (49.9%) | 4156 (49.6%) | 0.717 |
| Female | 9563 (50.1%) | 4219 (50.4%) | |
| Race | |||
| White | 10,577 (55.5%) | 6173 (73.7%) | <0.001 |
| Black | 6234 (32.7%) | 1115 (13.3%) | |
| Asian | 1270 (6.7%) | 718 (8.6%) | |
| Other | 992 (5.2%) | 369 (4.4%) | |
| Ethnicity | |||
| Non-Hispanic | 13,730 (74.0%) | 7466 (93.1%) | <0.001 |
| Hispanic | 4831 (26.0%) | 552 (6.9%) | |
| Charlson-Deyo Score † | 0.404 (0.761) | 0.367 (0.728) | <0.001 |
| Facility Type | |||
| Community Cancer Program | 1737 (10.1%) | 646 (8.5%) | <0.001 |
| Comprehensive Community Cancer Program | 5203 (30.2%) | 2711 (35.8%) | |
| Academic/Research Program | 7801 (45.3%) | 2455 (32.4%) | |
| Integrated Network Cancer Program | 2485 (14.4%) | 1758 (23.2%) | |
| Clinical Stage | |||
| 0 | 767 (8.5%) | 285 (7.3%) | 0.002 |
| 1 | 1466 (16.3%) | 724 (18.6%) | |
| 2 | 1253 (13.9%) | 553 (14.2%) | |
| 3 | 958 (10.6%) | 440 (11.3%) | |
| 4 | 4559 (50.6%) | 1893 (48.6%) | |
| Received Chemotherapy | |||
| No | 9697 (50.8%) | 4315 (51.5%) | 0.009 |
| Yes | 8669 (45.5%) | 3811 (45.5%) | |
| Unknown | 707 (3.7%) | 249 (3.0%) | |
| Received Immunotherapy | |||
| No | 17,793 (93.3%) | 7900 (94.3%) | 0.005 |
| Yes | 1103 (5.8%) | 411 (4.9%) | |
| Unknown | 176 (0.9%) | 64 (0.8%) | |
| Received Surgery | |||
| No | 3607 (19.0%) | 1442 (17.3%) | <0.001 |
| Yes | 15,415 (81.0%) | 6914 (82.7%) |
| Estimate (Ref: Low-Education) | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| Time to treatment initiation † | −3.926 | −5.643, −2.210 | <0.001 |
| Time to surgery † | −2.500 | −5.456, 0.457 | 0.097 |
| 30-day postoperative mortality ‡ | 1.047 | 0.734, 1.494 | 0.799 |
| 90-day postoperative mortality ‡ | 0.950 | 0.737, 1.225 | 0.694 |
| Overall survival § | 0.897 | 0.845, 0.951 | <0.001 |
| Comparison Group | Estimate (Ref: LI/LE) | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Time to treatment initiation † | LI/HE | −7.642 | −16.218, 0.933 | 0.081 |
| HI/LE | −1.465 | −6.376, 3.446 | 0.559 | |
| HI/HE | −4.419 | −6.485, −2.353 | <0.001 | |
| Time to surgery † | LI/HE | −4.816 | −19.990, 10.359 | 0.534 |
| HI/LE | −7.458 | −16.543, 1.626 | 0.108 | |
| HI/HE | −3.613 | −7.449, 0.224 | 0.065 | |
| 30-day postoperative mortality ‡ | LI/HE | 0.905 | 0.196, 4.183 | 0.898 |
| HI/LE | 0.963 | 0.277, 3.344 | 0.953 | |
| HI/HE | 1.057 | 0.677, 1.651 | 0.807 | |
| 90-day postoperative mortality ‡ | LI/HE | 0.772 | 0.244, 2.437 | 0.659 |
| HI/LE | 0.753 | 0.307, 1.848 | 0.307 | |
| HI/HE | 1.039 | 0.760, 1.422 | 0.809 | |
| Overall survival § | LI/HE | 0.958 | 0.717, 1.278 | 0.768 |
| HI/LE | 0.800 | 0.661, 0.968 | 0.022 | |
| HI/HE | 0.827 | 0.768, 0.890 | <0.001 |
| Comparison Group | Hazard Ratio (Ref: Non-Medicaid) | 95% Confidence Interval | p-Value |
|---|---|---|---|
| High-income Medicaid | 1.130 | 1.088, 1.173 | <0.001 |
| High-education Medicaid | 1.209 | 1.155, 1.265 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cummins, K.C.; El Moheb, M.; Shen, C.; Kim, S.J.; Witt, R.; Ruff, S.M.; Tsung, A. Outcomes for Medicaid Patients with Colorectal Cancer Are Improved in Affluent Neighborhoods, but Disparities Persist. Cancers 2025, 17, 1399. https://doi.org/10.3390/cancers17091399
Cummins KC, El Moheb M, Shen C, Kim SJ, Witt R, Ruff SM, Tsung A. Outcomes for Medicaid Patients with Colorectal Cancer Are Improved in Affluent Neighborhoods, but Disparities Persist. Cancers. 2025; 17(9):1399. https://doi.org/10.3390/cancers17091399
Chicago/Turabian StyleCummins, Kaelyn C., Mohamad El Moheb, Chengli Shen, Susan J. Kim, Russell Witt, Samantha M. Ruff, and Allan Tsung. 2025. "Outcomes for Medicaid Patients with Colorectal Cancer Are Improved in Affluent Neighborhoods, but Disparities Persist" Cancers 17, no. 9: 1399. https://doi.org/10.3390/cancers17091399
APA StyleCummins, K. C., El Moheb, M., Shen, C., Kim, S. J., Witt, R., Ruff, S. M., & Tsung, A. (2025). Outcomes for Medicaid Patients with Colorectal Cancer Are Improved in Affluent Neighborhoods, but Disparities Persist. Cancers, 17(9), 1399. https://doi.org/10.3390/cancers17091399







