Assessing the Clinical Effectiveness of Radioimmunotherapy with Combined Radionuclide/Monoclonal Antibody Conjugates in Cancer Treatment: Insights from Randomised Clinical Trials
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Design and Registration
- -
- Population (P): All patients receiving RIT for cancer treatment.
- -
- Interventions (I): RIT for cancer treatment.
- -
- Comparison (C): Conventional and/or emerging cancer therapies.
- -
- Outcomes (O): Treatment outcomes (e.g., overall survival, disease-free survival, progression-free survival) in cancer patients treated with RIT.
- -
- Study Design (S): This review focuses on RCTs to evaluate the overall effectiveness of RIT.
2.2. Eligibility Criteria
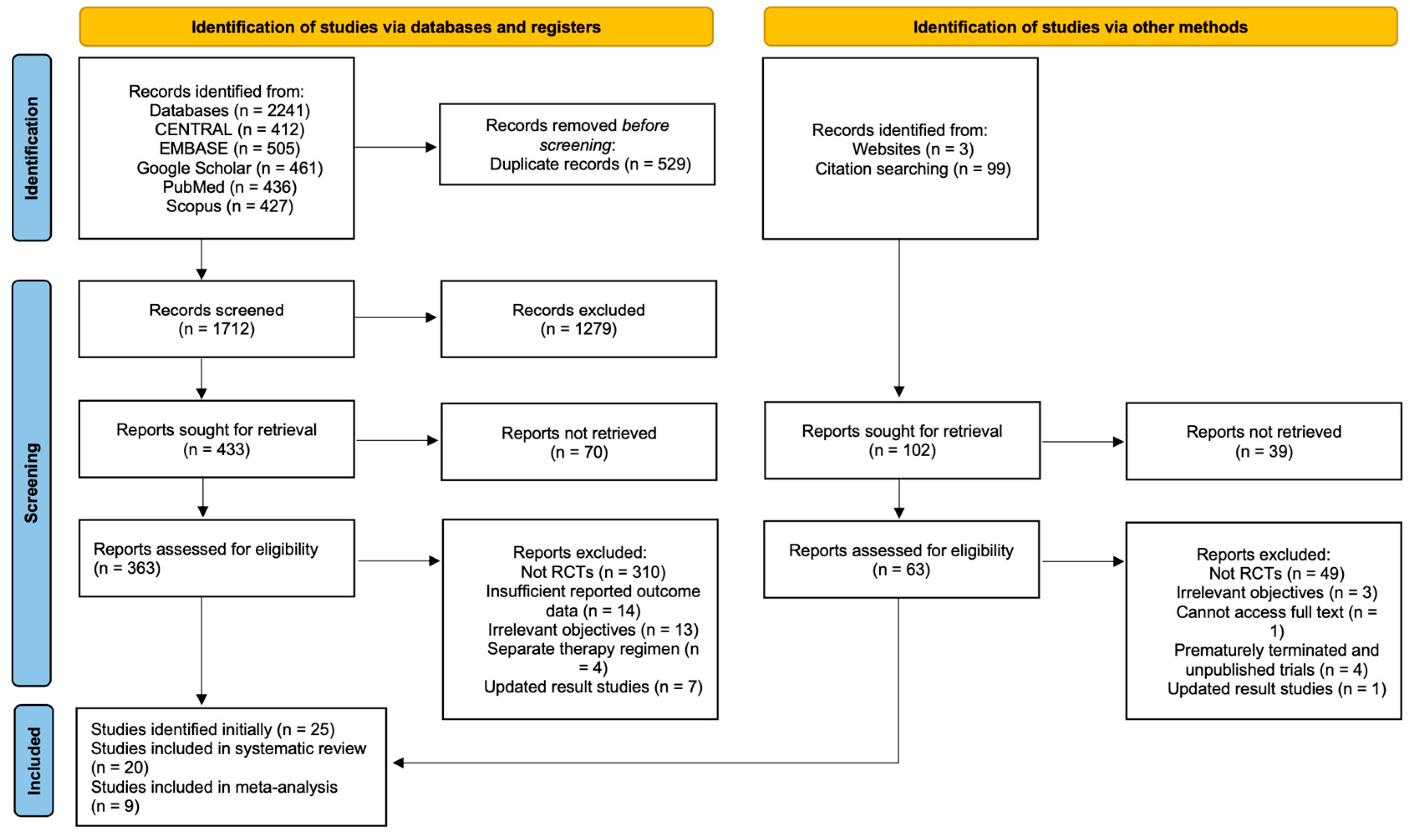
2.3. Search Strategy, Study Screening, and Selection
2.4. Data Extractions
2.5. Quality Assessment
2.6. Data Synthesis and Analysis
3. Results
3.1. Description of Studies Included
3.2. Characteristics of Included Clinical Trials
3.3. Reported Treatment Outcomes
3.4. Comparison of RIT Therapy with Other Therapies
3.4.1. Forest Plot for Progression-Related Outcomes
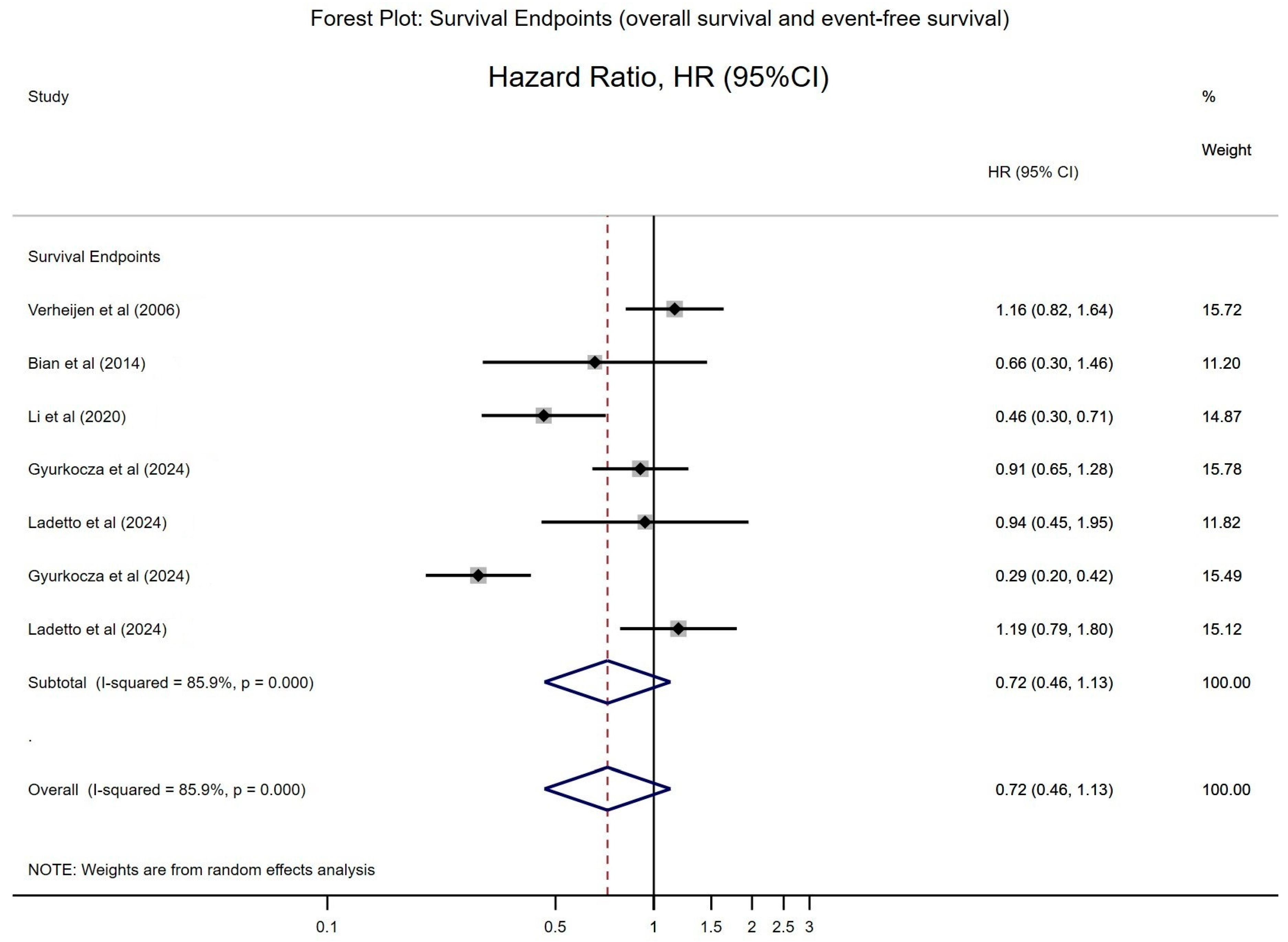
3.4.2. Forest Plot for Overall Survival (OS)
3.5. Safety and Toxicity
3.6. Quality of Included Studies
4. Discussion
4.1. Strengths and Limitations
4.2. Implications of Findings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Larson, S.M.; Carrasquillo, J.A.; Cheung, N.K.; Press, O.W. Radioimmunotherapy of human tumours. Nat. Rev. Cancer 2015, 15, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Rondon, A.; Rouanet, J.; Degoul, F. Radioimmunotherapy in Oncology: Overview of the Last Decade Clinical Trials. Cancers 2021, 13, 5570. [Google Scholar] [CrossRef]
- Pouget, J.P.; Navarro-Teulon, I.; Bardiès, M.; Chouin, N.; Cartron, G.; Pèlegrin, A.; Azria, D. Clinical radioimmunotherapy—The role of radiobiology. Nat. Rev. Clin. Oncol. 2011, 8, 720–734. [Google Scholar] [CrossRef]
- Zabor, E.C.; Kaizer, A.M.; Hobbs, B.P. Randomized Controlled Trials. Chest 2020, 158, S79–S87. [Google Scholar] [CrossRef]
- Durando, M.; Gopal, A.K.; Tuscano, J.; Persky, D. A Systematic Review of Clinical Applications of Anti-CD20 Radioimmunotherapy for Lymphoma. Oncologist 2024, 29, 278–288. [Google Scholar] [CrossRef]
- Goto, H.; Shiraishi, Y.; Okada, S. Recent preclinical and clinical advances in radioimmunotherapy for non-Hodgkin’s lymphoma. Explor. Target. Anti-Tumor Ther. 2024, 5, 208–224. [Google Scholar] [CrossRef]
- Hull, A.; Li, Y.; Bartholomeusz, D.; Hsieh, W.; Allen, B.; Bezak, E. Radioimmunotherapy of Pancreatic Ductal Adenocarcinoma: A Review of the Current Status of Literature. Cancers 2020, 12, 481. [Google Scholar] [CrossRef]
- Li, Y.; Marcu, L.G.; Hull, A.; Bezak, E. Radioimmunotherapy of glioblastoma multiforme—Current status and future prospects. Crit. Rev. Oncol./Hematol. 2021, 163, 103395. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell. Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Higgins, J.P.T. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed; Wiley-Blackwell: Hoboken, NJ, USA, 2019. [Google Scholar]
- Barker, T.H.; Stone, J.C.; Sears, K.; Klugar, M.; Tufanaru, C.; Leonardi-Bee, J.; Aromataris, E.; Munn, Z. The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid. Synth. 2023, 21, 494–506. [Google Scholar] [CrossRef]
- Akers, J.; Aguiar-Ibáñez, R.; Baba-Akbari, A. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care; University of New York, Center for Reviews and Dissemination: New York, NY, USA, 2009. [Google Scholar]
- Witzig, T.E.; Gordon, L.I.; Cabanillas, F.; Czuczman, M.S.; Emmanouilides, C.; Joyce, R.; Pohlman, B.L.; Bartlett, N.L.; Wiseman, G.A.; Padre, N.; et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J. Clin. Oncol. 2002, 20, 2453–2463. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Kaminski, M.S.; Leonard, J.P.; Hsu, F.J.; Wilkinson, M.; Zelenetz, A.; Wahl, R.L.; Kroll, S.; Coleman, M.; Goris, M.; et al. The radioisotope contributes significantly to the activity of radioimmunotherapy. Clin. Cancer Res. 2004, 10, 7792–7798. [Google Scholar] [CrossRef] [PubMed]
- Verheijen, R.H.; Massuger, L.F.; Benigno, B.B.; Epenetos, A.A.; Lopes, A.; Soper, J.T.; Markowska, J.; Vyzula, R.; Jobling, T.; Stamp, G.; et al. Phase III trial of intraperitoneal therapy with yttrium-90-labeled HMFG1 murine monoclonal antibody in patients with epithelial ovarian cancer after a surgically defined complete remission. J. Clin. Oncol. 2006, 24, 571–578. [Google Scholar] [CrossRef]
- Wygoda, Z.; Kula, D.; Bierzyńska-Macyszyn, G.; Larysz, D.; Jarzab, M.; Właszczuk, P.; Bazowski, P.; Wojtacha, M.; Rudnik, A.; Stepień, T.; et al. Use of monoclonal anti-EGFR antibody in the radioimmunotherapy of malignant gliomas in the context of EGFR expression in grade III and IV tumors. Hybridoma 2006, 25, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shen, Z.Y.; Chen, X.G.; Zhang, Q.; Bian, H.J.; Zhu, P.; Xu, H.Y.; Song, F.; Yang, X.M.; Mi, L.; et al. A randomized controlled trial of Licartin for preventing hepatoma recurrence after liver transplantation. Hepatology 2007, 45, 269–276. [Google Scholar] [CrossRef]
- Morschhauser, F.; Radford, J.; Van Hoof, A.; Vitolo, U.; Soubeyran, P.; Tilly, H.; Huijgens, P.C.; Kolstad, A.; D’Amore, F.; Diaz, M.G.; et al. Phase III trial of consolidation therapy with yttrium-90-ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. J. Clin. Oncol. 2008, 26, 5156–5164. [Google Scholar] [CrossRef]
- Goff, L.; Summers, K.; Iqbal, S.; Kuhlmann, J.; Kunz, M.; Louton, T.; Hagenbeek, A.; Morschhauser, F.; Putz, B.; Lister, A.; et al. Quantitative PCR analysis for Bcl-2/IgH in a phase III study of Yttrium-90 Ibritumomab Tiuxetan as consolidation of first remission in patients with follicular lymphoma. J. Clin. Oncol. 2009, 27, 6094–6100. [Google Scholar] [CrossRef]
- Sultana, A.; Shore, S.; Raraty, M.G.; Vinjamuri, S.; Evans, J.E.; Smith, C.T.; Lane, S.; Chauhan, S.; Bosonnet, L.; Garvey, C.; et al. Randomised Phase I/II trial assessing the safety and efficacy of radiolabelled anti-carcinoembryonic antigen I(131) KAb201 antibodies given intra-arterially or intravenously in patients with unresectable pancreatic adenocarcinoma. BMC Cancer 2009, 9, 66. [Google Scholar] [CrossRef]
- Shimoni, A.; Avivi, I.; Rowe, J.M.; Yeshurun, M.; Levi, I.; Or, R.; Patachenko, P.; Avigdor, A.; Zwas, T.; Nagler, A. A randomized study comparing yttrium-90 ibritumomab tiuxetan (Zevalin) and high-dose BEAM chemotherapy versus BEAM alone as the conditioning regimen before autologous stem cell transplantation in patients with aggressive lymphoma. Cancer 2012, 118, 4706–4714. [Google Scholar] [CrossRef]
- Press, O.W.; Unger, J.M.; Rimsza, L.M.; Friedberg, J.W.; LeBlanc, M.; Czuczman, M.S.; Kaminski, M.; Braziel, R.M.; Spier, C.; Gopal, A.K.; et al. Phase III randomized intergroup trial of CHOP plus rituximab compared with CHOP chemotherapy plus (131)iodine-tositumomab for previously untreated follicular non-Hodgkin lymphoma: SWOG S0016. J. Clin. Oncol. 2013, 31, 314–320. [Google Scholar] [CrossRef]
- Vose, J.M.; Carter, S.; Burns, L.J.; Ayala, E.; Press, O.W.; Moskowitz, C.H.; Stadtmauer, E.A.; Mineshi, S.; Ambinder, R.; Fenske, T.; et al. Phase III Randomized Study of Rituximab/Carmustine, Etopo-side, Cytarabine, and Melphalan (BEAM) Compared With Iodine-131 Tositumomab/BEAM With Autologous Hematopoietic Cell Transplantation for Relapsed Diffuse Large B-Cell Lymphoma: Results From the BMT CTN 0401 Trial. J. Clin. Oncol. 2013, 31, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Zheng, J.S.; Nan, G.; Li, R.; Chen, C.; Hu, C.X.; Zhang, Y.; Sun, B.; Wang, X.L.; Cui, S.C.; et al. Randomized trial of [131I] metuximab in treatment of hepatocellular carcinoma after percutaneous radiofrequency ablation. J. Natl. Cancer. Inst. 2014, 106, dju239. [Google Scholar] [CrossRef]
- Quackenbush, R.C.; Horner, T.J.; Williams, V.C.; Giampietro, P.; Lin, T.S. Patients with relapsed follicular lymphoma treated with rituximab versus tositumomab and iodine I-131 tositumomab. Leuk. Lymphoma 2015, 56, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Su, Z.; Zhang, W.; Luo, M.; Wang, H.; Huang, L. A randomized study comparing the effectiveness of microwave ablation radioimmunotherapy and postoperative adjuvant chemoradiation in the treatment of non-small cell lung cancer. J. Buon. 2016, 21, 326–332. [Google Scholar] [PubMed]
- Li, J.; Xing, J.; Yang, Y.; Liu, J.; Wang, W.; Xia, Y.; Yan, Z.; Wang, K.; Wu, D.; Wu, L.; et al. Adjuvant (131)I-metuximab for hepatocellular carcinoma after liver resection: A randomised, controlled, multicentre, open-label, phase 2 trial. Lancet. Gastroenterol. Hepatol. 2020, 5, 548–560. [Google Scholar] [CrossRef]
- López-Guillermo, A.; Canales, M.Á.; Dlouhy, I.; Mercadal, S.; Briones, J.; Martín García-Sancho, A.; Sancho, J.M.; Moraleda, J.M.; Terol, M.J.; Salar, A.; et al. A randomized phase II study comparing consolidation with a single dose of 90Y ibritumomab tiuxetan vs. maintenance with rituximab for two years in patients with newly diagnosed follicular lymphoma responding to R-CHOP. Long-term follow-up results. Leuk. Lymphoma 2022, 63, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, S.T.; Thomas, C.; Adra, N.; Zakharia, Y.; Philips, G.; Quinn, D.I.; Agarwal, N.; Nordquist, L.T.; Wulff-Burchfield, E.M.; Appleman, L.J.; et al. Randomized, double-blinded phase II study of ketoconazole (keto), hydrocortisone (HC), and anti-PSMA antibody J591 labeled with 177Lu or 111In in patients (pts) with high-risk non-metastatic (met) castration-resistant prostate cancer (M0 CRPC). J. Clin. Oncol. 2023, 41 (Suppl. S6), LBA21. [Google Scholar] [CrossRef]
- Gyurkocza, B.; Nath, R.; Seropian, S.; Choe, H.; Litzow, M.R.; Abboud, C.; Koshy, N.; Stiff, P.; Tomlinson, B.; Abhyankar, S.; et al. Randomized Phase III SIERRA Trial of <sup>131</sup>I-Apamistamab Before Allogeneic Hematopoietic Cell Transplantation Versus Conventional Care for Relapsed/Refractory AML. J. Clin. Oncol. 2024, 43, 201–213. [Google Scholar] [CrossRef]
- Ladetto, M.; Tavarozzi, R.; Zanni, M.; Evangelista, A.; Ferrero, S.; Tucci, A.; Botto, B.; Bolis, S.; Volpetti, S.; Zilioli, V.R.; et al. Radioimmunotherapy versus autologous hematopoietic stem cell transplantation in relapsed/refractory follicular lymphoma: A Fondazione Italiana Linfomi multicenter, randomized, phase III trial. Ann. Oncol. 2024, 35, 118–129. [Google Scholar] [CrossRef]
- Laoruangroj, C.; Atherton, P.J.; Wiseman, G.A.; Ansell, S.; Feldman, A.L.; Schumacher, P.; Witzig, T.E. The asymptomatic follicular lymphoma (AFL) trial: Single-agent rituximab immunotherapy versus 90Y-ibritumomab tiuxetan radioimmunotherapy (RIT) for patients with new, untreated follicular lymphoma. Leuk. Lymphoma 2024, 65, 333–338. [Google Scholar] [CrossRef]
- Gibson, A.D. Updated results of a Phase III trial comparing ibritumomab tiuxetan with rituximab in previously treated patients with non-Hodgkin’s lymphoma. Clin. Lymphoma 2002, 3, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.I.; Witzig, T.; Molina, A.; Czuczman, M.; Emmanouilides, C.; Joyce, R.; Vo, K.; Theuer, C.; Pohlman, B.; Bartlett, N.; et al. Yttrium 90-labeled ibritumomab tiuxetan radioimmunotherapy produces high response rates and durable remissions in patients with previously treated B-cell lymphoma. Clin. Lymphoma 2004, 5, 98–101. [Google Scholar] [CrossRef]
- Oei, A.L.; Verheijen, R.H.; Seiden, M.V.; Benigno, B.B.; Lopes, A.; Soper, J.T.; Epenetos, A.A.; Massuger, L.F. Decreased intraperitoneal disease recurrence in epithelial ovarian cancer patients receiving intraperitoneal consolidation treatment with yttrium-90-labeled murine HMFG1 without improvement in overall survival. Int. J. Cancer 2007, 120, 2710–2714. [Google Scholar] [CrossRef]
- Morschhauser, F.; Radford, J.; Van Hoof, A.; Botto, B.; Rohatiner, A.Z.; Salles, G.; Soubeyran, P.; Tilly, H.; Bischof-Delaloye, A.; van Putten, W.L.; et al. 90Yttrium-ibritumomab tiuxetan consolidation of first remission in advanced-stage follicular non-Hodgkin lymphoma: Updated results after a median follow-up of 7.3 years from the International, Randomized, Phase III First-LineIndolent trial. J. Clin. Oncol. 2013, 31, 1977–1983. [Google Scholar] [CrossRef]
- Shadman, M.; Li, H.; Rimsza, L.; Leonard, J.P.; Kaminski, M.S.; Braziel, R.M.; Spier, C.M.; Gopal, A.K.; Maloney, D.G.; Cheson, B.D.; et al. Continued Excellent Outcomes in Previously Untreated Patients With Follicular Lymphoma After Treatment With CHOP Plus Rituximab or CHOP Plus 131I-Tositumomab: Long-Term Follow-Up of Phase III Randomized Study SWOG-S0016. J. Clin. Oncol. 2018, 36, 697–703. [Google Scholar] [CrossRef]
- Sharkey, R.M.; Goldenberg, D.M. Cancer Radioimmunotherapy. Immunotherapy 2011, 3, 349–370. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H. Radioimmunotherapy: A specific treatment protocol for cancer by cytotoxic radioisotopes conjugated to antibodies. ScientificWorldJournal 2014, 2014, 492061. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, W.R.; Gupta, D.; Mahapatra, A.K.; Gopal, S.; Judy, K.; Patel, S.J.; Shan, J. Open-label, dose confirmation study of interstitial 131I-chTNT-1/b mab for the treatment of glioblastoma multiforme (GBM) at first relapse: Interim results. J. Clin. Oncol. 2011, 29 (Suppl. S15), 2035. [Google Scholar] [CrossRef]
- Shen, S.; Lustig, R.; Judy, K.; Shapiro, W.; Spicer, K.; Patel, S.; Fiveash, J.; Lai, J.; Shan, J. Dosimetry of phase I interstitial 131I-chTNT-1/B MAb (Cotara) for the treatment of recurrent glioma. J. Nucl. Med. 2009, 50, 445. [Google Scholar]
- Zhao, M.; Fu, X.; Zhang, Z.; Li, A.; Wang, X.; Li, X. Intracranial 131I-chTNT Brachytherapy in Patients with Deep-Seated Glioma: A Single-center Experience with 10-Year Follow-up from China. Nuklearmedizin 2021, 60, 283–288. [Google Scholar] [CrossRef]
- Euctr, P.L. Radioimmunotherapy with 90Y-Clivatizumab Tetraxetan Plus Low Dose Gemcitabine Versus Placebo Plus Low-Dose Gemcitabine. 2015. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2013-004516-21-PL (accessed on 17 September 2023).
- Li, L.; Quang, T.S.; Gracely, E.J.; Kim, J.H.; Emrich, J.G.; Yaeger, T.E.; Jenrette, J.M.; Cohen, S.C.; Black, P.; Brady, L.W. A Phase II study of anti-epidermal growth factor receptor radioimmunotherapy in the treatment of glioblastoma multiforme. J. Neurosurg. 2010, 113, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; de Bono, J.; Chi Kim, N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa Scott, T.; Nordquist Luke, T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Mody, V.V.; Singh, A.N.; Deshmukh, R.; Shah, S. Chapter 40—Thyroid Hormones, Iodine and Iodides, and Antithyroid Drugs. In Side Effects of Drugs Annual; Ray, S.D., Ed.; Elsevier: New York, NY, USA, 2015; Volume 37, pp. 513–519. [Google Scholar]
- Leonard, J.P.; Siegel, J.A.; Goldsmith, S.J. Comparative physical and pharmacologic characteristics of iodine-131 and yttrium-90: Implications for radioimmunotherapy for patients with non-Hodgkin’s lymphoma. Cancer Investig. 2003, 21, 241–252. [Google Scholar] [CrossRef] [PubMed]

| Study (Year) | Country | Sample Size (I/C) (n) | Gender Age (I/C) | Clinical Phase | Cancer Treated (Type) | Cancer Type | RIT Used | Antigen Targeted |
|---|---|---|---|---|---|---|---|---|
| Witzig et al. (2002) [13] | USA | 143 (73/70) | Both genders Median: 60/57 | III | NHL (Stage III/IV) | Non-solid | 90Y-ibritumomab tiuxetan | CD20 |
| Davis et al. (2004) [14] | USA | 78 (42/36) | Both genders Median: 56/55 | II | NHL (Low-grade or transformed low-grade) | Non-solid | 131I-tositumomab | CD20 |
| Verheijen et al. (2006) [15] | The Netherlands | 447 (224/223) | Female Median: 54.5/53.7 | III | EOC (Stage ≥ Ic a) | Solid | 90Y-muHMFG1 | MUC1 |
| Wygoda et al. (2006) [16] | Poland | 18 (8/10) | Both genders Median: 47/52 | N/A | Gliomas (Grade III/IV) | Solid | 125I-MAb425 | EGFR |
| Xu et al. (2007) [17] | China | 60 (30/30) | Both genders Median: 44.5/42.5 | N/A | HCC (Stage III/IV) | Solid | 131I-metuximab | CD147/HAb18G |
| Morschhauser et al. (2008) [18] | Germany | 414 (208/206) | Both genders Median: 55/53 | III | FL (Stage III/IV) | Non-solid | 90Y-ibritumomab tiuxetan | CD20 |
| Goff et al. (2009) [19] | United Kingdom | 186 (90/96) | Both genders Median: 54/51 | III | FL (Stage III/IV) | Non-solid | 90Y-ibritumomab tiuxetan | CD20 |
| Sultana et al. (2009) [20] | United Kingdom | 19 (10/9) | Both genders Median: 59/60 | I/II | Pancreatic adenocarcinoma (Stage IVa/IVb) | Solid | 131I-Kab201 | CEA |
| Shimoni et al. (2012) [21] | Israel | 43 (22/21) | Both genders Median: 58/51 | III | NHL (High-grade) | Non-solid | 90Y-ibritumomab tiuxetan | CD20 |
| Press et al. (2013) [22] | USA | 532(265/267) | Both genders Median: 53.3/54.5 | III | FL (Stage II, III, or IV) | Non-solid | 131I-tositumomab | CD20 |
| Vose et al. (2013) [23] | USA | 224 (111/113) | Both genders Median: 56.8/58.5 | III | DLBCL | Non-solid | 131I-tositumomab | CD20 |
| Bian et al. (2014) [24] | China | 127 (62/65) | Both genders Median: 57/57 | IV | HCC (Stage 0-B b) | Solid | 131I-metuximab | CD147/HAb18G |
| Quackenbush et al. (2015) [25] | USA | 14 (8/6) | Both genders Mean: 53.1/58.2 | III | FL | Non-solid | 131I-tositumomab | CD20 |
| Zhao et al. (2016) [26] | China | 96 (47/49) | Both genders Mean: 57 | N/A | NSCLC (Stage II and IIIa) | Solid | 131I-chTNT | DNA/Histone H1 |
| Li et al. (2020) [27] | China | 156 (78/78) | Both genders Median: 53/53 | II | HCC | Solid | 131I-metuximab | CD147/HAb18G |
| López-Guillermo et al. (2022) [28] | Spain | 126 (64/62) | Both genders Median: 52/53 | II | FL (Stages II, III, or IV) | Non-solid | 90Y-ibritumomab tiuxetan | CD20 |
| Tagawa et al. (2023) [29] | USA | 55 (38/17) | Male Median: 68 | II | Prostate cancer | Solid | 177Lu-J591 | PSMA |
| Gyurkocza et al. (2024) [30] | USA | 153 (76/77) | Both genders Median: 64/66 | III | AML | Non-solid | 131I-apamistamab | CD45 |
| Ladetto et al. (2024) [31] | Italy | 141 (71/70) | Both genders Median: 56/58 | III | FL (Stage III/IV) | Non-solid | 90Y-ibritumomab tiuxetan | CD20 |
| Laoruangroj et al. (2024) [32] | USA | 20 (10/10) | Both genders Median: 59/61 | III | FL (Stages I, II, III, IV) | Non-solid | 90Y-ibritumomab tiuxetan | CD20 |
| Clinical Trial/Study (Year) | Key Treatment Outcomes [Intervention (I) Versus Control (C)] | Key Findings | RIT Treatment with Other Therapy |
|---|---|---|---|
| Witzig et al. (2002) [13] | Median TTP(ITT):11.2 vs. 10.1 months (p = 0.173). | 90Y-ibritumomab tiuxetan was well tolerated and significantly improved ORR and CR vs. rituximab alone. | Rituximab |
| Gibson, A. D. (2002) [33] | Median TTP (ITT): 10.6 vs. 10.1 months (p = 0.425) | 90Y-ibritumomab tiuxetan is effective for R/R low-grade or follicular NHL vs. rituximab. | Rituximab |
| Gordon et al. (2004) [34] | Median TTP (ITT): 10.6 vs. 10.1 months (p = 0.41) | RIT with 90Y-ibritumomab tiuxetan is effective in follicular NHL, especially for patients achieving CR. | Rituximab |
| Davis et al. (2004) [14] | Median PFS: 6.3 vs. 5.5 months (p = 0.016) | Combination of 131I and tositumomab significantly improved OR, CR, and TTP in relapsed NHL. | None |
| Verheijen et al. (2006) [15] | RR of death (ITT): 1.159 (p = 0.4033); 31.3% vs. 27.4% deaths | A single intrapleural dose of 90Y-muHMFG1 did not prolong survival or time to relapse in EOC patients. | Chemotherapy |
| Oei et al. (2007) [35] | Intraperitoneal relapse-free survival (HR = 0.31; p = 0.002) | No survival benefit for intraperitoneal RIT as consolidation treatment in EOC. | Chemotherapy |
| Wygoda et al. (2006) [16] | Median OS: 14 months; no significant OS/DFS difference (p = 0.23) | Concomitant radiotherapy and RIT (with anti-EGFR 125I-Mab 425) was superior to radiotherapy alone in high-grade gliomas. | Teleradiotherapy |
| Xu et al. (2007) [17] | Median follow-up:12.3 (range = 2 to 13, mean = 10.99) months. 3-month OS: 100% vs. 93.1%; 6-month OS: 96.7% vs. 75.9%; 9-month OS: 90% vs. 69.0% (I); 12-month OS: 82.5% vs. 61.9% (p = 0.0289). | 131I-metuximab after liver transplantation was well tolerated, reduced recurrence, and prolonged survival in HCC. | OLT |
| Morschhauser et al. (2008) [18] | Median PFS: 36.5 vs. 13.3 months (HR = 0.465; p < 0.0001) | Consolidation with 90Y-ibritumomab tiuxetan after first-line therapy significantly prolonged 2-year PFS. | Rituximab |
| Morschhauser et al. (2013) [36] | Median PFS: 4.1 vs. 1.1 years (HR = 0.47; p < 0.001 | First-line consolidation therapy with 90Y-ibritumomab is valuable for patients with advanced FL, providing a durable PFS benefit. | Rituximab |
| Goff et al. (2009) [19] | Median PFS: 3 years vs. 13 months (p < 0.0001; HR = 0.465) | 90Y-ibritumomab consolidation deepened molecular response and prolonged PFS. | Rituximab |
| Sultana et al. (2009) [20] | Median OS: 5.2 months; OS difference not significant (p = 0.79) | 131I-Kab201 monotherapy was comparable to gemcitabine; combination therapy might improve survival. | N/A |
| Shimoni et al. (2012) [21] | 2-yr PFS: 48%; 2-yr OS: 77% overall; 91% vs. 62% (p = 0.05) | Adding 90Y-ibritumomab tiuxetan to BEAM chemotherapy may improve outcomes in ASCT conditioning. | BEAM, rituximab, ASCT |
| Press et al. (2013) [22] | 2-/5-yr PFS: 80%/66% vs. 76%/60% (p = 0.11) | No clear PFS benefit of CHOP-RIT over R-CHOP; both arms had excellent PFS/OS. | CHOP |
| Shadman et al. (2018) [37] | 10-yr PFS: 56% vs. 42% (p = 0.011); 10-yr OS: 75% vs. 81% (p = 0.12) | CHOP-RIT extended PFS but did not improve OS vs. CHOP-R alone. | CHOP |
| Vose et al. (2013) [23] | 2-yr PFS: ~48% vs. ~48% (p = 0.94); 2-yr OS: ~66% vs. ~61% (p = 0.38) | Adding RIT to AHCT did not show additional benefit in relapsed DLBCL. | BEAM |
| Bian et al. (2014) [24] | 1-/2-yr OS: 93.5%/84.7% vs. 90.1%/76.4% (HR = 0.66; p = 0.30) | 131I-metuximab post-RFA reduces recurrence in HCC; CD147-targeted strategy shows promise. | RFA |
| Quackenbush et al. (2015) [25] | Median PFS: NR vs. 9 months (p = 0.0705); OS: all vs. 3/6 alive (p = 0.0272) | 131I-tositumomab provided durable clinical benefit in relapsed FL with selected patients. | Tositumomab |
| Zhao et al. (2016) [26] | 1-/2-yr OS: ~83%/53% vs. ~80%/49% (p > 0.05); median survival: 29.1 vs. 23.0 months (p < 0.05) | 131I-chTNT RIT + PMCT improved survival in NSCLC, with efficacy comparable to adjuvant chemotherapy. | PMCT, follow-up chemotherapy |
| Li et al. (2020) [27] | 5-yr RFS: 43.4% vs. 21.7% (p < 0.0001); 5-yr OS: 61.3% vs. 35.9% (p < 0.0001) | 131I-metuximab adjuvant therapy significantly improved RFS/OS in CD147+ HCC after hepatectomy. | Hepatectomy |
| López-Guillermo et al. (2022) [28] | 10-yr PFS: 50% vs. 56% (p = 0.19); OS: 78% vs. 84.5% | In FL responding to R-CHOP, RIT did not significantly differ in PFS/OS, with potential late toxicities. | R-CHOP |
| Tagawa et al. (2023) [29] | Median MFS: 23.8 vs. 20.8 months; 18-mo MFS: 50% vs. 24% (p = 0.066) | 177Lu-J591 (anti-PSMA) showed longer MFS vs. ketone/HC in prostate cancer, though best radionuclide remains unclear. | Ketoconazole |
| Gyurkocza et al. (2024) [30] | Median OS: ~6.3 vs. ~5.9 months (p = 0.59); Median EFS: 3.2 vs. 0 month (p < 0.0001) | 131I-apamistamab showed higher durable CR in elderly R/R AML vs. standard care, addressing an unmet need. | alloHCT |
| Ladetto et al. (2024) [31] | Median PFS: 78 vs. 62 months (HR = 1.11, p = 0.6662); OS not reached in both arms | ASCT offered no advantage over RIT; RIT consolidation yields excellent disease control with less toxicity. | Rituximab, immunochemotherapy |
| Laoruangroj et al. (2024) [32] | Median PFS: ~29.9 months vs. NR (p = 0.431) | Both rituximab alone and rituximab + single-dose RIT were highly effective in asymptomatic FL. | Rituximab |
| Clinical Trial/Study (Year) | Comparator Treatment | RIT Treatment with Other Therapy | Outcome |
|---|---|---|---|
| Witzig et al. (2002) [13] | Rituximab alone | Rituximab | RIT combination therapy was superior to rituximab alone |
| Davis et al. (2004) [14] | Unlabeled tositumomab | None | Radiolabelled tositumomab improved treatment outcome |
| Verheijen et al. (2006) [15] | Chemotherapy alone | Chemotherapy | RIT combination therapy was similar to chemotherapy alone |
| Wygoda et al. (2006) [16] | Teleradiotherapy alone | Teleradiotherapy | RIT combination therapy was not superior to teleradiotherapy alone |
| Xu et al. (2007) [17] | Placebo (physiological saline) | OLT | RIT combination therapy was superior to placebo |
| Morschhauser et al. (2008) [18] | No consolidation treatment a | Rituximab | RIT combination therapy was superior to no consolidation therapy |
| Goff et al. (2009) [19] | No consolidation treatment a | Rituximab | RIT combination therapy was superior to no consolidation therapy |
| Sultana et al. (2009) [20] | N/A (compare different routes of RIT administration) | N/A | N/A |
| Shimoni et al. (2012) [21] | BEAM alone | BEAM, rituximab, ASCT | RIT combination therapy was superior to BEAM alone |
| Press et al. (2013) [22] | R-CHOP | CHOP | RIT combination therapy was similar to R-CHOP |
| Vose et al. (2013) [23] | R-BEAM | BEAM | RIT combination therapy was not superior to R-BEAM |
| Bian et al. (2014) [24] | RFA alone | RFA | RIT combination therapy was superior to RFA alone |
| Quackenbush et al. (2015) [25] | Rituximab alone | Tositumomab | RIT combination therapy was superior to rituximab alone |
| Zhao et al. (2016) [26] | Surgery, chemotherapy, radiotherapy | PMCT, follow-up chemotherapy | RIT combination therapy was superior to surgery, chemotherapy, and radiotherapy |
| Li et al. (2020) [27] | No adjuvant treatment a | Hepatectomy | RIT combination therapy was superior to no adjuvant treatment |
| López-Guillermo et al. (2022) [28] | Rituximab alone | R-CHOP | No significant difference between RIT combination therapy and rituximab alone |
| Tagawa et al. (2023) [29] | 111In-J591, ketoconazole | Ketoconazole | RIT combination therapy prolonged survival but with more toxicity |
| Gyurkocza et al. (2024) [30] | Salvage therapy followed by standard-of-care alloHCT | alloHCT | RIT combination therapy was superior to standard-of-care alloHCT |
| Ladetto et al. (2024) [31] | ASCT | Rituximab, immunochemotherapy | RIT combination therapy was superior to ASCT |
| Laoruangroj et al. (2024) [32] | Rituximab alone | Rituximab | No significant difference between RIT combination therapy and rituximab alone |
| Clinical Trial/Study (Year) | Nonhaematological AEs | Haematological AEs |
|---|---|---|
| Witzig et al. (2002) [13] a | Grades 1/2 (Intervention/Control): - Cough: 15%/7%; - Dyspnea: 15%/7%; - Nausea: 43%/19% - Vomiting: 19%/7%; - Anorexia: 11%/3% | Grade 3/4 (Intervention only): - ANC: 57%; - Platelets: 60% MDS: one case |
| Davis et al. (2004) [14] a | (Intervention/Control): - Nausea: 48%/17%; – Rash: 31%/14%; - Chills: 24%/19% - Pain: 21%/28% (also overall drug-related AEs: 100% vs. 89% [all grades], 71% vs. 31% [grade 3/4], serious: 33% vs. 14%) | Grade 3/4 (Intervention/Control): - ANC: 33%/8%; - Platelets: 33%/0% MDS/AML (Intervention): three cases (5%) |
| Verheijen et al. (2006) [15] a | (Intervention/Control): - Nausea: 40%/19%; - Fatigue: 34%/21%; - Arthralgia: 31%/19% - Myalgia: 25%/7%; - Abdominal pain: 25%/16% - Rash: 17%/5%; – Diarrhoea: 17%/8%; - Vomiting: 17%/8% | Grade 3/4: - Thrombocytopenia (Intervention only): 24.3% |
| Wygoda et al. (2006) [16] | Not Reported | Not Reported |
| Xu et al. (2007) [17] | Not Reported | Not Reported |
| Morschhauser et al. (2008) [18] a | Grade 1/2 (Intervention only): - Fatigue: 32.8%; - Nasopharyngitis: 19.1% - Nausea: 18.1%; - Asthenia: 14.2%; - Arthralgia: 11.8%; - Cough: 11.3%; - Headache: 11.3%; - Diarrhoea: 10.8%; - Pyrexia: 10.3% Grade 3/4 (Intervention/Control): - Infections: 7.9%/2.4%; - Pyrexia: 3%/0% - Hypertension: 2.9%/0.5% | Grade 3/4 (Intervention/Control): - Lymphopenia (60.3%/10.8%); - Neutropenia (66.7%/2.5%); - Thrombocytopenia (60.8%/0%); - Anaemia (3.4%/0%). AML (Intervention): one case |
| Goff et al. (2009) [19] | N/A | N/A |
| Sultana et al. (2009) [20] a | Not reported | Grade 3/4 drug-related: - Lymphopenia (n = 5); - Thrombocytopenia (n = 6) - Leukopenia (n = 4); - Neutropenia (n = 3) |
| Shimoni et al. (2012) [21] | Organ toxicity (≥Grade 3): - Mucositis: 15 (I) vs. 9 (C) - Pneumonia/fungal infection: 6 (I) vs. 1 (C) | Not reported |
| Press et al. (2013) [22] | Cardiovascular events (Grade 3–5): 3% (I) vs. 7% (C) | (Intervention/Control): - Thrombocytopenia: 18% vs. 2% - Febrile neutropenia: 10% vs. 16% - AML/MDS: 3% vs. 1% |
| Vose et al. (2013) [23] b | Any Grade 3–5 nonhaematological toxicity: 65% (I) vs. 43% (C) - Mucositis: 52% (I) vs. 18% (C) | MDS/AML: two cases (I) vs. one case (C) |
| Bian et al. (2014) [24] b | (Intervention/Control): - Pleural effusion: 20.0% vs. 9.4% - Increased ALT: 28.3% vs. 42.2% - Increased AST: 26.7% vs. 43.8% | (Intervention/Control): - Decreased WBC: 66.7% vs. 50.0% - Grade 3 decreased platelet count: 18.3% vs. 7.8% |
| Quackenbush et al. (2015) [25] b | Intervention: headache, nausea, vomiting, cough Control: pyrexia, infusion-related reactions | Intervention: - Neutropenia (n = 1), thrombocytopenia (n = 3), leukopenia (n = 4), lymphopenia (n = 4) Control: none reported (Serious AEs: four cases per arm) |
| Zhao et al. (2016) [26] | Radioactive esophagitis: 4.25% (I) vs. 20.4% (C) | Myelotoxicity: 14.89% (I) vs. 8.16% (C) |
| Li et al. (2020) [27] b | - Fever: 3%; - Fatigue: 3%; - Nausea/vomiting: 1% - Increased bilirubin/ALT/AST/ALP: 1% | Decreased WBC/platelet counts: 2% (Intervention) |
| López-Guillermo et al. (2022) [28] | (Intervention/Control): - Infectious complications: 2% vs. 13% - Second neoplasms: 14% vs. 3% | (Intervention/Control): - Neutropenia: 9% vs. 3% - Thrombocytopenia: 8% vs. 0% (p = 0.055) - MDS/AML/HL: 9% vs. 0% (p = 0.028) |
| Tagawa et al. (2023) [29] | (Intervention/Control): - Abdominal pain: 0% vs. 11%; - Increased ALT: 3.3% vs. 2% - Diarrhoea: 0% vs. 22%; - All-cause mortality: 34.21% vs. 29.41%; - Serious AEs: 5.26% vs. 0% | (Intervention/Control): - Neutropenia: 57% vs. 11% - Thrombocytopenia: 77% vs. 11% |
| Gyurkocza et al. (2024) [30] b | Not separately detailed | Febrile neutropenia: 18.1% (I) vs. 22.4% (C) - Treatment-related deaths: 4.2% (I) vs. 5.3% (C) (Serious AEs: 30.6% vs. 31.6%) |
| Ladetto et al. (2024) [31] b | Consolidation: - Nonhaematological AEs: 5% (I) vs. 37% (C) (mostly GI disorders) Maintenance: N/A | Consolidation: - Haematological AEs: 46% (I) vs. 93% (C) (p < 0.0001) Maintenance: - Haematological AEs: 13% (I) vs. 25% (C) (p = 0.168) - Secondary MDS/AML: two cases (I) vs. three cases (C) |
| Laoruangroj et al. (2024) [32] b | Not reported | Grade 3/4 haematological AEs: - Leukopenia: 40% (I) vs. 0% (C); - Neutropenia: 40% (I) vs. 0% (C) - Thrombocytopenia: 40% (I) vs. 0% (C) - Anaemia: 10% (I) vs. 0% (C) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Dahal, P.K.; Mosharaf, P.; Shahjalal, M.; Mahumud, R.A. Assessing the Clinical Effectiveness of Radioimmunotherapy with Combined Radionuclide/Monoclonal Antibody Conjugates in Cancer Treatment: Insights from Randomised Clinical Trials. Cancers 2025, 17, 1413. https://doi.org/10.3390/cancers17091413
Chen Y, Dahal PK, Mosharaf P, Shahjalal M, Mahumud RA. Assessing the Clinical Effectiveness of Radioimmunotherapy with Combined Radionuclide/Monoclonal Antibody Conjugates in Cancer Treatment: Insights from Randomised Clinical Trials. Cancers. 2025; 17(9):1413. https://doi.org/10.3390/cancers17091413
Chicago/Turabian StyleChen, Yifu, Padam Kanta Dahal, Parvez Mosharaf, Md. Shahjalal, and Rashidul Alam Mahumud. 2025. "Assessing the Clinical Effectiveness of Radioimmunotherapy with Combined Radionuclide/Monoclonal Antibody Conjugates in Cancer Treatment: Insights from Randomised Clinical Trials" Cancers 17, no. 9: 1413. https://doi.org/10.3390/cancers17091413
APA StyleChen, Y., Dahal, P. K., Mosharaf, P., Shahjalal, M., & Mahumud, R. A. (2025). Assessing the Clinical Effectiveness of Radioimmunotherapy with Combined Radionuclide/Monoclonal Antibody Conjugates in Cancer Treatment: Insights from Randomised Clinical Trials. Cancers, 17(9), 1413. https://doi.org/10.3390/cancers17091413








