Surgical Outcomes After Neoadjuvant Chemo-Immunotherapy for Stage III NSCLC: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
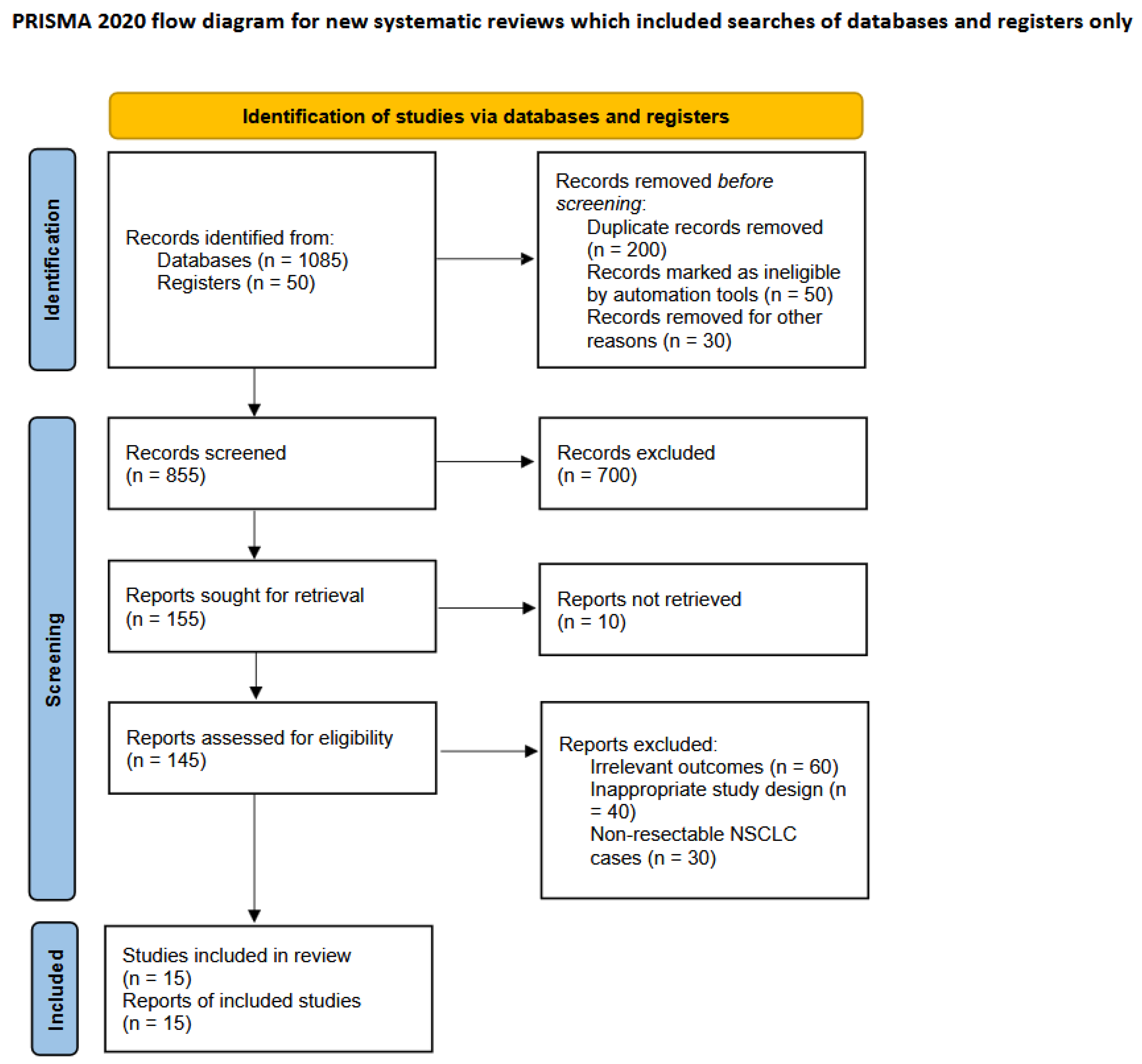
2. Material and Methods
Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, Z.; Wu, Z.; Qin, Y.; Zhao, Y.; Xuan, Y.; Qiu, T.; Liu, A.; Dong, Y.; Su, W.; Du, W.; et al. Perioperative safety and feasibility outcomes of stage IIIA-N2 non-small cell lung cancer following neoadjuvant immunotherapy or neoadjuvant chemotherapy: A retrospective study. Ann. Transl. Med. 2021, 9, 685. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Provencio, M.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal-Rubio, J.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; Carpeño, J.D.C.; et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Huang, J.; Jiang, S.; Rong, W.; Shen, Y.; Li, C.; Tian, Y.; Ning, J.; Chen, X.; Yang, Y.; et al. The surgical perspective in neoadjuvant immunotherapy for resectable non-small cell lung cancer. Cancer Immunol. Immunother. 2021, 70, 2313–2321. [Google Scholar] [CrossRef] [PubMed]
- Tong, B.C.; Gu, L.; Wang, X.; Wigle, D.A.; Phillips, J.D.; Harpole, D.H.; Klapper, J.A.; Sporn, T.; Ready, N.E.; D’Amico, T.A. Perioperative outcomes of pulmonary resection after neoadjuvant pembrolizumab in patients with non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2022, 163, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Li, N.; Gao, S.; Xue, Q.; Ying, J.; Wang, S.; Tao, X.; Zhao, J.; Mao, Y.; Wang, B.; et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J. Thorac. Oncol. 2020, 15, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Mynard, N.; Nasar, A.; Villena-Vargas, J.; Chow, O.; Harrison, S.; Stiles, B.; Port, J.; Altorki, N. Surgical resection after neoadjuvant durvalumab and radiation is feasible and safe in non-small cell lung cancer: Results from a randomized trial. J. Thorac. Cardiovasc. Surg. 2023, 165, 327–334.e2. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.K.; Torri, V.; Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998, 17, 2815–2834. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. he PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Wang, J.; Wu, J.; Chen, S.; Li, J.; Liu, J.; Chen, Q.; Jiang, Y. Neoadjuvant pembrolizumab with chemotherapy for the treatment of stage IIB-IIIB resectable lung squamous cell carcinoma. J. Thorac. Dis. 2021, 13, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.A.; Gainor, J.F.; Awad, M.M.; Chiuzan, C.; Grigg, C.M.; Pabani, A.; Garofano, R.F.; Stoopler, M.B.; Cheng, S.K.; White, A.; et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Bott, M.J.; Cools-Lartigue, J.; Tan, K.S.; Dycoco, J.; Bains, M.S.; Downey, R.J.; Huang, J.; Isbell, J.M.; Molena, D.; Park, B.J.; et al. Safety and Feasibility of Lung Resection After Immunotherapy for Metastatic or Unresectable Tumors. Ann. Thorac. Surg. 2018, 106, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-F.J.; McSherry, F.; Mayne, N.R.; Wang, X.; Berry, M.F.; Tong, B.; Harpole, D.H.; D’amico, T.A.; Christensen, J.D.; Ready, N.E.; et al. Surgical Outcomes After Neoadjuvant Chemotherapy and Ipilimumab for Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2018, 105, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ning, J.; Campisi, A.; Dell’amore, A.; Ciarrocchi, A.P.; Li, Z.; Song, L.; Huang, J.; Yang, Y.; Stella, F.; et al. Neoadjuvant PD-1 Inhibitors and Chemotherapy for Locally Advanced NSCLC: A Retrospective Study. Ann. Thorac. Surg. 2022, 113, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yan, B.; Xu, F.; Hui, Z.; Zhao, G.; Liu, J.; Zhang, H.; Zeng, Z.; Zhang, R.; Provencio, M.; et al. Neoadjuvant chemoimmunotherapy in resectable stage IIIA/IIIB non-small cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 2193–2204. [Google Scholar] [CrossRef] [PubMed]
- Altorki, N.K.; McGraw, T.E.; Borczuk, A.C.; Saxena, A.; Port, J.L.; Stiles, B.M.; Lee, B.E.; Sanfilippo, N.J.; Scheff, R.J.; Pua, B.B.; et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: A single-centre, randomised phase 2 trial. Lancet Oncol. 2021, 22, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Provencio, M.; Serna-Blasco, R.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Rubio, J.C.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; et al. Overall Survival and Biomarker Analysis of Neoadjuvant Nivolumab Plus Chemotherapy in Operable Stage IIIA Non-Small-Cell Lung Cancer (NADIM phase II trial). J. Clin. Oncol. 2022, 40, 2924–2933. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Tang, D.; Gao, W.; Zhang, H. Neoadjuvant Immuno-Chemotherapy: A New Perspective for Stage III NSCLC? Front. Surg. 2022, 9, 843987. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhuang, W.; Xu, C.; Jin, K.; Chen, B.; Tian, D.; Hiley, C.; Onishi, H.; Zhu, C.; Qiao, G. Surgery or Non-surgical Treatment of ≤8 mm Non-small Cell Lung Cancer: A Population-Based Study. Front. Surg. 2021, 8, 632561. [Google Scholar] [CrossRef] [PubMed]
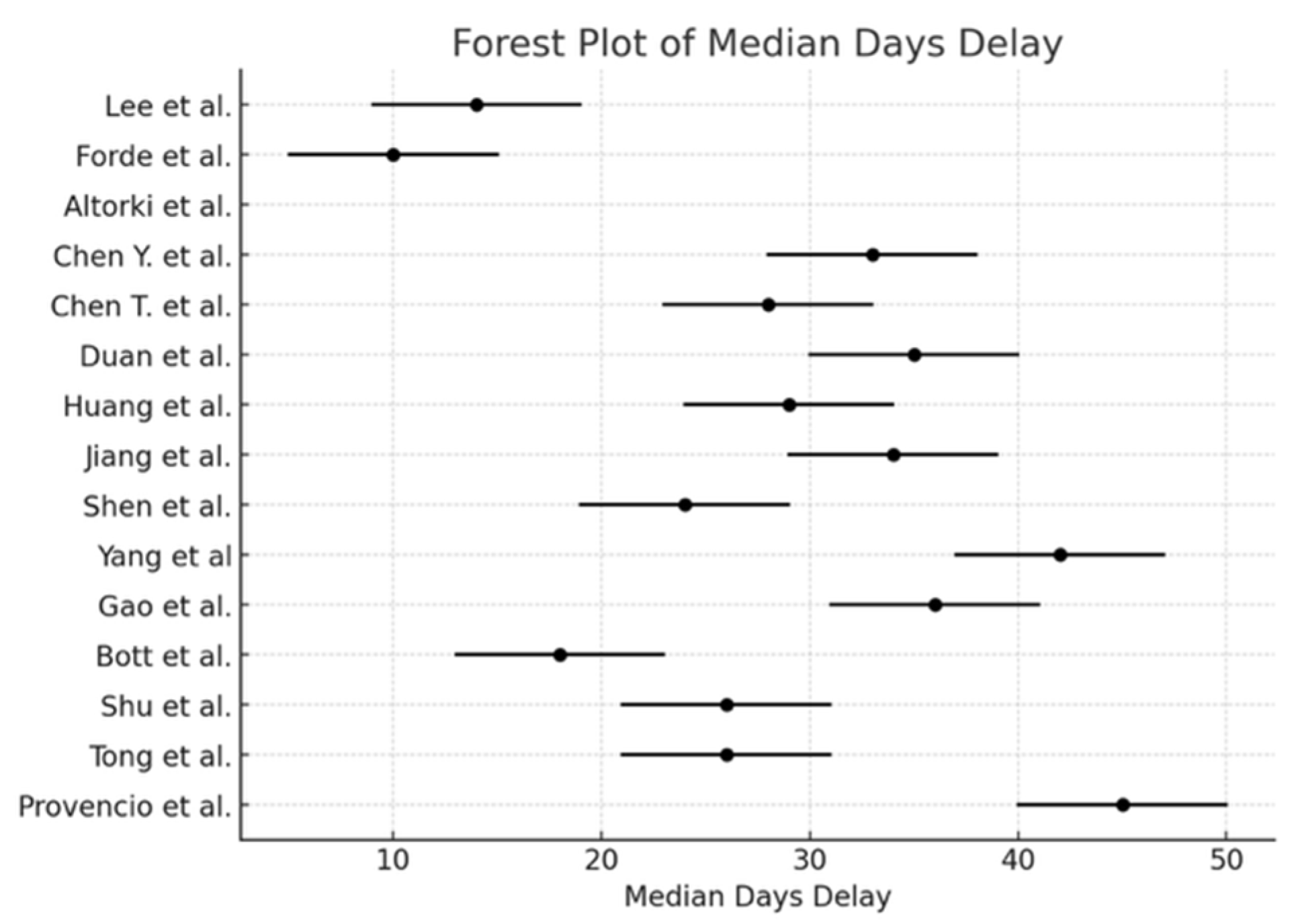
| Study | Reference | Lobectomy | Bilobectomy | Sleeve Lobectomy | Wedge | Pneumonectomy | Other | Exploratory Thoracotomy | Minimally-Invasive | Conversion to Open | R0 | R1 | R2 | Median Days Delay (n) | Time (min) | Blood Loss (mL) | Transfusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Huang et al. | [1] | 19 | 3 | - | - | 1 | 1 | 0 | 24 | - | 23 | 1 | 0 | 29 | 196 | 92 | 2 |
| Forde et al. | [2] | 115 | 3 | 2 | - | 25 | 24 | - | 44 | 17 | 124 | 16 | 5 | 10 | 185 | - | - |
| Jiang et al. | [4] | 18 | 4 | 7 | 0 | 2 | 0 | - | 9 | 1 | 24 | 4 | 3 | 34 | 158 | 200 | 2 |
| Tong et al. | [5] | 18 | 1 | 2 | - | 3 | 1 | - | 23 | 5 | 22 | 3 | 0 | 26 | 309 | - | 2 |
| Gao et al. | [6] | 18 | 5 | 1 | 0 | 13 | - | 2 | 29 | 10 | 36 | 0 | 1 | 36 | - | - | - |
| Lee et al. | [7] | 37 | 5 | 1 | - | 9 | - | - | 33 | 3 | 48 | 3 | 1 | 14 | 137 | 275 | - |
| Shen et al. | [10] | 22 | 7 | 6 | - | 2 | - | - | 25 | - | 37 | 0 | 0 | 24 | 184 | - | 2 |
| Shu et al. | [11] | 19 | 4 | 0 | 0 | 3 | - | 3 | 14 | 12 | 26 | - | - | 26 | - | - | - |
| Provencio et al. | [3] | 32 | 3 | 3 | 0 | 3 | - | 0 | 24 | 17 | 41 | 0 | 0 | 45 | - | 195 | - |
| Bott et al. | [12] | 15 | 1 | 1 | 1 | 2 | - | - | 14 | 7 | 20 | - | - | 18 | 228 | 100 | 0 |
| Yang et al. | [13] | 10 | 1 | 0 | 1 | 1 | - | - | 4 | 3 | 13 | 0 | 0 | 42 | - | - | 2 |
| Antonia et al. | [14] | 11 | 2 | 5 | 0 | 2 | - | 0 | 14 | 2 | 19 | 1 | 0 | 35 | 250 | 212 | - |
| Chen T. et al. | [15] | 8 | 1 | 3 | 0 | 0 | - | - | 9 | 3 | 12 | 0 | 0 | 28 | 140 | 200 | - |
| Chen Y. et al. | [16] | 9 | 9 | 14 | - | 3 | - | - | 1 | - | 35 | 0 | 0 | 33 | 0 | - | - |
| Altorki et al. | [17] | 38 | 5 | - | - | 9 | - | 4 | 35 | - | 48 | 1 | 3 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardoni, C.; Chiari, M.; Bertolaccini, L.; Diotti, C.; De Fabiani, A.; Nicolosi, G.; Mazzella, A.; Casiraghi, M.; Spaggiari, L. Surgical Outcomes After Neoadjuvant Chemo-Immunotherapy for Stage III NSCLC: A Systematic Review and Meta-Analysis. Cancers 2025, 17, 1426. https://doi.org/10.3390/cancers17091426
Bardoni C, Chiari M, Bertolaccini L, Diotti C, De Fabiani A, Nicolosi G, Mazzella A, Casiraghi M, Spaggiari L. Surgical Outcomes After Neoadjuvant Chemo-Immunotherapy for Stage III NSCLC: A Systematic Review and Meta-Analysis. Cancers. 2025; 17(9):1426. https://doi.org/10.3390/cancers17091426
Chicago/Turabian StyleBardoni, Claudia, Matteo Chiari, Luca Bertolaccini, Cristina Diotti, Alessia De Fabiani, Giuseppe Nicolosi, Antonio Mazzella, Monica Casiraghi, and Lorenzo Spaggiari. 2025. "Surgical Outcomes After Neoadjuvant Chemo-Immunotherapy for Stage III NSCLC: A Systematic Review and Meta-Analysis" Cancers 17, no. 9: 1426. https://doi.org/10.3390/cancers17091426
APA StyleBardoni, C., Chiari, M., Bertolaccini, L., Diotti, C., De Fabiani, A., Nicolosi, G., Mazzella, A., Casiraghi, M., & Spaggiari, L. (2025). Surgical Outcomes After Neoadjuvant Chemo-Immunotherapy for Stage III NSCLC: A Systematic Review and Meta-Analysis. Cancers, 17(9), 1426. https://doi.org/10.3390/cancers17091426














