Emerging Role of Chimeric Antigen Receptor-Natural Killer Cells for the Treatment of Hematologic Malignancies
Simple Summary
Abstract
1. Introduction
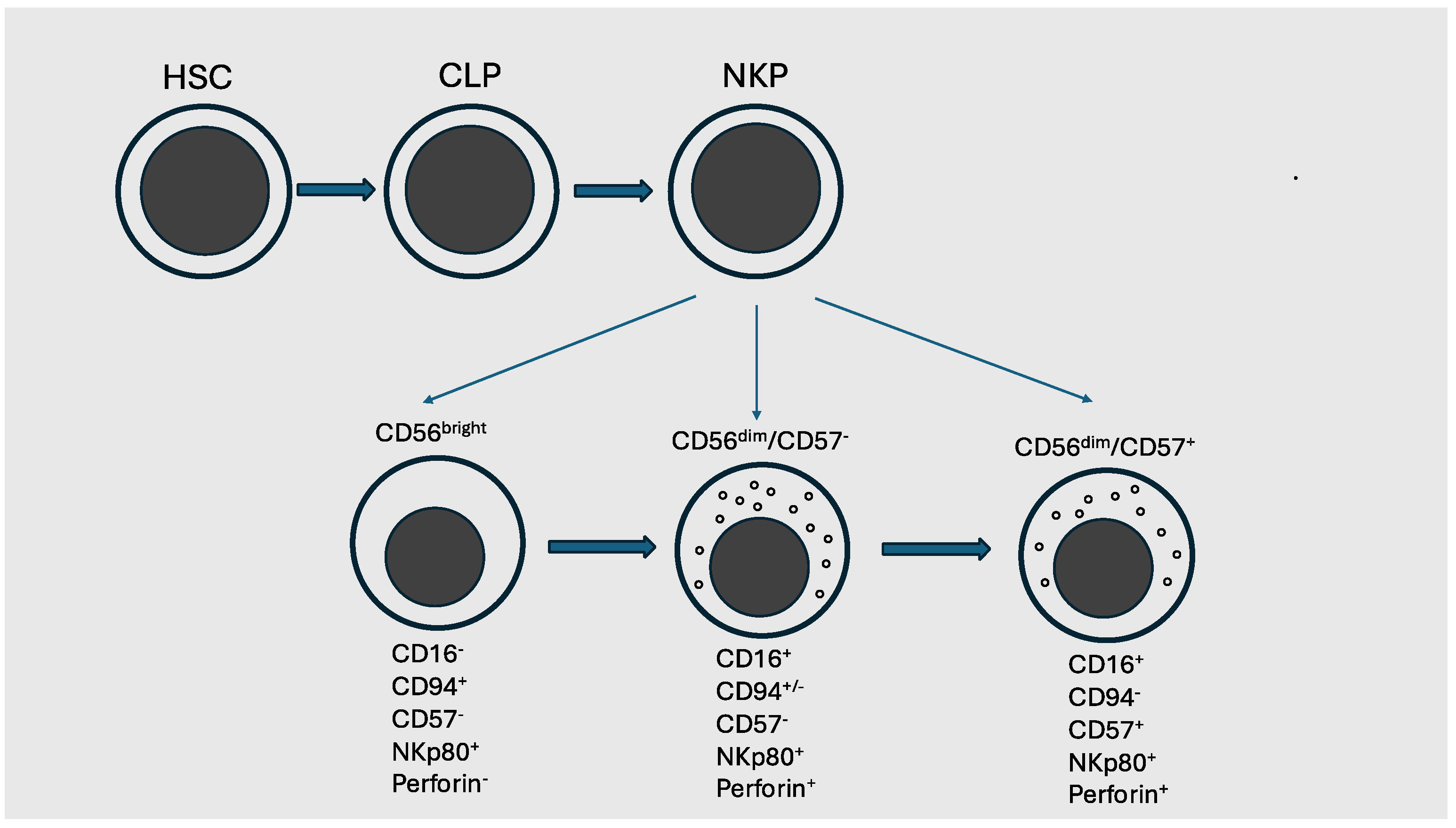
2. Basic Biology of Human NK Cells
3. NK Cell Engineering
4. CD19-CAR-NK
5. NK and CAR-NK Cells in AML Therapy
5.1. Studies Involving NK-Expanded Cells
5.2. Cytokine-Induced Memory-like Natural Killer Cells
5.3. NK-CAR in AML Patients
5.4. CD70 Targeting with CAR-NK Cells
5.5. CAR-NK Cells in Multiple Myeloma
5.6. Limitations of CAR-NK Cells
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Imai, Y. Novel treatment strategies for hematological malignancies in the immunotherapy era. Int. J. Hematol. 2024, 120, 3–5. [Google Scholar] [CrossRef]
- June, C.H.; Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med. 2018, 379, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Cappell, K.M.; Kochenderfer, J.N. Long-term outcomes following CAR-T cells therapy: What we know so far. Nat. Rev. Clin. Oncol. 2023, 20, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Santomasso, B.; Bachier, C.; Westin, J.; Rezvani, C.; Shpall, E.J. The other side of CAR-T cell therapy: Cytokine release syndrome, neurologic toxicity, and financial burden. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 433–444. [Google Scholar] [CrossRef]
- Hoffmann, M.S.; Hunter, B.D.; Cobb, P.W.; Varela, J.C.; Munoz, J. Overcoming barriers to referral for chimeric antigen receptor T cell therapy in patients with relapsed/refractory diffuse large B-cell lymphoma. Transpl. Cell. Ther. 2023, 29, 440–448. [Google Scholar] [CrossRef]
- Brudno, J.N.; Kochenderfer, J.N. Current understanding and management of CART cell-associated toxicities. Nat. Rev. Clin. Oncol. 2024, 21, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Arachchinge, A.S.P.M. Human NK cells: From development to effector functions. Innate Immun. 2021, 27, 212–229. [Google Scholar]
- Mace, E.M. Human natural killer cells: Form, function, and development. J. Allergy Clin. Immunol. 2023, 151, 371–385. [Google Scholar] [CrossRef]
- Rebuffet, L.; Melsen, J.E.; Escalière, B.; Basurto-Lozada, D.; Bhandoola, A.; Bjorkstrom, N.K.; Bryceson, Y.T.; Castriconi, R.; Chichocki, F.; Colonna, M.; et al. High-dimensional single-cell analysis of human natural killer cell heterogeneity. Nat. Immunol. 2024, 25, 1474–1488. [Google Scholar] [CrossRef]
- Dagra, P.; Roncan, C.; Ma, W.; Toth, M.; Senda, T.; Carpenter, D.J.; Kubota, M.; Matsumoto, R.; Thapa, P.; Szabo, P.A.; et al. Tissue determinants of human NK cell development, function, and residence. Cell 2020, 180, 749–763. [Google Scholar] [CrossRef]
- Ding, Y.; Lavaert, M.; Grassman, S.; Band, V.I.; Chi, L.; Das, A.; Harly, C.; Shissler, S.C.; Malin, J.; Peng, D.; et al. Distinct developmental pathways generate functionally distinct populations of natural killer cells. Nat. Immunol. 2024, 25, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, M.; Zhang, W.; Liu, N.; Wang, D.; Jing, L.; Xu, N.; Yang, N.; Ren, T. Chimeric antigen receptor-based natural killer immunotherapy in cancer: From bench to bedside. Cell Death Dis. 2024, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Liu, Y.G.; Huang, G.; Hao, L.; Wang, R. The development and application of chimeric antigen receptor natural killer (CAR-NK) cells for cancer therapy: Current states, challenges and emerging therapeutic advances. Exp. Hematol. Oncol. 2024, 8, 118. [Google Scholar] [CrossRef]
- Shah, N.; Martin-Antonio, B.; Yang, H.; Ku, S.; Lee, D.; Cooper, L.; Decker, W.; Li, S.; Robinson, S.N.; Sekine, T.; et al. Antigen presenting cell-mediated expansion of human umbilical cord blood yelds log-scale expansion of natural killer cells with anti-myeloma activity. PLoS ONE 2013, 8, e76781. [Google Scholar] [CrossRef]
- Shah, N.; McCarty, J.; Kaur, I.; Yvon, E.; Shaim, H.; Muftuoglu, M.; Liu, E.; Orlowski, R.Z.; Cooper, L.; Lee, D.; et al. Phase I study of cord blood-derived natural killer cells combined with autologous stem cell transplantation in multiple myeloma. Br. J. Haematol. 2017, 177, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, T.; Maeoka, R.; Morimoto, T.; Matsuda, R.; Nakamura, M.; Nishimura, F.; Yamada, S.; Nakagawa, I.; Park, Y.S.; Ito, T. An efficient feeder-free and chemically-defined expansion strategy for highly purified human natural killer cells derived from human cord blood. Regen. Ther. 2023, 24, 32–42. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Kuang, J.J.; Chen, X.; Liu, Z.; Li, J.; Dong, T.; Li, X.; Chen, Q.; Liu, T. A pilot study of cord blood derived natural killer cells as maintenance therapy after autologous hematopoietic stem cell transplantation. Ann. Hematol. 2023, 102, 3229–3237. [Google Scholar] [CrossRef]
- Wibowo, T.; Kogue, Y.; Ikeda, S.; Yaga, M.; Tachikawa, M.; Suga, M.; Kida, S.; Shibata, K.; Tsutsumi, K.; Murakami, H.; et al. CAR-NK cells derived from cord blood originate mainly from DC56−CD7+CD34−HLA-DR−Lin− NK progenitor cells. Methods Clin. Dev. 2024, 32, 1–5. [Google Scholar]
- Liu, E.; Marin, D.; Nanerjee, P.; Macalinpoc, H.A.; Thompson, P.; Basar, R.; Kerbany, L.N.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef]
- Marin, D.; Li, Y.; Basar, R.; Rafei, H.; Daher, M.; Dou, J.; Maohanty, V.; Dede, M.; Nieto, Y.; Uperty, N.; et al. Safety, efficacy and determinants of response of allogeneic CD19-specific CAR-NK cells in CD19+ B cell tumors: A phase 1-2 trial. Nat. Med. 2024, 30, 772–784. [Google Scholar] [CrossRef]
- Hamieh, M.; Dobrin, A.; Cabriolu, A.M.; dan der Stegen, S.; Giavridis, T.; Manilla-Soto, J.; Eyquem, J.; Zhao, Z.; Whitlock, B.M.; Miele, M.M.; et al. CAR T cell trogocytosis and cooperative killing regulate tumor antigen escape. Nature 2019, 668, 112–116. [Google Scholar] [CrossRef]
- Li, Y.; Basar, R.; Wang, G.; Liu, E.; Moyes, J.S.; Li, L.; Kerbauy, L.N.; Upretyy, N.; Fathi, M.; Rezvan, A.; et al. KIR-based inhibitory CARs overcome CAR-NK cell trogocytosis-mediated fratricide and tumor escape. Nat. Med. 2022, 28, 2133–2148. [Google Scholar] [CrossRef] [PubMed]
- Darrah, J.M.; Varadarajan, I.; Mehta, A.; Saultz, J.N.; McKinney, M.; Ghosh, M.; Gergis, U.; Bende, G.; Kasar, S.; Hupf, B.; et al. Efficacy and safety of TAK-007, cord blood-derived CD19 CAR-NK cells, in adult patients with relapsed/refractory (R/R) B-cell non-Hodgkin lymphoma (NHL). Blood 2024, 144 (Suppl. 1), 95–96. [Google Scholar] [CrossRef]
- Qian, W.; Lei, W.; Liu, H.; Chen, W.; Liang, Y.; Sun, Y.E.; Tong, X.; Liang, A. Safety and feasibility of a 41BB co-stimulated CD19 CAR-NK cell therapy in refractory/relapsed large B-cell lymphoma. Blood 2024, 144 (Suppl. 1), 2709. [Google Scholar] [CrossRef]
- Dickinson, M.; Hamada, N.; Bryant, C.; Kothari, N.; Ojeras, P.; Vohra, A.; Lin, M.; Tohme, M.; Trager, J.; Shook, D.; et al. First in human data of NKX019, an allogeneic CAR NK for the treatment of relapsed/refractory (R/R) B-cell malignancies. Hemasphere 2023, 7, 358–359. [Google Scholar] [CrossRef]
- Ghobadi, A.; Bachanova, V.; Patel, K.; Park, J.H.; Flinn, I.; Riedell, P.A.; Bachier, C.; Diefenbach, C.S.; Wong, C.; Bickers, C.; et al. Induced pluripotent stem-cell-derived CD19-directed chimeric antigen receptor natural killer cells in B-cell lymphoma: A phase 1, first-in human trial. Lancet 2025, 405, 127–136. [Google Scholar] [CrossRef]
- Koh, S.K.; Kim, H.; Han, B.; Jo, H.; Doh, J.; Park, J.; Nguyen, M.H.; Kim, H.Y.; Kim, H.; Lee, S.H.; et al. Anti-CD19 antibody co-treatment enhances serial killing activity of anti-CD19 CAR-T/-NK cells and reduces trogocytosis. Blood 2025, 145, 956–969. [Google Scholar] [CrossRef]
- He, B.; Chen, H.; Deng, S.; Li, C.; Xu, N.; Liu, X.; Zhou, H.; Liu, Q. CD19-specific CAR NK cells coexpressing IL-21 exhibit superior expansion and antitumor activity against CD19-positive lymphoma. Blood 2023, 142 (Suppl. 1), 6284–6285. [Google Scholar] [CrossRef]
- Carfagnini, C.; Singh, R.; Bechara, S.B.; Kandula, M. The efficacy and safety of CD19 directed CAR-NK therapy in adults with B-cell malignancies: A meta-analysis. Blood 2024, 144 (Suppl. 1), 7180. [Google Scholar] [CrossRef]
- Miller, J.S.; Soignier, Y.; Panoskalsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef]
- Curti, A.; Ruggeri, L.; D’Addio, A.; Bontadini, A.; Dan, E.; Motta, M.R. Successfull transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood 2011, 118, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Bachanova, V.; Cooley, S.; Defor, T.E.; Verneris, M.R.; Zhang, B.; McKenna, D.H. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphteria toxin fusion protein. Blood 2014, 123, 3855–3863. [Google Scholar] [CrossRef] [PubMed]
- Cooley, S.; He, F.; Bachanova, V.; Vercellotti, G.M.; DeFor, T.T.; Curtsinger, J.M.; Robertson, P.; Grxywach, B.; Conlon, K.C.; Waldmann, T.A.; et al. First-in-human trial of rhIL-15 and haploidentical naurl killer cell therapy for advanced acute myeloid leukemia. Blood Adv. 2019, 3, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Ciurea, S.O.; Kongtim, P.; Soebbing, D.; Trikhja, P.; Nehbehani, G.; Rondon, G.; Olson, A.; Bashir, Q.; Gubis, A.M.; Indreshpal, K.; et al. Decrease of post-transplant relapse using donor-derived expanded NK cells. Leukemia 2022, 36, 155–164. [Google Scholar] [CrossRef]
- Lee, K.H.; Yoon, S.R.; Gong, J.R.; Choi, E.J.; Kim, H.S.; Park, C.J.; Yun, S.C.; Park, S.Y.; Jung, S.J.; Kim, H. The infusion of ex vivo, interleukin-15 and -21-activated donor NK cells after haploidentical HCT in high-risk AML and MDS patients- a randomized trial. Leukemia 2023, 37, 807–819. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, S.; Park, Y.H.; Mun, Y.C.; Choi, E.J.; Choi, Y.; Park, H.S.; Lee, J.H.; Park, S.Y.; Yonn, S.R.; et al. Interleikin-15 and -21-activated, donor-derived NK cell infusion after haploidentical HCT in high-risk AML and MDS- a cohort analysis. Leukemia 2024, 38, 451–454. [Google Scholar] [CrossRef]
- Ciurea, S.O.; Kongtim, P.; Srour, S.; Chen, J.; Soebbing, D.; Shpall, E.; Rezvani, K.; Nakkula, R.; Thakkar, A.; Troy, E.C.; et al. Results of a phase I trial with haploidentical mbIL-21 ex vivo expanded NK cells for patients with multiply relapsed and refractory AML. Am. J. Hematol. 2024, 99, 890–899. [Google Scholar] [CrossRef]
- Berrien-Elliott, M.M.; Wagner, J.A.; Fehniger, T.A. Human cytokine-induced memory-like natural killer cells. J. Innate Immunol. 2015, 7, 563–571. [Google Scholar] [CrossRef]
- Romee, R.; Rosario, M.; Berrien-Elliott, M.M.; Wagner, J.A.; Jewell, B.A.; Schappe, T.; Leong, J.W.; Abdel-Latif, S.; Schneider, S.E.; Willey, S.; et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 2016, 8, 357ra123. [Google Scholar] [CrossRef]
- Berrien-Elliott, M.M.; Cashen, A.F.; Cubitt, C.C.; Neal, C.C.; Wong, P.; Wagner, J.A.; Foster, M.; Schappe, T.; Desai, S.; McClain, E.; et al. Multidimensional analysesx of donor memory-like NK cells reveal new associations with response after adoptive immunotherapy for leukemia. Cancer Discov. 2020, 10, 1854–1871. [Google Scholar] [CrossRef]
- Berrien-Elliott, M.M.; Foltz, J.A.; Russler-Germain, D.A.; Nael, C.C.; Tran, J.; Gang, M.; Wong, P.P.; Fisk, B. Hematopoietic cell transplantation donor-derived memory-like NK cells functionally persist after transfer into patients with leukemia. Sci. Transl. Med. 2022, 14, eabm1375. [Google Scholar] [CrossRef] [PubMed]
- Bednarski, J.J.; Zimmermann, C.; Berrien-Elliott, M.M.; Foltz, J.A.; Becker-Hapak, M.; Neal, C.C.; Foster, M.; Schappe, T.; McClain, E.; Pence, P.P.; et al. Donor memory-like NK cells persist and induce remissions in pediatric patients with relapsed AML after transplant. Blood 2022, 139, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- Rutella, S.; Vadakekolathu, J.; Cashen, A.F.; Mahajan, N.; Ruiz-Heredia, Y.; Martin-Munoz, A.; Barrio, S.; Berrien-Elliott, M.M.; Davidson-Moncada, J.; Fehniger, T.A. Adoptively infused memory-like natural killer cells impact adaptive immune responses in patients with acute myeloid leukemia. Blood 2023, 142 (Suppl. 1), 4813–4814. [Google Scholar] [CrossRef]
- Bhatnagar, N.; Petit, V.; Vadakekolathu, J.; Pinset, C.; Mahajan, N.; Dean, J.; Spingola, C.; Arthur, L.; Boocock, D.; Coveney, C.; et al. WU-NK-101 (W-NK), a memoty-like (ML) NK cell, naturally overcomes tumor microenvironment (TME) metabolic challenges, retaining anti-tumor potency. Blood 2023, 142 (Suppl. 1), 4834. [Google Scholar] [CrossRef]
- Leedom, T.; Magee, K.; Vadakekolathu, J.; Arthur, L.; Tran, M.; Luukkonene, L.; Mahajan, N.; Hamil, A.; Muth, J.; Berrien-Elliott, M.; et al. W-NK1 choreographs innate and adaptive immune responses to provide a robust and durable anti-AML response. Blood 2024, 144 (Suppl. 1), 916–917. [Google Scholar] [CrossRef]
- Cashen, A.F.; Al Malki, M.; Stevens, D.A.; Muiffly, L.; Edwin, N.; Abadir, E.; Tan, P.; Niyongere, S.; Bajel, A.; Arthur, L.; et al. WUN101-01: First in human (FIH) phase 1 study of WU-NK-101 (W-NK1) in patients with relapsed or refractory (R/R) acute myeloid leukemia (AML). Blood 2024, 144 (Suppl. 1), 4257–4258. [Google Scholar] [CrossRef]
- Albinger, N.; Pfeifer, R.; Nitsche, M.; Merlitz, S.; Campe, J.; Stein, K.; Kreynberg, H.; Schubert, R.; Quadflieg, M.; Schneider, D.; et al. Primary CD33-targeting CAR-NK cells for the treatment of acute myeloid leukemia. Blood Cancer J. 2022, 12, 61. [Google Scholar] [CrossRef]
- Huang, R.; Wang, X.; Yan, H.; Tan, X.; Ma, Y.; Wang, M.; Han, X.; Liu, J.; Gao, L.; Jing, G.; et al. Safety and efficacy of CD33-targeted CAR-NK cell therapy for relapsed/refractory AML: Preclinical evaluation and phase I trial. Exp. Hematol. Oncol. 2025, 14, 1. [Google Scholar] [CrossRef]
- Bexte, T.; Albinger, N.; Al Ajami, A.; Wendel, P.; Buchinger, L.; Gessner, A.; Alzubi, J.; Sarchen, V.; Vogler, M.; Rasheed, H.M.; et al. CRISPR/Cas9 editing of NKG2A improves the efficacy of primary CD33-directed chimeric antigen receptor natural killer cells. Nat. Commun. 2024, 15, 8439. [Google Scholar] [CrossRef]
- Frankel, N.W.; Deng, H.; Yucel, G.; Gainer, M.; Leemqns, N.; Lam, A.; Li, Y.; Hung, M.; Lee, D.; Banicki, A.; et al. Precision off-the-shelf natural killer cell therapies for oncology with logic-gated gene circuits. Cell Rep. 2024, 43, 114145. [Google Scholar] [CrossRef]
- Li, Y.R.; Zhou, Y.; Yu, J.; Kim, Y.J.; Li, M.; Lee, D.; Zhou, K.; Chen, Y.; Zhu, Y.; Wang, Y.C.; et al. Generation of allogeneic CAR-NKT cells from hematopoietic stem and progenitor cells using a clinically guided culture method. Nat. Biotechnol. 2024, 43, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Fang, Y.; Niu, S.; Zhu, Y.; Chen, Y.; Lyu, Z.; Zhu, E.; Tian, Y.; Huang, J.; Rezek, V.; et al. Allogeneic CD33-directed CAR-NKT cells for the treatment of bone marrow-resident myeloid malignancies. Nat. Commun. 2025, 16, 1248. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.G.; Teng, K.Y.; Li, Z.; Zhu, Z.; Chen, H.; Tian, L.; Ali, A.; Zhang, J.; Lu, T.; Ma, S.; et al. Off-the-shelf CAR-engineered natural killer cells targeting FLT3 enhance killing of acute myeloid leukemia. Blood Adv. 2023, 7, 6225–6253. [Google Scholar] [CrossRef] [PubMed]
- Paczulla, A.M.; Rothfelder, K.; Raffel, S.; Konantz, M.; Steinbacher, J.; Wang, H.; Tandler, C.; Mbarga, M.; Schaefer, T.; Falcone, M.; et al. Absence of NKG2D ligands defines leukemia stem cells and mediates their immune evasion. Nature 2019, 592, 254–259. [Google Scholar] [CrossRef]
- Sauter, C.S.; Borthakur, G.; Muntjoy, L.; Rotta, M.; Liu, H.; Murthy, H.S.; Lin, M.; Trager, J.; Chang, C.; Kothari, N.; et al. A phase 1 study of NKX101, a chimeric antigen receptor natural killer (CAR-NK) cell therapy, with fludarabine and cytarabine in patients with acute myeloid leukemic. Blood 2023, 142 (Suppl. 1), 2097. [Google Scholar] [CrossRef]
- Pelosi, E.; Castelli, G.; Testa, U. CD123 a therapeutic target for acute myeloid leukemia and blastic plasmocytoid dendritic neoplasm. Int. J. Med. Sci. 2023, 24, 2718. [Google Scholar]
- Caruso, S.; De Angelis, B.; Del Bufalo, F.; Ciccone, R.; Donsante, S.; Volpe, G.; Manni, S.; Guercio, M.; Pezzella, M.; Iaffaldano, L.; et al. Safe and effective off-the-shelf immunotherapy based on CAR.CD123-NK cells for the treatment of acute myeloid leukemia. J. Hematol. Oncol. 2022, 15, 163. [Google Scholar] [CrossRef]
- Gauthier, L.; Virone-Oddos, A.; Beninga, J.; Rossi, B.; Nicolazzi, C.; Amara, C.; Blanchard-Alvarez, A.; Gourdin, N.; Courta, J.; Basset, A.; et al. Control of acute myeloid leukemia by a trifunctional NKp46-CD16a-NK cell engager targeting CD123. Nat. Biotechnol. 2023, 41, 1296–1306. [Google Scholar] [CrossRef]
- Mendfelowitz, A.S.; Chu, Y.Y.; Felices, M.; Miller, J.; Forman, S.J.; Bhjbehani, G.; Bonifant, C.; Lee, D.A.; Cairo, M. Anti-CD123 CAR NK cells and cam161533 trispecific killer have synergistic activity against acute myeloid leukemia. Blood Cancer Discov. 2024, 5 (Suppl. 2), P20. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, H.; Xiao, X.; Bai, X.; Liu, P.; Pu, Y.; Meng, J.; Zhu, H.; Wang, Z.; Zhang, H.; et al. A phase I clinical trial of CLL-1 CAR-T cells for the treatment of relapsed/refractory acute myeloid leukemia in adults. Blood 2023, 142 (Suppl. 1), 2106–2108. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Y.; Chai, X.; Wang, Y.; Zhao, M.; Guo, S.; Zahng, Y.; Zhso, M. Modified CD16/CD16-CLL1 inhibitory CAR-T cells for mitigating granulocytopenia toxicities in the treatment of acute myeloid leukemia. Transl. Oncol. 2025, 52, 102225. [Google Scholar] [CrossRef] [PubMed]
- Sedloev, D.N.; Chen, Q.; Liglaub, J.M.; Schmidt, A.; Muller-Tidow, C.; Schmidt, M.; Sauer, T. Structurally optimized CLL-1 CAR-NK cells are highly potent effectors against AML without Hpsc toxicity. Blood 2024, 144 (Suppl. 1), 3440–3441. [Google Scholar] [CrossRef]
- Sauer, T.; Parikh, K.; Omer, B.; Omer, B.; Sedloev, D.; Chen, Q.; Angenendt, L.; Schliemann, C.; Schmitt, M.; Muller-Tidow, C.; et al. CD70-specific CAR T cells have potent activity against acute myeloid leukemia without HSC toxicity. Blood 2021, 138, 318–330. [Google Scholar] [CrossRef]
- Wu, G.; Guo, S.; Luo, Q.; Wang, X.; Deng, W.; Ouyang, G.; Pu, J.J.; Lei, W.; Qian, W. Preclinical evaluation of CD70-specific CAR T cells targeting acute myeloid leukemia cells. Front. Immunol. 2023, 14, 1093750. [Google Scholar] [CrossRef]
- Silva, H.J.; Martin, G.; Birocchi, F.; Wehrli, M.; Kann, M.C.; Supper, V.; Parker, A.; Graham, C.; Bratt, A.; Bouffard, A.; et al. CD70 CAR T cells secreting an anti-CD33/anti-CD3 dual-targeting antibody overcome antigen heterogeneity. Blood 2025, 145, 720–731. [Google Scholar] [CrossRef]
- Guo, S.; Lei, W.; Jin, X.; Liu, H.; Wang, J.Q.; Deng, W.; Qian, W. CD70-specific CAR NK cells expressing IL-15 for the treatment of CD19-negative malignancy. Blood Adv. 2024, 8, 2635–2645. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; He, X.; Mo, Z.; Zhao, M.; Liang, X.; Hu, K.; Wang, K.; Yue, Y.; Mo, G.; et al. CD70-targeted iPSC-derived CAR-NK cells display potent function against tumors and alloreactive T cells. Cell Rep. Med. 2025, 6, 101889. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Reyes Silva, F.C.; Lin, P.; Gilbert, A.L.; Acharya, S.; Nunez Cortes, A.K.; Banerjee, P.; Fang, D.; Melo Garcia, L.; Daher, M.; et al. CD70 CAR NK cells in the treatment of multiple myeloma. Blood 2023, 142 (Suppl. 1), 3463–3465. [Google Scholar] [CrossRef]
- Ren, Q.; Zu, Y.; Su, H.; Lu, Q.; Xiang, B.; Luo, Y.; Zhang, J.; Song, Y. Single VHH-directed BCMA CAR-NK cells for multiple myeloma. Exp. Hematol. Oncol. 2023, 12, 989. [Google Scholar] [CrossRef]
- Talarico, L.; Wong, C.; Pang, C.; Hickman, T.; Hu, C.; Shaw, A.; Wasniewski, E.; La, S.C.; Sharma, P.; Moore, S.; et al. A cryopreserved allogeneic anti-BCMA CAR-NK cellular therapy exhibits both innate and CAR-mediated MM cell killing in vitro and in vivo. Cancer Res. 2024, 84 (Suppl. 6), 1322. [Google Scholar] [CrossRef]
- Motais, B.; Charvatova, S.; Herdinka, M.; Hajek, R.; Bago, J.R. Anti-BCMA-CAR NK cells expressing soluble TRAIL: Promising therapeutic approach for multiple myeloma in combination with bortezomib and γ-secretase inhibitors. Blood 2022, 140 (Suppl. 1), 12683–12684. [Google Scholar] [CrossRef]
- Park, E.; Mun, H.J.; Seo, E.; Hwang, S.; Lee, J.H.; Song, S.; Sung, H.; Kim, H.Y.; Kwon, M.J. CARNK92 tergeting BCMA can effectively kill multiple myeloma cells both in vitro and in vivo. Biomedicines 2024, 12, 248. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, B.; Bardeja, J.G.; Gregory, T.; Ly, T.; Bickers, C.; Zong, X.; Wong, L.; Goodridge, J.P.; Cooley, S.; Valamehr, B.; et al. Interim phase I clinical data of FT576 as monotherapy and in combination with daratumumab in subjects with relapsed/refractory multiple myeloma. Blood 2022, 140 (Suppl. 1), 4586–4587. [Google Scholar] [CrossRef]
- Cao, Z.; Yang, C.; Wang, Y.; Wang, C.; Wang, Q.; Ye, G.; Liu, T.; Wang, Q.; Wang, H.; Gong, Y.; et al. Allogeneic CAR-NK cell therapy targeting both BCMA and GPRC5D for the treatment of multiple myeloma. Blood 2022, 140 (Suppl. 1), 7378. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, L.; Zhu, Z.; Yan, Y.; Fu, J.; Wei, M. CIB315: An allogeneic, off-the-shelf anti-GPRC5D iPSC-derived CAR-NK product targeting multiple myeloma. Blood 2024, 144 (Suppl. 1), 2055. [Google Scholar] [CrossRef]
- Wu, X.; Matosevic, S. Gene-edited and CAR-NK cells: Opportunities and challenges with engineering of NK cells for immunotherapy. Mol. Ther. Oncolytics 2022, 27, 224–232. [Google Scholar] [CrossRef]
- Maia, A.; Tarannum, M.; Lérias, J.R.; Piccinelli, S.; Borrego, L.M.; Mauerer, M.; Romee, R.; Castillo-Martin, M. Building a better defense: Expanding and improving natural killer cells for adoptive cell therapy. Cells 2024, 13, 451. [Google Scholar] [CrossRef]
- Liu, E.; Tong, Y.; Dotti, G.; Shaim, H.; Savoldo, B.; Mukherjee, M.; Orange, J.; Wan, X.; Lu, X.; Reynolds, A.; et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 2018, 32, 520–531. [Google Scholar] [CrossRef]
- Li, L.; Mohanty, V.; Dou, J.; Huang, Y.; Banerjee, P.P.; Mia, Q.; Lohr, J.G.; Vijekkumar, T.; Frede, J.; Knoechel, B.; et al. Loss of metabolic fitness drives tumor resistance after CAR-NK cell therapy and can be overcome by cytokine engineering. Sci. Adv. 2023, 9, eadd6997. [Google Scholar] [CrossRef]
- Berrien-Elliott, M.M.; Becker-Hapak, M.; Cashen, A.F.; Jacobs, M.; Wong, P.; Foster, M.; McClain, E.; Deasai, S.; Pence, P.; Cooley, S.; et al. Systemic IL-15 promotes allogeneic cell rejection in patients treated with natural killer cell adoptive therapy. Blood 2022, 131, 1177–1183. [Google Scholar] [CrossRef]
- Felices, M.; Lenvik, A.J.; McElmurry, R.; Chu, S.; Hinerlie, P.; Bendzick, L.; Geller, M.A.; Tolar, J.; Blazar, B.R.; Miller, J.S. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight 2018, 3, e96219. [Google Scholar] [CrossRef]
- Luevano, M.; Daryuzeh, M.; Alnabhan, R.; Querol, S.; Khakoo, S.; Madrigal, A.; Saudemont, A. The unique profile of cord blood natural killer cells balances incomplete maturation and effective killing function upon activation. Hum. Immunol. 2012, 73, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Corredera, M.M.; Paillet, J.; Gaudeaux, P.; Blein, T.; Sadek, H.; Rault, P.; Berriche, A.; Roche-Naude, J.; Lagreste-Peyrou, C.; Soheili, T.S.; et al. Feder-cell-free system for ex vivo production of natural killer cells from cord blood hematopoietic stem and progenitor cells. Front. Immunol. 2025, 16, 1531736. [Google Scholar]
- Kunkanjanawan, H.; Somrendgan, S.; Kunkanjanawan, T.; Wongtrakoongate, P.; Wongsakmanee, W.; Khemarangsan, V.; Masuayma, I.; Parnpai, R. Efficient large-scale expansion of cord blood-derived NK cells: Leveraging lipopolysaccharide for enhanced NK cell production. Cytotherapyy, 2025; in press. [Google Scholar]
- Quintarelli, C.; Sivori, S.; Caruso, S.; Carlomagno, S.; Falco, M.; Boffa, I.; Orlando, D.; Guercio, M.; Abbaszadeh, Z.; Sinibaldi, M.; et al. Efficacy of third-party chimeric antigen receptor modified peripheral blood natural killer cells for adoptive cell therapy of B-cell precursor acute lymphoblastic leukemia. Leukemia 2020, 34, 1102–1115. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Kaczmarek, S.; Shin, O.; Wang, L.; Cowan, J.; McComb, S.; Lee, S.H. simultaneous engineering of natural killer cells for CAR transgenesis and CRSPR-Cas9 knockout using retroviral particles. Methods Clin. Dev. 2023, 29, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Sakemura, R.; Hefazi, M.; Siegler, E.R.; Cox, M.J.; Larson, D.P.; Hansen, M.J.; Manriquez Roman, C.; Schick, K.J.; Can, I.; Tapper, E.E.; et al. Targeting cancer-associated fibroblasts in the bone marrow prevents resistance to CART-cell therapy in multiple myeloma. Blood 2022, 139, 3708–3721. [Google Scholar] [CrossRef]
- Kilgour, M.K.; Bastin, D.J.; Lee, S.H.; Ardolino, M.; McComb, S.; Visram, A. Advancements in CAR-NK therapy: Lessons to be learned from CAR-T therapy. Front. Immunol. 2023, 14, 1166038. [Google Scholar] [CrossRef]
- Rouce, R.H.; Shaim, H.; Sekine, T.; Weber, G.; Ballard, B.; Ku, S.; Barese, C.; Murali, V.; Wu, M.F.; Liu, H.; et al. The TGF-beta/SNAD pathway is an important mechanism for NK cell immune evasion in childhood B-acute lymphoblastic leukemia. Leukemia 2016, 30, 800–811. [Google Scholar] [CrossRef]
| Source | Advantages | Limitations |
|---|---|---|
| PB-NK | Mature phenotype High cytotoxic activity | Low presence in PB Limited |
| UCB-NK | High proliferation Favorable safety profile | Poor differentiation Suboptimal cytotoxic activity |
| hPSC | Abundant availability Low immunologic risk | Safety to be evaluated Incomplete differentiation Reduced cytotoxic activity |
| NK-92 cell line | Abundant availability | Potential tumorigenic risk Lack expression of CD16 |
| NKT | Mature phenotype High cytotoxic activity | Limited presence in PB Limited proliferation and expansion |
| CAR-NK Cell Therapy | NCT Identifier | Patients (Disease and Number) | Membrane Target | NK Cell Source | Efficacy | Toxicity |
|---|---|---|---|---|---|---|
| CAR19/IL-15 NK cells | NCT03056339 | B-cell tumors (LG-NHL, DLBCL, CLL) 37 | CD19 | Umbilical cord blood | ORR 49% CR 30% LG-NHL (100%; 83%) CLL (67%; 50%) DLBCL (41%; 29%) | One case CRS, no ICANS |
| TAK-007 | NCT050220015 | B-cell tumors (LBCL, NHL) 27 | CD19 | Umbilical cord blood | LBCL (ORR 50%; CR 21%) iNHL (ORR 78%; CR 56%) | Three cases CRS, no ICANS |
| CAR19bBBz NK cells | NCT05472558 | B-cell tumors (R/R LBCL) 9 | CD19 | Umbilical cord blood | ORR 66.7% CR 55.6% mPFS 9 mo OS at 12 mon 58.3% | No CRS, no ICANS |
| NKX019 | NCT05020678 | B-cell tumors (14 NHL, 5ALL, CLL) | CD19 | Peripheral blood | ORR 71% NHL ORR 77%; CR 57% | Five cases CRS, no ICANS |
| FT596 | NCT04245722 | B-cell and CLL Regimen A and B (without or with Rituximab, respectively) | CD19 | iPSC | fNHL ORR 100%; CR 85% LBCL ORR 38%; CR 25% | CRS 6% reg A 13% reg B |
| FT576 | NCT05182073 | Multiple myeloma 9 | BCMA | iPSC | ORR 22% | No CRS; no ICANS |
| NKX101 | NCT04623944 | AML and MDS 6 | NKG2D | Peripheral blood | CR 66% CR with MRD negative 50% | No CRS; no ICANS |
| CD33CAR-NK cells | NCT05008575 | AML | CD33 | Umbilical cord blood | CR 60% | No CRS; no ICANS |
| Limitations | Possible Solutions |
|---|---|
| Short persistence in vivo | Lymphodepleting conditioning Multiple infusions of CAR-NK cells Exogenous cytokine support Autonomous cytokine production (e.g., IL-15) Use of CIML (cytokine-induced memory-like cells) Design of activation-inhibitory CARs |
| Suboptimal antitumor cytotoxicity | Dual or multi-specific CAR-NK cells Gene editing of CAR-NK cells using CRISPR CAR with different affinities |
| Manufacturing (limited expansion) | Improvement of NK cell expansion using cytokines or feeder cells |
| Tumor microenvironment (TME) | Targeting of inhibitory cells in the TME Targeting of inhibitory pathways in the TME Targeting of specific NK inhibitory checkpoints |
| Autologous CAR-T | Allogeneic CAR-T | Allogeneic CAR-NK | |
|---|---|---|---|
| Mechanism of antitumor effects | Specific (CAR-dependent) MHC-independent | Specific (CAR-dependent) MHC-independent | Specific and non-specific (CAR-dependent and CAR-independent) MHC independent |
| Antitumor efficacy | High | High | High |
| In vivo persistence | Long | Limited due to host-mediated immune rejection | Short |
| Risk of CRS or ICANS | Moderate/High | Moderate/High | Low |
| Risk of GvHD | No | High | Low |
| Off-the-shelf | No potential | High potential | High potential |
| Time for production | Long | Short | Short |
| Economic cost | High | Low | Low |
| Clinical efficacy | High clinical activity against hematological malignancies | High clinical activity against hematological malignancies | Promising clinical activity in some hematological malignancies |
| Regulatory status | Six approved products | Still under evaluation in clinical trials | Still under evaluation in early clinical use |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Testa, U.; Castelli, G.; Pelosi, E. Emerging Role of Chimeric Antigen Receptor-Natural Killer Cells for the Treatment of Hematologic Malignancies. Cancers 2025, 17, 1454. https://doi.org/10.3390/cancers17091454
Testa U, Castelli G, Pelosi E. Emerging Role of Chimeric Antigen Receptor-Natural Killer Cells for the Treatment of Hematologic Malignancies. Cancers. 2025; 17(9):1454. https://doi.org/10.3390/cancers17091454
Chicago/Turabian StyleTesta, Ugo, Germana Castelli, and Elvira Pelosi. 2025. "Emerging Role of Chimeric Antigen Receptor-Natural Killer Cells for the Treatment of Hematologic Malignancies" Cancers 17, no. 9: 1454. https://doi.org/10.3390/cancers17091454
APA StyleTesta, U., Castelli, G., & Pelosi, E. (2025). Emerging Role of Chimeric Antigen Receptor-Natural Killer Cells for the Treatment of Hematologic Malignancies. Cancers, 17(9), 1454. https://doi.org/10.3390/cancers17091454






