Differential Cytotoxicity of Surface-Functionalized Silver Nanoparticles in Colorectal Cancer and Ex-Vivo Healthy Colonocyte Models
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
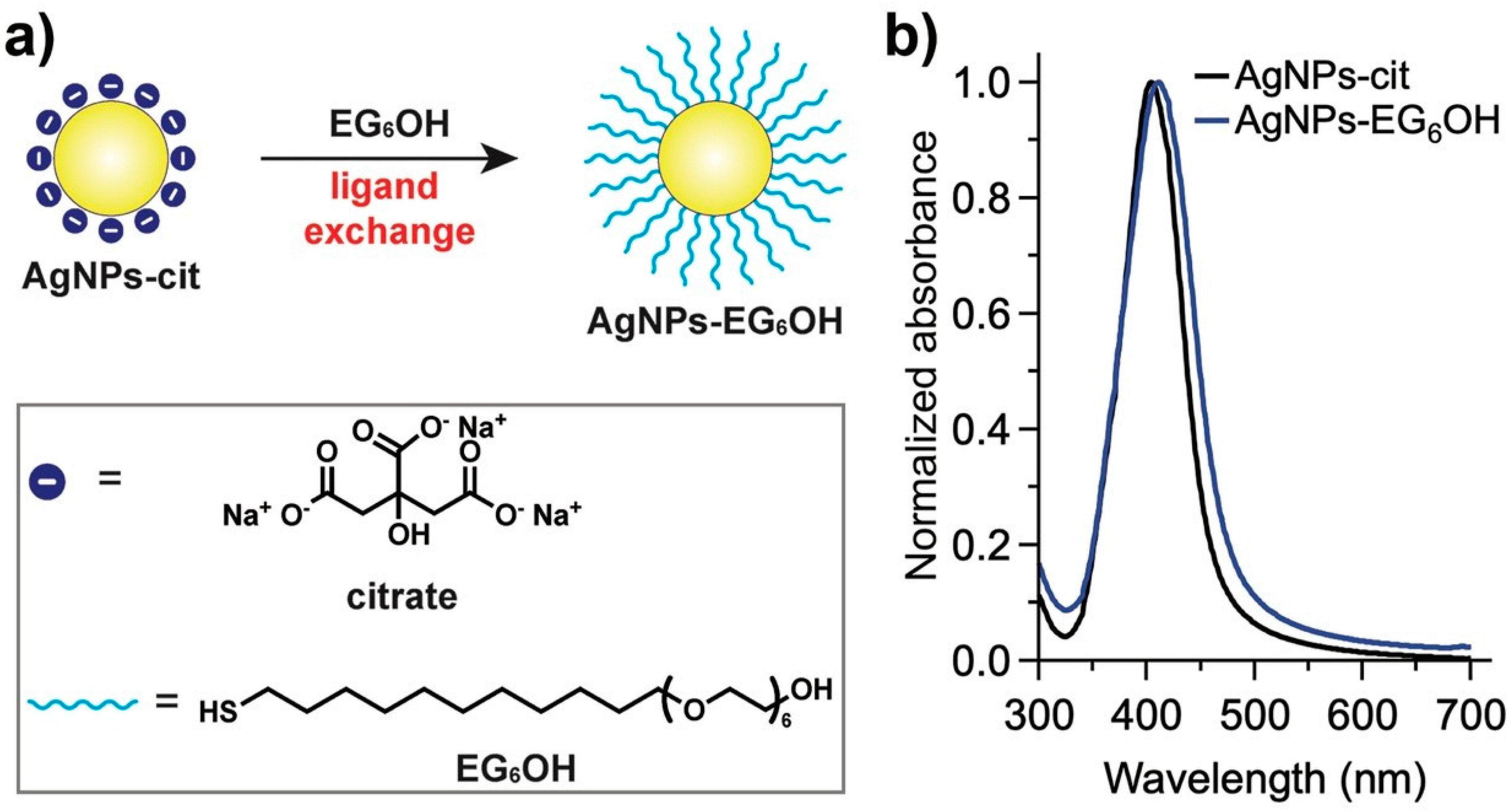
3.1. Synthesis and Functionalization of AgNPs
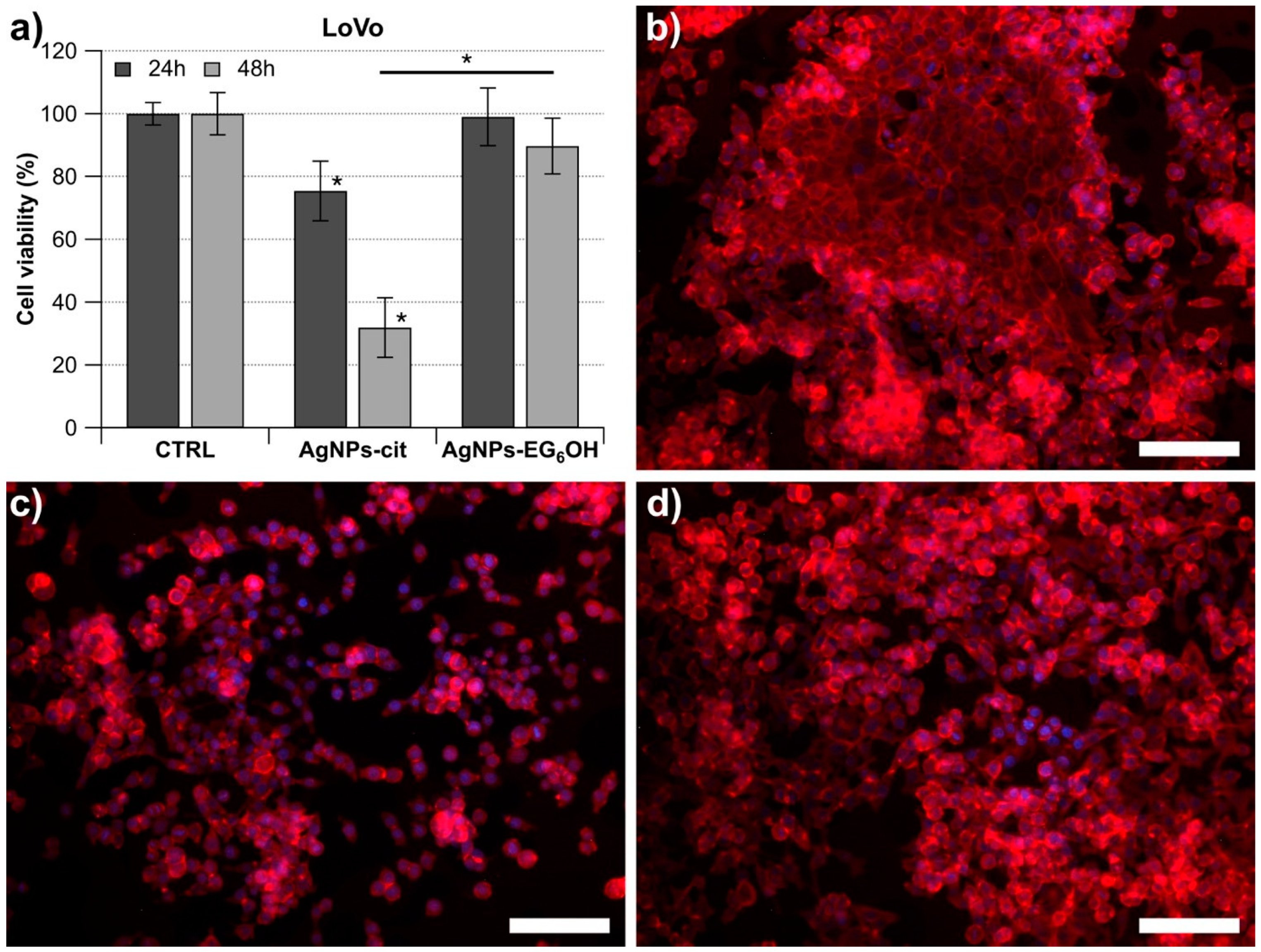
3.2. AgNPs-Cit and AgNPs-EG6OH Exhibit Distinct Cytotoxic Effects on LoVo Cells
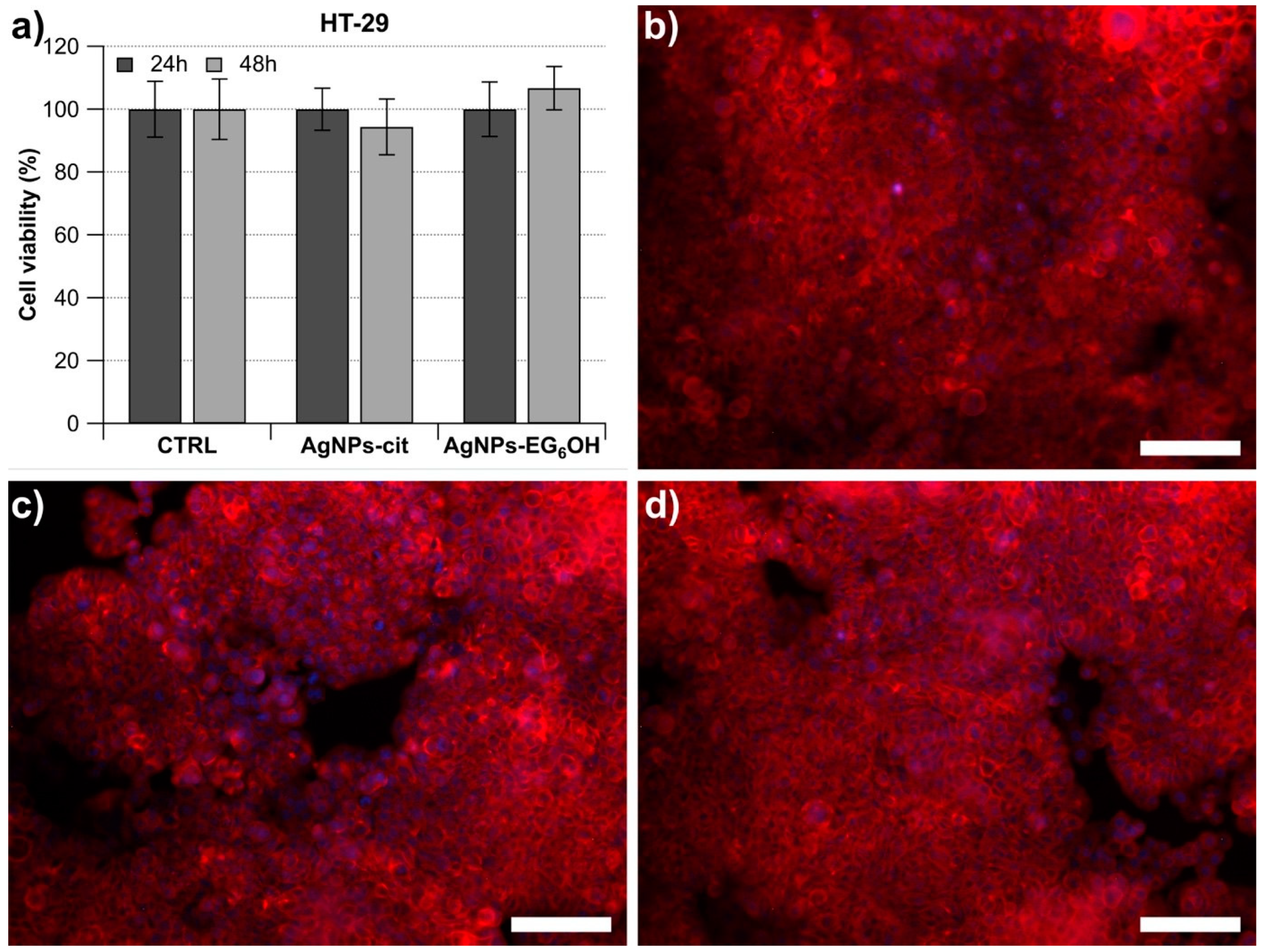
3.3. AgNPs-Cit and AgNPs-EG6OH Do Not Exert a Negative Effect on the Cell Viability of HT-29 Cells
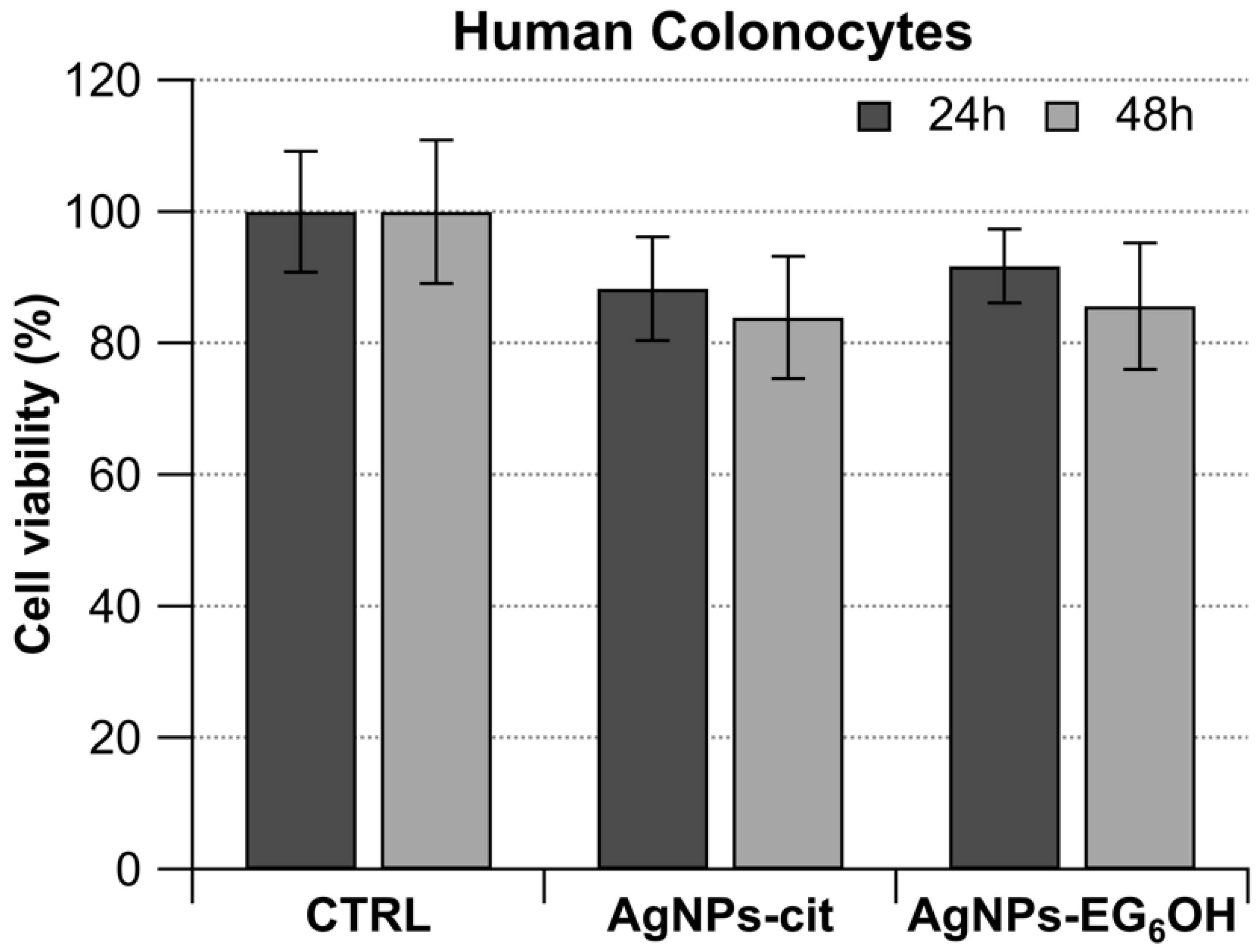
3.4. AgNPs-Cit and AgNPs-EG6OH Do Not Show Any Antiproliferative Activity Against Human Colonocytes from Healthy Donors
3.5. Morphological Changes Due to the Cytotoxic Effect of AgNPs-Cit on LoVo Cells Are Clearly Observable
3.6. Internalization of Both AgNPs-Cit and AgNPs-EG6OH in Colonocytes Assessed Using Confocal Microscopy Techniques
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, C.; Cheng, Z.; Tan, H.; Wang, Y.; Sun, S.; Zhang, M.; Wang, J. Nanomaterials: Leading immunogenic cell death-based cancer therapies. Front. Immunol. 2024, 15, 1447817. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Wang, X.; Wei, M.; Wang, Q.; Li, J.; Bi, L.; Deng, X.; Wang, Z. First-line cetuximab versus bevacizumab for RAS and BRAF wild-type metastatic colorectal cancer: A systematic review and meta-analysis. BMC Cancer 2019, 19, 280. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Kaneko, M.K.; Yoshida, Y.; Takashima, A.; Kato, Y.; Kawada, M. Current Targeted Therapy for Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 1702. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Noga, M.; Milan, J.; Frydrych, A.; Jurowski, K. Toxicological Aspects, Safety Assessment, and Green Toxicology of Silver Nanoparticles (AgNPs)—Critical Review: State of the Art. Int. J. Mol. Sci. 2023, 24, 5133. [Google Scholar] [CrossRef]
- Ibrahim, S.; Ahmad, Z.; Manzoor, M.Z.; Mujahid, M.; Faheem, Z.; Adnan, A. Optimization for biogenic microbial synthesis of silver nanoparticles through response surface methodology, characterization, their antimicrobial, antioxidant, and catalytic potential. Sci. Rep. 2021, 11, 770. [Google Scholar] [CrossRef]
- Nallanthighal, S.; Chan, C.; Bharali, D.J.; Mousa, S.A.; Vásquez, E.; Reliene, R. Particle coatings but not silver ions mediate genotoxicity of ingested silver nanoparticles in a mouse model. NanoImpact 2017, 5, 92–100. [Google Scholar] [CrossRef]
- Rodriguez-Garraus, A.; Azqueta, A.; Vettorazzi, A.; López de Cerain, A. Genotoxicity of Silver Nanoparticles. Nanomaterials 2020, 10, 251. [Google Scholar] [CrossRef]
- Barbalinardo, M.; Caicci, F.; Cavallini, M.; Gentili, D. Protein Corona Mediated Uptake and Cytotoxicity of Silver Nanoparticles in Mouse Embryonic Fibroblast. Small 2018, 14, 1801219. [Google Scholar] [CrossRef]
- Barbalinardo, M.; Bertacchini, J.; Bergamini, L.; Magaro, M.S.; Ortolani, L.; Sanson, A.; Palumbo, C.; Cavallini, M.; Gentili, D. Surface properties modulate protein corona formation and determine cellular uptake and cytotoxicity of silver nanoparticles. Nanoscale 2021, 13, 14119–14129. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, R.; Li, J.; Zeng, H.; Li, L.; Chen, R.; Sun, K.; Han, B.; Bray, F.; Wei, W.; et al. Global, regional, and national lifetime risks of developing and dying from gastrointestinal cancers in 185 countries: A population-based systematic analysis of GLOBOCAN. Lancet Gastroenterol. Hepatol. 2024, 9, 229–237. [Google Scholar] [CrossRef]
- Shejawal, K.P.; Randive, D.S.; Bhinge, S.D.; Bhutkar, M.A.; Todkar, S.S.; Mulla, A.S.; Jadhav, N.R. Green synthesis of silver, iron and gold nanoparticles of lycopene extracted from tomato: Their characterization and cytotoxicity against COLO320DM, HT29 and Hella cell. J. Mater. Sci. Mater. Med. 2021, 32, 19. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Zhang, W.; He, T.; Shu, M.; Deng, J.; Wang, J.; Li, W.; Bai, J.; Lin, Q.; Luo, F.; et al. Evaluation of the Genotoxic and Oxidative Damage Potential of Silver Nanoparticles in Human NCM460 and HCT116 Cells. Int. J. Mol. Sci. 2020, 21, 1618. [Google Scholar] [CrossRef] [PubMed]
- Raja, G.; Jang, Y.K.; Suh, J.S.; Kim, H.S.; Ahn, S.H.; Kim, T.J. Microcellular Environmental Regulation of Silver Nanoparticles in Cancer Therapy: A Critical Review. Cancers 2020, 12, 664. [Google Scholar] [CrossRef] [PubMed]
- Barbalinardo, M.; Chiarini, F.; Teti, G.; Paganelli, F.; Mercadelli, E.; Bartoletti, A.; Migliori, A.; Piazzi, M.; Bertacchini, J.; Sena, P.; et al. Surface charge overrides protein corona formation in determining the cytotoxicity, cellular uptake, and biodistribution of silver nanoparticles. ChemRxiv 2025. [Google Scholar] [CrossRef]
- Sena, P.; Mancini, S.; Pedroni, M.; Reggiani Bonetti, L.; Carnevale, G.; Roncucci, L. Expression of Autophagic and Inflammatory Markers in Normal Mucosa of Individuals with Colorectal Adenomas: A Cross-Sectional Study among Italian Outpatients Undergoing Colonoscopy. Int. J. Mol. Sci. 2022, 23, 5211. [Google Scholar] [CrossRef]
- Gentili, D.; Ori, G.; Ortolani, L.; Morandi, V.; Cavallini, M. Cooperative and Reversible Anisotropic Assembly of Gold Nanoparticles by Modulation of Noncovalent Interparticle Interactions. ChemNanoMat 2017, 3, 874–878. [Google Scholar] [CrossRef]
- Decataldo, F.; Barbalinardo, M.; Gentili, D.; Tessarolo, M.; Calienni, M.; Cavallini, M.; Fraboni, B. Organic Electrochemical Transistors for Real-Time Monitoring of In Vitro Silver Nanoparticle Toxicity. Adv. Biosyst. 2020, 4, e1900204. [Google Scholar] [CrossRef]
- Aoki, K.; Satoi, S.; Harada, S.; Uchida, S.; Iwasa, Y.; Ikenouchi, J. Coordinated changes in cell membrane and cytoplasm during maturation of apoptotic bleb. Mol. Biol. Cell 2020, 31, 833–844. [Google Scholar] [CrossRef]
- Bouafia, A.; Laouini, S.E.; Ahmed, A.S.A.; Soldatov, A.V.; Algarni, H.; Feng Chong, K.; Ali, G.A.M. The Recent Progress on Silver Nanoparticles: Synthesis and Electronic Applications. Nanomaterials 2021, 11, 2318. [Google Scholar] [CrossRef]
- Varna, D.; Christodoulou, E.; Gounari, E.; Apostolidou, C.P.; Landrou, G.; Papi, R.; Koliakos, G.; Coutsolelos, A.G.; Bikiaris, D.N.; Angaridis, P.A. Pegylated-Polycaprolactone Nano-Sized Drug Delivery Platforms Loaded with Biocompatible Silver(I) Complexes for Anticancer Therapeutics. RSC Med. Chem. 2022, 13, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, P.; An, X.; Xiang, J.; Kalva, N.; Shen, Y.; Li, C. Facile Synthesis of Noncytotoxic PEGylated Dendrimer Encapsulated Silver Sulfide Quantum Dots for NIR-II Biological Imaging. Nanoscale 2020, 12, 5678–5684. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Aberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 12, 779–786. [Google Scholar] [CrossRef]
- Tejeda-Muñoz, N.; Albrecht, L.V.; Bui, M.H.; De Robertis, E.M. Wnt canonical pathway activates macropinocytosis and lysosomal degradation of extracellular proteins. Proc. Natl. Acad. Sci. USA 2019, 116, 10402–10411. [Google Scholar] [CrossRef] [PubMed]
- Moravčík, R.; Olejárová, S.; Zlacká, J.; Herichová, I. Effect of miR-34a on the Expression of Clock and Clock-Controlled Genes in DLD1 and Lovo Human Cancer Cells with Different Backgrounds with Respect to p53 Functionality and 17β-Estradiol-Mediated Regulation. PLoS ONE 2023, 18, e0292880. [Google Scholar] [CrossRef] [PubMed]
- Khare, V.; Paul, G.; Movadat, O.; Frick, A.; Jambrich, M.; Krnjic, A.; Marian, B.; Wrba, F.; Gasche, C. IL10R2 Overexpression Promotes IL22/STAT3 Signaling in Colorectal Carcinogenesis. Cancer Immunol. Res. 2015, 3, 1227–1235. [Google Scholar] [CrossRef]
- Wang, X.; Cai, Q.; Ping, J.; Diaz-Zabala, H.; Xia, Y.; Guo, X. The Putative Oncogenic Role of WDTC1 in Colorectal Cancer. Carcinogenesis 2022, 43, 594–600. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Zhang, Y.; Fang, S.; Zhang, M.; Li, H.; Xu, F.; Liu, L.; Liu, J.; Zhao, Q.; et al. Subtyping of Microsatellite Stability Colorectal Cancer Reveals Guanylate Binding Protein 2 (GBP2) as a Potential Immunotherapeutic Target. J. Immunother. Cancer 2022, 10, e004302. [Google Scholar] [CrossRef]
- He, Y.; Lu, F.; Jiang, C.; Gong, F.; Wu, Z.; Ostrikov, K. Cold Atmospheric Plasma Stabilizes Mismatch Repair for Effective, Uniform Treatment of Diverse Colorectal Cancer Cell Types. Sci. Rep. 2024, 14, 3599. [Google Scholar] [CrossRef]
- Miethling-Graff, R.; Rumpker, R.; Richter, M.; Verano-Braga, T.; Kjeldsen, F.; Brewer, J.; Hoyland, J.; Rubahn, H.G.; Erdmann, H. Exposure to Silver Nanoparticles Induces Size- and Dose-Dependent Oxidative Stress and Cytotoxicity in Human Colon Carcinoma Cells. Toxicol. In Vitro 2014, 28, 1280–1289. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Ghosh, M.; Dev, A. Signalling and Molecular Pathways, Overexpressed Receptors of Colorectal Cancer and Effective Therapeutic Targeting Using Biogenic Silver Nanoparticles. Gene 2024, 936, 149099. [Google Scholar] [CrossRef]
- Tamura, S.; Tazawa, H.; Hori, N.; Li, Y.; Yamada, M.; Kikuchi, S.; Kuroda, S.; Urata, Y.; Kagawa, S.; Fujiwara, T. p53-Armed Oncolytic Adenovirus Induces Autophagy and Apoptosis in KRAS and BRAF-Mutant Colorectal Cancer Cells. PLoS ONE 2023, 18, e0294491. [Google Scholar] [CrossRef] [PubMed]
- Hazar-Rethinam, M.; Kleyman, M.; Han, G.C.; Liu, D.; Ahronian, L.G.; Shahzade, H.A.; Chen, L.; Parikh, A.R.; Allen, J.N.; Clark, J.W.; et al. Convergent Therapeutic Strategies to Overcome the Heterogeneity of Acquired Resistance in BRAFV600E Colorectal Cancer. Cancer Discov. 2018, 8, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Gu, R.; Fang, H.; Wang, R.; Dai, W.; Cai, G. A Comprehensive Overview of the Molecular Features and Therapeutic Targets in BRAFV600E-Mutant Colorectal Cancer. Clin. Transl. Med. 2024, 14, e1764. [Google Scholar] [CrossRef]
- Saito-Diaz, K.; Benchabane, H.; Tiwari, A.; Tian, A.; Li, B.; Thompson, J.J.; Hyde, A.S.; Sawyer, L.M.; Jodoin, J.N.; Santos, E.; et al. APC Inhibits Ligand-Independent Wnt Signaling by the Clathrin Endocytic Pathway. Dev. Cell 2018, 44, 566–581.e8. [Google Scholar] [CrossRef]
- Sritharan, S.; Sivalingam, N. Curcumin-Induced Apoptosis Is Mediated through Oxidative Stress in Mutated p53 and Wild Type p53 Colon Adenocarcinoma Cell Lines. J. Biochem. Mol. Toxicol. 2021, 35, e22616. [Google Scholar] [CrossRef]
- Ozer, M.; Vigivinti, C.T.R.; Syed, M.; Ferrell, M.E.; Goez, C.G.; Cheng, S.; Holder-Murray, J.; Bruno, T.; Saeed, A.; Sahin, I.H. Neoadjuvant Immunotherapy for Patients with dMMR/MSI-High Gastrointestinal Cancers: A Changing Paradigm. Cancers 2023, 15, 3833. [Google Scholar] [CrossRef]
- Artiukh, L.; Povnitsa, O.; Zahorodnia, S.; Pop, C.V.; Rizun, N. Effect of Coated Silver Nanoparticles on Cancerous vs. Healthy Cells. J. Toxicol. 2022, 2022, 1519104. [Google Scholar] [CrossRef]
- Stati, G.; Rossi, F.; Trakoolwilaiwan, T.; Tung, L.D.; Mourdikoudis, S.; Thanh, N.T.K.; Di Pietro, R. Development and Characterization of Curcumin-Silver Nanoparticles as a Promising Formulation to Test on Human Pterygium-Derived Keratinocytes. Molecules 2022, 27, 282. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbalinardo, M.; Benvenuti, E.; Gentili, D.; Chiarini, F.; Bertacchini, J.; Roncucci, L.; Sena, P. Differential Cytotoxicity of Surface-Functionalized Silver Nanoparticles in Colorectal Cancer and Ex-Vivo Healthy Colonocyte Models. Cancers 2025, 17, 1475. https://doi.org/10.3390/cancers17091475
Barbalinardo M, Benvenuti E, Gentili D, Chiarini F, Bertacchini J, Roncucci L, Sena P. Differential Cytotoxicity of Surface-Functionalized Silver Nanoparticles in Colorectal Cancer and Ex-Vivo Healthy Colonocyte Models. Cancers. 2025; 17(9):1475. https://doi.org/10.3390/cancers17091475
Chicago/Turabian StyleBarbalinardo, Marianna, Emilia Benvenuti, Denis Gentili, Francesca Chiarini, Jessika Bertacchini, Luca Roncucci, and Paola Sena. 2025. "Differential Cytotoxicity of Surface-Functionalized Silver Nanoparticles in Colorectal Cancer and Ex-Vivo Healthy Colonocyte Models" Cancers 17, no. 9: 1475. https://doi.org/10.3390/cancers17091475
APA StyleBarbalinardo, M., Benvenuti, E., Gentili, D., Chiarini, F., Bertacchini, J., Roncucci, L., & Sena, P. (2025). Differential Cytotoxicity of Surface-Functionalized Silver Nanoparticles in Colorectal Cancer and Ex-Vivo Healthy Colonocyte Models. Cancers, 17(9), 1475. https://doi.org/10.3390/cancers17091475







