Particle Therapy to Overcome Cancer Radiation Resistance: “ARCHADE” Consortium Updates in Radiation Biology
Simple Summary
Abstract
1. Introduction
2. Radiobiology of Radioresistant Cancer Models: Advantages of Protons and C-Ions Relative to X-Rays
2.1. Determination of the RBE of Irradiations with Particles
2.1.1. Chondrosarcoma (CHS) Models
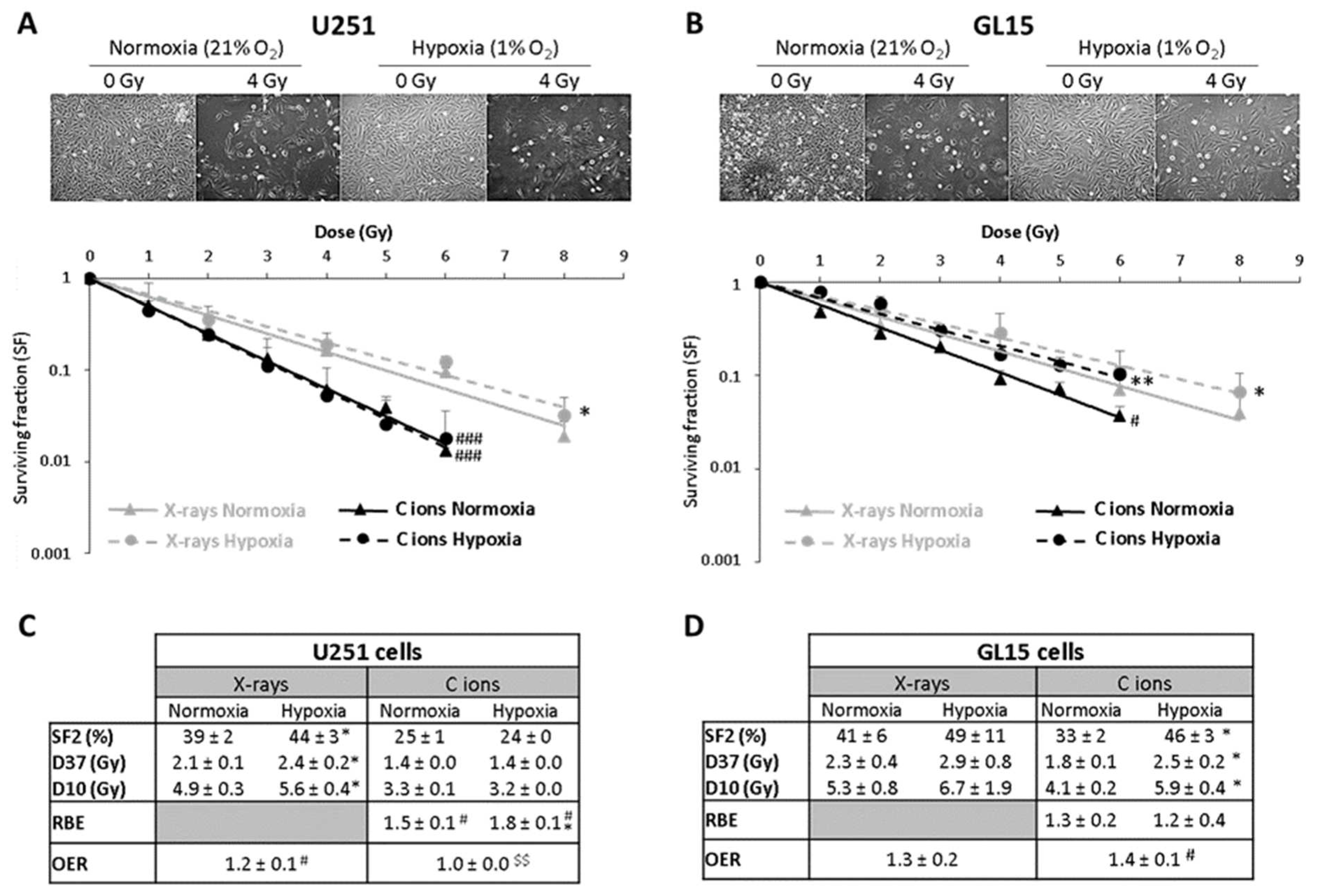
2.1.2. Glioblastoma (GB) Models
2.2. Impact of Hypoxia on Radioresistance
2.2.1. GB Models
2.2.2. Lung Cancer Cells Models
2.3. The Impact of CSCs on Radioresistance
2.3.1. GB Models
2.3.2. CHS Models
2.4. Radiosensitization of Resistant Cancer Models with Targeted Drug: Combination with Protons or C-Ions Versus X-Rays
2.4.1. PARP Inhibitor on CHS
2.4.2. PARP Inhibitor on GB
2.4.3. Inhibitors of Proliferation: KRAS G12C Inhibitor on Lung Cancer
2.4.4. Nanoparticles with a CHS Model
3. Radiotoxicity of Protons and C-Ions Relative to X-Rays on Normal Tissues
3.1. Radiation Side Effects on Healthy Brain
3.1.1. Cognitive Deficits and Fatigue After Brain Irradiation
3.1.2. Systemic Inflammation After Brain Irradiation
3.2. Radiation Side-Effects on the Cartilage
3.3. Radiation Side-Effects on the Skin
3.4. Radiation Side-Effects on the Stem Cells: Focus on the Role of Oxidative Stress in the Response of Normal Adipose-Derived Stem Cells
4. Non-Targeted Effects of High LET Particles (C-Ions and Protons) Compared to X-Rays: Radiation Induced Bystander Effects
4.1. Analysis of Bystander Effects
4.2. Analysis of Bystander Factors Secreted by CHS Cells
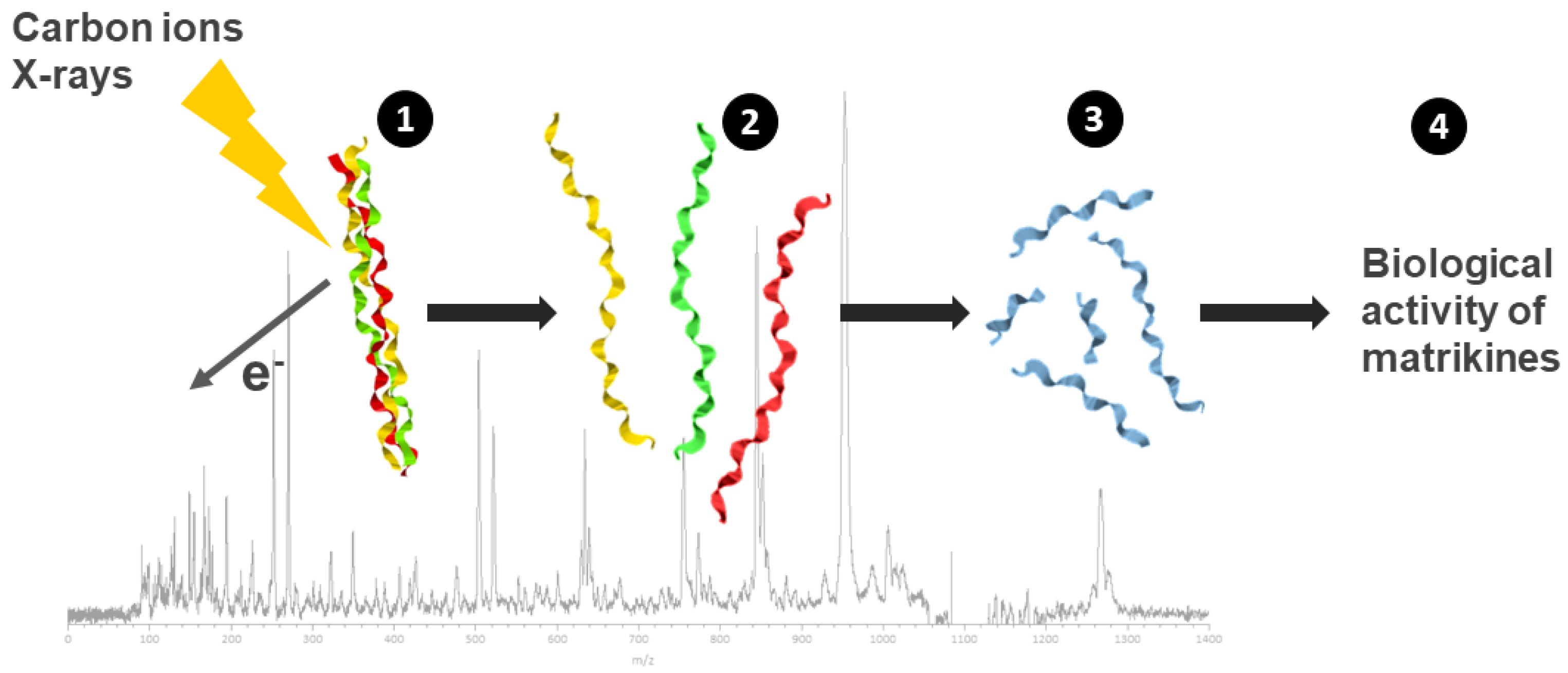
4.3. Bystander Activity of Matrikines from Collagen Irradiation
5. Discussion
6. Conclusions
Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chevalier, F. Counteracting Radio-Resistance Using the Optimization of Radiotherapy. Int. J. Mol. Sci. 2020, 21, 1767. [Google Scholar] [CrossRef]
- Thariat, J.; Valable, S.; Laurent, C.; Haghdoost, S.; Pérès, E.A.; Bernaudin, M.; Sichel, F.; Lesueur, P.; Césaire, M.; Petit, E.; et al. Hadrontherapy Interactions in Molecular and Cellular Biology. Int. J. Mol. Sci. 2020, 21, 133. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, F.; Lesueur, P.; Gaubert, G. CYCLHAD: A French Facility Dedicated for Research and Treatment in Hadrontherapy. Nucl. Phys. News 2022, 32, 27–31. [Google Scholar] [CrossRef]
- Chevalier, F.; Hamdi, D.H.; Saintigny, Y.; Lefaix, J.-L. Proteomic Overview and Perspectives of the Radiation-Induced Bystander Effects. Mutat. Res. Rev. Mutat. Res. 2014, 763, 280–293. [Google Scholar] [CrossRef]
- Lesueur, P.; Chevalier, F.; Austry, J.-B.; Waissi, W.; Burckel, H.; Noël, G.; Habrand, J.-L.; Saintigny, Y.; Joly, F. Poly-(ADP-Ribose)-Polymerase Inhibitors as Radiosensitizers: A Systematic Review of Pre-Clinical and Clinical Human Studies. Oncotarget 2017, 8, 69105–69124. [Google Scholar] [CrossRef]
- Césaire, M.; Thariat, J.; Candéias, S.M.; Stefan, D.; Saintigny, Y.; Chevalier, F. Combining PARP Inhibition, Radiation, and Immunotherapy: A Possible Strategy to Improve the Treatment of Cancer? Int. J. Mol. Sci. 2018, 19, 3793. [Google Scholar] [CrossRef]
- Césaire, M.; Montanari, J.; Curcio, H.; Lerouge, D.; Gervais, R.; Demontrond, P.; Balosso, J.; Chevalier, F. Radioresistance of Non-Small Cell Lung Cancers and Therapeutic Perspectives. Cancers 2022, 14, 2829. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, A.; Tudor, M.; Montanari, J.; Commenchail, K.; Savu, D.I.; Lesueur, P.; Chevalier, F. Chondrosarcoma Resistance to Radiation Therapy: Origins and Potential Therapeutic Solutions. Cancers 2023, 15, 1962. [Google Scholar] [CrossRef]
- Rødland, G.E.; Temelie, M.; Eek Mariampillai, A.; Hauge, S.; Gilbert, A.; Chevalier, F.; Savu, D.I.; Syljuåsen, R.G. Potential Benefits of Combining Proton or Carbon Ion Therapy with DNA Damage Repair Inhibitors. Cells 2024, 13, 1058. [Google Scholar] [CrossRef]
- Chevalier, F.; Hamdi, D.H.; Lepleux, C.; Temelie, M.; Nicol, A.; Austry, J.B.; Lesueur, P.; Vares, G.; Savu, D.; Nakajima, T.; et al. High LET Radiation Overcomes In Vitro Resistance to X-Rays of Chondrosarcoma Cell Lines. Technol. Cancer Res. Treat. 2019, 18, 1533033819871309. [Google Scholar] [CrossRef]
- Hamdi, D.H.; Barbieri, S.; Chevalier, F.; Groetz, J.-E.; Legendre, F.; Demoor, M.; Galera, P.; Lefaix, J.-L.; Saintigny, Y. In Vitro Engineering of Human 3D Chondrosarcoma: A Preclinical Model Relevant for Investigations of Radiation Quality Impact. BMC Cancer 2015, 15, 579. [Google Scholar] [CrossRef] [PubMed]
- Valable, S.; Gérault, A.N.; Lambert, G.; Leblond, M.M.; Anfray, C.; Toutain, J.; Bordji, K.; Petit, E.; Bernaudin, M.; Pérès, E.A. Impact of Hypoxia on Carbon Ion Therapy in Glioblastoma Cells: Modulation by LET and Hypoxia-Dependent Genes. Cancers 2020, 12, 2019. [Google Scholar] [CrossRef]
- Calipel, A.; Lux, A.; Guérin, S.; Lefaix, J.-L.; Laurent, C.; Bernaudin, M.; Mouriaux, F. Differential Radiosensitivity of Uveal Melanoma Cell Lines After X-Rays or Carbon Ions Radiation. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3085–3094. [Google Scholar] [CrossRef] [PubMed]
- Nisar, H.; Labonté, F.M.; Roggan, M.D.; Schmitz, C.; Chevalier, F.; Konda, B.; Diegeler, S.; Baumstark-Khan, C.; Hellweg, C.E. Hypoxia Modulates Radiosensitivity and Response to Different Radiation Qualities in A549 Non-Small Cell Lung Cancer (NSCLC) Cells. Int. J. Mol. Sci. 2024, 25, 1010. [Google Scholar] [CrossRef] [PubMed]
- Nisar, H.; Sanchidrián González, P.M.; Labonté, F.M.; Schmitz, C.; Roggan, M.D.; Kronenberg, J.; Konda, B.; Chevalier, F.; Hellweg, C.E. NF-κB in the Radiation Response of A549 Non-Small Cell Lung Cancer Cells to X-Rays and Carbon Ions under Hypoxia. Int. J. Mol. Sci. 2024, 25, 4495. [Google Scholar] [CrossRef]
- Lesueur, P.; Chevalier, F.; El-Habr, E.A.; Junier, M.-P.; Chneiweiss, H.; Castera, L.; Müller, E.; Stefan, D.; Saintigny, Y. Radiosensitization Effect of Talazoparib, a Parp Inhibitor, on Glioblastoma Stem Cells Exposed to Low and High Linear Energy Transfer Radiation. Sci. Rep. 2018, 8, 3664. [Google Scholar] [CrossRef]
- Vares, G.; Ahire, V.; Sunada, S.; Ho Kim, E.; Sai, S.; Chevalier, F.; Romeo, P.-H.; Yamamoto, T.; Nakajima, T.; Saintigny, Y. A Multimodal Treatment of Carbon Ions Irradiation, miRNA-34 and mTOR Inhibitor Specifically Control High-Grade Chondrosarcoma Cancer Stem Cells. Radiother. Oncol. 2020, 150, 253–261. [Google Scholar] [CrossRef]
- Césaire, M.; Ghosh, U.; Austry, J.-B.; Muller, E.; Cammarata, F.P.; Guillamin, M.; Caruso, M.; Castéra, L.; Petringa, G.; Cirrone, G.A.P.; et al. Sensitization of Chondrosarcoma Cells with PARP Inhibitor and High-LET Radiation. J. Bone Oncol. 2019, 17, 100246. [Google Scholar] [CrossRef]
- Gilbert, A.; Tudor, M.; Delaunay, A.; Leman, R.; Levilly, J.; Atkinson, A.; Castéra, L.; Dinischiotu, A.; Savu, D.I.; Valable, S.; et al. Radiosensitizing Effect of PARP Inhibition on Chondrosarcoma and Chondrocyte Cells Is Dependent on Radiation LET. Biomolecules 2024, 14, 1071. [Google Scholar] [CrossRef]
- Rødland, G.E.; Temelie, M.; Mariampillai, A.E.; Serban, A.M.; Edin, N.F.J.; Malinen, E.; Lindbergsengen, L.; Gilbert, A.; Chevalier, F.; Savu, D.I.; et al. Interferon Signaling Is Enhanced by ATR Inhibition in Glioblastoma Cells Irradiated with X-Rays, Protons or Carbon Ions. Radiother. Oncol. 2025, 203, 110669. [Google Scholar] [CrossRef]
- Tudor, M.; Popescu, R.C.; Negoita, R.D.; Gilbert, A.; Ilisanu, M.A.; Temelie, M.; Dinischiotu, A.; Chevalier, F.; Mihailescu, M.; Savu, D.I. In Vitro Hyperspectral Biomarkers of Human Chondrosarcoma Cells in Nanoparticle-Mediated Radiosensitization Using Carbon Ions. Sci. Rep. 2023, 13, 14878. [Google Scholar] [CrossRef] [PubMed]
- Tudor, M.; Popescu, R.C.; Irimescu, I.N.; Rzyanina, A.; Tarba, N.; Dinischiotu, A.; Craciun, L.; Esanu, T.R.; Vasile, E.; Hotnog, A.T.; et al. Enhancing Proton Radiosensitivity of Chondrosarcoma Using Nanoparticle-Based Drug Delivery Approaches: A Comparative Study of High- and Low-Energy Protons. Int. J. Mol. Sci. 2024, 25, 11481. [Google Scholar] [CrossRef]
- Pham, T.-N.; Coupey, J.; Toutain, J.; Candéias, S.M.; Simonin, G.; Rousseau, M.; Touzani, O.; Thariat, J.; Valable, S. Early Effects of Different Brain Radiotherapy Modalities on Circulating Leucocyte Subpopulations in Rodents. Int. J. Radiat. Biol. 2024, 100, 744–755. [Google Scholar] [CrossRef]
- Pham, T.-N.; Coupey, J.; Rousseau, M.; Thariat, J.; Valable, S. Revealing the Effect of X-Ray or Proton Brain Irradiation on Systemic Inflammation and Leukocyte Subpopulation Interplay in Rodents. J. Leukoc. Biol. 2024, 116, 1530–1543. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, D.H.; Chevalier, F.; Groetz, J.-E.; Durantel, F.; Thuret, J.-Y.; Mann, C.; Saintigny, Y. Comparable Senescence Induction in Three-Dimensional Human Cartilage Model by Exposure to Therapeutic Doses of X-Rays or C-Ions. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Leduc, A.; Pottier, I.; Prévost, V.; Sichel, F.; Lefaix, J.-L. Dramatic Increase in Oxidative Stress in Carbon-Irradiated Normal Human Skin Fibroblasts. PLoS ONE 2013, 8, e85158. [Google Scholar] [CrossRef]
- Prevost, V.; Sichel, F.; Pottier, I.; Leduc, A.; Lagadu, S.; Laurent, C. Production of Early and Late Nuclear DNA Damage and Extracellular 8-oxodG in Normal Human Skin Fibroblasts after Carbon Ion Irradiation Compared to X-Rays. Toxicol. In Vitro 2018, 52, 116–121. [Google Scholar] [CrossRef]
- Chaouni, S.; Leduc, A.; Pouzoulet, F.; De Marzi, L.; Megnin-Chanet, F.; Stefan, D.; Habrand, J.-L.; Sichel, F.; Laurent, C. Biological Effects of Scattered Versus Scanned Proton Beams on Normal Tissues in Total Body Irradiated Mice: Survival, Genotoxicity, Oxidative Stress and Inflammation. Antioxidants 2020, 9, 1170. [Google Scholar] [CrossRef]
- Leduc, A.; Chaouni, S.; Pouzoulet, F.; De Marzi, L.; Megnin-Chanet, F.; Corre, E.; Stefan, D.; Habrand, J.-L.; Sichel, F.; Laurent, C. Differential Normal Skin Transcriptomic Response in Total Body Irradiated Mice Exposed to Scattered versus Scanned Proton Beams. Sci. Rep. 2021, 11, 5876. [Google Scholar] [CrossRef]
- Godoy, P.R.D.V.; Pour Khavari, A.; Rizzo, M.; Sakamoto-Hojo, E.T.; Haghdoost, S. Targeting NRF2, Regulator of Antioxidant System, to Sensitize Glioblastoma Neurosphere Cells to Radiation-Induced Oxidative Stress. Oxidative Med. Cell. Longev. 2020, 2020, 2534643. [Google Scholar] [CrossRef]
- Hammad, M.; Salma, R.; Balosso, J.; Rezvani, M.; Haghdoost, S. Role of Oxidative Stress Signaling, Nrf2, on Survival and Stemness of Human Adipose-Derived Stem Cells Exposed to X-Rays, Protons and Carbon Ions. Antioxidants 2024, 13, 1035. [Google Scholar] [CrossRef]
- Lepleux, C.; Marie-Brasset, A.; Temelie, M.; Boulanger, M.; Brotin, É.; Goldring, M.B.; Hirtz, C.; Varès, G.; Nakajima, T.; Saintigny, Y.; et al. Bystander Effectors of Chondrosarcoma Cells Irradiated at Different LET Impair Proliferation of Chondrocytes. J. Cell. Commun. Signal. 2019, 13, 343–356. [Google Scholar] [CrossRef]
- Gilbert, A.; Payet, V.; Bernay, B.; Chartier-Garcia, E.; Testard, I.; Candéias, S.M.; Chevalier, F. Label-Free Direct Mass Spectrometry Analysis of the Bystander Effects Induced in Chondrocytes by Chondrosarcoma Cells Irradiated with X-Rays and Carbon Ions. Front. Biosci. Landmark 2022, 27, 277. [Google Scholar] [CrossRef]
- Chailapakul, P.; Maloney, O.; Hirakawa, H.; Fujimori, A.; Kitamura, H.; Kato, T.A. The Contribution of High-LET Track to DNA Damage Formation and Cell Death for Monoenergy and SOBP Carbon Ion Irradiation. Biochem. Biophys. Res. Commun. 2024, 696, 149500. [Google Scholar] [CrossRef] [PubMed]
- Durantel, F.; Balanzat, E.; Cassimi, A.; Chevalier, F.; Ngono-Ravache, Y.; Madi, T.; Poully, J.-C.; Ramillon, J.-M.; Rothard, H.; Ropars, F.; et al. Dosimetry for Radiobiology Experiments at GANIL. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2016, 816, 70–77. [Google Scholar] [CrossRef]
- Boissonnat, G.; Fontbonne, J.M.; Balanzat, E.; Boumard, F.; Carniol, B.; Colin, J.; Cussol, D.; Etasse, D.; Fontbonne, C.; Frelin, A.M.; et al. Characterization and Performances of DOSION, a Dosimetry Equipment Dedicated to Radiobiology Experiments Taking Place at GANIL. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2017, 856, 1–6. [Google Scholar] [CrossRef]
- Gérard, M.; Corroyer-Dulmont, A.; Lesueur, P.; Collet, S.; Chérel, M.; Bourgeois, M.; Stefan, D.; Limkin, E.J.; Perrio, C.; Guillamo, J.-S.; et al. Hypoxia Imaging and Adaptive Radiotherapy: A State-of-the-Art Approach in the Management of Glioma. Front. Med. 2019, 6, 117. [Google Scholar] [CrossRef] [PubMed]
- Pérès, E.A.; Gérault, A.N.; Valable, S.; Roussel, S.; Toutain, J.; Divoux, D.; Guillamo, J.-S.; Sanson, M.; Bernaudin, M.; Petit, E. Silencing Erythropoietin Receptor on Glioma Cells Reinforces Efficacy of Temozolomide and X-Rays through Senescence and Mitotic Catastrophe. Oncotarget 2014, 6, 2101–2119. [Google Scholar] [CrossRef]
- Pérès, E.A.; Valable, S.; Guillamo, J.-S.; Marteau, L.; Bernaudin, J.-F.; Roussel, S.; Lechapt-Zalcman, E.; Bernaudin, M.; Petit, E. Targeting the Erythropoietin Receptor on Glioma Cells Reduces Tumour Growth. Exp. Cell Res. 2011, 317, 2321–2332. [Google Scholar] [CrossRef]
- Aury-Landas, J.; Pérès, E.A.; Pasquet, N.; Hélaine, C.; Lebhertz, D.; Taylor, J.; Toutain, J.; Bernaudin, M.; Samuel, V. Hadrontherapy to Target Glioblastoma Stem Cells to Overcome Radioresistance. In Proceedings of the Assemblée générale du GDR Mi2B (Outils et Méthodes Nucléaires Pour la Lutte Contre le Cancer), Bordeaux, France, 4 October 2023. [Google Scholar]
- Ben Diouf, O.; Gilbert, A.; Bernay, B.; Syljuåsen, R.G.; Tudor, M.; Temelie, M.; Savu, D.I.; Soumboundou, M.; Sall, C.; Chevalier, F. Phospho-Proteomics Analysis of Early Response to X-Ray Irradiation Reveals Molecular Mechanism Potentially Related to U251 Cell Radioresistance. Proteomes 2025, 13, 1. [Google Scholar] [CrossRef]
- Montanari, J.; Césaire, M.; Gilbert, A.; Chevalier, F. Role of KRAS Mutation on NSCLC Resistance to X-Rays and Protons. In Proceedings of the ERRS 2022—47th Annual Meeting of the European Radiation Research Society, Catania, Italy, 21 September 2022. [Google Scholar]
- Popescu, R.C.; Savu, D.; Dorobantu, I.; Vasile, B.S.; Hosser, H.; Boldeiu, A.; Temelie, M.; Straticiuc, M.; Iancu, D.A.; Andronescu, E.; et al. Efficient Uptake and Retention of Iron Oxide-Based Nanoparticles in HeLa Cells Leads to an Effective Intracellular Delivery of Doxorubicin. Sci. Rep. 2020, 10, 10530. [Google Scholar] [CrossRef] [PubMed]
- Bécam, J.; Ropars, G.; Dwiri, F.-A.; Brunaud, C.; Toutain, J.; Chazalviel, L.; Naveau, M.; Valable, S.; Bernaudin, M.; Touzani, O.; et al. Physical Activity Attenuates Brain Irradiation-Associated Skeletal Muscle Damage in the Rat. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Brunaud, C.; Valable, S.; Ropars, G.; Dwiri, F.-A.; Naveau, M.; Toutain, J.; Bernaudin, M.; Freret, T.; Léger, M.; Touzani, O.; et al. Deformation-Based Morphometry: A Sensitive Imaging Approach to Detect Radiation-Induced Brain Injury? Cancer Imaging 2024, 24, 95. [Google Scholar] [CrossRef]
- Le Deroff, C.; Pérès, E.A.; Ledoux, X.; Toutain, J.; Frelin-Labalme, A.-M. In Vivo Surface Dosimetry with a Scintillating Fiber Dosimeter in Preclinical Image-Guided Radiotherapy. Med. Phys. 2020, 47, 234–241. [Google Scholar] [CrossRef]
- Dwiri, F.-A.; Bécam, J.; Carole, B.; Chazalviel, L.; Samuel, V.; Bernaudin, M.; Pérès, E.A.; Touzani, O. Impact of Targeted Irradiation on the Healthy Brain Tissue and Cognition in the Rat. In Proceedings of the 46th Annual Meeting for the European Radiation Research Society, Caen, France, 26 November 2021. [Google Scholar]
- Beaufils, V.; Bécam, J.; Dwiri, F.-A.; Touzani, O.; Pérès, E.A. Optimisation Study of a Whole-Brain Irradiation Model in the Rat: Comparison between Vertical and Horizontal X-Ray Beams. In Proceedings of the 16th International Workshop “Optimizing Imaging and Dose-Response in Radiotherapies” (Cancéropôle Grand-Ouest), Erquy, France, 4 October 2023. [Google Scholar]
- Pham, T.-N.; Coupey, J.; Thariat, J.; Valable, S. Lymphocyte Radiosensitivity: An Extension to the Linear-Quadratic Model? Radiother. Oncol. 2024, 198, 110406. [Google Scholar] [CrossRef]
- Bian, X.; Piipponen, M.; Liu, Z.; Luo, L.; Geara, J.; Chen, Y.; Sangsuwan, T.; Maselli, M.; Diaz, C.; Bain, C.A.; et al. Epigenetic Memory of Radiotherapy in Dermal Fibroblasts Impairs Wound Repair Capacity in Cancer Survivors. Nat. Commun. 2024, 15, 9286. [Google Scholar] [CrossRef]
- Sangsuwan, T.; Khavari, A.P.; Blomberg, E.; Romell, T.; Godoy, P.R.D.V.D.; Harms-Ringdahl, M.; Haghdoost, S. Oxidative Stress Levels and DNA Repair Kinetics in Senescent Primary Human Fibroblasts Exposed to Chronic Low Dose Rate of Ionizing Radiation. Front. Biosci. Landmark 2023, 28, 296. [Google Scholar] [CrossRef]
- Hammad, M.; Raftari, M.; Cesário, R.; Salma, R.; Godoy, P.; Emami, S.N.; Haghdoost, S. Roles of Oxidative Stress and Nrf2 Signaling in Pathogenic and Non-Pathogenic Cells: A Possible General Mechanism of Resistance to Therapy. Antioxidants 2023, 12, 1371. [Google Scholar] [CrossRef] [PubMed]
- Tudor, M.; Gilbert, A.; Lepleux, C.; Temelie, M.; Hem, S.; Armengaud, J.; Brotin, E.; Haghdoost, S.; Savu, D.; Chevalier, F. A Proteomic Study Suggests Stress Granules as New Potential Actors in Radiation-Induced Bystander Effects. Int. J. Mol. Sci. 2021, 22, 7957. [Google Scholar] [CrossRef]
- Montanari, J.; Schwob, L.; Marie-Brasset, A.; Vinatier, C.; Lepleux, C.; Antoine, R.; Guicheux, J.; Poully, J.-C.; Chevalier, F. Pilot Screening of Potential Matrikines Resulting from Collagen Breakages through Ionizing Radiation. Radiat. Environ. Biophys. 2024, 63, 337–350. [Google Scholar] [CrossRef]
- Lalande, M.; Schwob, L.; Vizcaino, V.; Chirot, F.; Dugourd, P.; Schlathölter, T.; Poully, J.-C. Direct Radiation Effects on the Structure and Stability of Collagen and Other Proteins. ChemBioChem 2019, 20, 2972–2980. [Google Scholar] [CrossRef] [PubMed]
- Tinganelli, W.; Durante, M. Carbon Ion Radiobiology. Cancers 2020, 12, 3022. [Google Scholar] [CrossRef]
- Durante, M.; Debus, J. Heavy Charged Particles: Does Improved Precision and Higher Biological Effectiveness Translate to Better Outcome in Patients? Semin. Radiat. Oncol. 2018, 28, 160–167. [Google Scholar] [CrossRef]
- Vischioni, B.; Barcellini, A.; Magro, G.; Rotondi, M.; Durante, M.; Facoetti, A.; Thariat, J.; Orlandi, E. RAdioresistant, RAre, REcurrent, and RAdioinduced: 4Rs of Hadrontherapy for Patients Selections. Int. J. Part. Ther. 2025, 15, 100737. [Google Scholar] [CrossRef]
- Gelderblom, H.; Hogendoorn, P.C.W.; Dijkstra, S.D.; van Rijswijk, C.S.; Krol, A.D.; Taminiau, A.H.M.; Bovée, J.V.M.G. The Clinical Approach towards Chondrosarcoma. Oncologist 2008, 13, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Lohberger, B.; Barna, S.; Glänzer, D.; Eck, N.; Kerschbaum-Gruber, S.; Stasny, K.; Leithner, A.; Georg, D. Cellular and Molecular Biological Alterations after Photon, Proton, and Carbon Ions Irradiation in Human Chondrosarcoma Cells Linked with High-Quality Physics Data. Int. J. Mol. Sci. 2022, 23, 11464. [Google Scholar] [CrossRef]
- Mery, B.; Espenel, S.; Guy, J.-B.; Rancoule, C.; Vallard, A.; Aloy, M.-T.; Rodriguez-Lafrasse, C.; Magné, N. Biological Aspects of Chondrosarcoma: Leaps and Hurdles. Crit. Rev. Oncol. Hematol. 2018, 126, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Wakatsuki, M.; Magpayo, N.; Kawamura, H.; Held, K.D. Differential Bystander Signaling between Radioresistant Chondrosarcoma Cells and Fibroblasts after X-Ray, Proton, Iron Ion and Carbon Ion Exposures. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e103–e108. [Google Scholar] [CrossRef]
- Nakano, T.; Suzuki, Y.; Ohno, T.; Kato, S.; Suzuki, M.; Morita, S.; Sato, S.; Oka, K.; Tsujii, H. Carbon Beam Therapy Overcomes the Radiation Resistance of Uterine Cervical Cancer Originating from Hypoxia. Clin. Cancer Res. 2006, 12, 2185–2190. [Google Scholar] [CrossRef]
- Tinganelli, W.; Durante, M.; Hirayama, R.; Krämer, M.; Maier, A.; Kraft-Weyrather, W.; Furusawa, Y.; Friedrich, T.; Scifoni, E. Kill-Painting of Hypoxic Tumours in Charged Particle Therapy. Sci. Rep. 2015, 5, 17016. [Google Scholar] [CrossRef]
- Wozny, A.-S.; Vares, G.; Alphonse, G.; Lauret, A.; Monini, C.; Magné, N.; Cuerq, C.; Fujimori, A.; Monboisse, J.-C.; Beuve, M.; et al. ROS Production and Distribution: A New Paradigm to Explain the Differential Effects of X-Ray and Carbon Ion Irradiation on Cancer Stem Cell Migration and Invasion. Cancers 2019, 11, 468. [Google Scholar] [CrossRef]
- Miszczyk, J.; Rawojć, K.; Panek, A.; Borkowska, A.; Prasanna, P.G.S.; Ahmed, M.M.; Swakoń, J.; Gałaś, A. Do Protons and X-Rays Induce Cell-Killing in Human Peripheral Blood Lymphocytes by Different Mechanisms? Clin. Transl. Radiat. Oncol. 2018, 9, 23–29. [Google Scholar] [CrossRef]
- Durante, M.; Formenti, S. Harnessing Radiation to Improve Immunotherapy: Better with Particles? Br. J. Radiol. 2020, 93, 20190224. [Google Scholar] [CrossRef]
- Durante, M. Kaplan Lecture 2023: Lymphopenia in Particle Therapy. Int. J. Radiat. Biol. 2024, 100, 669–677. [Google Scholar] [CrossRef]
- Lesueur, P.; Clarisse, B.; Lequesne, J.; Licaj, I.; Feuvret, L.; Stefan, D.; Ricard, D.; Noel, G.; Balosso, J.; Lange, M.; et al. Proton Therapy versus Conventional Radiotherapy for the Treatment of Cavernous Sinus Benign Meningioma, a Randomized Controlled Phase III Study Protocol (COG-PROTON-01). BMC Cancer 2024, 24, 1594. [Google Scholar] [CrossRef]
- Lesueur, P.; Joly, F.; Clarisse, B.; Lequesne, J.; Stefan, D.; Balosso, J.; Lange, M.; Thureau, S.; Capel, A.; Castera, M.; et al. Neurocognitive Impact of Different Irradiation Modalities for Patients with Grade I-II Skull Base Meningioma: A Prospective Multi-Arm Cohort Study (CANCER COG). Radiat. Oncol. 2025, 20, 16. [Google Scholar] [CrossRef]
- Dosanjh, M.; Jones, B.; Pawelke, J.; Pruschy, M.; Sørensen, B.S. Overview of Research and Therapy Facilities for Radiobiological Experimental Work in Particle Therapy. Report from the European Particle Therapy Network Radiobiology Group. Radiother. Oncol. 2018, 128, 14–18. [Google Scholar] [CrossRef]
- Malouff, T.D.; Mahajan, A.; Krishnan, S.; Beltran, C.; Seneviratne, D.S.; Trifiletti, D.M. Carbon Ion Therapy: A Modern Review of an Emerging Technology. Front. Oncol. 2020, 10, 82. [Google Scholar] [CrossRef]
- Riva, G.; Cavallo, I.; Gandini, S.; Ingargiola, R.; Pecorilla, M.; Imparato, S.; Rossi, E.; Mirandola, A.; Ciocca, M.; Orlandi, E.; et al. Particle Radiotherapy for Skull Base Chondrosarcoma: A Clinical Series from Italian National Center for Oncological Hadrontherapy. Cancers 2021, 13, 4423. [Google Scholar] [CrossRef]
- Permata, T.B.M.; Sato, H.; Gu, W.; Kakoti, S.; Uchihara, Y.; Yoshimatsu, Y.; Sato, I.; Kato, R.; Yamauchi, M.; Suzuki, K.; et al. High Linear Energy Transfer Carbon-Ion Irradiation Upregulates PD-L1 Expression More Significantly than X-Rays in Human Osteosarcoma U2OS Cells. J. Radiat. Res. 2021, 62, 773–781. [Google Scholar] [CrossRef]
- Kaneko, T.; Suefuji, H.; Koto, M.; Demizu, Y.; Saitoh, J.-I.; Tsuji, H.; Okimoto, T.; Ohno, T.; Shioyama, Y.; Nemoto, K.; et al. Multicenter Study of Carbon-Ion Radiotherapy for Oropharyngeal Non-Squamous Cell Carcinoma. In Vivo 2021, 35, 2239–2245. [Google Scholar] [CrossRef]
- Chen, J.; Mao, J.; Ma, N.; Wu, K.-L.; Lu, J.; Jiang, G.-L. Definitive Carbon Ion Radiotherapy for Tracheobronchial Adenoid Cystic Carcinoma: A Preliminary Report. BMC Cancer 2021, 21, 734. [Google Scholar] [CrossRef]
- Konings, K.; Vandevoorde, C.; Baselet, B.; Baatout, S.; Moreels, M. Combination Therapy with Charged Particles and Molecular Targeting: A Promising Avenue to Overcome Radioresistance. Front. Oncol. 2020, 10, 128. [Google Scholar] [CrossRef]
- Veuger, S.J.; Curtin, N.J.; Richardson, C.J.; Smith, G.C.M.; Durkacz, B.W. Radiosensitization and DNA Repair Inhibition by the Combined Use of Novel Inhibitors of DNA-Dependent Protein Kinase and Poly(ADP-Ribose) Polymerase-1. Cancer Res. 2003, 63, 6008–6015. [Google Scholar]
- Bache, M.; Kappler, M.; Said, H.M.; Staab, A.; Vordermark, D. Detection and Specific Targeting of Hypoxic Regions within Solid Tumors: Current Preclinical and Clinical Strategies. Curr. Med. Chem. 2008, 15, 322–338. [Google Scholar] [CrossRef]
- Ghorai, A.; Sarma, A.; Chowdhury, P.; Ghosh, U. PARP-1 Depletion in Combination with Carbon Ion Exposure Significantly Reduces MMPs Activity and Overall Increases TIMPs Expression in Cultured HeLa Cells. Radiat. Oncol. 2016, 11, 126. [Google Scholar] [CrossRef]
- Park, S.; Choi, C.; Kim, H.; Shin, Y.J.; Oh, Y.; Park, W.; Cho, W.K.; Kim, N. Olaparib Enhances Sensitization of BRCA-Proficient Breast Cancer Cells to x-Rays and Protons. Breast Cancer Res. Treat. 2024, 203, 449–461. [Google Scholar] [CrossRef]
- Kawanishi, M.; Fujita, M.; Karasawa, K. Combining Carbon-Ion Irradiation and PARP Inhibitor, Olaparib Efficiently Kills BRCA1-Mutated Triple-Negative Breast Cancer Cells. Breast Cancer Basic Clin. Res. 2022, 16, 11782234221080553. [Google Scholar] [CrossRef]
- Zhou, C.; Fabbrizi, M.R.; Hughes, J.R.; Grundy, G.J.; Parsons, J.L. Effectiveness of PARP Inhibition in Enhancing the Radiosensitivity of 3D Spheroids of Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2022, 12, 940377. [Google Scholar] [CrossRef]
- Wang, L.; Cao, J.; Wang, X.; Lin, E.; Wang, Z.; Li, Y.; Li, Y.; Chen, M.; Wang, X.; Jiang, B.; et al. Proton and Photon Radiosensitization Effects of Niraparib, a PARP-1/-2 Inhibitor, on Human Head and Neck Cancer Cells. Head Neck 2020, 42, 2244–2256. [Google Scholar] [CrossRef]
- Kageyama, S.-I.; Junyan, D.; Hojo, H.; Motegi, A.; Nakamura, M.; Tsuchihara, K.; Akimoto, T. PARP Inhibitor Olaparib Sensitizes Esophageal Carcinoma Cells to Fractionated Proton Irradiation. J. Radiat. Res. 2020, 61, 177–186. [Google Scholar] [CrossRef]
- Waissi, W.; Nicol, A.; Jung, M.; Rousseau, M.; Jarnet, D.; Noel, G.; Burckel, H. Radiosensitizing Pancreatic Cancer with PARP Inhibitor and Gemcitabine: An In Vivo and a Whole-Transcriptome Analysis after Proton or Photon Irradiation. Cancers 2021, 13, 527. [Google Scholar] [CrossRef]
- Ghorai, A.; Bhattacharyya, N.P.; Sarma, A.; Ghosh, U. Radiosensitivity and Induction of Apoptosis by High LET Carbon Ion Beam and Low LET Gamma Radiation: A Comparative Study. Scientifica 2014, 2014, 438030. [Google Scholar] [CrossRef]
- Hong, J.H.; Gatti, R.A.; Huo, Y.K.; Chiang, C.S.; McBride, W.H. G2/M-Phase Arrest and Release in Ataxia Telangiectasia and Normal Cells after Exposure to Ionizing Radiation. Radiat. Res. 1994, 140, 17–23. [Google Scholar] [CrossRef]
- Eek Mariampillai, A.; Hauge, S.; Øynebråten, I.; Rødland, G.E.; Corthay, A.; Syljuåsen, R.G. Caspase Activation Counteracts Interferon Signaling after G2 Checkpoint Abrogation by ATR Inhibition in Irradiated Human Cancer Cells. Front. Oncol. 2022, 12, 981332. [Google Scholar] [CrossRef]
- Bright, S.J.; Manandhar, M.; Flint, D.B.; Kolachina, R.; Ben Kacem, M.; Martinus, D.K.; Turner, B.X.; Qureshi, I.; McFadden, C.H.; Marinello, P.C.; et al. ATR Inhibition Radiosensitizes Cells through Augmented DNA Damage and G2 Cell Cycle Arrest Abrogation. JCI Insight 2024, 9, e179599. [Google Scholar] [CrossRef]
- Lohberger, B.; Glänzer, D.; Eck, N.; Stasny, K.; Falkner, A.; Leithner, A.; Georg, D. The ATR Inhibitor VE-821 Enhances the Radiosensitivity and Suppresses DNA Repair Mechanisms of Human Chondrosarcoma Cells. Int. J. Mol. Sci. 2023, 24, 2315. [Google Scholar] [CrossRef]
- Zhou, Q.; Howard, M.E.; Tu, X.; Zhu, Q.; Denbeigh, J.M.; Remmes, N.B.; Herman, M.G.; Beltran, C.J.; Yuan, J.; Greipp, P.T.; et al. Inhibition of ATM Induces Hypersensitivity to Proton Irradiation by Upregulating Toxic End Joining. Cancer Res. 2021, 81, 3333–3346. [Google Scholar] [CrossRef]
- Mostafavi, M.; Ghazi, F.; Mehrabifard, M.; Alivirdiloo, V.; Hajiabbasi, M.; Rahimi, F.; Mobed, A.; Taheripak, G.; Ramezani Farani, M.; Huh, Y.S.; et al. State-of-the-Art Application of Nanoparticles in Radiotherapy: A Platform for Synergistic Effects in Cancer Treatment. Strahlenther. Onkol. 2024, 1–12. [Google Scholar] [CrossRef]
- Ternad, I.; Penninckx, S.; Lecomte, V.; Vangijzegem, T.; Conrard, L.; Lucas, S.; Heuskin, A.-C.; Michiels, C.; Muller, R.N.; Stanicki, D.; et al. Advances in the Mechanistic Understanding of Iron Oxide Nanoparticles’ Radiosensitizing Properties. Nanomaterials 2023, 13, 201. [Google Scholar] [CrossRef]
- Ibáñez-Moragues, M.; Fernández-Barahona, I.; Santacruz, R.; Oteo, M.; Luján-Rodríguez, V.M.; Muñoz-Hernando, M.; Magro, N.; Lagares, J.I.; Romero, E.; España, S.; et al. Zinc-Doped Iron Oxide Nanoparticles as a Proton-Activatable Agent for Dose Range Verification in Proton Therapy. Molecules 2023, 28, 6874. [Google Scholar] [CrossRef] [PubMed]
- Khoei, S.; Mahdavi, S.R.; Fakhimikabir, H.; Shakeri-Zadeh, A.; Hashemian, A. The Role of Iron Oxide Nanoparticles in the Radiosensitization of Human Prostate Carcinoma Cell Line DU145 at Megavoltage Radiation Energies. Int. J. Radiat. Biol. 2014, 90, 351–356. [Google Scholar] [CrossRef]
- Klein, S.; Sommer, A.; Distel, L.V.R.; Hazemann, J.-L.; Kröner, W.; Neuhuber, W.; Müller, P.; Proux, O.; Kryschi, C. Superparamagnetic Iron Oxide Nanoparticles as Novel X-Ray Enhancer for Low-Dose Radiation Therapy. J. Phys. Chem. B 2014, 118, 6159–6166. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.K.; Mitov, M.I.; Daley, E.F.; McGarry, R.C.; Anderson, K.W.; Hilt, J.Z. Targeted Iron Oxide Nanoparticles for the Enhancement of Radiation Therapy. Biomaterials 2016, 105, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Russell, E.; Dunne, V.; Russell, B.; Mohamud, H.; Ghita, M.; McMahon, S.J.; Butterworth, K.T.; Schettino, G.; McGarry, C.K.; Prise, K.M. Impact of Superparamagnetic Iron Oxide Nanoparticles on in Vitro and in Vivo Radiosensitisation of Cancer Cells. Radiat. Oncol. 2021, 16, 104. [Google Scholar] [CrossRef]
- Shetake, N.G.; Kumar, A.; Pandey, B.N. Iron-Oxide Nanoparticles Target Intracellular HSP90 to Induce Tumor Radio-Sensitization. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 857–869. [Google Scholar] [CrossRef]
- Shapiro, C.L.; Recht, A. Side Effects of Adjuvant Treatment of Breast Cancer. N. Engl. J. Med. 2001, 344, 1997–2008. [Google Scholar] [CrossRef]
- Yanagi, T.; Kamada, T.; Tsuji, H.; Imai, R.; Serizawa, I.; Tsujii, H. Dose-Volume Histogram and Dose-Surface Histogram Analysis for Skin Reactions to Carbon Ion Radiotherapy for Bone and Soft Tissue Sarcoma. Radiother. Oncol. 2010, 95, 60–65. [Google Scholar] [CrossRef]
- Kralik, S.F.; Ho, C.Y.; Finke, W.; Buchsbaum, J.C.; Haskins, C.P.; Shih, C.-S. Radiation Necrosis in Pediatric Patients with Brain Tumors Treated with Proton Radiotherapy. Am. J. Neuroradiol. 2015, 36, 1572–1578. [Google Scholar] [CrossRef]

| Type of Particle | Facility | Energy of Native Beam | LET of Particles (keV/µm) | Biological Model | Study (1) | References |
|---|---|---|---|---|---|---|
| carbon ions | GANIL | 95 MeV/A | 28 and 73 | CHS cell lines (SW1353, CH2879, OUMS27, L835) | RBE, cell cycle, g-H2AX | [10] |
| oxygen ions | GANIL | 50 MeV/A | 103 | CHS cell line SW1353 | Ki67, g-H2AX | [11] |
| carbon ions | GANIL | 95 MeV/A | 28, 50 and 100 | GBM cell lines (U251-MG, GL15) | RBE, CFE, cell cycle, p-ERK | [12] |
| carbon ions | GANIL | 75 MeV/A | 34 | Uveal melanoma (92.1, MEL270, SP6.5, MKT-BR, μ2, and TP17) | RBE, CFE, p-ERK | [13] |
| carbon ions | GANIL | 95 MeV/A | 75 | NSCLC A549 | CFE, cell cycle, gene analysis | [14] |
| carbon ions | GANIL | 95 MeV/A | 75 | NSCLC A549 | CFE, cell cycle, gene analysis, cytokines | [15] |
| carbon ions | GANIL | 95 MeV/A | 50 | GBM (R633, TG1) | proliferation, cell cycle, gene analysis, cytokines, GSC | [16] |
| carbon ions | HIMAC | 290 MeV/A | 50 | CHS (CH2879) | CFE, cell cycle, gene analysis, spheres, in vivo (mice) | [17] |
| carbon ions | GANIL | 95 MeV/A | 73 | CHS (CH2879) | proliferation, CFE, WB PARPi | [18] |
| proton | INFN-LNS | 62 MeV | 11 | CHS (CH2879) | proliferation, CFE, WB PARPi | [18] |
| carbon ions | GANIL | 95 MeV/A | 73 | CHS cell lines (OUMS27, JJ012) | proliferation, CFE, gene analysis, WB PARPi | [19] |
| carbon ions | GANIL | 95 MeV/A | 28 and 73 | GBM (U-251, T98G) | CFE, cell cycle, ELISA, ATMi, ATRi | [20] |
| proton | OCL | 15.5 MeV | 5; 42 | GBM (U-251, T98G) | CFE, cell cycle, ELISA, ATMi, ATRi | [20] |
| carbon ions | GANIL | 95 MeV/A | 73 | CHS cell lines (SW1353) | proliferation, CFE, MN, hyperspectral images | [21] |
| proton | IFIN-HH | 18 MeV | 12,6 | CHS cell lines (SW1353) | CFE, MN, G-H2AX, Nano-P | [22] |
| proton | CYRCé | 25 MeV | 2–3 | lymphocyte | circulating LY count | [23] |
| proton | CYRCé | 25 MeV | 2–3 | lymphocyte | leucocyte interplay | [24] |
| carbon ions | GANIL | 95 MeV/A | 28 | chondrocytes | CFE, WB, 3D senescence | [25] |
| carbon ions | GANIL | 75 MeV/A | 34 | NHDF | CFE, comet, stress ox, cytokines | [26] |
| carbon ions | GANIL | 75 MeV/A | 34 | NHDF | CFE, MN, 8-oxodG | [27] |
| proton | CPO | 190 MeV | 1.2 | C57Bl/6 mice | survival, LY MN, SOD, LPO, cytokines | [28] |
| proton | CPO | 190 MeV | 1.2 | C57Bl/6 mice | body weight, RNAseq | [29] |
| carbon ions | GANIL | 95 MeV/A | 28 | GBM cell lines (U87-MG, T98G, LN18, M059K, M059J) | Spheres, WB, 8-oxo-dG, NRF2 KO cells | [30] |
| carbon ions | GANIL | 95 MeV/A | 28, 33 | ADSCs | CFE, NRF2i, WB, differentiation | [31] |
| carbon ions | GANIL | 95 MeV/A | 28, 73 | CHS (SW1353), chondrocytes | CFE, bystander medium transfer, MN, cytokines | [32] |
| carbon ions | HIMAC | 290 MeV/A | 50 | CHS (SW1353), chondrocytes | CFE, bystander medium transfer, MN, cytokines | [32] |
| carbon ions | GANIL | 95 MeV/A | 73 | CHS (SW1353), chondrocytes | mass spectrometry | [33] |
| Cell survival | Clonogenicity | 3.28 |
| DNA damage | OTM | 0.38 |
| Half time repair | 0.31 | |
| 8-oxo-Gua | 0.83 | |
| Protein damage | Carbonyls | 0.21 |
| lipid damage | peroxidation | 0.49 |
| Antioxidant enzymes | SOD | 1.72 |
| Catalase | 2.63 | |
| GPx | 1.69 | |
| Oxidative status | GSH/GSSG | 0.48 |
| Inflammation | TNF-a | 0.79 |
| IL-6 | 0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valable, S.; Césaire, M.; Lecrosnier, K.; Gilbert, A.; Tudor, M.; Vares, G.; Hamdi, D.H.; Diouf, O.B.; Nguyen Pham, T.; Coupey, J.; et al. Particle Therapy to Overcome Cancer Radiation Resistance: “ARCHADE” Consortium Updates in Radiation Biology. Cancers 2025, 17, 1580. https://doi.org/10.3390/cancers17091580
Valable S, Césaire M, Lecrosnier K, Gilbert A, Tudor M, Vares G, Hamdi DH, Diouf OB, Nguyen Pham T, Coupey J, et al. Particle Therapy to Overcome Cancer Radiation Resistance: “ARCHADE” Consortium Updates in Radiation Biology. Cancers. 2025; 17(9):1580. https://doi.org/10.3390/cancers17091580
Chicago/Turabian StyleValable, Samuel, Mathieu Césaire, Kilian Lecrosnier, Antoine Gilbert, Mihaela Tudor, Guillaume Vares, Dounia Houria Hamdi, Ousseynou Ben Diouf, Thao Nguyen Pham, Julie Coupey, and et al. 2025. "Particle Therapy to Overcome Cancer Radiation Resistance: “ARCHADE” Consortium Updates in Radiation Biology" Cancers 17, no. 9: 1580. https://doi.org/10.3390/cancers17091580
APA StyleValable, S., Césaire, M., Lecrosnier, K., Gilbert, A., Tudor, M., Vares, G., Hamdi, D. H., Diouf, O. B., Nguyen Pham, T., Coupey, J., Thariat, J., Lesueur, P., Pérès, E. A., Aury-Landas, J., Nikitaki, Z., Haghdoost, S., Laurent, C., Poully, J.-C., Balosso, J., ... Chevalier, F. (2025). Particle Therapy to Overcome Cancer Radiation Resistance: “ARCHADE” Consortium Updates in Radiation Biology. Cancers, 17(9), 1580. https://doi.org/10.3390/cancers17091580









