Gelatinase B/MMP-9 in Tumour Pathogenesis and Progression
Abstract
:1. Introduction
2. The Gelatinase B/MMP-9 Gene and mRNA
Gelatinase B/MMP-9 SNPs
3. The Gelatinase B/MMP-9 Protein
3.1. Gelatinase B/MMP-9 Catalytic Site
3.2. Gelatinase B/MMP-9 Hemopexin Domain
3.3. Gelatinase B/MMP-9 O-Glycosylation Domain
3.4. Truncated Gelatinase B/MMP-9 Isoforms
4. Gelatinase B/MMP-9 Substrates
| Class | Substrate | Substrate/MMP-9 source | [Refs] |
|---|---|---|---|
| ECM Substrates | Collagen type I | (bo/mu sub/hu MMP-9) | [55] |
| Collagen type II | (hu sub/MMP-9) | [56] | |
| Collagen III | (bo sub/hu MMP-9) | [55] | |
| Collagen IV | (hu/mu sub/MMP-9) | [8,57,58,66,67,68,69,70] | |
| Collagen V | (hu sub/MMP-9) | [4,8,68] | |
| Collagen VI | (hu sub/MMP-9) | [70] | |
| Collagen α1 and α2 (VI) | (hu sub/MMP-9) | [62] | |
| Collagen α1 (XI) | (hu sub/MMP-9) | [71] | |
| Collagen α1 (XVIII) | (hu sub/MMP-9) | [70,72] | |
| Procollgen lysine-2-oxygluterate-5 dioxygenase-1 | (hu sub/MMP-9) | [62] | |
| Periostin | (hu sub/MMP-9) | [70] | |
| Galectin-1 | (hu sub/MMP-9) | [62,65] | |
| Galectin 3 | (hu sub/MMP-9) | [73] | |
| Fibronectin | (hu sub/MMP-9) | [68,70,74] | |
| Laminin | (mu sub/hu MMP-9) | [60,62,68] | |
| Tenascin C | (hu sub/MMP-9) | [70,74,75] | |
| Tenascin X | (hu sub/MMP-9) | [70] | |
| Thrombospondin-2 | (hu sub/MMP-9) | [65] | |
| Insulin growth factor binding protein 4 | (hu sub/MMP-9) | [65] | |
| Cystatin C | (hu sub/MMP-9) | [65] | |
| Elastin | (Bo/mu sub/hu/mu MMP-9) | [76,77] | |
| Vitronectin | (hu sub/MMP-9) | [78] | |
| Entactin | (mu sub/hu MMP-9) | [79] | |
| Heparan sulphate | (hu sub/MMP-9) | [80] | |
| Cell surface substrates | ICAM-1 | (hu sub/MMP-9) | [81,82] |
| uPAR | (hu sub/MMP-9) | [83] | |
| Laminin receptor | (Xenopus sub/hu MMP-9) | [84] | |
| IL2Rα | (hu sub/MMP-9) | [85,86] | |
| proTNFα | (hu sub/MMP-9) | [87] | |
| IL-1β | (hu sub/MMP-9) | [88,89] | |
| Kit ligand | (mu/hu sub/MMP-9) | [90,91] | |
| β2 integrin subunit | (mu sub/MMP-9) | [92] | |
| proTGFβ | (mu sub/MMP-9) | [93] | |
| HB-EGF | (hu sub/MMP-9) | [94] | |
| Occludin tight junction protein | (bo sub/huMMP-9) | [95] | |
| Syndecan 1 and 4 | (mu sub/MMP-9) | [96,97] | |
| Serpin α-1 proteinase inhibitor | (mu sub/MMP-9) | [98] | |
| myelin basic protein | (hu sub/MMP-9) | [99] | |
| NG2 Proteoglycan | (hu sub/MMP-9) | [100] | |
| β-distroglycan | (mouse substrate/MMP-9 ?) | [101] | |
| Soluble beta amyloid protein | (hu sub/MMP-9) | [102,103] | |
| Fibrilar beta amyloid protein | (mu sub/MMP-9 ?) | [103] | |
| ADAMTS-4 (aggrecanase-1) | (hu sub/MMP-9) | [104] | |
| Candidate cell surface substrates | Angiopoetin 1 receptor Tie2 | (hu sub/MMP-9) | [62] |
| Neuropilin 1 | (hu sub/MMP-9) | [62] | |
| Integrin α3 | (hu sub/MMP-9) | [62] | |
| Clatherin heavy chain CLH17 | (hu sub/MMP-9) | [62] | |
| CD166/ALCAM | (hu sub/MMP-9) | [62] | |
| Saposin A | (hu sub/MMP-9) | [62] | |
| Semaphorin 7A | (hu sub/MMP-9) | [62] | |
| CC Chemokines | CCL7 | (mu sub/MMP-9) | [105] |
| CCL11 (Eotaxin) | (mu sub/MMP-9) | [105] | |
| CCL17 (TARC) | (mu sub/MMP-9) | [105] | |
| CXC Chemokines | CXCL1/NAP-3 | (hu sub/MMP-9) | [106] |
| CXCL4/PF4 | (hu sub/MMP-9) | [106] | |
| CXCL8/IL-8 | (hu sub/MMP-9) | [106] | |
| CXCL7/CTAP-III | (hu sub/MMP-9) | [106] | |
| CXCL9/ MIG | (hu sub/MMP-9) | [107] | |
| CXCL10/IP-10 | (hu sub/MMP-9) | [107] | |
| CXCL6/GCP-2 | (hu/mu sub/hu MMP-9) | [107] | |
| CXCL5/ENA78 | (hu sub/MMP-9) | [107] | |
| CXCL11/ITAC | (hu sub/MMP-9) | [108] | |
| CXCL12/SDF-1 | (hu sub/MMP-9) | [109] | |
| Other Substrates | Leukaemia inhibitory factor (LIF) | (hu sub/MMP-9) | [62] |
| Protease nexin-1 | (hu sub/MMP-9) | [62] | |
| Granulins precursor acrogranin | (hu sub/MMP-9) | [62] | |
| Hsp90 | (hu sub/MMP-9) | [62] | |
| uPA precursor | (hu sub/MMP-9) | [62] | |
| tPA precursor | (hu sub/MMP-9) | [62] | |
| C1q | (hu sub/MMP-9) | [110] | |
| C1r-A | (mu sub/hu MMP-9) | [65] | |
| Pyruvate kinase isoenzymes M1/M2 | (ra sub/hu MMP-9) | [65] | |
| Collagenase 3 (MMP-13) | (hu sub/MMP-9) | [62] | |
| Dickkopf-1 | (hu sub/MMP-9) | [111] | |
| Dickkopf-3 tumour suppressor | (hu sub/MMP-9) | [65] | |
| DJ-1 oncogene | (hu sub/MMP-9) | [111] | |
| Follistain-like 3 | (hu sub/MMP-9) | [111] | |
| Neuron specific enolase | (hu sub/MMP-9) | [111] | |
| Nieman-Pick C2 | (hu sub/MMP-9) | [111] | |
| Proglanulins | (hu sub/MMP-9) | [111] | |
| Ym 1 | (mu sub/MMP-9) | [105] | |
| S100A8 proinflammatory protein | (mu sub/MMP-9) | [105] | |
| S100A9 proinflammatory protein | (mu sub/MMP-9) | [105] | |
| Plasminogen | (hu sub/MMP-9) | [112,113] | |
| Mature NGF | (mu sub/MMP-9) | [114] | |
| Interferon-β | (hu sub/MMP-9) | [115] | |
| KISS-1 metastasis suppressor | (hu sub/MMP-9) | [116] | |
| Tau | (hu sub/MMP-9) | [117] | |
| VEGF | (mu sub/MMP-9 not hu MMP-9) | [80,118] |
5. Gelatinase B/MMP-9 Transcription and Translation
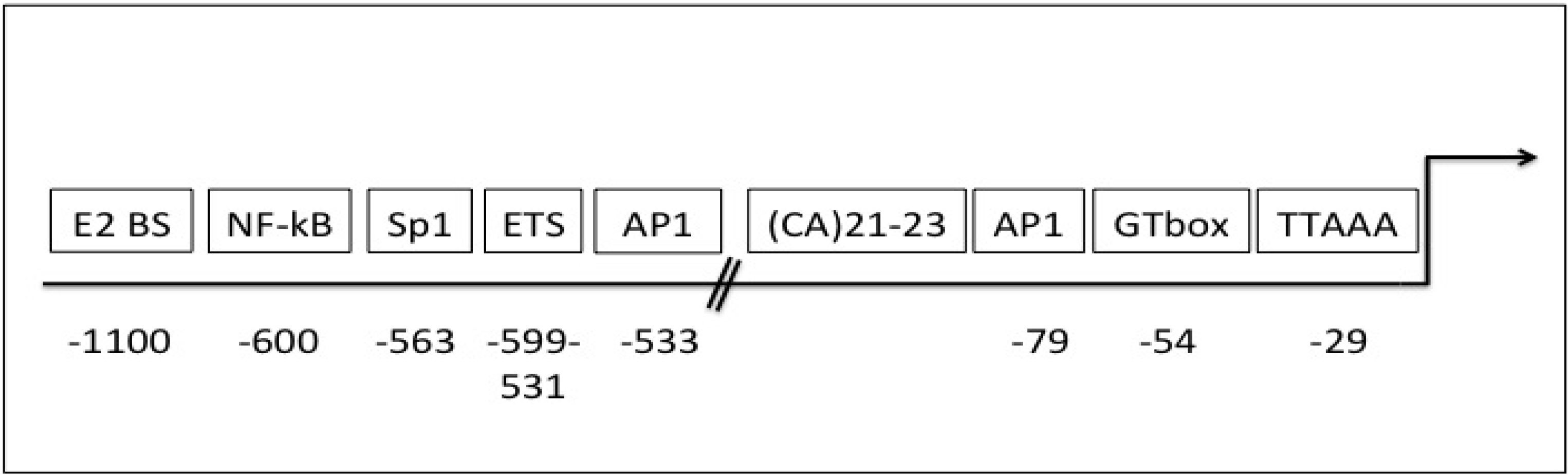
6. Gelatinase B/MMP-9 Expression, Bioavailability, Activity and Endogenous Inhibitors
The Gelatinase B/MMP-9/TIMP-1 Protease-Antiprotease Equilibrium
7. Gelatinase B/MMP-9, Tumour Initiation/Promotion and Genetic Instability

8. Gelatinase B/MMP-9 and Tumour Initiating Cell Proliferation and Expansion
9. Gelatinase B/MMP-9, Stem Cells and the Cancer Stem Cell Niche
10. Gelatinase B/MMP-9 and Epithelial to Mesenchymal Transition (EMT)

11. Gelatinase B/MMP-9 and Cancer-Related Inflammation
12. Gelatinase B/MMP-9 and Angiogenesis

Gelatinase B/MMP-9 and Lymphangiogenesis
13. Gelatinase B/MMP-9 and Disruption of Tissue Architecture
14. Gelatinase B/MMP-9 Induction of Intracellular Signalling
15. Gelatinase B/MMP-9, Tumour Cell Invasion and Motility

Gelatinase B/MMP-9 and Primary Tumour Cell Escape
16. Gelatinase B/MMP-9 and Immunological Surveillance
17. Gelatinase B/MMP-9 Haematogenous and Lymphatic Metastatic Dispersal

18. Gelatinase B/MMP-9 and Extravasation
19. Gelatinase B/MMP-9 and the Metastatic Niche
20. Gelatinase B/MMP-9, Apoptosis, Survival and the Mitochondria
21. Lessons from Gelatinase B/MMP-9 Knockout and Transgenics
22. Gelatinase B/MMP-9 Inhibitors and Future Directions
23. Conclusions
Acknowledgments
Conflicts of Interest
References
- Sopata, I.; Dancewicz, A.M. Presence of a gelatin-specific proteinase and its latent form in human leukocytes. Biochim. Biophys. Acta 1974, 370, 510–523. [Google Scholar] [CrossRef]
- Murphy, G.; Bretz, U.; Baggiolini, M.; Reynolds, J.J. The latent collagenase and gelatinase of human polymorphonuclear neutrophil leukocytes. Biochem. J. 1980, 192, 517–525. [Google Scholar]
- Dewald, B.; Bretz, U.; Baggiolini, M. Release of gelatinase from a novel secretory compartment of human neutrophils. J. Clin. Investig. 1982, 70, 518–525. [Google Scholar] [CrossRef]
- Hibbs, M.A.; Hasty, K.A.; Seyer, J.M.; Kang, A.H.; Mainardi, C.L. Biochemical characterisation of the secreted forms of human neutrophil gelatinase. J. Biol. Chem. 1985, 260, 2493–2500. [Google Scholar]
- Hibbs, M.A.; Hoidal, J.R.; Kang, A.H. Expression of a metalloproteinase that degrades native type V collagen and denatured collagens by cultured human alveolar macrophages. J. Clin. Investig. 1987, 80, 1644–1650. [Google Scholar] [CrossRef]
- Ballin, M.; Gomez, D.E.; Sinha, C.C.; Thorgeirsson, U.P. Ras oncogene mediated induction of a 92kDa metalloproteinase; strong correlation with the malignant phenotype. Biochem. Biophys. Res. Commun. 1988, 154, 832–838. [Google Scholar] [CrossRef]
- Ballin, M.; Mackay, A.R.; Hartzler, J.L.; Nason, A.; Pelina, M.D.; Thorgeirsson, U.P. Ras levels and metalloproteinase activity in normal versus neoplastic rat mammary tissues. Clin. Exp. Metastasis 1991, 9, 179–189. [Google Scholar] [CrossRef]
- Mackay, A.R.; Hartzler, J.L.; Pelina, M.D.; Thorgeirsson, U.P. Studies on the ability of 65-kDa and 92-kDa tumor cell gelatinases to degrade type IV collagen. J. Biol. Chem. 1990, 265, 21929–21934. [Google Scholar]
- Bernhardt, E.J.; Gruber, S.B.; Muschel, R.J. Direct evidence linking expression of matrix metalloproteinase 9 (92 kDa gelatinase/collagenase) to the metastatic phenotype in transformed rat embryo cells. Proc. Natl. Acad. Sci. USA 1994, 91, 4293–4297. [Google Scholar] [CrossRef]
- Bernhardt, E.J.; Muschel, R.J.; Hughes, E.N. Mr 92,000 gelatinase release correlates with the metastatic phenotype in transformed rat embryo cells. Cancer Res. 1990, 50, 3872–3877. [Google Scholar]
- Wilhelm, S.M.; Collier, I.M.; Marmer, B.L.; Eisen, A.Z.; Grant, G.A.; Goldberg, G.I. SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase, which is identical to that secreted by normal human macrophages. J. Biol. Chem. 1989, 264, 17213–17221. [Google Scholar]
- Van den Steen, P.E.; Dubois, B.; Nelissen, I.; Rudd, P.M.; Dwek, R.A.; Opdenakker, G. Biochemistry and Molecular biology of gelatinase B or matrix metalloproteinase-9 (gelatinase B/MMP-9). Crit. Rev. Biochem. Mol. Biol. 2002, 37, 375–536. [Google Scholar] [CrossRef]
- Vandooren, J.; van den Steen, P.E.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (gelatinase B/MMP-9): The next decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef]
- Hutala, P.; Tuuttila, A.; Chow, L.T.; Lohi, J.; Keski, O.J.; Tryggvason, K. Complete structure of the human gene for 92-kDa type IV collagenase. Divergent regulation of expression of the 92- and 72- kilodalton enzyme genes in HT-180 cells. J. Biol. Chem. 1991, 266, 16485–16490. [Google Scholar]
- Mackay, A.R.; Ballin, M.; Pelina, M.D.; Farina, A.R.; Nason, A.M.; Hartzler, J.L.; Thorgeirsson, U.P. Effect of phorbol ester and cytokines on matrix metalloproteinase expression and tissue inhibitor of metalloproteinase expression in tumor and normal cell lines. Invasion Metastasis 1992, 12, 168–184. [Google Scholar]
- Masure, S.; Billiau, A.; van Damme, J.; Opdenakker, G. Human hepatoma cells produce an 85 kDa gelatinase regulated by phorbol 12-myristate 13-acetate. Biochim. Biophys. Acta 1990, 1054, 317–325. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, W.; Sun, L.; Liu, L.; You, G.; Wang, Y.; Kang, C.; You, Y.; Jiang, T. Identification of gelatinase B/MMP-9 specific microRNA expression profile as potential targets of anti-invasion therapy in glioblastoma multiforme. Brain Res. 2011, 1411, 108–115. [Google Scholar]
- Asuthkar, S.; Velpula, K.K.; Chetty, C.; Gorantla, B.; Rao, J.S. Epigenetic regulation of miRNA-211 by gelatinase B/MMP-9 governs glioma cell apoptosis, chemosensitivity and radiosensitivity. Oncotarget 2012, 3, 1439–1454. [Google Scholar]
- Zhang, B.; Ye, S.; Herrmann, S.M.; Eriksson, P.; de Maat, M.; Evans, A.; Arveiler, D.; Luc, G.; Cambien, F.; Hamsten, A.; et al. Functional polymorphism in the regulatory region of gelatinase B gene in relation to severity of coronary atherosclerosis. Circulation 1999, 99, 1788–1794. [Google Scholar] [CrossRef]
- Matsumura, S.; Oue, N.; Nakayama, H.; Kitadai, Y.; Yoshida, K.; Yamaguchi, Y.; Imai, K.; Nakachi, K.; Matsusaki, K.; Chayama, K.; et al. A single nucleotide polymorphism in the gelatinase B/MMP-9 promoter affects tumor progression and invasive phenotype of gastric cancer. J. Cancer Res. Clin. Oncol. 2005, 131, 19–25. [Google Scholar] [CrossRef]
- Tu, H.F.; Wu, C.H.; Kao, S.Y.; Liu, C.J.; Liu, T.Y.; Liu, M.T. Functional -1562 C to T polymorphism in matrix metalloproteinase-9 (MMP-9) promoter is associated with the risk for oral squamous cell carcinoma in younger male area users. J. Oral Pathol. Med. 2007, 36, 409–414. [Google Scholar] [CrossRef]
- Vairaktaris, E.; Vassiliou, S.; Nkenke, E.; Serefoglou, Z.; Derka, S.; Tsigris, C.; Vylliotis, A.; Yapijakis, C.; Neukam, F.W.; Patsouris, E. A metalloproteinase-9 polymorphism which affects expression is associated with increased risk of oral squamous cell carcinoma. Eur. J. Surg. Oncol. 2008, 34, 450–455. [Google Scholar] [CrossRef]
- Vairaktaris, E.; Serefoglou, Z.; Avgoustidis, D.; Yapijakis, C.; Critselis, E.; Vylliotis, A.; Spyridonidou, S.; Derka, S.; Vassiliou, S.; Nkenke, E.; et al. Gene polymorphisms related to angiogenesis, inflammation and thrombosis that influence risk for oral cancer. Oral Oncol. 2009, 45, 247–253. [Google Scholar] [CrossRef]
- Nasr, H.B.; Mestiri, S.; Chahed, K.; Bounaouina, N.; Gabbouj, S.; Jalbout, M.; Chouchane, L. Matrix metalloproteinase-1 (-16076) 1G/2G and -9 (-1562) C/T promoter polymorphisms: Susceptibility and prognostic implications in nasopharyngeal carcinomas. Clin. Chim. Acta 2007, 384, 57–63. [Google Scholar] [CrossRef]
- Rollin, J.; Regina, S.; Vourc’h, P.; Lochman, S.; Blechet, C.; Reverdiau, P.; Gruel, Y. Influence of MMP-2 and MMP-9 promoter polymorphisms on gene expression and clinical outcome of non-small cell lung cancer. Lung Cancer 2007, 56, 273–280. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, L.; Luo, H.; Zhu, Z.; Zhang, C.; Hou, Y. Association of matrix metalloproteinase-9 gene polymorphisms with genetic susceptibility to oesophageal squamous cell carcinoma. DNA Cell. Biol. 2008, 27, 553–557. [Google Scholar] [CrossRef]
- Liu, D.; Guo, H.; Li, Y.; Xu, X.; Yang, K.; Bai, Y. Association between polymorphisms in the promoter regions of matrix metalloproteinases (MMPs) and risk of cancer metastasis: A meta-analysis. PLoS One 2012, 7, e31251. [Google Scholar]
- Shimajiri, S.; Arima, N.; Tanimoto, A.; Murata, Y.; Hamada, T.; Wang, K.Y.; Sasaguri, Y. Shortened microsatellite d(CA)21 sequence down-regulates promoter activity of matrix metalloproteinase 9 gene. FEBS Lett. 1999, 455, 70–74. [Google Scholar]
- Maeda, S.; Haneda, M.; Guo, B.; Koya, D.; Hayashi, K.; Sugimoto, T.; Isshiki, K.; Yasuda, H.; Kashiwagi, A.; Kikkawa, R. Dinucleotide repeat polymorphism of matrix metalloproteinase-9 gene is associated with diabetic nephropathy. Kidney Int. 2001, 60, 1428–1434. [Google Scholar] [CrossRef]
- Peters, D.G.; Kassam, A.; St. Jean, P.L.; Yonas, H.; Ferrell, R.E. Functional polymorphism in the matrix metalloproteinase-9 promoter as a potential risk factor for intracranial aneurysm. Stroke 1999, 30, 2612–2616. [Google Scholar] [CrossRef]
- Yuan, M.; Zhan, Q.; Duan, X.; Song, B.; Zeng, S.; Chen, X.; Yang, Q.; Xia, J. A functional polymorphism at miR-491–5p binding site in the 3'-UTR of gelatinase B/MMP-9 gene confers increased risk for atherosclerotic cerebral infarction in a Chinese population. Atherosclerosis 2013, 226, 447–452. [Google Scholar] [CrossRef]
- Hu, Z.; Huo, X.; Lu, D.; Qian, J.; Zhou, J.; Chen, Y.; Xu, L.; Ma, H.; Zhu, J.; Wei, Q.; et al. Functional polymorphisms of matrix metalloproteinase-9 are associated with risk of occurrence and metastasis of lung cancer. Clin. Cancer Res. 2005, 11, 5433–5439. [Google Scholar] [CrossRef]
- Liu, H.; Huang, P.Y.; Tang, L.Q.; Chen, Q.Y.; Zhang, H.; Zhang, L.; Guo, L.; Luo, D.H.; Mo, H.Y.; Xiang, Y.Q.; et al. Functional polymorphisms of matrix metalloproteinase-9 and survival in patients with locoregionally advanced nasopharyngeal carcinoma treated with radiotherapy. Med. Oncol. 2013, 30, 685. [Google Scholar] [CrossRef]
- Liu, Z.; Li, L.; Yang, Z.; Luo, W.; Li, X.; Yang, H.; Yao, K.; Wu, B.; Feng, W. Increased expression of gelatinase B/MMP-9 is correlated with poor prognosis of nasopharyngeal carcinoma. BMC Cancer 2010, 10, 270. [Google Scholar] [CrossRef]
- Tang, Y.; Zhu, J.; Chen, L.; Chen, L.; Zhang, S.; Lin, J. Associations of matrix metalloproteinase-9 protein polymorphisms with lymph node metastasis but not invasion of gastric cancer. Clin. Cancer Res. 2008, 14, 2870–2877. [Google Scholar] [CrossRef]
- Sharma, K.L.; Misra, S.; Kumar, A.; Mittal, B. Higher risk of matrix metalloproteinase (MMP-2, 7, 9) and tissue inhibitor of metalloproteinase (TIMP-2) genetic variants in gallbladder cancer. Liver Int. 2012, 32, 1278–1286. [Google Scholar] [CrossRef]
- Yang, Z.H.; Li, S.N.; Liu, J.X.; Guo, Q.X.; Sun, X.W. MMP-9 polymorphisms are related to serum lipids levels but not associated with colorectal cancer susceptibility in Chinese population. Mol. Biol. Rep. 2012, 39, 9399–9404. [Google Scholar] [CrossRef]
- Jin, G.; Miao, R.; Hu, Z.; Xu, L.; Huang, X.; Chen, Y.; Tian, T.; Wei, Q.; Boffetta, P.; Shen, H. Putative functional polymorphisms of MMP-9 predict survival of NSCLC in a Chinese population. Int. J. Cancer 2009, 124, 2172–2178. [Google Scholar] [CrossRef]
- O’Farrell, T.J.; Pourmotabbed, T. Identification of structural elements important for matrix metalloproteinase type V collagenolytic activity as revealed by chimeric enzymes. Role of fibronectin-like domain and active site of gelatinase B. J. Biol. Chem. 2000, 275, 27964–27972. [Google Scholar]
- O’Farrell, T.J.; Guo, R.; Hasegawa, H.; Pourmotabbed, T. Matrix metalloproteinase-1 takes advantage of the induced fit mechanism to cleave the triple-helical type I collagen molecule. Biochemistry 2006, 45, 15411–15418. [Google Scholar] [CrossRef]
- Farina, A.R.; Cappabianca, L.; di Ianni, N.; Ruggeri, P.; Ragone, M.; Merolla, S.; Gulino, A.; Mackay, A.R. Alendronate promotes plasmin-mediated MMP-9 inactivation by exposing cryptic plasmin degradation sites within the MMP-9 catalytic domain. FEBS Lett. 2012, 586, 2366–2374. [Google Scholar] [CrossRef]
- Triebel, S.; Blaser, J.; Reinke, H.; Knauper, V.; Tschesche, H. Mercurial activation of human PMN leukocyte type IV procollagenase (gelatinase). FEBS Lett. 1992, 298, 280–284. [Google Scholar] [CrossRef]
- Okamoto, T.; Akaike, T.; Sawa, T.; Miyamoto, Y.; van del Vliet, A.; Maeda, H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutothiolation via disulphide S-oxide formation. J. Biol. Chem. 2001, 276, 29596–29602. [Google Scholar]
- Gu, Z.; Kaul, M.; Yan, B.; Kridel, S.J.; Cui, J.; Strongin, A.; Smith, J.W.; Liddington, R.C.; Lipton, S.A.S. nitrosylation of matrix metalloproteinases: Signalling pathway to neuronal cell death. Science 2002, 297, 1186–1190. [Google Scholar] [CrossRef]
- Kahn, M.M.G.; Simizu, S.; Suzuli, T.; Masuda, A.; Kawatani, M.; Muroi, M.; Dohmae, N.; Nad Osada, H. Protein disulphide isomerase-mediated disulphide binds regulate gelatinolytic activity and secretion of matrix metalloproteinase-9. Exp. Cell Res. 2012, 318, 904–911. [Google Scholar] [CrossRef]
- Dufour, A.; Sampson, N.S.; Li, J.; Kuscu, C.; Rizzo, R.C.; Deleon, J.L.; Zhi, J.; Jaber, N.; Liu, E.; Zucker, S.; et al. Small-molecule anticancer compounds selectively target the hemopexin domain of matrix metalloproteinase-9. Cancer Res. 2011, 71, 4977–4988. [Google Scholar] [CrossRef]
- Geurts, N.; Martens, E.; van Aelst, I.; Proost, P.; Opdenakker, G.; van den Steen, P.E. Beta-haematin interaction with the hemopexin domain of gelatinase B/MMP-9 provokes autocatalytic processing of the propeptide, thereby priming activation by MMP-3. Biochemistry 2008, 47, 2689–2699. [Google Scholar] [CrossRef]
- Dufour, A.; Zucker, S.; Sampson, N.S.; Kuscu, C.; Cao, J. Role of matrix metalloproteinase-9 dimers in cell migration: Design of inhibitory peptides. J. Biol. Chem. 2010, 285, 35944–35956. [Google Scholar] [CrossRef]
- Van den Steen, P.E.; van Aelst, I.; Hvidberg, V.; Piccard, H.; Fiten, P.; Jacobsen, C.; Moestrup, S.K.; Fry, S.; Royle, L.; Wormald, M.R.; et al. The hemopexin and O-glycosylated domains tune gelatinase B/MMP-9 bioavailability via inhibition of binding to cargo receptors. J. Biol. Chem. 2006, 281, 18626–18637. [Google Scholar] [CrossRef]
- Bellini, T.; Trentini, A.; Manfrinato, M.C.; Tamborino, C.; Volta, C.A.; di Foggia, V.; Fainardi, E.; Dallocchio, F.; Castellazzi, M. Matrix metalloproteinase-9 activity detected in body fluids is the result of two different enzyme forms. J. Biochem. 2012, 151, 493–499. [Google Scholar] [CrossRef]
- Geurts, N.; Becker-Pauly, C.; Martens, E.; Proost, P.; van den Steen, P.E.; Stoker, W.; Opdenakker, G. Meprins process matrix metalloproteinase-9 (gelatinase B/MMP-9)/gelatinase B and enhance the activation kinetics by MMP-3. FEBS Lett. 2012, 586, 4264–4269. [Google Scholar] [CrossRef]
- Ramani, V.C.; Kaushal, G.P.; Haun, R.S. Proteolytic activation of kallikrien-related peptidase 7 produces unique active matrix metalloproteinase-9 lacking the C-terminal domains. Biochim. Biophys. Acta 2011, 1813, 1525–1531. [Google Scholar] [CrossRef]
- Reis, C.; Lottspeich, F.; Dittmann, K.H.; Petrides, P.E. HL60 leukemia cells produce an autocatalytically truncated form of matrix metalloproteinase-9 with impaired sensitivity to inhibition by tissue inhibitors of metalloproteinases. Leukemia 1996, 10, 1520–1526. [Google Scholar]
- Reis, C.; Pitsch, T.; Mentele, R.; Zahler, S.; Egea, V.; Nagase, H.; Jochum, M. Identification of a novel 82 kDa proMMP-9 species associated with the surface of leukaemic cells: (Auto-) catalytic activation and resistance to inhibition by TIMP-1. Biochem. J. 2007, 405, 547–558. [Google Scholar] [CrossRef] [Green Version]
- Bigg, H.F.; Rowan, A.D.; Barker, M.D.; Cawston, T.E. Activity of matrix metalloproteinase-9 against native collagen I and II. FEBS J. 2007, 274, 1246–1255. [Google Scholar] [CrossRef]
- Van den Steen, P.E.; Proost, P.; Brand, D.D.; Kang, A.H.; van Damme, J.; Opdenakker, G. Generation of glycosylated remnant epitopes from human type II collagen by gelatinase B. Biochemistry 2004, 43, 10809–10816. [Google Scholar] [CrossRef]
- Eble, J.A.; Ries, A.; Lichy, A.; Mann, K.; Stanton, H.; Gavrilovic, J.; Murphy, G.; Kuhn, K. The recognition sites of the integrins α1β1 and α2β1 within collagen IV are protected against gelatinase A attack in the native protein. J. Biol. Chem. 1996, 271, 30964–30970. [Google Scholar]
- Shoji, A.; Kabeya, M.; Sugawara, M. Real-time monitoring of matrix metalloproteinase-9 collagenolytic activity with a surface plasmon resonance biosensor. Anal. Biochem. 2011, 419, 53–60. [Google Scholar] [CrossRef]
- Mackay, A.R.; Corbitt, R.H.; Hartzler, J.L.; Thorgeirsson, U.P. Basement membrane type IV collagen degradation: Evidence for the involvement of a proteolytic cascade independent of metalloproteinases. Cancer Res. 1990, 50, 5997–6001. [Google Scholar]
- Beliveau, A.; Mott, J.D.; Lo, A.; Chen, E.I.; Koller, A.A.; Yaswen, P.; Muschler, J.; Bissel, M.J. Raf-induced MMP-9 disrupts tissue architecture of human breast cells in three-dimensional culture and is necessary for tumor growth in vitro. Genes Dev. 2010, 24, 2800–2811. [Google Scholar] [CrossRef]
- Gu, Z.; Cui, J.; Brown, S.; Fridman, R.; Mobashery, S.; Strongin, A.Y.; Lipton, S.A. A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J. Neurosci. 2005, 25, 6401–6408. [Google Scholar] [CrossRef]
- Xu, D.; Suenaga, N.; Edelman, J.; Fridman, R.; Muschel, R.; Kessler, B.M. Novel MMP-9 substrates in cancer cells revealed by a label-free quantitative proteomics approach. Mol. Cell. Proteomics 2008, 7, 2215–2228. [Google Scholar] [CrossRef]
- Cauwe, B.; van den Steen, P.E.; Opdenakker, G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 113–115. [Google Scholar] [CrossRef] [Green Version]
- Cauwe, B.; Opdenakker, G. Intracellular substrate cleavage: A novel dimension in the biochemistry, biology and pathology of matrix metalloproteinases. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 351–423. [Google Scholar] [CrossRef]
- Prudova, A.; auf dem Keller, U.; Butler, G.S.; Overall, C.M. Multiplex N-terminome analysis of MMP-2 and MMP-9 substrate degradomes by iTRAQ-TAILS quantitative proteomics. Mol. Cell. Proteomics 2010, 9, 894–911. [Google Scholar] [CrossRef]
- Gioia, M.; Monaco, S.; van den Steen, P.E.; Sbardella, D.; Grasso, G.; Marini, S.; Overall, C.M.; Opdenakker, G.; Coletta, M. The collagen binding domain of gelatinase A modulates degradation of collagen IV by gelatinase B. J. Mol. Biol. 2009, 386, 419–434. [Google Scholar] [CrossRef] [Green Version]
- Murphy, G.; Reynolds, J.J.; Bretz, U.; Baggiolini, M. Partial purification of collagenase and gelatinase from human polymorphonuclear leucocytes. Analysis of their actions on soluble and insoluble collagens. Biochem. J. 1982, 203, 209–221. [Google Scholar]
- Morodomi, T.; Ogata, Y.; Sasaguri, Y.; Morimatsu, M.; Nagase, H. Purification and characterization of matrix metalloproteinase 9 from U937 monocytic leukaemia and HT-1080 fibrosarcoma cells. Biochem. J. 1992, 285, 603–611. [Google Scholar]
- Kridel, S.J.; Chen, E.; Kotra, L.P.; Howard, E.W.; Mobashery, S.; Smith, J.W. Substrate hydrolysis by matrix metalloproteinase-9. J. Biol. Chem. 2001, 276, 20572–20578. [Google Scholar]
- Stegemann, C.; Didangelos, A.; Ballarobre-Barriero, J.; Langley, S.R.; Mandal, K.; Jahangiri, M.; Mayr, M. Proteomic identification of matrix metalloproteinase substrates in the human vasculature. Circ. Cardiovasc. Genet. 2013, 6, 106–117. [Google Scholar] [CrossRef]
- O’Farrell, T.J.; Pourmattabbed, T. The fibronectin-like domain is required for the type V and XI collagenolytic activity of gelatinase B. Arch. Biochem. Biophys. 1998, 354, 24–30. [Google Scholar] [CrossRef]
- Ferreras, M.; Felbor, U.; Lenhard, T.; Olsen, B.R.; Delaissé, J. Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett. 2000, 486, 247–251. [Google Scholar] [CrossRef]
- Ochieng, J.; Fridman, R.; Nagia-Makker, P.; Kleiner, D.E.; Liotta, L.A.; Stetler-Stevenson, W.G.; Raz, A. Galectin-3 is a novel substrate for human matrix metalloproteinase-2 and 9. Biochemistry 1994, 33, 14109–14114. [Google Scholar] [CrossRef]
- Zampila, R.; Lopez, E.F.; Chiao, Y.A.; Dai, Q.; Escobar, G.P.; Hakala, K.; Weintraub, S.T.; Lindsey, M.L. Proteomic analysis identifies in vivo candidate matrix metalloproteinase-9 substrates in the left ventrical post-myocardial infaction. Proteomics 2010, 10, 2214–2223. [Google Scholar] [CrossRef]
- Siri, A.; Knauper, V.; Veirana, N.; Caocci, F.; Murphy, G.; Zardi, L. Different susceptibility of small and large human tenascin-C isoforms to degradation by matrix metalloproteinases. J. Biol. Chem. 1995, 270, 8650–8654. [Google Scholar]
- Katsuda, S.; Okada, Y.; Okada, Y.; Imai, K.; Nakanishi, I. Matrix metalloproteinase-9 (92-kd gelatinase/type IV collagenase equals gelatinase B) can degrade arterial elastin. Am. J. Pathol. 1994, 145, 1208–1218. [Google Scholar]
- Lau, A.C.; Duong, T.T.; Ito, S.; Yeung, R.S. Matrix metalloproteinase 9 activity leads to elastin breakdown in an animal model of Kawasaki disease. Arthritis Rheum. 2008, 58, 854–863. [Google Scholar] [CrossRef]
- Imai, K.; Shikata, H.; Okada, Y. Degradation of vitronectin by matrix metalloproteinases-1, -2, -3, -7 and 9. FEBS Lett. 1995, 369, 249–251. [Google Scholar] [CrossRef]
- Sires, U.I.; Griffin, G.L.; Broekelmann, T.J.; Mecham, R.P.; Murphy, G.; Chung, A.E.; Welgus, H.G.; Senior, R.M. Degradation of entactin by matrix metalloproteinases. J. Biol. Chem. 1993, 268, 2069–2074. [Google Scholar]
- Hawinkels, L.J.A.C.; Ziudwijk, K.; Verspaget, H.W.; de Jong-Muller, E.S.M.; van Duijin, W.; Ferreira, V.; Fontijn, R.D.; David, G.; Hommes, D.W.; Lamers, C.B.H.W.; et al. VEGF release by MMP-9 mediated heparin sulphate cleavage induces colorectal cancer angiogenesis. Eur. J. Cancer 2008, 44, 1904–1913. [Google Scholar] [CrossRef]
- Fiore, E.; Fusco, C.; Romero, P.; Stamenkovic, I. Matrix metalloproteinase 9 (MMP-9/gelatinase B) proteolytically cleaves ICAM-1 and participates in tumor cell resistance to natural killer cell-mediated cytotoxicity. Oncogene 2002, 21, 5213–5223. [Google Scholar] [CrossRef]
- Sultan, S.; Gosling, M.; Nagase, H.; Powell, J.T. Shear stress-induced shedding of soluble intercellular adhesion molecule-1 from saphenous vein endothelium. FEBS Lett. 2004, 564, 161–165. [Google Scholar] [CrossRef]
- Andolfo, A.; English, W.R.; Resnati, M.; Murphy, G.; Blasi, F.; Sidenius, N. Metalloproteinase cleave the urokinase-type plasminogen activator receptor in the D1-D2 linker region and expose epitopes not present in the intact soluble receptor. Thromb. Haemost. 2002, 88, 298–306. [Google Scholar]
- Amano, T.; Kwak, O.; Fu, L.; Marshak, A.; Shi, Y.B. The matrix metalloproteinase stromelysin-3 cleaves laminin receptor at two distinct sites between the transmembrane domain and laminin binding sequence within the extracellular domain. Cell Res. 2005, 15, 150–159. [Google Scholar] [CrossRef]
- Sheu, B.C.; Hsu, S.M.; Ho, H.N.; Lien, H.C.; Huang, S.C.; Lin, R.H. A novel role of metalloproteinase in cancer-mediated immunosuppression. Cancer Res. 2001, 61, 237–242. [Google Scholar]
- De Paiva, C.S.; Yoon, K.-C.; Pangelinan, S.B.; Pham, S.; Puthenparambi, L.M.; Chuang, E.Y.; Farley, W.J.; Stern, M.E.; Li, D.-C.; Pflugfelder, S.C. Cleavage of functional IL-2 receptor alpha chain (CD25) from murine corneal and conjunctival epithelial by MMP-9. J. Inflamm. Lond. 2009, 6, 31. [Google Scholar] [CrossRef]
- Mohan, M.J.; Seaton, T.; Mitchell, J.; Howe, A.; Blackburn, K.; Burkhart, W.; Moyer, M.; Patel, I.; Waitt, G.M.; Becherer, J.D.; et al. The tumor necrosis factor-alpha converting enzyme (TACE): A unique metalloproteinase with highly defined substrate selectivity. Biochemistry 2002, 41, 9462–9469. [Google Scholar] [CrossRef]
- Ito, A.; Mukaiyama, A.; Itoh, Y.; Nagase, H.; Thogersen, I.B.; Enghild, J.J.; Sasaguri, Y.; Mori, Y. Degradation of interleukin 1 beta by matrix metalloproteinases. J. Biol. Chem. 1996, 271, 14657–1460. [Google Scholar]
- Schonbeck, U.; Mach, F.; Libby, P. Generation of biologically active IL-1 beta by matrix metalloproteinases: A novel caspase-1-independent pathway of IL-1 beta processing. J. Immunol. 1998, 161, 3340–3346. [Google Scholar]
- Heissig, B.; Hattori, K.; Dias, S.; Friedrich, M.; Ferris, B.; Hackett, N.R.; Crystal, R.G.; Besmer, P.; Lyden, D.; Moore, M.A.; et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediate release of kit-ligand. Cell 2002, 109, 625–637. [Google Scholar] [CrossRef]
- Hollenbeck, S.T.; Sakakibara, K.; Faries, P.L.; Workhu, B.; Liu, B.; Kent, K.C. Stem cell factor and c-kit are expressed by and may affect vascular SMCs through an autocrine pathway. J. Surg. Res. 2004, 120, 288–294. [Google Scholar] [CrossRef]
- Vaisar, T.; Kassim, S.Y.; Gomez, I.G.; Green, P.S.; Hargarten, S.; Gough, P.J.; Parks, W.C.; Wilson, C.L.; Raines, E.W.; Heinecke, J.W. MMP-9 sheds the beta2 integrin subunit (CD18) from macrophages. Mol. Cell. Proteomics 2009, 8, 1044–1060. [Google Scholar] [CrossRef]
- Yu, Q.; Stamenkovic, I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD-44-mediated tumor invasion. Genes Dev. 1999, 13, 35–48. [Google Scholar] [CrossRef]
- Lue, H.-W.; Yang, X.; Wang, R.; Qian, W.; Xu, R.Z.H.; Lyles, R.; Osunkoya, A.O.; Zhou, B.P.; Vessella, R.L.; Zayzafoon, M.; et al. LIV-1 promotes prostate cancer epithelial-to-mesenchymal transition and metastasis through HB-EGF shedding and EGFR-mediated Erk signalling. PLoS One 2011, 6, e27720. [Google Scholar] [CrossRef]
- Giebel, J. S.; Menicucci, G.; McGuire, P. G.; Das, A. Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood-retinal barrier. Lab. Invest. 2005, 85, 597–607. [Google Scholar] [CrossRef]
- Fitzgerald, M.L.; Wang, Z.; Park, P.W.; Murphy, G.; Bernfield, M. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signalling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J. Cell. Biol. 2000, 148, 811–824. [Google Scholar] [CrossRef]
- Brule, S.; Charnaux, N.; Sutton, A.; Ledoux, D.; Chaigneau, T.; Saffar, L.; Gattegno, L. The shedding of syndecan-4 and syndecan-1 from HeLa cells and human primary macrophages is accelerated by SDF-1/CXCL12 and mediated by the matrix metalloproteinase-9. Glycobiology 2006, 16, 488–501. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, X.; Shapiro, S.D.; Shipley, J.M.; Twining, S.S.; Diaz, L.A.; Senior, R.M.; Werb, Z. The serpin alpha-1 proteinase inhibitor is a critical substrate for gelatinase B/MMP-9 in vivo. Cell 2000, 102, 647–655. [Google Scholar] [CrossRef]
- Proost, P.; van Damme, J.; Opdenakker, G. Leukocyte gelatinase B cleavage releases encephalitogens from human myelin basic protein. Biochem. Biophys. Res. Commun. 1993, 192, 1175–1181. [Google Scholar] [CrossRef]
- Larsen, P.H.; Wells, J.E.; Stallcup, W.B.; Opdenakker, G.; Young, V.W. Matrix metalloproteinase-9 facilitates remyelination in part by processing the inhibitory NG2 proteoglycan. J. Neurosci. 2003, 23, 11127–11135. [Google Scholar]
- Agrawal, S.; Anderson, P.; Durbeej, M.; van Rooijen, N.; Ivars, F.; Opdenakker, G.; Sorokin, L.M. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J. Exp. Med. 2006, 203, 1007–1019. [Google Scholar] [CrossRef]
- Backstrom, J.R.; Lim, G.P.; Cullen, M.J.; Tokes, Z.A. Matrix metalloproteinase-9 (MMP-9) is synthesized in neurons of the human hippocampus and is capable of degrading amyloid-beta peptide (1–40). J. Neurosci. 1996, 16, 7910–7919. [Google Scholar]
- Yan, P.; Hu, X.; Song, H.; Yin, K.; Bateman, R.J.; Cirrito, J.R.; Xiao, Q.; Hsu, F.F.; Turk, J.W.; Xu, J.; Hsu, C.Y.; Holtzman, D.M.; Lee, J.M. Matrix metalloproteinase-9 degrades amyloid-beta fibrils in vitro and compact plaques in situ. J. Biol. Chem. 2006, 281, 24566–24574. [Google Scholar] [CrossRef]
- Tortorella, M.D.; Arner, E.C.; Hills, R.; Gormley, J.; Fok, K.; Pegg, L.; Munie, G.; Malfait, A.-M. ADAMTS-4 (aggreganase-1): N-terminal activation mechanisms. Arch. Biochem. Biophys. 2005, 444, 34–44. [Google Scholar] [CrossRef]
- Greenlee, K.J.; Corry, D.B.; Engler, D.A.; Matsunami, R.K.; Tessier, P.; Cook, R.G.; Werb, Z.; Kheradmand, F. Proteomic identification of in vivo substrates from matrix metalloproteinase 2 and 9 reveals a mechanism for resolution of inflammation. J. Immunol. 2006, 177, 7312–7321. [Google Scholar]
- Van den Steen, P.E.; Proost, P.; Wuyts, A.; van Damme, J.; Opdenakker, G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4 and GRO-alpha and leaves RANTES and MCP-2 intact. Blood 2000, 275, 34335–34343. [Google Scholar]
- Van den Steen, P.E.; Wuyts, A.; Husson, S.J.; Proost, P.; van Damme, J.; Opdenakker, G. Gelatinase B/MMP-9 and neutrophil collagenase/MMP-8 process the chemokines human GCP-2/CXCL6, ENA-78/CXCL5 and mouse GCP-2/LIX and modulate their physiological activities. Eur. J. Biochem. 2003, 270, 3739–3749. [Google Scholar] [CrossRef]
- Cox, J.H.; Dean, R.A.; Roberts, C.R.; Overall, C.M. Matrix metalloproteinase processing of CXCL11/I-TAC results in loss of chemoattractant activity and altered glycosaminoglycan binding. J. Biol. Chem. 2008, 283, 19389–19399. [Google Scholar] [CrossRef]
- Jin, F.; Zhai, Q.; Qui, L.; Meng, H.; Zou, D.; Wang, Y.; Li, Q.; Yu, Z.; Han, J.; Li, Q.; et al. Degradation of BM SDF-1 by MMP-9: The role in G-CSF-induced hematopoietic stem/progenitor cell mobilization. Bone Marrow Transplant. 2008, 42, 581–588. [Google Scholar] [CrossRef]
- Ruiz, S.; Henschen-Edman, A.H.; Nagase, H.; Tenner, A.J. Digestion of C1q collagen like domains by MMPs-1, -2, -3, and -9 further defines the sequence involved in the stimulation of neutrophil superoxide production. J. Leukoc. Biol. 1999, 66, 416–422. [Google Scholar]
- Butler, G.S.; Dean, R.A.; Tam, E.M.; Overall, C.M. Pharmacoproteomics of a metalloproteinase hydroxamates inhibitor in breast cancer cells: Dynamics of membrane type 1 matrix metalloproteinase-mediated protein shedding. Mol. Cell. Biol. 2008, 28, 4896–4914. [Google Scholar] [CrossRef]
- Patterson, B.C.; Sang, Q.A. Angiostatin-converting enzyme activities of human matrilysin (MMP-7) and gelatinase B/type IV collagenase (MMP-9). J. Biol. Chem. 1997, 272, 28823–28825. [Google Scholar] [CrossRef]
- Farina, A.R.; Tacconelli, A.; Cappabianca, L.; Gulino, A.; Mackay, A.R. Inhibition of human MDA-MB-231 breast cancer invasion by matrix metalloproteinase 3 involves degradation of plasminogen. Eur. J. Biochem. 2002, 269, 4476–4483. [Google Scholar] [CrossRef]
- Bruno, M.A.; Cuello, C.A. Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proc. Natl. Acad. Sci. USA 2006, 103, 6735–6740. [Google Scholar] [CrossRef]
- Nelissen, I.; Martens, E.; van den Steen, P.E.; Proost, P.; Ronsse, I.; Opdenakker, G. Gelatinase B/Matrix metalloproteinase-9 cleaves interferon-b and is a target for immunotherapy. Brain 2003, 126, 1371–1381. [Google Scholar] [CrossRef]
- Takino, T.; Koshikawa, N.; Miyamori, H.; Tanaka, M.; Sasaki, T.; Okada, Y.; Seiki, M.; Sato, H. Cleavage of metastasis suppressor gene product KiSS-1 protein/metastin by matrix metalloproteinases. Oncogene 2003, 4617–4626. [Google Scholar]
- Nubling, G.; Levin, J.; Brader, B.; Isreal, L.; Bitzel, K.; Lorenzi, S.; Giese, A. Limited cleavage of tau with matrix metalloproteinase gelatinase B/MMP-9, but not MMP-3, enhances tau oligomer formation. Exp. Neurol. 2012, 237, 470–476. [Google Scholar] [CrossRef]
- Lee, S.; Jilani, S.M.; Nikolova, G.V.; Carpizo, D.; Iruela-Arispe, M.L. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumor. J. Cell. Biol. 2005, 169, 681–691. [Google Scholar] [CrossRef]
- Sato, H.; Kita, M.; Seiki, M. v-Src activates the expression of 92-kDa type IV collagenase gene through the AP-1 site and the GT box homologous to retinoblastoma control elements. A mechanism regulating gene expression independent of that by inflammatory cytokines. J. Biol. Chem. 1993, 268, 23460–23468. [Google Scholar]
- Gum, R.; Lengyel, E.; Juarez, J.; Chen, J.H.; Seiki, M.; Boyd, D. Stimulation of 92-kDa gelatinase B promoter activity by ras is mitogen-activated protein kinase 1-independent and requires multiple transcription factor binding sites including closely speced PEA3/ets and AP-1 sequences. J. Biol. Chem. 1996, 271, 10672–10680. [Google Scholar] [CrossRef]
- Han, Y.P.; Tuan, T.L.; Hughes, M.; Wu, H.; Garner, W.L. Transforming growth factor-beta-and tumor necrosis factor-alpha-mediated induction and activation of MMP-9 in human skin. J. Biol. Chem. 2001, 276, 22341–22350. [Google Scholar]
- Schwarzt, B.; Melnikova, V.O.; Tellez, C.; Mourad-Zeidan, A.; Blehm, K.; Zhao, Y.-J.; McCarty, M.; Adam, L.; Bar-Eli, M. Loss of AP-2a results in deregulation of E-caherin and MMP-9 and an increase in tumorigenicity of colon cancer cells in vivo. Oncogene 2007, 26, 4049–4058. [Google Scholar] [CrossRef]
- Farina, A.R.; Tacconelli, A.; Vacca, A.; Maroder, M.; Gulino, A.; Mackay, A.R. Transcriptional up-regulation of matrix metalloproteinase-9 expression during spontaneous epithelial to neuroblast phenotype conversion by SH-N-SH neuroblastoma cells, involved in enhanced invasivity, depends upon GT-box and nuclear factor kappaB elements. Cell Growth Differ. 1999, 10, 353–367. [Google Scholar]
- Farina, A.R.; Masciulli, M.-P.; Tacconelli, A.; Cappabianca, L.; de Santis, G.; Gulino, A.; Mackay, A.R. All-trans-retinoic acid induces nuclear factor κB activation and matrix metalloproteinase-9 expression and enhances basement membrane invasivity of differentiation-resistant human SK-N-BE 9N neuroblastoma cells. Cell Growth Differ. 2002, 13, 343–354. [Google Scholar]
- Hozumi, A.; Nishimura, Y.; Nishiuma, T.; Kotani, Y.; Yokoyama, M. Induction of MMP-9 in normal human bronchial epithelial cells by TNF-alpha via NF-kappa B-mediated pathway. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 281, L1444–L1452. [Google Scholar]
- Bond, M.; Chase, A.J.; Baker, A.H.; Newby, A.C. Inhibition of transcription factor NF-kappaB reduces matrix metalloproteinase-1, -3 and -9 production by vascular smooth muscle cells. Cardiovasc. Res. 2001, 50, 556–565. [Google Scholar]
- Yokoo, T.; Kitamura, M. Dual regulation of Il-1 beta-mediated matrix metalloproteinase-9 expression in mesangial cells by NF-kappaB and AP-1. Am. J. Physiol. 1996, 270, F123–F130. [Google Scholar]
- Ricca, A.; Biroccio, A.; Del Bufalo, D.; Mackay, A.R.; Santoni, A.; Cipitelli, M. Bcl-2 over-expression enhances NF-kappaB activity and induces mmp-9 transcription in human MCF7 (ADR) breast-cancer cells. Int. J. Cancer 2000, 86, 188–196. [Google Scholar] [CrossRef]
- Kumar, A.; Dhawan, S.; Mukhopadhyay, A.; Arrarwal, B.B. Human immunodeficiency virus-1-tat induces matrix metalloproteinase-9 in monocytes through protein tyrosine phosphatase-mediated activation of nuclear transcription factor NF-kappaB. FEBS Lett. 1999, 462, 140–144. [Google Scholar] [CrossRef]
- Yan, L.; Borregaard, N.; Kjeldsen, L.; Moses, M.A. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J. Biol. Chem. 2001, 276, 37258–37265. [Google Scholar] [CrossRef]
- Bond, M.; Fabunmi, R.P.; Baker, A.H.; Newby, A.C. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: An absolute requirement for transcription factor NF-kappa B. FEBS Lett. 1998, 435, 29–34. [Google Scholar] [CrossRef]
- Farina, A.R.; Cappabianca, L.; DeSantis, G.; di Ianni, N.; Ruggeri, P.; Ragone, M.; Merolla, S.; Tonissen, K.F.; Gulino, A.; Mackay, A.R. Thioredoxin stimulates MMP-9 expression, de-regulates the MMP-9/TIMP-1 equilibrium and promotes MMP-9 dependent invasion by human MDA-MB-231 breast cancer cells. FEBS Lett. 2011, 585, 3328–3336. [Google Scholar] [CrossRef]
- Himelstein, B.P.; Lee, E.J.; Sato, H.; Seike, M.; Muschel, R.J. Transcriptional activation of the matrix metalloproteinase-9 gene in an H-ras and v-myc transformed rat embryo cell lines. Oncogene 1997, 14, 1995–1998. [Google Scholar]
- Akgul, B.; Garcia-Escudero, R.; Ekechi, C.; Steger, G.; Navsaria, H.; Pfister, H.; Storey, A. he E2 protein of human papillomavirus type 8 increases the expression of matrix metalloproteinase-9 in human keratinocytes and organotypic skin cultures. Med. Microbiol. Immunol. 2011, 200, 127–135. [Google Scholar] [CrossRef]
- Akool, el-S.; Kleinert, H.; Hamada, F.M.; Abdelwahab, M.H.; Forstermann, U.; Pfeilschifter, J.; Eberhardt, W. Nitric oxide increases the decay of matrix metalloproteinase 9 mRNA by inhibiting the expression of mRNA-stabilizing factor HuR. Mol. Cell. Biol. 2003, 23, 4901–4916. [Google Scholar] [CrossRef]
- Eberhardt, W.; Akool, el-S.; Rebhan, J.; Frank, S.; Beck, K.F.; Franzen, R.; Hamada, F.M.; Pfeilschifter, J. Inhibition of cytokine-induced matrix metalloproteinase 9 expression by peroxisome proliferator-activated receptor alpha agonists is indirect and due to a NO-mediated reduction of mRNA stability. J. Biol. Chem. 2012, 277, 33518–33528. [Google Scholar]
- Jiang, Y.; Muschel, R.J. Regulation of matrix metalloproteinase-9 (MMP-9) by translational efficiency in murine prostate carcinoma cells. Cancer Res. 2002, 62, 1910–1914. [Google Scholar]
- Morini, M.; Mottolese, M.; Ferrari, N.; Ghiorzo, F.; Buglioni, S.; Mortarini, R.; Noonan, D.M.; Natali, P.G.; Albini, A. The alpha 3 beta 1 integrin is associated with mammary carcinoma cell metastasis, invasion and gelatinase B (MMP-9) activity. Int. J. Cancer 2000, 87, 336–342. [Google Scholar] [CrossRef]
- Sehgal, I.; Thompson, T.C. Novel regulation of type IV collagenase (matrix metalloproteinase-9 and -2) activities by transforming growth factor-beta1 in human prostate cancer cells. Mol. Biol. Cell 1999, 10, 407–416. [Google Scholar] [CrossRef]
- Thant, A.A.; Nawa, A.; Nikkawa, F.; Ichigotani, Y.; Zhang, Y.; Sein, T.T.; Amin, A.R.; Hamaguchi, M. Fibronectin activates matrix metalloproteinase-9 secretion via the MEK-1-MAPK and the PI3K-Akt pathways in ovarian cancer cells. Clin. Exp. Metastasis 2000, 18, 423–428. [Google Scholar] [CrossRef]
- Iyer, V.; Pumiglia, K.; DiPersio, C.M. Alpha3beta1 integrin regulates MMP-9 mRNA stability in immortalized keratinocytes: A novel mechanism of integrin-mediated MMP gene expression. J. Cell Sci. 2005, 118, 1185–1195. [Google Scholar] [CrossRef]
- Zhang, S.; Qi, L.; Li, M.; Zhang, D.; Xu, S.; Wang, N.; Sun, B.J. Chemokine CXCL12 and its receptor CXCR4 expression are associated with perineural invasion of prostate cancer. J. Exp. Clin. Cancer Res. 2008, 27, 62. [Google Scholar] [CrossRef]
- Farina, A.R.; Coppa, A.; Tiberio, A.; Tacconelli, A.; Turco, A.; Colletta, G.; Gulino, A.; Mackay, A.R. Transforming growth factor-beta1 enhances the invasiveness of human MDA-MB-231 breast cancer cells by up-regulating urokinase activity. Int. J. Cancer 1998, 75, 721–730. [Google Scholar] [CrossRef]
- Festuccia, C.; Bologna, M.; Vicentini, C.; Tacconelli, A.; Miano, R.; Violini, S.; Mackay, A.R. Increased matrix metalloproteinase-9 secretion in short term tissue cultures of prostatic tumor cells. Int. J. Cancer 1996, 69, 386–393. [Google Scholar] [CrossRef]
- Shima, I.; Sasaguri, Y.; Kusukawa, J.; Nakano, R.; Yamana, H.; Fujita, H.; Kagegawa, T.; Morimatsu, M. Production of matrix metalloproteinase 9 (92 kDa gelatinase) by human oesophageal squamous cell carcinoma in response to epidermal growth factor. Br. J. Cancer 1993, 67, 721–727. [Google Scholar] [CrossRef]
- Price, T.J.; Wilson, H.M.; Haites, N.E. Epidermal growth factor (EGF) increases the in vitro invasion, motility and adhesion interactions of the primary renal carcinoma cell line, A704. Eur. J. Cancer 1996, 32A, 1977–1982. [Google Scholar] [CrossRef]
- Uchiyama, A.; Essner, R.; Dol, F.; Nguyen, T.; Ramming, K.P.; Nakamura, T.; Morton, D.L.; Hoon, D.S. Interleukin 4 inhibits hepatocyte growth factor-induced invasion and migration of colon carcinomas. J. Cell Biochem. 1996, 62, 443–453. [Google Scholar] [CrossRef]
- Horie, S.; Aruga, S.; Kawamata, H.; Okui, N.; Kakizoe, T.; Kitamura, T. Biological role of HGF/MET pathway in renal cell carcinoma. J. Urol. 1999, 161, 990–997. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, W.; Lu, J.; He, F.; Yang, X. Invasiveness of hepatocellular carcinoma cell lines: Contribution of hepatocyte growth factor, c-met, and transcription factor Est-1. Biochem. Biophys. Res. Commun. 2001, 286, 1123–1130. [Google Scholar] [CrossRef]
- Harvey, P.; Clark, I.M.; Jaurand, M.C.; Warn, R.M.; Edwards, D.R. Hepatocyte growth factor/scatter factor enhances the invasion of mesothelioma cell lines and the expression of matrix metalloproteinases. Br. J. Cancer 2000, 83, 1147–1153. [Google Scholar] [CrossRef]
- To, Y.; Dohi, M.; Matsumoto, K.; Tanaka, R.; Sato, A.; Nakagome, K.; Nakamura, T.; Yamamoto, K. A two-way interaction between hepatocyte growth factor and interleukin-6 in tissue invasion of lung cancer cell line. Am. J. Resp. Cell. Mol. Biol. 2002, 27, 220–226. [Google Scholar] [CrossRef]
- Lee, K.H.; Hyun, M.S.; Kim, J.R. Growth factor-dependent activation of the MAPK pathway in human pancreatic cancer: MEK/ERK and p38 MAP kinase interaction in uPA synthesis. Clin. Exp. Metastasis 2003, 20, 499–505. [Google Scholar] [CrossRef]
- Kurogi, T.; Nabeshima, K.; Kataoka, H.; Okada, Y.; Koono, M. Stimulation of gelatinase B and tissue inhibitor of metalloproteinase (TIMP) production in co-culture of human osteosarcoma cells and human fibroblasts: Gelatinase B production was stimulated via up-regulation of fibroblast growth factor (FGF) receptor. Int. J. Cancer 1996, 66, 82–90. [Google Scholar] [CrossRef]
- Miyake, H.; Yoshimura, K.; Hara, I.; Eto, H.; Arakawa, S.; Kamidono, S. Basic fibroblast growth factor regulates matrix metalloproteinases production and in vitro invasiveness in human bladder cancer cell lines. J. Urol. 1997, 157, 2351–2355. [Google Scholar] [CrossRef]
- Hazan, R.B.; Phillips, G.R.; Qiao, R.F.; Norton, L.; Aaronson, S.A. Exogenous expression of N-Cadherin in breast cancer cells induces cell migration, invasion and metastasis. J. Cell Biol. 2000, 148, 779–790. [Google Scholar] [CrossRef]
- Suyama, K.; Shapiro, I.; Guttman, M.; Hazan, R.B. A signalling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell 2002, 2, 301–314. [Google Scholar]
- Sehgal, G.; Hua, J.; Bernhard, E.J.; Sehgal, I.; Thompson, T.C.; Muschel, R.J. Requirement for matrix metalloproteinase-9 (gelatinase B) expression in metastasis by murine prostate carcinoma. Am. J. Pathol. 1998, 152, 591–596. [Google Scholar]
- Siddiqui, F.A.; Siddiqui, T.F.; Francis, J.L. Haemoglobin induces the production and release of matrix metalloproteinase-9 from human malignant cells. Blood Coagul. Fibrinolysis 2003, 14, 449–455. [Google Scholar] [CrossRef]
- Masure, S.; Proost, P.; van Damme, J.; Opdenakker, G. Purification and identification of 91-kDa neutrophil gelatinase. Release by the activating peptide interleukin-8. Eur. J. Biochem. 1991, 198, 391–398. [Google Scholar] [CrossRef]
- Rehman, A.A.; Ahsan, H.; Kahn, F.H. α-2-Macroglobulin: A physiological guardian. J. Cell. Physiol. 2013, 228, 1665–1675. [Google Scholar] [CrossRef]
- Gomez, D.E.; Alonso, D.F.; Yoshiji, H.; Thorgeirsson, U.P. Tissue inhibitors of metalloproteinases: Structure, regulation and biological functions. Eur. J. Cell Biol. 1997, 74, 111–122. [Google Scholar]
- Murphy, G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011, 12, 233. [Google Scholar] [CrossRef]
- Roeb, E.; Schleinkofer, K.; Kernebeck, T.; Potsch, S.; Jensen, B.; Behrmann, I.; Matern, S.; Grotzinger, J. The matrix metalloproteinase-9 (mmp-9) hemopexin domain is a novel gelatin-binding domain and acts as an antagonist. J. Biol. Chem. 2002, 277, 50326–50332. [Google Scholar] [CrossRef]
- Farina, A.R.; Tacconelli, A.; Cappabianca, L.; Masciulli, M.P.; Holmgren, A.; Beckett, G.J.; Gulino, A.; Mackay, A.R. Thioredoxin alters the matrix metalloproteinase/tissue inhibitors of metalloproteinase balance and stimulates human SK-N-SH neuroblastoma cell invasion. Eur. J. Biochem. 2001, 268, 405–413. [Google Scholar] [CrossRef]
- Hahn-Dantona, E.; Ruiz, J.F.; Bornstein, P.; Strickland, D.K. The low-density lipoprotein receptor-related protein modulates levels of matrix metalloproteinase 9 (MMP-9) by mediating its cellular catabolism. J. Biol. Chem. 2001, 276, 15498–15503. [Google Scholar]
- Triebel, S.; Blaser, J.; Reinke, H.; Tschesche, H. A 25 kDa alpha 2-microglobulin-related protein is a component of the 125 kDa form of human gelatinase. FEBS Lett. 1992, 314, 386–388. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kaur, S.; Guha, S.; Batra, S.K. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim. Biophys. Acta 2012, 1826, 129–169. [Google Scholar]
- Ardi, V.C.; Kupriyanova, T.A.; Deryugina, E.L.; Quigley, J.P. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 20262–20267. [Google Scholar] [CrossRef]
- Ardi, V.C.; van den Steen, P.E.; Opdenakker, G.; Schweighofer, B.; Deryugina, E.I.; Quigley, J.P. Neutrophil MMP-9 proenzyme, unencumbered by TIMP-1, undergoes efficient activation in vivo and catalytically induces angiogenesis via a basic fibroblast growth factor (FGF-2)/FGFR-2 pathway. J. Biol. Chem. 2009, 284, 25854–25866. [Google Scholar]
- Huang, S.; van Arsdall, M.; Tedjarati, S.; McCarty, M.; Wu, W.; Langley, R.; Fidler, I.J. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J. Natl. Cancer Inst. 2002, 94, 1134–1142. [Google Scholar] [CrossRef]
- O’Grady, A.; Dunne, C.; O’Kelly, P.; Murphy, G.M.; Leader, M.; Kay, E. Differential expression of matrix metalloproteinase (MMP)-2, MMP-9 and tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2 in non-melanoma skin cancer: Implications for tumour progression. Histopathology 2008, 51, 793–804. [Google Scholar]
- Heissenberg, M.C.; Gorogh, T.; Lippert, B.M.; Werner, J.A. Metalloproteinases and their inhibitors in squamous cell carcinoma of the hypopharynx: Indicators of individual tumor aggressiveness. Otolaryngol. Pol. 1998, 52, 521–526. [Google Scholar]
- Roeb, E.; Dietrich, C.G.; Winograd, R.; Arndt, M.; Breuer, B.; Fass, J.; Schumpelick, V.; Matern, S. Activity and cellular origin of gelatinases in patients with colon and rectal carcinoma differential activity of matrix metalloproteinase-9. Cancer 2001, 92, 2680–2691. [Google Scholar] [CrossRef]
- Asai, M.; Kato, M.; Asai, N.; Iwashita, T.; Murakami, H.; Kawai, K.; Nakashima, I.; Takahashi, M. Differential regulation of MMP-9 and TIMP-2 expression in malignant melanoma developed in metallothionin/RET transgenic mice. Jpn. J. Cancer Res. 1999, 90, 86–92. [Google Scholar] [CrossRef]
- Dong, Z.; Nemeth, J.A.; Cher, M.L.; Palmer, K.C.; Bright, R.C.; Fridman, R. Differential regulation of matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1 (TIMP-1) and TIMP-2 expression in co-cultures of prostate cancer and stromal cells. Int. J. Cancer 2001, 93, 507–515. [Google Scholar] [CrossRef]
- Schonherr, E.; Schaefer, L.; O’Connel, B.C.; Kresse, H. Matrix metalloproteinase expression by endothelial cells in collagen lattices changes during co-culture with fibroblasts and upon induction of decorin expression. J. Cell Physiol. 2001, 187, 37–47. [Google Scholar] [CrossRef]
- Smola-Hess, S.; Schnitzler, R.; Hadaschik, D.; Smola, H.; Mauch, C.; Krieg, T.; Pfister, H. CD40L induces matrix metalloproteinase-9 but not tissue inhibitor of metalloproteinase-1 in cervical carcinoma cells: Imbalance between NF-kappaB and STAT3 activation. Exp. Cell Res. 2001, 267, 205–215. [Google Scholar] [CrossRef]
- O-charoenrat, P.; Rhys-Evans, P.; Court, W.J.; Box, G.M.; Eccles, S.A. Differential modulation of proliferation, matrix metalloproteinase expression and invasion of human head and neck squamous carcinoma cells by c-erbB ligands. Clin. Exp. Metastasis 1999, 17, 631–639. [Google Scholar] [CrossRef]
- Chang, X.Z.; Li, D.Q.; Hou, Y.F.; Wu, J.; Lu, J.S.; Di, G.H.; Jin, W.; Ou, Z.L.; Shen, Z.Z.; Shao, Z.M. Identification of the functional role of peroxiredoxin 6 in the progression of breast cancer. Breast Cancer Res. 2007, 9, R76. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Cho, C.S.; Jun, H.O.; Kim, D.H.; Yu, Y.S.; Kim, K.-W. Differential roles of matrix metalloproteinase-9 and -2, depending on proliferation or differentiation of retinoblastoma cells. Invest. Opthalmol. Vis. Sci. 2010, 51, 1783–1788. [Google Scholar] [CrossRef]
- Yu, G.; Wang, X.; Wu, T.; Zhu, J.; Huang, S.; Wan, Y.; Tang, J. MicroRNA-19a targets tissue factor to inhibit colon cancer cells migration and invasion. Mol. Cell. Biochem. 2013, 380, 239–247. [Google Scholar] [CrossRef]
- Li, S.; Guo, J.; Wu, J.; Sun, Z.; Han, M.; Shan, S.W.; Deng, Z.; Yang, B.B.; Weisel, R.D.; Li, R.K. miR-17 targets tissue inhibitor of metalloproteinase-1 and 2 to modulate cardiac matrix remodelling. FASEB J. 2013, 27, 4254–4265. [Google Scholar] [CrossRef]
- Oh, J.H.; Chung, A.S.; Steinbrenner, H.; Sies, H.; Brenneisen, P. Thioredoxin secreted upon ultraviolet A irradiation modulates the activities of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 in human dermal fibroblasts. Arch. Biochem. Biophys. 2004, 423, 218–226. [Google Scholar] [CrossRef]
- Shabani, F.; McNeil, J.; Tippett, L. The oxidative inactivation of tissue inhibitor of metalloproteinase-1 (TIMP-1) by hypochlorous acid (HOCl), is suppressed by anti-rheumatic drugs. Free Radic. Res. 1998, 28, 115–123. [Google Scholar] [CrossRef]
- Wang, Y.; Rosen, H.; Madtes, D.K.; Shao, B.; Martin, T.R.; Heinecke, J.W.; Fu, X. Myeloperoxidase inactivates TIMP-1 by oxidising its N-terminal Cystein residue. J. Biol. Chem. 2007, 282, 31826–31834. [Google Scholar] [CrossRef]
- Thompson, E.W.; Mackay, A.R. Review of: Opposing effects for specific TIMPs in breast cancer. Breast Cancer Online 2005, 8, e5. [Google Scholar]
- Okada, Y.; Watanabe, S.; Nakanishi, I.; Kishi, J.; Hayakawa, T.; Watorek, W.; Travis, J.; Nagase, H. Inactivation of tissue inhibitor of metalloproteinases by neutrophil elastase and other serine proteinases. FEBS Lett. 1988, 229, 157–160. [Google Scholar] [CrossRef]
- Ferry, G.; Lonchampt, M.; Pennel, L.; de Nanteil, G.; Canet, E.; Tucker, G.C. Activation of MMP-9 by neutrophil elastase in an in vivo model of acute lung injury. FEBS Lett. 1997, 402, 111–115. [Google Scholar] [CrossRef]
- Itkonen, O. Human trypsinogens in the pancreas and in cancer. Scand. J. Clin. Lab. Invest. 2010, 70, 136–143. [Google Scholar] [CrossRef]
- Tsai, J.R.; Wang, H.M.; Liu, P.L.; Chen, Y.H.; Yang, M.C.; Chou, S.H.; Cheng, Y.J.; Yin, W.H.; Hwang, J.J.; Chong, I.W. High expression of heme oxygenase-1 is associated with tumor invasiveness and poor clinical outcome in non-small cell lung cancer patients. Cell. Oncol. 2012, 35, 461–471. [Google Scholar] [CrossRef]
- Yeghiazaryan, M.; Zybura-Broda, K.; Cabaj, A.; Wlodarczyk, J.; Slaeinska, U.; Rylski, M.; Wilczynski, G.M. Fine-structural distribution of MMP-2 and MMP-9 activities in the rat skeletal muscle upon training: A study of high resolution in situ zymography. Histochem. Cell Biol. 2012, 138, 75–87. [Google Scholar] [CrossRef]
- Yang, Y.; Candelario-Jalil, E.; Thompson, J.F.; Cuadrado, E.; Estrada, E.Y.; Rosell, A.; Montaner, J.; Rosenberg, G.A. Increased intranuclear metalloproteinase activity in neurons interferes with oxidative DNA repair in focal cerebral ischemia. J. Neurochem. 2010, 112, 134–149. [Google Scholar] [CrossRef]
- Kwan, J.A.; Schulze, C.J.; Wang, W.; Leon, H.; Sariahmetoglu, M.; Sung, M.; Sawicka, J.; Simms, D.E.; Sawicki, G.; Schulz, R. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J. 2004, 18, 690–692. [Google Scholar]
- Hill, J.W.; Poddar, R.; Thompson, J.F.; Rosenberg, G.A.; Yang, Y. Intranuclear matrix metalloproteinases promote DNA damage and apoptosis induced by oxygen-glucose deprivation in neurons. Neuroscience 2012, 18, 277–290. [Google Scholar]
- Mannello, F.; Luchetti, F.; Falcieri, E.; Papa, S. Multiple roles of matrix metalloproteinases during apoptosis. Apoptosis 2005, 10, 19–24. [Google Scholar]
- Monferran, S.; Paupert, J.; Dauvillier, S.; Salles, B.; Muller, C. The membrane form of the DNA repair protein Ku interacts at the cell surface with metalloproteinase 9. EMBO J. 2004, 23, 3758–3768. [Google Scholar] [CrossRef]
- Sans-Fons, G.M.; Sole, S.; Sanfeliu, C.; Planas, A.M. Matrix metalloproteinase-9 and cell division in neuroblastoma cells and bone marrow macrophages. Am. J. Pathol. 2010, 177, 2870–2885. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.J.; Zhang, W.; Li, G.L.; Cui, Y.; Shi, Z.F.; Yuan, F. Differential expression of MMP-9 and AQP4 in human glioma samples. Folia Neuropathol. 2012, 50, 176–186. [Google Scholar]
- Moran, A.; Iniesta, P.; de Juan, C.; Gonzales-Quevedo, R.; Sanchez-Pernaute, A.; Diaz-Rubio, E.; Ramon y Cajal, S.; Torres, A.; Balibrea, J.L.; Benito, M. Stromelysin-1 promoter mutations impair gelatinase B activation in high microsatellite instability sporadic colorectal tumors. Cancer Res. 2002, 62, 3855–3860. [Google Scholar]
- Moran, A.; Iniesta, P.; de Juan, C.; Garcia-Aranda, C.; Benito, M. Impairment of stromelysin-1 transcriptional activity by promoter mutations in high microsatellite instability colorectal tumors. Cancer Res. 2005, 65, 3811–3814. [Google Scholar] [CrossRef]
- Thiefin, G.; Dupont, A.; Guillou, P.J.; Vitry, F.; Bouche, O.; Yaziji, N.; Lagarde, S.; Maquart, F.X.; Palot, J.P.; Hornebeck, W.; et al. Beneficial influence of microsatellite instability on gelatinase-tissue inhibitors of metalloproteinase balance in colorectal cancer. Anticancer Res. 2007, 27, 583–588. [Google Scholar]
- Sinnamon, M.J.; Carter, K.J.; Fingleton, B.; Matrisian, L.M. Matrix metalloproteinase-9 contributes to intestinal tumourigenesis in the adenomatous polyposis coli multiple intestinal neoplasia mouse. Int. J. Exp. Pathol. 2008, 89, 466–475. [Google Scholar] [CrossRef]
- Opdenakker, G.; van den Steen, P.E.; Dubois, B.; Nielssen, I.; van Coillie, E.; Masure, S.; Proost, P.; van Damme, J. Gelatinase B functions as regulator and effector in leukocyte biology. J. Leukoc. Biol. 2001, 69, 851–859. [Google Scholar]
- Radisky, D.C.; Levy, D.D.; Litllepage, L.E.; Liu, H.; Nelson, C.M.; Fata, J.E.; Leake, D.; Godden, E.L.; Albertson, D.G.; Nieto, M.A.; et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 2005, 436, 123–127. [Google Scholar] [CrossRef]
- Samper, E.; Nicholls, D.G.; Melov, S. Mitochondrial oxidative stress causes chromosomal instability of mouse embryonic fibroblasts. Aging Cell 2003, 2, 277–285. [Google Scholar] [CrossRef]
- Thieringer, F.R.; Maass, T.; Anthon, B.; Meyer, E.; Schirmacher, P.; Longerich, T.; Galle, P.R.; Kanzler, S.; Teufel, A. Liver-specific overexpression of matrix metalloproteinase 9 (MMP-9) in transgenic mice accelerates development of hepatocellular cancer. Mol. Carcinog. 2012, 51, 439–448. [Google Scholar] [CrossRef]
- Fatunmbi, M.; Shelton, J.; Aronica, S.M. gelatinase B/MMP-9 increases HER2/neu expression and alters apoptosis levels in human mammary epithelia cells. Breast Cancer Res. Treat. 2012, 135, 519–530. [Google Scholar] [CrossRef]
- Ponnala, S.; Veeravalli, K.K.; Chetty, C.; Dinh, D.H.; Rao, J.S. Regulation of DNA repair mechanism in human glioma xenograft cells both in vitro and in vivo in nude mice. PLoS One 2011, 6, e26191. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef]
- Bergers, G.; Brekken, R.; McMahon, G.; Vu, T.H.; Itoh, T.; Tamaki, K.; Tanzawa, K.; Thorpe, P.; Itohara, S.; Werb, Z.; et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell. Biol. 2000, 2, 737–744. [Google Scholar] [CrossRef]
- Belotti, D.; Paganoni, P.; Manetti, L.; Garofalo, A.; Marchini, S.; Taraboletti, G.; Giavazzi, R. Matrix metalloproteinases (MMP-9 and MMP-2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: Implications for ascites formation. Cancer Res. 2003, 63, 5224–5229. [Google Scholar]
- Mott, J.D.; Werb, Z. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 2004, 16, 558–564. [Google Scholar] [CrossRef]
- Brauer, P.R. MMPs—Role in cardiovascular development and disease. Front Biosci. 2006, 11, 447–478. [Google Scholar] [CrossRef]
- Rorive, S.; Berton, A.; D’haene, N.; Takacs, C.N.; Debeir, O.; Decaestecker, C.; Salmon, I. Matrix metalloproteinase-9 interplays with the IGFBP2-IGFII complex to promote cell growth and motility in astrocytomas. Glia 2008, 56, 1679–1690. [Google Scholar] [CrossRef]
- Alferez, D.; Wilkinson, R.W.; Watkins, J.; Poulsom, R.; Mandir, N.; Wedge, S.R.; Pyrah, I.T.; Smith, N.R.; Jackson, L.; Ryan, A.J.; et al. Dual inhibition of VEGFR and EGFR signalling reduces the incidence and size of intestinal adenomas in Apc(min/+) mice. Mol. Cancer Ther. 2008, 7, 590–598. [Google Scholar] [CrossRef]
- Ingraham, C.A.; Park, G.C.; Makarenkova, H.P.; Crossin, K.L. Matrix metalloproteinase (MMP)-9 induced by Wnt signalling increases the proliferation and migration of embryonic neural stem cells at low O2 levels. J. Biol. Chem. 2011, 286, 17649–17657. [Google Scholar]
- Aguilar-Gallardo, C.; Simon, C. Cells, stem cells and cancer stem cells. Semin. Reprod. Med. 2013, 31, 5–13. [Google Scholar] [CrossRef]
- Beck, B.; Blanpain, C. Unravelling cancer stem cell potential. Nat. Rev. Cancer 2013, 13, 727–738. [Google Scholar] [CrossRef]
- Santamaria-Martinez, A.; Huelsken, J. The niche under siege: Novel targets for metastasis therapy. J. Intern. Med. 2013, 274, 127–136. [Google Scholar] [CrossRef]
- Seidel, S.; Garvalov, B.K.; Wirta, W.; von Stechow, L.; Schanzer, A.; Meletis, K.; Wolter, M.; Sommerlad, D.; Henze, A.T.; Nister, M.; et al. A hypoxic niche regulates glioblastoma stem cells through hypoxia inducible factor 2 alpha. Brain 2010, 133, 983–995. [Google Scholar] [CrossRef]
- Wang, J.; Loberg, R.; Taichman, R.S. The pivotal role of CXCL12 (SDF-1)/CXCR4 axis in bone metastasis. Cancer Met. Rev. 2006, 25, 573–587. [Google Scholar]
- Wels, J.; Kaplan, R.N.; Rafii, S.; Lyden, D. Migratory neighbors and distant invaders: Tumor-associated niche cells. Genes Dev. 2008, 22, 559–574. [Google Scholar] [CrossRef]
- Chiang, A.C.; Massague, J. Molecular basis of metastasis. N. Engl. J. Med. 2008, 359, 2814–2823. [Google Scholar] [CrossRef]
- Van Amerongen, R.; Nusse, R. Towards an integrated view of Wnt signalling in development. Development 2009, 136, 3205–3214. [Google Scholar] [CrossRef]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2011, 414, 105–111. [Google Scholar]
- Brabletz, T.; Hlubek, F.; Spaderna, S.; Schmalhofer, O.; Hiendlmeyer, E.; Jung, A.; Kirchner, T. Invasion and metastasis in colorectal cancer: Epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and beta catenin. Cells Tissues Organs 2005, 179, 56–65. [Google Scholar] [CrossRef]
- Pardal, R.; Clarke, M.F.; Morrison, S.J. Applying principles of stem-cell biology to cancer. Nat. Rev. Cancer 2003, 3, 895–902. [Google Scholar] [CrossRef]
- Margetts, P.J. Twist: A new player in the epithelial-mesenchymal transition of the peritoneal mesothelial cells. Nephrol. Dial. Transplant. 2012, 27, 3978–3981. [Google Scholar] [CrossRef]
- Lin, C.Y.; Tsai, P.H.; Kandaswami, C.C.; Lee, P.P.; Huang, C.J.; Lee, M.T. Matrix metalloproteinase-9 cooperates with transcription factor snail to induce epithelial-mesenchymal transition. Cancer Sci. 2011, 102, 815–827. [Google Scholar] [CrossRef]
- Asuthkar, S.; Nalla, A.K.; Gondi, G.S.; Dinh, D.H.; Gujrati, M.; Mohanam, S.; Rao, J.S. Gadd45a sensitizes medulloblastoma cells to irradiation and suppresses MMP-9-mediated EMT. Neuro. Oncol. 2011, 13, 1059–1073. [Google Scholar] [CrossRef]
- Gao, X.H.; Yang, X.Q.; Wang, B.C.; Liu, S.P.; Wang, F.B. Overexpression of twist and matrix metalloproteinase-9 with metastasis and prognosis in gastric cancer. Asian Pac. J. Cancer Prev. 2013, 14, 5055–5060. [Google Scholar]
- Zhao, J.; Guan, J.L. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009, 28, 35–49. [Google Scholar] [CrossRef]
- Li, J.; Li, F.; Wang, H.; Wang, X.; Jiang, Y.; Li, D. Wortmannin reduces metastasis and angiogenesis of human breast cancer cells via nuclear factor-kappaB-dependent matrix metalloproteinase-9 and interleukin-8 pathways. J. Int. Med. Res. 2012, 40, 867–876. [Google Scholar] [CrossRef]
- Zhao, J.; Guan, J.L. Focal adhesion kinase and its signalling pathways in cell migration and angiogenesis. Adv. Drug Deliv. Rev. 2011, 63, 610–615. [Google Scholar] [CrossRef]
- Yoo, Y.A.; Kang, M.H.; Lee, H.J.; Kim, B.H.; Park, J.K.; Kim, H.K.; Kim, J.S.; Oh, S.C. Sonic hedgehog pathway promotes metastasis and lymphangiogenesis via activation of Akt, EMT, and MMP-9 pathway in gastric cancer. Cancer Res. 2011, 71, 7061–7070. [Google Scholar] [CrossRef]
- Opdenakker, G.; van den Steen, P.E.; van Damme, J. Gelatinase B: A tuner and amplifier of immune functions. Trends Immunol. 2001, 22, 571–579. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Patel, K.D. Regulation of matrix metalloproteinase release from IL-8-stimulated human neutrophils. J. Leukoc. Biol. 2005, 78, 279–288. [Google Scholar] [CrossRef]
- Chinni, S.R.; Sivalogan, S.; Dong, Z.; Filho, J.C.; Deng, X.; Bonfil, R.D.; Cher, M.L. CXCL12/CXCR4 signaling activates Akt and MMP-9 expression in prostate cancer cells: The role of bone microenvironment-associated CXCL12. Prostate 2006, 66, 32–48. [Google Scholar] [CrossRef]
- Kwak, Y.E.; Jeon, N.K.; Kim, J.; Lee, E.J. The cyclooxygenase-2 selective inhibitor celecoxib suppresses proliferation and invasiveness in the human oral squamous carcinoma. Ann. NY Acad. Sci. 2007, 1095, 99–112. [Google Scholar] [CrossRef]
- Ishizaki, T.; Katsumata, K.; Tsuchida, A.; Wada, T.; Mori, Y.; Hisada, M.; Kawakita, H.; Aoki, T. Etodolac, a selective cyclooxygenase-2 inhibitor, inhibits liver metastasis of colorectal cancer cells via the suppression of gelatinase B/MMP-9 activity. Int. J. Mol. Med. 2006, 17, 357–362. [Google Scholar]
- Kim, Y.H.; Kwon, H.J.; Kim, D.S. Matrix metalloproteinase 9 (MMP-9)-dependent processing of Big-h3 protein regulates cell migration, invasion, and adhesion. J. Biol. Chem. 2012, 287, 38957–38969. [Google Scholar] [CrossRef]
- Leifer, K.S.; Svensson, S.; Abrahamsson, A.; Bendrick, C.; Robertson, J.; Gauldie, J.; Olsson, A.-K.; Dabrosin, C. Inflammation induced by MMP-9 enhances tumor regression of experimental breast cancer. J. Immunol. 2013, 190, 4420–4430. [Google Scholar] [CrossRef]
- Farnsworth, R.H.; Lackmann, M.; Achen, M.G.; Stacker, S.A. Vascular remodelling in cancer. Oncogene 2013. [Google Scholar] [CrossRef]
- Van Hinsbergh, V.W.; Engelse, M.A.; Quax, P.H. Pericellular proteases in angiogenesis and vasculogenesis. Aterioscler. Thromb. Vasc. Biol. 2006, 26, 716–728. [Google Scholar] [CrossRef]
- Joyce, J.A. Therapeutic targeting of the tumor microenvironment. Cancer Cell 2005, 7, 513–520. [Google Scholar] [CrossRef]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef]
- Coussens, L.M.; Tinkle, C.L.; Hanahan, D.; Web, Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 2000, 103, 481–490. [Google Scholar] [CrossRef]
- Heissig, B.; Werb, Z.; Rafii, S.; Hattori, K. Role of c-kit/Kit ligand signalling in regulating vasculogenesis. Thromb. Haemost. 2003, 90, 570–576. [Google Scholar]
- Mira, E.; Lacalle, R.A.; Buesa, J.M.; de Buitrago, G.G.; Jimenez-Baranda, S.; Gòmez-Moutòn, C.; Mrtinaz, A.C.; Manes, S. Secreted MMP-9 promotes angiogenesis more efficiently than constitutive active MMP-9 bound to the tumour cell surface. J. Cell Sci. 2004, 117, 1847–1857. [Google Scholar] [CrossRef]
- Gao, D.; Nolan, D.; McDonnell, K.; Vahdat, L.; Benezra, R.; Attorki, N.; Mittal, V. Bone marrow-derived endothelial progenitor cells contribute to the angiogenic switch in tumor growth and metastatic progression. Biochim. Biophys. Acta 2009, 1796, 33–40. [Google Scholar]
- Giraudo, E.; Inoue, M.; Hanahan, D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical angiogenesis. J. Clin. Investig. 2004, 114, 623–633. [Google Scholar]
- Nosawa, H.; Chiu, C.; Hanahan, D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 12493–12498. [Google Scholar] [CrossRef]
- Melani, C.; Sangaletti, S.; Barazzetta, F.M.; Werb, Z.; Colombo, M.P. Amino-bisphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 2007, 67, 11438–11446. [Google Scholar] [CrossRef]
- Hagemann, T.; Robinson, S.C.; Schultz, M.; Trumper, L.; Balkwill, F.R.; Binder, C. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up-regulation of matrix metalloproteinases. Carcinogenesis 2004, 25, 1543–1549. [Google Scholar] [CrossRef]
- Morales, J.K.; Kmeiciak, M.; Knutson, K.L.; Bear, H.D.; Manjili, M.H. GM-CSF is one of the main breast tumor-derived soluble factors involved in the differentiation of CD11b-Gr1-bone marrow progenitor cells into myeloid-derived suppressor cells. Breast Cancer Res. Treat. 2010, 123, 39–49. [Google Scholar]
- Marigo, I.; Dolcetti, L.; Serafini, P.; Zanovello, P.; Bronte, V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol. Rev. 2008, 222, 162–179. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Urbich, C.; Dimmeler, S. Endothelial progenitor cells: Characterisation and role in vascular biology. Circ. Res. 2004, 95, 343–353. [Google Scholar] [CrossRef]
- Rafii, S.; Lyden, D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat. Med. 2003, 9, 702–712. [Google Scholar] [CrossRef]
- Inoue, T.; Taguchi, I.; Abe, S.; Toyoda, S.; Nakajima, K.; Sakuma, M.; Node, K. Activation of matrix metalloproteinase-9 is associated with mobilization of bone marrow-derived cells after coronary stent implantation. Int. J. Cardiol. 2011, 152, 332–336. [Google Scholar] [CrossRef]
- Inoue, T.; Sata, M.; Hikichi, Y.; Sohma, R.; Fukuda, D.; Uchida, T.; Shimizu, M.; Komoda, H.; Node, K. Mobilization of CD34-positive bone marrow-derived cells after coronary stent implantation: Impact on restenosis. Circulation 2007, 115, 553–561. [Google Scholar] [CrossRef]
- Jodele, S.; Chantrain, C.F.; Blavier, L.; Lutzko, C.; Crooks, G.M.; Shimada, H.; Coussens, L.M.; DeClerck, Y.A. The contribution of bone marrow-derived cells to the tumor vasculature in neuroblastoma is matrix metalloproteinase-9 dependent. Cancer Res. 2005, 65, 3200–3208. [Google Scholar]
- Tacconelli, A.; Farina, A.R.; Cappabianca, L.; de Santis, G.; Tessitore, A.; Vetuschi, A.; Sferra, R.; Rucci, N.; Argenti, B.; Screpanti, I.; et al. TrkA alternative splicing: A regulated tumor-promoting switch in human neuroblastoma. Cancer Cell 2004, 6, 347–360. [Google Scholar] [CrossRef]
- Chantrain, C.F.; Shimada, H.; Jodele, S.; Groshen, S.; Ye, W.; Shalinsky, D.R.; Werb, Z.; Coussens, L.M.; DeClerck, Y.A. Stromal matrix metalloproteinase-9 regulates the vascular architecture in neuroblastoma by promoting pericyte recruitment. Cancer Res. 2004, 64, 1675–1686. [Google Scholar] [CrossRef]
- Nielsen, B.S.; Sehested, M.; Kjeldsen, L.; Borregaard, N.; Rygaard, J.; Dano, K. Expression of matrix metalloproteinase-9 in vascular pericytes in human breast cancer. Lab. Invest. 1997, 77, 345–355. [Google Scholar]
- Vu, T.H.; Shipley, J.M.; Bergers, G.; Berger, J.E.; Helms, J.A.; Hanahan, D.; Shapiro, S.D.; Senior, R.M.; Werb, Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 1998, 93, 411–422. [Google Scholar] [CrossRef]
- Ahn, G.O.; Brown, J.M. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: Role of bone marrow-derived myelomonocytic cells. Cancer Cell 2008, 13, 195–205. [Google Scholar]
- Johnson, C.; Sung, H.J.; Lessner, S.M.; Fini, M.E.; Galis, Z.S. Matrix metalloproteinase-9 is required for adequate angiogenic revascularization of ischemic tissues: Potential role in capillary branching. Circ. Res. 2004, 94, 262–268. [Google Scholar] [CrossRef]
- Shekhar, M.P.; Werdell, J.; Santner, S.J.; Pauley, R.J.; Tait, L. Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: Implications for tumor development and progression. Cancer Res. 2001, 61, 1320–1326. [Google Scholar]
- Nakamura, T.; Kuwai, T.; Kim, J.S.; Fan, D.; Kim, S.J.; Fidler, I.J. Stromal metalloproteinase-9 is essential to angiogenesis and progressive growth of orthotopic human pancreatic cancer in parabiont nude mice. Neoplasia 2007, 9, 979–986. [Google Scholar] [CrossRef]
- He, J.Z.; Quan, A.; Xu, Y.; Teoh, H.; Wang, G.; Fish, J.E.; Steer, B.M.; Itohara, S.; Marsden, P.A.; Davidge, S.T.; et al. Induction of matrix metalloproteinase-2 enhances systemic arterial contraction after hypoxia. Am. J. Physiol. 2007, 292, 684–693. [Google Scholar]
- Nakano, D.; Hyashi, T.; Tazawa, N.; Yamashita, C.; Inamoto, S.; Okuda, N.; Mori, T.; Sohmiya, K.; Kitaura, Y.; Okada, Y.; et al. Chronic hypoxia accelerates the progression of atherosclerosis in apoliprotein E-knockout mice. Hypertens. Res. 2005, 28, 837–845. [Google Scholar] [CrossRef]
- Zalba, G.; Fortuno, A.; Orbe, J.; San Jose, G.; Moreno, M.U.; Belzunce, M.; Rodriguez, J.A.; Beloqui, O.; Paramo, J.A.; Diez, J. Phagocytic NADPH oxidase-dependent superoxide production stimulates metalloproteinase-9: Implications for human atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 587–593. [Google Scholar] [CrossRef]
- Jadhav, U.; Chigurupati, S.; Lakka, S.S.; Mohanam, S. Inhibition of matrix metalloproteinase-9 reduces in vitro invasion and angiogenesis in human microvascular endothelial cells. Int. J. Oncol. 2004, 25, 1407–1414. [Google Scholar]
- Tallant, C.; Marrero, A.; Gomis-Ruth, F.X. Matrix metalloproteinases: Fold and function of their catalytic domains. Biochim. Biophys. Acta 2010, 1803, 20–28. [Google Scholar] [CrossRef]
- Li, H.; Liang, J.; Castrillon, D.H.; DePinho, R.A.; Olson, E.N.; Liu, Z.P. FoxO4 regulates tumor necrosis factor alpha-directed smooth muscle cell migration by activating matrix metalloproteinase 9 gene expression. Mol. Cell. Biol. 2007, 27, 2676–2686. [Google Scholar] [CrossRef]
- Chandrasekar, B.; Mummidi, S.; Mahimainathan, L.; Patel, D.N.; Bailey, S.R.; Imam, S.Z.; Greene, W.C.; Valente, A.J. Interleukin-18-induced human coronary artery smooth muscle cell migration is dependent on NF-kappaB and AP-1-mediated matrix metalloproteinase-9 expression and is inhibited by atorvastatin. J. Biol. Chem. 2006, 281, 15099–15109. [Google Scholar] [CrossRef]
- Cheng, G.; Wei, L.; Xiurong, W.; Xiangzhen, L.; Shiguang, Z.; Songbin, F. IL-17 stimulates migration of carotid artery smooth muscle cells in an MMP-9 dependent manner via p38 MAPK and ERK1/2-dependent NF-KappaB and AP-1 activation. Cell. Mol. Neurobiol. 2009, 29, 1161–1168. [Google Scholar] [CrossRef]
- Galis, Z.S.; Johnson, C.; Godin, D.; Magid, R.; Shipley, J.M.; Senior, R.M.; Ivan, E. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodelling. Circ. Res. 2002, 91, 852–859. [Google Scholar] [CrossRef]
- Jenkins, G.M.; Crow, M.T.; Bilato, C.; Gluzband, Y.; Ryu, W.S.; Li, Z.; Stetler-Stevenson, W.; Nater, C.; Froehlich, J.P.; Lakatta, E.G.; et al. Increased expression of membrane type matrix metalloproteinase and preferential localization of matrix metalloproteinase-2 to the neointima of balloon-injured rat carotid arteries. Circulation 1998, 97, 82–90. [Google Scholar] [CrossRef]
- Xu, J.; Rodriguez, D.; Petitclerc, E.; Kim, J.J.; Hangai, M.; Moon, Y.S.; Davis, G.E.; Brooks, P.C. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J. Cell Biol. 2001, 154, 1069–1080. [Google Scholar] [CrossRef]
- Hamano, Y.; Zeisberg, M.; Sugimoto, H.; Lively, J.C.; Maeshima, Y.; Yang, C.; Hynes, R.O.; Werb, Z.; Sudhakar, A.; Kalluri, R. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell 2003, 3, 589–601. [Google Scholar] [CrossRef]
- Wahl, M.L.; Kenan, D.J.; Gonzalez-Gronow, M.; Pizzo, S.V. Angiostatin’s molecular mechanism: Aspects of specificity and regulation elucidated. J. Cell. Biochem. 2005, 96, 242–261. [Google Scholar] [CrossRef]
- Kalhuri, R. Basement membranes: Structure, assembly and role in tumor angiogenesis. Nat. Rev. Cancer 2003, 3, 422–433. [Google Scholar] [CrossRef]
- Sudhakar, A.; Sugimoto, H.; Yang, C.; Lively, J.; Zeisberg, M.; Kalluri, R. Human tumstatin and human endostatin exhibit distinct anti-angiogenic activities mediated by alpha V beta 3 and alpha 5 beta 1 integrins. Proc. Natl. Acad. Sci. USA 2003, 100, 4766–4771. [Google Scholar]
- Kim, Y.M.; Jang, J.W.; Lee, O.H.; Yeon, J.; Choi, E.Y.; Kim, K.W.; Lee, S.T.; Kwon, Y.G. Endostatin inhibits endothelial and tumor cellular invasion by blocking the activation and catalytic activity of matrix metalloproteinase. Cancer Res. 2000, 60, 5410–5413. [Google Scholar]
- Nyberg, P.; Heikkila, P.; Sorsa, T.; Luostarinen, J.; Heljasvaara, R.; Stenman, U.H.; Pihlajaniemi, T.; Salo, T. Endostatin inhibits human tongue carcinoma cell invasion and intravasation and blocks the activation of matrix metalloproteinase-2, -9, and -13. J. Biol. Chem. 2003, 278, 22404–22411. [Google Scholar] [CrossRef]
- Guan, K.P.; Ye, H.Y.; Yan, Z.; Wang, Y.; Huo, S.K. Serum levels of endostatin and matrix metalloproteinase-9 associated with high stage and grade primary transitional cell carcinoma of the bladder. Urology 2003, 61, 719–723. [Google Scholar] [CrossRef]
- Ezhilarasan, R.; Jadhav, U.; Mohaman, I.; Rao, J.S.; Gujrati, M.; Mohaman, S. The hemopexin domain of MMP-9 inhibits angiogenesis and retards the growth of intracranial glioblastoma xenograft in nude mice. Int. J. Cancer 2009, 124, 306–315. [Google Scholar] [CrossRef]
- Qian, X.; Wang, T.N.; Rothman, V.L.; Nicosia, R.F.; Tuszynski, G.P. Thrombospondin-1 modulates angiogenesis in vitro by up-regulation of matrix metalloproteinase-9 in epithelial cells. Exp. Cell Res. 1997, 235, 403–412. [Google Scholar] [CrossRef]
- Asuthkar, S.; Velpula, K.K.; Nalla, A.K.; Gogineni, V.R.; Gondi, C.S.; Rao, J.S. Irradiation-induced angiogenesis is associated with an MMP-9-miR-494-syndecan-1 regulatory loop on medulloblastoma cells. Oncogene 2013. [Google Scholar] [CrossRef]
- Wang, X.; Lee, S.O.; Xia, S.; Jiang, Q.; Luo, J.; Li, L.; Yeh, S.; Chang, C. Endothelial cells enhance prostate cancer metastasis via IL-6-androgen receptor-TGF-β-MMP-9 signals. Mol. Cancer Ther. 2013, 12, 1026–1037. [Google Scholar] [CrossRef]
- Loukovaara, S.; Robciuc, A.; Holopainen, J.M.; Lehti, K.; Pessi, T.; Liinamaa, J.; Kukkonen, K.T.; Jauhiainen, M.; Koli, K.; Keski-Oja, J.; et al. Ang-2 upregulation correlates with increased levels of MMP-9, VEGF, EPO and TGFβ in diabetic eyes undergoing vitrectomy. Acta Opthalmol. 2013, 91, 531–539. [Google Scholar] [CrossRef]
- Hiratsuka, S.; Nakamura, K.; Iwai, S.; Murakami, M.; Itoh, T.; Kijima, H.; Shipley, J.M.; Senior, R.M.; Shibuya, M. MMP9 induction by vascular endothelial cell growth factor receptor-1 is involved in lung specific metastasis. Cancer Cell 2002, 2, 289–300. [Google Scholar]
- Wang, H.; Keiser, J.A. Vascular endothelial growth factor upregulates the expression of matrix metalloproteinases in vascular smooth muscle cells: Role of flt-1. Circ. Res. 1998, 83, 832–840. [Google Scholar] [CrossRef]
- Ghosh, S.; Basu, M.; Roy, S.S. ETS-1 protein regulates vascular endothelial cell growth factor-induced matrix metalloproteinase-9 and matrix metalloproteinase-13 expression in human ovarian carcinoma cell line SKOV-3. J. Biol. Chem. 2012, 287, 15001–15015. [Google Scholar] [CrossRef]
- Christoffersson, G.; Vagesjo, E.; Vandooren, J.; Liden, M.; Massena, S.; Reinert, R.B.; Brissova, M.; Powers, A.C.; Opdenakker, G.; Phillipson, M. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood 2012, 120, 4653–4662. [Google Scholar] [CrossRef]
- Lungu, G.; Covaleda, L.; Mendes, O.; Martini-Stoica, H.; Stoica, G. FGF-1-induced matrix metalloproteinase-9 expression in breast cancer cells is mediated by increased activities of NF-kappaB and activating protein-1. Mol. Carcinogen. 2008, 47, 424–435. [Google Scholar] [CrossRef]
- Mohan, R.; Sivak, J.; Ashton, P.; Russo, L.A.; Pham, B.Q.; Kasahara, N.; Raizman, M.B.; Fini, E.M. Curcuminoids inhibit the angiogenic response stimulated by fibroblast growth factor-2, including expression of matrix metalloproteinase gelatinase B. J. Biol. Chem. 2000, 275, 10405–10412. [Google Scholar]
- Tang, L.; Ma, X.; Tian, Q.; Cheng, Y.; Yao, H.; Liu, Z.; Qu, X.; Han, X. Inhibition of angiogenesis and invasion by DMBT is mediated by down regulation of VEGF and MMP-9 through Akt pathway in MDA-MB-231 breast cancer cells. Food Chem. Toxicol. 2013, 56, 204–213. [Google Scholar] [CrossRef]
- Xu, Y.B.; Du, Q.H.; Zhang, M.Y.; Yun, P.; He, C.Y. Propofol suppresses proliferation, invasion and angiogenesis by down regulating ERK/VEGF/MMP-9 signaling in Eca-109 esophageal squamous cell carcinoma cells. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2486–2494. [Google Scholar]
- Gorantla, B.; Bhoopathi, P.; Chetty, C.; Gogineni, V.R.; Sailaja, G.S.; Gondi, C.S.; Rao, J.S. Notch signalling regulates tumor-induced angiogenesis in SPARC overexpressed neuroblastoma. Angiogenesis 2013, 16, 85–100. [Google Scholar] [CrossRef]
- Jia, W.; Gao, X.J.; Zhang, Z.X.; Zhang, G. S100A4 silencing suppresses proliferation, angiogenesis and invasion of thyroid cancer cells through down regulation of MMP-9 and VEGF. Eur. Rev. Med. Pharm. Sci. 2013, 17, 1495–1508. [Google Scholar]
- Yi, E.Y.; Kim, Y.J. Xylitol inhibits in vitro and in vivo angiogenesis by suppressing the NF-κB and Akt signalling pathways. Int. J. Oncol. 2013, 43, 315–320. [Google Scholar]
- Xu, J.; Zhu, D.; Sonoda, S.; He, S.; Spee, C.; Ryan, S.J.; Hinton, D.R. Over-expression of BMP4 inhibits choroidal neovascularization by modulating VEGF and MMP-9. Angiogenesis 2012, 15, 213–227. [Google Scholar] [CrossRef]
- Suboj, P.; Babykutty, S.; Valiyaparambil Gopi, D.R.; Nair, R.S.; Srinivas, P.; Gopala, S. Aloe emodin inhibits colon cancer cell migration/angiogenesis by down regulating MMP-2/9, RhoB and VEGF via reduced DNA binding activity of NF-κB. Eur. J. Pharm. Sci. 2012, 45, 581–591. [Google Scholar] [CrossRef]
- Hendrix, M.J.C.; Seftor, E.A.; Meltzer, P.S.; Gardner, L.M.G.; Hess, A.R.; Kirschmann, D.A.; Schatteman, G.C.; Seftor, R.E.B. Expression and function of VE-cadherin in aggressive human melanoma cells: Role invasculogenic mimicry. Proc. Natl. Acad. Sci. USA 2001, 98, 8018–8023. [Google Scholar] [CrossRef]
- Karoum, A.; Mirshahi, P.; Faussat, A.-M.; Therwath, A.; Mirshahi, M.; Hatmi, M. Tubular network formation by adriamycin-resistant MCF-7 breast cancer cells is closely linked to MMP-9 and VEGFR-2/VEGFR-3 over-expression. Mol. Cell. Pharmacol. 2012, 685, 1–7. [Google Scholar]
- Wong, S.Y.; Hynes, R.O. Lymphatic or hematogenous dissemination: How does a metastatic tumor cell decide? Cell Cycle 2006, 5, 812–817. [Google Scholar] [CrossRef]
- Christiansen, A.; Detmar, M. Lymphangiogenesis and cancer. Genes Cancer 2011, 2, 1146–1158. [Google Scholar] [CrossRef]
- Rutkowski, J.M.; Boardman, K.C.; Swarz, M.A. Characterisation of lymphangiogenesis in a model of adult skin regeneration. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, 1402–1410. [Google Scholar] [CrossRef]
- Tan, K.W.; Chong, S.Z.; Wong, F.H.; Evrard, M.; Tan, S.M.; Keeble, J.; Kemeny, M.D.; Ng, L.G.; Abastado, J.P.; Angeli, V. Neutrophils contribute to inflammatory lymphangiogenesis by increasing VEGF-A bioavailability and secreting VEGF-D. Blood 2013, 122, 3666–3677. [Google Scholar] [CrossRef]
- Zheng, S.Q.; Huang, R.Q.; Zhang, Y.J. Role of matrix metalloproteinases (MMP)-2 and -9 and vascular endothelial growth factor C in lymph node metastasis of breast cancer. Zhonghua Bing Li Xue Za Zhi 2010, 39, 240–244. [Google Scholar]
- Elston, C.W.; Ellis, I.O. Pathological prognostic factors in breast cancer. 1. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 2002, 41, 154–161. [Google Scholar] [CrossRef]
- Peterson, O.W.; Ronnov-Jessen, L.; Howlett, A.R.; Bissel, M.J. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl. Acad. Sci. USA 1992, 89, 9064–9068. [Google Scholar] [CrossRef]
- Redondo-Munoz, J.; Ugarte-Berzal, E.; Garcia-Marco, J.A.; del Cerro, M.H.; van den Steen, P.E.; Opdenakker, G.; Terol, M.J.; Garcia-Pardo, A. Alpha4Beta1 integrin and 190-kDa CD44v constitute a cell surface docking complex fro gelatinase B/MMP-9 in chronic leukemia but not in normal B cells. Blood 2008, 112, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Brooks, P.C.; Silletti, S.; von Schalscha, T.L.; Friedlander, M.; Cheresh, D. Disruption of angiogenesis by Pex, a non catalytic fragment with integrin binding activity. Cell 1998, 92, 683–693. [Google Scholar]
- Dufour, A.; Sampson, N.S.; Zucker, S.; Cao, J. Role of the hemopexin domain of matrix metalloproteinases in cell migration. J. Cell Physiol. 2008, 217, 643–651. [Google Scholar] [CrossRef]
- Paupert, J.; Mansat-De Mas, V.; Demur, C.; Salles, B.; Muller, C. Cell-surface MMP-9 regulates the invasive capacity of leukemia blast cells with Monocytic features. Cell Cycle 2008, 7, 1047–1053. [Google Scholar] [CrossRef]
- Redondo-Munoz, J.; Ugarte-Berzal, E.; Terol, M.J.; van den Steen, P.E.; Hernandez del Cerro, M.; Roderfeld, M.; Roeb, E.; Opdenakker, G.; Garcia-Marco, J.A.; Garcia-Pardo, A. Matrix metalloproteinase-9 promotes chronic lymphocytic leukemia B cell survival through its hemopexin domain. Cancer Cell 2010, 17, 160–172. [Google Scholar] [CrossRef]
- Hu, X.; Paik, P.K.; Chen, J.; Yarilina, A.; Kockeritz, L.; Lu, T.T.; Woodgett, J.R.; Ivashkiv, L.B. IFN-gamma suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity 2006, 24, 563–574. [Google Scholar] [CrossRef]
- Rao, J.S.; Bhoopathi, P.; Chetty, C.; Gujrati, M.; Lakka, S.S. MMP-9 short interfering RNA induced senescence resulting in inhibition of medulloblastoma growth via p16 (INK4a) and mitogen-activated protein kinase pathway. Cancer Res. 2007, 67, 4956–4964. [Google Scholar] [CrossRef]
- Bhoopathi., P.; Chetty, C.; Kunigal., S.; Vanamala, S.K.; Rao, J.S.; Lakka, S.S. Blockage of tumour growth due to matrix metalloproteinase-9 inhibition is mediated by sequential activation of β1-integrin, ERK, and NF-κB. J. Biol. Chem. 2008, 283, 1545–1552. [Google Scholar]
- Wheeler, D.L.; Dunn, E.F.; Harari, P.M. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat. Rev. Clin. Oncol. 2010, 7, 493–507. [Google Scholar] [CrossRef]
- Frame, M.C.; Patel, H.; Serrels, B.; Lietha, D.; Eck, M.J. The FERM domain: Organizing the structure and function of FAK. Nat. Rev. Mol. Cell. Biol. 2010, 11, 802–814. [Google Scholar] [CrossRef]
- Stuelten, C.H.; DaCosta Byfield, S.; Arany, P.R.; Karpova, T.S.; Stetler-Stevenson, W.G.; Roberts, A.B. Breast cancer cells induce stromal fibroblasts to express MMP-9 via secretion of TNF-alpha and TGF-beta. J. Cell Sci. 2005, 118, 2143–2153. [Google Scholar] [CrossRef]
- Mook, O.R.; Frederiks, W.M.; van Noorden, C.J. The role of gelatinases in colorectal cancer progression and metastasis. Biochim. Biophys. Acta 2004, 1705, 69–89. [Google Scholar]
- Jandova, J.; Janda, J.; Sligh, J.E. Changes in mitochondrial DNA alter expression of nuclear encoded genes associated with tumorigenesis. Exp. Cell. Res. 2012, 318, 2215–2225. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef]
- Sabeh, F.; Shimizu-Hirota, R.; Weiss, S.J. Protease-dependent versus -ndependent cancer cell invasion programs: Three-dimensional amoeboid movement revisited. J. Cell Biol. 2009, 185, 11–19. [Google Scholar] [CrossRef]
- Legrand, C.; Gilles, C.; Zahm, J.M.; Polette, M.; Buisson, A.C.; Kaplan, H.; Birembaut, P.; Tournier, J.M. Airway epithelial cell migration dynamics. MMP-9 role in cell-extracellular matrix remodelling. J. Cell Biol. 1999, 146, 517–529. [Google Scholar] [CrossRef]
- Rolli, M.; Fransvea, E.; Pilch, J.; Saven, A.; Felding-Habermann, B. Activated integrin aplphavbeta3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 9482–9487. [Google Scholar]
- Shibata, K.; Kikkawa, F.; Nawa, A.; Thant, A.A.; Naruse, K.; Mizutani, S.; Hamaguchi, M. Both focal adhesion kinase and c-Ras are required for the enhanced matrix metalloproteinase 9 secretion by fibronectin in ovarian cancer cells. Cancer Res. 1998, 58, 900–903. [Google Scholar]
- Ozanne, B.W.; McGarry, L.; Spence, H.J.; Johnston, I.; Winnie, J.; Meagher, L.; Stapleton, G. Transcriptional regulation of cell invasion: AP-1 regulation of a multigenic invasion programme. Eur. J. Cancer 2000, 36, 1640–1648. [Google Scholar] [CrossRef]
- Shin, E.Y.; Ma, E.K.; Kim, C.K.; Kwak, S.J.; Kim, E.G. Src/ERK but not phospholipase D is involved in keratinocyte growth factor-stimulated secretion of matrix metalloproteinase-9 and urokinase-type plasminogen activator in SNU-16 human stomach cancer cells. J. Cancer Res. Clin. Oncol. 2002, 128, 596–602. [Google Scholar] [CrossRef]
- Thomas, G.J.; Poomsawat, S.; Lewis, M.P.; Hart, I.R.; Speight, P.M.; Marshall, J.F. Alpha v beta 6 integrin upregulates matrix metalloproteinase 9 and promotes migration of normal oral keratinocytes. J. Invest. Dermatol. 2001, 11, 898–904. [Google Scholar]
- Thomas, G.J.; Lewis, M.P.; Hart, I.R.; Marshall, J.F.; Speight, P.M. AlphaVbeta6 integrin promotes invasion of squamous carcinoma cells through up-regulation of matrix metalloproteinase-9. Int. J. Cancer 2001, 92, 641–650. [Google Scholar] [CrossRef]
- Sil, H.; Sen, T.; Chatterjee, A. Fibronectin-integrin (alpha5beta1) modulates migration and invasion of murine melanoma cell line B16F10 by involving MMP-9. Oncol. Res. 2011, 19, 335–348. [Google Scholar] [CrossRef]
- Mackay, A.R.; Gomez, D.E.; Nason, A.M.; Thorgeirsson, U.P. Sudies on the effects of laminin, E-8 fragment of laminin and synthetic laminin peptides PA22–2 and YIGRS on matrix metalloproteinase and tissue inhibitor of metalloproteinase expression. Lab. Invest. 1994, 70, 800–806. [Google Scholar]
- Kahn, K.M.; Falcone, D.J. Role of laminin in matrix induction of macrophage urokinase-type plasminogen activator and 92-kDa metalloproteinase expression. J. Biol. Chem. 1997, 272, 8270–8275. [Google Scholar] [CrossRef]
- Anderson, R.B. Matrix metalloproteinase-2 is involved in the migration and network formation of enteric neural crest-derived cells. Int. J. Dev. Biol. 2010, 54, 63–69. [Google Scholar] [CrossRef]
- Fortier, A.M.; Asselin, E.; Cadrin, M. Keratin 8 and 18 loss in epithelial cancer cells increases collective cell migration and cisplatin sensitivity through claudin up-regulation. J. Biol. Chem. 2013, 288, 11555–11571. [Google Scholar] [CrossRef]
- Xu, D.; McKee, C.M.; Cai, Y.; Ding, Y.; Kessler, B.M.; Muschel, R.J. Matrix metalloproteinase-9 regulates tumor cell invasion through cleavage of protease nexin-1. Cancer Res. 2010, 70, 6988–6998. [Google Scholar] [CrossRef]
- Pal-Ghosh, S.; Blanco, T.; Tadvalkar, G.; Pajoohesh-Ganji, A.; Parthasarathy, A.; Zieske, J.D.; Stepp, M.A. MMP-9 cleavage of the β4 integrin ectodomain leads to recurrent epithelial erosions in mice. J. Cell Sci. 2011, 124, 2666–2675. [Google Scholar] [CrossRef]
- Taddei, M.L.; Parri, M.; Angelucci, A.; Bianchini, F.; Marconi, C.; Giannoni, E.; Raugei, G.; Bologna, M.; Calorini, L.; Chiarugi, P. EphA2 induces metastatic growth regulating amoeboid motility and clonogenic potential in prostate carcinoma cells. Mol. Cancer Res. 2011, 9, 149. [Google Scholar] [CrossRef]
- Wyckoff, J.; Wang, W.; Lin, E.Y.; Wang, Y.; Pixley, F.; Stanley, E.R.; Graf, T.; Pollard, J.W.; Segall, J.; Condeelis, J. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004, 64, 7022–7029. [Google Scholar] [CrossRef]
- Gherardi, E.; Birchmeier, W.; Birchmeier, C.; vande Woude, G. Tageting MET in cancer: Rational and progress. Nat. Rev. Cancer 2012, 12, 89–103. [Google Scholar] [CrossRef]
- Wang, M.; Qin, X.; Mudgett, J.S.; Ferguson, T.A.; Senior, R.M.; Welgus, H.G. Matrix metalloproteinase deficiencies affect contact hypersensitivity: Stromelysin-1 deficiency prevents the response and gelatinase B deficiency prolongs the response. Proc. Natl. Acad. Sci. USA 1999, 96, 6885–6889. [Google Scholar]
- Creighton, C.; Hanash, S. Expression of matrix metalloproteinase 9 (MMP-9/gelatinase B) in adenocarcinomas strongly correlated with expression of immune response genes. In Silico Biol. 2003, 3, 301–311. [Google Scholar]
- Zhu, X.S.; Shi, W.; An, G.Y.; Zhang, H.M.; Song, Y.G.; Li, Y.B. Matrix metalloproteinase-9 was involved in the immune-modulatory defect of mesenchymal stem cell from chronic myeloid leukemia patients. Chin. Med. J. 2011, 124, 2423. [Google Scholar]
- Bratcher, P.E.; Weathington, N.M.; Nick, H.J.; Jackson, P.L.; Snelgrove, R.J.; Gaggar, A. Gelatinase B/MMP-9 cleaves SP-D and abrogates its innate immune functions in vitro. PLoS One 2012, 7, e41881. [Google Scholar]
- Geiger, T.; Rordorf, C.; Galakatos, N.; Seligmann, B.; Henn, R.; Lazdins, J.; Vosbeck, K. Recombinant human C5a induces transcription but not translation of interleukin-1 beta mRNA in human monocytes. Res. Immunol. 1992, 143, 117–123. [Google Scholar] [CrossRef]
- Wu, G.; Chen, T.; Shahsafaei, A.; Hu, W.; Bronson, R.T.; Shi, G.P.; Halperin, J.A.; Aktas, H.; Qin, X. Complement regulator CD59 protects against angiotensin II-induced abdominal aortic aneurisms in mice. Circulation 2010, 121, 1338–1346. [Google Scholar] [CrossRef]
- Voskoboinik, I.; Dunstone, M.A.; Baran, K.; Whisstock, J.C.; Trapani, J.A. Perforin: Structure function and role in human immunopathology. Immunol. Rev. 2010, 235, 35–54. [Google Scholar]
- Nishikawa, H.; Sakaguchi, S. Regulatory T cells in tumor immunity. Int. J. Cancer 2010, 127, 759–767. [Google Scholar]
- Wang, J.; Ke, X.Y. The four types of Tregs in malignant lymphomas. J. Hematol. Oncol. 2011, 4, 50. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, W.; Strasner, A.; Grivennikov, S.; Cheng, J.Q.; Hoffman, R.M.; Karin, M. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL.RANK signalling. Nature 2011, 470, 548–553. [Google Scholar] [CrossRef]
- Shah, W.; Yan, X.; Jing, L.; Zhou, Y.; Chen, H.; Wang, Y. A reversed CD4/CD8 ratio of tumor-infiltrating lymphocytes and a high percentage of CD4(+)FOXP3(+) regulatory T cells are significantly associated with clinical outcome in squamous cell carcinoma of the cervix. Cell. Mol. Immunol. 2011, 8, 59–66. [Google Scholar]
- Piersma, S.J.; Welters, M.J.; van den Burg, S.H. Tumor-specific regulatory T cells in cancer patients. Hum. Immunol. 2008, 69, 241–249. [Google Scholar] [CrossRef]
- Kim, J.; Yu, W.; Kovalski, K.; Ossowski, L. Requirement for specific proteases in cancer cell intravasation as revealed by a novel semi-quantitative PCR-based assay. Cell 1998, 94, 353–362. [Google Scholar] [CrossRef]
- Bekes, E.M.; Schweighofer, B.; Kupriyanova, T.A.; Zajac, E.; Ardi, V.C.; Quigley, J.P.; Deryugina, E.L. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate co-ordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am. J. Pathol. 2011, 179, 1455–1470. [Google Scholar] [CrossRef]
- Cho, K.; Matsuda, Y.; Ueda, J.; Uchida, E.; Naito, Z.; Ishiwata, T. Keratinocyte growth factor induces matrix metalloproteinase-9 expression and correlates with venous invasion in pancreatic cancer. Int. J. Oncol. 2012, 40, 1040–1048. [Google Scholar]
- Li, J.; Sun, R.; Tao, K.; Wang, G. The CCL21/CCR7 pathway plays a key role in human colon cancer metastasis through regulation of matrix metalloproteinase-9. Dig. Liver Dis. 2011, 43, 40–47. [Google Scholar] [CrossRef]
- Sugino, T.; Kusakabe, T.; Hoshi, N.; Yamaguchi, T.; Kawaguchi, T.; Goodison, S.; Sekimata, M.; Homma, Y.; Suzuki, T. An invasion-independent pathway of blood-borne metastasis: A new murine mammary tumor model. Am. J. Pathol. 2002, 160, 1973–1980. [Google Scholar] [CrossRef]
- Mook, O.R.; van Marle, J.; Vreeling-Sindelarova, H.; Jonges, R.; Frederiks, W.M.; van Noorden, C.J. Visualization of early events in tumor formation of eGFP-transfected rat colon cancer cells in liver. Hepatology 2003, 38, 295–304. [Google Scholar]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef]
- Rundhaug, J.E. Matrix metalloproteinases and angiogenesis. J. Cell Mol. Med. 2005, 9, 267–285. [Google Scholar] [CrossRef]
- Kopp, H.G.; Ramos, C.A.; Rafii, S. Contribution of endothelial progenitors and proangiogenic haematopoietic cells to vascularization of tumors and ischemic tissue. Curr. Opin. Hematol. 2006, 13, 175–181. [Google Scholar] [CrossRef]
- Kollet, O.; Dar, A.; Shivtiel, S.; Kalinkovich, A.; Lapid, K.; Sztainberg, Y.; Tesio, M.; Samstein, R.M.; Goichberg, P.; Spiegel, A.; et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat. Med. 2006, 12, 657–664. [Google Scholar] [CrossRef]
- Chen, X.; Su, Y.; Fingleton, B.; Acuff, H.; Matrisian, L.M.; Zent, R.; Pozzi, A. An orthotopic model of lung cancer to analyse primary and metastatic NSCL growth in integrin alpha1-null mice. Clin. Exp. Metastasis 2005, 22, 185–193. [Google Scholar] [CrossRef]
- Kaplan, R.N.; Psaila, B.; Lyden, D. Niche-to-niche migration of bone-marrow-derived cell. Trends Mol. Med. 2007, 13, 72–81. [Google Scholar] [CrossRef]
- Van Kempen, L.C.L.; Coussens, L.M. MMP-9 potentiates pulmonary metastasis formation. Cancer Cell 2002, 2, 251–252. [Google Scholar] [CrossRef]
- Grange, C.; Tapparo, M.; Collino, F.; Vitillo, L.; Damasco, C.; Deregibus, M.C.; Tetta, C.; Bussolati, B.; Camussi, G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung pre-metastatic niche. Cancer Res. 2011, 71, 5346–5356. [Google Scholar] [CrossRef]
- Van Deventer, H.W.; Palmieri, D.A.; Wu, Q.P.; McCook, E.C.; Serody, J.S. Circulating fibrocytes prepare the lung for cancer metastasis by recruiting Ly-6C+ monocytes via CCL2. J. Immunol. 2013, 190, 4861–4867. [Google Scholar] [CrossRef]
- Kucia, M.; Reca, R.; Miekus, K.; Wanzeck, J.; Wojakowski, W.; Janowska-Wieczorek, A.; Ratajczak, J.; Ratajczak, M.Z. Trafficking of normal stem cells and cancer stem cells involve similar mechanisms: Pivotal role for the SDF-1/CXCR4 axis. Stem Cells 2005, 23, 879–894. [Google Scholar] [CrossRef]
- Kaplan, R.N.; Rifil, S.; Lyden, D. Preparing the “soil”: The pre-metastatic niche. Cancer Res. 2006, 66, 11089–11093. [Google Scholar] [CrossRef]
- Mannello, F.; Tonti, G.A.; Bagnara, G.P.; Papa, S. Role and function of matrix metalloproteinases in the differentiation and biological characterisation of mesenchymal stem cells. Stem Cells 2005, 24, 475–481. [Google Scholar]
- Lee, R.; Kermani, P.; Teng, K.K.; Hempstead, B.L. Regulation of cell survival by secreted proneurotrophins. Science 2001, 294, 1945–1948. [Google Scholar] [CrossRef]
- Vaillant, C.; Meissirel, C.; Mutin, M.; Belin, F.; Lund, L.R.; Thomasset, N. MMP-9 deficiency affects axonal outgrowth, migration, and apoptosis in the developing cerebellum. Mol. Cell. Neurosci. 2003, 24, 395–408. [Google Scholar] [CrossRef]
- Chintala, S.K.; Zhang, X.; Austin, J.S.; Fini, M.E. Deficiency in matrix metalloproteinase gelatinase B (MMP-9) protects against retinal ganglion cell death after optic nerve ligation. J. Biol. Chem. 2002, 277, 47461–47468. [Google Scholar] [CrossRef]
- Gazzanelli, G.; Luchetti, F.; Burattini, S.; Mannello, F.; Falcieri, E.; Papa, S. Matrix metalloproteinases expression in HL-60 promyelocytic leukaemia cell during apoptosis. Apoptosis 2000, 5, 165–172. [Google Scholar] [CrossRef]
- Moshal., K.S.; Tipparaju, S.M.; Vacek, T.P.; Kumar, M.; Singh, M.; Franke, I.E.; Patibandla, P.K.; Tyagi, N.; Rai, J.; Metreveli, N.; et al. Mitochondrial matrix metalloproteinase activation decreases myocyte contractility in hyperhomocysteinemia. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H890–H897. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Mohammad, G.; dos Santos, J.M.; Zhong, Q. Abrogation of gelatinase B/MMP-9 gene protects against the development of retinopathy in diabetic mice by preventing mitochondrial damage. Diabetes 2011, 60, 3023–3033. [Google Scholar] [CrossRef]
- Overchin, A.V.; Tyagi, N.; Rodriguez, W.E.; Hyden, M.R.; Moshal, K.S.; Tyagi, S.C. Role of matrix metalloproteinase-9 in endothelial apoptosis in chronic heart failure in mice. J. Appl. Physiol. 2005, 99, 2398–2405. [Google Scholar] [CrossRef]
- Chetty, C.; Lakka, S.S.; Bhoopathi, P.; Gondi, C.S.; Veeravalli, K.K.; Fassett, D.; Klopfenstein, J.D.; Dinh, D.H.; Gujrati, M.; Rao, J.S. Urokinase plasminogen activator and /or matrix metalloproteinase-9 inhibition induces apoptosis signalling through lipid rafts in glioblastoma xenograft cells. Mol. Cancer Ther. 2010, 9, 2605–2617. [Google Scholar] [CrossRef]
- Shchors, K.; Nozawa, H.; Xu, J.; Rostker, F.; Swigart-Brown, L.; Evan, G.; Hanahan, D. Increased invasiveness of MMP-9-deficient tumors in two mouse models of neuroendocrine tumorigenesis. Oncogene 2013, 32, 502–513. [Google Scholar] [CrossRef]
- Deryugina, E.L.; Quigley, J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef]
- Acuff, H.B.; Carter, K.J.; Fingleton, B.; Gorden, D.L.; Matrisian, L.M. Matrix metalloproteinase-9 from bone marrow-derived cells contributes to survival but not growth of tumor cells in lung microenvironment. Cancer Res. 2006, 66, 259–266. [Google Scholar] [CrossRef]
- Itoh, T.; Tanioka, M.; Matsuda, H.; Nishimoto, H.; Yoshioka, T.; Suzuki, R.; Uehira, M. Experimental metastasis is suppressed in MMP-9-deficient mice. Clin. Exp. Metastasis 1999, 17, 177–181. [Google Scholar] [CrossRef]
- Pozzi, A.; Moberg, P.E.; Miles, L.A.; Wagner, S.; Soloway, P.; Gardner, H.A. Elevated matrix metalloproteinase and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc. Natl. Acad. Sci. USA 2000, 97, 2202–2207. [Google Scholar]
- Pozzi, A.; LeVine, W.F.; Gardner, H.A. Low plasma levels of matrix metalloproteinase 9 permit increased tumor angiogenesis. Oncogene 2002, 21, 272–281. [Google Scholar] [CrossRef]
- Chen, X.; Su, Y.; Fingleton, B.; Acuff, H.; Matrisian, L.M.; Zent, R.; Pozzi, A. Increased plasma MMP9 in integrin alpha1-null mice enhances lung metastasis of colon carcinoma cells. Int. J. Cancer 2005, 116, 52–61. [Google Scholar] [CrossRef]
- Buck, T.B.; Yoshiji, H.; Harris, S.R.; Bunce, O.R.; Thorgeirsson, U.P. The effects of sustained elevated levels of circulating tissue inhibitor of metalloproteinase-1 on the development of breast cancer in mice. Ann. NY Acad. Sci. USA 1999, 878, 732–735. [Google Scholar] [CrossRef]
- De Lornzo, M.S.; Ripoll, G.V.; Yoshiji, H.; Yamazaki, M.; Thorgeirsson, U.P.; Alonso, D.F.; Gomez, D.E. Altered tumor angiogenesis and metastasis of B16 melanoma in transgenic mice overexpressing tissue inhibitor of metalloproteinase-1. In Vivo 2003, 17, 45–50. [Google Scholar]
- Yoshiji, H.; Kuriyama, S.; Miyamoto, Y.; Thorgeirsson, U.P.; Gomez, D.E.; Kawata, M.; Yoshii, J.; Ikenaka, Y.; Noguchi, R.; Tsujinoue, H.; et al. Tissue inhibitor of metalloproteinase-1 promotes liver fibrosis development in a transgenic mouse model. Hepatology 2000, 32, 1248–1254. [Google Scholar] [CrossRef]
- Dziembowska, M.; Wlodarczyk, J. MMP9: Novel function in synaptic plasticity. Int. J. Biochem. Cell Biol. 2012, 44, 709–713. [Google Scholar] [CrossRef]
- Zucker, S.; Cao, J.; Chen, W.-T. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene 2001, 19, 6642–6650. [Google Scholar] [CrossRef]
- Pavlaki, M.; Zucker, S. Matrix metalloproteinase inhibitors (MMPIs): The beginning of phase I or the termination of phase III clinical trials. Cancer Metastasis Rev. 2003, 22, 177–203. [Google Scholar] [CrossRef]
- Overall, C.M.; Kleifeld, O. Validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat. Rev. Cancer 2006, 6, 227–239. [Google Scholar] [CrossRef]
- McCawley, L.J.; Wright, J.; LaFleur, B.J.; Crawford, H.C.; Matrisian, L.M. Keratinocyte expression of MMP-3 enhances differentiation and prevents tumor establishment. Am. J. Pathol. 2008, 173, 1528–1529. [Google Scholar] [CrossRef]
- McCawley, L.J.; Crawford, H.C.; King, L.E., Jr.; Mudgett, J.; Matrisian, L.M. A protective role for matrix metalloproteinase-3 in squamous cell carcinoma. Cancer Res. 2004, 64, 6965–6972. [Google Scholar]
- Kopitz, C.; Gerg, M.; Bandapalli, O.R.; Ister, D.; Pennington, C.J.; Hauser, S.; Flecgsig, C.; Krell, H.W.; Antolovic, D.; Brew, K.; et al. Tissue inhibitor of metalloproteinases-1 promotes liver metastasis by inducing hepatocyte growth factor signalling. Cancer Res. 2007, 67, 8615–8623. [Google Scholar] [CrossRef]
- Wolf, K.; Mazo, I.; Leung, H.; Engelke, K.; von Andrian, U.H.; Deryugina, E.L.; Strongin, A.Y.; Brocker, E.B.; Friedl, P. Compensation mechanism in tumor cell migration: Mesenchymal-amoeboid transition after blocking of peri-cellular proteolysis. J. Cell Biol. 2003, 160, 267–277. [Google Scholar] [CrossRef]
- Hu, J.; van den Steen, P.E.; Sang, Q.X.; Opdenakker, G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat. Rev. Drug Discov. 2007, 6, 480–498. [Google Scholar] [CrossRef]
- Martens, E.; Leyssen, A.; van Aelst, I.; Fiten, P.; Piccard, H.; Hu, J.; Descamps, F.J.; van den Steen, P.E.; Proost, P.; van Damme, J.; et al. A monoclonal antibody inhibits gelatinase B/MMP-9 by selective binding to part of the catalytic domain and not to the fibronectin or zinc binding domains. Biochim. Biophys. Acta 2007, 1770, 178–186. [Google Scholar] [CrossRef]
- Stefanidakis, M.; Karjalainen, K.; Jaalouk, D.E.; Gahmberg, C.G.; O’Brien, S.; Pasqualini, R.; Arap, W.; Koivunen, E. Role of leukemia cell invadosome in extramedullary infiltration. Blood 2009, 114, 3008–3017. [Google Scholar] [CrossRef]
- Suojanen, J.; Vilen, S.-T.; Nyberg, P.; Heikkila, P.; Penate-Medina, O.; Saris, P.E.J.; Hagstrom, J.; Ranta, T.-M.; Salo, T.; Sorsa, T.; et al. Selective gelatinase inhibitor peptide is effective in targeting tongue carcinoma cell tumors in vivo. Anticancer Res. 2011, 31, 3659–3664. [Google Scholar]
- Burg-Roderfeld, M.; Roderfeld, M.; Wagner, S.; Henkel, C.; Grotzinger, J.; Roeb, E. MMP-9-hemopexin domain hampers adhesion and migration of colorectal cancer cells. Int. J. Oncol. 2007, 30, 985–992. [Google Scholar]
- Urgate-Berzal, E.; Bailon, E.; Amigo-Jiminez, I.; Vituri, C.L.; Hernandez del Cerro, M.; Terol, M.J.; Albar, J.P.; Rivas, G.; Barcia-Marco, J.A.; Garcia-Pardo, A. A 17-residue sequence from the matrix metalloproteinase-9 (MMP-9) hemopexin domain binds α4β1 integrin and inhibits MMP-9-induced functions in chronic Lymphocytic Leukaemia B cells. J. Biol. Chem. 2012, 287, 27601–27613. [Google Scholar] [CrossRef] [Green Version]
- Bjorklund, M.; Heikkila, P.; Koivunen, E. Peptide inhibition of catalytic and non-catalytic activities of matrix metalloproteinase-9 blocks tumor cell migration and invasion. J. Biol. Chem. 2004, 279, 29589–29597. [Google Scholar] [CrossRef]
- Vandooren, J.; Geurts, N.; Opdenakker, G. Gelatin degradation assay reveals MMP-9 inhibitors and function of O-glycosylated domain. World J. Biol. Chem. 2011, 26, 14–24. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Farina, A.R.; Mackay, A.R. Gelatinase B/MMP-9 in Tumour Pathogenesis and Progression. Cancers 2014, 6, 240-296. https://doi.org/10.3390/cancers6010240
Farina AR, Mackay AR. Gelatinase B/MMP-9 in Tumour Pathogenesis and Progression. Cancers. 2014; 6(1):240-296. https://doi.org/10.3390/cancers6010240
Chicago/Turabian StyleFarina, Antonietta Rosella, and Andrew Reay Mackay. 2014. "Gelatinase B/MMP-9 in Tumour Pathogenesis and Progression" Cancers 6, no. 1: 240-296. https://doi.org/10.3390/cancers6010240




